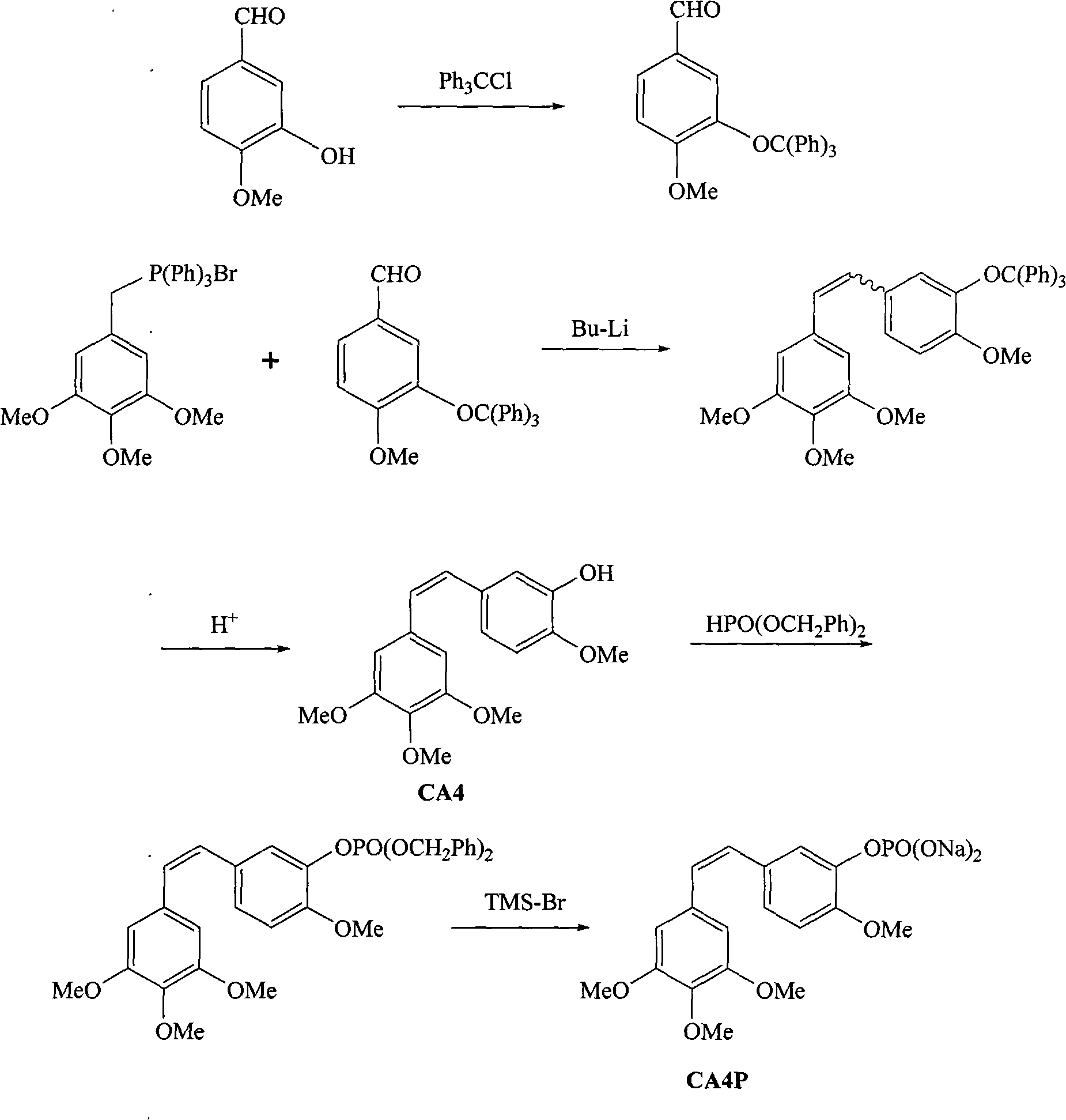
Method for synthesizing CA4P
The technology of conbutastatin and mixed solvent, which is applied to a preparation field of conbutastatin, can solve the problems of complicated operation, harsh conditions and high reagent price, and achieves the advantages of cheap and easy-to-obtain raw materials, mild reaction conditions, and simple and feasible operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1.1 3-trityloxy-4-methoxybenzaldehyde
[0021] Into a 500 mL flask, 50 g of isovanillin, 104 g of trityl chloride, 65 mL of triethylamine, and 180 mL of dry tetrahydrofuran were added. Raise the temperature to reflux, and react for 4 to 5 hours. TLC showed the reaction was complete. Add 100 mL of water to the reaction system to stop the reaction, and lower the temperature to 20-25°C. Ethyl acetate / n-heptane (1:1) 80 mL was added, and a granular light yellow solid was formed. The precipitate was filtered. The filter cake was washed with purified water. After vacuum drying, 100 g of 3-trityloxy-4-methoxybenzaldehyde was obtained with a yield of 77%.
[0022] 1.2 3'-trityloxy-3,4,4',5-tetramethoxystilbene
[0023] In a 500 mL four-necked flask, under argon protection, 65 g of trimethoxyphenylmethylenetriphenylphosphine bromide and 112 mL of anhydrous tetrahydrofuran were added. Cool to -25°C, add 70mL of n-butyl lithium in n-hexane solution (2.4M) dropwise to the sy...
Embodiment 2
[0040] 2.13-trityloxy-4-methoxybenzaldehyde
[0041] Into a 500 mL flask, 50 g of isovanillin, 104 g of trityl chloride, 65 mL of triethylamine, and 180 mL of dry methylene chloride were added. The temperature was raised to reflux, and the reaction was followed by TLC. Add 100 mL of water to the reaction system to stop the reaction, and lower the temperature to normal temperature 20-25°C. Ethyl acetate / n-heptane (1:1) 80 mL was added, and a granular light yellow solid was formed. The precipitate was filtered, and the filter cake was washed with purified water. After vacuum drying, 95 g of 3-trityloxy-4-methoxybenzaldehyde was obtained with a yield of 74%.
[0042] 2.23'-trityloxy-3,4,4',5-tetramethoxystilbene
[0043] In a 500 mL four-necked flask, under argon protection, 65 g of trimethoxyphenylmethylenetriphenylphosphine bromide and 112 mL of anhydrous tetrahydrofuran were added. Cool to -15°C, add 70mL of n-butyl lithium in n-hexane solution (2.4M) dropwise to the syst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com