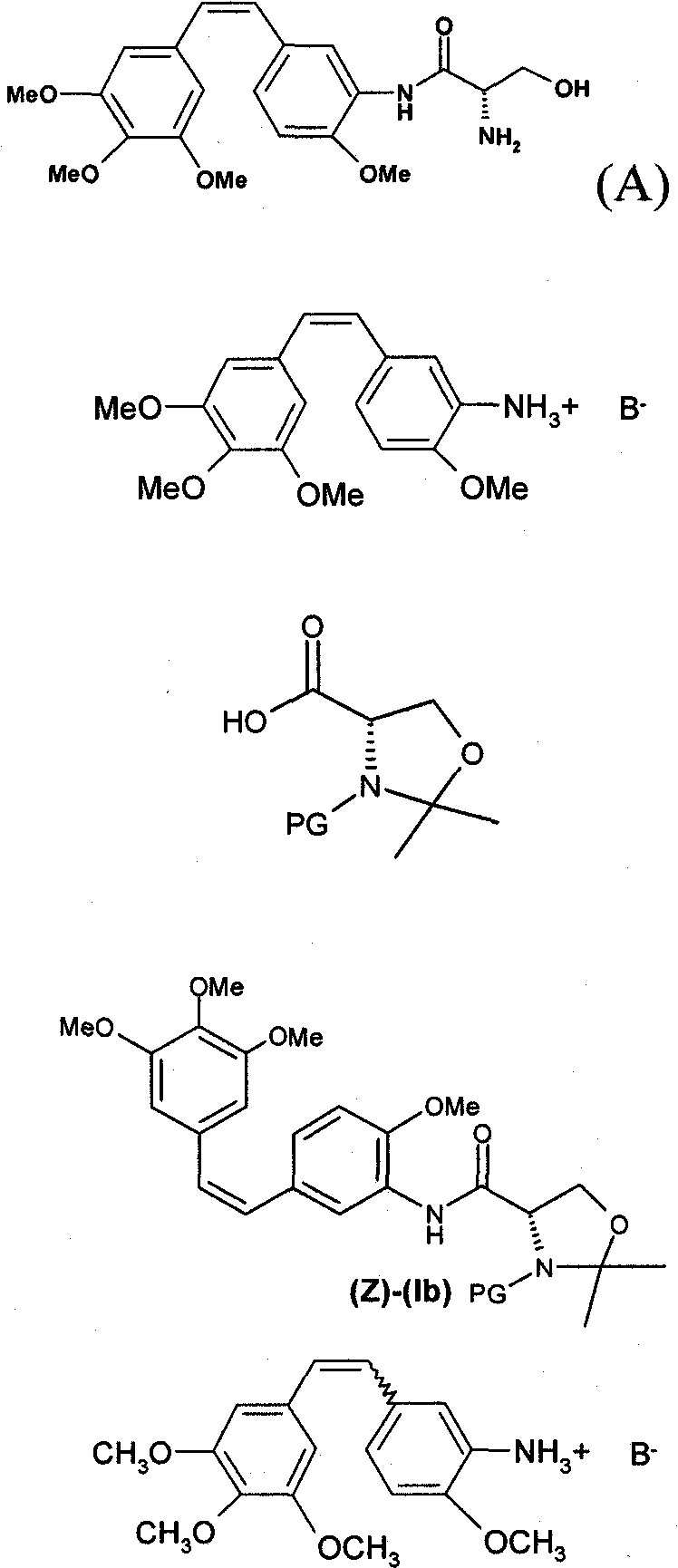
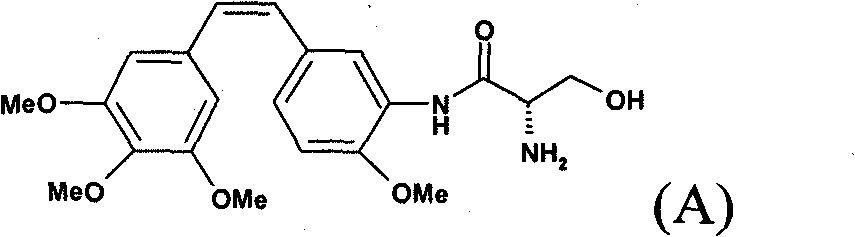
Method for preparing combretastatin
A technology of combretastatin and form, which is applied in the field of preparing combretastatin in base form or acid addition salt form, and can solve problems such as poor yield, multiple epimerization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1 (according to the present invention)
[0066] Step (i):
[0067] At a temperature of 5-10° C., sodium methoxide solution (5.66 kg, 29.34 mol) was flowed into a mixture containing toluene (91.1 liters), trimethoxybenzylphosphonium bromide (15.35 kg, 29.33 mol) and 4- Methoxy-3-nitrobenzaldehyde (5.06 kg, 27.93 mol). At the end of the reaction, 0.32 liters (5.59 mol) of acetic acid flowed in. After the medium was kept at 20°C, it was filtered. The filter cake was washed with toluene (11.1 L). The filtrate was washed several times with water (20.2 L), then concentrated under vacuum. Isopropanol (87.6 L) was then introduced, the medium was concentrated and then cooled. The suspension was then filtered at 10°C. The isolated product (6.46 kg, 52.2%) was dried under vacuum. The purity of the isolated product was of the order of 78% for (Z) and 22% for (E).
[0068] Step (ii):
[0069] At 50°C, water (80ml) was flowed into a medium comprising methano...
Embodiment 2
[0073] Embodiment 2 (according to the present invention)
[0074]In a jacketed 1.6-liter reactor, (Z)-amino compound-Z-aminostilbene HCl (Z-aminostil, HCl, 50.0 g), N-BOC-acetonate (41.8 g) and 500ml of DCM. Then 53.4 ml of triethylamine (2.7 equiv) was flowed in at 22±3°C, followed by a 50% solution of T3P in DCM (1.7 equiv). The mixture was stirred under reflux of DCM for 1 hour. The mixture was then cooled to 22±3°C and 500 ml of demineralized water was added. The mixture was separated by settling and the phases were separated. The DCM phase was washed with 500 ml of a 6 wt % (30 g) aqueous solution of sodium hydrogen phosphate and then with 500 ml of demineralized water. The DCM phase was concentrated at about 40-50° C. under reduced pressure of 360 to 150 mbar. A 3 mol / l solution of HCl in methanol (379 ml, 8 equivalents) was then flowed in, followed by 300 ml of demineralized water. The mixture was separated by settling and the phases were separated. The DCM / met...
Embodiment 3
[0076] Embodiment 3 (according to the present invention)
[0077] At 50° C., water (60 ml) was flowed into a medium containing methanol (50 ml), (Z)- and (E)-nitro compounds to be reduced (15 g, 0.043 mol) and sodium dithionite (27.2 g, 0.133mol). Once the reaction was complete, hydrochloric acid (26.2ml, 0.314mol) was added. Work up by adding water and DCM followed by sodium hydroxide to pH=7. Hydrogen chloride in methanol (18.9 ml, 0.0586 mol) was added to the DCM phase, and the solvent was then replaced with acetonitrile. Then get the broth. Benzyl alcohol (31 ml) was then added to the suspension at 50°C and kept at 65°C for 2 hours. After cooling, the medium is then filtered and washed. The isolated product was then dried under vacuum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com