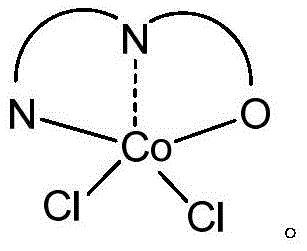
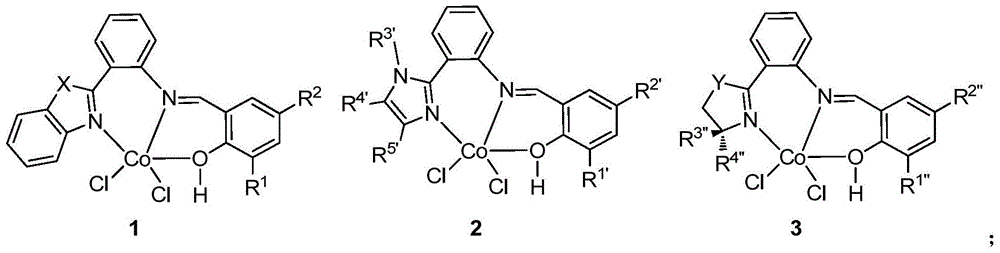
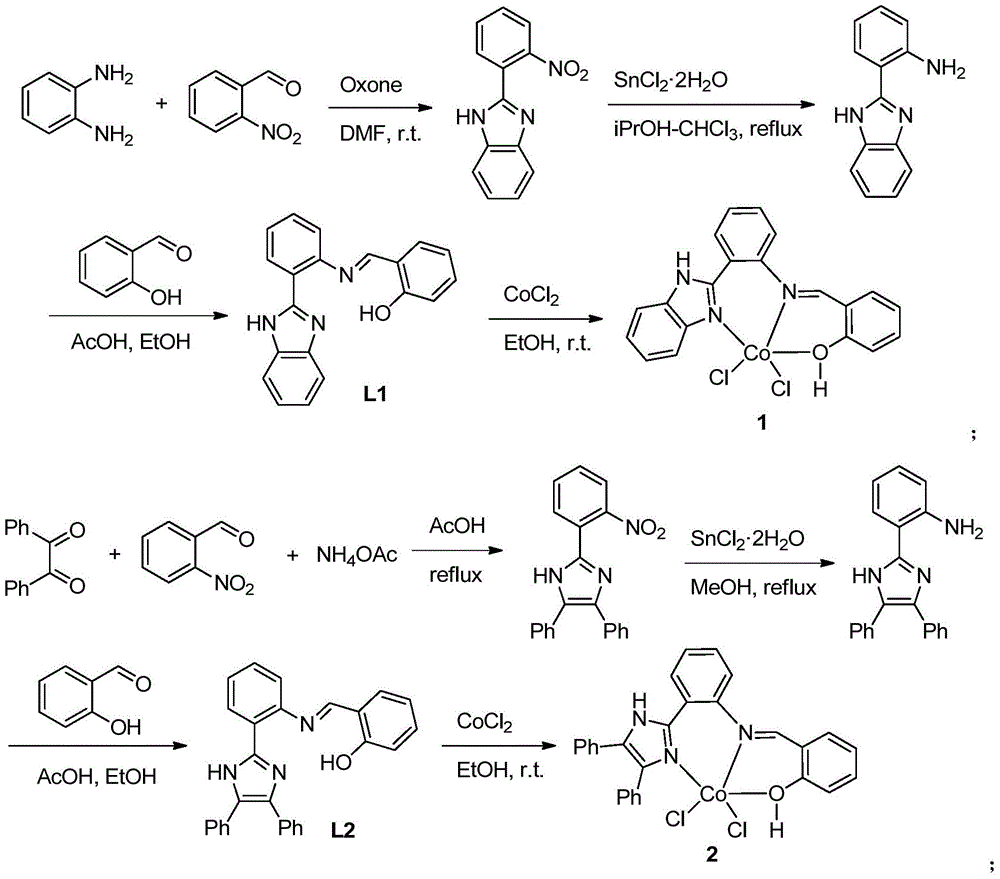
A kind of cobalt series catalyst and its application in 1,3-butadiene polymerization reaction
A polymerization reaction and catalyst technology, which is applied to cobalt-based catalysts and its application in 1,3-butadiene polymerization, can solve the problems of polybutadiene molecular weight and molecular weight distribution that are not easy to control, and achieve excellent catalytic performance. Active, low price, low environmental requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. The preparation of 2-(2-nitrophenyl)benzimidazole: Add o-nitrobenzaldehyde (3.045g, 20.1mmol), potassium hydrogen persulfate (3.381g, 11.0mmol), o-benzene Diamine (1.984g, 18.4mmol) and solvent 50ml DMF gave a yellow transparent liquid, and the reaction mixture was stirred at room temperature for 2h. Then add K dropwise 2 CO 3 (1M, 25mL) and distilled water (250mL), a milky white solid precipitated. Then, it was extracted three times with ethyl acetate (3 x 50 mL), and the organic layer was separated. They were then washed once with distilled water and twice with saturated saline. After drying with anhydrous sodium sulfate, filter and remove the solvent to obtain a brown solid crude product, wash it on a silica gel column with chloroform, collect and remove the solvent to obtain a light brown solid, which is 2-(2-nitrophenyl) Benzimidazole (3.266 g), 72.4% yield.
[0051] 1 HNMR (400MHz, CDCl 3 ):δ8.16(dd,J 1 =7.6Hz,J 2 =1.2Hz,1H,Ar-H),7.97(dd,J 1 =7.6Hz,J ...
Embodiment 2
[0063] 1. The preparation of complex 1 is the same as in Example 1.
[0064] 2. Polymerization of butadiene solution: under the protection of nitrogen, take complex 1 (2.2 mg, 5.0 μmol) in a polymerization reaction bottle, seal it with a rubber stopper, vacuumize and vent nitrogen for three times, and add 3.4 ml of purified toluene solvent . Then, add cocatalyst sesquiethylaluminum chloride (EASC, 0.4mol / L normal hexane solution) 1.0ml with syringe, make [Al] / [Co] be 160, this catalyst composition is stirred at 30 ℃ for two Minutes, then add 5.6ml of butadiene solution (1.85mol / L toluene solution) with a syringe, make [BD] / [Co] be 2000 (molar ratio, the same below), and the polymerization reaction starts. Unless otherwise stated, the total volume of the polymerization reaction was controlled at 10 ml by adjusting the amount of solvent toluene. Two hours later, the reaction solution was poured into 200ml of terminator to terminate the polymerization, and the terminator was 5%...
Embodiment 3
[0066] 1. The preparation of complex 1 is the same as in Example 1.
[0067] 2. Polymerization of butadiene solution: under the protection of nitrogen, take complex 1 (2.2 mg, 5.0 μmol) in a polymerization reaction bottle, seal it with a rubber stopper, vacuumize and vent nitrogen for three times, and add 3.9 ml of purified toluene solvent . Then, add cocatalyst sesquiethylaluminum chloride (EASC, 0.4mol / L normal hexane solution) 0.5ml with syringe, make [Al] / [Co] be 80, this catalyst composition is stirred at 30 ℃ for two Minutes, then add 5.6ml of butadiene solution (1.85mol / L toluene solution) with a syringe, make [BD] / [Co] be 2000 (molar ratio, the same below), and the polymerization reaction starts. Unless otherwise stated, the total volume of the polymerization reaction was controlled at 10 ml by adjusting the amount of solvent toluene. Two hours later, the reaction solution was poured into 200ml of terminator to terminate the polymerization, and the terminator was 5% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com