Cobalt catalyst and application thereof in 1,3-butadiene polymerization reaction
A polymerization reaction and catalyst technology, which is applied to cobalt-based catalysts and the application field of cobalt-based catalysts in 1,3-butadiene polymerization, can solve problems such as transition metal complexes that have not yet been found, and achieve broad industrial application prospects, The effect of high product cis selectivity and excellent catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
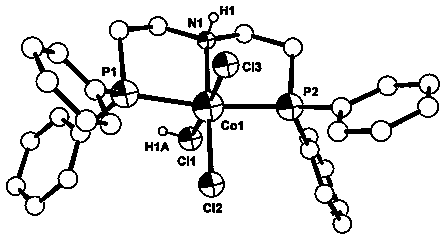
[0072] 1. Bis[2-(diphenylphosphino)ethyl]amine hydrochloride (ligand L1·HCl ) preparation: under nitrogen protection, diphenylphosphine (2.8 ml, 3.0 g, 16.1 mmol), potassium tert-butoxide (4.68 g, 41.7 mmol) were added into dry tetrahydrofuran (100 ml), stirred for 20 minutes, The reaction solution changed from dark red to orange red, and then powdered bis(2-chloroethyl)amine hydrochloride (1.42 g, 8.0 mmol) was added, and heated to reflux for 28 hours. Then add 100 ml of 1 mol / L dilute hydrochloric acid solution, separate the organic layer, dry with anhydrous sodium sulfate, remove the solvent to obtain a white precipitate, and the separated white precipitate is recrystallized from dichloromethane-ether and washed with acetonitrile , and dried to obtain 1.605 g of white solid with a yield of 42%.
[0073] FT-IR (KBr disc, cm -1 ): 3422 (ν N–H ), 1585, 1480, 1433, 1388, 748, 738, 695, 513.
[0074] 1 H NMR (400 MHz, CDCl 3 ), δ (ppm): 9.95 (s, 2 H, N H 2 Cl), 7.38–...
Embodiment 2
[0081] 1. Complex 1a The preparation is with embodiment 1.
[0082] 2. Butadiene solution polymerization: under the protection of nitrogen, take the complex 1a (3.0 mg, 5.0 μmol) in a polymerization vial, sealed with a rubber stopper, vacuumed and vented with nitrogen three times, and dissolved by adding 4.34 ml of purified toluene. Then, 0.063 ml of cocatalyst sesquiethylaluminum chloride (EASC, 0.4 mol / L n-hexane solution) was added with a syringe, so that [Al] / [Co] was 10, and the catalyst composition was stirred at 25 °C After two minutes, 5.6 ml of butadiene (1.78 mol / L toluene solution) was added with a syringe, so that [BD] / [Co] was 2000, and the polymerization reaction started. Unless otherwise stated, the total volume of the polymerization reaction was controlled at 10 ml by adjusting the amount of solvent toluene. After one hour, the reaction solution was poured into 200 ml of terminator to terminate the polymerization. The terminator was 5% hydrochloric acid eth...
Embodiment 3
[0084] 1. Complex 1a The preparation is with embodiment 1.
[0085] 2. Butadiene solution polymerization: under the protection of nitrogen, take the complex 1a (3.0 mg, 5.0 μmol) in a polymerization vial, sealed with a rubber stopper, vacuumed and vented with nitrogen three times, and dissolved by adding 4.275 ml of purified toluene. Then, 0.125 ml of cocatalyst sesquiethylaluminum chloride (EASC, 0.4 mol / L n-hexane solution) was added with a syringe, so that [Al] / [Co] was 20, and the catalyst composition was stirred at 25 °C After two minutes, 5.6 ml of butadiene (1.78 mol / L toluene solution) was added with a syringe, so that [BD] / [Co] was 2000, and the polymerization reaction started. Unless otherwise stated, the total volume of the polymerization reaction was controlled at 10 ml by adjusting the amount of solvent toluene. After one hour, the reaction solution was poured into 200 ml of terminator to terminate the polymerization. The terminator was 5% hydrochloric acid et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com