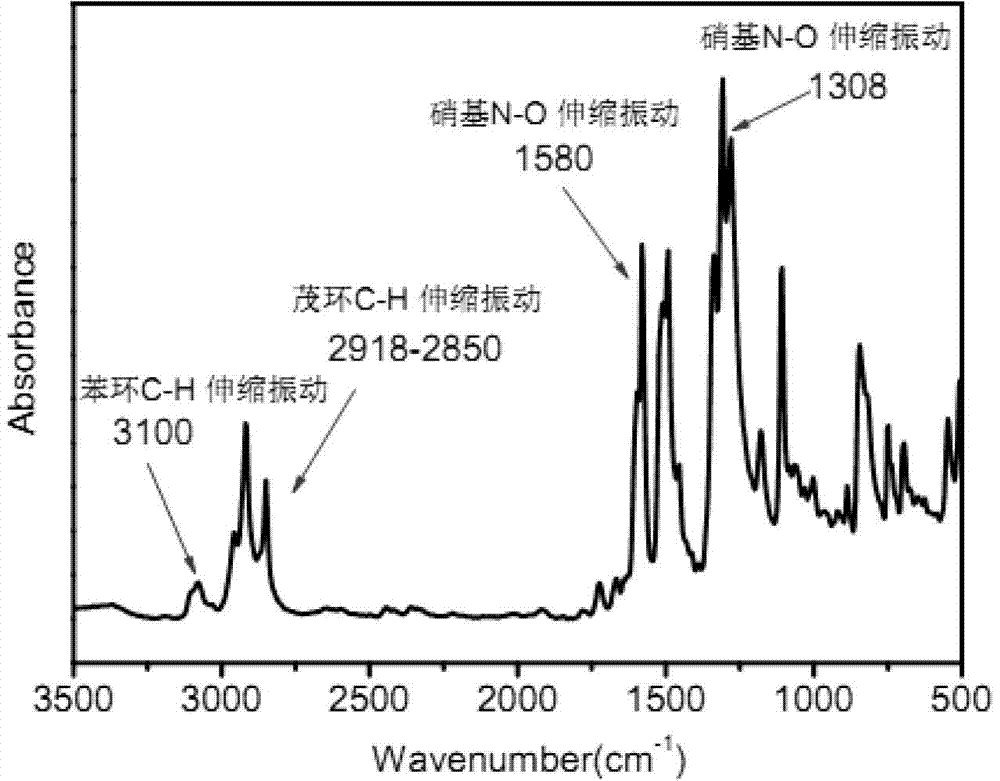
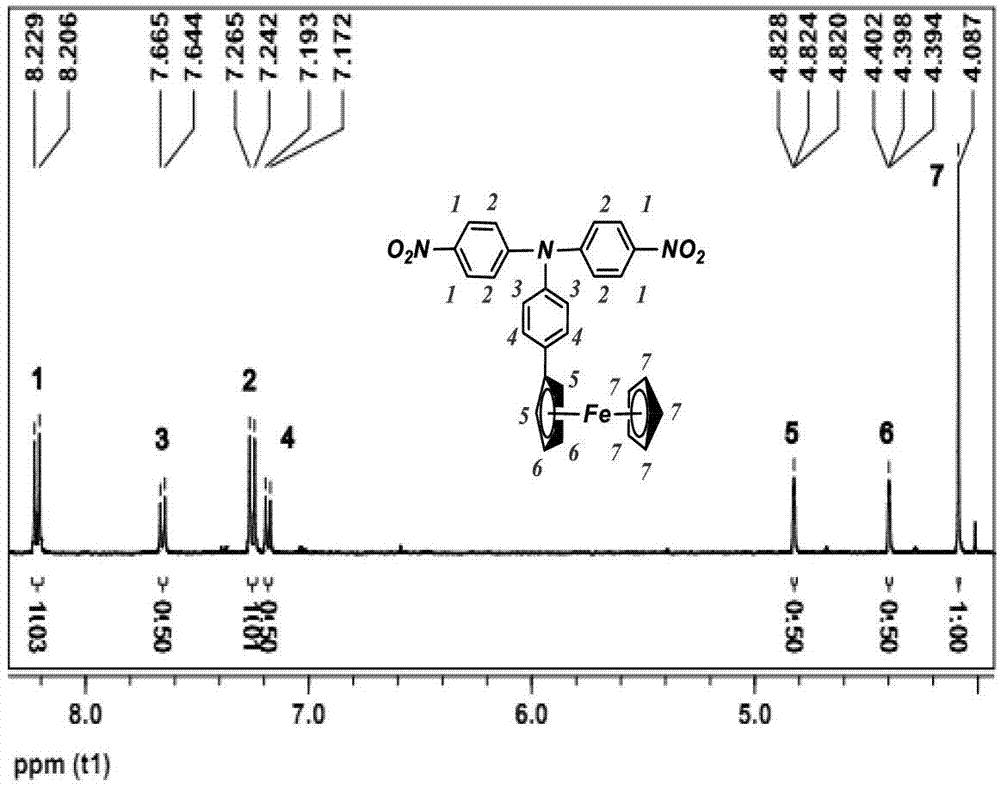
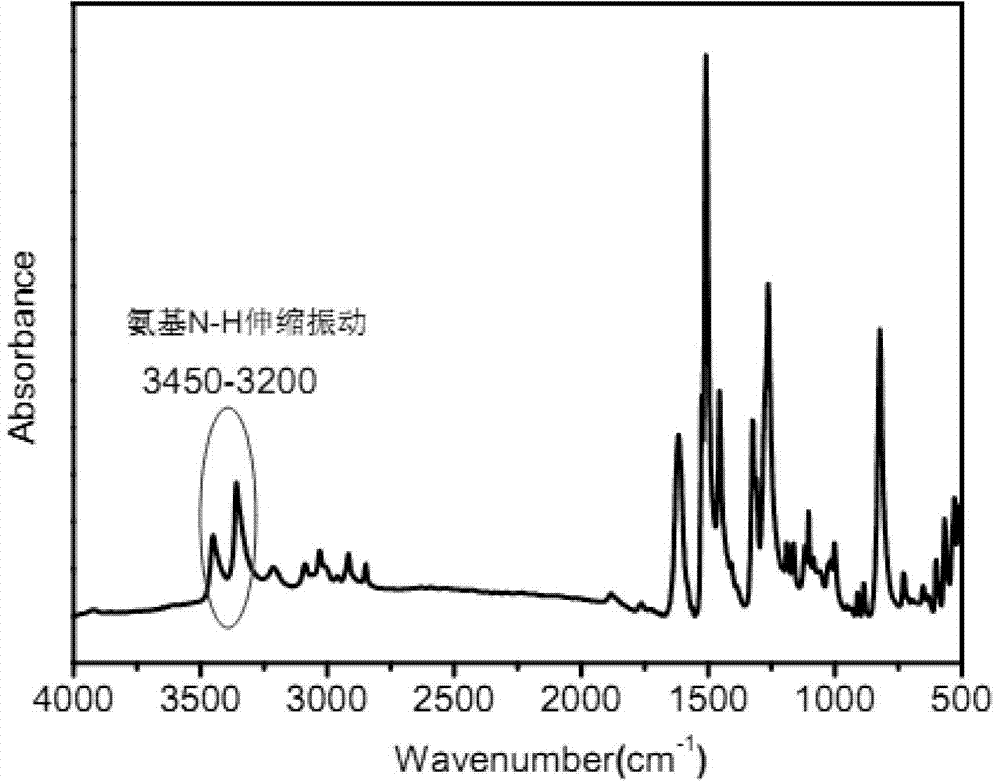
4,4'-diamido-4''-ferrocenyl triphenylamine and preparation method thereof
A technology of ferrocenyl aniline and ferrocenyl, which is applied in the field of bisaminoaromatic organic compounds and their preparation, and achieves the effects of simple operation, simple synthesis process and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] a) Preparation of 4,4'-dinitro-4"-ferrocenyltriphenylamine:
[0036] In a 100ml single-necked flask equipped with reflux condensation and a magnet, add 2.21g of 4-ferrocenylaniline, 2.26g of p-nitrofluorobenzene and 4.42g of anhydrous potassium carbonate, and add 30ml of DMSO as a solvent, and start the magnetic stirring and condensed water, and heated to 130°C with a constant temperature oil bath, reacted for 24 hours, cooled to room temperature after the reaction, poured the reaction solution into 800ml deionized water and precipitated for 24 hours, poured off the supernatant, and obtained a dark red viscous Precipitate. Purified by column separation method, the eluent is ethyl acetate and petroleum ether, the ratio is 1:5, after being concentrated by rotary evaporation, it is dried in a vacuum oven at 60°C for 12 hours, and the red solid powder is obtained as the final product 4,4' - Dinitro-4"-ferrocenyltriphenylamine. The mass of product obtained was 2.90 g.
[0...
Embodiment 2
[0042] a) Preparation of 4,4’-dinitro-4”-ferrocenyltriphenylamine
[0043] In a 250ml single-necked flask equipped with reflux condensation and a magnet, add 6.9g of 4-ferrocenylaniline, 10.58g of p-nitrofluorobenzene and 20.7g of anhydrous potassium carbonate, and add 100ml of DMSO as a solvent, and start the magnetic stirring and condensed water, and heated to 150°C with a constant temperature oil bath to react for 48 hours, cooled to room temperature after the reaction, poured the reaction solution into 1600ml deionized water and precipitated for 24 hours, poured off the supernatant, and obtained a dark red viscous precipitation. Purified by column separation method, the eluent is ethyl acetate and petroleum ether, the ratio is 1:5, after being concentrated by rotary evaporation, it is dried in a vacuum oven at 60°C for 12 hours, and the red solid powder is obtained as the final product 4,4' -Dinitro-4 "-ferrocenyl triphenylamine. The product quality obtained is 9.76g;
...
Embodiment 3
[0049] a) Preparation of 4,4'-dinitro-4"-ferrocenyltriphenylamine:
[0050] In a 100ml single-necked flask equipped with reflux condensation and a magnet, add 2.21g of 4-ferrocenylaniline, 2.26g of p-nitrofluorobenzene and 3.65g of cesium fluoride, and add 30ml of DMF as a solvent, start magnetic stirring and Condensed water, heated to 130°C with a constant temperature oil bath, reacted for 24 hours, cooled to room temperature after the reaction, poured the reaction solution into 800ml deionized water and precipitated for 24 hours, poured off the supernatant, and obtained a dark red viscous precipitation. Purified by column separation method, the eluent is ethyl acetate and petroleum ether, the ratio is 1:5, after being concentrated by rotary evaporation, it is dried in a vacuum oven at 60°C for 12 hours, and the red solid powder is obtained as the final product 4,4' -Dinitro-4 "-ferrocenyltriphenylamine. The quality of the product obtained is 2.84g;
[0051] Yield 68.4%, me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com