Chiral platinum complex and preparation method thereof
A platinum complex and chiral technology, applied in the application field of chiral platinum complexes and their preparation, and preparation of anti-tumor drugs, achieving strong anti-tumor activity, great application potential, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] One, preparation method of the present invention:
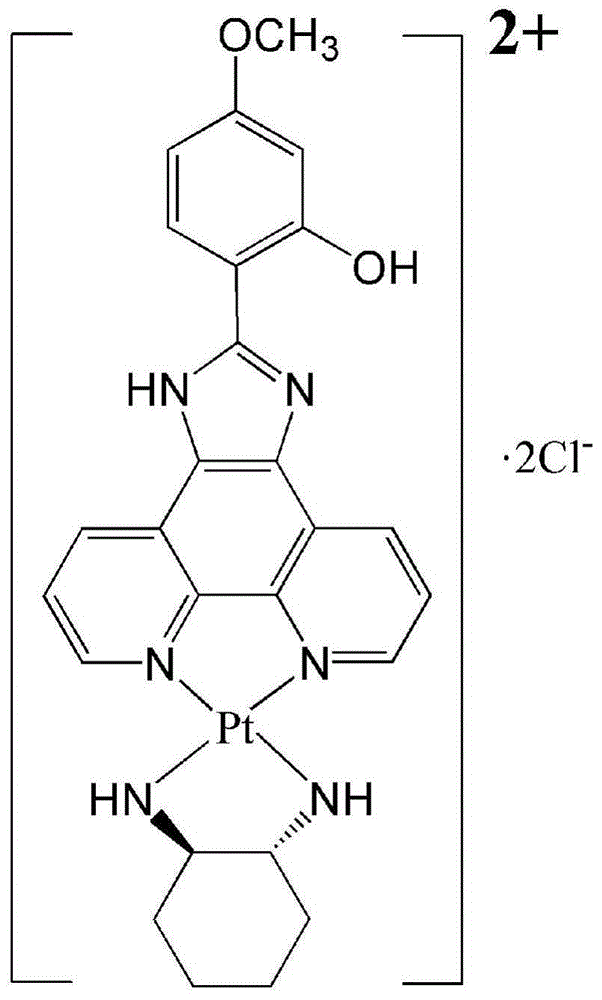
[0040](1) Preparation of compound 2: Weigh 2 mol of phenanthroline-5,6-dione (compound 1) and 2 mol of 2-hydroxy-4-methoxybenzaldehyde, add glacial acetic acid 16mL and ammonium acetate 3.1 g, under 120°C oil bath, heat to reflux for 6h, cool to room temperature; under ice bath conditions, stir, slowly add 16mL of concentrated ammonia water (25-28%) to neutrality to obtain 2-[2-(hydroxyl) - yellow precipitate of 4-(methoxy)-phenyl]imidazo[4,5-f][1,10]-phenanthroline (compound 2);
[0041] (2) Preparation of complex 3: Prepare potassium chloroplatinite solution in advance, add 0.8mL water to 2.4mmol potassium chloroplatinite, heat to dissolve at 60°C, add 2mL dimethyl sulfoxide, heat and stir at 60°C for 5min That's it.
[0042] Add 10mL of dimethyl sulfoxide to 2.4mmol of compound 2, heat the oil bath to 140°C to dissolve compound 2, slowly drop the pre-prepared potassium chloroplatinite solution into the compound 2 ...
Embodiment 2
[0064] One, preparation method of the present invention:
[0065] (1) Preparation of compound 2: Weigh 2 mol of phenanthroline-5,6-dione (compound 1) and 4 mol of 2-hydroxy-4-methoxybenzaldehyde, add glacial acetic acid 20mL and ammonium acetate 3.6 g, in an oil bath at 130°C, heat to reflux for 7 hours, and cool to room temperature; in an ice bath, stir, and slowly add 20 mL of concentrated ammonia water (25-28%) dropwise to neutral, and a yellow precipitate (compound 2) is obtained;
[0066] (2) Preparation of complex 3: Prepare potassium chloroplatinite solution in advance, add 1 mL of water to 2.4 mmol potassium chloroplatinite, heat to dissolve at 50 ° C, add 3 mL of dimethyl sulfoxide, heat and stir at 50 ° C for 10 min Can.
[0067] Add 12mL of dimethyl sulfoxide to 2.4mmol of compound 2, heat the oil bath to 150°C to dissolve compound 2, slowly drop the pre-prepared potassium chloroplatinite solution into the compound 2 solution, and a yellow precipitate precipitates ...
Embodiment 3
[0083] One, preparation method of the present invention:
[0084] (1) Preparation of compound 2: Weigh 2 mol of phenanthroline-5,6-dione (compound 1) and 4 mol of 2-hydroxy-4-methoxybenzaldehyde, add glacial acetic acid 18mL and ammonium acetate 3.6 g, in an oil bath at 125°C, heated to reflux for 5h, cooled to room temperature; in an ice bath, stirred, and slowly added 18mL of concentrated ammonia water (25-28%) dropwise until neutral, and a yellow precipitate (compound 2) was obtained;
[0085] (2) Preparation of complex 3: Prepare potassium chloroplatinite solution in advance, add 1 mL of water to 2.4 mmol potassium chloroplatinite, heat to dissolve at 55 ° C, add 2.5 mL of dimethyl sulfoxide, heat and stir at 55 ° C for 8 min That's it.
[0086] Add 11mL of dimethyl sulfoxide to 2.4mmol of compound 2, heat the oil bath to 145°C to dissolve compound 2, slowly drop the pre-prepared potassium chloroplatinite solution into the compound 2 solution, and a yellow precipitate pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com