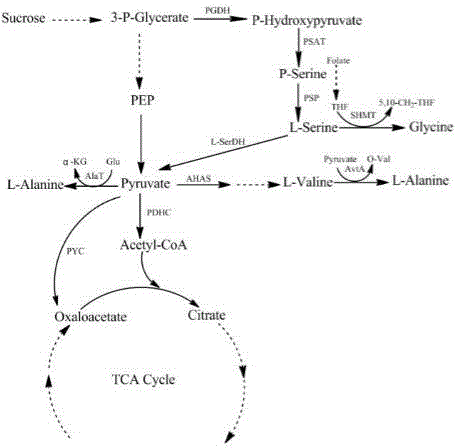
Methods for constructing and fermenting L-serine high-yielding recombinant corynebacterium glutamicum
A Corynebacterium glutamicum, construction method technology, applied in the direction of microorganism-based methods, fermentation, recombinant DNA technology, etc., can solve the problems of slow bacterial growth, reduced production efficiency, reduced production rate, etc., to achieve increased yield and sugar Effects of acid conversion, increased growth rate, and increased production intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Recombinant bacteria Corynebacterium glutamicum SYPS-062-33a SS avtA build
[0024] Based on the genome sequence information of Corynebacterium glutamicum ATCC13032, it was designed to construct avtA The primers of the knockout recombinant knockout plasmid, the primer sequence is as follows:
[0025] avtA -1: 5’-GAAAAGCTTAAGGCCCTGCAGTAGTG-3’ ( Hin dIII)
[0026] avtA -2: 5’- CCCATCTGTTAAACTTAAACCAAACGGCTGAACATTGCTT-3’
[0027] avtA -3: 5’- GGTTTAAGTTTAACAGATGGGGGTGTGCGCAAAATCGG-3’
[0028] avtA -4: 5’- ATTCTAGACCTGCGCTGCCACGTTGT-3’ ( Xba I)
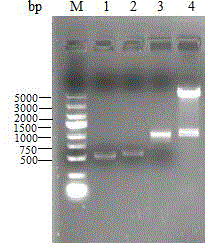
[0029] primers avtA -1 and avtA -2 to amplify the upstream fragment with primers avtA -3 and avtA -4 Amplify the downstream fragments, respectively purify the upstream and downstream fragments by agarose gel electrophoresis, see figure 2 , the lengths are 507bp and 548bp respectively; the upstream fragment and the downstream fragment are used as templates respectively, and the primers avt...
Embodiment 2
[0030] Embodiment 2: Recombinant bacteria Corynebacterium glutamicum SYPS-062-33a SS avtA C-T wxya build
[0031] Using the recombinant strain constructed in Example 1 as the starting strain, the coding regulatory gene of acetohydroxyacid synthase (AHAS) that catalyzes pyruvate to generate α-acetolactate wxya The 249bp of the C-terminus of the C-terminus was precisely deleted, so that the enzyme activity of acetohydroxyacid synthase was reduced. Design and synthesize the following primers for knockout wxya The recombinant plasmid:
[0032] wxya -1: 5’-CCCAAGCTTGCTGTTTCCAGATGACCAACC-3’ ( Hin dIII)
[0033] wxya -2: 5’-GGCGATAGTGGTCTCTTCATCAAGTCGCACGACTTTGAGC-3’
[0034] wxya -3: 5’-GAAGAGACCACTATCGCCACAGCAATTAATCTGATTGC-3’
[0035] wxya -4: 5’-CGCGGATCCCGTTCAGGTTTGGCTCGATG-3’ ( Bam HI)
[0036] Specific experimental steps refer to Example 1, knockout plasmid pK18mob sacB C-T wxya The construction diagram see Figure 4 . Recombinant bacteria Coryneba...
Embodiment 3
[0040] Embodiment 3: the enzyme activity assay method of acetohydroxyacid synthase (AHAS)
[0041] Reaction system: 100 mM potassium phosphate buffer (pH 7.3), 50 mM sodium pyruvate, 10 mM MgCl 2 and 100 μM flavin adenine dinucleotide (FAD). Take the Corynebacterium glutamicum cells in the logarithmic phase, centrifuge to remove the supernatant, wash with 0.2% ice KCl for 3 times, ultrasonically break and centrifuge to obtain the supernatant, take a certain amount of supernatant and add it to the reaction system, Add 100 μL of 50 % H after incubation at 37oC for 20 minutes 2 SO 4 Stop the reaction. After 30 minutes of incubation at 37oC, acetoin was produced from α-acetolactic acid, and the content of acetoin in the reaction solution was determined by gas chromatography. One unit of enzyme activity is set as: 1 nmol of α-acetolactate produced per milligram of protein per minute. Protein concentration was determined by the Bradford method. Acetohydroxyacid synthase enzym...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com