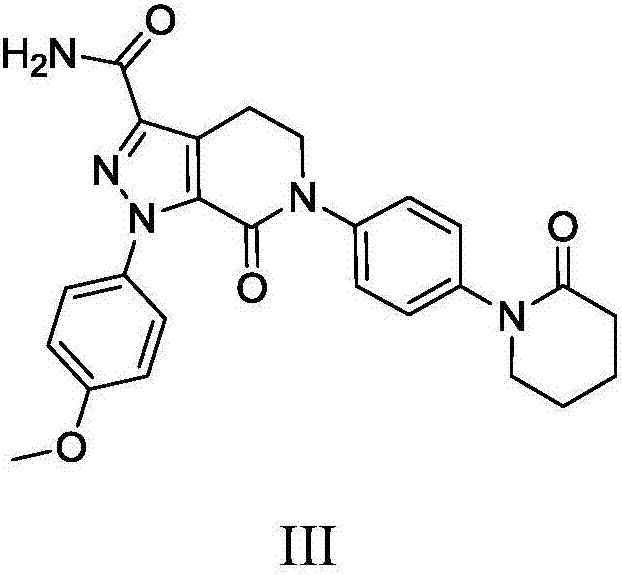
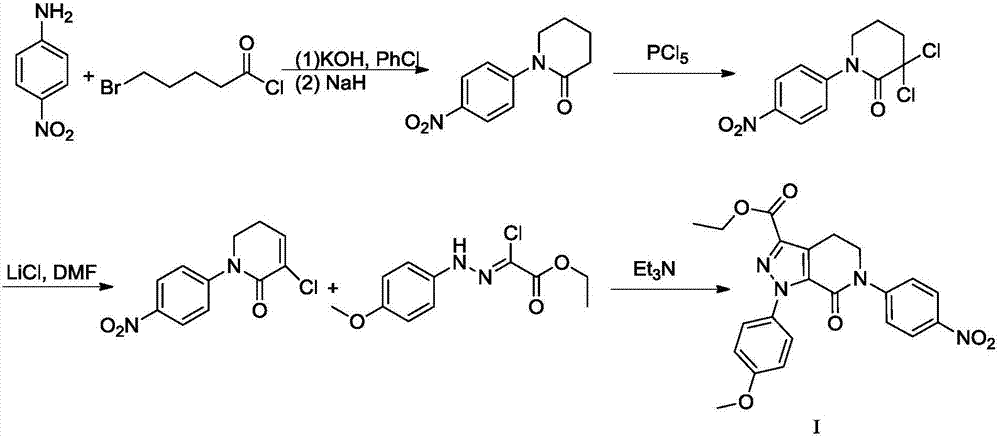
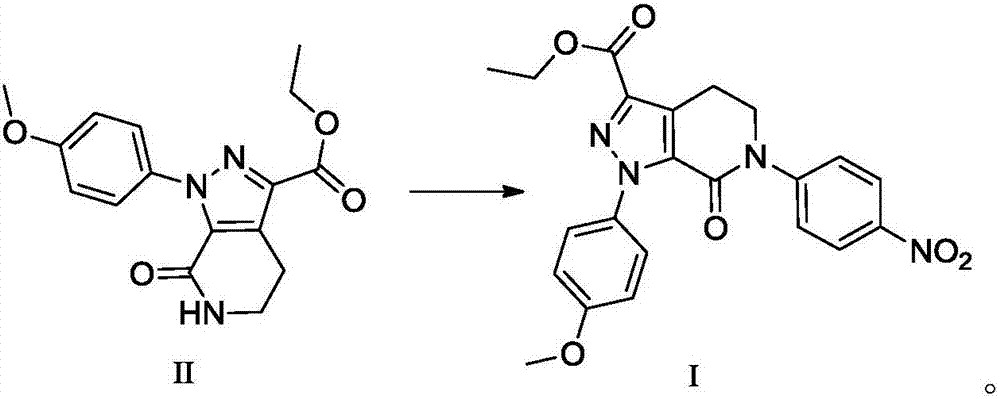
Preparation method of apixaban and intermediates thereof
A technology for apixaban and its intermediates, applied in the field of preparation of apixaban and its intermediates, capable of solving problems such as high production costs, high requirements for production equipment, and unsuitability for industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: Preparation of Apixaban Intermediate II
[0083]
[0084] Under nitrogen protection, 18.2 g of 5,6-dihydro-3-(4-morpholinyl)-2(1H)-pyridone and [(4-methoxyphenyl)hydrazine were added to 540 mL of ethyl acetate ] Ethyl chloroacetate 38.5g, add triethylamine 20.3g under stirring, heat the reaction to 75-85°C and stir for 16 hours. Cool to 15-25°C, filter and add 32 mL of trifluoroacetic acid dropwise, and stir at 15-25°C for 2 hours. Add 10% sodium bicarbonate aqueous solution to quench (the said mass percent refers to the percentage of the mass of sodium bicarbonate in the total mass of sodium bicarbonate aqueous solution), and then extract once with ethyl acetate. Organic phase is that 10% sodium bicarbonate aqueous solution and mass percentage are 15% salt water washings (described mass percentage is meant that the quality of sodium chloride accounts for the percentage of the total mass of saline solution) with mass percentage, without water and sodi...
Embodiment 2
[0085] Embodiment 2: Preparation of Apixaban Intermediate II
[0086]
[0087] Under nitrogen protection, 18.2 g of 5,6-dihydro-3-(4-morpholinyl)-2(1H)-pyridone and [(4-methoxyphenyl)hydrazine were added to 360 mL of ethyl acetate ] 30.8 g of ethyl chloroacetate, 19.4 g of diisopropylethylamine was added under stirring, and the reaction was heated to 85-95° C. and stirred for 20 hours. Cool to 15-25°C, filter and add 35 mL of trifluoroacetic acid dropwise, and stir at 15-25°C for 2 hours. Adding mass percentage composition is that 10% sodium bicarbonate aqueous solution is quenched (the described mass percentage composition refers to the percentage that the quality of sodium bicarbonate accounts for the total mass of sodium bicarbonate aqueous solution, the same below), then extracts with ethyl acetate for 1 Second-rate. Organic phase is that 10% sodium bicarbonate aqueous solution and mass percentage are 15% salt water washings (described mass percentage is meant that th...
Embodiment 3
[0089] Embodiment 3: Preparation of Apixaban Intermediate II
[0090]
[0091] Under nitrogen protection, 18.2 g of 5,6-dihydro-3-(4-morpholinyl)-2(1H)-pyridone and [(4-methoxyphenyl)hydrazino]chloride were added to 720 mL of toluene 64.1 g of ethyl acetate, 92.6 g of tri-n-butylamine was added under stirring, the reaction was heated to 95-105°C and stirred for 12 hours. Cool to 15-25°C, filter and add 50 mL of trifluoroacetic acid dropwise, and stir at 15-25°C for 2 hours. Adding mass percentage composition is that 10% sodium bicarbonate aqueous solution is quenched (the described mass percentage composition refers to the percentage that the quality of sodium bicarbonate accounts for the total mass of sodium bicarbonate aqueous solution, the same below), then extracts with ethyl acetate for 1 Second-rate. Organic phase is that 10% sodium bicarbonate aqueous solution and mass percentage are 15% salt water washings (described mass percentage is meant that the quality of so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com