Method for producing p-fluoroaniline by solvent-free catalytic hydrogenation method
A technology for p-fluoroaniline and catalytic hydrogenation, applied in chemical instruments and methods, preparation of organic compounds, chemical recovery and other directions, can solve problems such as difficulties in industrialization, and achieve the effects of reducing production costs, simple preparation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Put 200g of p-fluoronitrobenzene, 1g of 1%Pt / C catalyst, and 1g of sodium hypophosphite into the autoclave, and replace with nitrogen and hydrogen three times respectively.
[0026] (2) Turn on the stirring, raise the temperature to 60°C, pass in hydrogen gas at 0.9Mpa, start the reaction, and control the reaction temperature to 85°C.
[0027] (3) During the reaction process, measure the instantaneous reaction speed of the reaction, expressed as the hydrogen pressure drop per minute, and the reaction speed is: 0.12Mpa / min.
[0028] (4) The reaction time is 3 hours. At the end of the reaction, the reaction pressure increases, the reaction temperature decreases, and the reaction speed is zero.
[0029] (5) The catalyst is separated from the reaction solution by suction filtration, the reaction solution is simply separated from water, and the final product p-fluoroaniline is obtained after vacuum distillation. The conversion rate of the reaction is 100%, the product pu...
Embodiment 2
[0031] Under the premise of embodiment 1, carry out the mechanical experiment of catalyst (that is, catalyst recycles):
[0032] (1) Put 200g of p-fluoronitrobenzene, add 0.2g of 1%Pt / C catalyst, and 1g of sodium hypophosphite into the autoclave, and replace with nitrogen and hydrogen three times respectively.
[0033] (2) Turn on the stirring, raise the temperature to 60°C, pass in hydrogen gas at 0.9Mpa, start the reaction, and control the reaction temperature to 85°C.
[0034] (3) During the reaction process, measure the instantaneous reaction speed of the reaction, expressed as the hydrogen pressure drop per minute, and the reaction speed is: 0.13Mpa / min.
[0035] (4) The reaction time is 2 hours and 45 minutes. At the end of the reaction, the reaction pressure increases, the reaction temperature decreases, and the reaction speed is zero.
[0036] (5) The catalyst is separated from the reaction solution by suction filtration, the reaction solution is simply separated from...
Embodiment 3
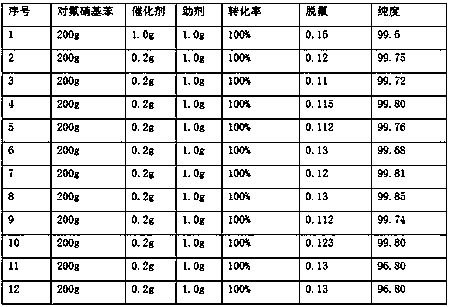
[0038] Under the premise of embodiment 1, embodiment 2, continue to apply mechanically 10 batches and obtain result as follows:
[0039] Wherein sequence number 8 is to change the sodium hypophosphite in embodiment 1 into sodium phosphate, other conditions are constant;
[0040] Sequence number 9 is to change the sodium hypophosphite in embodiment 1 into disodium hydrogen phosphate, and other conditions are constant;
[0041] Sequence number 10 is to change the sodium hypophosphite in embodiment 1 into sodium biphosphite, and other conditions are constant;
[0042] Sequence number 11 is that the control reaction temperature in embodiment 1 is 30 ℃, other conditions are unchanged;
[0043] No. 12 is to control the reaction temperature in Example 1 to 150° C., and keep other conditions unchanged.
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com