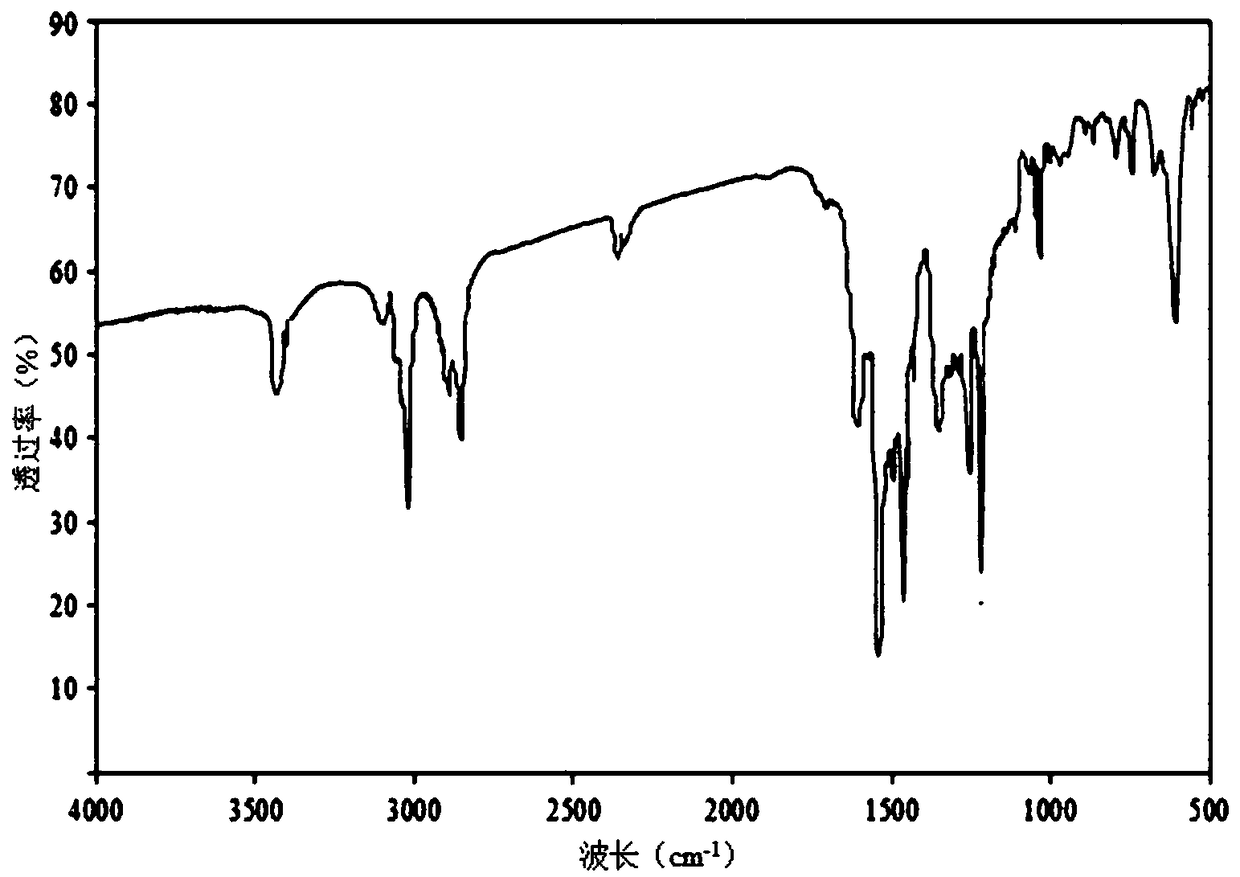
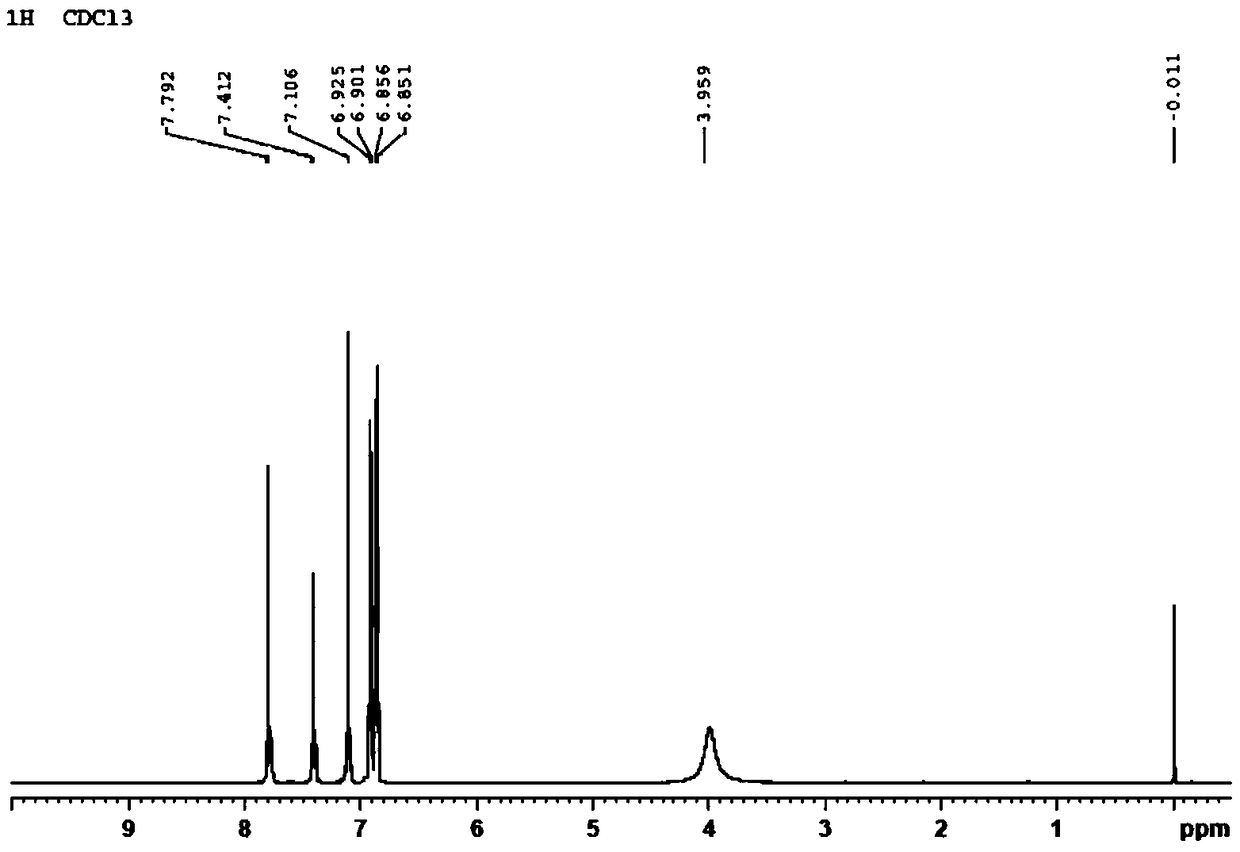
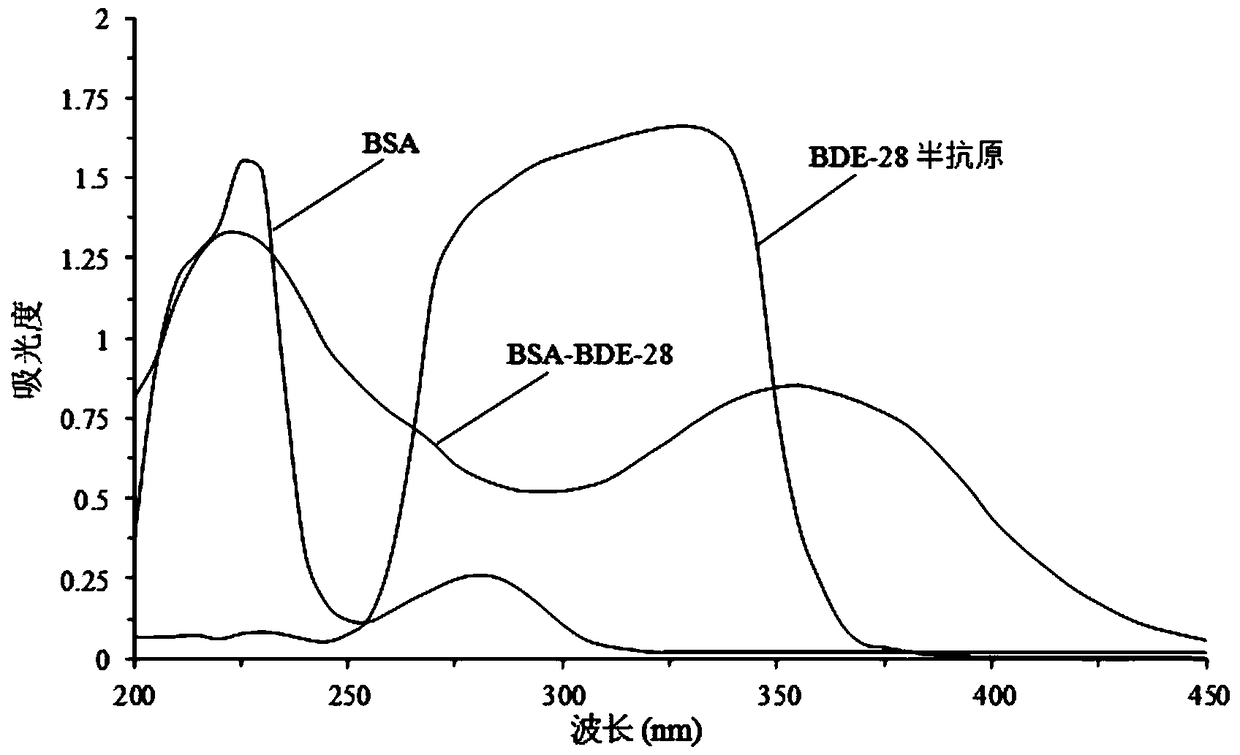
Preparation method and application of 2,4,4'-tribromobiphenyl ether immunogen
A technology of tribrominated diphenyl ether and dibrominated diphenyl ether, applied in the field of biochemistry, can solve problems such as less research, and achieve the effects of simple synthesis method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1BD
[0032] The preparation of embodiment 1BDE-28 immunogen
[0033] Step 1: Dissolve 1.04g of 2,4-dibromophenol (4.13mmol) in 8mL of dry DMF, cool to 0°C in an ice bath; add 0.199g of sodium hydride (8.28mmol) in batches, and stir in an ice bath After 20 minutes, the reaction mixture was left at room temperature for 10 minutes; then 0.583 g of p-fluoronitrobenzene (4.13 mmol) was slowly added, heated and stirred at 90°C for 2 hours; 10 mL of ultrapure water (ddH 2 O), after slightly shaking several times, use CH 2 Cl 2 Extraction (25mL × 3), combined organic extracts, with anhydrous MgSO 4 After drying and concentrating by rotary evaporation, after separation and purification by silica gel column chromatography (eluent: n-hexane: ether = 2:3 (v / v)), the orange-yellow oily product 4-nitro-2',4' was obtained - Dibromodiphenyl ether (1.018 g).
[0034] In the second step, 0.485g of the above-mentioned 4-nitro-2', 4'-dibromodiphenyl ether (1.30mmol) was dissolved in 8mL of n-butyl...
Embodiment 2BD
[0043] The preparation of embodiment 2BDE-28 polyclonal antibody
[0044] Healthy New Zealand white rabbits were immunized with the immunogen BSA-BDE-28 prepared in Example 1 to prepare BDE-28 polyclonal antibody. For the initial immunization of rabbits, an equal proportion of Freund's complete adjuvant (CFA) and the immunogen was used (the protein concentration was 1 mg / mL), and for subsequent booster immunizations, an equal proportion of Freund's incomplete adjuvant (IFA) and the immunogen was used Mixed (protein concentration is 1mg / mL), the specific immunization scheme of white rabbit is shown in Table 2, adopts indirect competitive ELISA method to measure antiserum titer, the change of BDE-28 antibody titer is shown in the appendix Figure 5 As shown, the change of antiserum titer is the process of immune response again in purebred male New Zealand white rabbits. Both rabbits No. 1 and No. 2 used BSA-BDE-28 as the immunogen. With the increase of immunization times, rabbit...
Embodiment 3BD
[0047] The specificity of embodiment 3BDE-28 polyclonal antibody
[0048] 2,2',4,4'-tetrabromodiphenyl ether (CAS: 5436-43-1), 2,3',4,4'-tetrabromodiphenyl ether (CAS: 189084-61-5 ), 2,3,3',4,4'-pentabromodiphenyl ether (CAS: 373594-78-6), 2,2',4,4',5-pentabromodiphenyl ether (CAS: 60348 -60-9), 2,2',4,4',6-pentabromodiphenyl ether (CAS: 189084-64-8), 2,2',4,4',5,5'-hexabromodiphenyl ether Diphenyl ether (CAS: 67774-32-7), 2,2',3,4,4',5',6-heptabromodiphenyl ether (CAS: 68928-80-3), decabromodiphenyl ether (CAS: 1163-19-5) As a structural analogue of BDE-28, the specificity of the BDE-28 polyclonal antibody was determined by indirect competition ELISA, and the cross-reaction between pAb-BDE-28 and BDE-28 analogues was calculated ( CR) to evaluate the specificity of pAb-BDE-28, wherein, the chemical structure and cross-reactivity results of BDE-28 analogues are shown in Table 3. It can be concluded that the cross-reactivity rates between pAb-BDE-28 and compounds similar to B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com