Synthetic analysis and detection method for industrially producing p-fluoronitrobenzene
A technology for the synthesis and analysis of p-fluoronitrobenzene, which is applied in the direction of chemical instruments and methods, analytical materials, and the preparation of organic compounds, etc., can solve the problems that the conversion rate and impurity content of fluoronitrobenzene cannot be effectively controlled, and the detection time can be reached Effects of short, high accuracy and sensitivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
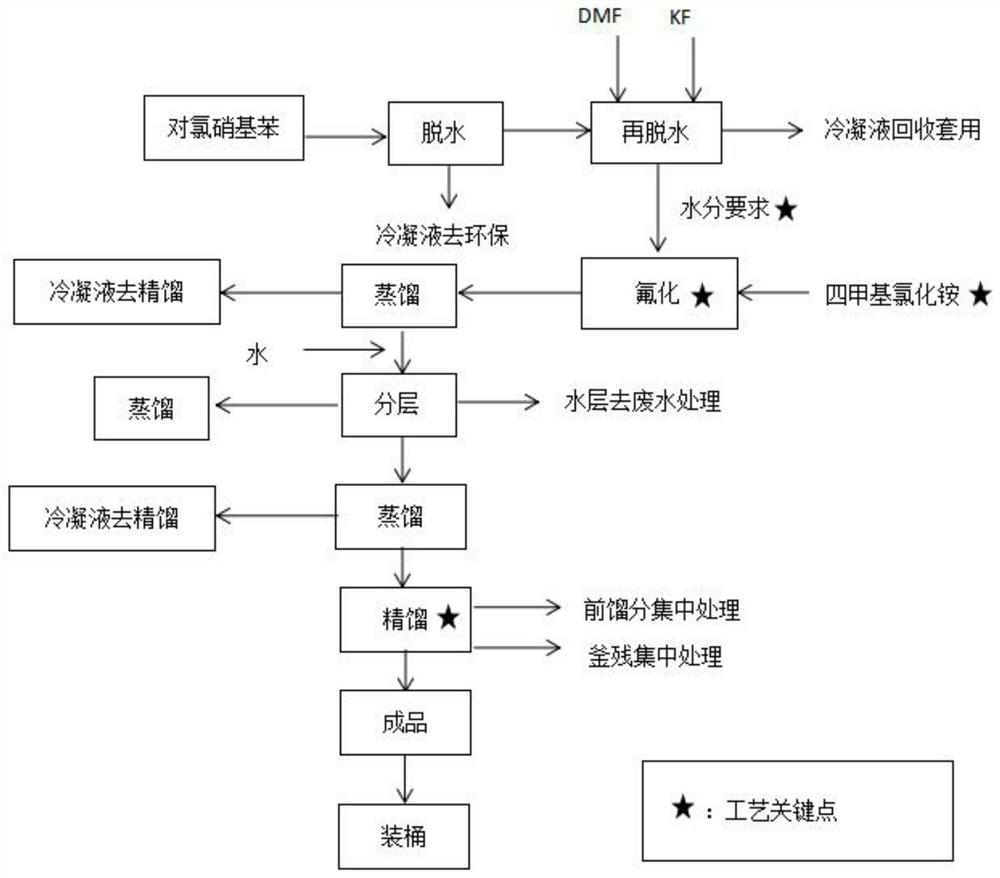
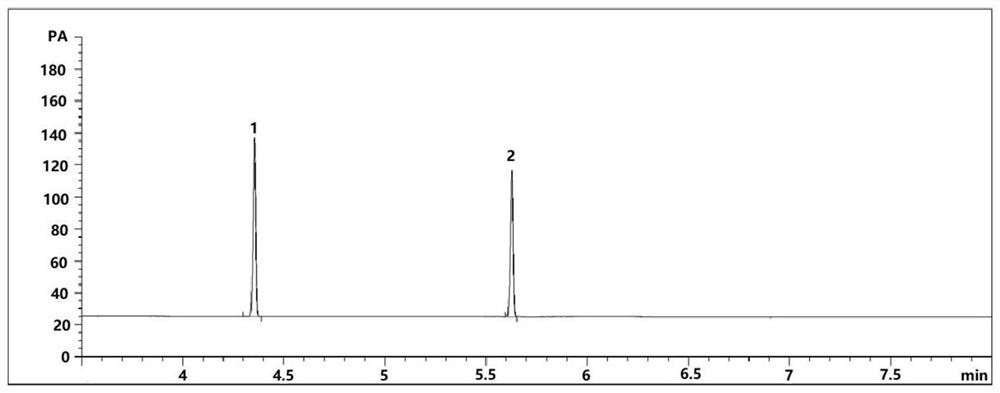
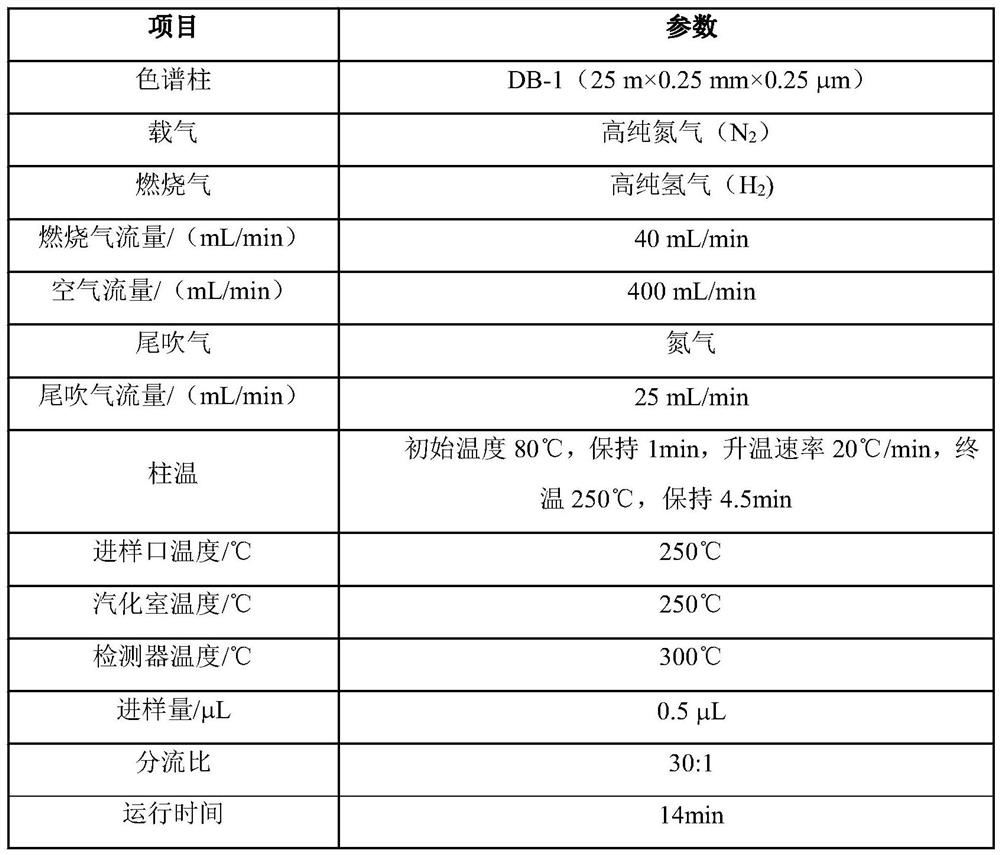
[0047] (1) Open the feed valve of the fluorination kettle, turn on the vacuum pump to suck in 1500 kg of raw material p-chloronitrobenzene, heat up less than 120° C., dehydrate under negative pressure for 3 hours, until no water is evaporated, and the water content is ≤0.05%. Cool down to 50°C with nitrogen gas, add 2000L of N,N-dimethylformamide (DMF), turn on the vacuum system, and vacuum dehydrate at 90-120°C for about 2 hours at a vacuum of -0.075Mpa. 0.05% qualified, filled with nitrogen to normal pressure, opened the inlet and put in the first batch of potassium fluoride 250kg. After reacting and dehydrating for 2 hours, fill it with nitrogen gas to normal pressure, open the inlet and put in the second batch of potassium fluoride 200kg respectively; after reacting and dehydrating for 2 hours, fill it with nitrogen gas to normal pressure, open the inlet and put in the third batch of potassium fluoride respectively 150kg, turn on the vacuum system, vacuum-0.075Mpa, vacuum ...
Embodiment 2
[0050](1) Open the feed valve of the fluorination kettle, turn on the vacuum pump to suck in 1500 kg of raw material p-chloronitrobenzene, heat up less than 120° C., dehydrate under negative pressure for 3 hours, until no water is evaporated, and the water content is ≤0.05%. Cool down to 50°C with nitrogen gas, add 2000L of N,N-dimethylformamide (DMF), turn on the vacuum system, and vacuum dehydrate at 90-120°C for about 2 hours at a vacuum of -0.075Mpa. 0.05% qualified, filled with nitrogen to normal pressure, opened the inlet and put in the first batch of potassium fluoride 350kg. After reacting and dehydrating for 2 hours, fill it with nitrogen gas to normal pressure, open the inlet and put in the second batch of potassium fluoride 200kg respectively; after reacting and dehydrating for 2 hours, fill it with nitrogen gas to normal pressure, open the inlet and put in the third batch of potassium fluoride respectively 150kg, turn on the vacuum system, vacuum-0.075Mpa, vacuum d...
Embodiment 3
[0053] (1) Open the feed valve of the fluorination kettle, turn on the vacuum pump to suck in 1500 kg of raw material p-chloronitrobenzene, heat up less than 120° C., dehydrate under negative pressure for 3 hours, until no water is evaporated, and the water content is ≤0.05%. Cool down to 50°C with nitrogen gas, add 1500L of N,N-dimethylformamide (DMF), turn on the vacuum system, and vacuum dehydrate at 90-120°C for about 2 hours at a vacuum of -0.075Mpa. 0.05% qualified, filled with nitrogen to normal pressure, opened the inlet and put in the first batch of potassium fluoride 350kg. After reacting and dehydrating for 2 hours, fill it with nitrogen gas to normal pressure, open the inlet and put in the second batch of potassium fluoride 200kg respectively; after reacting and dehydrating for 2 hours, fill it with nitrogen gas to normal pressure, open the inlet and put in the third batch of potassium fluoride respectively 150kg, turn on the vacuum system, vacuum-0.075Mpa, vacuum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com