Diamine monomer containing diphenylamine-fluorene, preparation method and application of same in polyimide preparation
A polyimide and dimethyl fluorene diamine technology, which is applied in the field of polyimide preparation, can solve the problems of single polyimide species, and achieve the effects of improving solubility, large application prospects, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
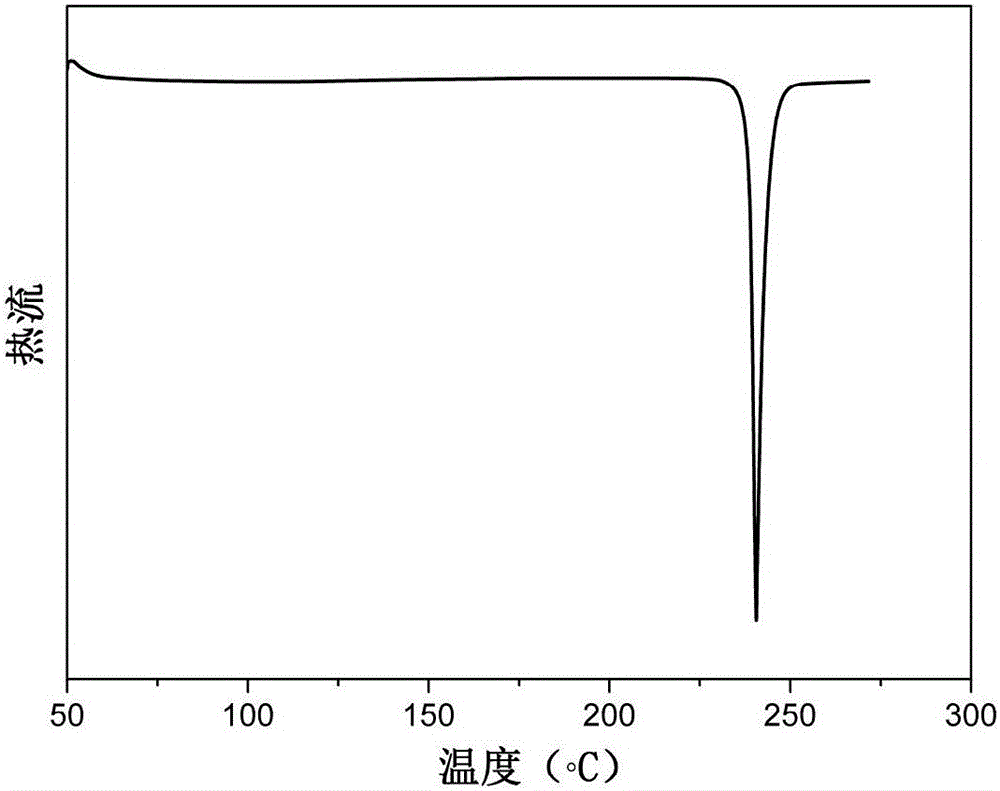
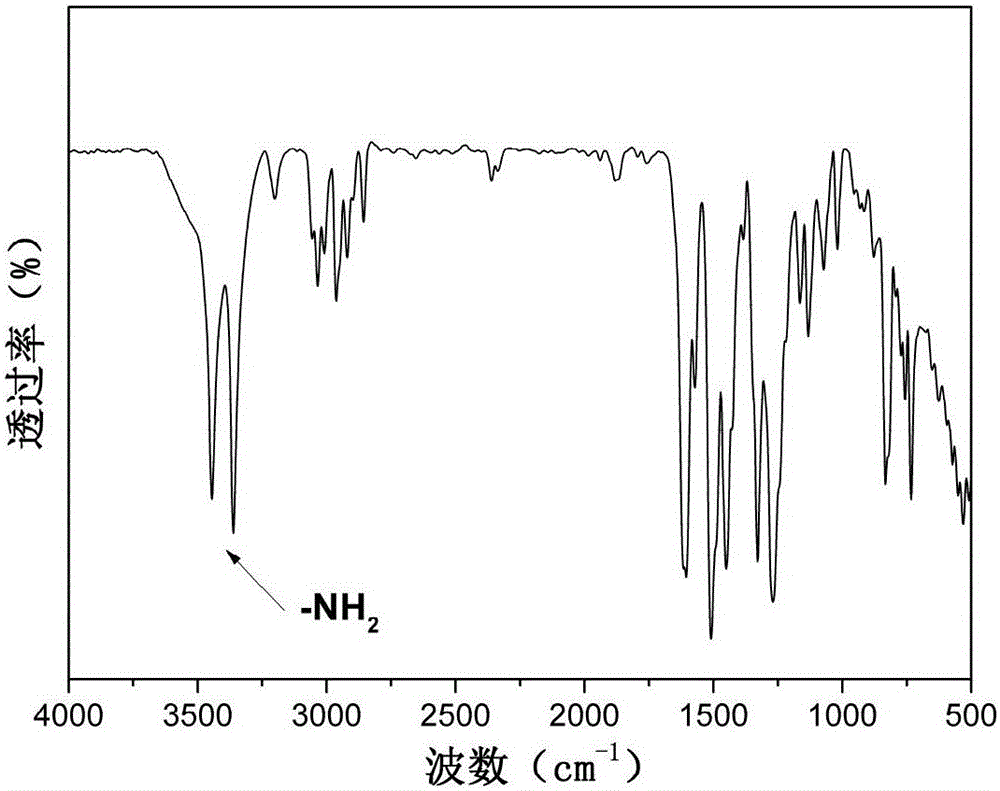
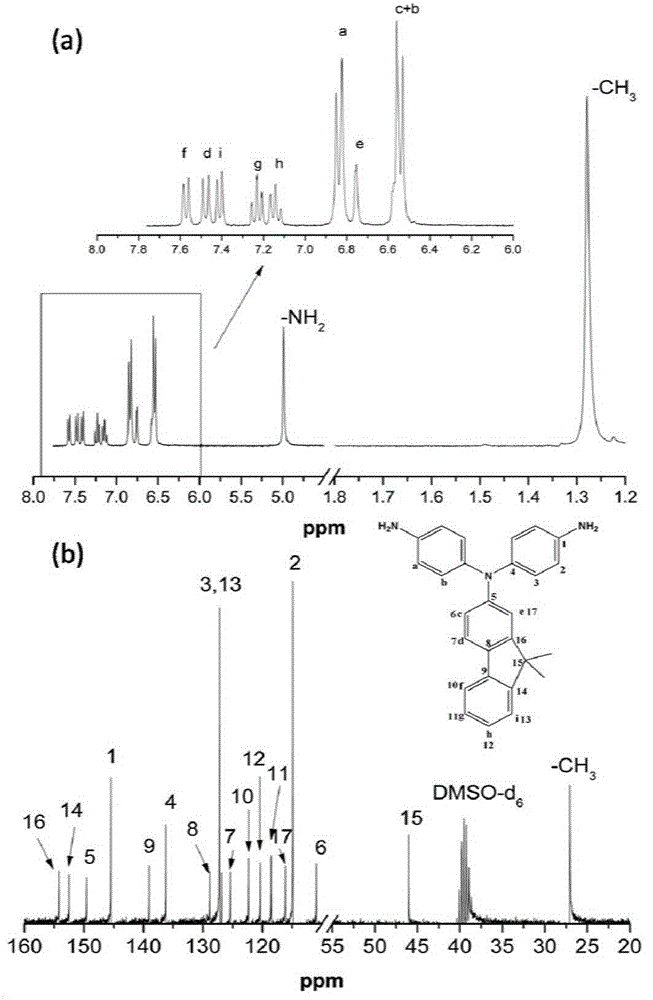
[0030] Example 1: Preparation of N,N-bis(4-aminophenyl)-9,9-dimethylfluorene
[0031] Step 1: Add 12g (57mmol) of 2-amino-9,9-dimethylfluorene, 12.2g (114mmol) of p-fluoronitrobenzene, 13g (114mmol) of fluorine to a 250mL three-necked flask equipped with mechanical stirring Cesium chloride, add 100mL dimethyl sulfoxide as a solvent, react at 160°C for 20h, discharge in ethanol, and recrystallize with glacial acetic acid to obtain yellow N,N-bis(4-nitrophenyl)-9,9- Dimethylfluorene 21.2g, yield rate is 80%;
[0032] The second step reaction: add 20g of N,N-bis(4-nitrophenyl)-9,9-dimethyl prepared in the first step to a 500mL three-necked flask equipped with a magnet, a thermometer and a condenser Base fluorene, 2g of Pd / C with a mass fraction of 10%, add 200mL of ethanol as a solvent, and stir evenly to obtain a suspension. After heating to reflux, slowly dropwise add N,N-bis(4-nitrophenyl)-9,9-dimethylfluorene with a molar ratio of 20:1 and 85% hydrated solution to the suspe...
Embodiment 2
[0033] Example 2: Preparation of polyimide by polymerization of N,N-bis(4-aminophenyl)-9,9-dimethylfluorene and pyromellitic anhydride
[0034]Feed nitrogen into the three-necked flask equipped with magneton, nitrogen gas inlet and outlet, and thermometer, add 0.3912g (1mmol) N,N-bis(4-aminophenyl)-9,9-bis Add 8mL of DMAc solvent to dissolve methylfluorene, slowly add 0.2181g (1mmol) pyromellitic anhydride, stir at room temperature for 6h, add 2mL pyridine and 4mL acetic anhydride, heat to 100°C and continue the reaction for 1.5h, after the reaction is completed, cool to room temperature , discharged into ethanol to obtain a dark yellow powder, reflux washed with ethanol three times, and dried in a vacuum oven at 100° C. to obtain a pyromellitic anhydride polyimide with a mass of 0.5913 g.
Embodiment 3
[0035] Example 3: Preparation of polyimide by polymerization of N,N-bis(4-aminophenyl)-9,9-dimethylfluorene and 3,3',4,4'-biphenyltetracarboxylic anhydride
[0036] Feed nitrogen into the three-necked flask equipped with magneton, nitrogen gas inlet and outlet, and thermometer, add 0.3912g (1mmol) N,N-bis(4-aminophenyl)-9,9-bis Add 10mL of DMAc solvent to dissolve methylfluorene, slowly add 0.2942g (1mmol) 3,3',4,4'-biphenyltetracarboxylic anhydride, stir at room temperature for 6h, add 2mL pyridine and 4mL acetic anhydride, heat to 100°C Continue the reaction for 1.5h, cool to room temperature after the reaction is complete, discharge the material into ethanol to obtain a dark yellow powder, reflux wash with ethanol three times, and dry in a vacuum oven at 100°C to obtain biphenyltetracarboxylic anhydride polyimide with a mass of 0.6431g .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com