Method for treating hematopoietic neoplasms
a hematopoietic neoplasm and neoplasm technology, applied in the field of hematopoietic neoplasm treatment, can solve the problems of poor prognosis of leukemic cells, reduced five-year survival rate, and low treatment efficacy of cancer treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A. Example 1
Subcutaneous In Vivo Leukemia Model
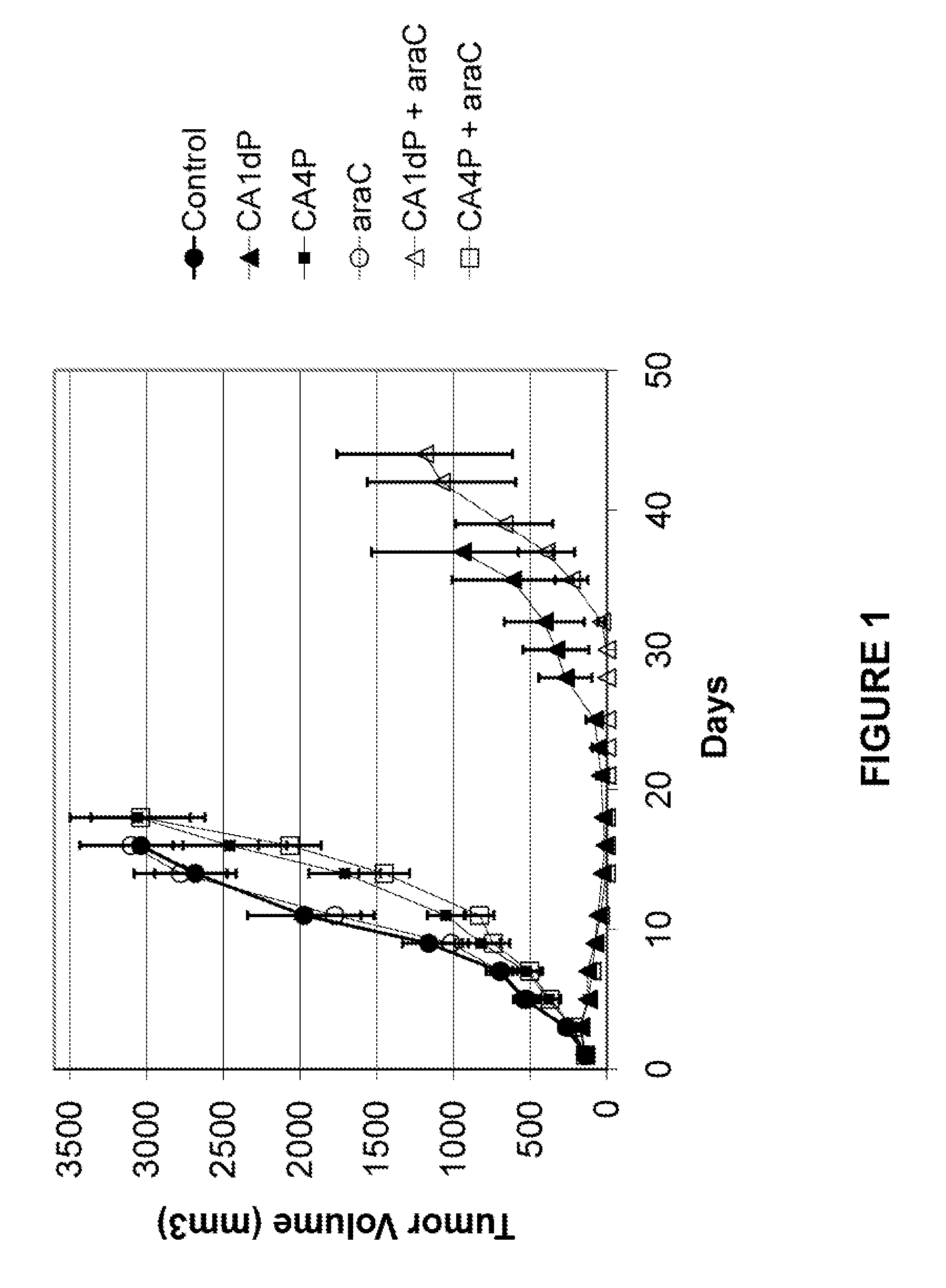
[0079]HL60 (1×107 cells in 0.1 ml) were injected subcutaneously into the dorsa of 4-6 week old athymic nu / nu mice (Southern Research Institute). When palpable tumors were evident (average tumor volume 100 mm3; i.e. approximately 12 days after inoculation), 6 experimental groups of mice were randomized, each with 10 animals. Treatment was initiated according to the following regimens:
Ani-TreatmentGroupmalsCompoundDoseSchedule110Control0Q4D × 2 / 2 wks(PBS)(SD) − Q3H × 3 ( )210CA1dP75 mg / kg / injQ7D × 2(SD + 1)310CA4P75 mg / kg / injQ7D × 2(SD + 1)410Ara-C20 mg / kg / injQ4D × 2 / 2 wks(SD) − Q3H × 3 ( )510CA1dP75 mg / kg / injQ7D × 2Ara-C20 mg / kg / inj(SD + 1)Q4D × 2 / 2 wks(SD) − Q3H × 3 ( )610CA4P75 mg / kg / injQ7D × 2Ara-C20 mg / kg / inj(SD + 1)Q4D × 2 / 2 wks(SD) − Q3H × 3 ( )
[0080]Tumor volume was measured three times a week until the endpoint was reached (tumor volume >3000 mm3), or for 90 days post-treatment. Treatment with Ara-C alone showed little improvemen...
example 2
A. Example 2
Dose Response Activity
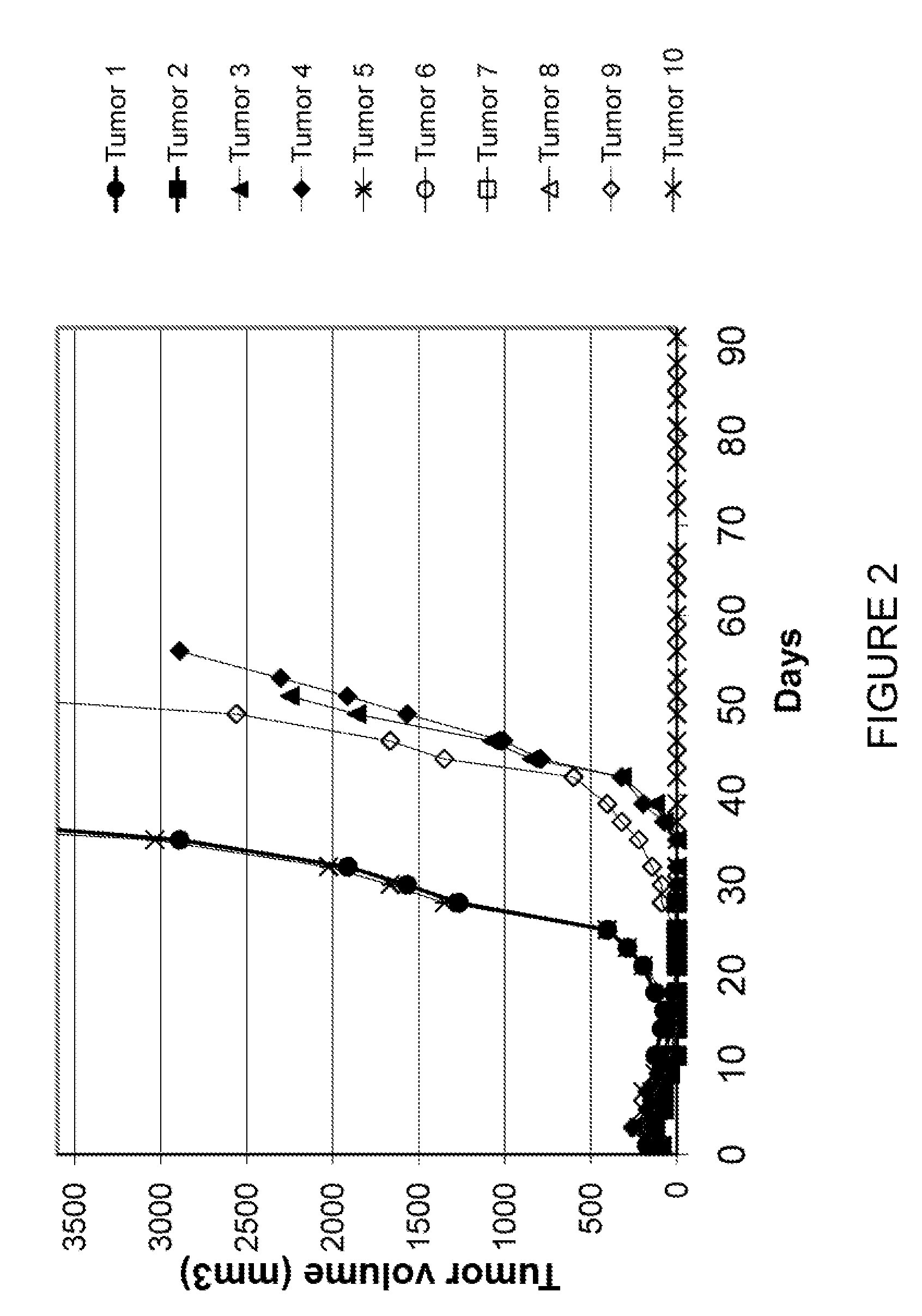
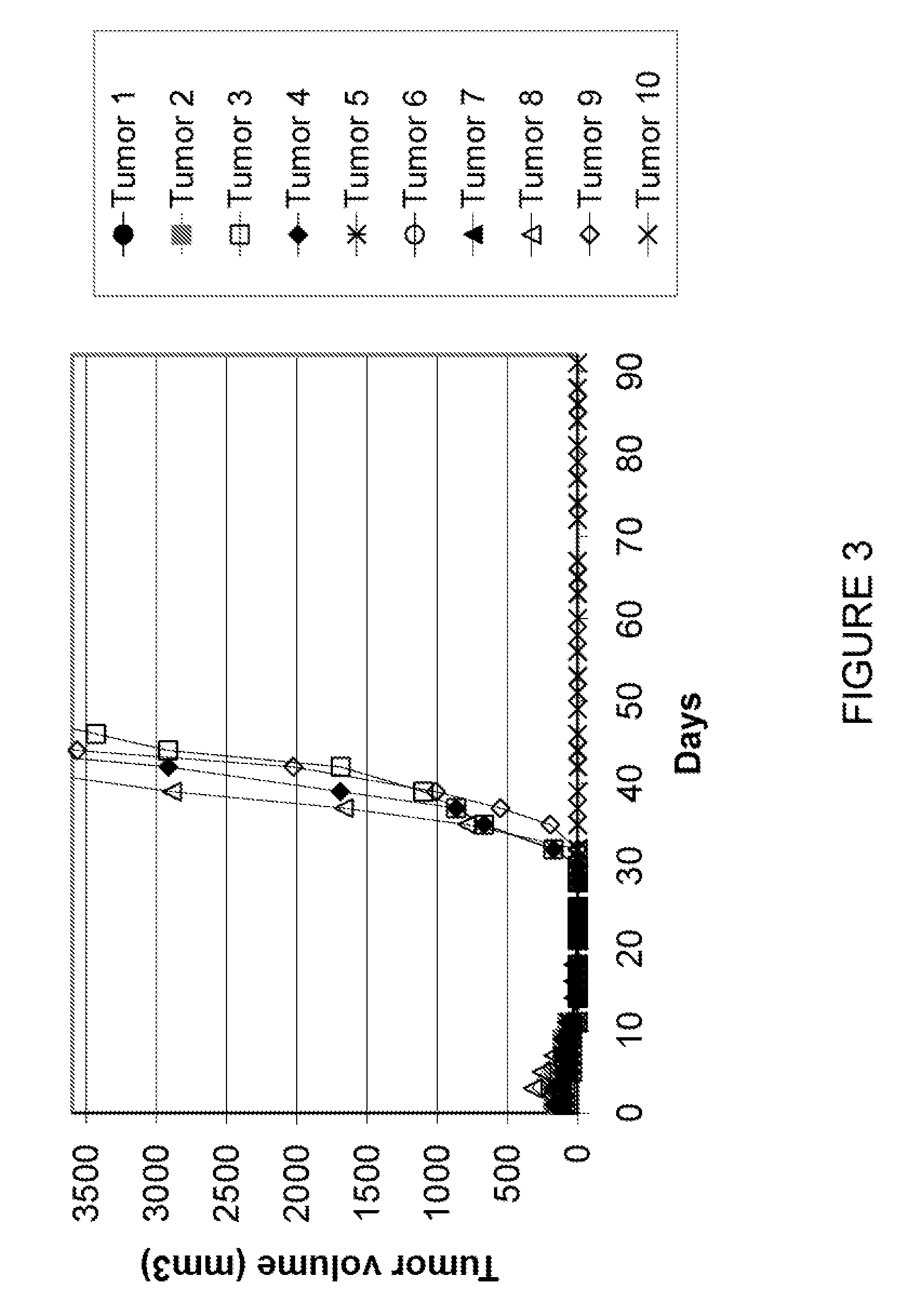
[0081]HL60 (1×107 cells in 0.1 ml) were injected subcutaneously into the dorsa of 4-6 week old athymic nu / nu mice (Southern Research Institute). When palpable tumors were evident (average tumor volume 100 mm3; i.e. approximately 12 days after inoculation), ten experimental groups of mice were randomized, each with 10 animals. Treatment was initiated according to the following regimens:
GroupCompoundDoseTreatment Schedule1Control0mg / kg / inj2CA1dP75mg / kg / injday 3 and 103CA1dP25mg / kg / injday 3 and 104CA1dP10mg / kg / injday 3 and 105CA1dP2.5mg / kg / injday 3 and 106Ara-C20mg / kg / injtid day 1, 4, 8 and 127Ara-C +20mg / kg / injtid day 1, 4, 8 and 12CA1dP75mg / kg / injday 3 and 108Ara-C +20mg / kg / injtid day 1, 4, 8 and 12CA1dP25mg / kg / injday 3 and 109Ara-C +20mg / kg / injtid day 1, 4, 8 and 12CA1dP10mg / kg / injday 3 and 1010Ara-C +20mg / kg / injtid day 1, 4, 8 and 12CA1dP2.5mg / kg / injday 3 and 10
[0082]Tumor volume was measured three times per week. The decrease in tumor volume showe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Chemotherapeutic properties | aaaaa | aaaaa |
| Refractory | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com