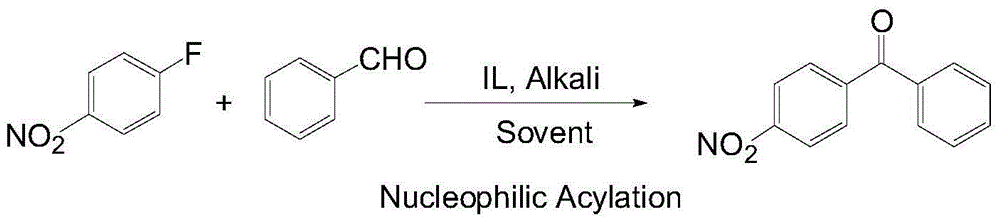
Novel method for synthesizing p-nitrobenzophenone
A technology for p-nitrobenzophenone and p-fluoronitrobenzene is applied in the field of nucleophilic acylation to synthesize p-nitrobenzophenone, and can solve the problems of difficulty in separation, recovery and reuse, large environmental pollution and poor stability. and other problems, to achieve the effect of simple operation and equipment requirements, high yield, and realization of recycling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
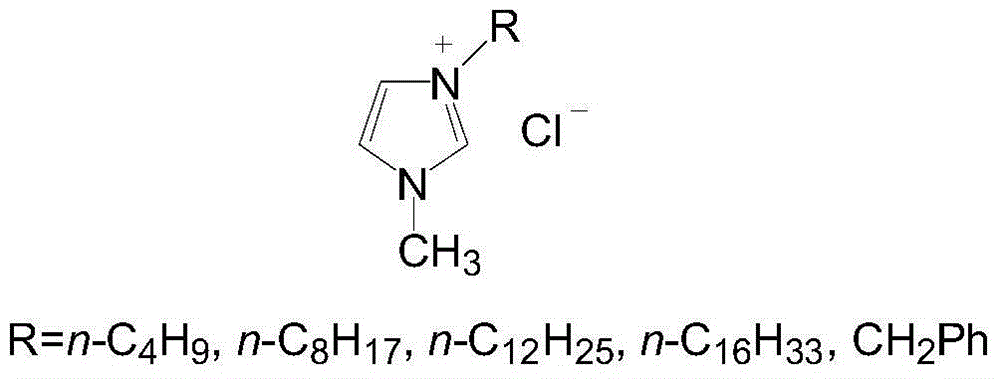
[0017] Example 1: Add 0.05 mol of N-methylimidazole, 0.06 mol of n-chlorobutane and 10 mL of methanol into a 100 mL single-necked flask equipped with a reflux condenser. Raise the temperature to 70-80°C, and reflux for 24 hours. The solvent was evaporated under reduced pressure, and unreacted n-chlorobutane was absorbed with paraffin. The residue was washed twice with ethyl acetate, and the ethyl acetate was removed by rotary evaporation. Dried to obtain a yellow viscous ionic liquid (R=n-C 4 h 9 ).
Embodiment 2~5
[0018] Embodiment 2~5: change the n-butane chloride in embodiment 1 into n-octane chloride, n-dodecane chloride, n-octadecane chloride and benzyl chloride respectively, obtain imidazoles ionic liquid ( R=n-C 8 h 17 ,n-C 12 h 25 ,n-C 16 h 33 ,CH 2 Ph).
Embodiment 6
[0019] Embodiment 6: Add 3mmol p-fluoronitrobenzene, 3.6mmol benzaldehyde, 1mmol imidazoles ionic liquid (R=n-C 4 h 9 ) and 20 mL of solvent DMSO. After stirring for 5 min, 4 mmol potassium tert-butoxide was added to the three-necked flask. The reaction was stirred at 30 °C for 6 h. After the reaction was completed, ice water was added to the reaction solution. The organic matter was extracted twice with ethyl acetate, and the extracts were combined. Wash with water, wash with salt, dry and distill off the solvent under reduced pressure. The product was separated by column chromatography (petroleum ether: ethyl acetate = 25:1), and the crude product was recrystallized from ethanol to obtain colorless needle-like crystals. The conversion rate of p-fluoronitrobenzene was 85.3%, and the yield of p-nitrobenzophenone was 75.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com