Benzofuryl-containing 1, 3, 4-oxadiazole compound
A technology based on benzofuryl and oxadiazoles, which is applied in the fields of organic chemistry and acaricides, can solve the problems of many residues, the preparation method is not environmentally friendly, and the insecticidal activity of oxadiazoles has not been reported, so as to achieve the utilization of atoms high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of 2-((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)acetylhydrazide
[0031] Put 1.64g (0.10mol) of furanol and 80mL of anhydrous acetone in a reaction flask, add 27.6g (0.20mol) of potassium carbonate, stir for 0.5h, add 2.45g (0.20mol) of ethyl chloroacetate, reflux for 4.0h, cool , remove acetone by precipitation, add 60mL hydrazine hydrate to the residue, heat up to reflux, react for 2.0h, cool, remove part of hydrazine hydrate under reduced pressure, leave still to precipitate a white solid, suction filter, recrystallize from ethanol to obtain a white powder 2-(( 2,2-Dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)acetylhydrazide, yield 82.0%.
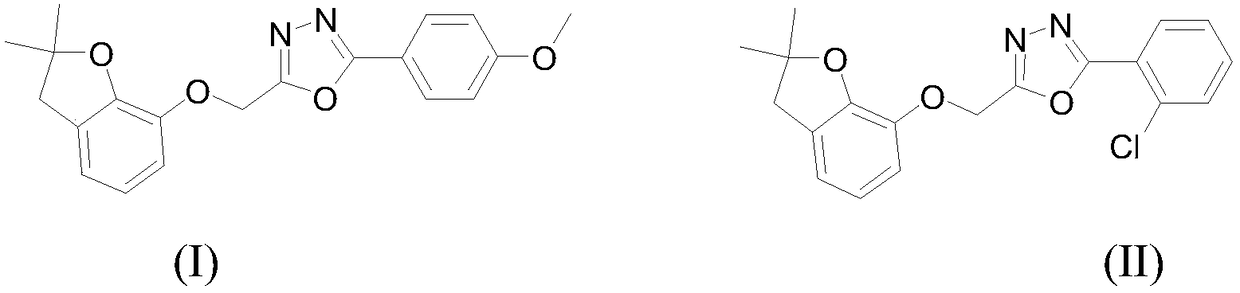
[0032] (2) Dissolve 1mmol 2-((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)acetylhydrazide in 30ml ethanol, and add 1mmmol 4-methanol in batches under stirring Oxybenzaldehyde and 0.1mmol acetic acid were heated to reflux, reacted for 2.0h, and a white solid was precipitated after cooling, and N-(4-methoxyphenyl)-2-(...
Embodiment 2
[0036] (1) Preparation of 2-((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)acetylhydrazide
[0037] Put 1.75g (0.10mol) of furanol and 82mL of anhydrous acetone in a reaction flask, add 28.6g (0.20mol) of potassium carbonate, stir for 0.5h, add 2.64g (0.20mol) of ethyl chloroacetate, reflux for 4.0h, cool , remove acetone by precipitation, add 65mL hydrazine hydrate to the residue, heat up to reflux, react for 2.0h, cool down, remove part of hydrazine hydrate under reduced pressure, leave still to precipitate a white solid, filter with suction, and recrystallize from ethanol to obtain a white powder 2-(( 2,2-Dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)acetohydrazide, yield 84.0%.
[0038] (2) Dissolve 1mmol 2-((2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)oxy)acetylhydrazide in 30ml ethanol, add 1mmol 2-chloro Benzaldehyde and 0.1mmol acetic acid were heated to reflux, reacted for 2.0h, and a white solid was precipitated after cooling, and N-(2-chlorophenyl)-2-((2,2-dimethyl-2,3- Di...
experiment example
[0042] Determination of Acaricidal Activity of a 1,3,4-Oxadiazole Compound Containing Benzofuryl
[0043] Referring to the "National Southern Pesticide Creation Center Bioassay Standard Procedure", accurately weigh (accurate to 0.0001g) a certain mass of target compound, add N,N-dimethylformamide (DMF) containing 0.1% Tween-80, and prepare The mother solution with a compound mass fraction of 5% was diluted with distilled water to form a 600mg / L mass concentration of the drug solution. DMF was used as the blank control. The acaricidal activity of the target compounds against Tetranychus cinnabarinus was tested by living pot method.
[0044] After sowing broad beans in small pots (diameter 5 cm), when the seedlings were about 5-8 cm high, each seedling was inoculated with quantitative adult mites (20 heads); into the spray tower, sprayed with prepared different concentrations of liquid medicine and DMF respectively. Observe for 5 to 10 days, investigate the number of live inse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com