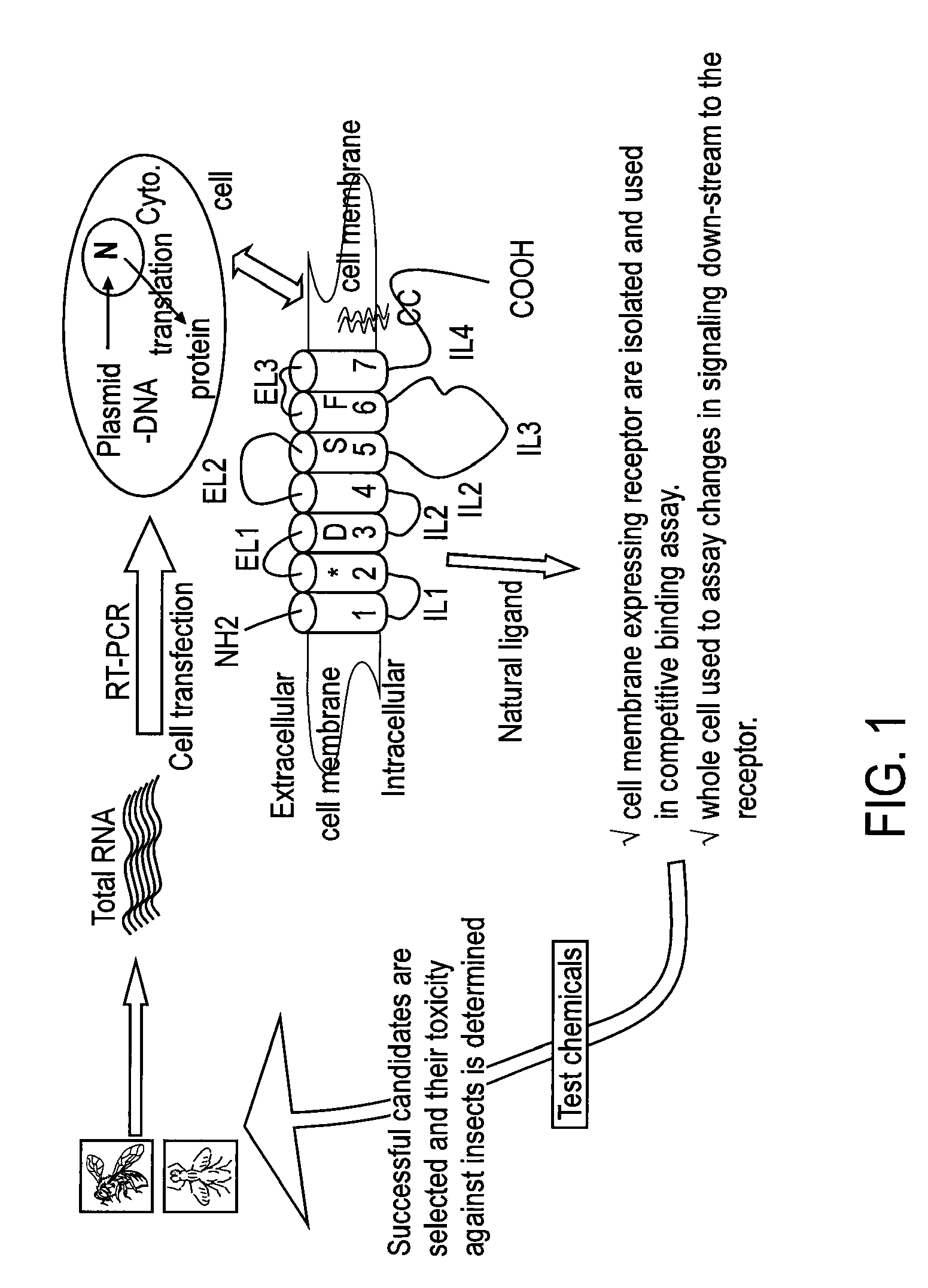
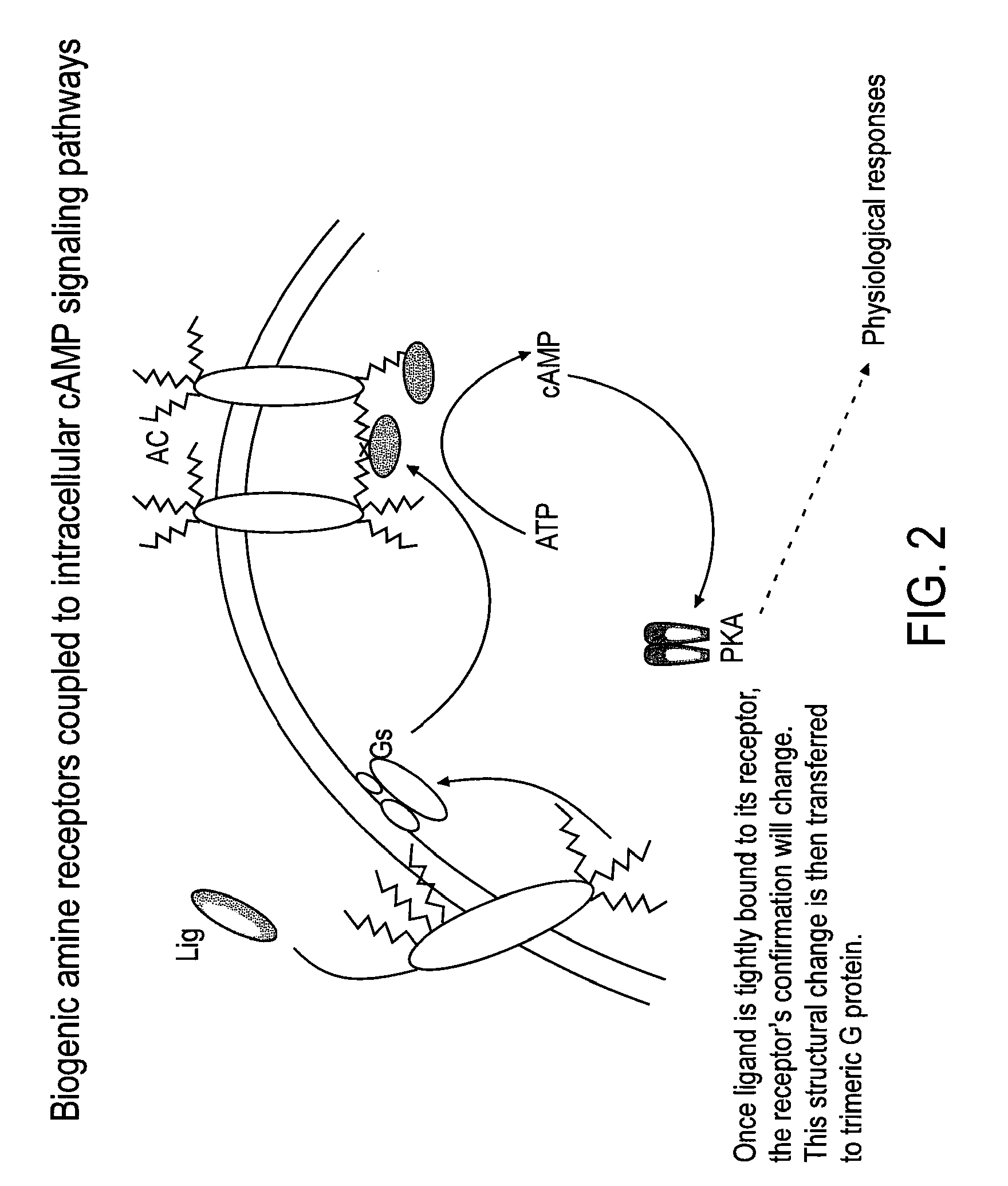
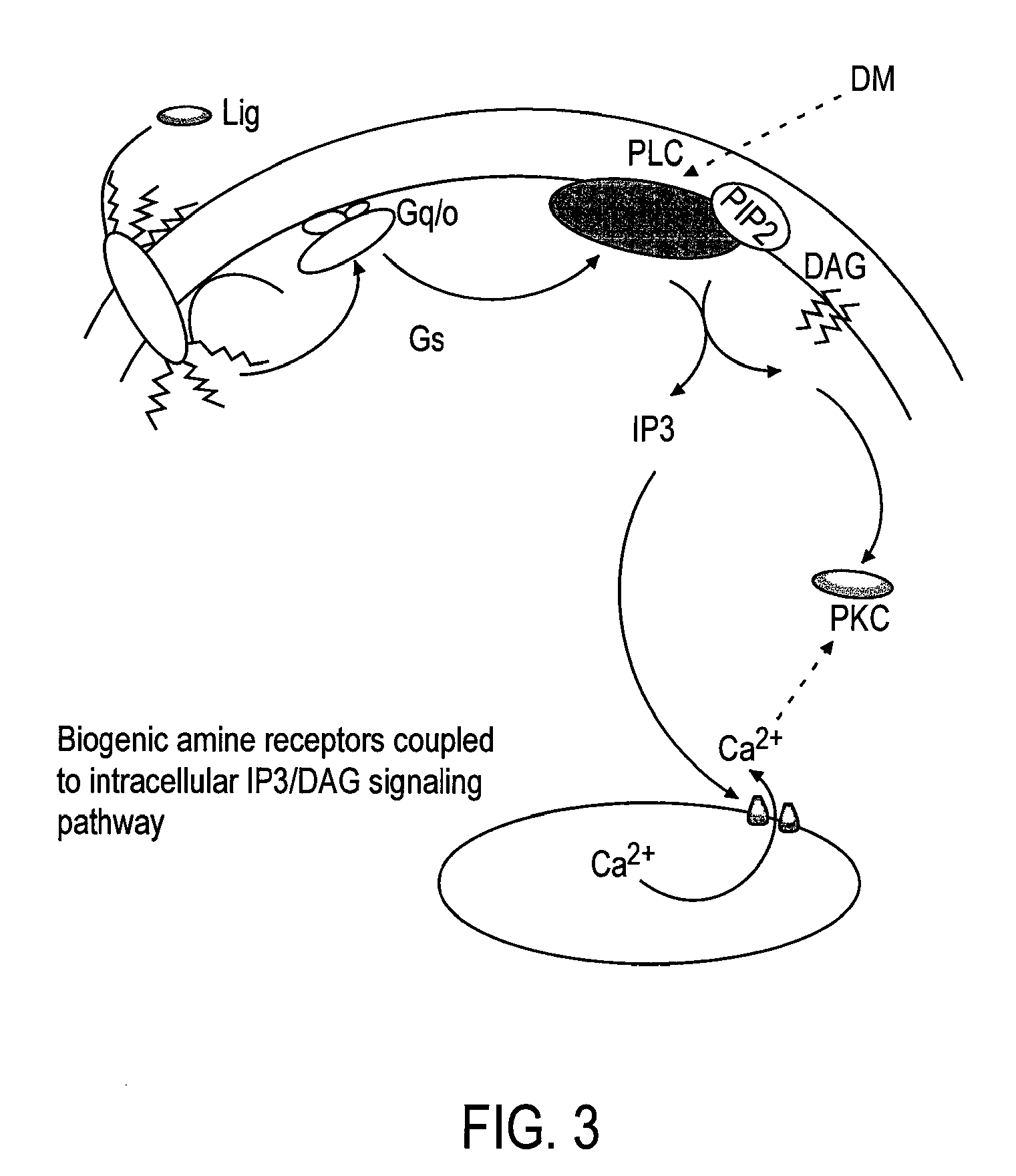
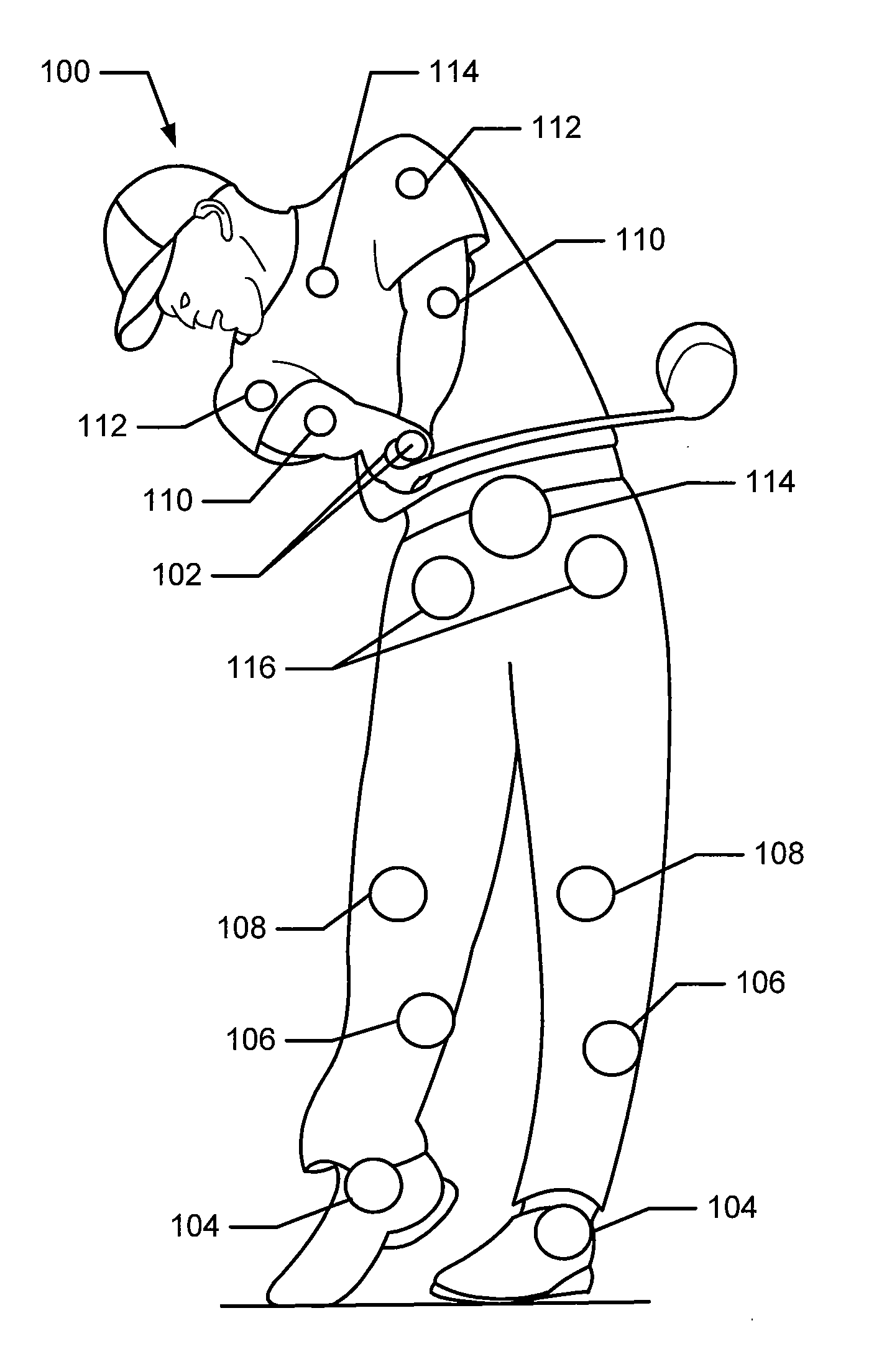
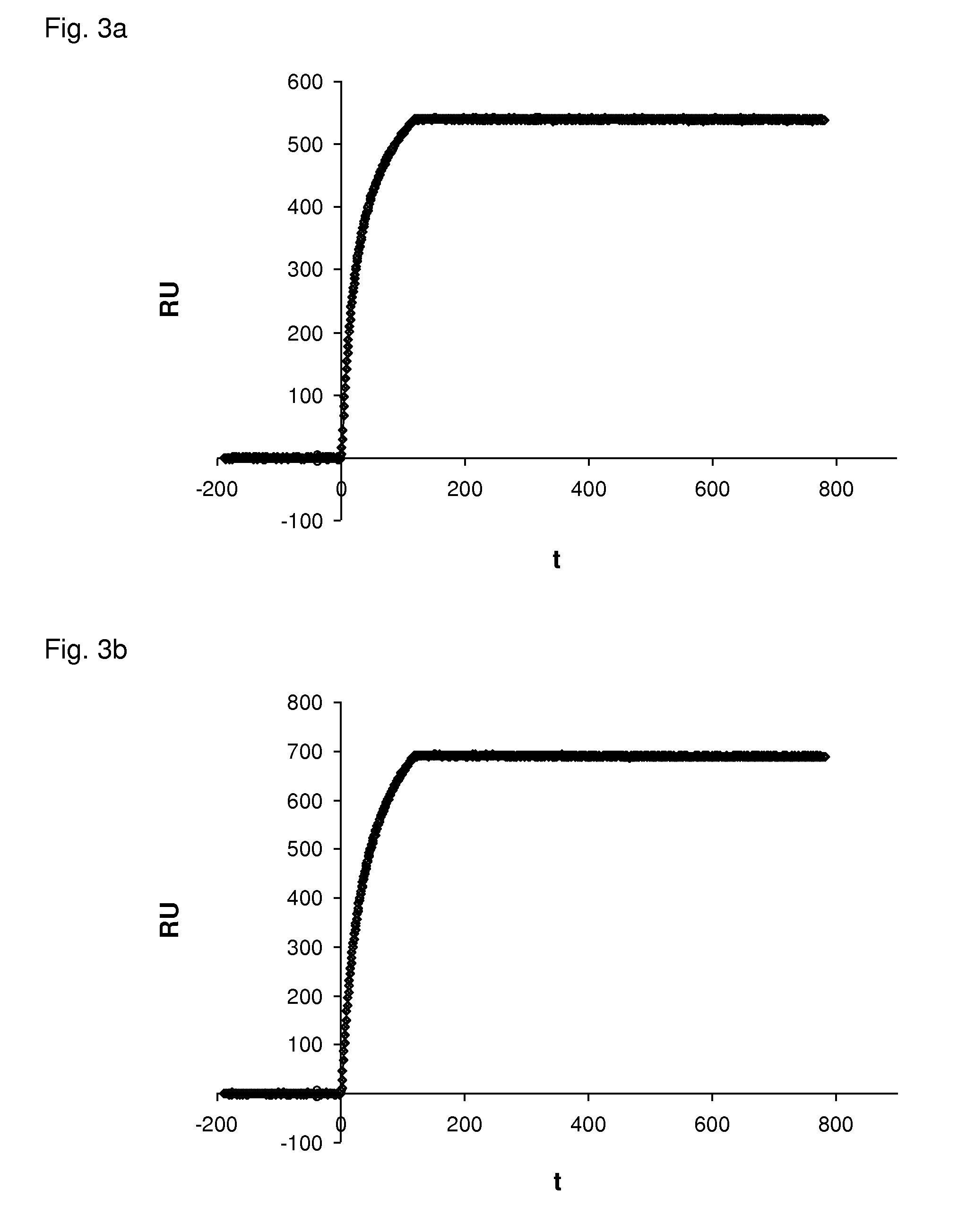
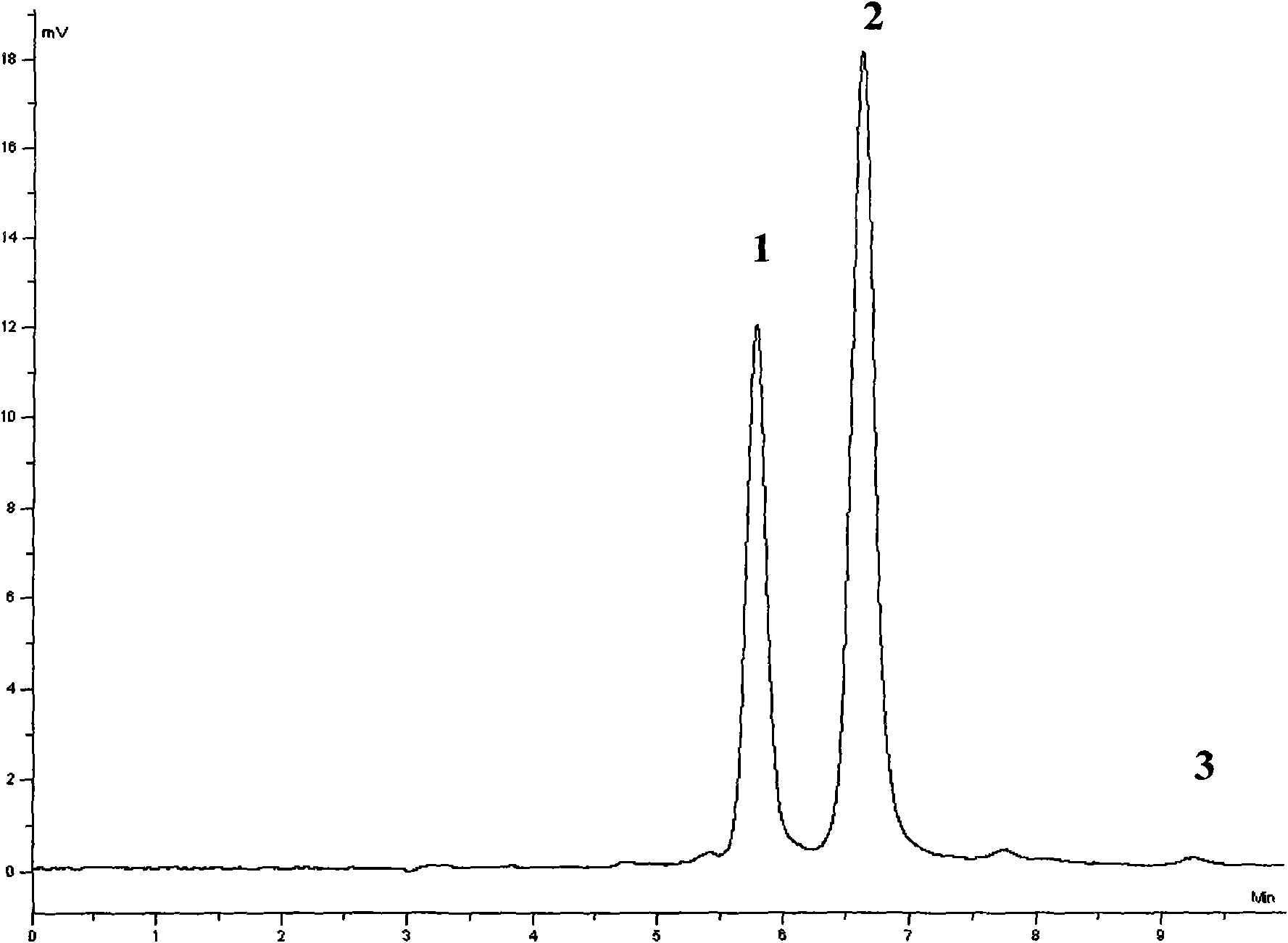
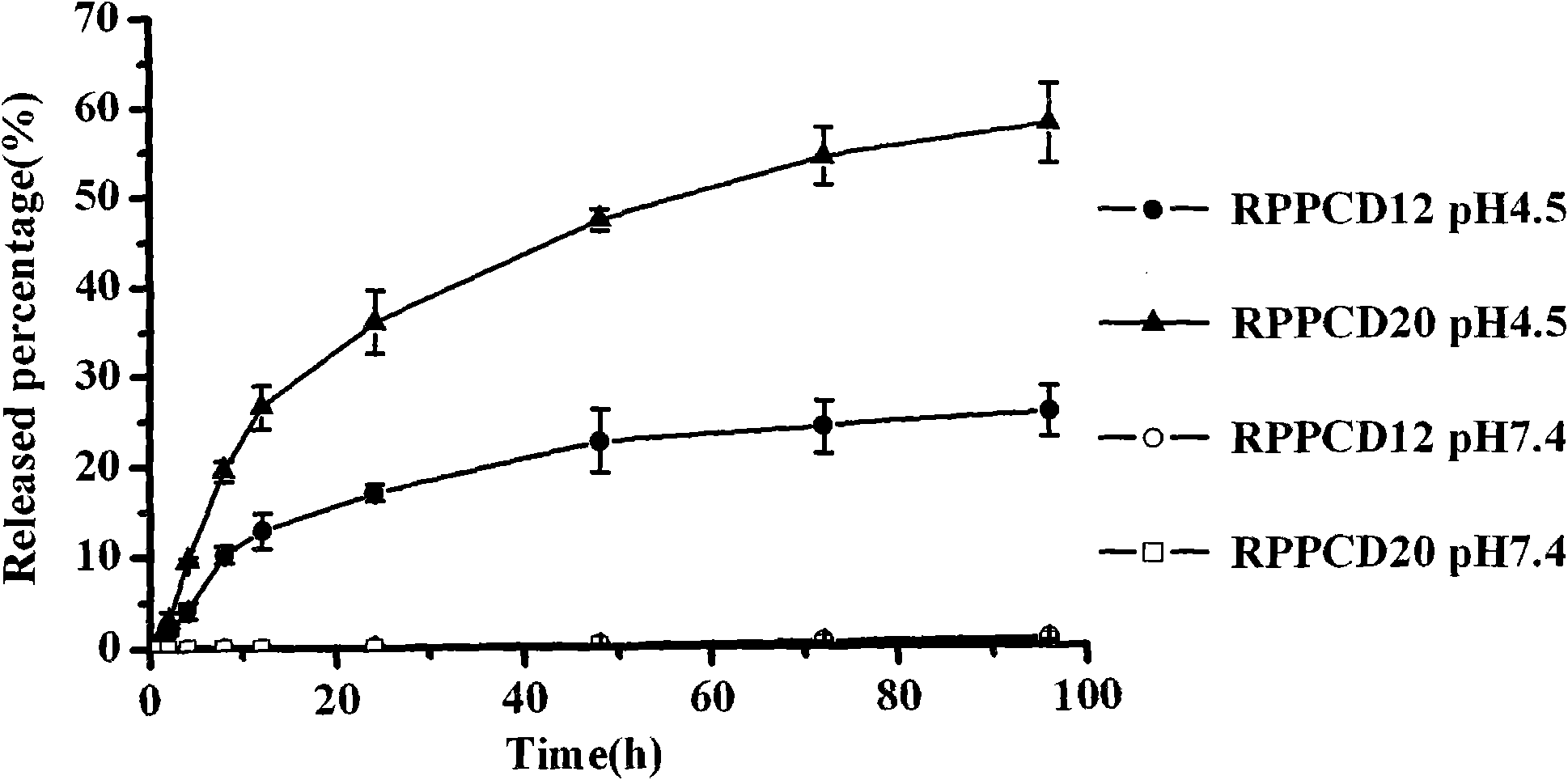
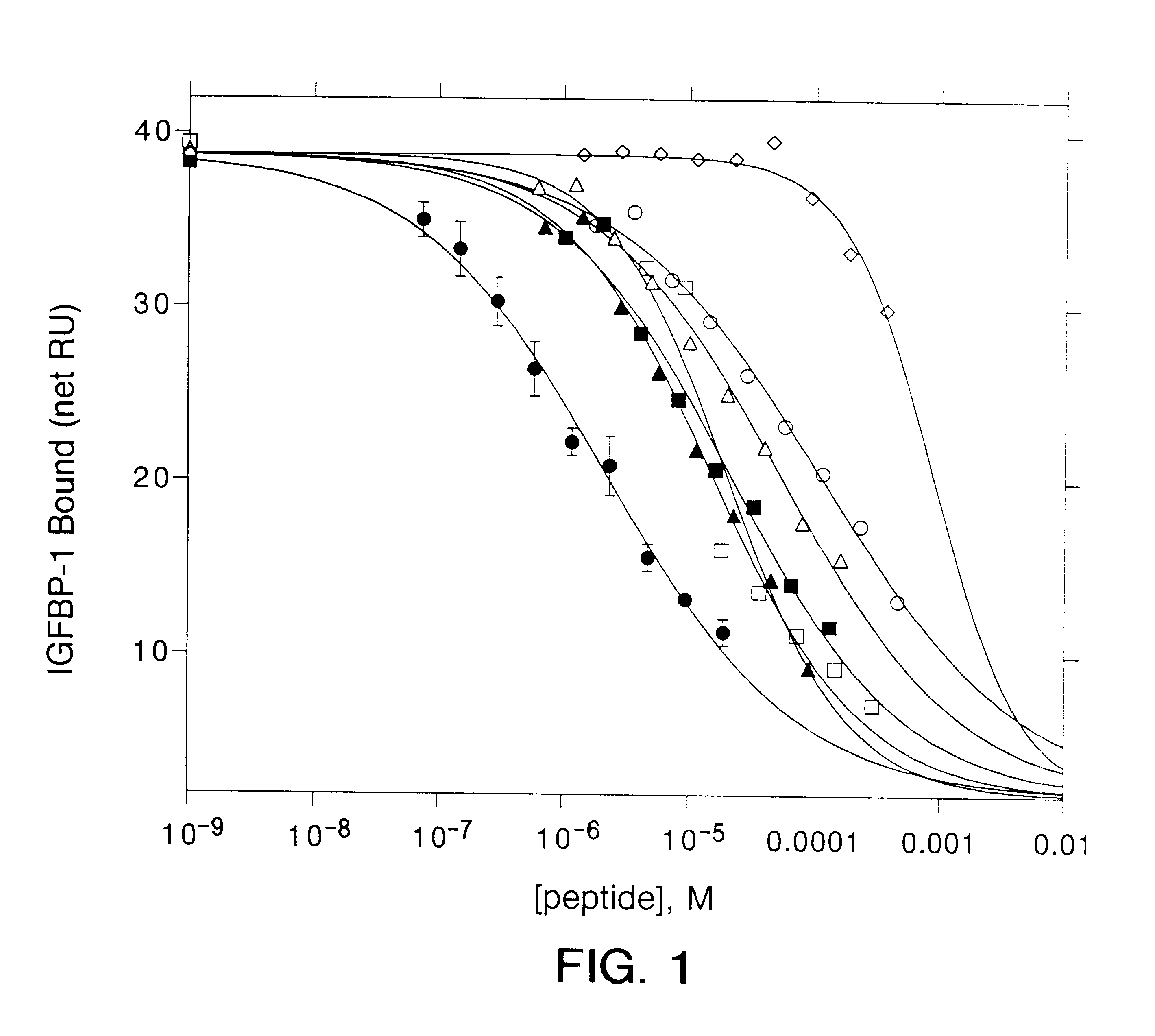
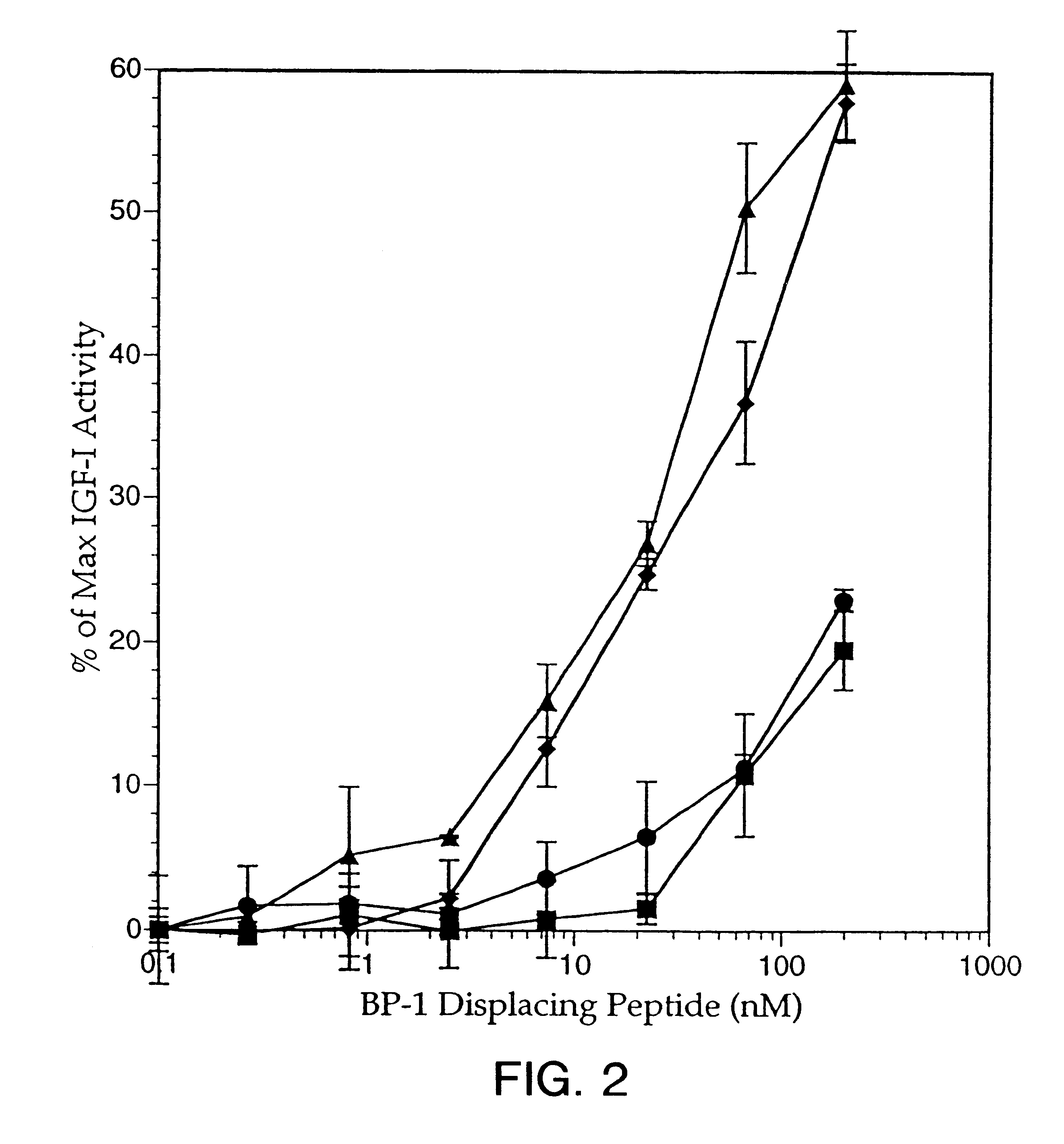
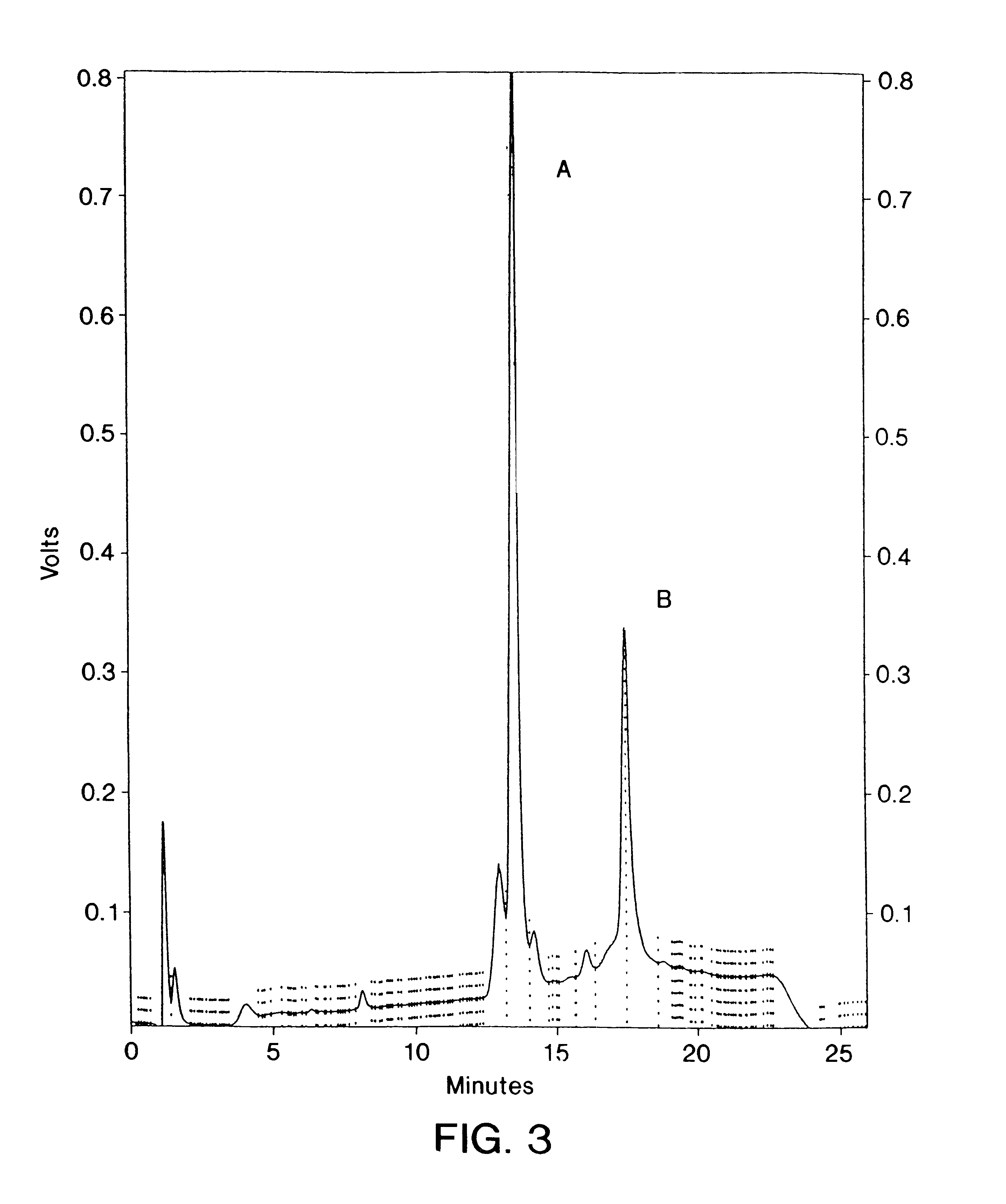
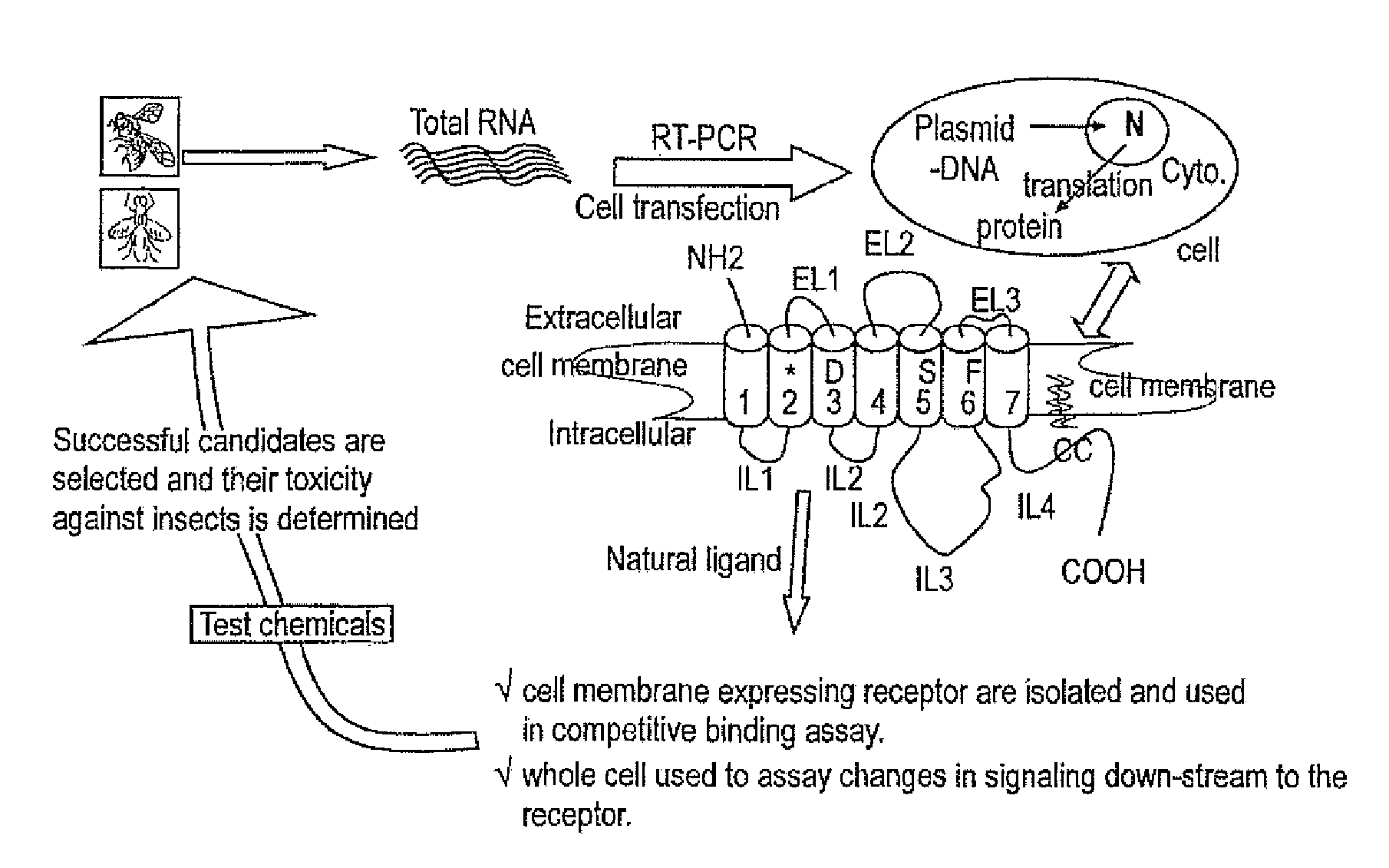
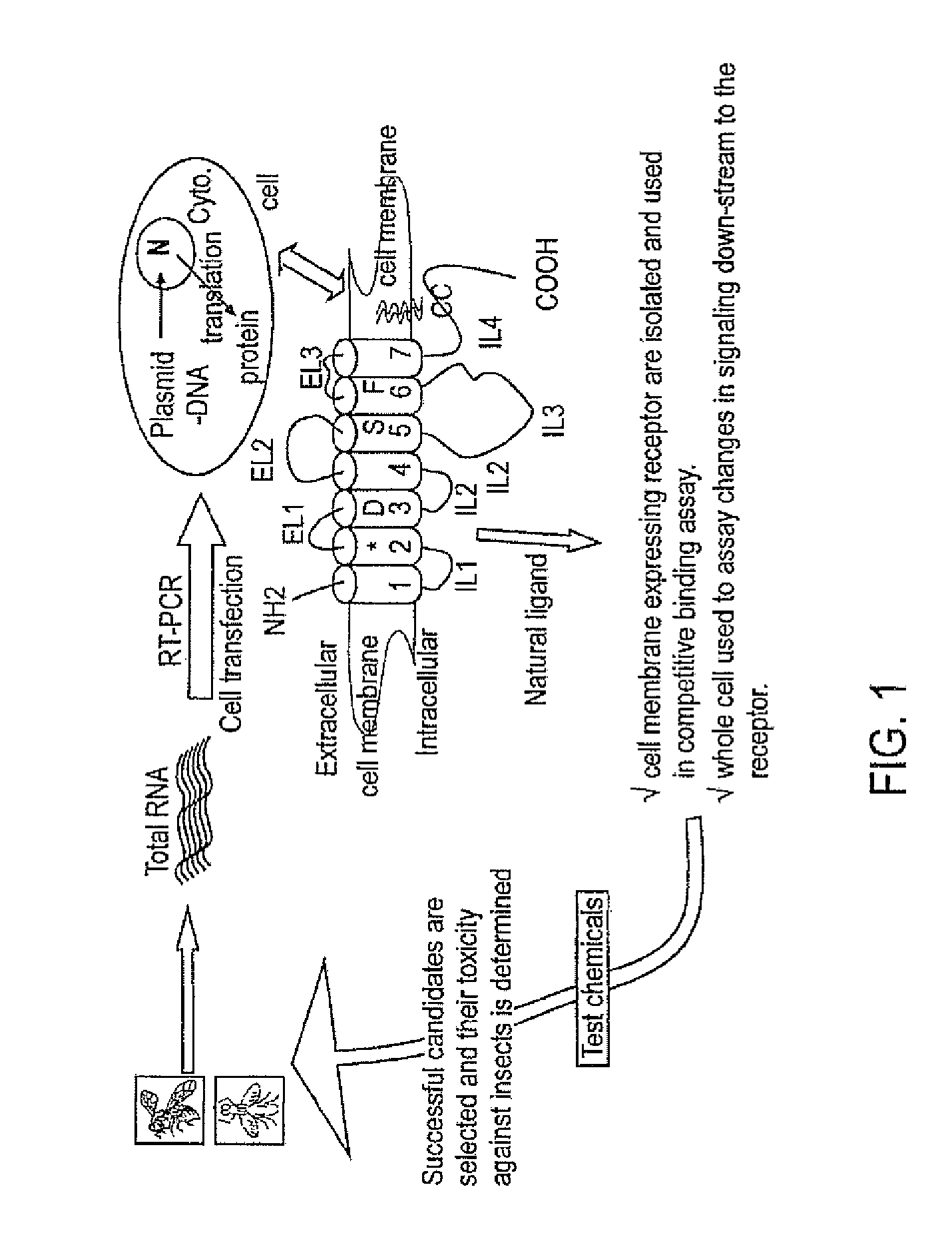
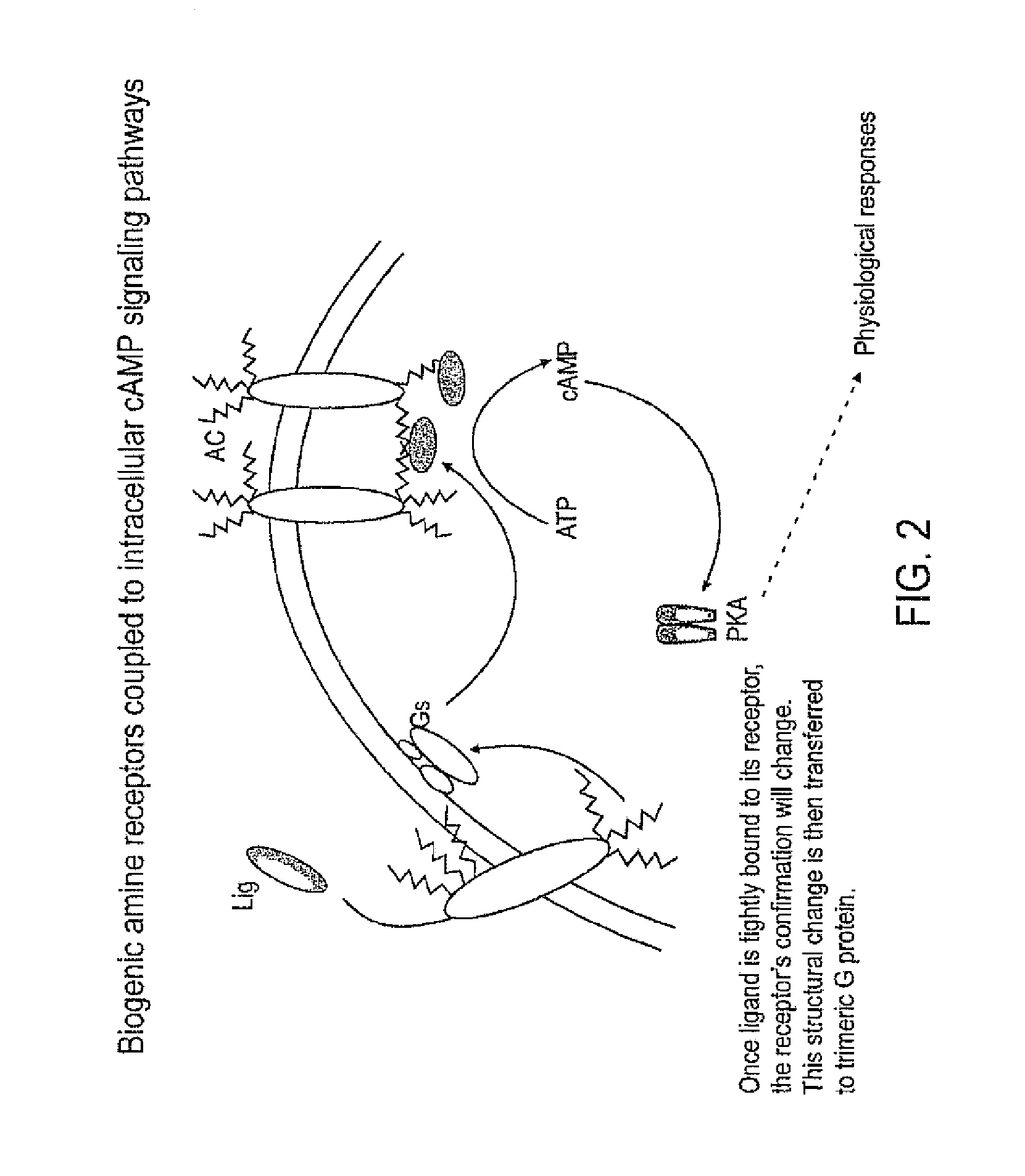
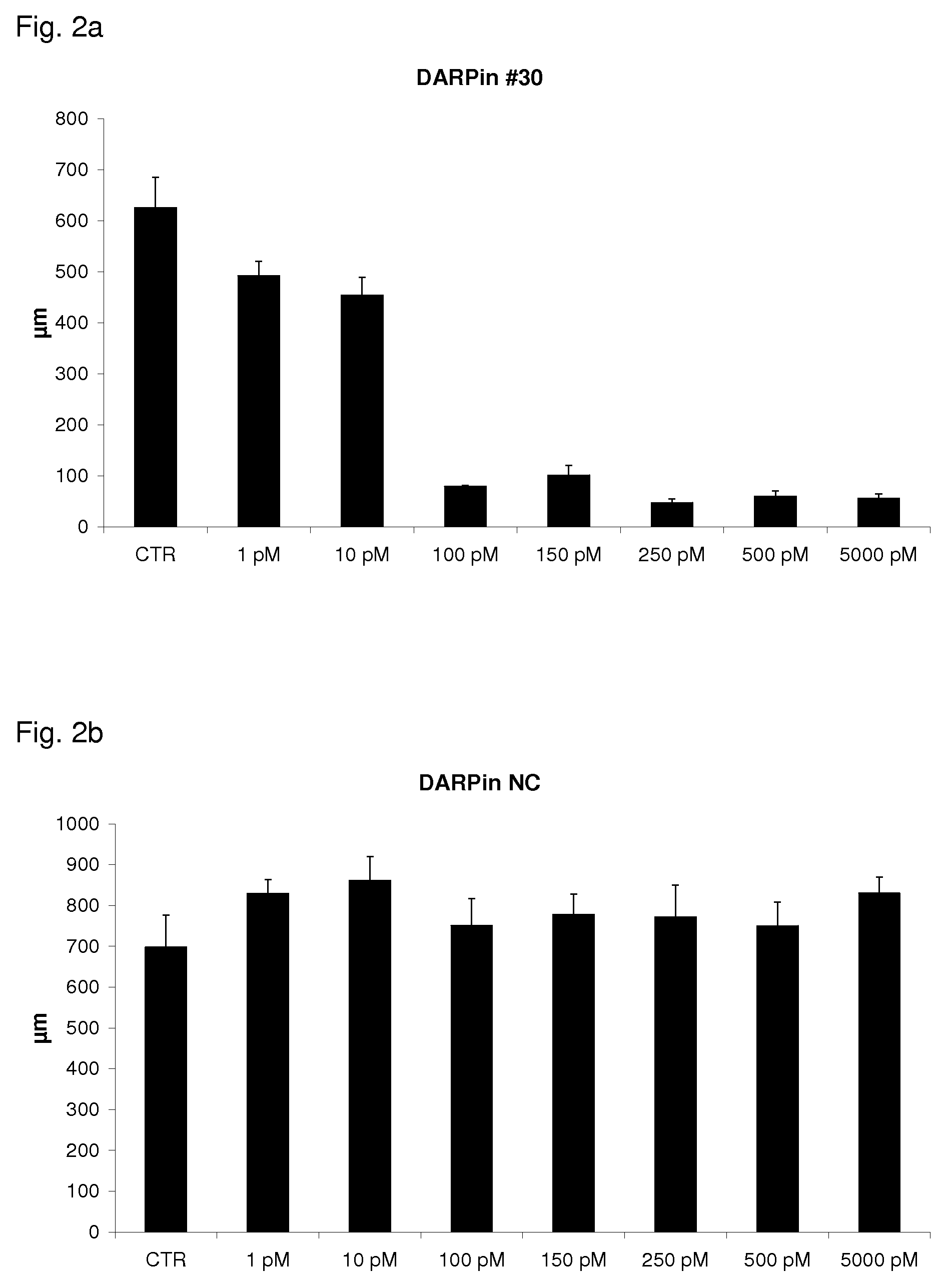
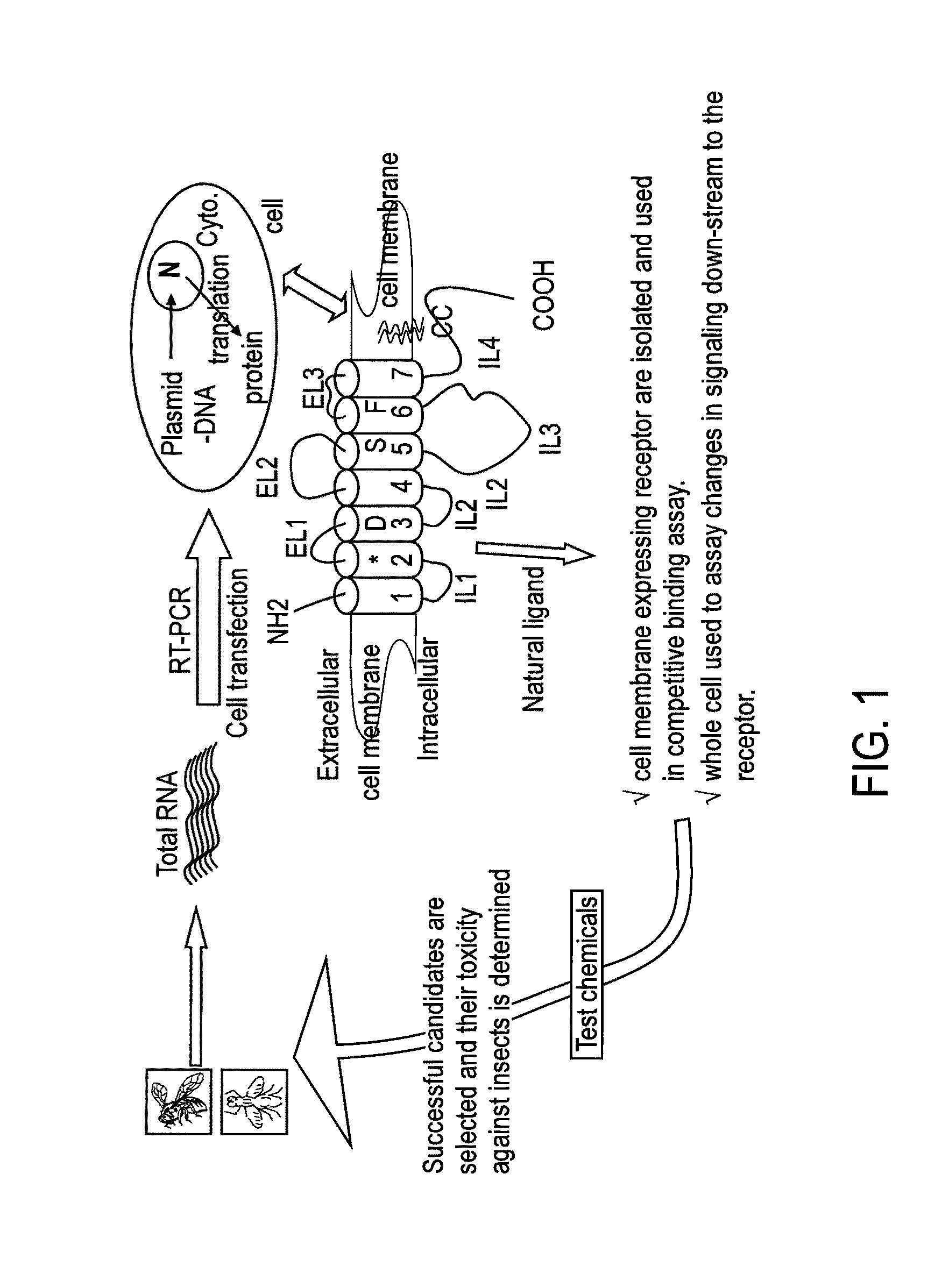
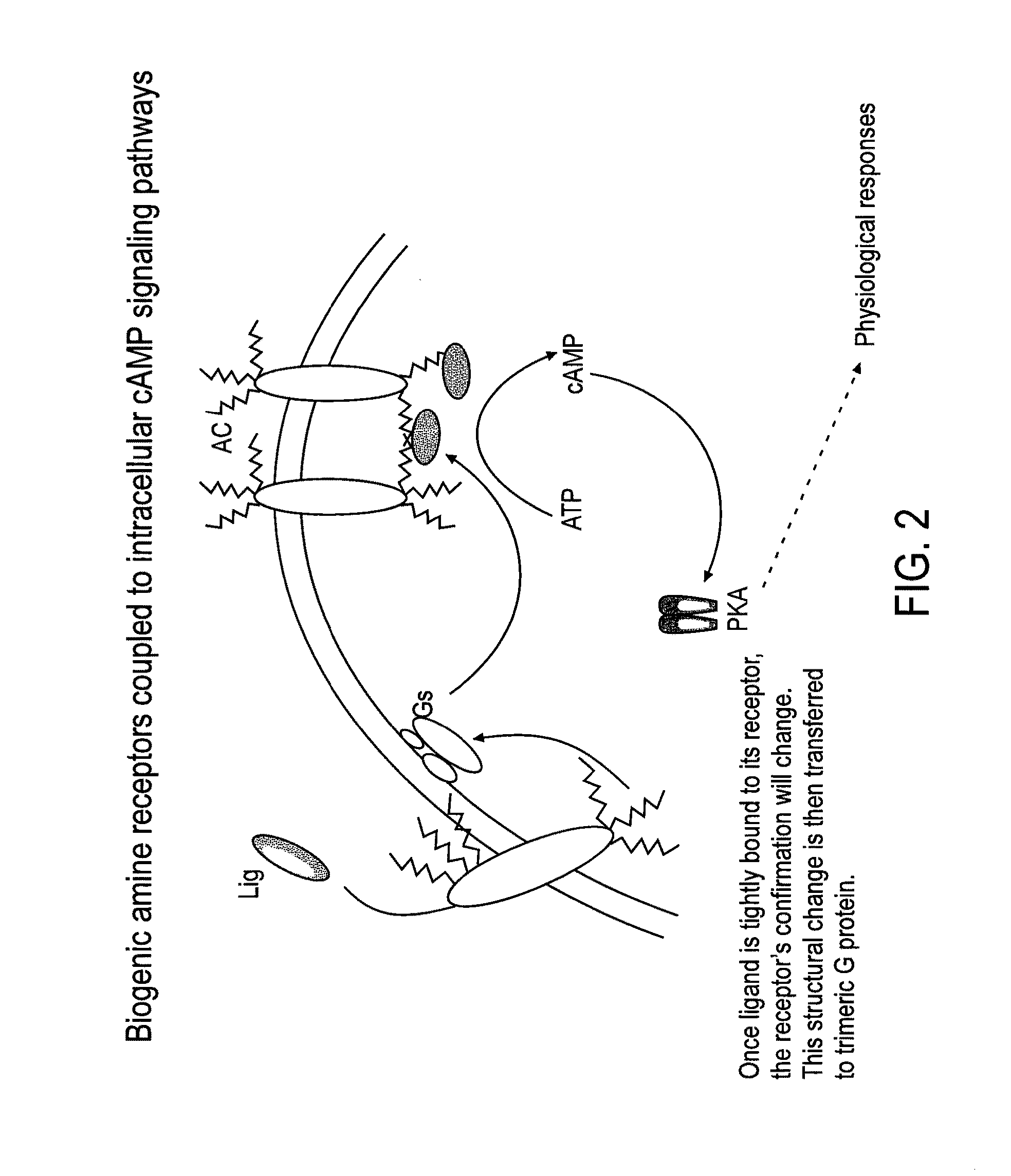
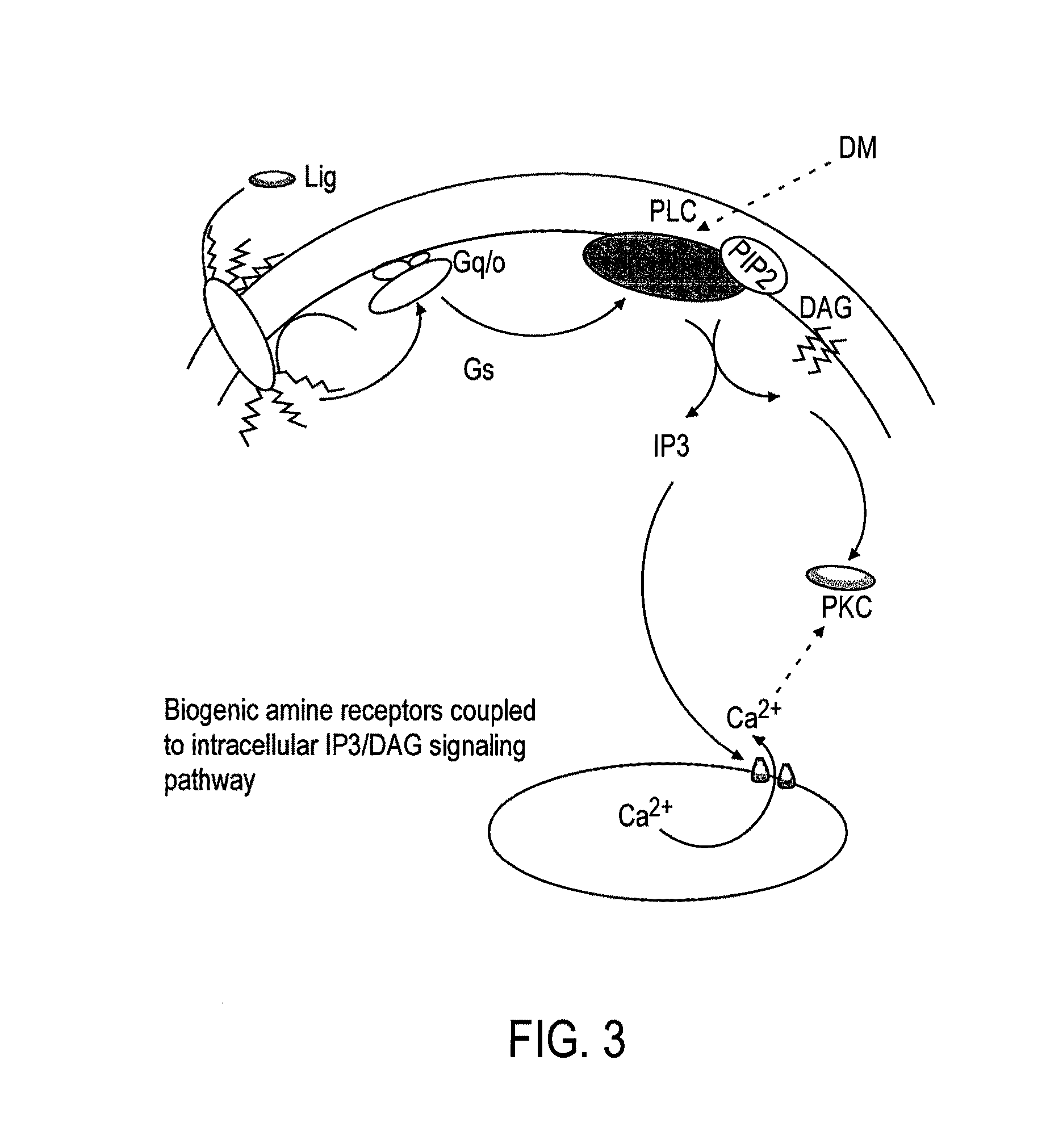
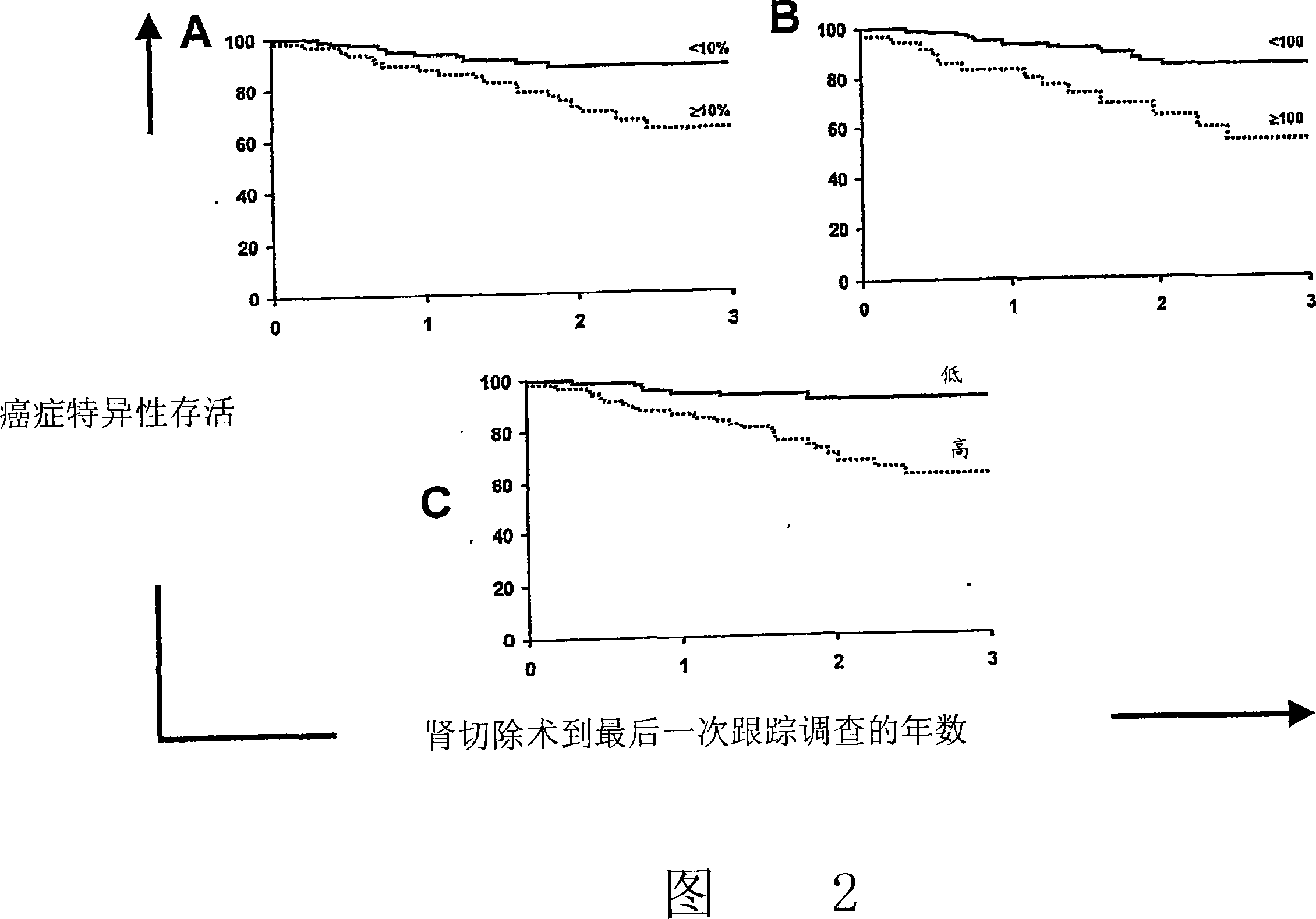
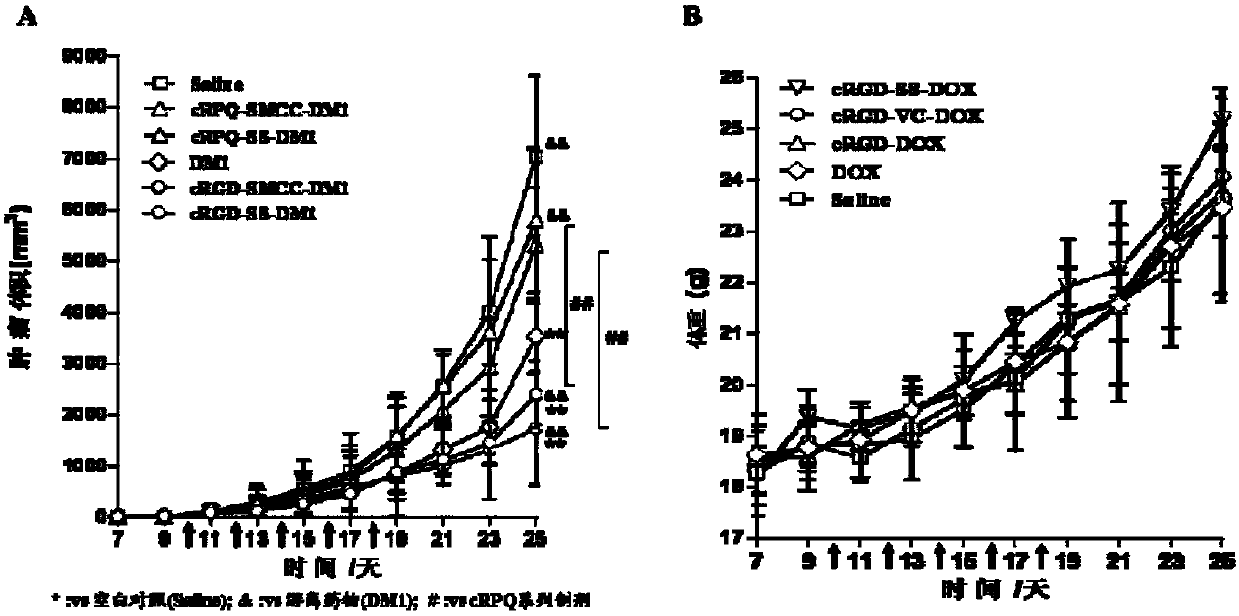
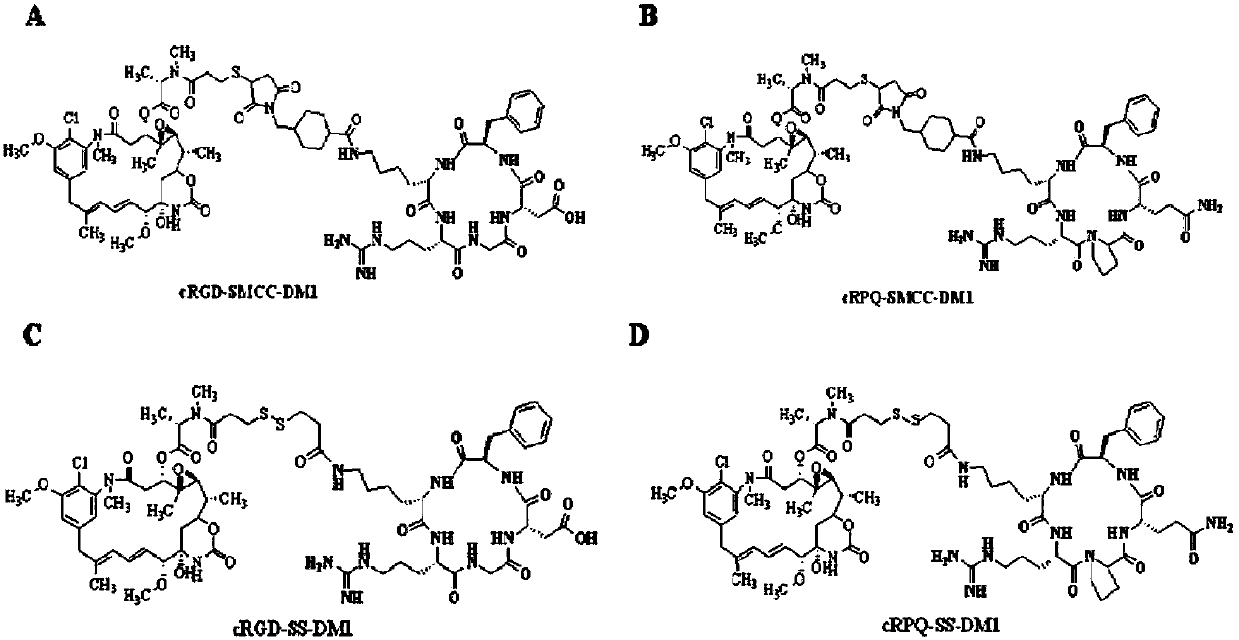
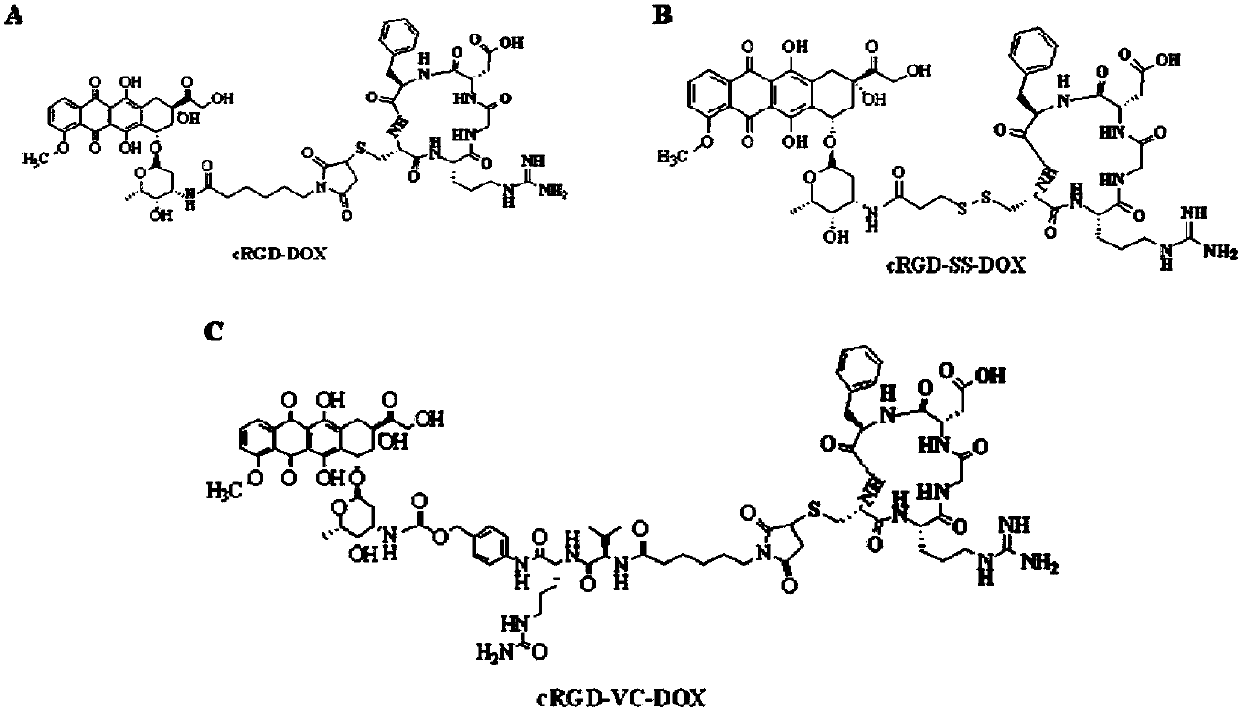
Patents
Literature
168 results about "Receptor interaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
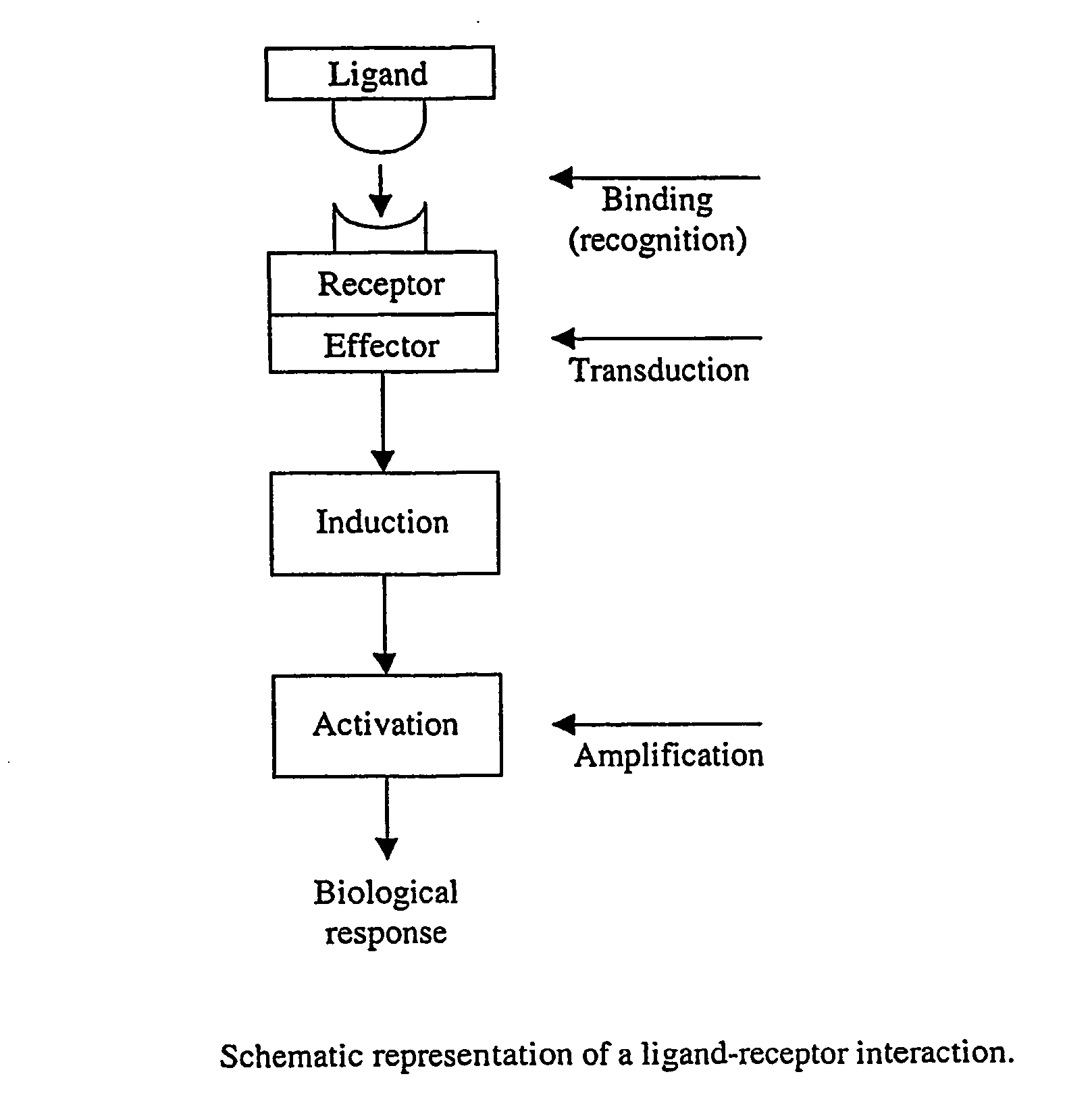
Interaction of receptors with ligands involves the formation of chemical bonds, most commonly electrostatic and hydrogen bonds, as well as weak interactions involving van der Waals forces. These bonds are important in determining the selectivity of receptors, because the strength...
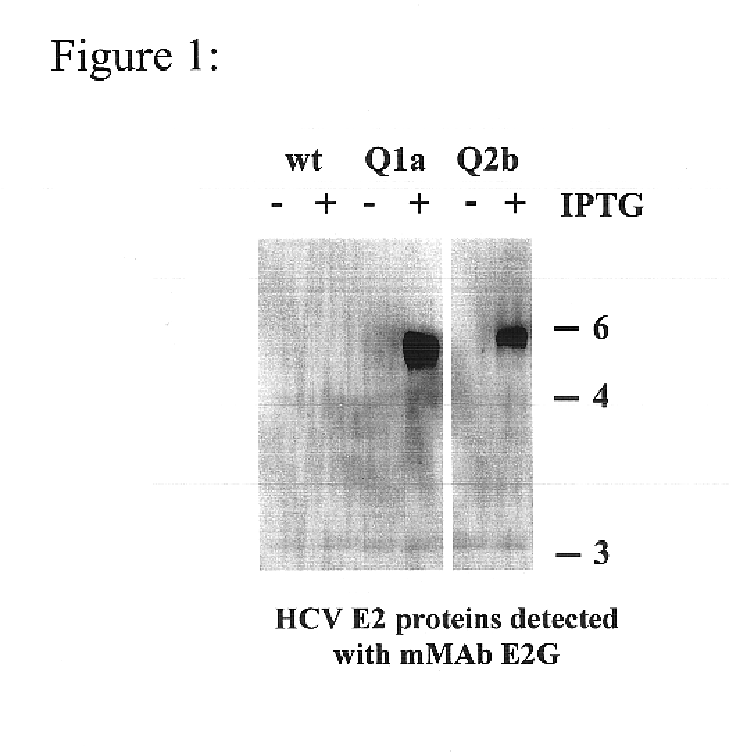
Prevention and treatment of HCV infection employing antibodies that inhibit the interaction of HCV virions with their receptor
Human monoclonal antibodies binding to epitopes common to type 1 and 2 HCV are provided, as well as conformationally conserved HCV E2 2a and 2b proteins. Compositions comprising the antibodies find use in diagnosis and therapy. The antibodies recognize conformational epitopes that are conserved across multiple genotypes of HCV. Thus the antibodies have the potential to be useful in the prevention and treatment of the majority of HCV infections. A subset of the antibodies (CBH-2, CBH-5, CBH-7, CBH-8C, CBH-8E, and CBH-11) have the ability to prevent the binding of HCV E2 proteins of multiple genotypes to human CD81, a possible co-receptor for HCV infection. A subset of the antibodies (CBH-2 and CBH-5) have been shown to inhibit the binding of HCV virions (as opposed to purified E2 protein) to human CD81. A further subset of the antibodies (CBH-4D, CBH4B, CBH-8C, and CBH-9) have been shown to prevent HCV envelope mediated fusion using an HCV psuedotype system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Polymers for analyte detection
InactiveUS20060127929A1Microbiological testing/measurementChemiluminescene/bioluminescenceEscherichia coliFluorescence
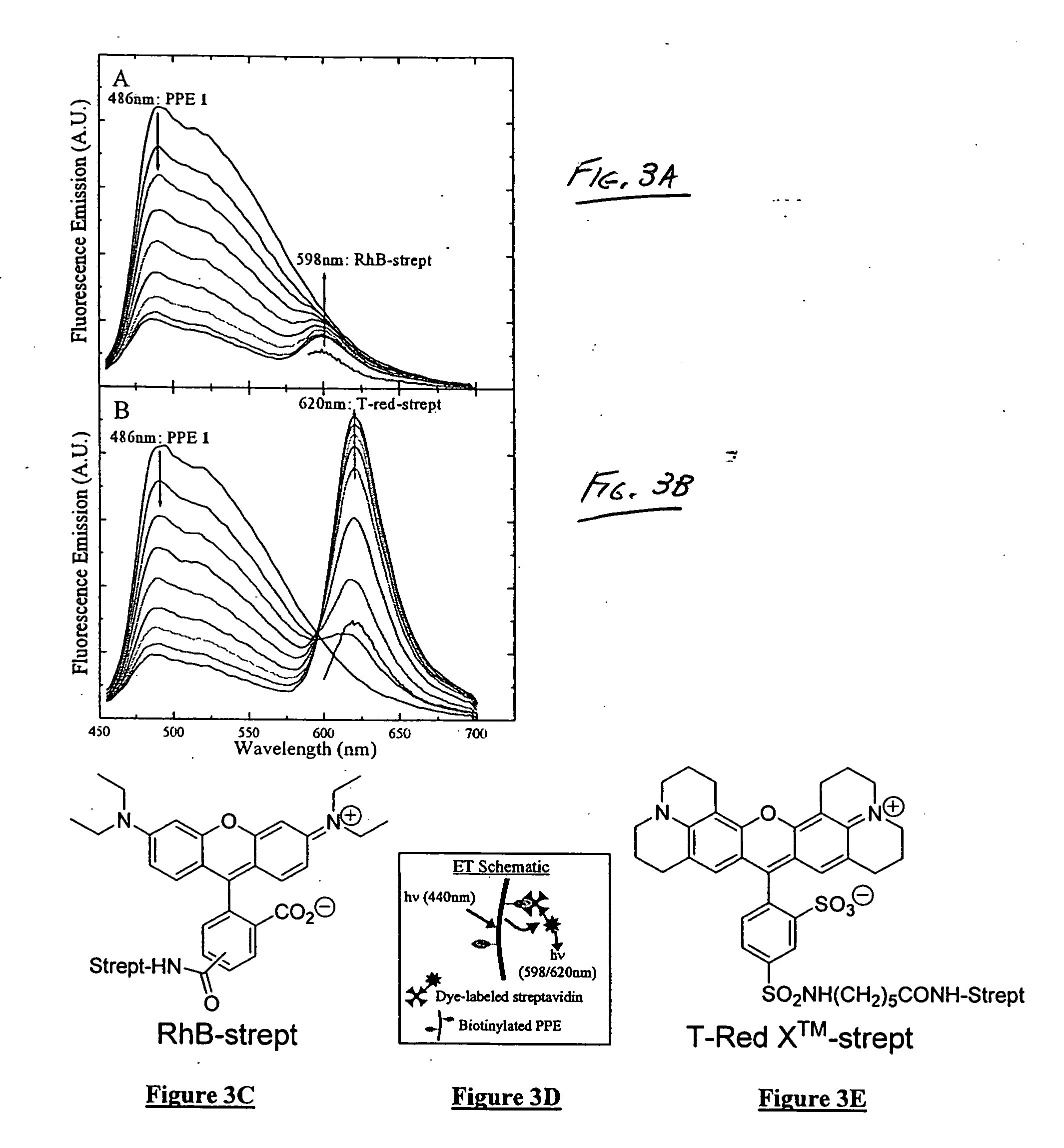
The present invention generally relates to organic polymers able to participate in an analyte-recognition process, where an analyte facilitates an energy transfer between an energy donor and an energy acceptor. Certain embodiments of the invention make use of fluorescent conjugated polymers, such as poly(phenylene ethynylene)s and other polymers comprising pi-conjugated backbones. For example, one aspect of the invention provides a fluorescent conjugated polymer and an indicator that can interact with each other in the presence of an analyte to produce an emissive signal. In some cases, the interaction may include energy exchange mechanisms, such as Dexter energy transfer or the strong coupling effect. The interaction of the conjugated polymer and the indicator, in some instances, may be facilitated through specific interactions, such as a protein / carbohydrate interaction, a ligand / receptor interaction, etc. Another aspect of the invention provides for the detection of biological entities, for example, pathogenic bacteria such as E. coli, or viruses such as influenza virus. In some cases, biological recognition elements may be used to determine the biological entity, for instance, carbohydrates that can be used to specifically interact with at least part of the biological entity, such as a protein in the cell membrane of a bacterium. Still other aspects of the invention involve articles, devices, and kits using any of the above-described systems.
Owner:MASSACHUSETTS INST OF TECH
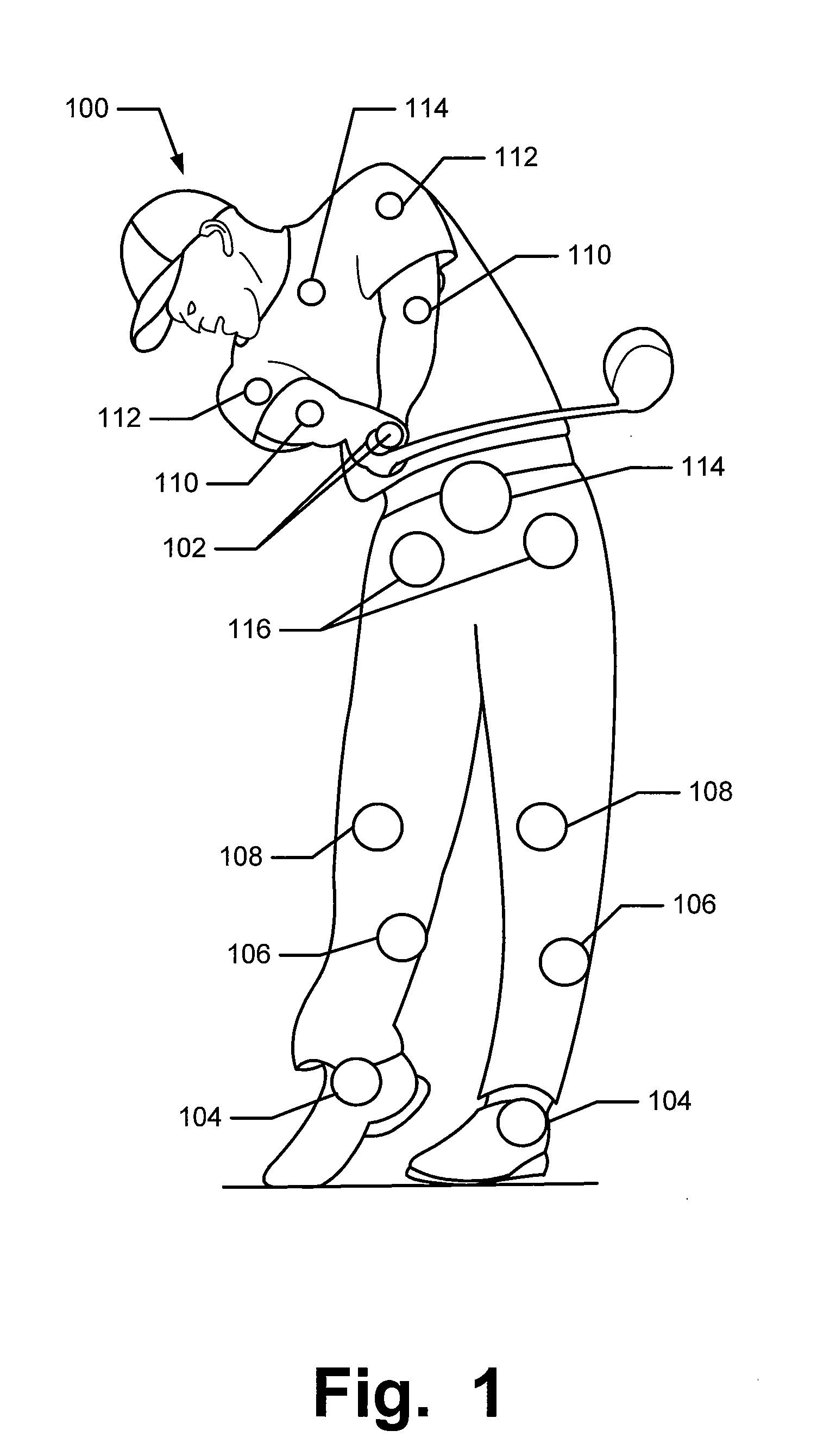
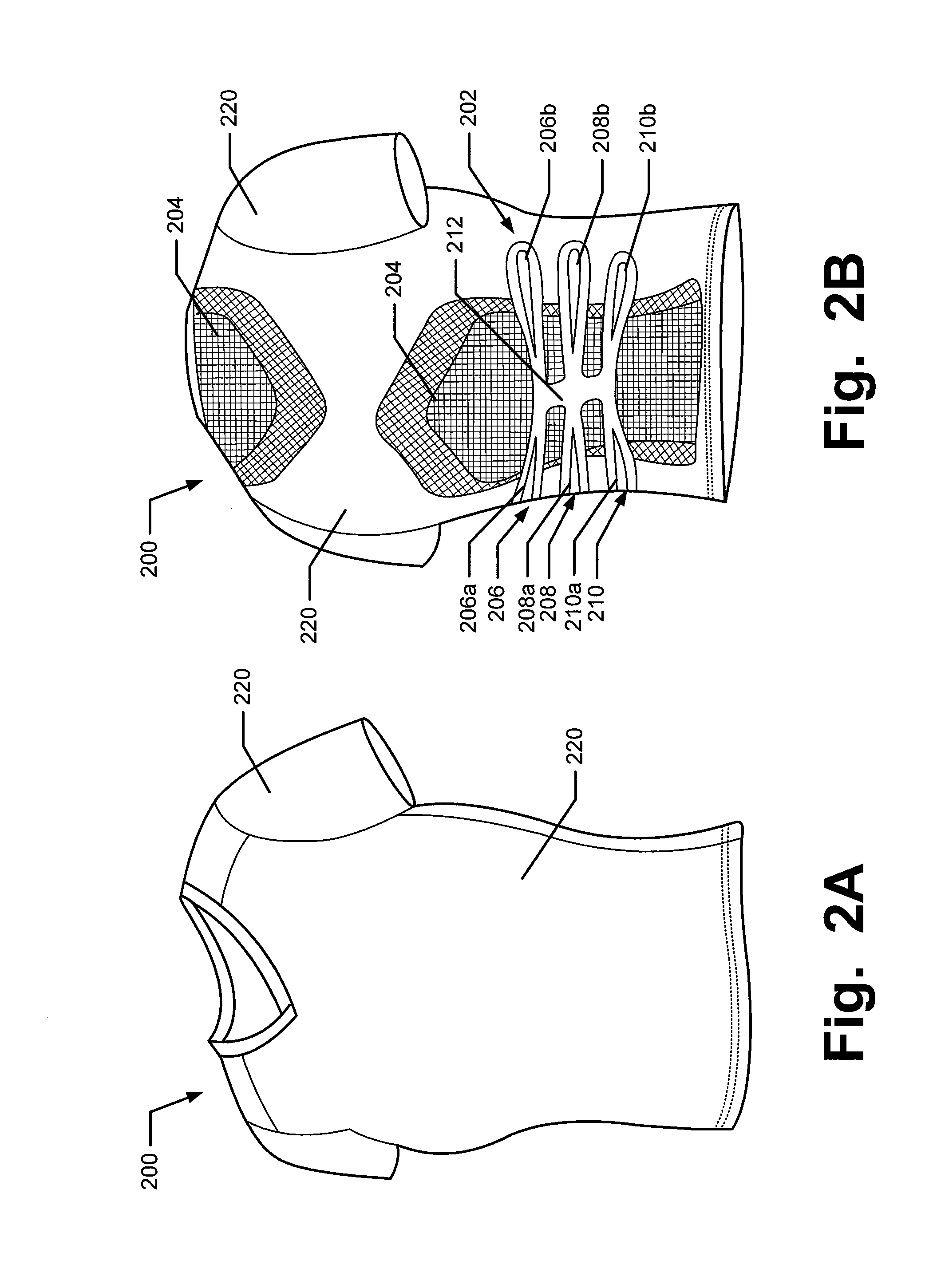
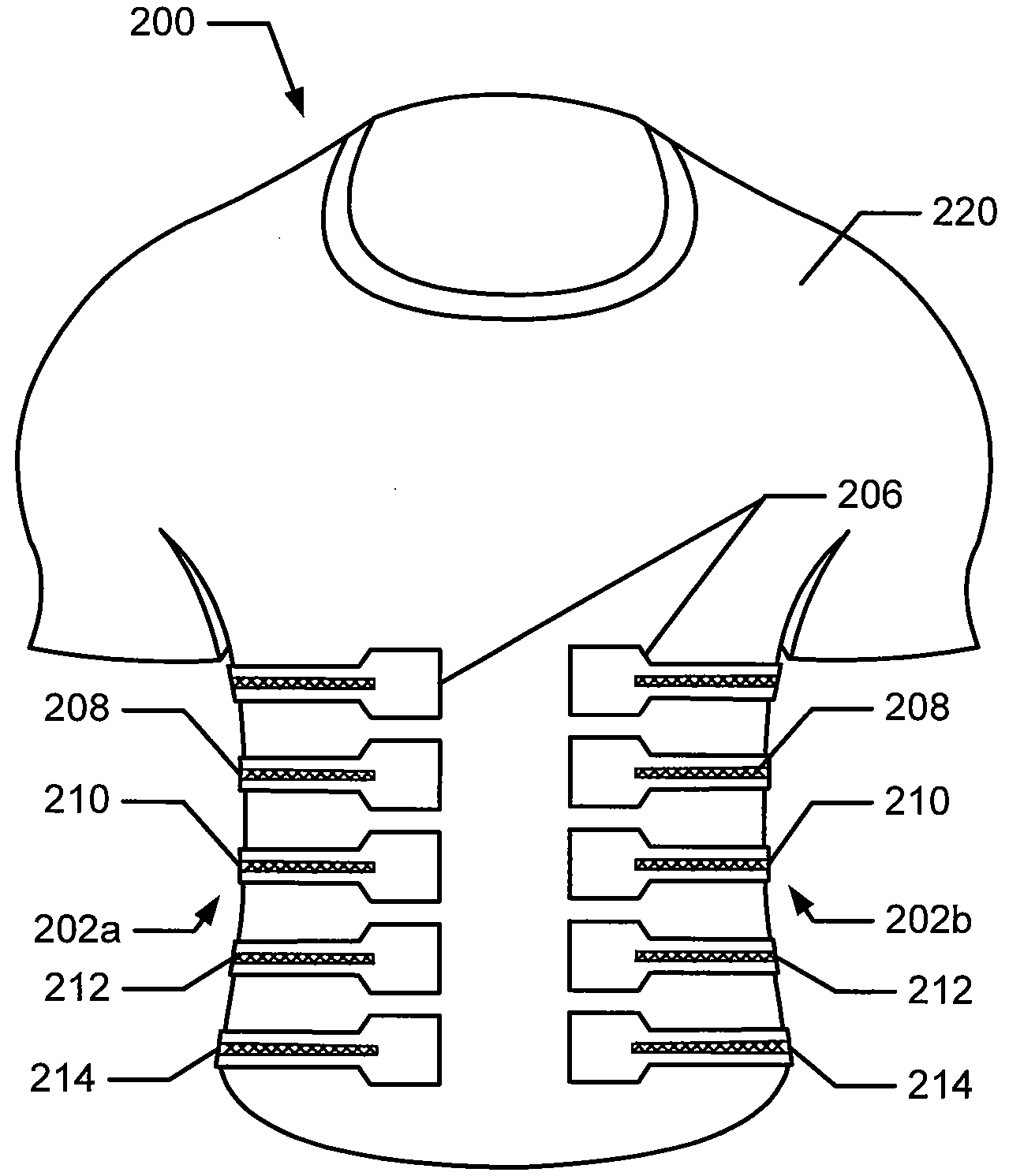
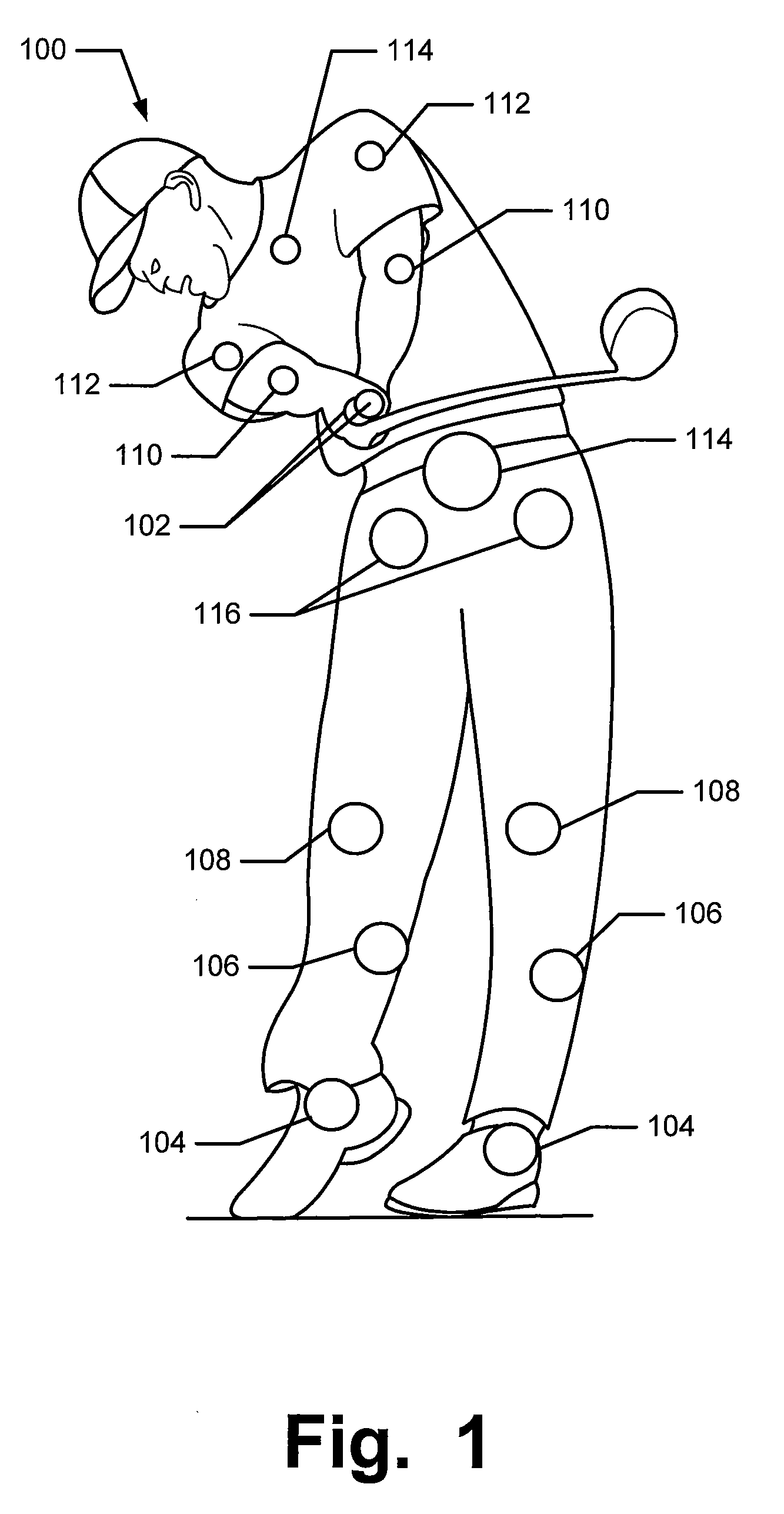
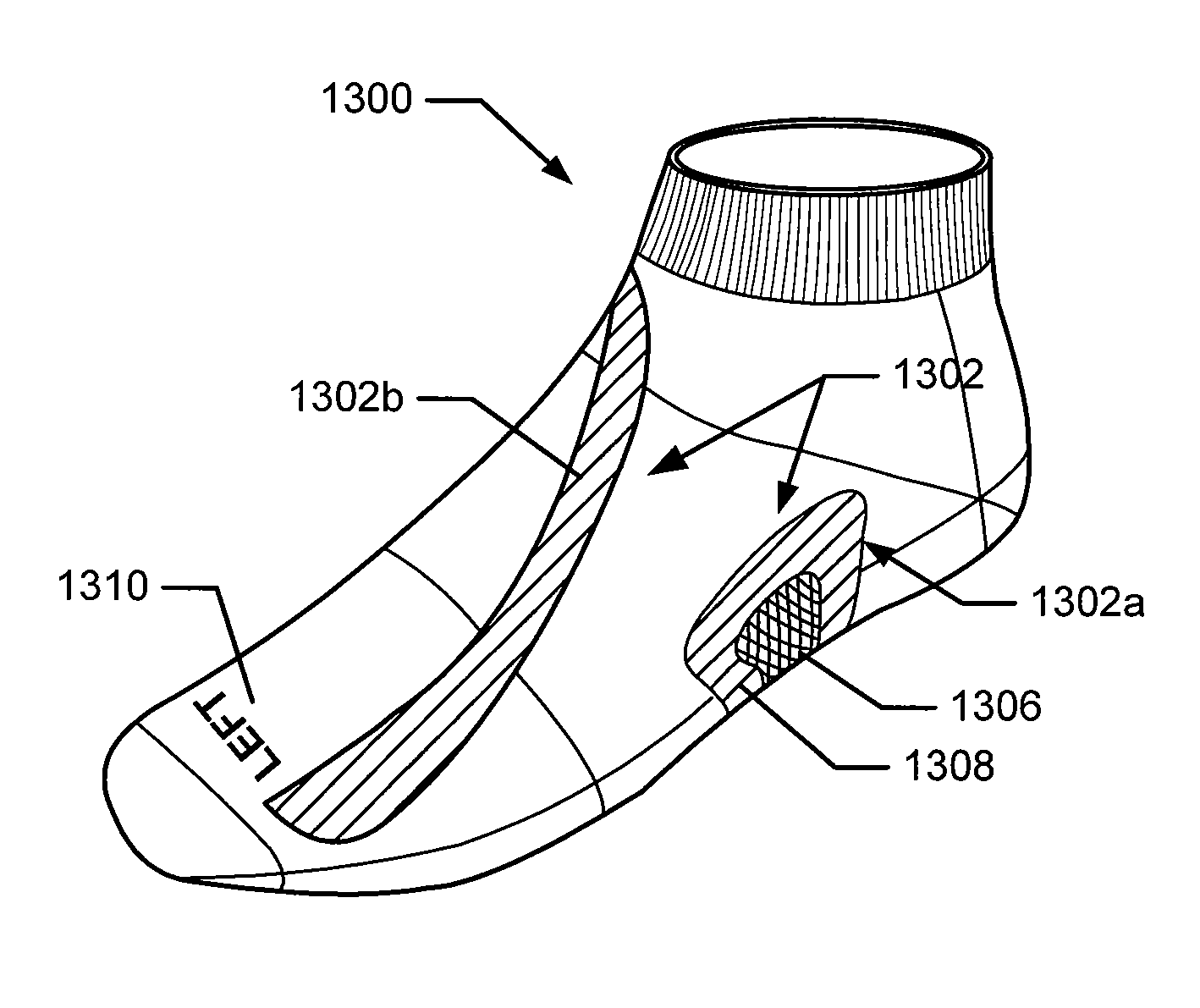
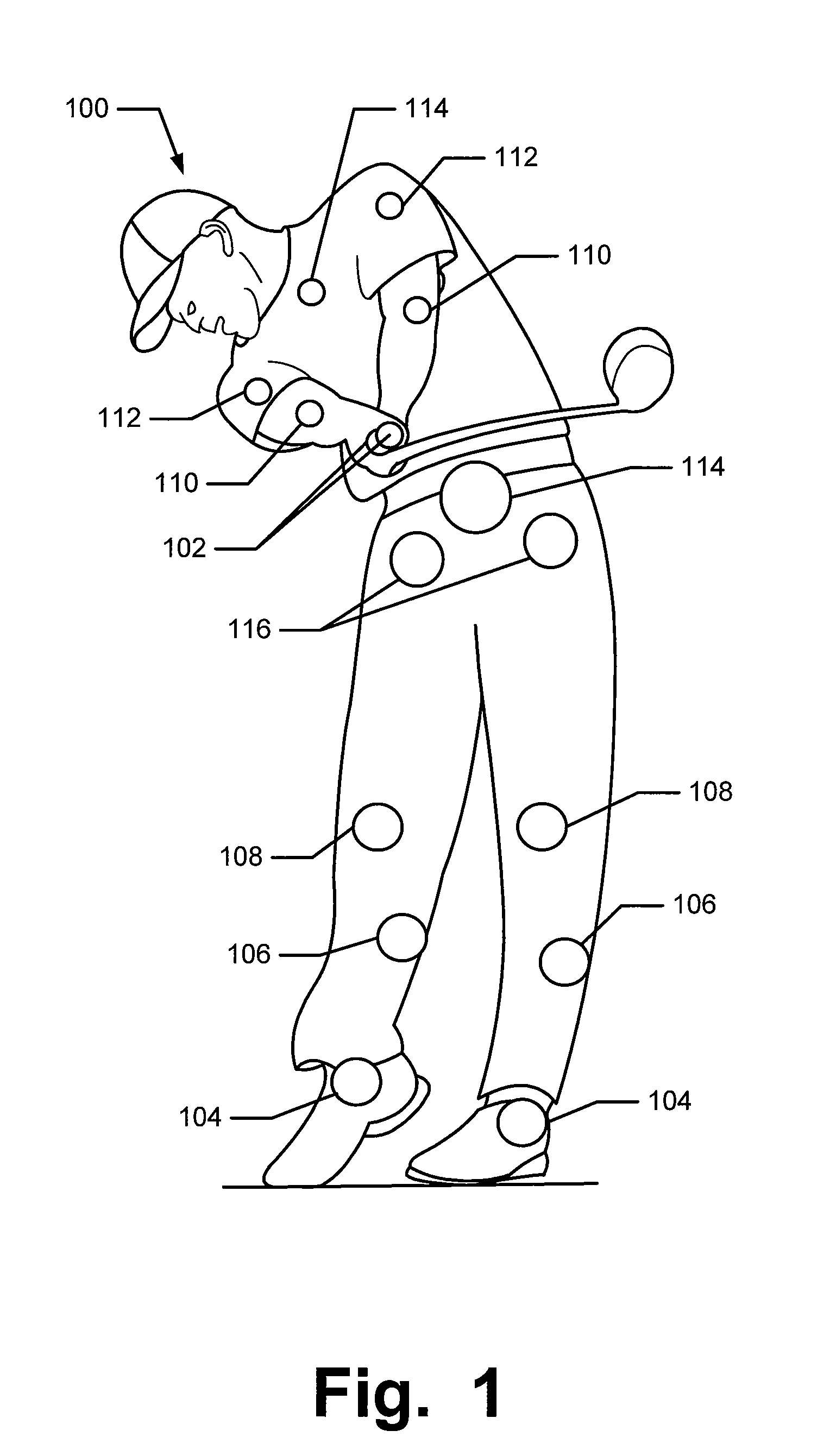
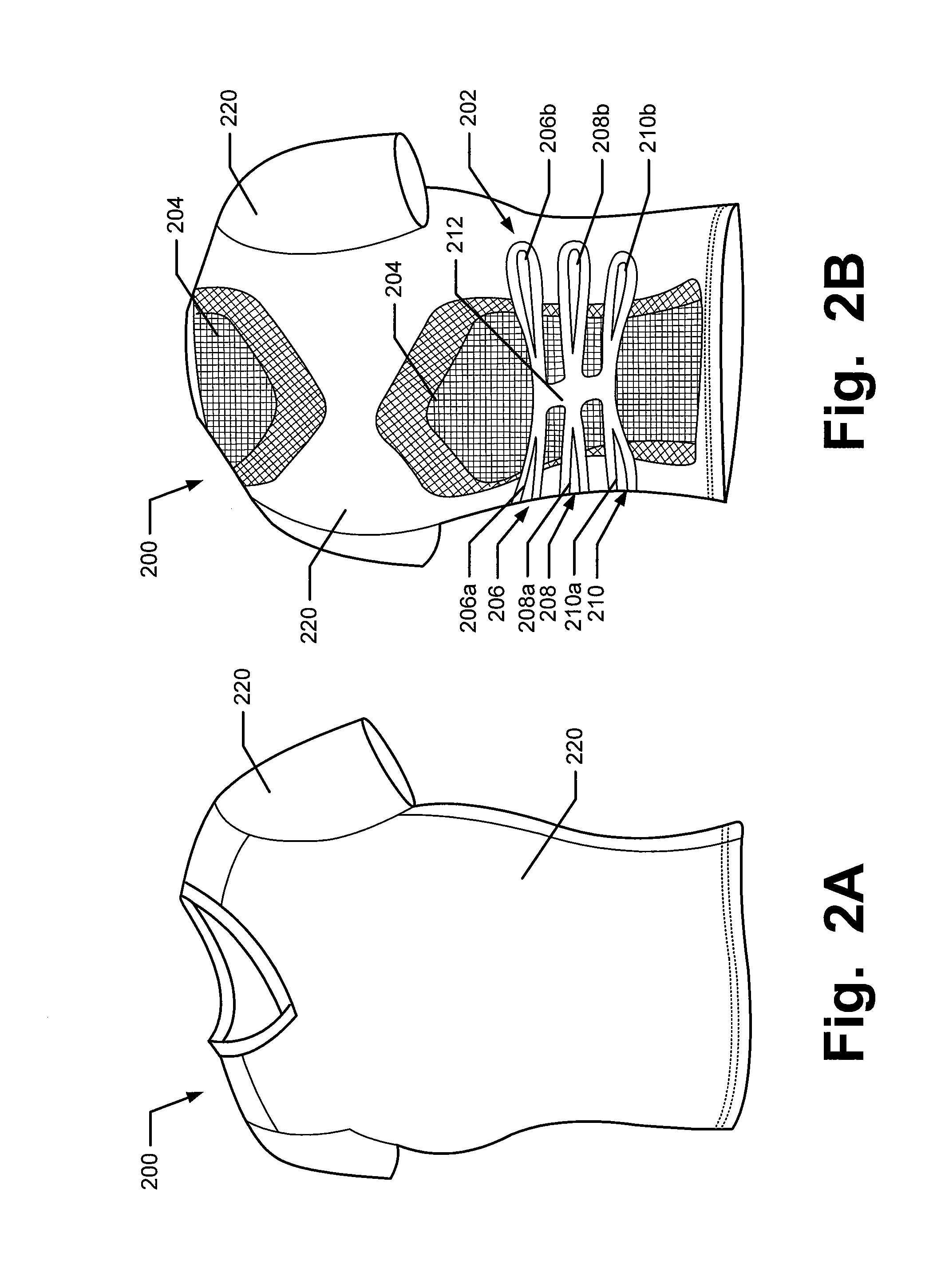
Articles of Apparel Providing Enhanced Body Position Feedback
ActiveUS20080295216A1Improved sensory feedbackEasy to understandInsolesSport apparatusSensory FeedbacksMuscle memory
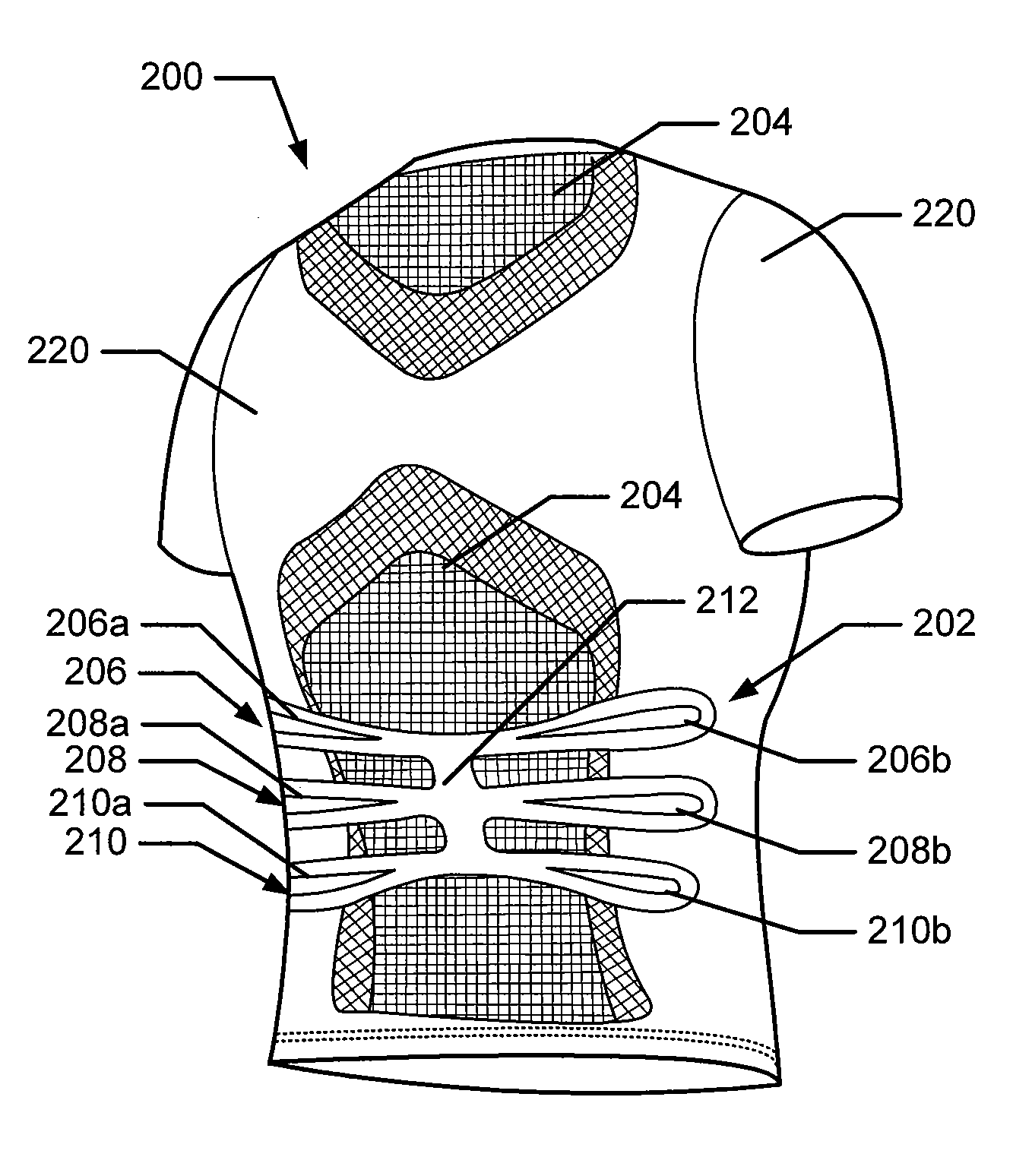
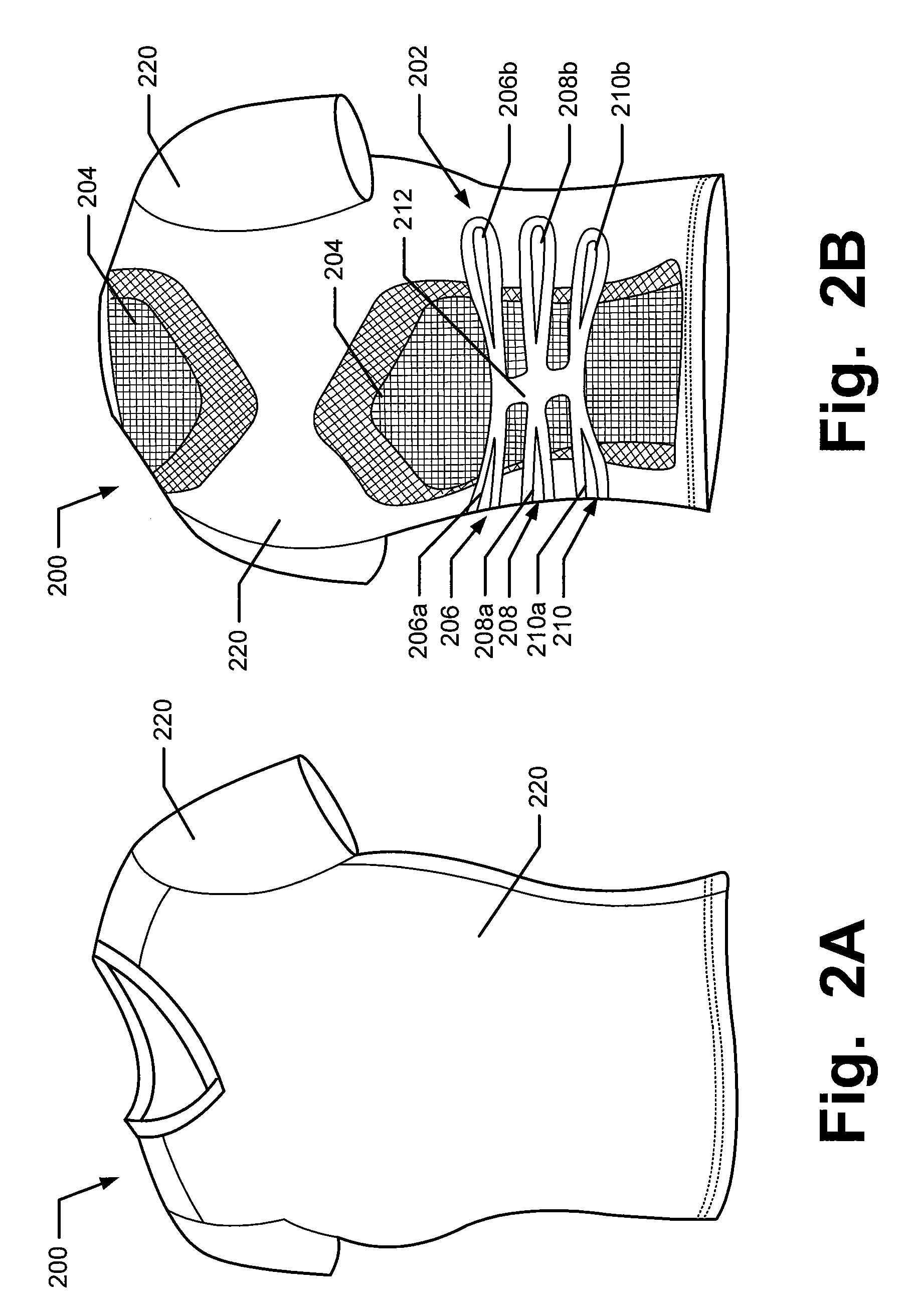
Articles of apparel include: (a) a garment structure having one or more fabric elements structured and arranged to provide a close fit to at least one predetermined portion of a body (e.g., area(s) of the body for which enhanced position sensing and / or feedback are desired, such as the lower back, the arch of the foot, etc.); and (b) a body position feedback system engaged with or integrally formed as part of the garment structure. The body position feedback system may apply higher tensile or constricting (compressive) forces to selected portions of the wearer's body, which can help stimulate or interact with nerves and deep tissue receptors located in various portions of the body. The increased forces at selected locations of the body give the wearer sensory feedback regarding the position or orientation of these parts of the body and can improve or accelerate development of “muscle memory.”
Owner:NIKE INC
Pest control compositions and methods
InactiveUS20090099135A1Good effectHigh activityBiocideHalogenated hydrocarbon active ingredientsActive agentPest control
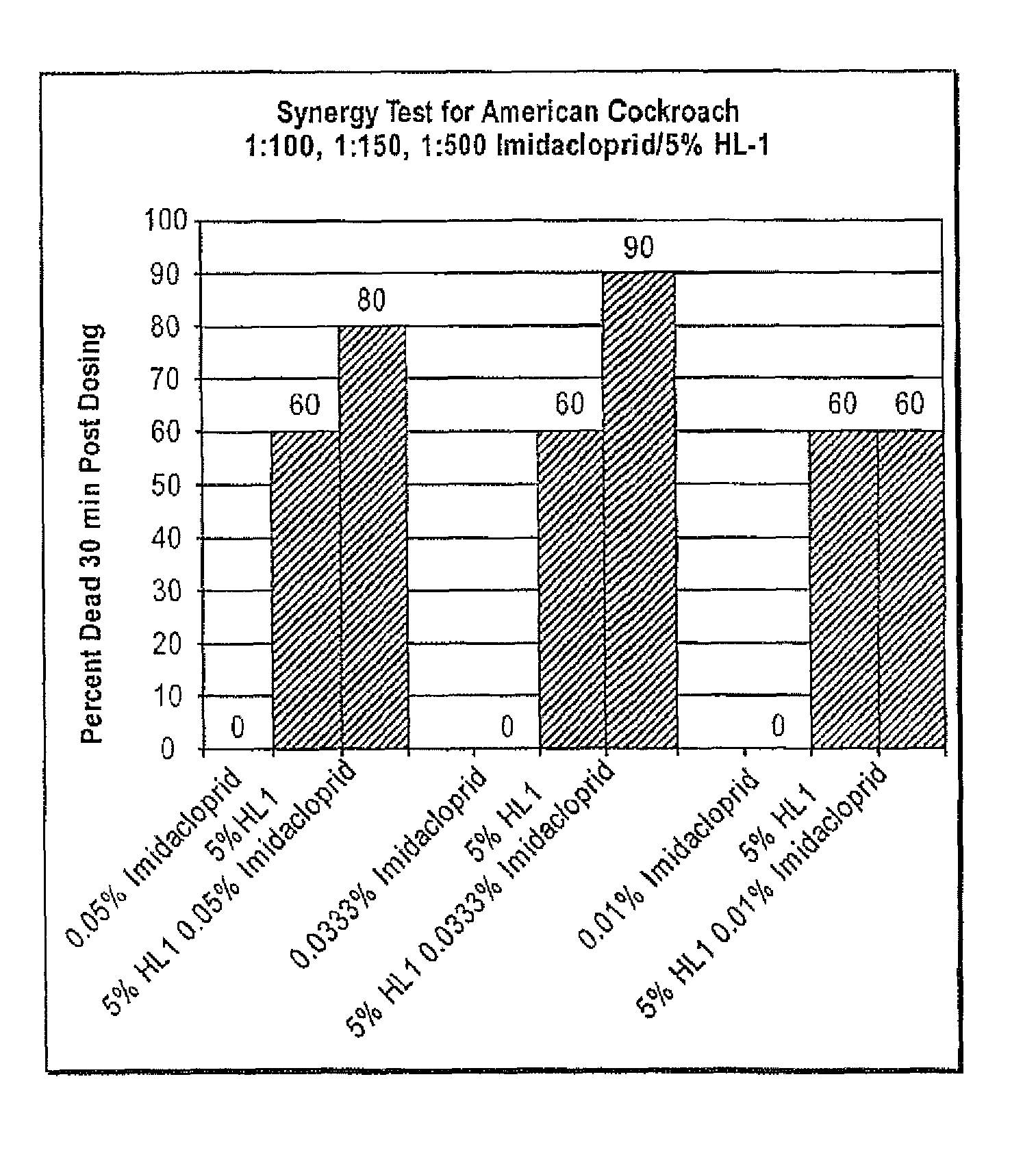
Embodiments of the present invention provide compositions for controlling a target pest including a pest control product and at least one active agent, wherein: the active agent can be capable of interacting with a receptor in the target pest; the pest control product can have a first activity against the target pest when applied without the active agent and the compositions can have a second activity against the target pest; and the second activity can be greater than the first activity.
Owner:TYRATECH
Articles of Apparel Providing Enhanced Body Position Feedback
ActiveUS20080295230A1Improved sensory feedbackEasy to understandChemical protectionGarment special featuresSensory FeedbacksMuscle memory
Articles of apparel include: (a) a garment structure having one or more fabric elements structured and arranged to provide a close fit to at least one predetermined portion of a body (e.g., area(s) of the body for which enhanced position sensing and / or feedback are desired, such as the lower back, the arch of the foot, etc.); and (b) a body position feedback system engaged with or integrally formed as part of the garment structure. The body position feedback system may apply higher tensile or constricting (compressive) forces to selected portions of the wearer's body, which can help stimulate or interact with nerves and deep tissue receptors located in various portions of the body. The increased forces at selected locations of the body give the wearer sensory feedback regarding the position or orientation of these parts of the body and can improve or accelerate development of “muscle memory.”
Owner:NIKE INC
Articles of apparel providing enhanced body position feedback
ActiveUS20090133181A1Heightened tactile feelEnhances wearer 's “ feel ”UndergarmentsProtective garmentSensory FeedbacksMuscle memory
Articles of apparel include: (a) a garment structure having one or more fabric elements structured and arranged to provide a close fit to at least one predetermined portion of a body (e.g., area(s) of the body for which enhanced position sensing and / or feedback are desired); and (b) a body position feedback system engaged with or integrally formed as part of the garment structure. The body position feedback system may apply higher tensile or constricting (compressive) forces to selected portions of the wearer's body and / or stretch resistance, which can help stimulate or interact with nerves and deep tissue receptors located in various portions of the body. The increased forces at selected locations of the body give the wearer sensory feedback regarding the position or orientation of these parts of the body and can improve or accelerate development of “muscle memory.”
Owner:NIKE INC
Haemopoietin receptor and genetic sequences encoding same
InactiveUS6911530B1Avoid interactionPeptide/protein ingredientsAntibody mimetics/scaffoldsAgonistFhit gene
The present invention relates generally to a novel haemopoietin receptor or components or part thereof and to genetic sequences encoding same. The receptor molecules and their components and / or parts and the genetic sequences encoding same of the present invention are useful in the development of a wide range of agonists, antagonists, therapeutics and diagnostic reagents based on ligand interaction with its receptor.
Owner:AMRAD OPERATIONS PTY LTD
Compositions and methods for modulating immune responses
InactiveUS20070054360A1Modulate activityHigh protein yieldPeptide/protein ingredientsAntibody mimetics/scaffoldsLymphocyteT cell
The present invention provides a newly identified B7 receptor, zB7R1 that functions as lymphocyte inhibitory receptor, which is a PD-1-like molecule and is expressed on T cells. The present invention also provides the discovery of zB7R1's ability to bind to CD155. Methods and compositions for modulating zB7R1-mediated negative signaling and interfering with the interaction of its counter-receptor for therapeutic, diagnostic and research purposes are also provided.
Owner:ZYMOGENETICS INC
Articles of apparel providing enhanced body position feedback
ActiveUS7996924B2Easy to FeedbackEasy to understandChemical protectionGarment special featuresSensory FeedbacksMuscle memory
Articles of apparel include: (a) a garment structure having one or more fabric elements structured and arranged to provide a close fit to at least one predetermined portion of a body (e.g., area(s) of the body for which enhanced position sensing and / or feedback are desired, such as the lower back, the arch of the foot, etc.); and (b) a body position feedback system engaged with or integrally formed as part of the garment structure. The body position feedback system may apply higher tensile or constricting (compressive) forces to selected portions of the wearer's body, which can help stimulate or interact with nerves and deep tissue receptors located in various portions of the body. The increased forces at selected locations of the body give the wearer sensory feedback regarding the position or orientation of these parts of the body and can improve or accelerate development of “muscle memory.”
Owner:NIKE INC
Smart aerogel
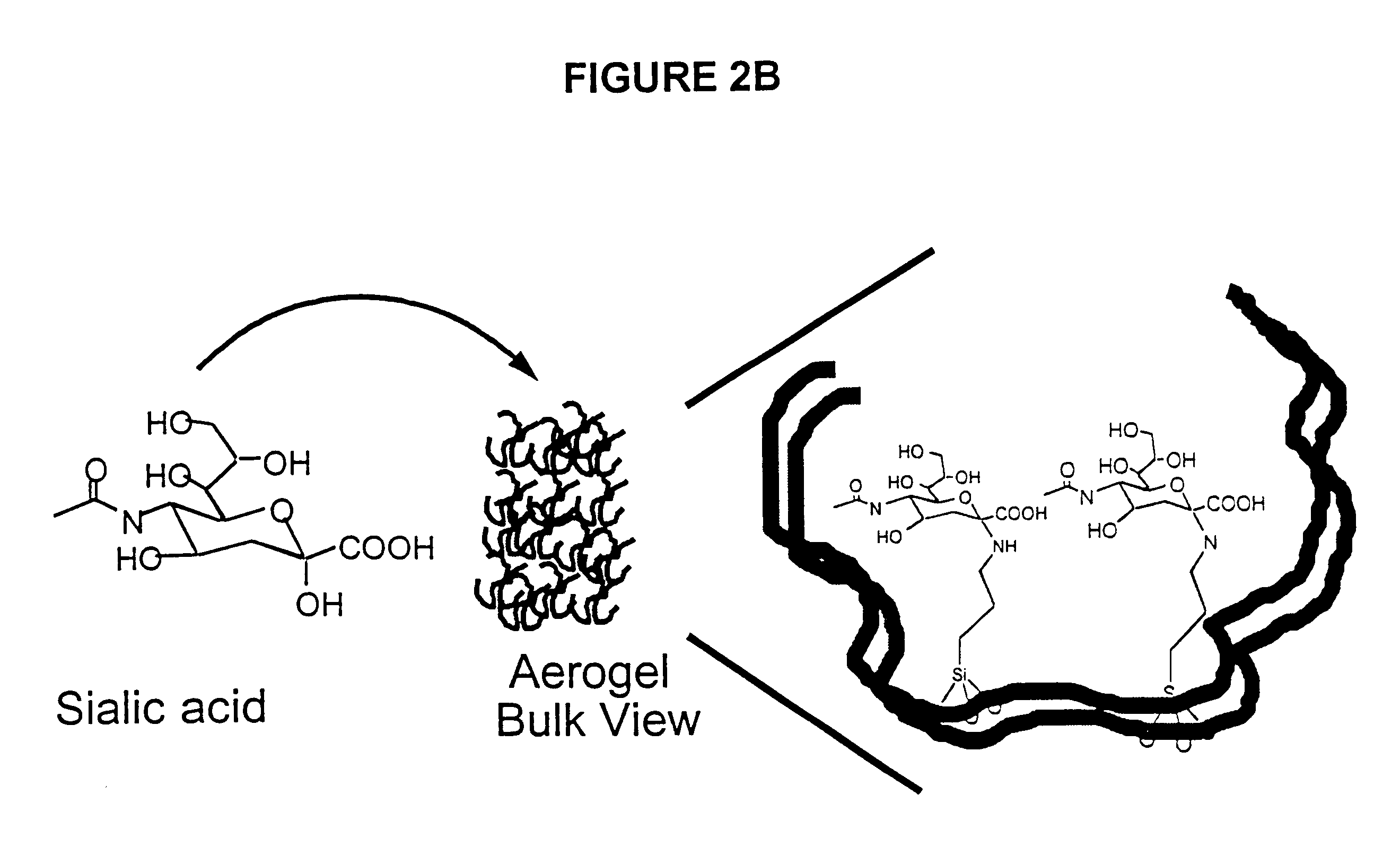
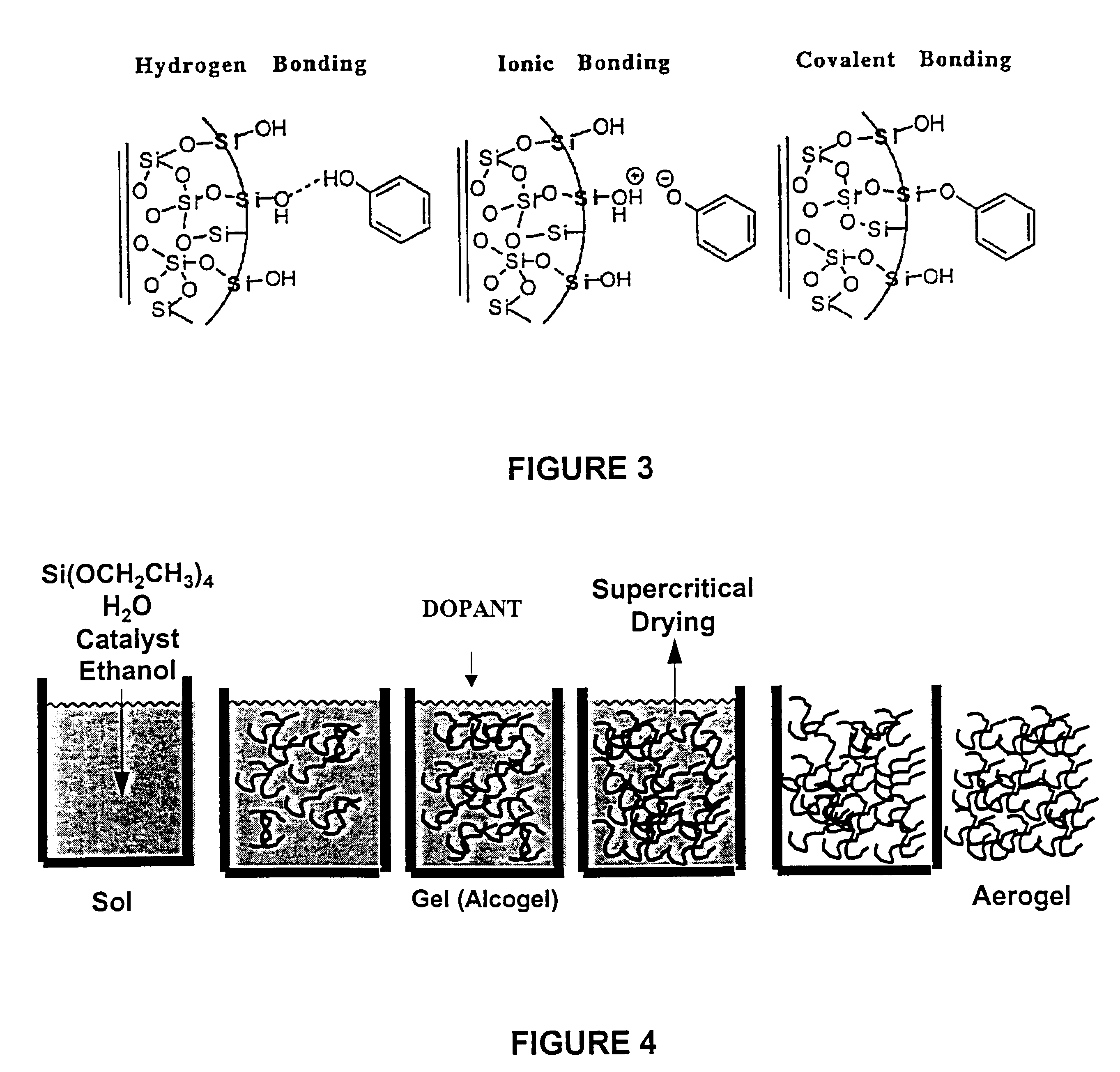
Smart aerogel, an aerogel material doped with special bio-affinity compounds to providing means of unique collection, detection and identification of bioaerosols, including bacteria, viruses, toxins, and other bioaerosols. Aerogels, extremely low density and highly porous materials with a complex pore structure, are used as an intelligent detection material by incorporating specific bioaffinity pharmaceuticals directly into the matrix. The complex pore structure contains micropores, mesopores, and macropores in an open pore structure. The opening pore structure of the aerogel is used to create docking sites by linking high affinity pharmaceuticals that specifically bind only to certain bioaerosols. The high internal surface area of the aerogel and the extremely low density provides abundant receptor sites per unit mesopore for a high bioaerosol-receptor interaction, yet in a manner which will reduce possible damage and destruction to the bioaerosols captured.
Owner:VIRGINIA PATENT FOUND UNIV OF +2
Binding proteins inhibiting the vegf-a receptor interaction
ActiveUS20110207668A1Inhibit bindingSenses disorderPeptide/protein ingredientsWAS PROTEINAnkyrin Repeat Protein
The present invention relates to binding proteins specific for VEGF-A, in particular to recombinant binding proteins comprising a binding domain, which inhibits VEGF-Axxx binding to VEGFR-2. Examples of such binding proteins are proteins which comprise an ankyrin repeat domain with the desired binding specificity. The binding proteins are useful in the treatment of cancer and other pathological conditions, e.g. eye diseases such as age-related macular degeneration.
Owner:MOLECULAR PARTNERS AG
Resonance driven changes in chain molecule structure
InactiveUS6060293AEfficient inductionPeptide preparation methodsElectrical/wave energy microorganism treatmentChemical industryDisease
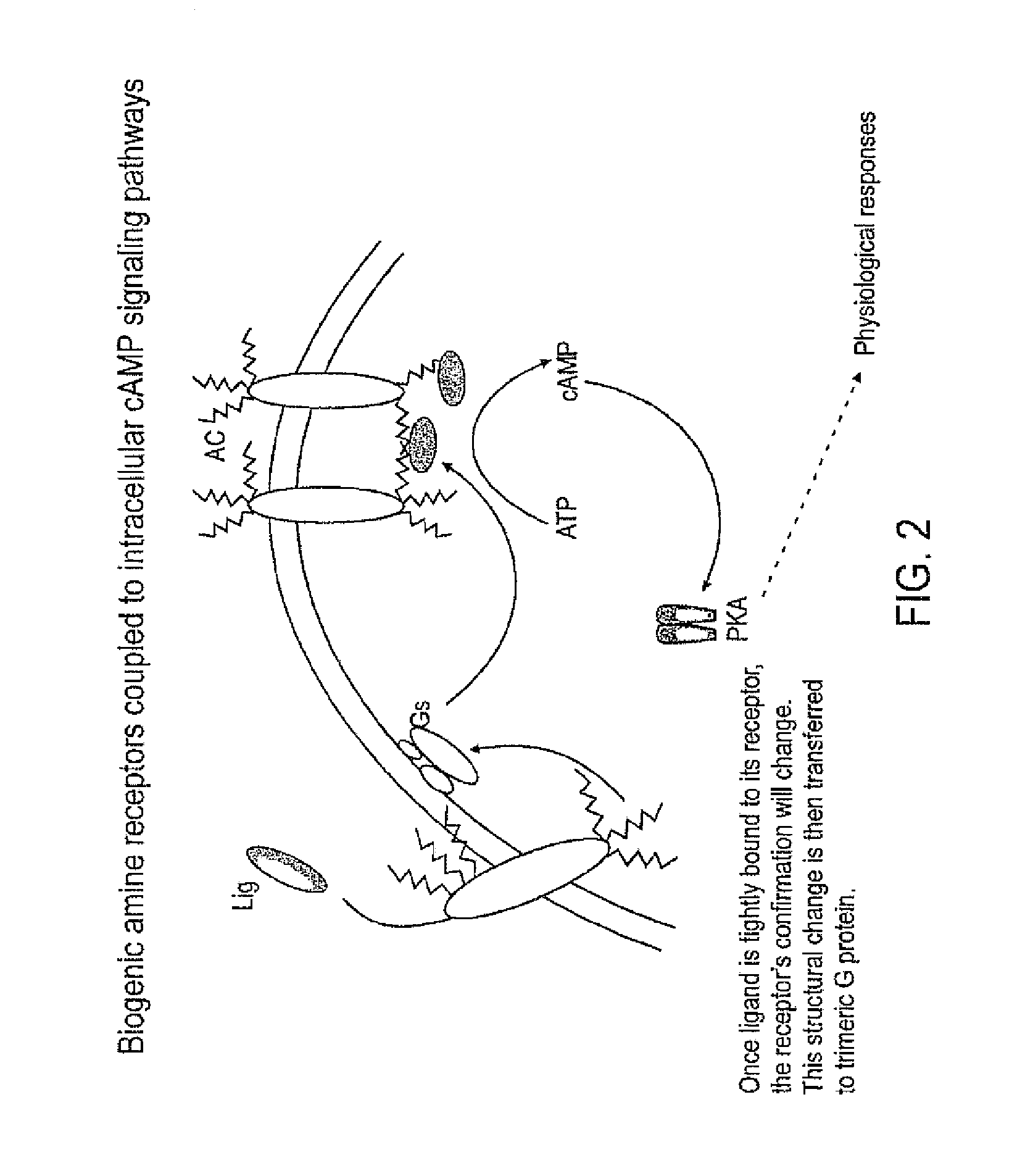
PCT No. PCT / DK96 / 00158 Sec. 371 Date Nov. 26, 1997 Sec. 102(e) Date Nov. 26, 1997 PCT Filed Apr. 1, 1996 PCT Pub. No. WO96 / 30394 PCT Pub. Date Oct. 3, 1996The invention relates to the technical application of electromagnetic radiation such as microwaves and radiowaves and application of ultra sound to chain molecules. In particular, the present invention relates to the utilization of topological excitations such as wring, twist and torsional modes, e.g., for generating structure, such as in folding, refolding or renaturation, and denaturation or unfolding of peptides, polypeptides, proteins, and enzymes; for generating changes in molecular affinity; for stimulating drug receptor interactions; and for changing molecular communication, is described. The technique is based on a new understanding of the underlying physical phenomenon and can also be applied to other chain molecules and biologically active biomolecules and tailored polymers such as glucoproteins, antibodies, genomic chain molecules such as DNA and RNA as well as PNA, carbohydrates, and synthetic and natural organic polymers. The invention is especially applicable for solving problems related to inclusion bodies and aggregation when using recombinant DNA and protein engineering techniques. Furthermore, the invention can be utilized in therapeutic treatment and in development and production of pharmaceuticals. The area of applicability ranges from biotechnological industry, food industry, drug industry, pharmacological industry, chemical industry, and concerns, e.g., the treatment of conditions and diseases related to influenza, hepatitis, polio, malaria, borrelia, diabetes, Alzheimer's disease, Creutzfeldt Jakob disease, other prion related diseases, multiple sclerosis, cataract, heart diseases, cancer, and aging.
Owner:PROKYON
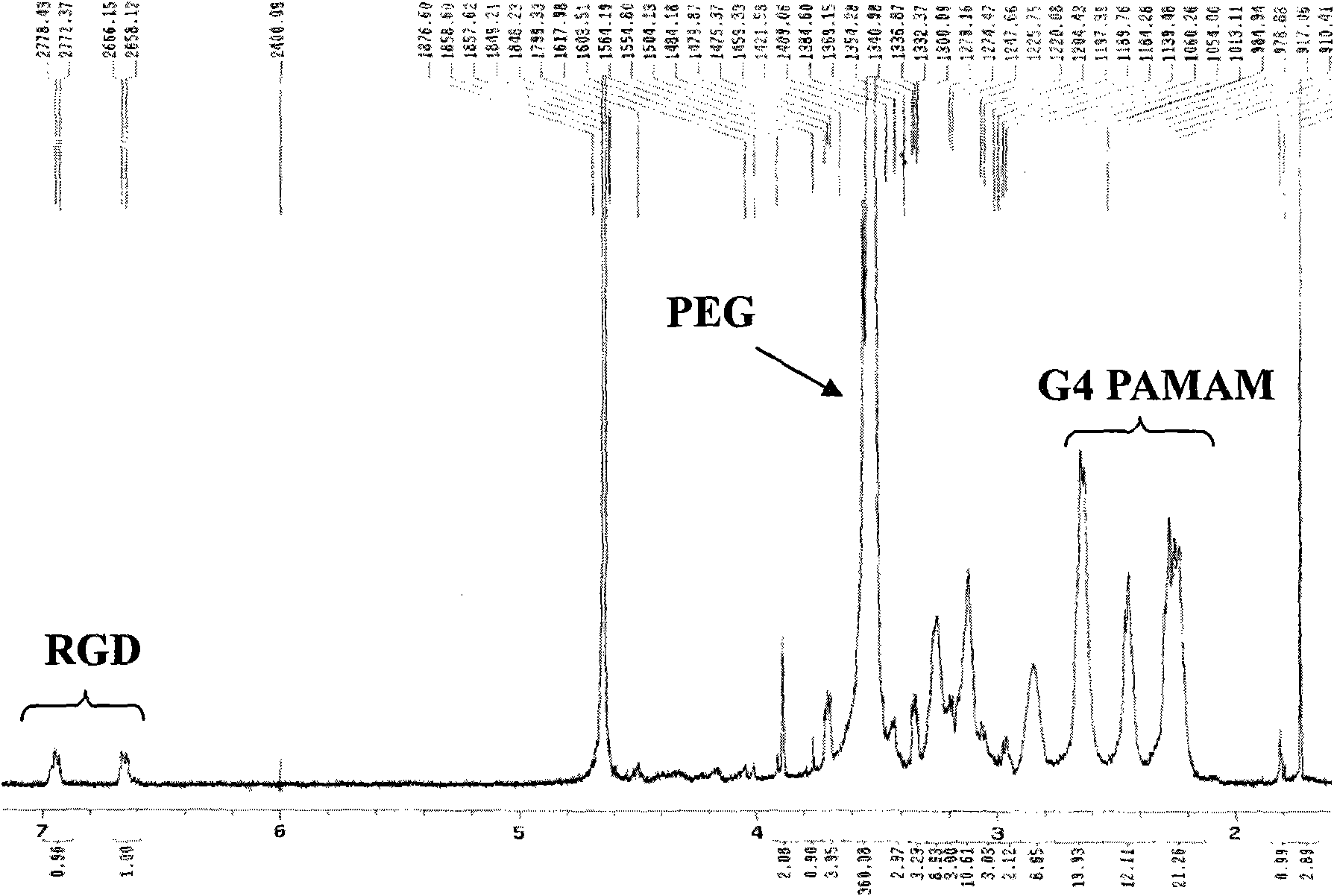
Anti-tumor nano prodrug system based on dendrimer and preparation method thereof
InactiveCN101879313AHigh molecular weightImprove hydrophilicityOrganic active ingredientsPharmaceutical non-active ingredientsDendrimerCyclic peptide
The invention relates to a multi-function nano prodrug system based on a dendrimer, which belongs to the technical field of biomedicines and nano medical science. The nano system comprises four function parts: an outermost layer RGD (arginyl-glycocoll-aspartate) cyclic peptide as an active targeting head group (1), a polyethylene glycol (PEG) hydrophilic chain segment (2) connected with the targeting head group and the dendrimer, an anti-tumour drug (3) connected with the dendrimer by an acid sensitive chemical bond and a dendrimer kernel (4). The general formula of the nano system is RGD-PEG-Dendrimer-DOX (doxorubicine), wherein the macromolecule attribute of a dendrimer vector makes a vector system passively target to tumor tissues through an EPR (Enhanced Permeability and retention) effect; the RGD cyclic peptide makes the vector system actively target to the tumor tissues through the interaction of a ligand and a receptor and promotes the endocytosis of the tumor tissues; the acid sensitive chemical bond ensures that a drug-carrying system is stable in systemic circulation and releases active drugs after arriving tumor positions and entering an acid organelle to perform the function of cytotoxicity.
Owner:FUDAN UNIV
Human monoclonal antibody against coreceptors for human immunodeficiency virus
InactiveUS7005503B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCXCR4Immunodeficiency virus
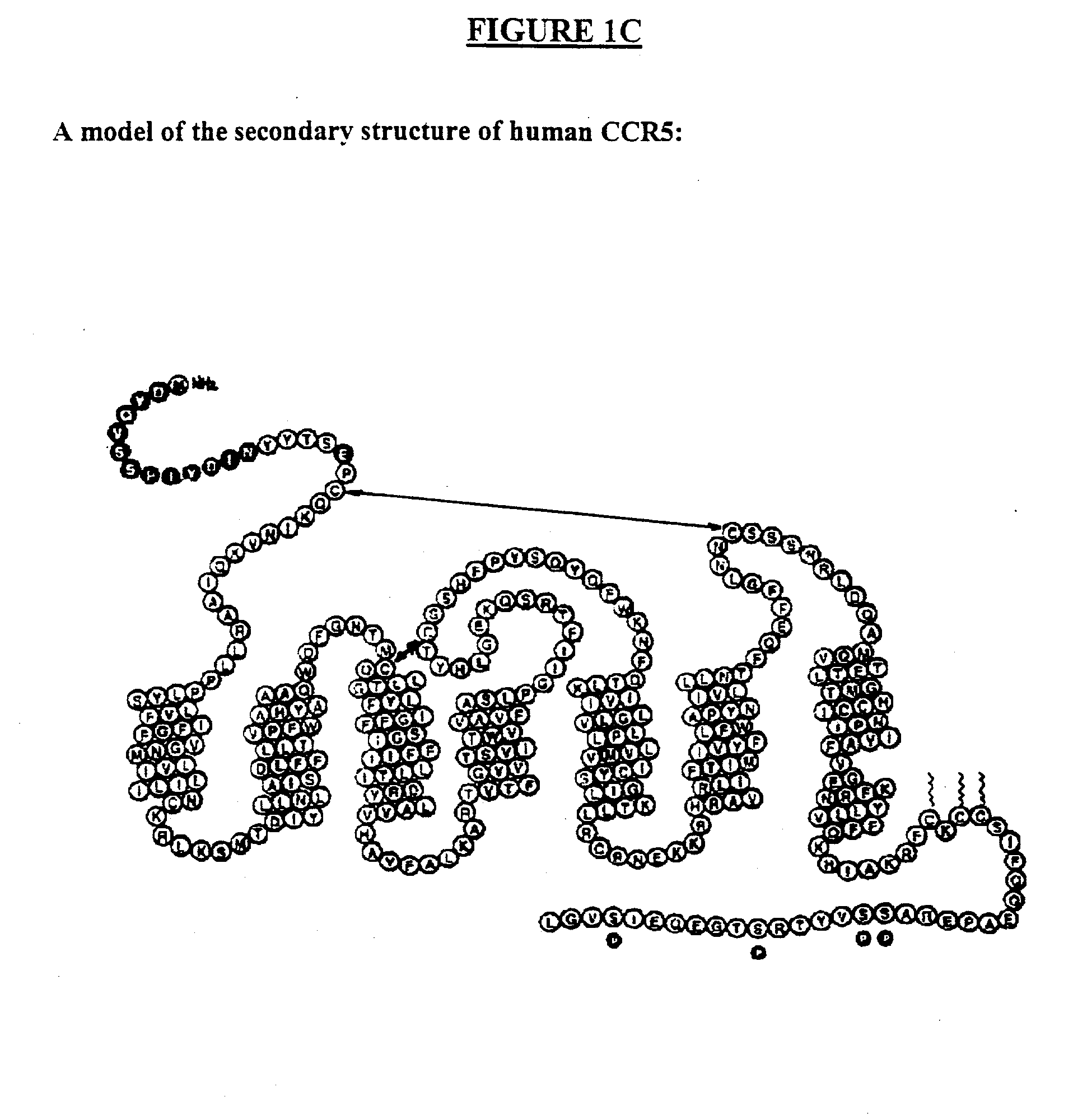
Compositions are provided that comprise antibody against coreceptors for human immunodeficiency virus such as CCR5 and CXCR4. In particular, monoclonal human antibodies against human CCR5 are provided that bind to CCR5 with high affinity and are capable of inhibiting HIV infection at low concentrations. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection, for screening drugs, and for diagnosing diseases or conditions associated with interactions with HIV coreceptors.
Owner:GENETASTIX CORP
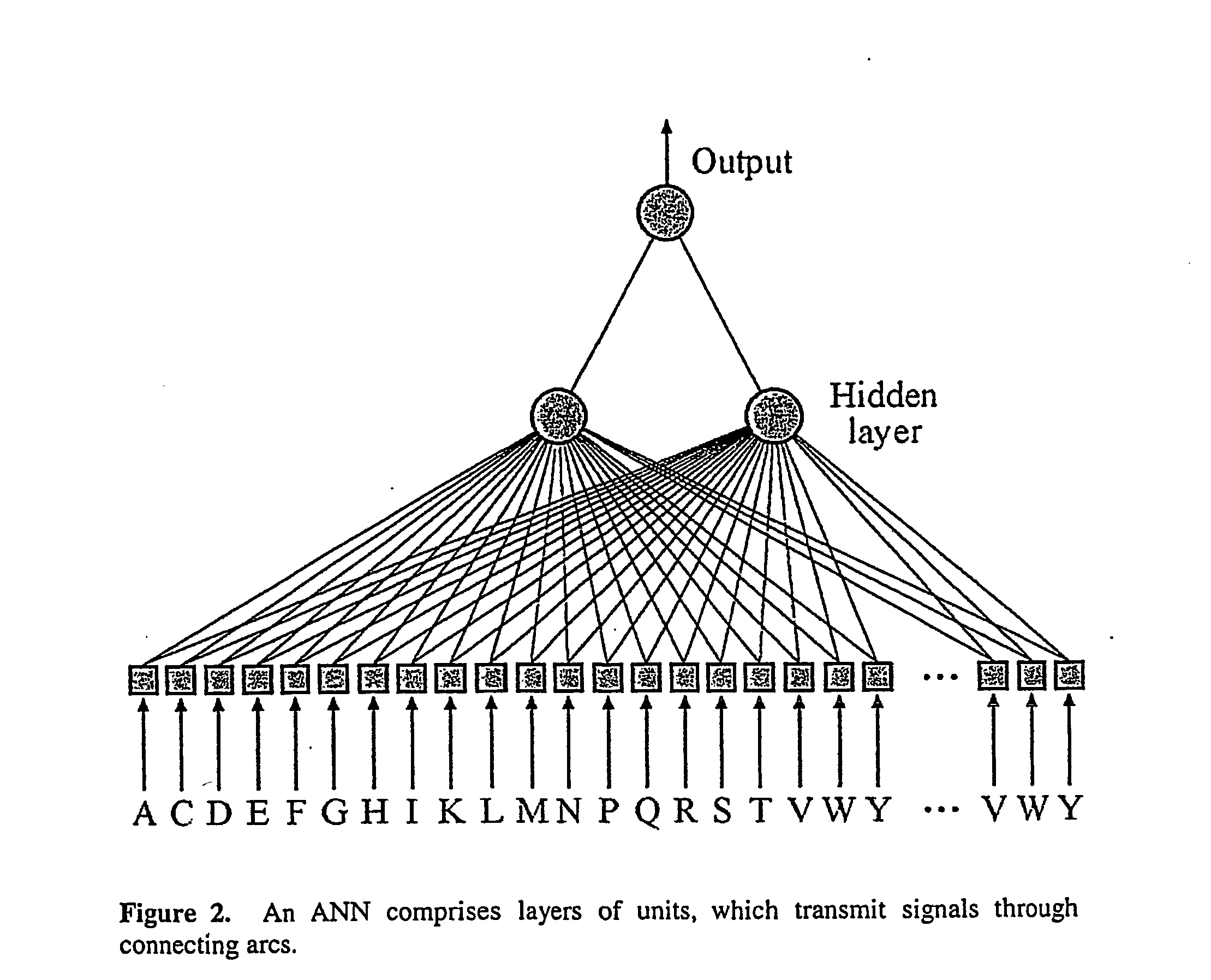
System and method for systematic prediction of ligand/receptor activity
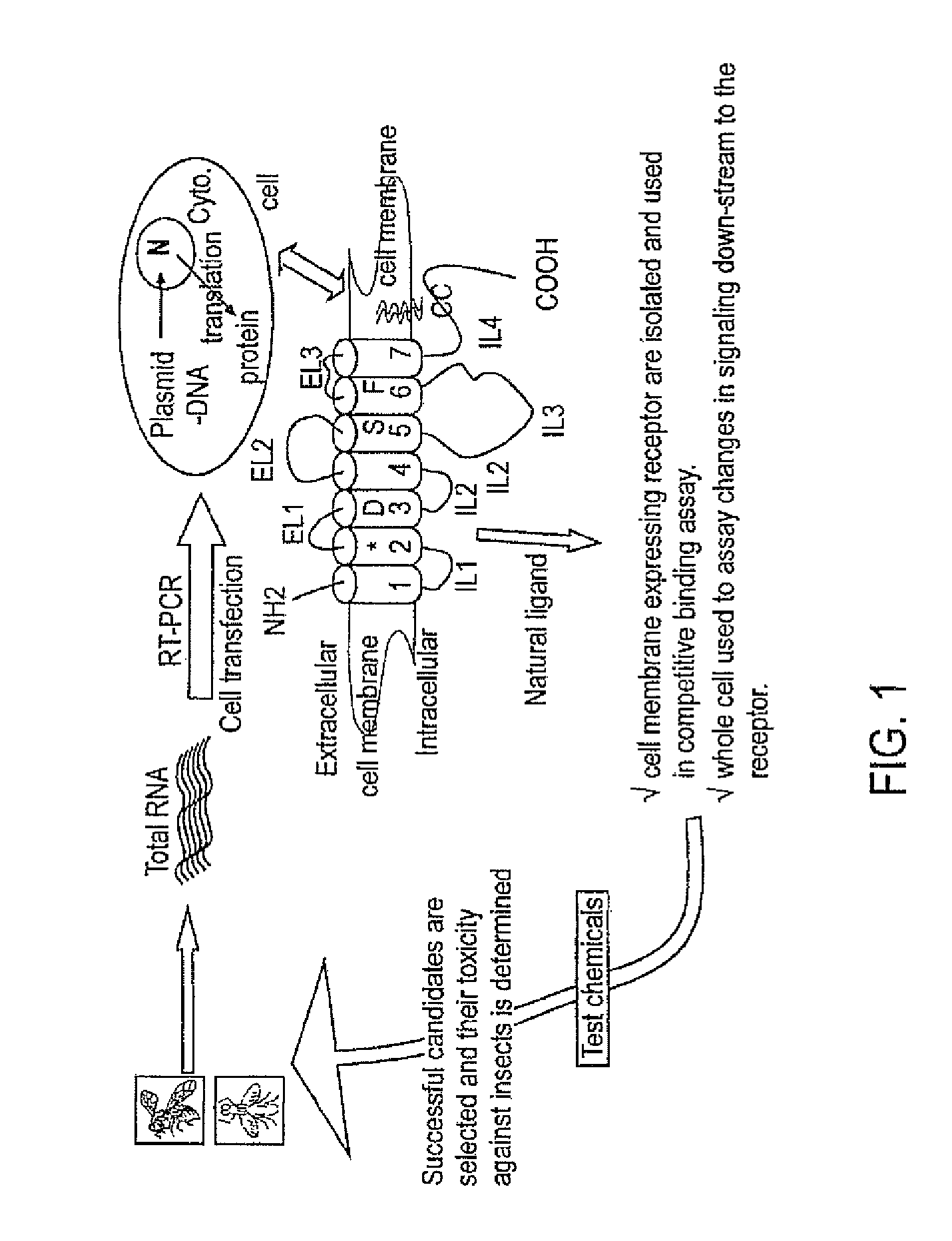
InactiveUS20050074809A1Easy to useAnalogue computers for chemical processesBiological testingMajor histocompatibilityComputer science
Disclosed is a general system and method, for prediction of binding of peptide-like ligands (peptides) to peptide-like receptors (receptors). Specifically this invention uses non-linear prediction models (including, but not limited to, artificial neural networks), sequence data form ligands and their respective receptors, and known ligand-receptor binding affinities. The representation of ligand-receptor interaction used along with the binding affinity of said interaction is used to train a determining means in a form of a predictive model. Prediction of binding affinity of a novel (not used for training of a predictive model) ligand-receptor interaction, involving a peptide and a particular receptor, involves the combining of representations of both peptide and receptor and presenting that representation to a previously trained predictive model. The system and method can be used as a single predictive model for determination of ligand binding to an individual receptor, or to a group of related receptors. This system and method was validated using data on peptide binding to major histocompatibility complex molecules (MHC) and artificial neural networks (ANN).
Owner:AGENCY FOR SCI TECH & RES
Modified binding proteins inhibiting the VEGF-A receptor interaction
ActiveUS8710187B2Senses disorderPeptide/protein ingredientsAnkyrin Repeat ProteinPolyethylene glycol
The present invention relates to binding proteins specific for Vascular Endothelial Growth Factor A (VEGF-A), in particular to recombinant binding proteins comprising a polyethylene glycol moiety and a binding domain, which inhibits VEGF-Axxx (wherein xxx denotes the amino acid length of the VEGF-A mature protein) binding to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2). Examples of such recombinant binding proteins are proteins which comprise an ankyrin repeat domain with the desired binding specificity, and a polyethylene glycol moiety. The binding proteins are useful in the treatment of cancer and other pathological conditions, e.g. eye diseases such as age-related macular degeneration.
Owner:MOLECULAR PARTNERS AG
Use of IL-20 Antagonists for Treating Rheumatoid Arthritis and Osteoporosis
ActiveUS20110064731A1Treating and delaying onset of and preventing osteoporosisMetabolism disorderAntibody ingredientsOsteopoikilosisAnti-IL-20
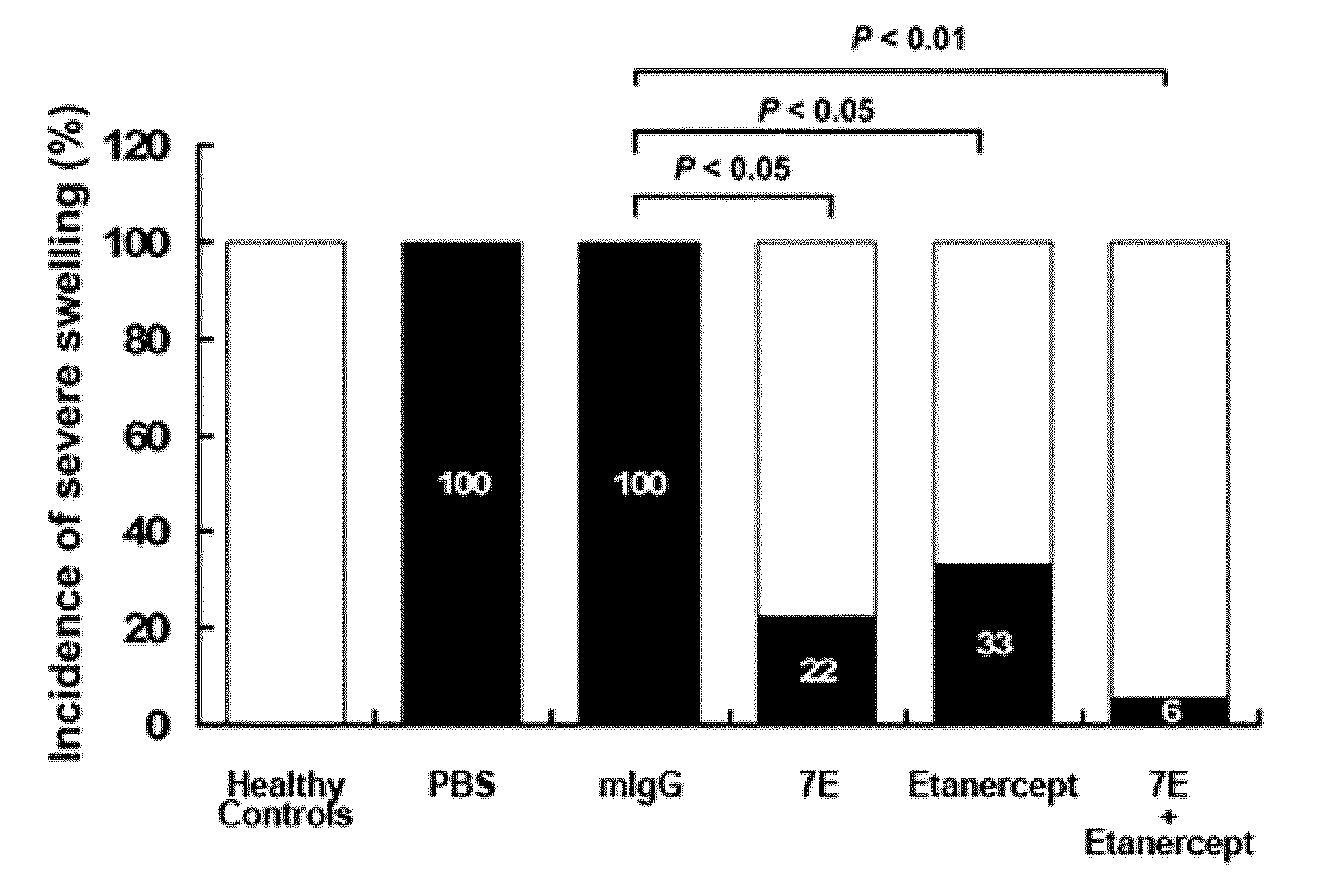
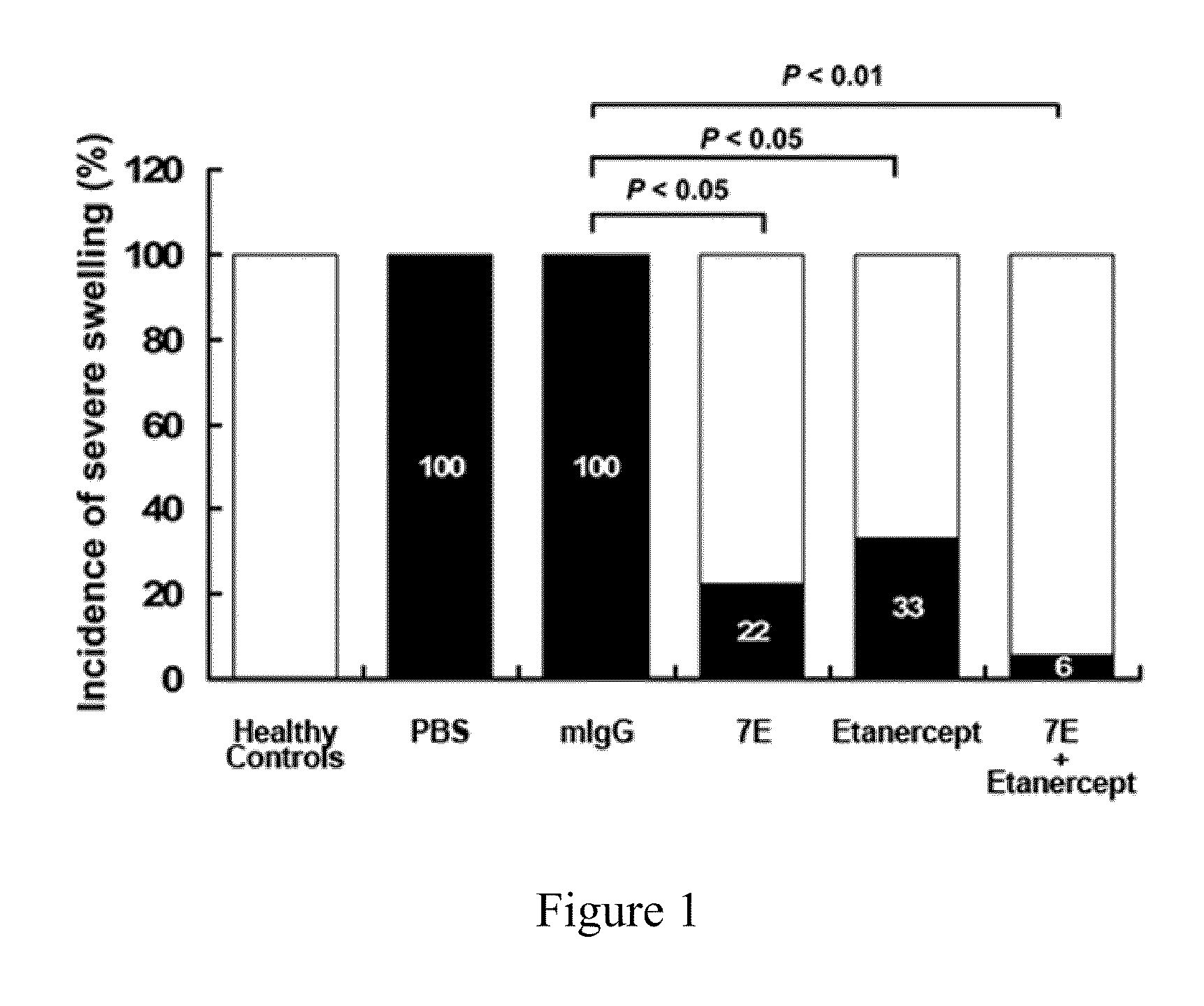
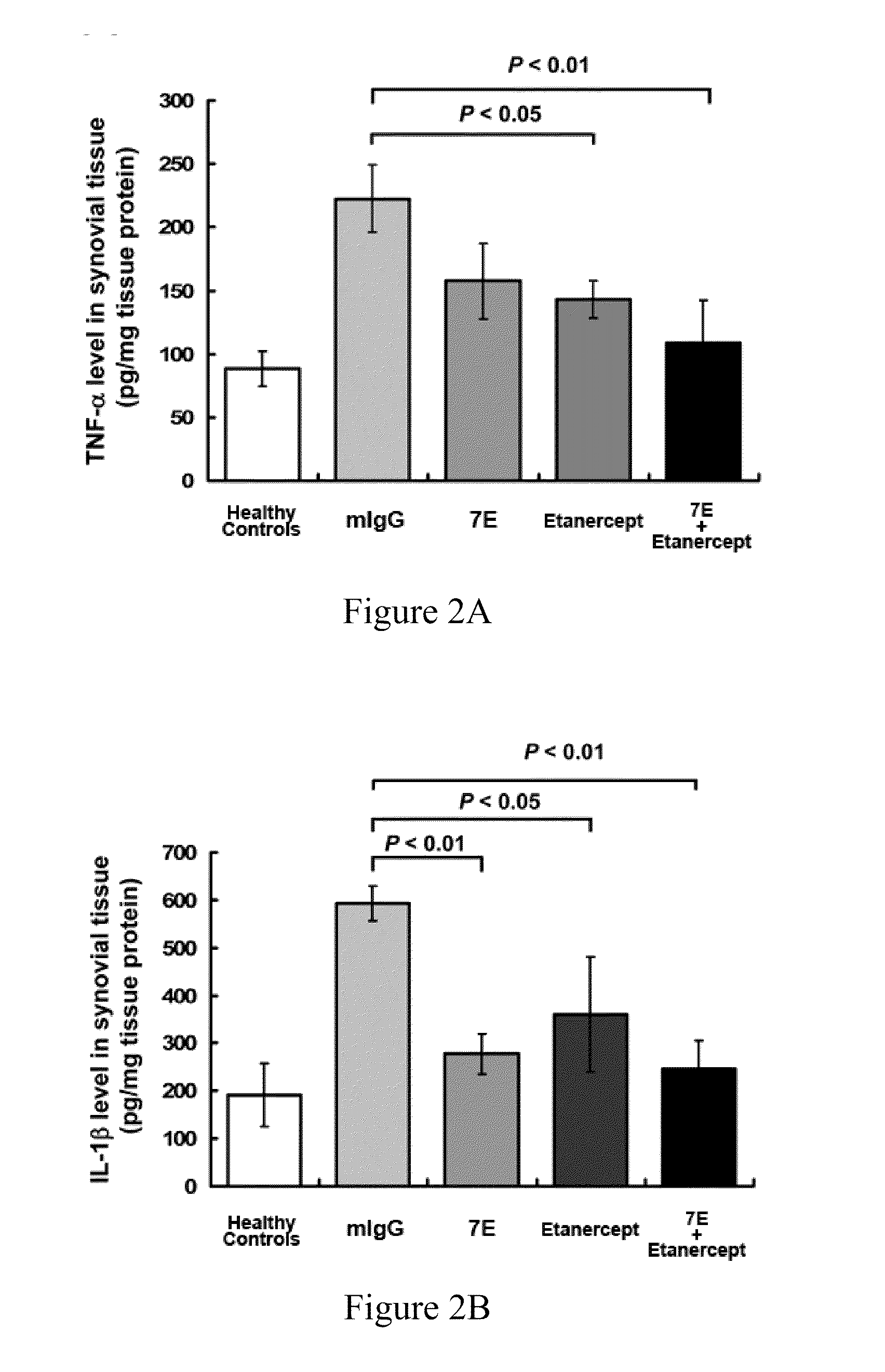
The invention features methods and compositions for preventing or treating rheumatoid arthritis and osteoporosis by administering an antagonist of IL-20. The IL-20 antagonist may be an anti-IL-20 antibody, such as mAB 7E, that is capable of binding human IL-20 and blocking IL-20 interaction with its receptors.
Owner:LBL BIOTECH INC
Human monoclonal antibody against coreceptors for human immunodeficiency virus
InactiveUS20030152913A1Microbiological testing/measurementImmunoglobulins against virusesCXCR4Immunodeficiency virus
Compositions are provided that comprise antibody against coreceptors for human immunodeficiency virus such as CCR5 and CXCR4. In particular, monoclonal human antibodies against human CCR5 are provided that bind to CCR5 with high affinity and are capable of inhibiting HIV infection at low concentrations. The antibodies can be used as prophylactics or therapeutics to prevent and treat HIV infection, for screening drugs, and for diagnosing diseases or conditions associated with interactions with HIV coreceptors.
Owner:GENETASTIX CORP
Active protease-resistant antibody FC mutants
InactiveUS8871204B2Improve abilitiesHigh activityAntibacterial agentsPeptide/protein ingredientsProteinase activityProtease resistant
The present invention relates to modified Fc-containing molecules including modified antibodies characterized by increased resistance to host and pathogen-derived proteases, ability to interact with FcγR receptors except with FcγRI, and lack of induction of IL-10 secretion by macrophages, and methods of using and making them.
Owner:JANSSEN BIOTECH INC
Pest control compositions and methods
Embodiments of the present invention provide compositions for controlling a target pest including a first agent and a second agent comprising a pest control product or a signal cascade modulator, wherein the first agent and the second agent act synergistically to control the target pest. The first agent can be capable of interacting with a receptor in the target pest. The pest control product can have a first activity against the target pest when applied without the active agent and the compositions can have a second activity against the target pest; and the second activity can be greater than the first activity. Embodiments of the invention can include compositions that modulate the signal cascade initiated by the binding of ligands to, for example, cell surface receptors. Methods of screening such compositions are also disclosed.
Owner:TYRATECH
Binding proteins inhibiting the VEGF-A receptor interaction
The present invention relates to binding proteins specific for VEGF-A, in particular to recombinant binding proteins comprising a binding domain, which inhibits VEGF-Axxx binding to VEGFR-2. Examples of such binding proteins are proteins which comprise an ankyrin repeat domain with the desired binding specificity. The binding proteins are useful in the treatment of cancer and other pathological conditions, e.g. eye diseases such as age-related macular degeneration.
Owner:MOLECULAR PARTNERS AG
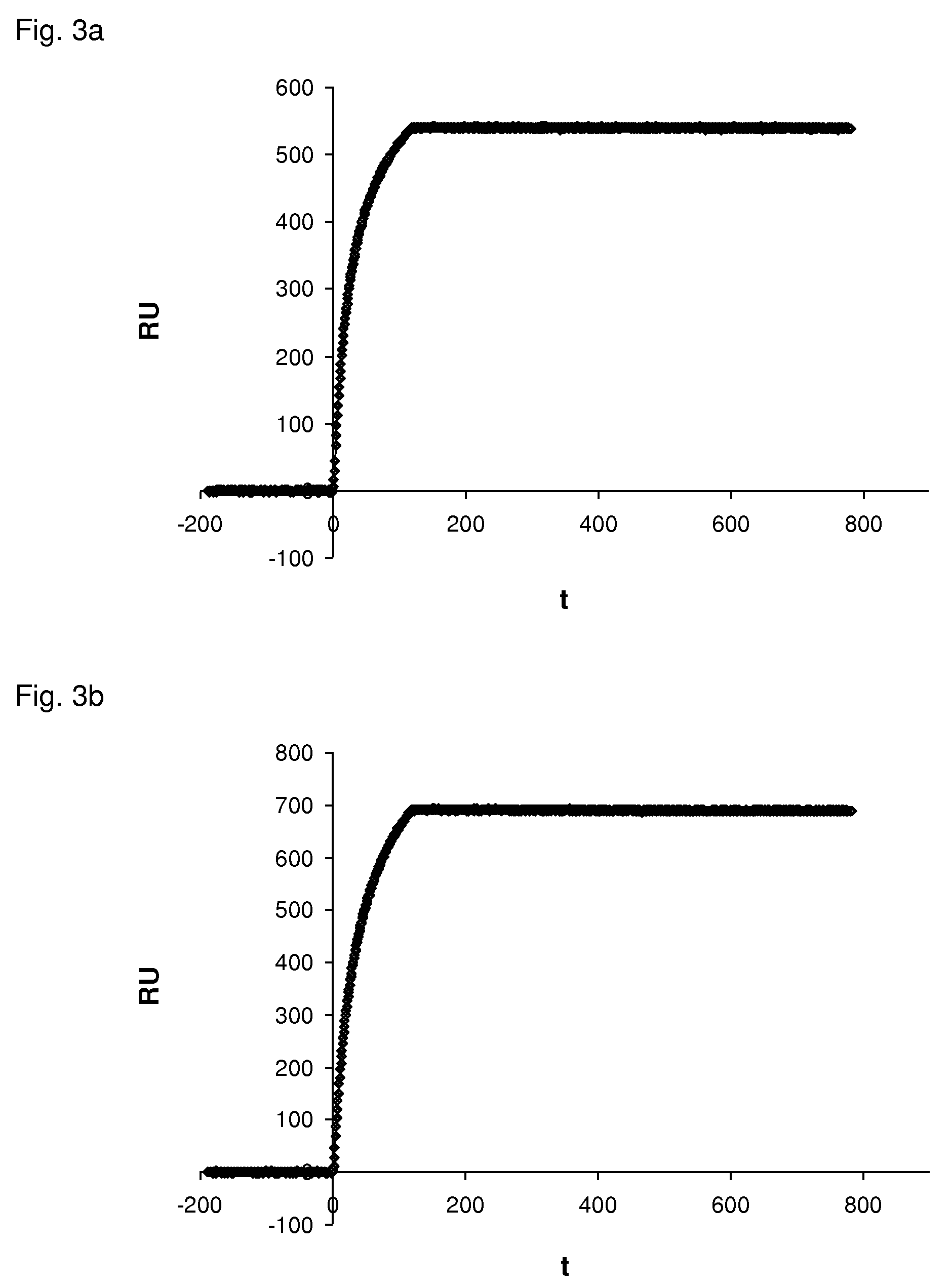
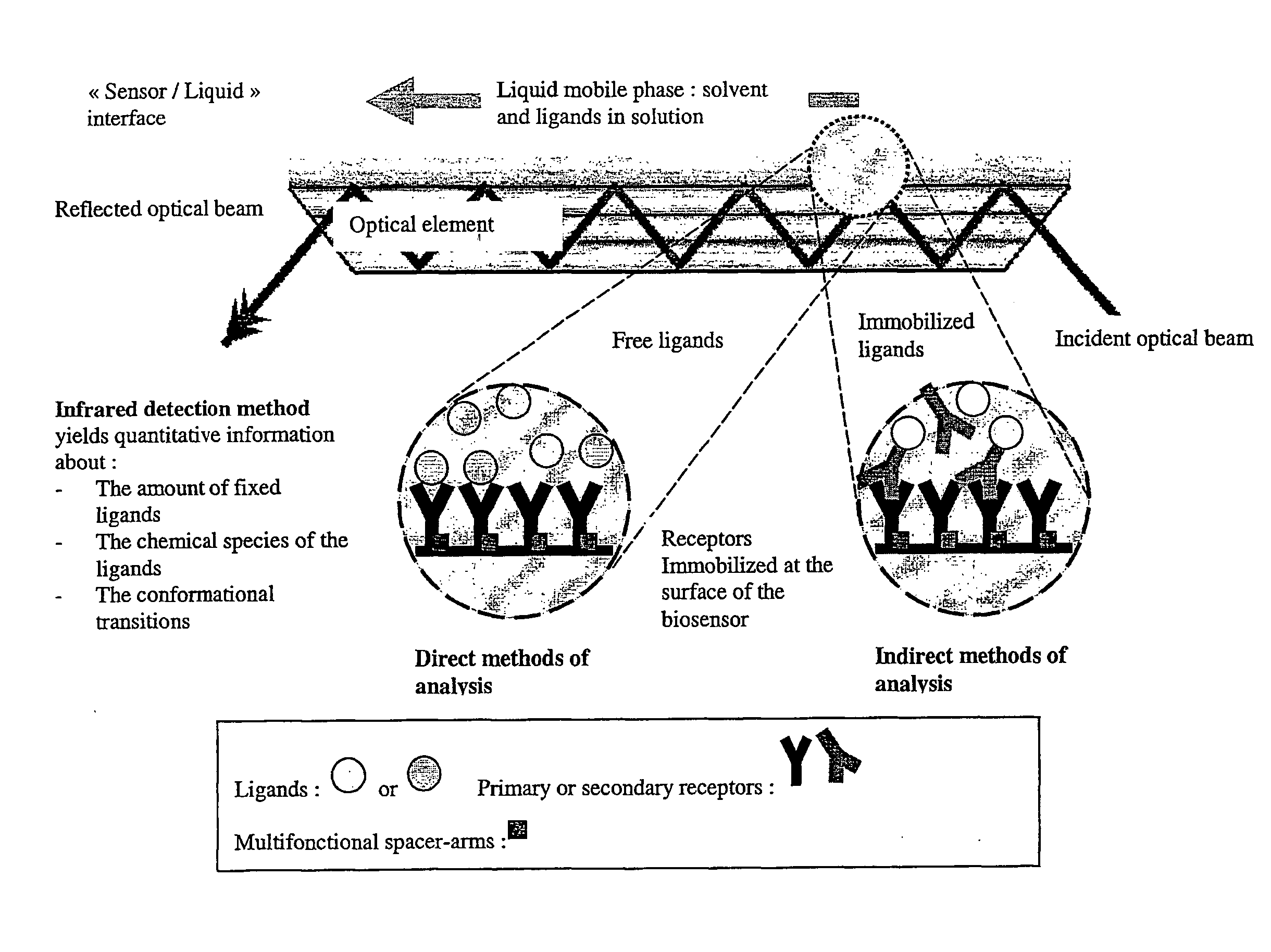
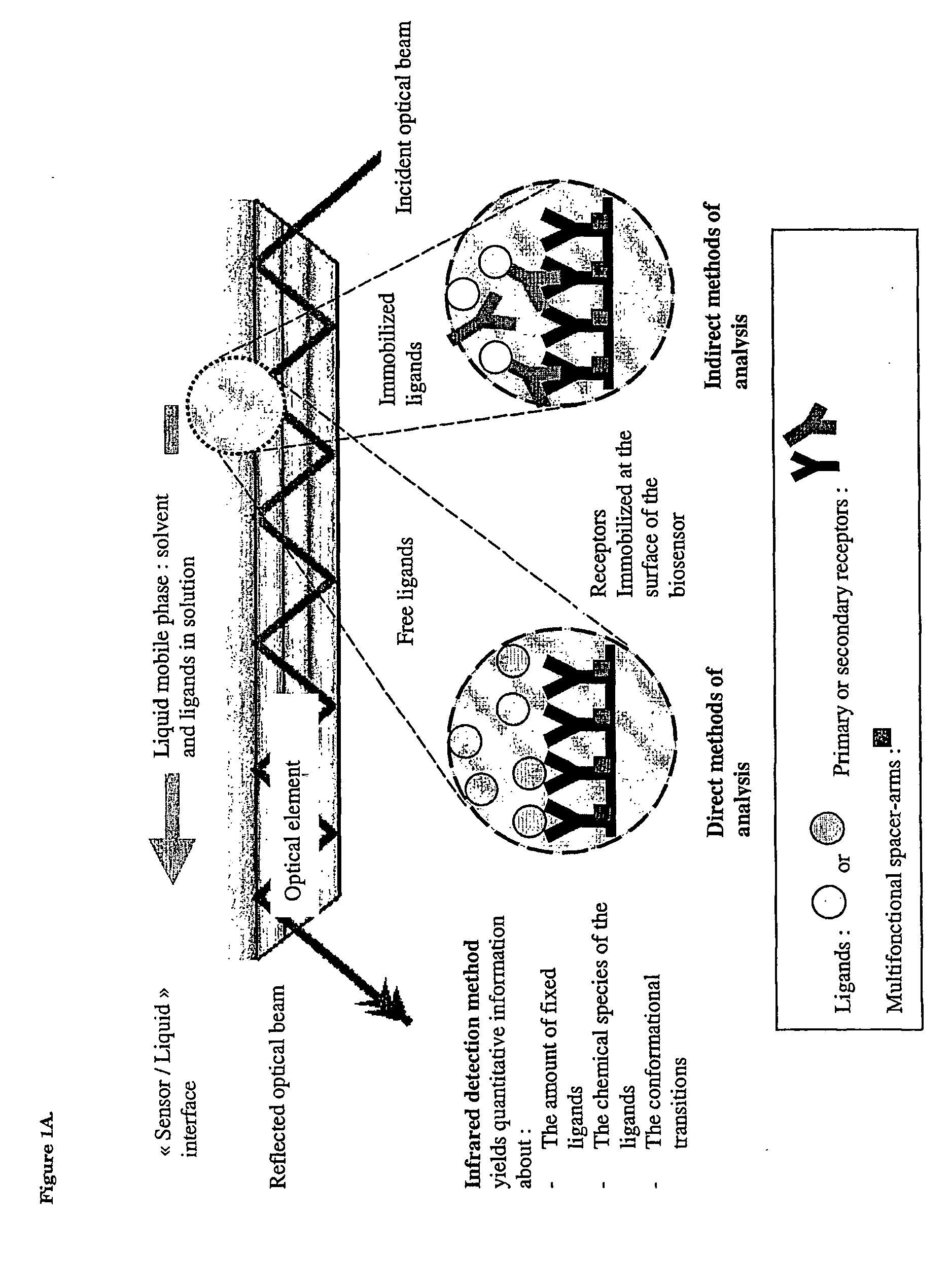
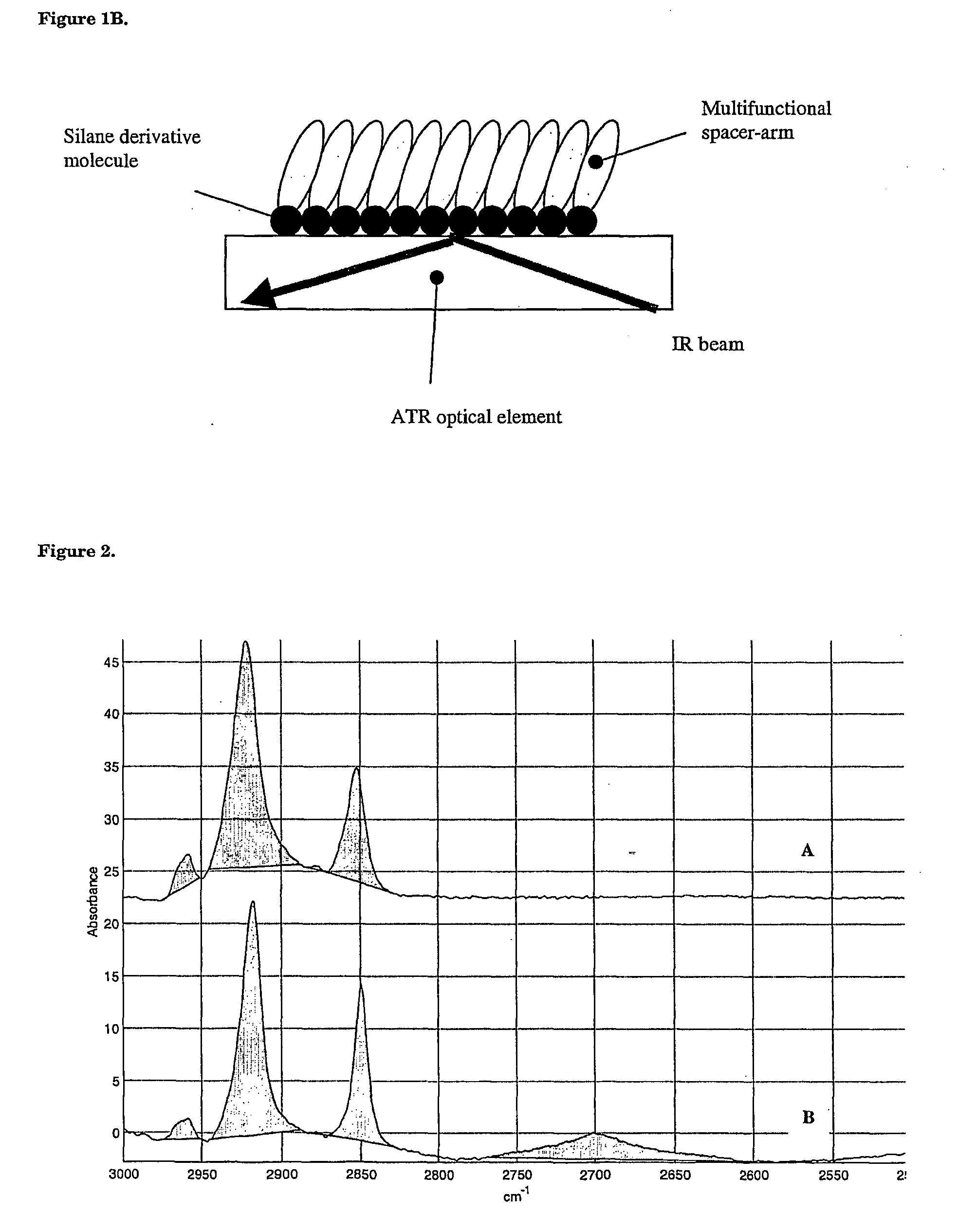
Surface chemical modification of optical elements for the spectroscopic detection of molecules and organic components
InactiveUS20040121491A1High reactivityImprove wettabilityRadiation pyrometryScattering properties measurementsInfraredBiological activation
The invention relates to a device suitable for the investigation of ligand-receptor interactions, in particular for the investigation of an analyte target interaction such as biological and chemical molecules and organic components and their interaction with surfaces, consisting of an attenuated total internal reflection element, transparent in the infra-red and of which at least one surface is chemically activated and covalently grafted with an organic molecule able to immobilize the receptor. The invention further relates to the use of said device and a method for the construction of said device comprising the steps of: surface activation of at least one surface of an attenuated total internal reflection element; surface grafting with an organic molecule of the activated surface obtained in the previous step; and coupling a receptor via covalent fixation on the organic molecule.
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN +2
Pest control compositions and methods
Embodiments of the present invention provide compositions for controlling a target pest including a pest control product and at least one active agent, wherein: the active agent can be capable of interacting with a receptor in the target pest; the pest control product can have a first activity against the target pest when applied without the active agent and the compositions can have a second activity against the target pest; and the second activity can be greater than the first activity.
Owner:TYRATECH
B7-h1 and methods of diagnosis, prognosis, and treatment of cancer
The invention features methods of diagnosis by assessing B7-H1 expression in a tissue from a subject that has, or is suspected of having, cancer, methods of treatment with agents that interfere with B7-H1-receptor interaction, methods of selecting candidate subjects likely to benefit from cancer immunotherapy, and methods of inhibiting expression of B7-H1.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Methods of prevention and treatment of asthma, and allergic conditions
InactiveUS20050013799A1Avoid allergic reactionsEffective preventionPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsInterferon alphaReceptor Inhibition
The present invention relates to allergy vaccines and methods of treating and / or preventing asthma, and allergic conditions. The invention is based on the discovery that inhibiting the ligand / receptor interactions involving, e.g., IgE, IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, interferon-alpha, histamine, leukotriene, and their respective receptors, inhibits production of IgE thereby treating or preventing such diseases or conditions. Competitive inhibition of such receptor / ligand interactions is accomplished by immunizing a human or veterinary patient with the interleukin, interferon-alpha, histamine, leukotriene, their receptors, in any combination. Also, the invention relates to inhibiting receptor / ligand interactions involved in IgE production by competitively inhibiting such interactions by administering antibodies to the ligands, receptors, or both, as well as by administering analogs of the receptors (e.g., soluble receptors not associated with a cell).
Owner:ADVANCED BIOTHERAPY
Methods of prevention and treatment of asthama, and allergic conditions
InactiveUS20050013800A1Avoid allergic reactionsEffective preventionPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsInterferon alphaReceptor Inhibition
The present invention relates to allergy vaccines and methods of treating and / or preventing asthma, and allergic conditions. The invention is based on the discovery that inhibiting the ligand / receptor interactions involving, e.g., IgE, IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, interferon-alpha, histamine, leukotriene, and their respective receptors, inhibits production of IgE thereby treating or preventing such diseases or conditions. Competitive inhibition of such receptor / ligand interactions is accomplished by immunizing a human or veterinary patient with the interleukin, interferon-alpha, histamine, leukotriene, their receptors, in any combination. Also, the invention relates to inhibiting receptor / ligand interactions involved in IgE production by competitively inhibiting such interactions by administering antibodies to the ligands, receptors, or both, as well as by administering analogs of the receptors (e.g., soluble receptors not associated with a cell).
Owner:ADVANCED BIOTHERAPY
Micromolecular conjugate based on RGD polypeptide-chemotherapy drug and nanometer prodrug system thereof
InactiveCN107335060AHigh drug loadingImprove solubilityOrganic active ingredientsPharmaceutical non-active ingredientsCathepsin BWilms' tumor
The invention relates to a micromolecular conjugate based on RGD polypeptide-chemotherapy drug and a self-assembly nanometer prodrug system thereof, and belongs to the technical field of biological medicine and nanometer medicine. The nanometer prodrug system has the main advantages that (1) the system is formed by RGD polypeptide and an anti-tumor drug through direct covalent connection via micromolecular connecting arms, and the drug loading capacity of the system is improved; (2) the conjugate is self-assembled into a nanometer prodrug using the drug as a hydrophobic inner core and the RGD polypeptide as a hydrophilic outer shell, the active targeting on tumor cells and tumor new vessels is realized through the ligand-receptor mutual action, and the endocytosis is promoted; (3) the stability of the nanometer prodrug system in the body circulation can be ensured through thioether bonds, reduction sensitive bonds and cathepsin B sensitive bonds, and the cytotoxic drug is released when the nanometer prodrug system reaches the tumor microenvironment; and (4) the micromolecular conjugate instead of a macromolecular material is used as a carrier, so that the nanometer prodrug system can favorably and better penetrate into the tumor tissues and cells to achieve better anti-tumor effects.
Owner:PEKING UNIV
Pest control compositions and methods
Embodiments of the present invention provide compositions for controlling a target pest including a first agent and a second agent comprising a pest control product or a signal cascade modulator, wherein the first agent and the second agent act synergistically to control the target pest. The first agent can be capable of interacting with a receptor in the target pest. The pest control product can have a first activity against the target pest when applied without the active agent and the compositions can have a second activity against the target pest; and the second activity can be greater than the first activity. Embodiments of the invention can include compositions that modulate the signal cascade initiated by the binding of ligands to, for example, cell surface receptors. Methods of screening such compositions are also disclosed.
Owner:TYRATECH
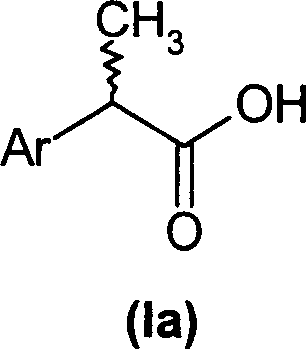
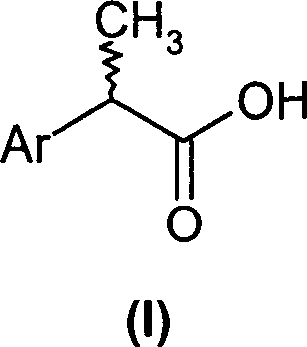
2-aryl-propionic acid and medicine composition containing same
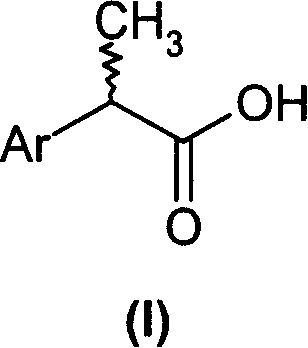
(R,S)-2-Aryl-propionic acids are useful as inhibitors of interleukin-8 induced human polymorphonucleated neutrophils (PMN) chemotaxis. (R,S)-2-Aryl-propionic acids of formula (I), their single (R) and (S) enantiomers or salts are useful as inhibitors of interleukin-8 (IL-8) induced human polymorphonucleated neutrophils (PMN) chemotaxis. [Image] Ar : phenyl ring (substituted by T in meta position, T1 in para position or T2 in ortho position); T : linear or branched 1-5C alkyl, 2-5C alkenyl, or 2-5C alkynyl (optionally substituted by 1-5C alkoxycarbonyl, optionally substituted phenyl, linear or branched 1-5C hydroxyalkyl or arylhydroxymethyl); T1benzoyloxy, benzoylamino, benzenesulfonyloxy, benzenesulfonylamino, benzenesulfonylmethyl (all optionally substituted), 1-5C acyloxy, 1-5C acylamino, 1-5C sulfonyloxy, 1-5C alkanesulfonylamino, 1-5C alkanesulfonylmethyl, 3-6C cycloalkyl, 2-furyl, 3-tetrahydrofuryl, 2- thiophenyl, 2-tetrahydrothiophenyl or ((1-8C)-alkanoyl, -cycloalkanoyl or -arylalkanoyl)-1-5C-alkylamino (preferably acetyl-N-methyl-amino or pivaloyl-N-ethyl-amino); and T2arylmethyl, aryloxy or acylamino (all optionally substituted by 1-4C alkyl, 1-4C-alkoxy, chlorine, fluorine or trifluoromethyl). The meta linear or branched 1-5C alkyl together with a substituent in ortho or para position and the benzene ring forms optionally saturated or optionally substituted bicyclo aryls. INDEPENDENT CLAIM are included for the following: (1) preparation of (I); and (2) use of (I) in the preparation of a medicament for the treatment of e.g. psoriasis, ulcerative colitis, melanoma and chronic obstructive pulmonary disease (COPD). - ACTIVITY : Antipsoriatic; Antiulcer; Respiratory-Gen.; Antirheumatic; Antiarthritic; Nephrotropic; Vasotropic; Antiinflammatory; Gastrointestinal-Gen.; Cytostatic. - MECHANISM OF ACTION : Interleukin-8 (IL-8) inhibitor; GROalpha inhibitor; CXCR2 agonist / antagonist. The ability of (R,S) 2-[(3'-isopropyl)phenyl]propionic acid (A) to inhibit IL-8 induced chemotaxis of human monocytes was tested as described in Van Damme J. et al. (Eur. J. Immunol., 19, 2367, 1989). (A) showed inhibition (%) of 51+-12.
Owner:DOMPE FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com