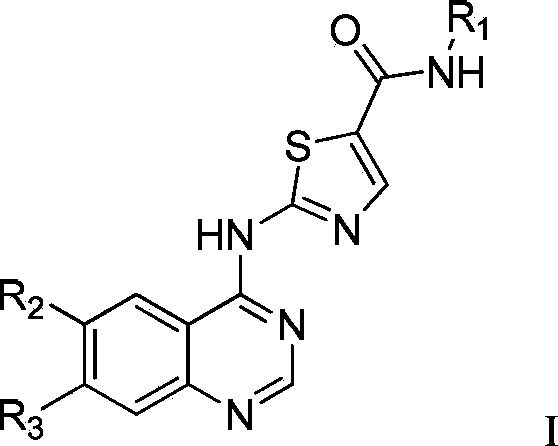
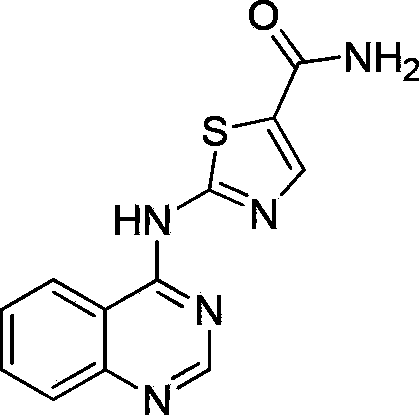
2-(quinazoline-4-amino)-5-thiazole carboxamide derivatives and biological medicine use thereof
A technology of thiazole carboxamide and quinazoline, applied in the field of biopharmaceuticals, can solve the problems of difficult to overcome tumor drug resistance, low structural diversity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Virtual screening based on DOCK program and GOLD program
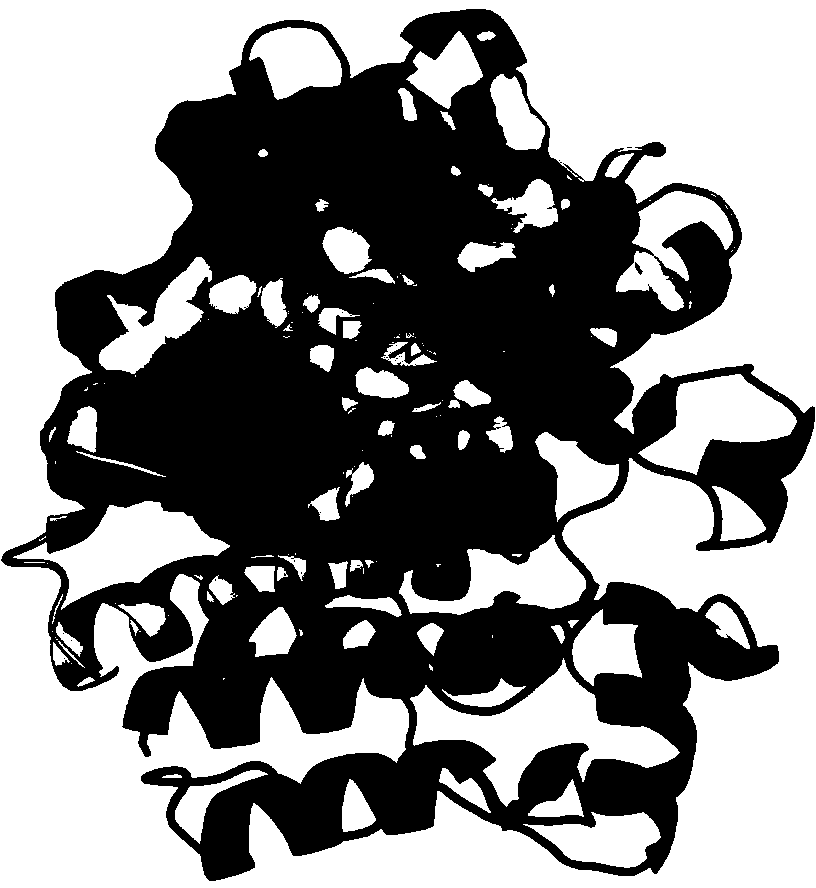
[0019] The crystal structure of Src kinase is taken from the Protein Data Bank protein database (PDB Code: 2H8H)
[0020] , And use the Chimera program to preprocess the complex crystal structure, that is, delete saracatinib and water molecules, and add hydrogen atoms. The active pocket of Src kinase is defined as around saracatinib Space within.
[0021] The main steps of virtual screening include:
[0022] 1) Screen the known chemical database Enamine with the GOLD program, and use the scoring function E int =E vdw +E elec (E int : Ligand-receptor interaction energy; E vdw : Van der Waals action energy; Eelec: electrostatic action energy to score the screening results;
[0023] 2) Select the top 500 compounds in the score;
[0024] 3) Use the more accurate scoring function GOLD Score in the GOLD program to re-evaluate the preliminary screening results of DOCK; 4) Select compounds with a GOLD Score great...
Embodiment 2
[0025] Example 2 Biological Activity Test
[0026] Select candidate compounds from the Enamine small molecule database (http: / / www.asinex.com), purchase them, and conduct activity tests.
[0027] The selected compounds were tested for their inhibition of Src kinase activity by Electrophoretic Mobility Shift Assay (EMSA), and the experiment was performed on a 384-well plate. The positive control substance of Src kinase inhibitor is Staurosporine. Both the test drug and the positive drug are dissolved in DMSO and mixed with kinase buffer (20mM HEPES, pH 7.5, 0.01% Triton X-100, 5mM MnCl2, 2mM DTT), and shaken Mix for 10 minutes on the vessel, then add Src kinase to the well and incubate for 10 minutes at room temperature. The phosphoacceptor peptide FAM-P4 and ATP containing tyrosine were added to the wells to finally obtain an enzyme reaction system of 25uL / well, and incubated at 28°C for 60min. At the end of the incubation, add 25uL of stop buffer (100mM HEPES, pH 7.5, 0.015% Bri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com