Preparation method of multi-receptor tyrosine kinase inhibitor and intermediate thereof
A tyrosine kinase and receptor technology, applied in the field of drug synthesis, can solve the problems of unsuitability for industrial production, high reaction temperature, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
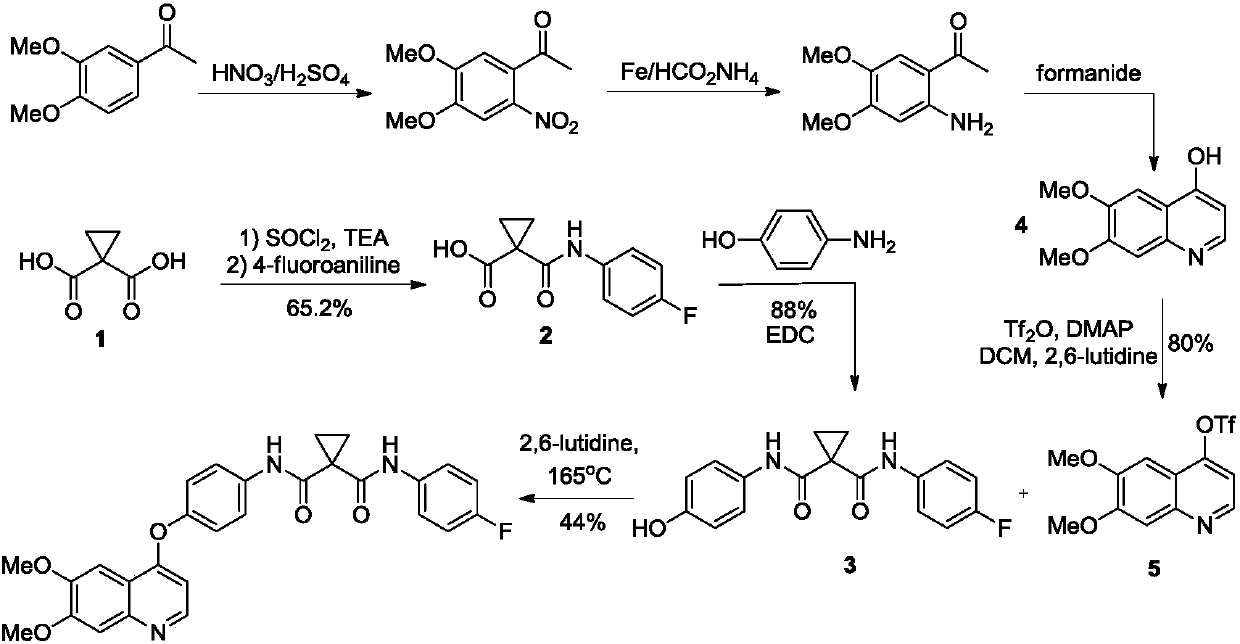
Examples
Embodiment 1
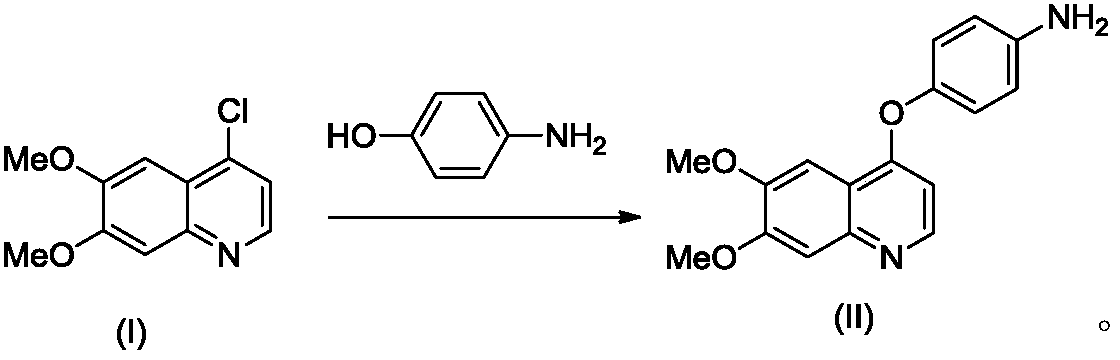
[0041] Example 1 Preparation of 4-(6,7-dimethoxy-quinolin-4-yloxy)aniline (II)
[0042]
[0043] Add compound (I) (1000g, 4.47mol) and 4-aminophenol (690g, 6.32mol) into the reaction flask, add N,N-dimethylacetamide (6L), cool down to 0-5°C, add dropwise Sodium tert-butoxide (860g) in N,N-dimethylacetamide (4L) suspension, after dropping, raise the temperature to 100-110°C and keep it warm for 4-5 hours. Cool down to -5-0°C, add ice water (20 L), stir and crystallize for 15-16 hours. After filtering, the filter cake was washed with a small amount of water, and air-dried at 50° C. for 15-16 hours to obtain 1180 g of a light yellow solid with a yield of 89.0% and a purity of 99.7% by HPLC.
Embodiment 2
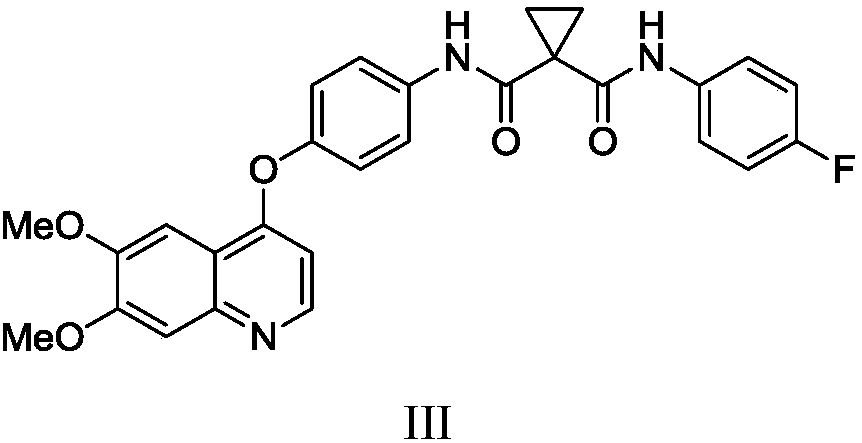
[0044] Example 2N-(4-{[6,7-bis(methoxy)quinolin-4-yl]oxy}phenyl)-N 1 Preparation of -(4-fluorophenyl)cyclopropane-1,1-diacid amide (III)
[0045]
[0046] 1-(4-fluorophenylcarbamoyl)cyclopropanecarboxylic acid (500g, 2.24mol), 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethylurea Hexafluorophosphate (850g, 2.24mol), triethylamine (380g) and N,N-dimethylacetamide (2L) were added to the reaction flask, and compound (II) (550g, 1.86mol) was added under stirring, and After completion, the reaction was stirred at 60°C for 5-6 hours. Add water (16L) to the reaction solution, stir and crystallize at 10-20°C for 1-2 hours, filter, and vacuum-dry the filter cake at 40-50°C for 23-24 hours to obtain 867g of off-white solid, yield 93.1%, HPLC purity 99.5 %.
[0047]1 H NMR (400MHz, d 6 -DMSO): δ10.2 (s, 1H), 10.08 (s, 1H), 8.4 (s, 1H), 7.8 (m, 2H), 7.65 (m, 2H), 7.5 (s, 1H), 7.4 ( s, 1H), 7.24 (m, 2H), 7.15 (m, 2H), 6.4 (s, 1H), 4.0 (d, 6H), 1.5 (s, 4H).
[0048] MS(ESI): m / z 502[...
Embodiment 3
[0050] 1-(4-fluorophenylcarbamoyl)cyclopropanecarboxylic acid (200g, 0.90mol), O-benzotriazole-tetramethyluronium hexafluorophosphate (307g, 0.81mol), DIEA (50ml) Add N,N-dimethylacetamide (3 L) into the reaction flask, add compound (II) (220 g, 0.74 mol) under stirring, after the addition is complete, stir and react at 70°C for 3-4 hours. Add water (6L) to the reaction solution, stir and crystallize at 10-20°C for 1-2 hours, filter, and vacuum-dry the filter cake at 40-50°C for 23-24 hours to obtain 334g of off-white solid, yield 90.1%, HPLC purity 98.5 %, nuclear magnetic data is basically the same as embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com