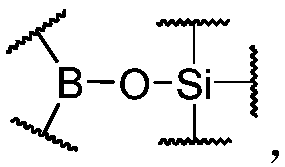Dynamic polymer having non-covalent crosslinking structure and application thereof
A technology of cross-linking structures and polymers, applied in the field of intelligent polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0157] In the preparation process of dynamic polymers, after the compounds as raw materials participate in the reaction with each other, the raw material components can be polymerized with organoborate silicate bonds or common covalent bonds as link points to obtain dynamic polymers with higher molecular weight . Among them, the functional groups contained in the raw material components can be completely reacted or partially reacted, and it is not required that all the organic boronic acid groups and / or organic boronic acid ester groups and the silanol and / or silanol precursors completely react with each other to form The organoborate silicate bond, as long as the organoborate silicate bond formed is sufficient to maintain the dynamic polymer structure.
[0158] In the present invention, the preparation process of the dynamic polymer prepared by the above two embodiments is simple, easy to operate, and highly controllable, so it is a preferred embodiment of the present invention....
Embodiment 1
[0265] A linear structure dynamic polymer is prepared by using a small molecule organoboron compound (I) containing a bifunctional group and a small molecule silicon-containing compound (II) containing a bifunctional group.
[0266]
[0267] Weigh a certain amount of organoboron compound (a) (Methyl vinyl boric acid is prepared by the reaction of methyl lithium, vinyl lithium and trimethyl borate; AIBN is used as initiator, triethylamine is used as catalyst, and methyl ethylene is used. Boronic acid and 1,6-hexanedithiol are prepared by thiol-ene click reaction) dissolved in tetrahydrofuran solvent to prepare a 0.8mol / L solution; weigh a certain amount of silicon-containing compound (b) (using dimethyl Allyl chlorosilane, 1,10-decanedithiol as raw materials, AIBN as initiator, triethylamine as catalyst, prepared by thiol-ene click reaction) dissolved in tetrahydrofuran solvent to prepare 0.8mol / L Solution: Take 20ml of tetrahydrofuran solution with organic boron compound dissolve...
Embodiment 2
[0269] The macromolecular organoboron compound (I) containing bifunctional groups and the macromolecular silicon-containing compound (II) containing bifunctional groups are used to prepare linear structure dynamic polymers.
[0270]
[0271] Weigh 7.5 g of boric acid-terminated polyether (a) in a dry and clean beaker (using 1-hydroxyborole-3-ene as a raw material, and reacting it with hydrobromic acid to obtain 3-bromo- 1-Hydroxyborolane is prepared by hydrocarbylation reaction with polyetheramine using potassium carbonate as a catalyst), 60ml of deionized water is added to it, and it is continuously stirred and dissolved at 50°C. After the dissolution is complete, add a small amount of 1mol / L NaOH solution dropwise; weigh 5.2g of silane-terminated polyether (b) (using dihydroxy-terminated polyethylene glycol as raw material, and esterify it with acrylic acid After the intermediate product is prepared, it is then slowly added to the boric acid-terminated polyether solution with m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| shear viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com