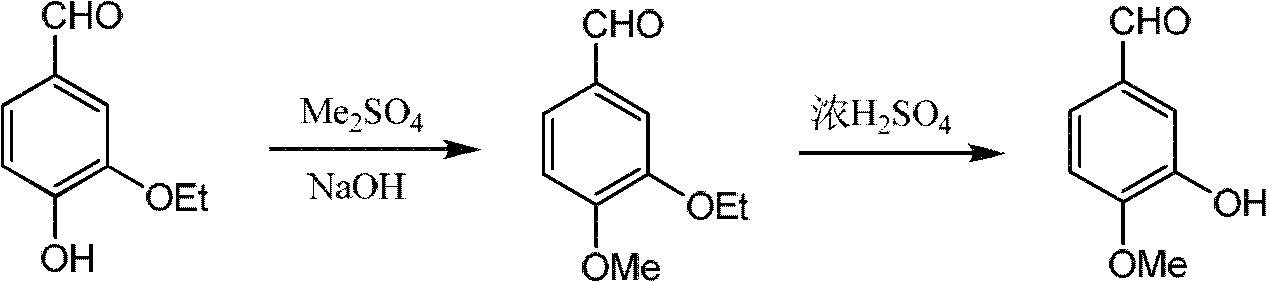
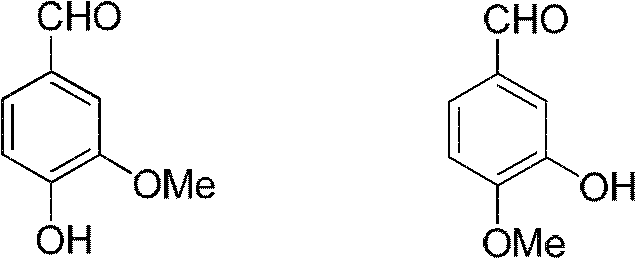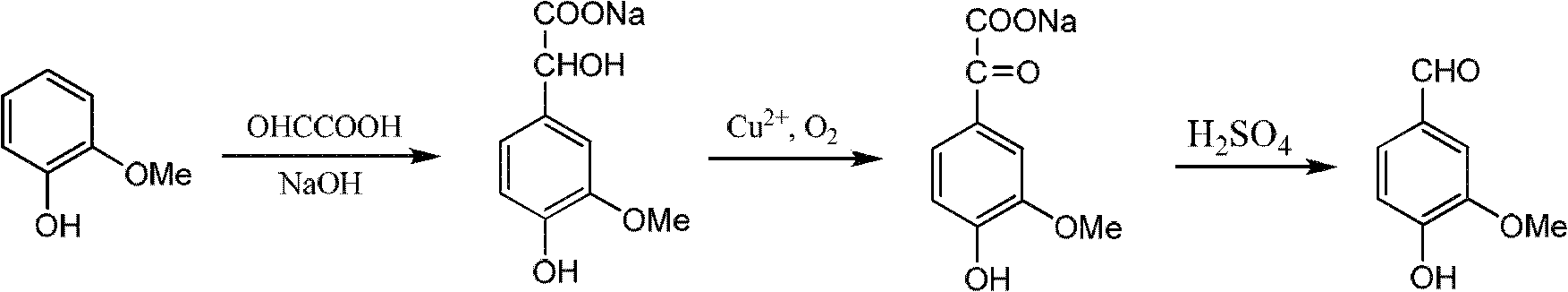Sharing synthesis method for vanillin and isovanillin
A technology for isovanillin and vanillin, which is applied in the field of fragrance and organic intermediate synthesis, can solve the problems of poor reaction selectivity, expensive raw materials, difficult to waste sulfuric acid and the like, and achieves the effects of high yield, simple operation and low environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Use 1-methoxy-2-isopropoxybenzene 1a as raw material to prepare 3-methoxy-4-isopropoxybenzaldehyde 2a and 3-isopropoxy-4-methoxy Benzaldehyde 3a.
[0042]
[0043] Mix 1-methoxy-2-isopropoxybenzene 1a (20.0g, 0.12mol) and N,N-methylformylaniline (22.0g, 0.16mol), and slowly dissolve oxygen trichloride in an ice-water bath Phosphorus (27.5 g, 0.18 mol) was added dropwise to the above mixture, and after the addition was complete, the reaction was stirred at room temperature to 100° C. and continued to be heated for 20 h. After the reaction, toluene (200 mL) was added for dilution, and then water (100 mL) was added for hydrolysis under an ice-water bath, and stirred for 1 h. The organic layer was separated, washed with 2% sodium hydroxide solution, concentrated and desolvated, and then distilled under reduced pressure to collect the yellow liquid fraction (20.3g, yield 86.9%) at 115-119°C / 2mmHg.
[0044] Spectral data:
[0045] EI-MS (m / z): 194.1 [M + ];
[004...
Embodiment 2
[0064] (1) Use 1-methoxy-2-n-butoxybenzene 1b as raw material to prepare 3-methoxy-4-n-butoxybenzaldehyde 2b and 3-n-butoxy-4-methoxy Benzaldehyde 3b.
[0065]
[0066] Mix 1-methoxy-2-n-butoxybenzene 1b (25.2g, 0.14mol) and N,N-methylformylaniline (25.5g, 0.19mol), and slowly dissolve oxygen trichloride in an ice-water bath Phosphorus (30.6 g, 0.20 mol) was added dropwise to the above mixture, and after the addition was complete, the reaction was stirred at room temperature to 100° C. and continued to be heated for 20 h. After the reaction was completed, toluene (220 mL) was added for dilution, and then water (120 mL) was added for hydrolysis under an ice-water bath, and stirred for 1 h. The organic layer was separated, washed with 2% sodium hydroxide solution, concentrated and desolvated, and then distilled under reduced pressure to collect a yellow liquid fraction (25.1 g, yield 86.2%) at 140-144° C. / 2 mmHg.
[0067] Spectral data:
[0068] EI-MS (m / z): 208.2[M + ]; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com