Novel use of chiral diphosphonic diamide oxazoline
A bisphosphonamide oxazoline, a new application technology, applied in the field of known compounds, can solve the problems of complicated operation and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] R / S-a-Phenylethylamine: Changzhou Kerunda Technology Co., Ltd.
[0024] (1) Preparation of chiral catalyst
[0025] 1. Preparation of intermediate 2-[(4S)-4,5-dihydro-4-(1',1'-dimethylethyl)-2-oxazolinyl]aniline
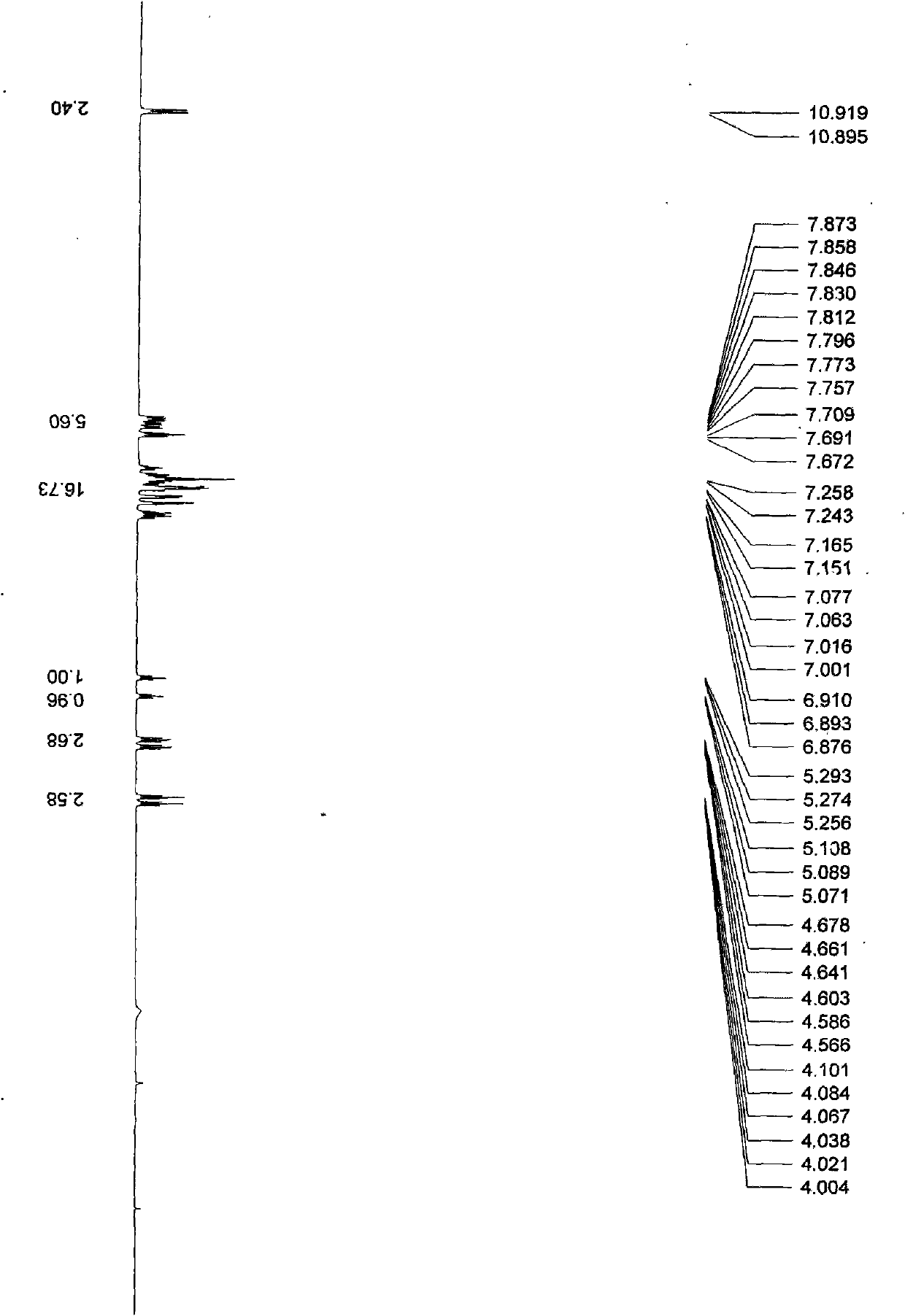
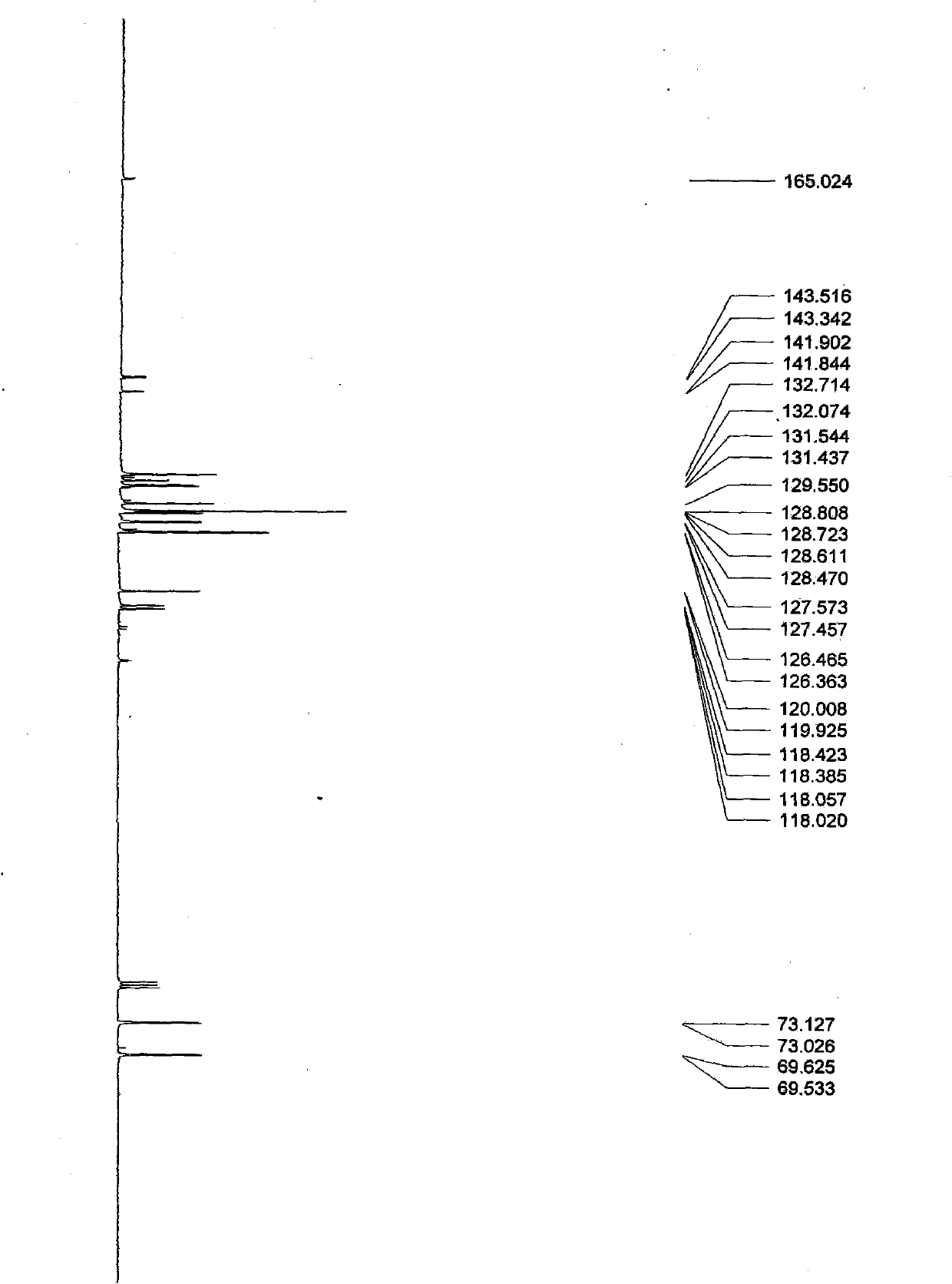
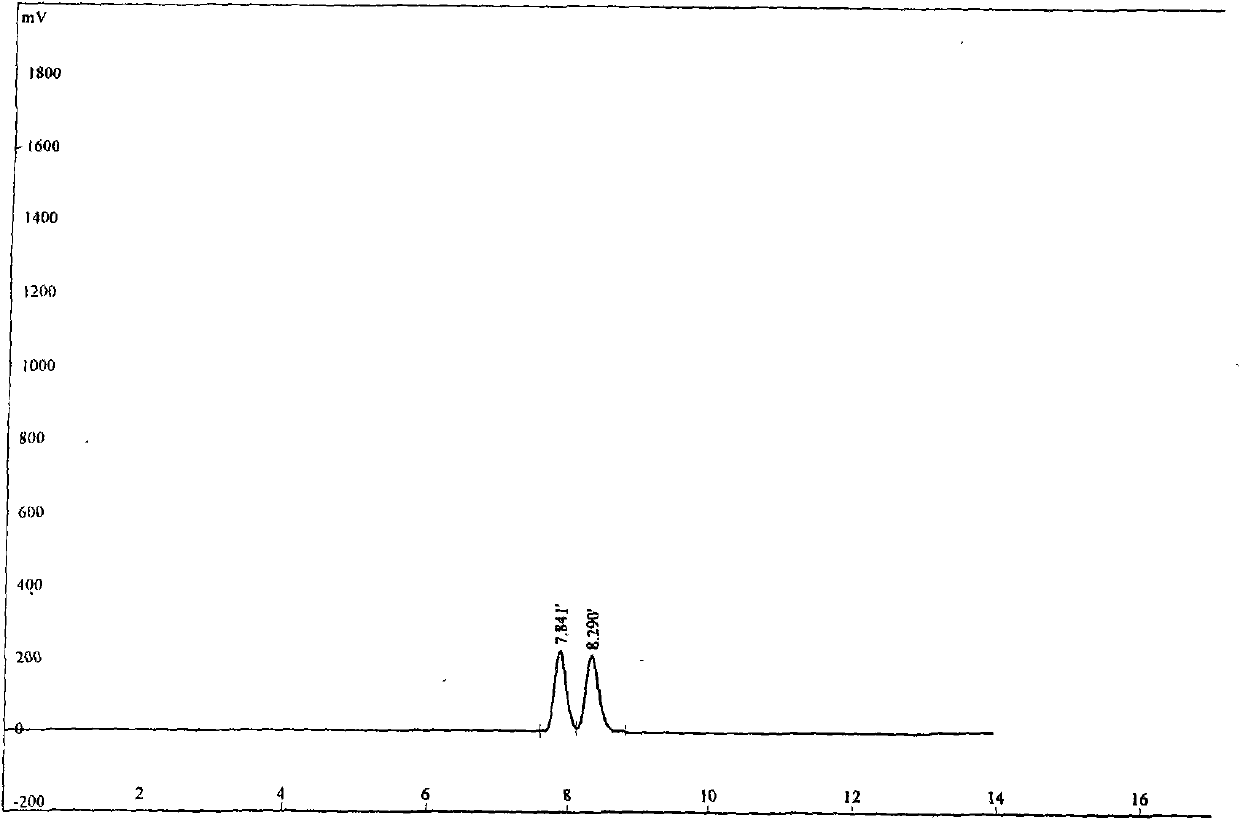
[0026] In a 100mL two-necked bottle, under anhydrous and anaerobic conditions, add anhydrous ZnCl 2 60mg (0.37mmol), 40mL chlorobenzene, 1.0g (8.47mmol) of 2-cyanoaniline, L-phenylglycinol 3g, the mixture was refluxed at high temperature for 24h, the reaction was stopped, and the solvent was removed under reduced pressure, and the remaining The substance was dissolved in water and washed with CHCl 3 (20mLx2) extraction, the organic phase was dried with anhydrous sodium sulfate, and the solvent was removed by rotation, and the crude product was subjected to column chromatography with petroleum ether / dichloromethane (4:1) to obtain a colorless oily liquid with a yield of 58%; [a ] 5 D =195.8° (c=0.25, CHCl 3 ): 1 HNMR (500MHz, CDCl 3 , 27℃), δ(ppm)=7.74(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com