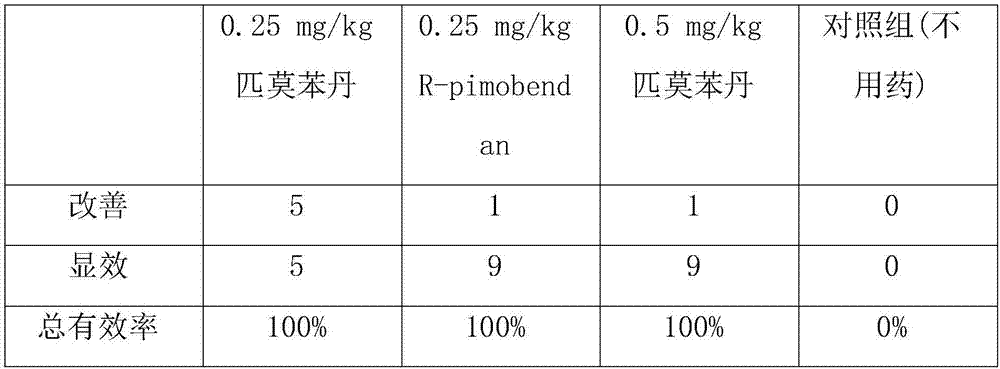
Preparation method and application of chiral pimobendan
A pimobendan and chiral technology, which is applied in the field of preparation of chiral pimobendan, can solve the problems of lengthy steps and poor economy, and achieve the effect of simple process and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
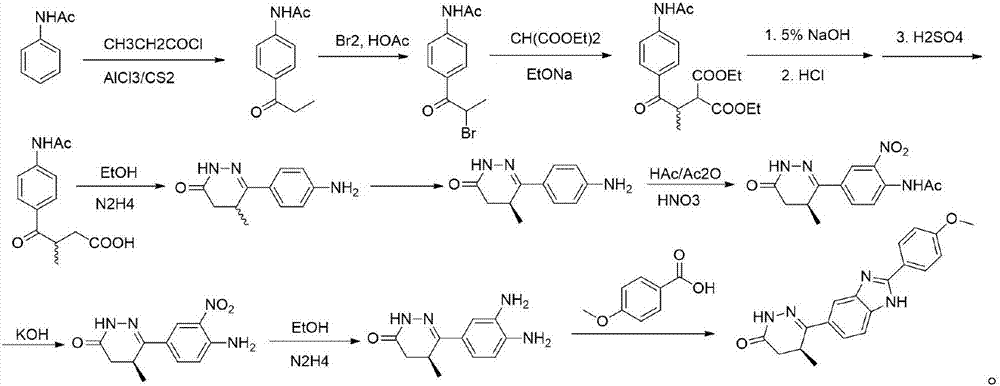
[0035] The invention provides a preparation method of chiral pimobendan, comprising the following steps:
[0036] Step S1: prepare chiral intermediate 1 with chiral reagent, diammonium phthalate and aluminum trichloride, including step S1a: add the chiral reagent dropwise to the mixture of diammonium phthalate and aluminum trichloride In the solution, the obtained mixture was refluxed for one hour, and then cooled to 30 degrees to obtain the first mixture; step S1b: adding alcohol and water dropwise to the first mixture, then adding dichloromethane for extraction, washing with water, drying over anhydrous sodium sulfate, and filtering , spin dry, add petroleum ether, filter to obtain chiral intermediate 1, in this step, prepare chiral intermediate 1 with 2-chloropropionyl chloride, diammonium phthalate under the action of Lewis acid and aluminum trichloride , which is not limited to the use of aluminum trichloride, but also aluminum trichloride, ferric chloride, zinc chloride,...
Embodiment 1
[0047] Step S1: add dropwise in the mixed solution of starting material (26.7 grams) and aluminum trichloride (22 grams) with chiral reagent S-2-chloropropionyl chloride (20g), the resulting mixture is refluxed for one hour, then Cool to 30 degrees, slowly add methanol and water to the mixture, then add dichloromethane to extract, wash with water, dry with anhydrous sodium sulfate, filter, spin dry, add petroleum ether, and filter to obtain 15.6 grams of the key chiral Intermediate R-1.
[0048] Step S2: In a three-necked flask, add 400 mg of NaH and N,N-dimethylformamide (5 mL), slowly add 19 g of methylbenzyl malonate dropwise under ice cooling (half an hour), After the dropwise addition, the mixture was stirred at room temperature for 12 hours, and the N,N-dimethylformamide solution of intermediate 1 (15.6 g dissolved in 30 ml) was added to the above reaction mixture at one time, and the obtained suspension continued to Stir overnight at room temperature, quench with water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com