Patents
Literature
101 results about "Protease resistant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Studies indicate that protease resistance is least likely to develop when these agents are given in combination with two nucleosides. Protease inhibitors should always be administered at optimal therapeutic doses; suboptimal doses of these drugs merely promote the development of high-level protease resistance.
Adhesin as immunogen against enterotoxigenic Escherichia coli
ActiveUS20060153878A1Inhibition of colonizationAvoid stickingAntibacterial agentsPeptide/protein ingredientsEscherichia coliDiarrhea
The inventive subject matter relates to the methods for the induction of immunity and prevention of diarrhea resulting from Escherichia coli. The inventive subject matter also relates to the use Escherichia coli adhesins as immunogens and to the construction of conformationally stability and protease resistant Escherichia coli adhesin constructs useful for inducing immunity to Escherichia coli pathogenic bacteria. The methods provide for the induction of B-cell mediated immunity and for the induction of antibody capable of inhibiting the adherence and colonization of Escherichia coli including enterotoxigenic Escherichia coli, to human cells.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Genes encoding plant protease-resistant pesticidal proteins and method of their use
InactiveUS7462760B2High expressionImprove the immunitySugar derivativesClimate change adaptationProteinase activityPlant cell
Compositions and methods for protecting a plant from an insect pest are provided. The invention provides mutagenized nucleic acids that have been engineered to encode pesticidal polypeptides having increased resistance to proteolytic degradation by a plant protease. In particular, nucleic acid sequences encoding pesticidal polypeptides modified to comprise a proteolytic protection site that confers resistance to degradation or proteolytic inactivation by a plant protease are provided. Particular embodiments of the invention provide expression cassettes and transformed plants, plant cells, and seeds. These compositions find use in methods for protecting a plant from a pest.
Owner:EI DU PONT DE NEMOURS & CO
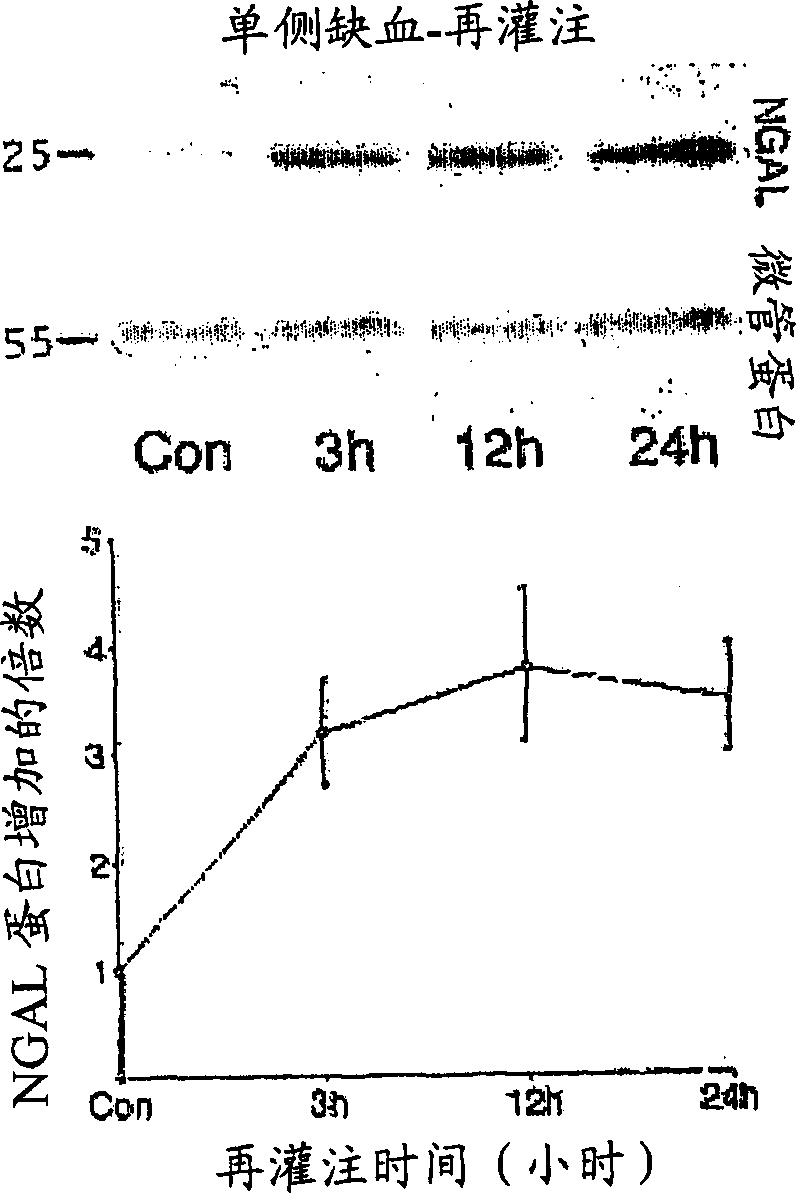
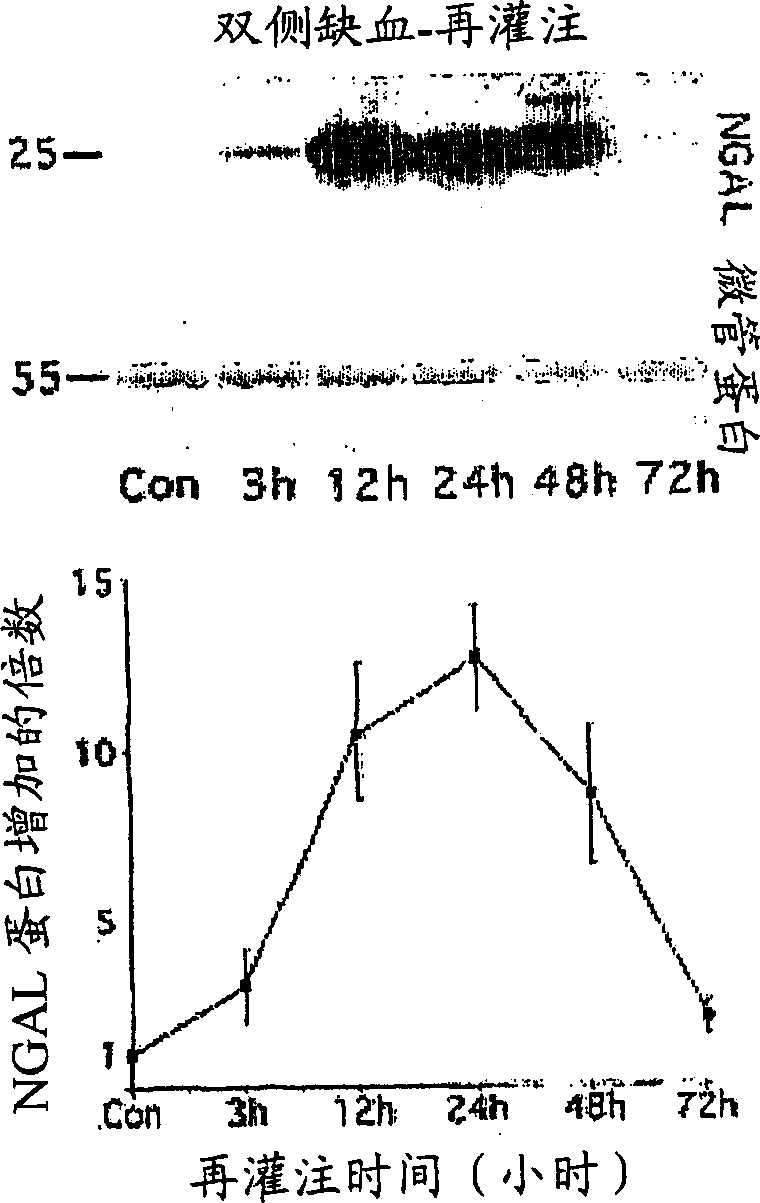
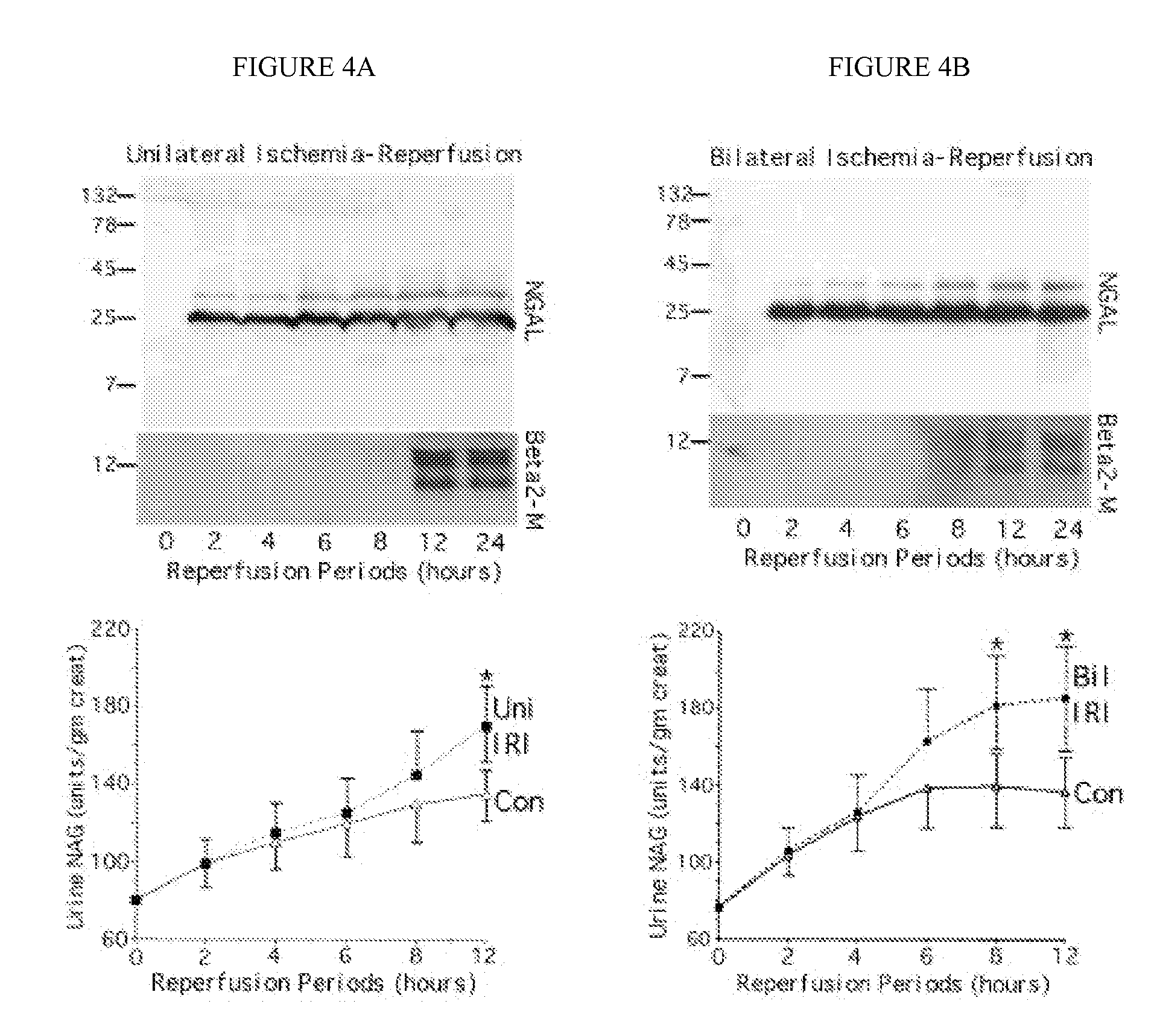
Method for the early detection of renal injury
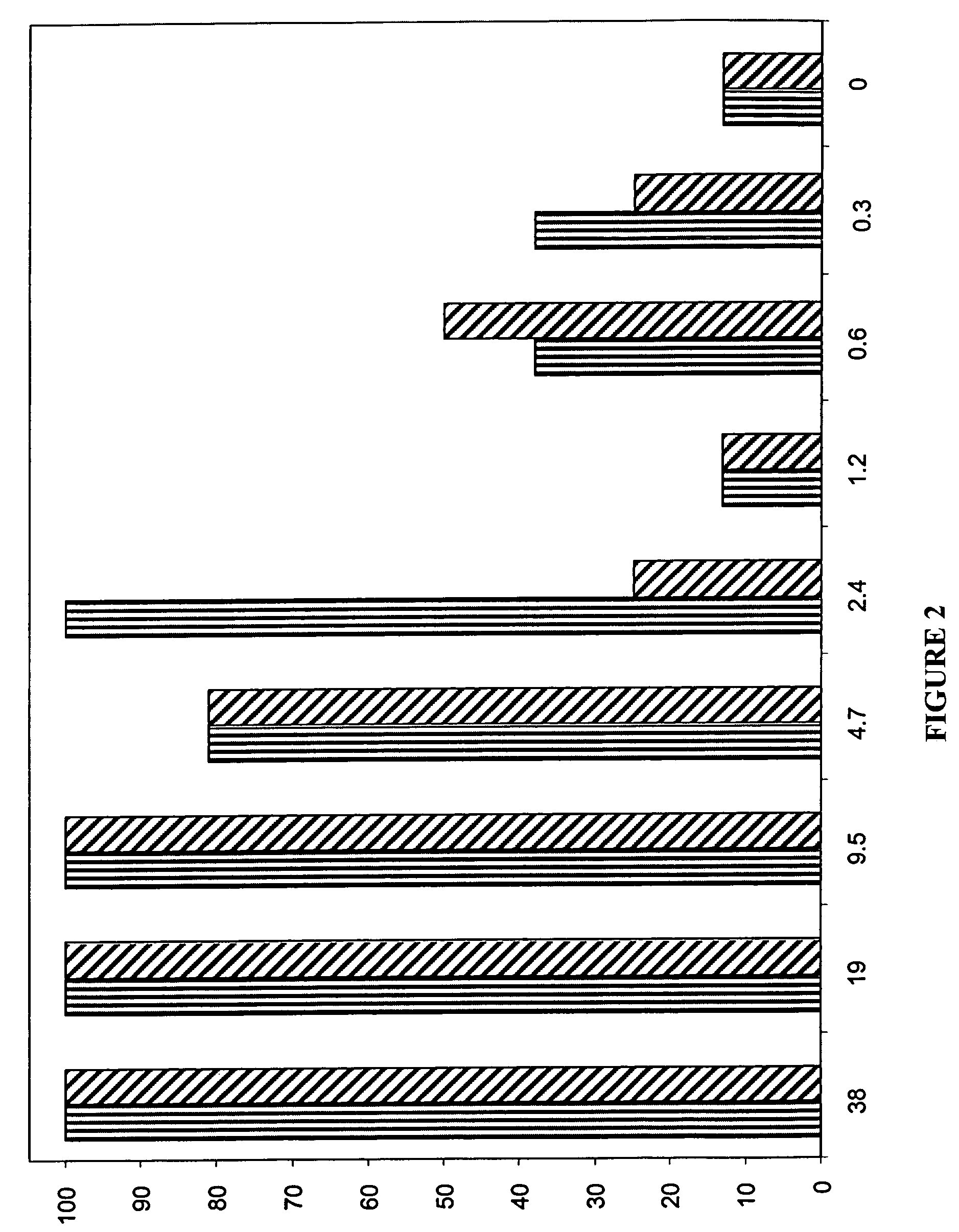
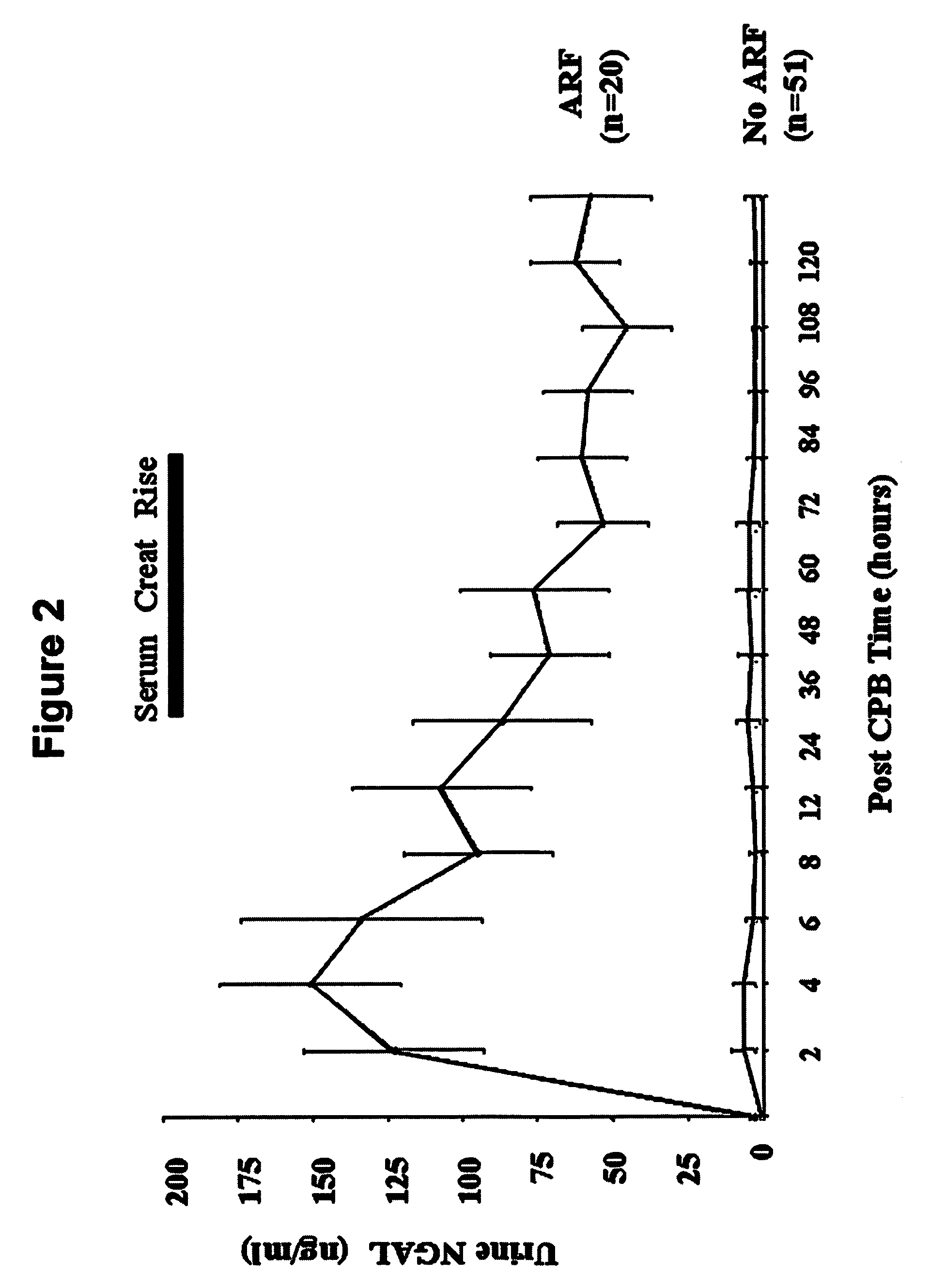
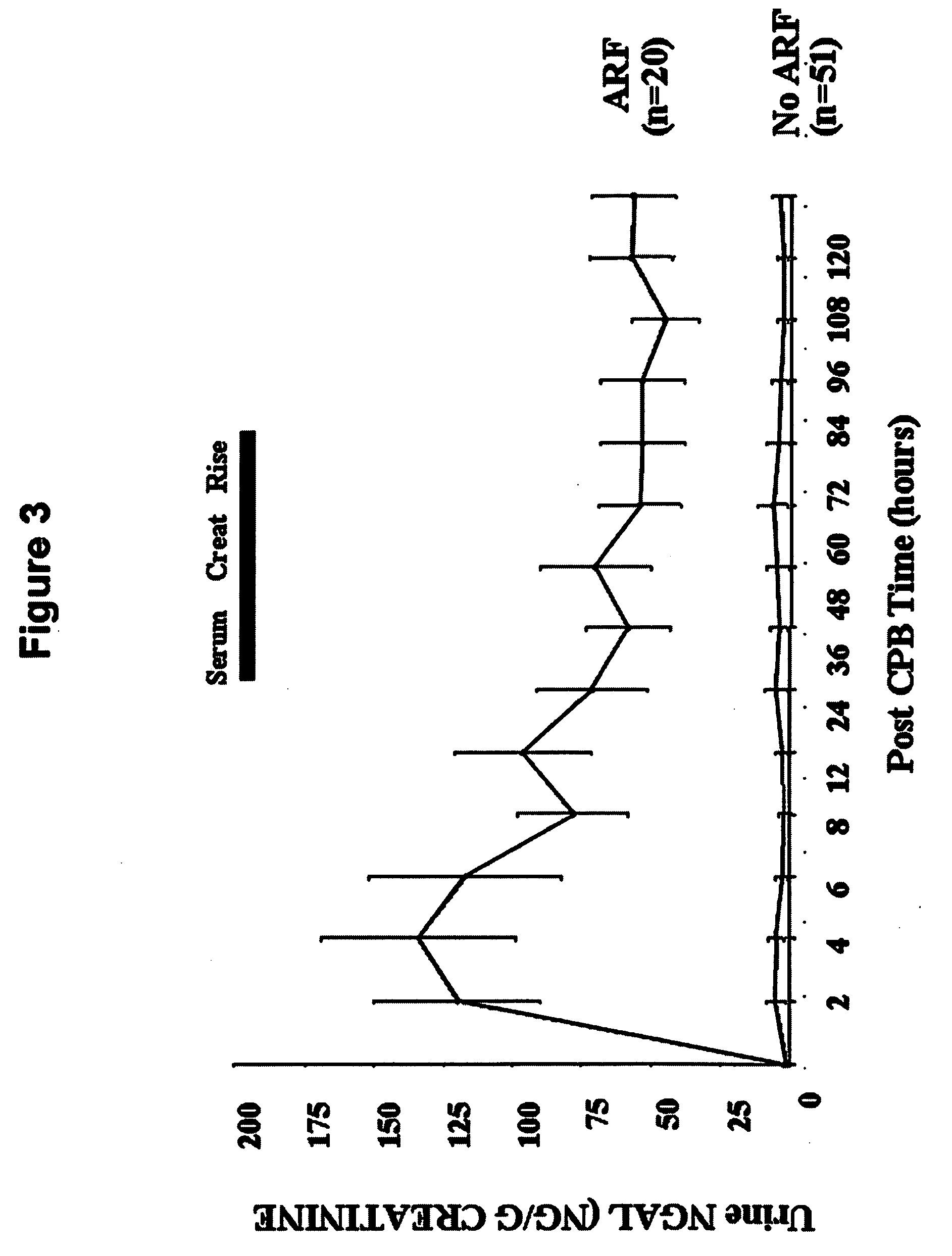
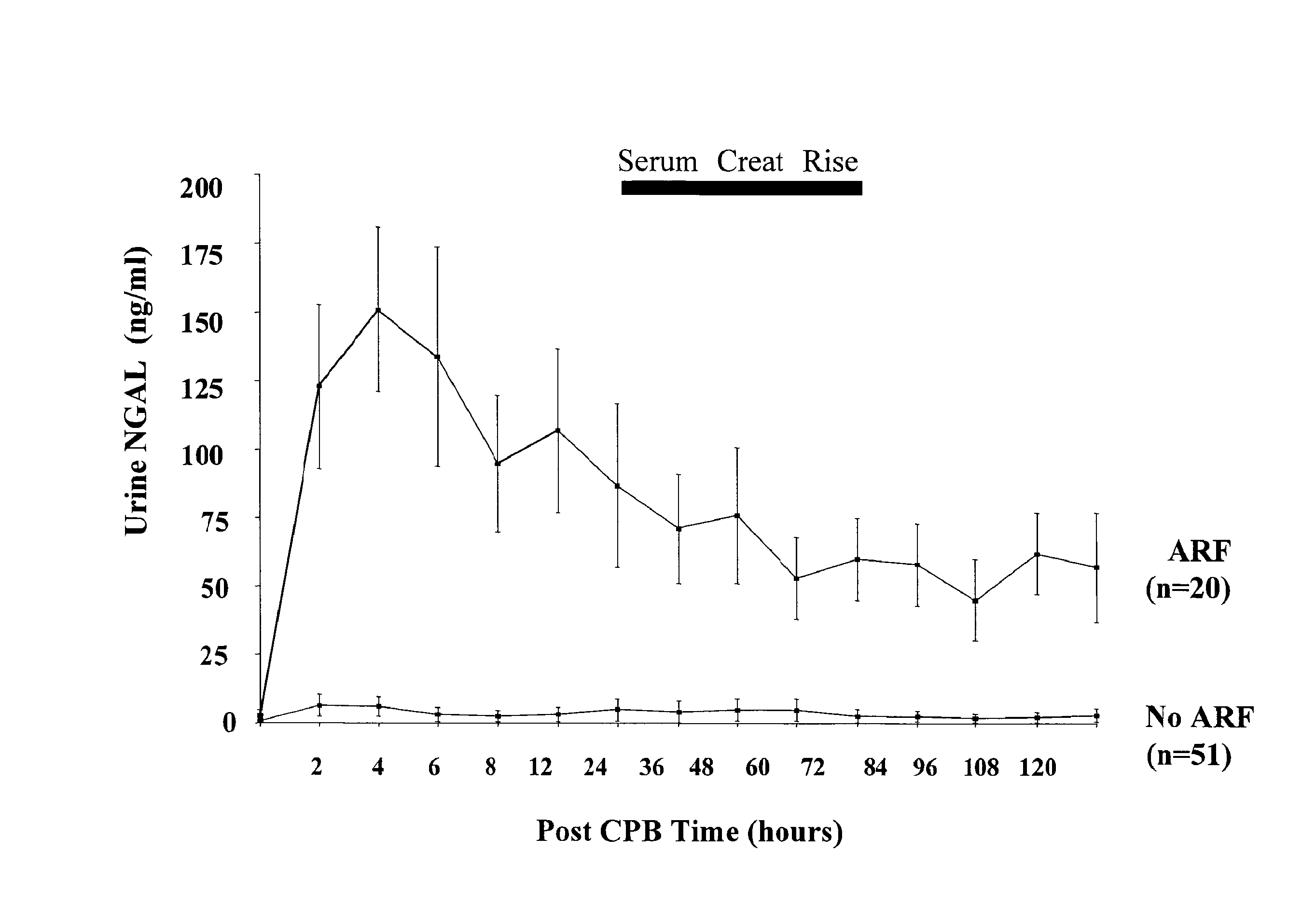
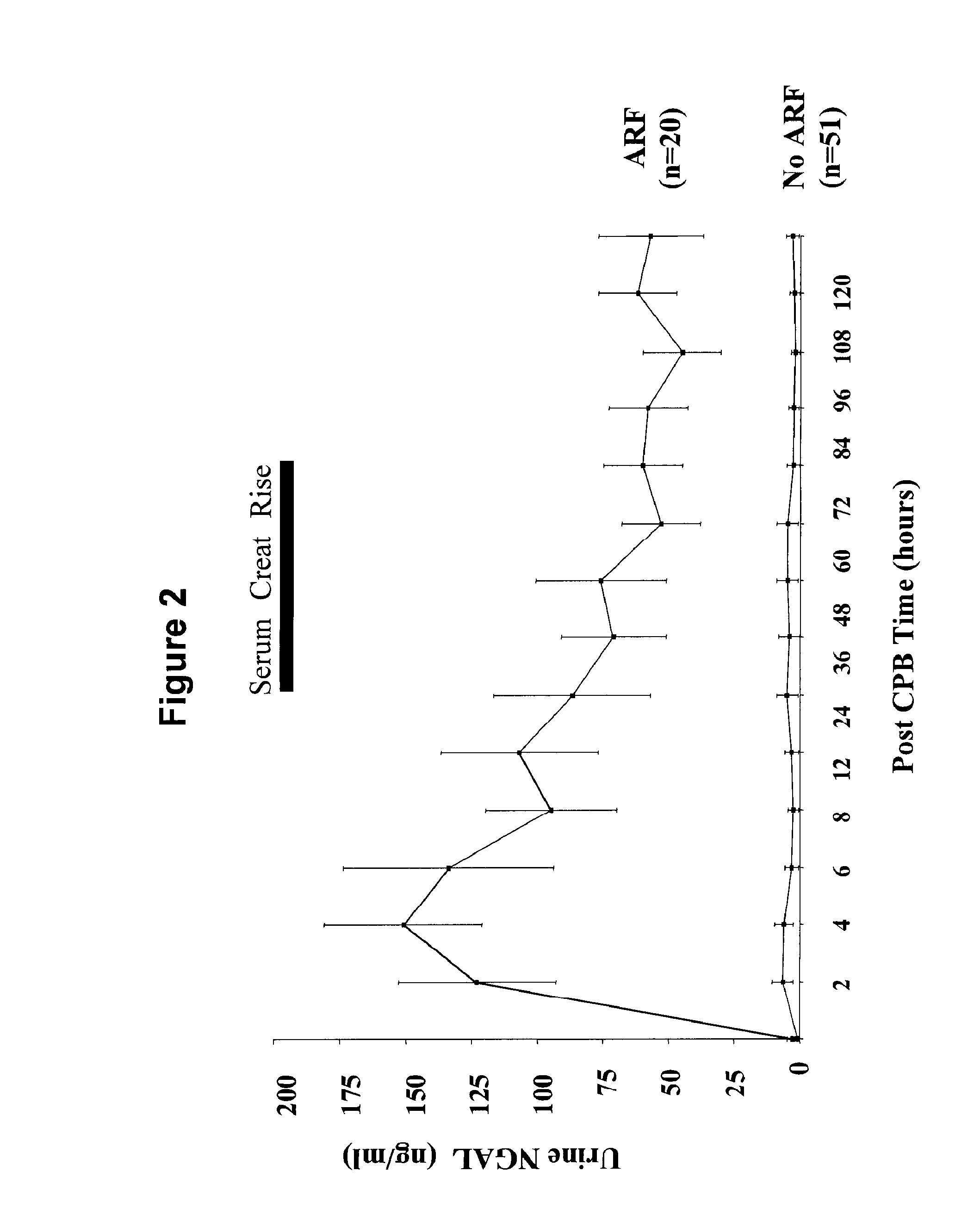
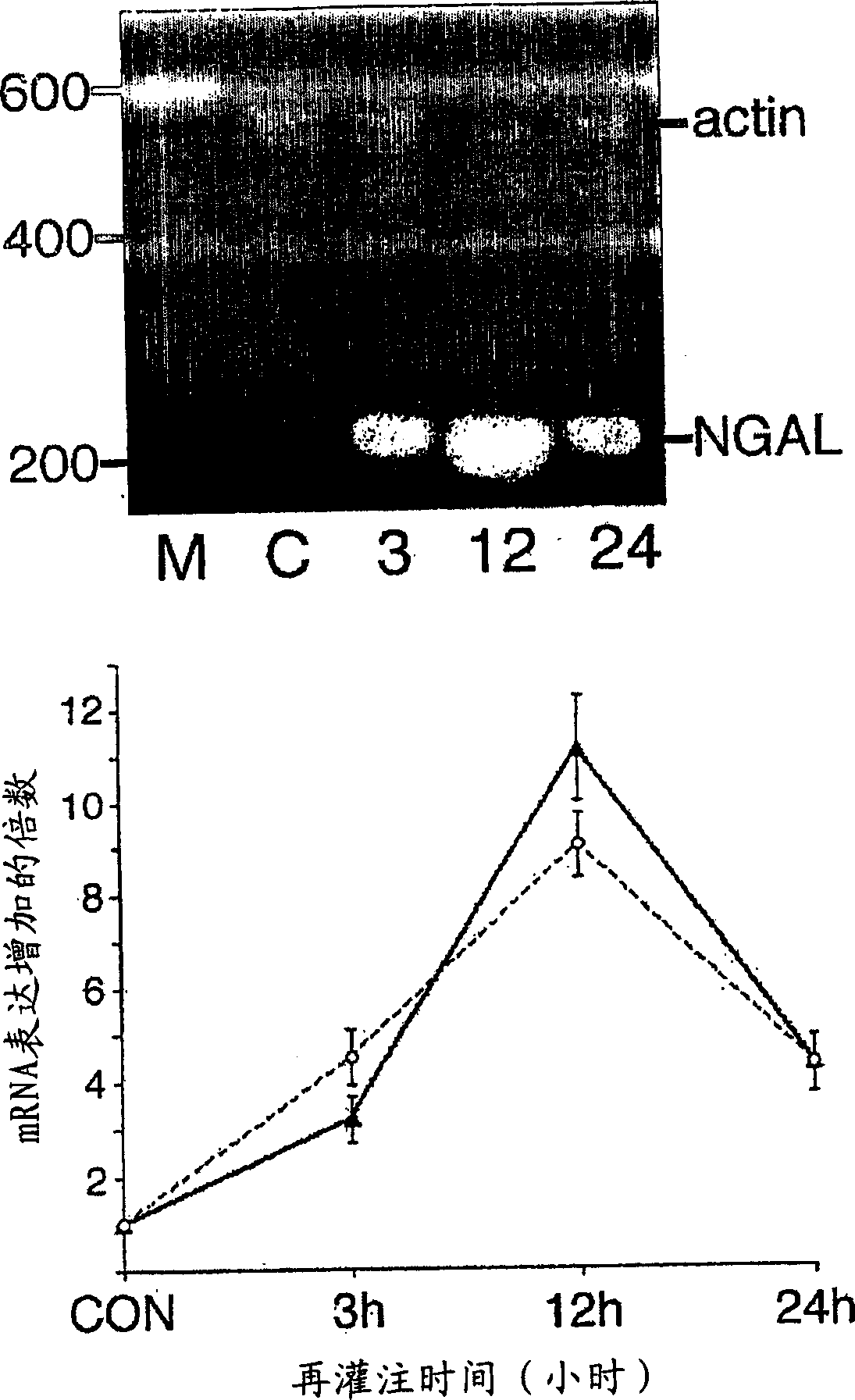
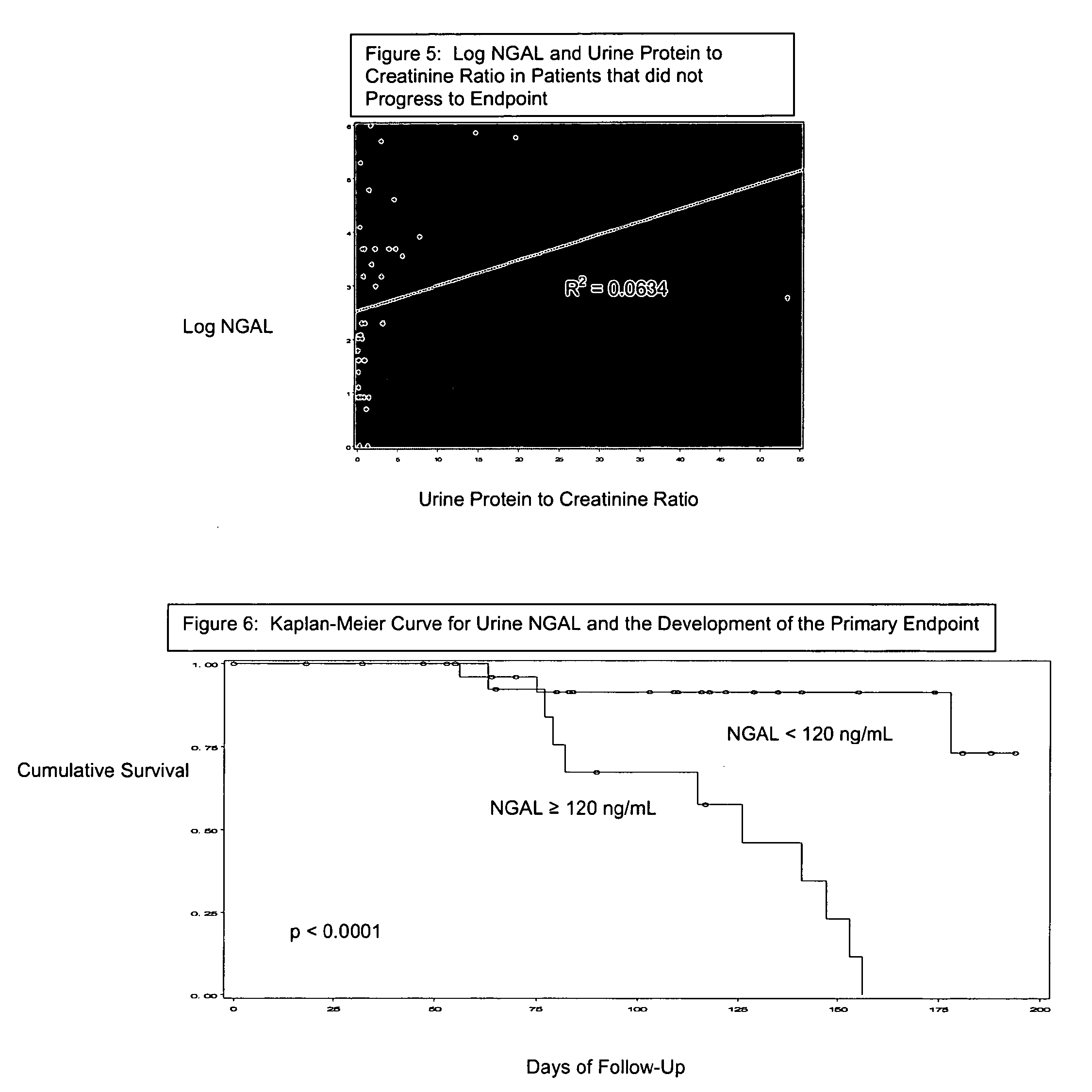
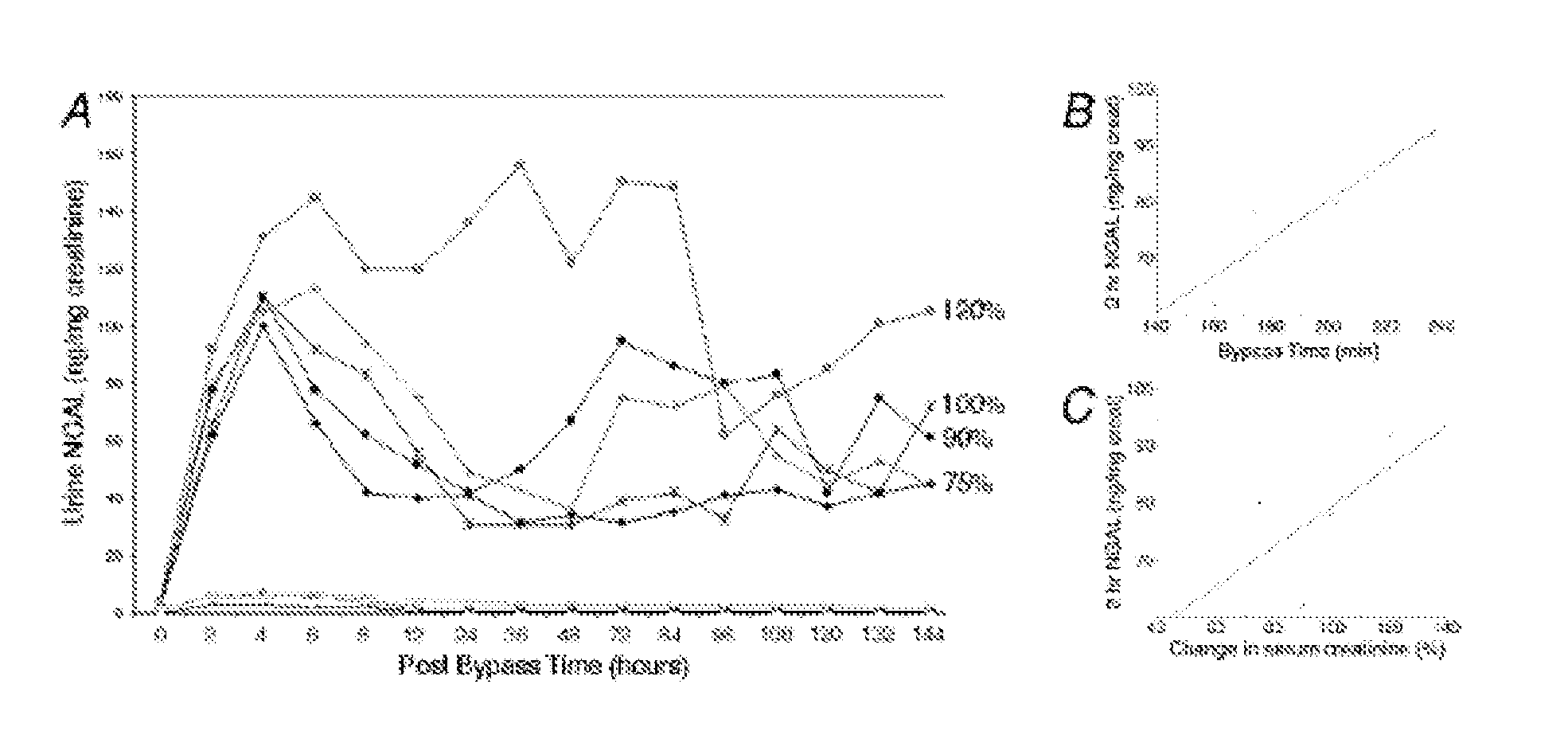
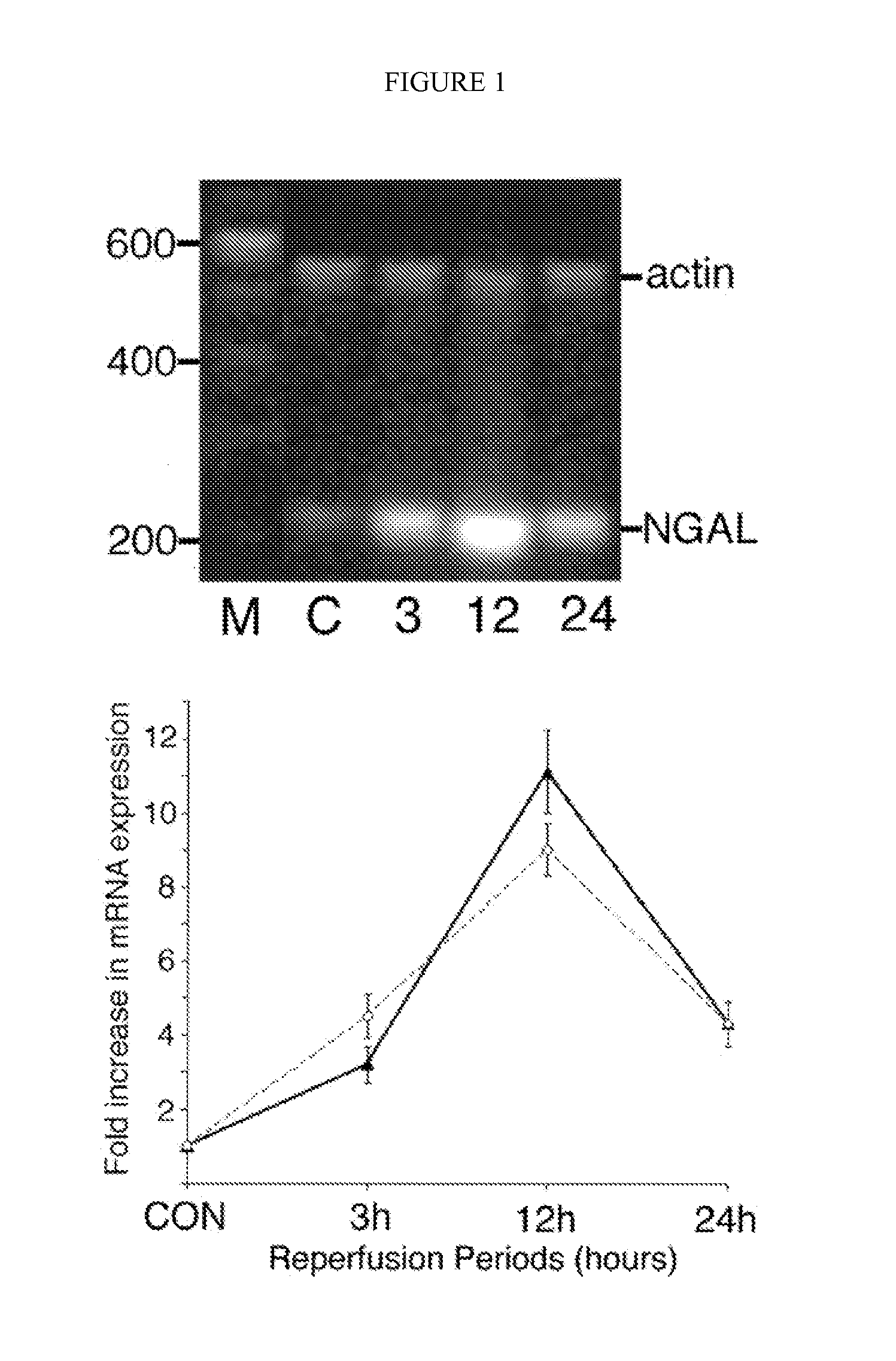
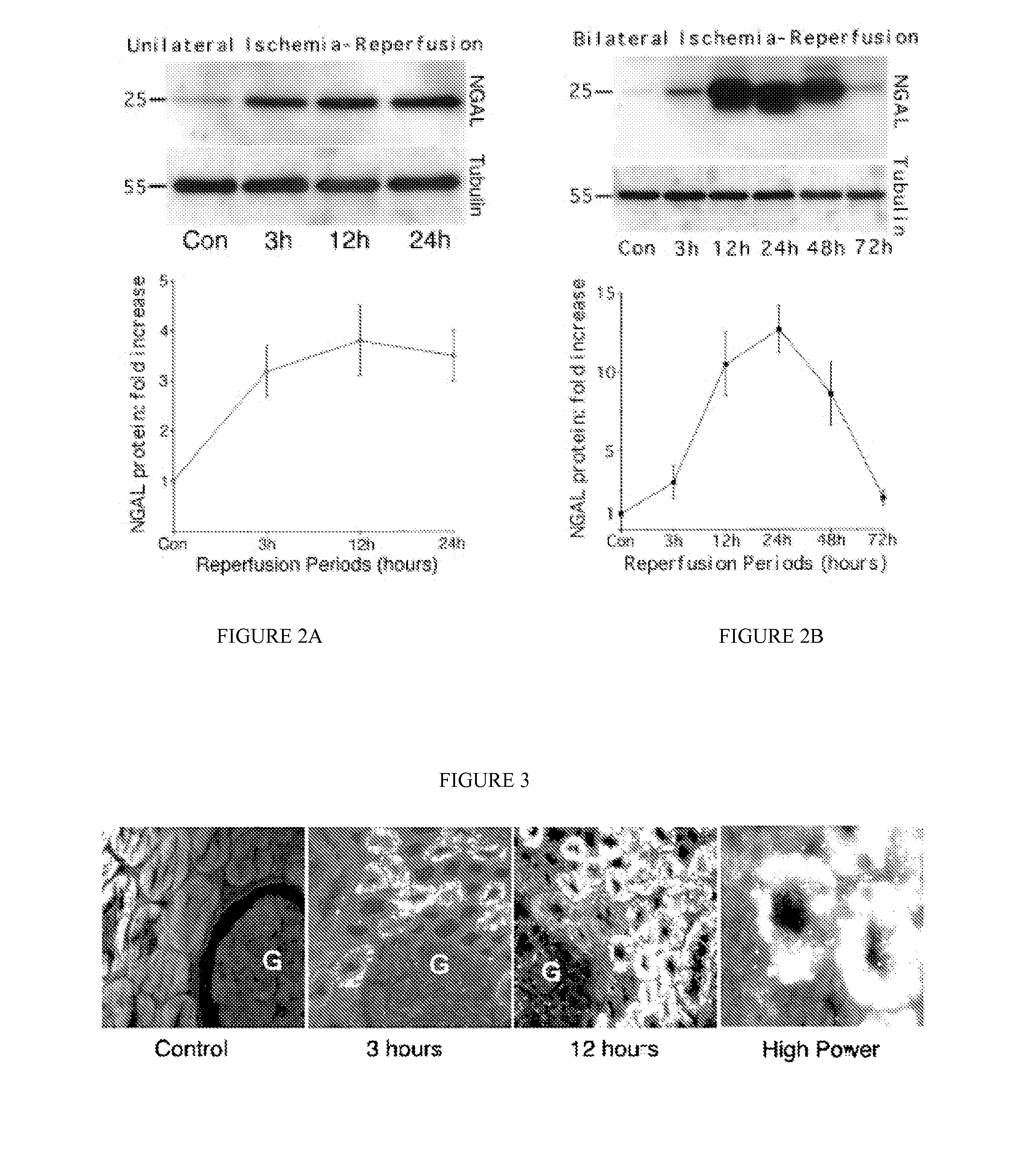
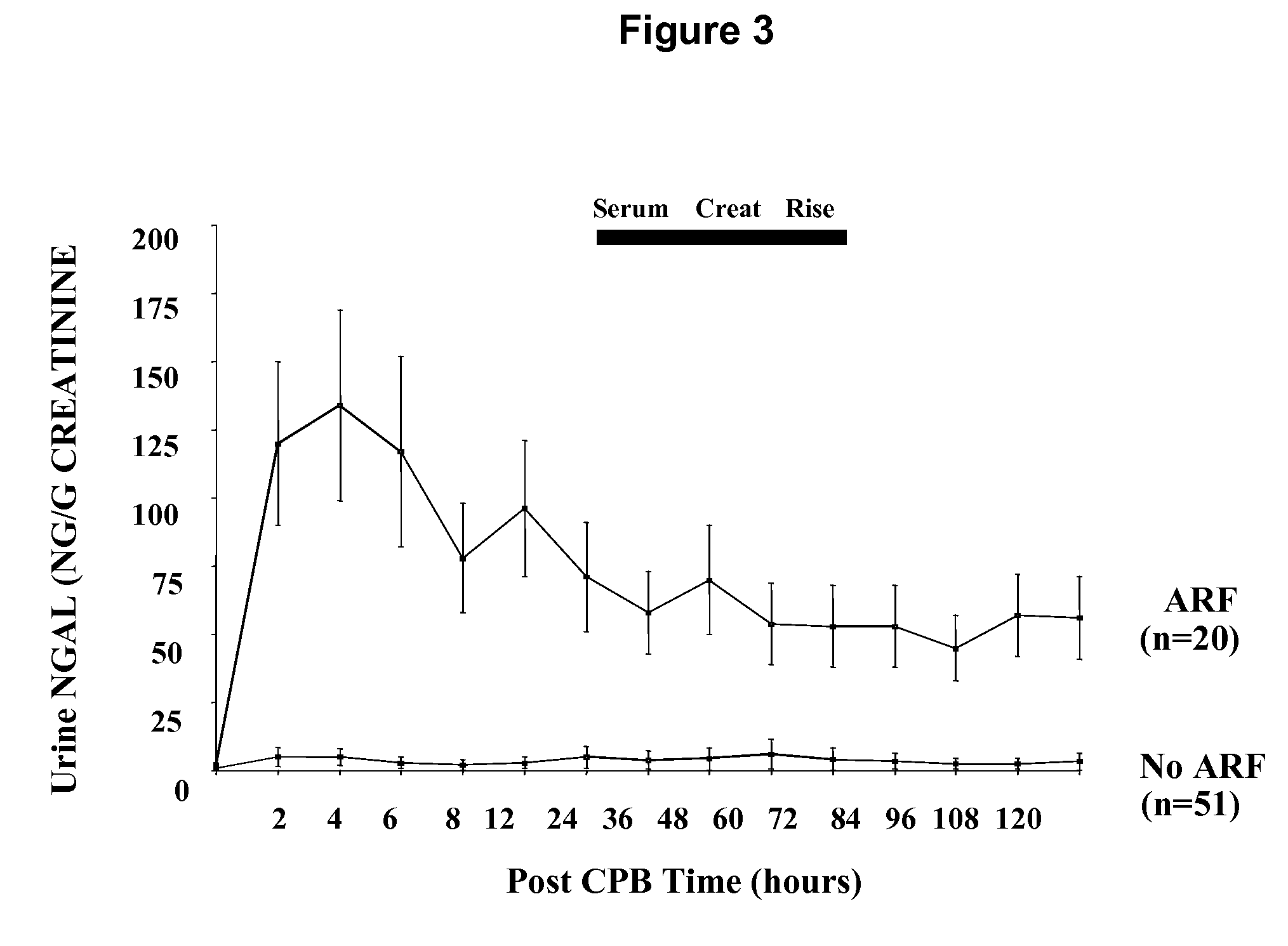
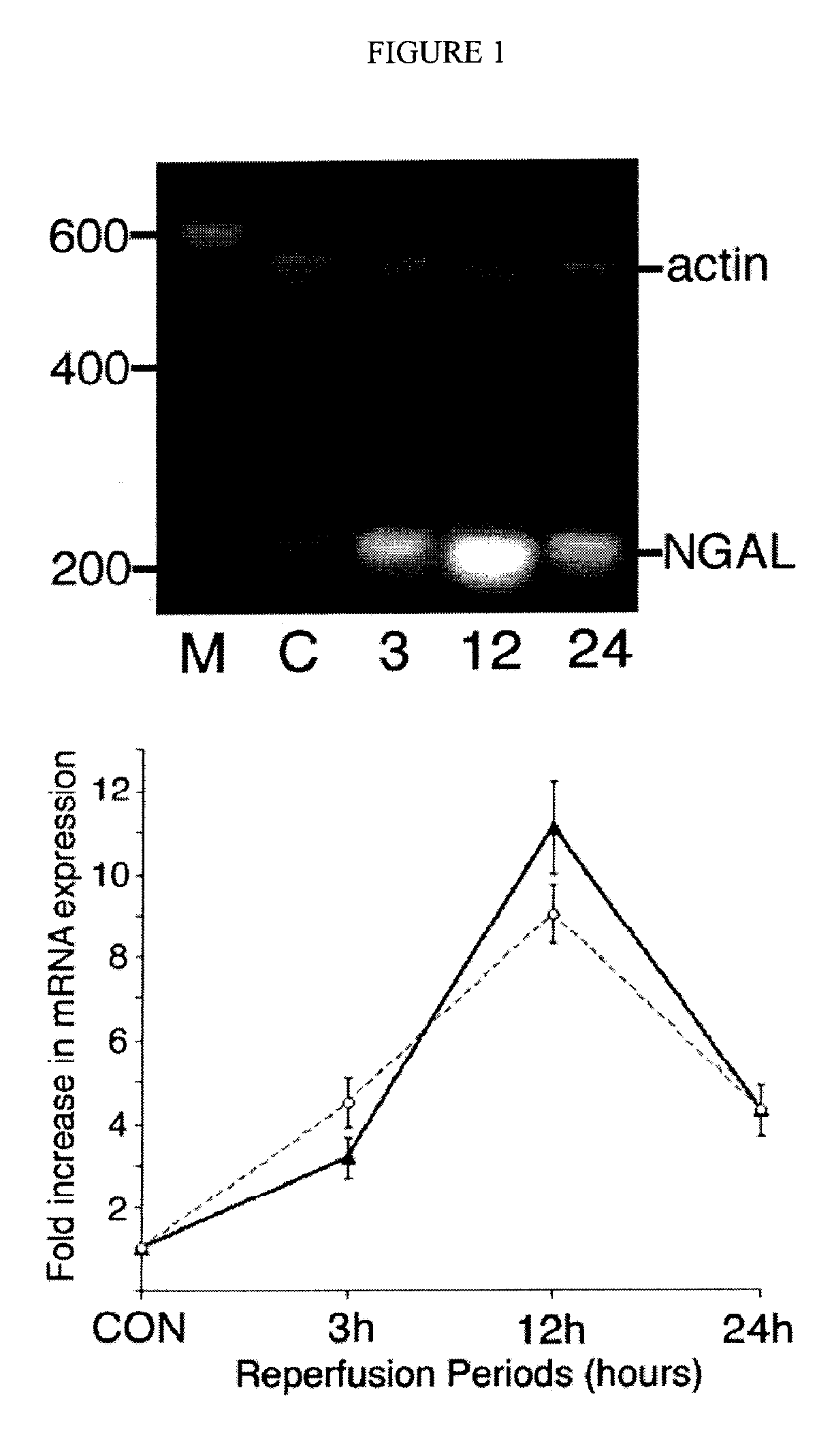
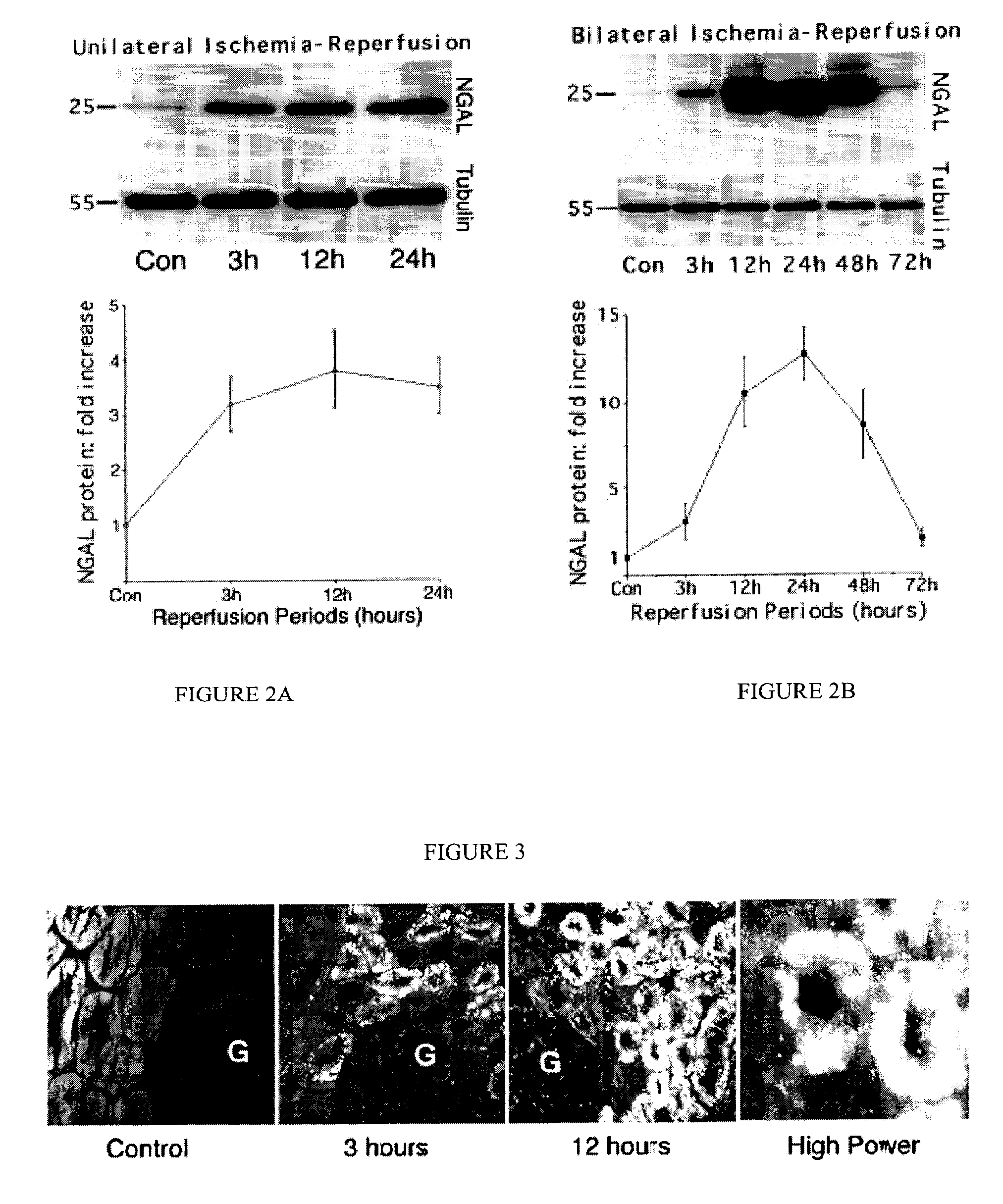
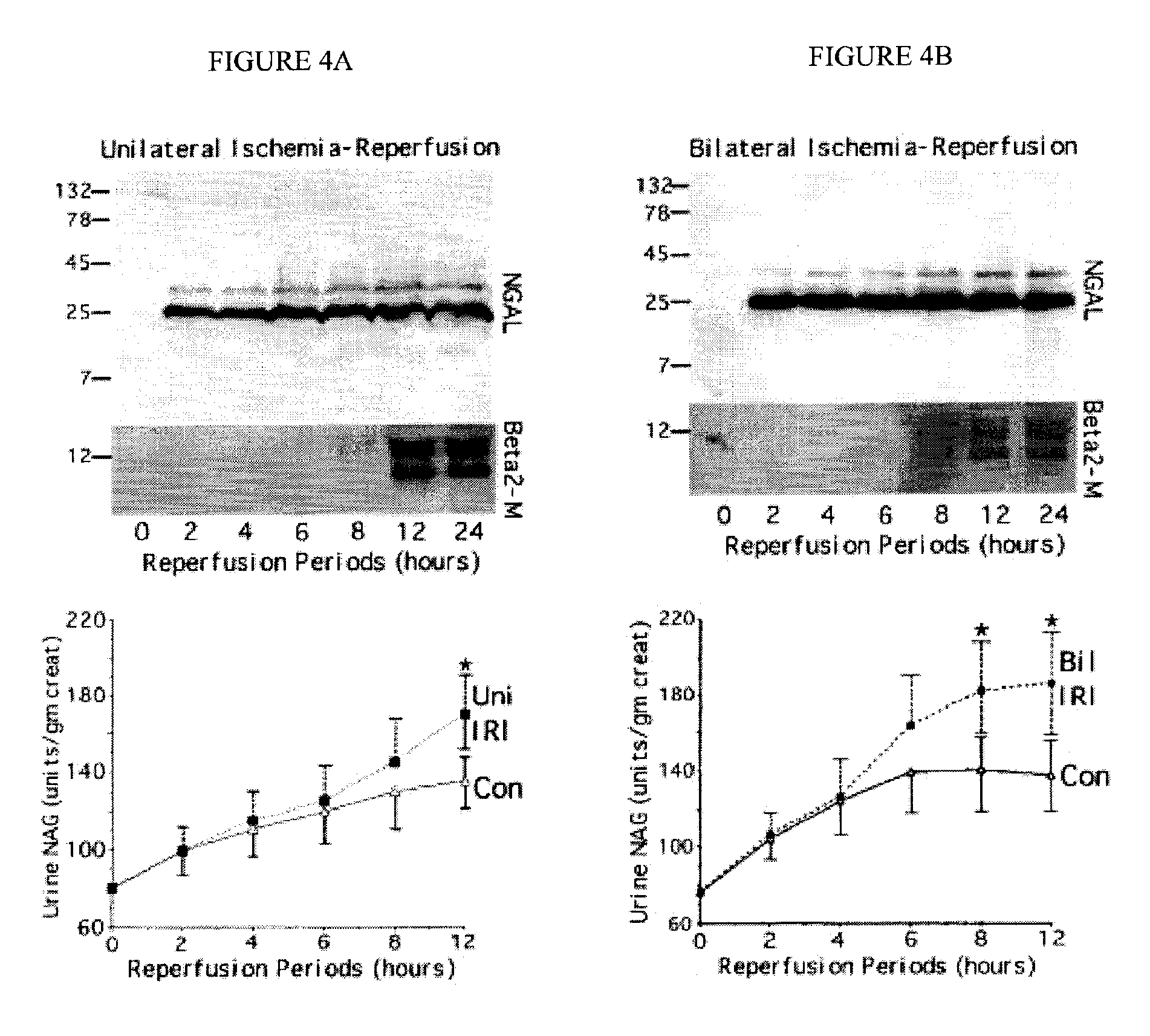
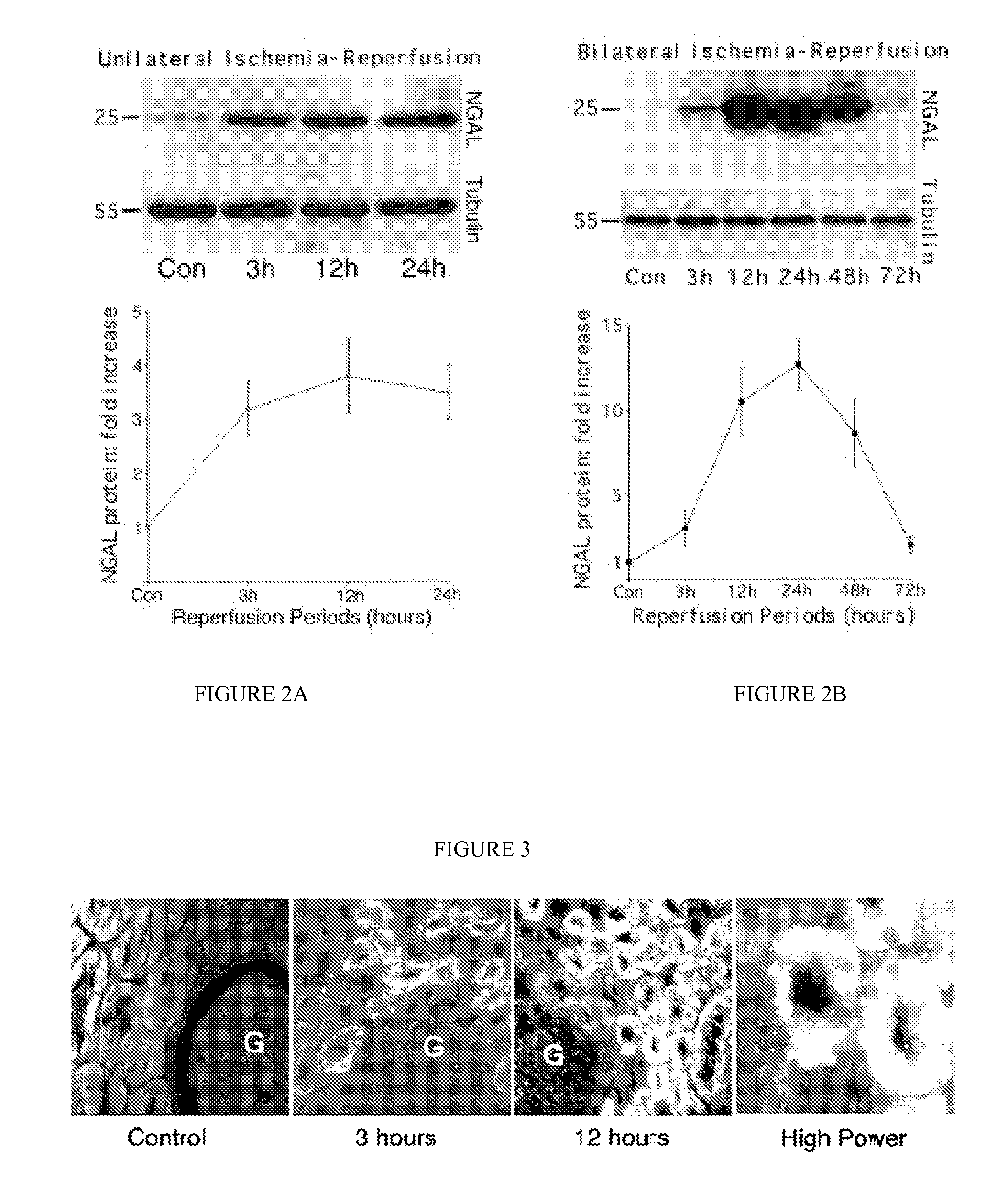
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Method for the early detection of renal injury
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:DEVARAJAN PRASAD +1
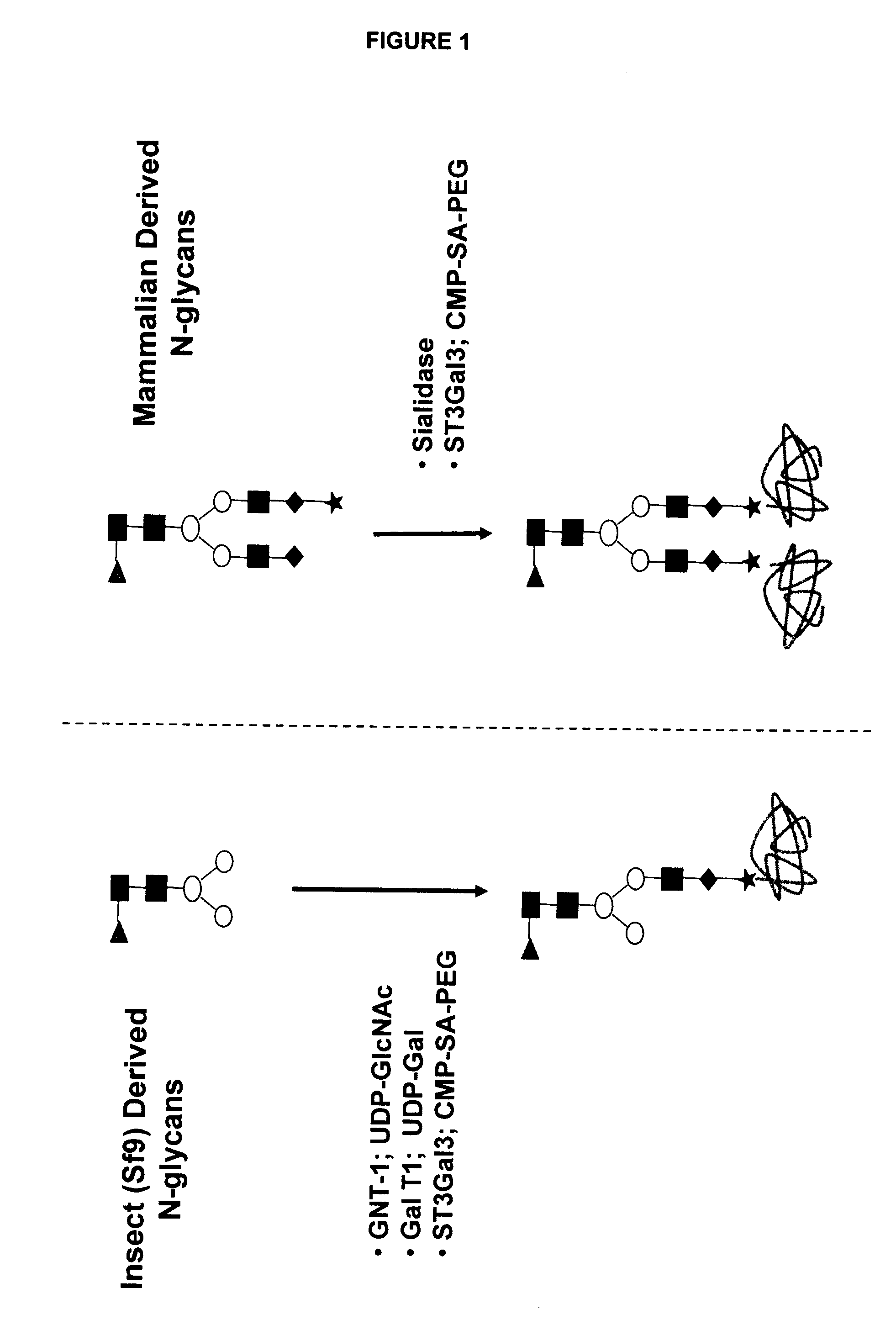
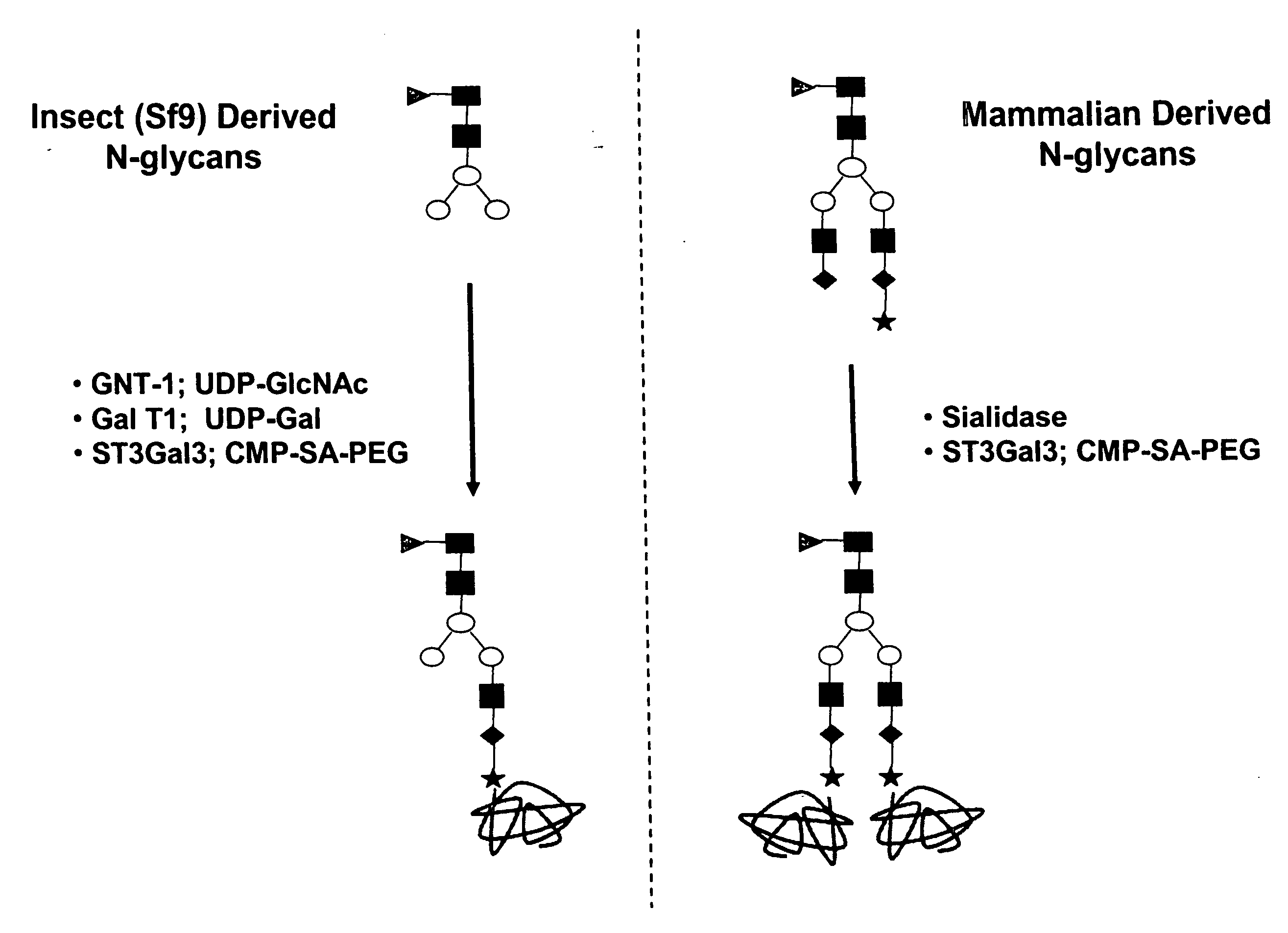
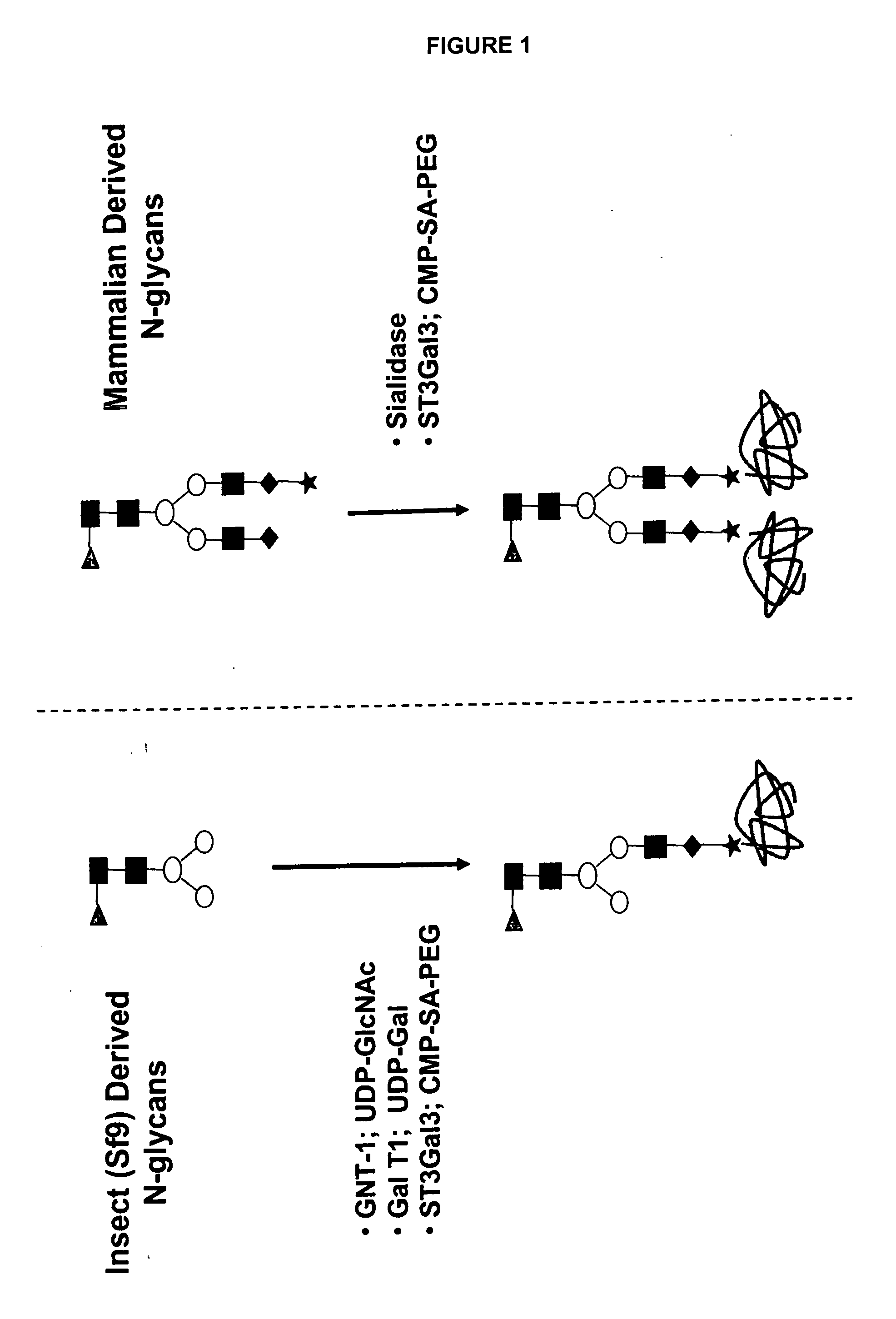
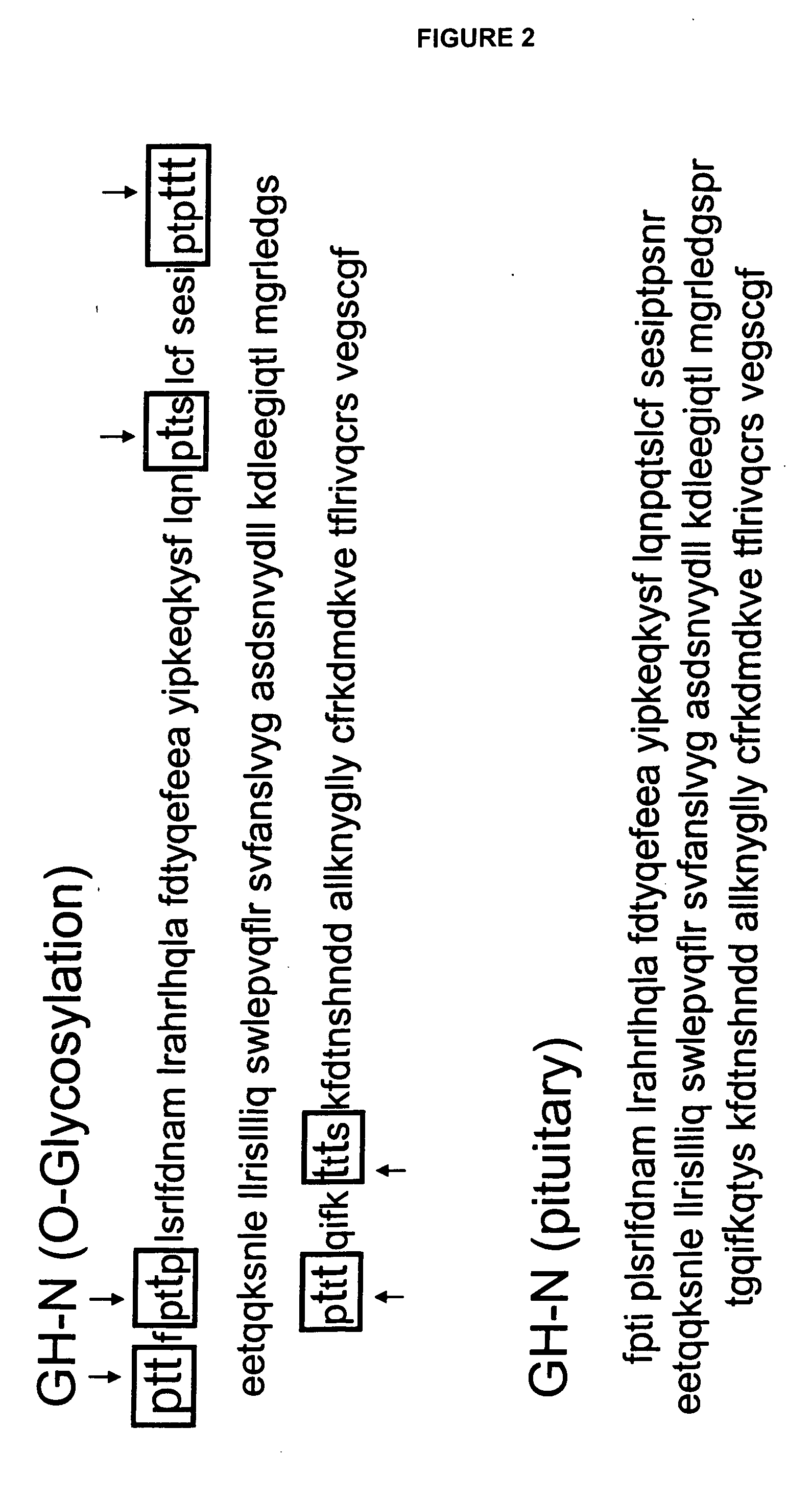
Compositions and methods for the preparation of protease resistant human growth hormone glycosylation mutants
The present invention relates to protease resistant mutants of human growth hormone, which contain newly introduced proteolysis resistant mutations and N-linked or O-linked glycosylation site(s), such that these recombinantly produced polypeptides have glycosylation patterns distinctly different from that of the naturally occurring human growth hormone. The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:NOVO NORDISK AS +1
A method and kit for detecting the early onset of renal tubular cell injury
An early-onset method and kit for detecting renal tubular cell injury, using NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and thus readily detected in urine following tubular cell injury. NGAL protein expression was mainly detected in the proximal tubule cells, which resembled a secreted protein with a punctate distribution in the cytoplasm. Apparent NGAL in urine correlates with the amount and duration of renal ischemia and nephrotoxicemia and is a diagnostic feature of tubular cell injury and renal failure. NGAL detection is also a useful marker for monitoring nephrotoxic side effects of drugs or other therapeutic agents.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
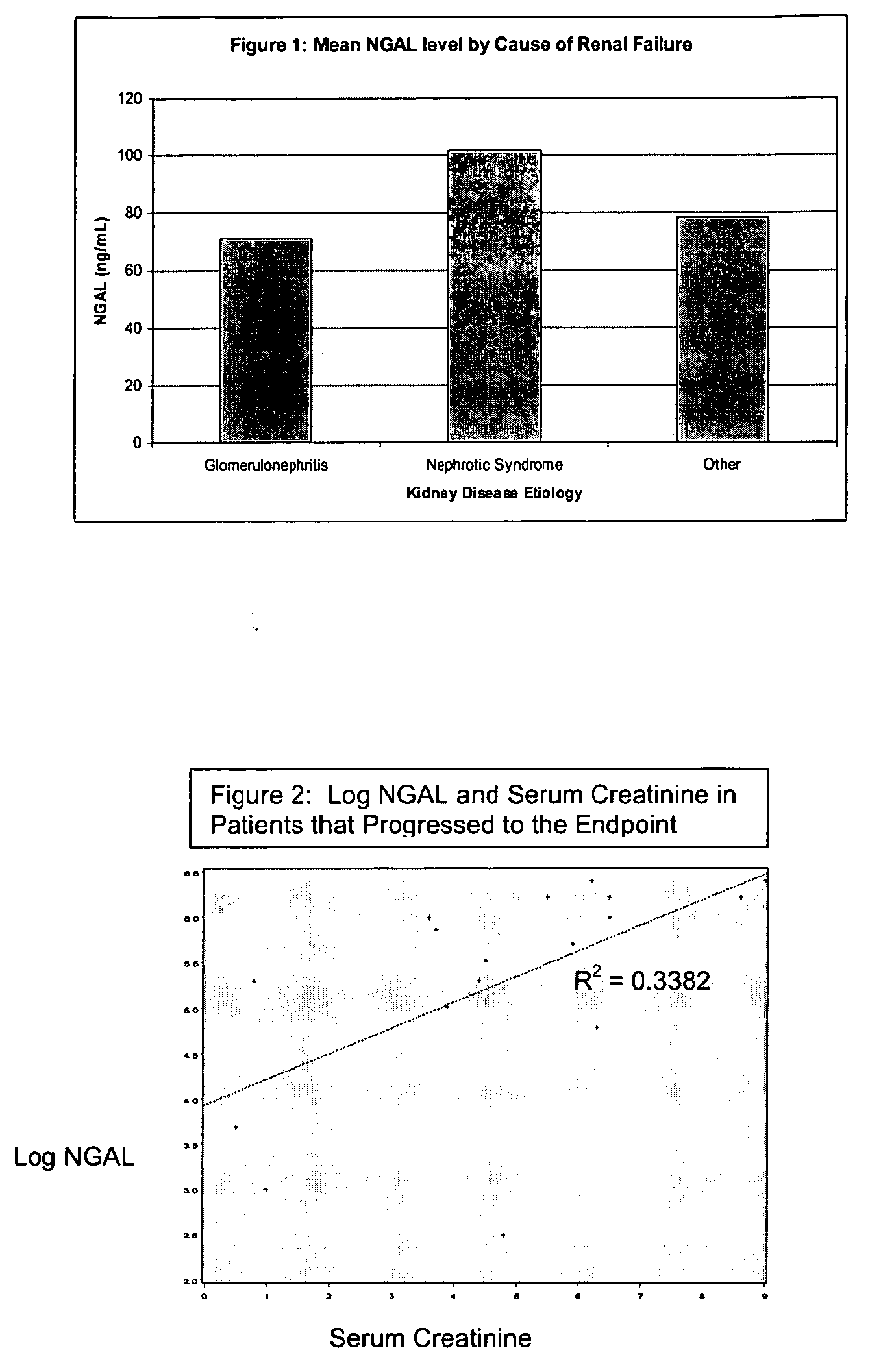
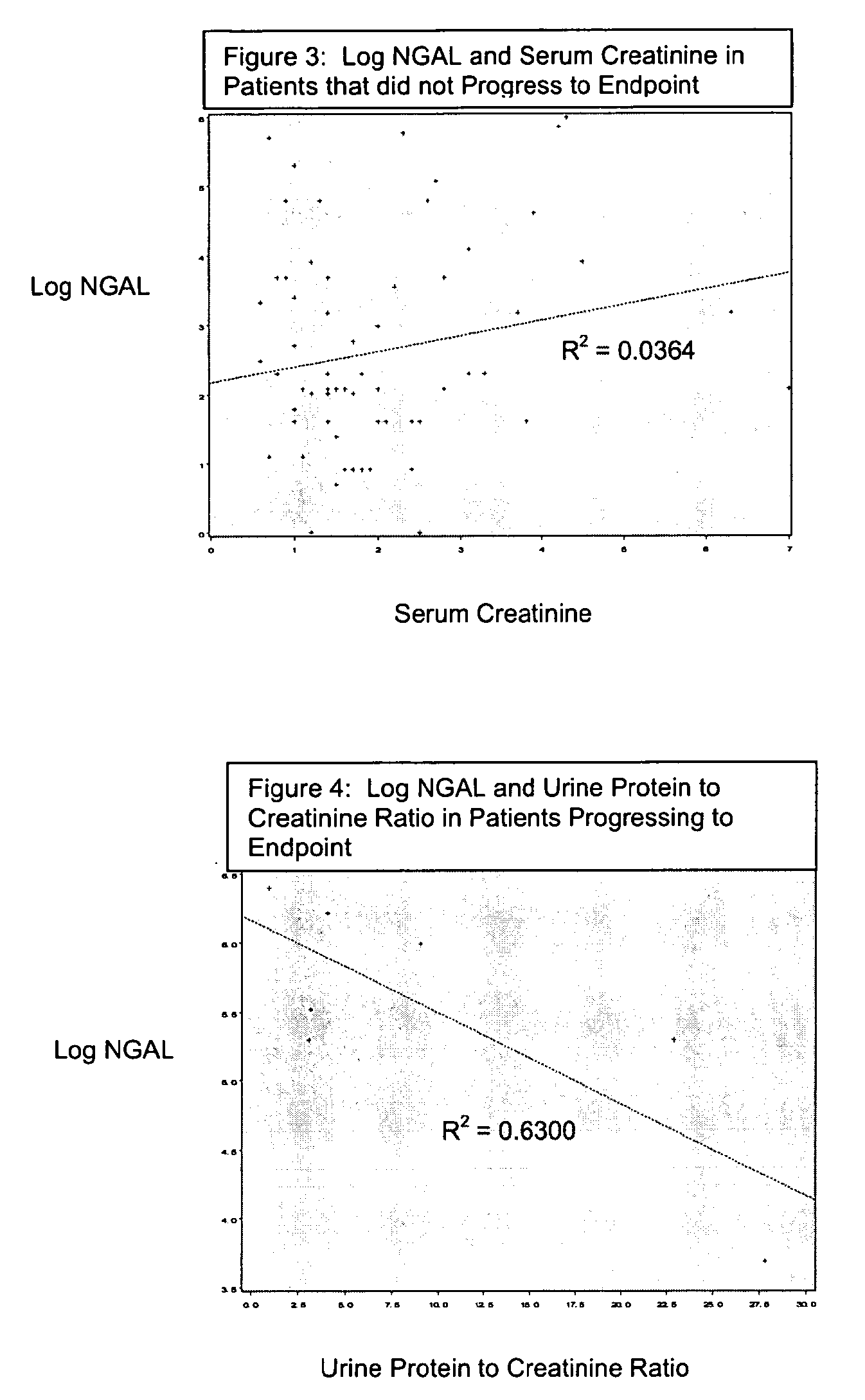
Detection of NGAL in chronic renal disease
Methods of assessing the ongoing kidney status in a subject afflicted with chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in fluid samples over time is disclosed. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine and serum as a result of chronic renal tubule cell injury. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Compositions and methods for the preparation of protease resistant human growth hormone glycosylation mutants
InactiveUS20070154992A1Sugar derivativesPeptide/protein ingredientsHuman growth hormoneProtease resistant
The present invention relates to protease resistant mutants of human growth hormone, which contain newly introduced proteolysis resistant mutations and N-linked or O-linked glycosylation site(s), such that these recombinantly produced polypeptides have glycosylation patterns distinctly different from that of the naturally occurring human growth hormone. The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:NOVO NORDISK AS
Protease resistant mutants of stromal cell derived factor-1 in the repair of tissue damage
ActiveUS20080095758A1Increase concentrationObviates abilityNervous disorderPeptide/protein ingredientsDipeptidyl peptidaseMutant
The present invention is directed stromal cell derived factor-1 peptides that have been mutated to make them resistant to digestion by the proteases dipeptidyl peptidase IV (DPPIV) and matrix metalloproteinase-2 (MMP-2) but which maintain the ability of native SDF-1 to attract T cells. The mutants may be attached to membranes formed by self-assembling peptides and then implanted at sites of tissue damage to help promote repair.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Method and kit for detecting the early onset of renal tubular cell injury
InactiveUS20070254370A1Organic active ingredientsMicrobiological testing/measurementSide effectNGAL Protein
A method and kit for detecting the early onset of renal tubular cell injury, utilizing NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the urine is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Synthetic hyperglycosylated, and hyperglycosylated protease-resistant polypeptide variants, oral formulations and methods of using the same
ActiveUS20060204473A1Increased protease resistanceNervous disorderPeptide/protein ingredientsHybrid typeInterferon receptor
The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites. The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites, as well as erythropoietin and darbepoetin alfa, each of which are linked to a penetrating peptide that facilitates translocation of a substance across a biological barrier as well as pharmaceutical compositions, including oral formulations, of the same. The present invention further provides oral formulations of hyperglycosylated or protease-resistant, hyperglycosylated polypeptide variants, which polypeptide variants lack at least one protease cleavage site found in a parent polypeptide, and thus exhibit increased protease resistance compared to the parent polypeptide, which polypeptide variants further include (1) a carbohydrate moiety covalently linked to at least one non-native glycosylation site not found in the parent protein therapeutic or (2) a carbohydrate moiety covalently linked to at least one native glycosylation site found but not glycosylated in the parent protein therapeutic. The present invention further provides compositions, including oral pharmaceutical compositions, comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides containers, devices, and kits comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides therapeutic methods involving administering an effective amount of an oral pharmaceutical composition comprising a synthetic Type I interferon receptor polypeptide agonist, a hyperglycosylated polypeptide variant, or a hyperglycosylated, protease-resistant polypeptide variant to an individual in need thereof.
Owner:ALIOS BIOPHARMA INC +1
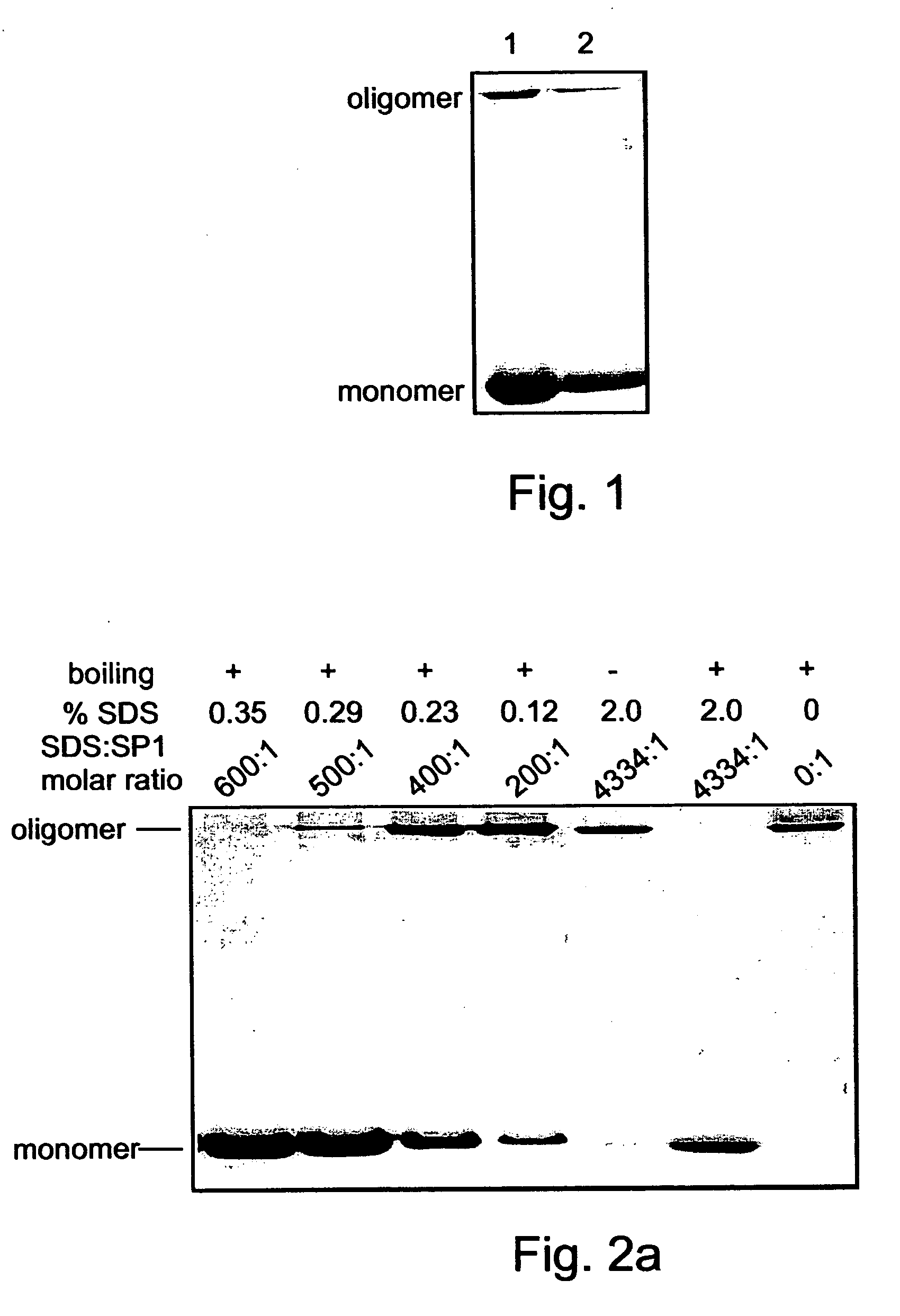
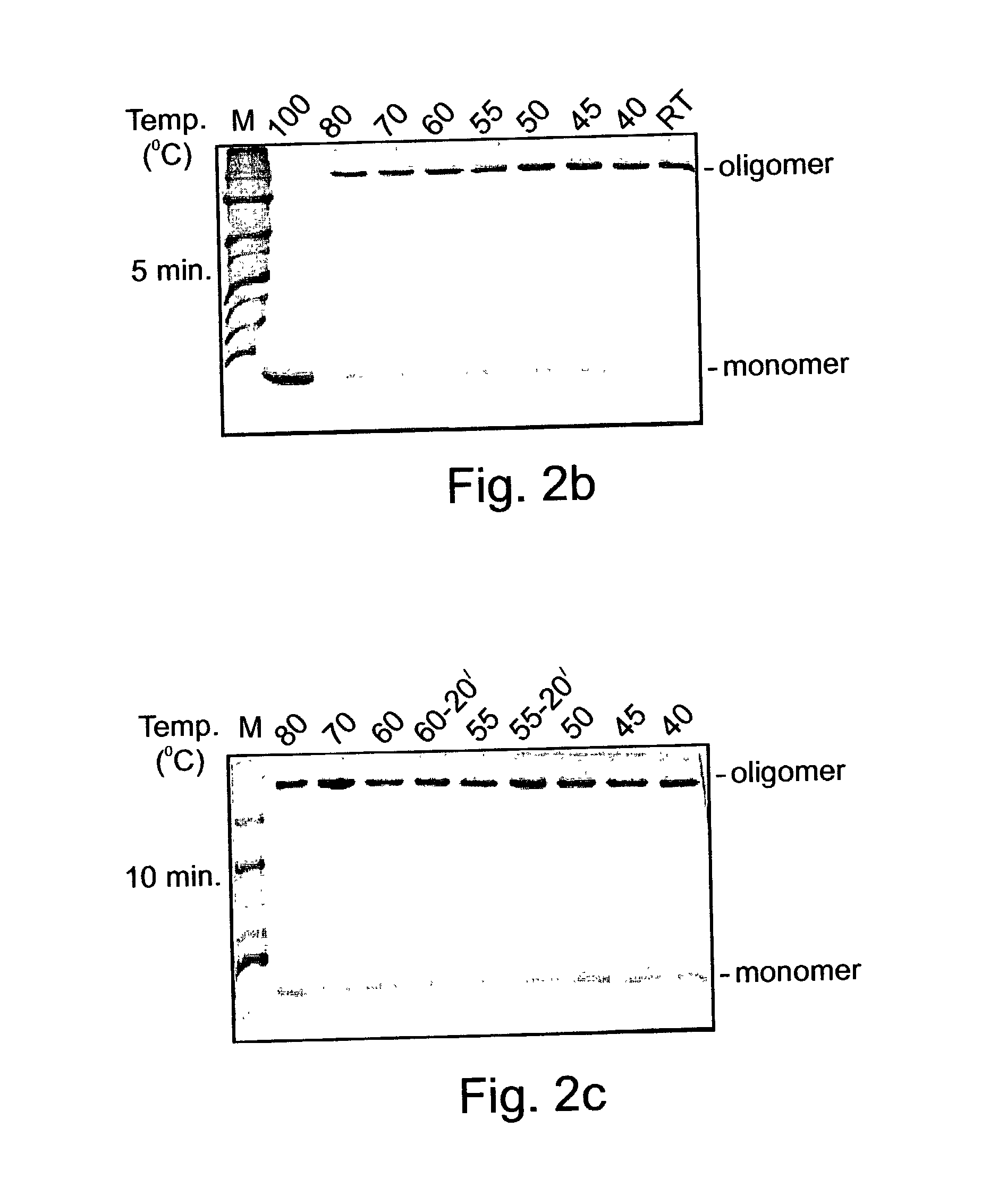
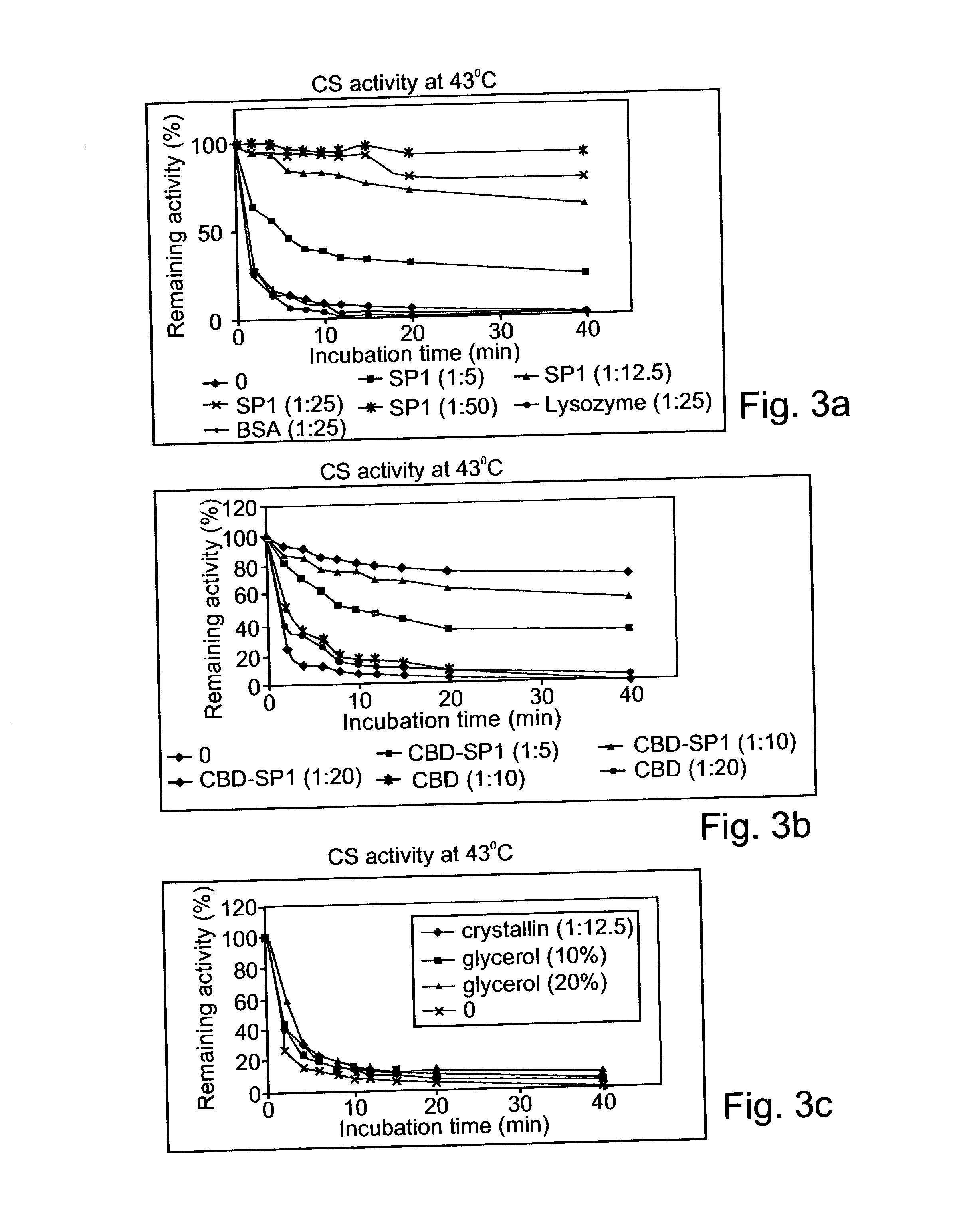
Denaturat stable and/or protease resistant, chaperone-like oligomeric proteins, polynucleotides encoding same and their uses
InactiveUS20050074763A1Effective activityHigh affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsPurification methodsNucleotide
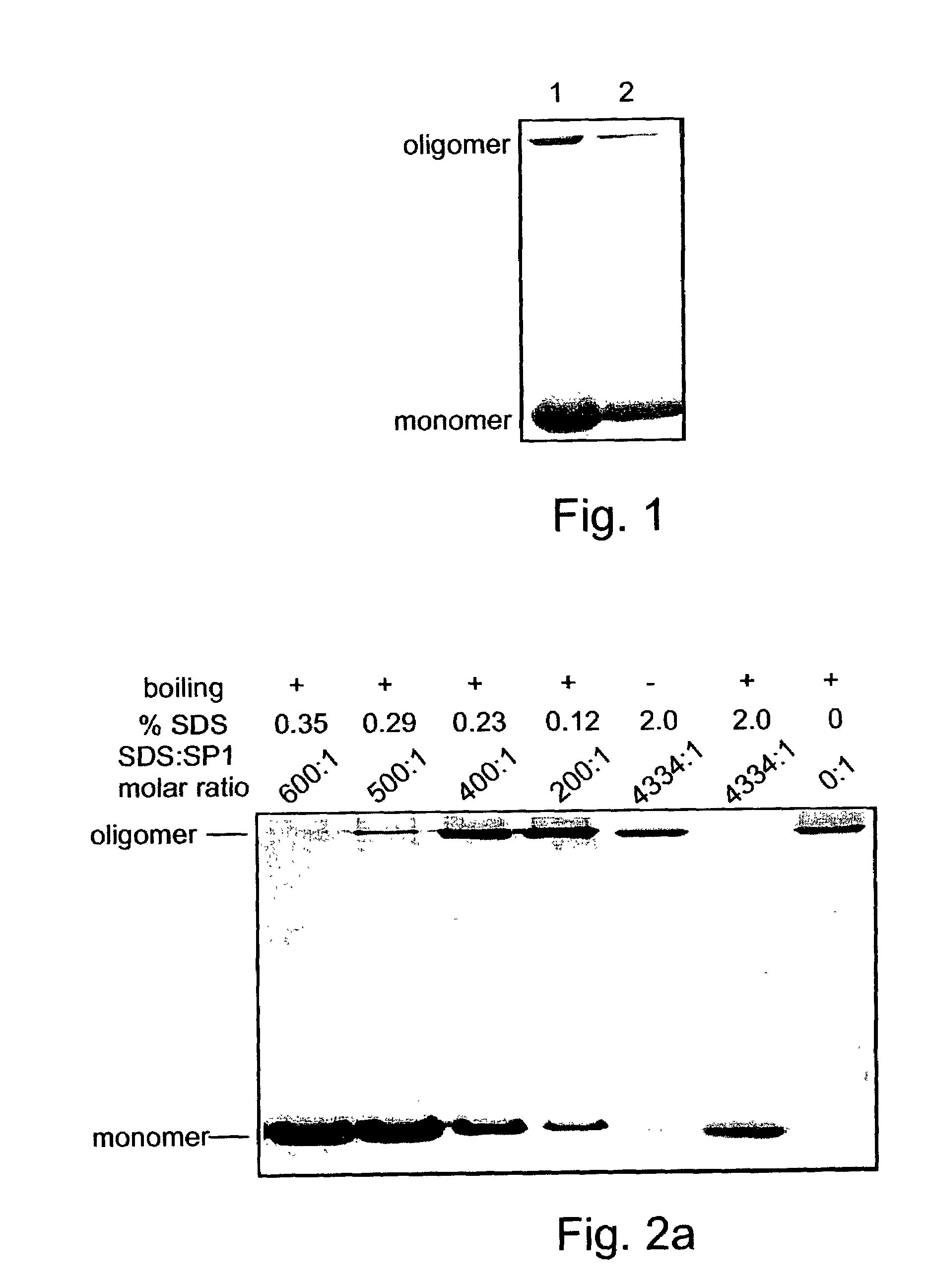
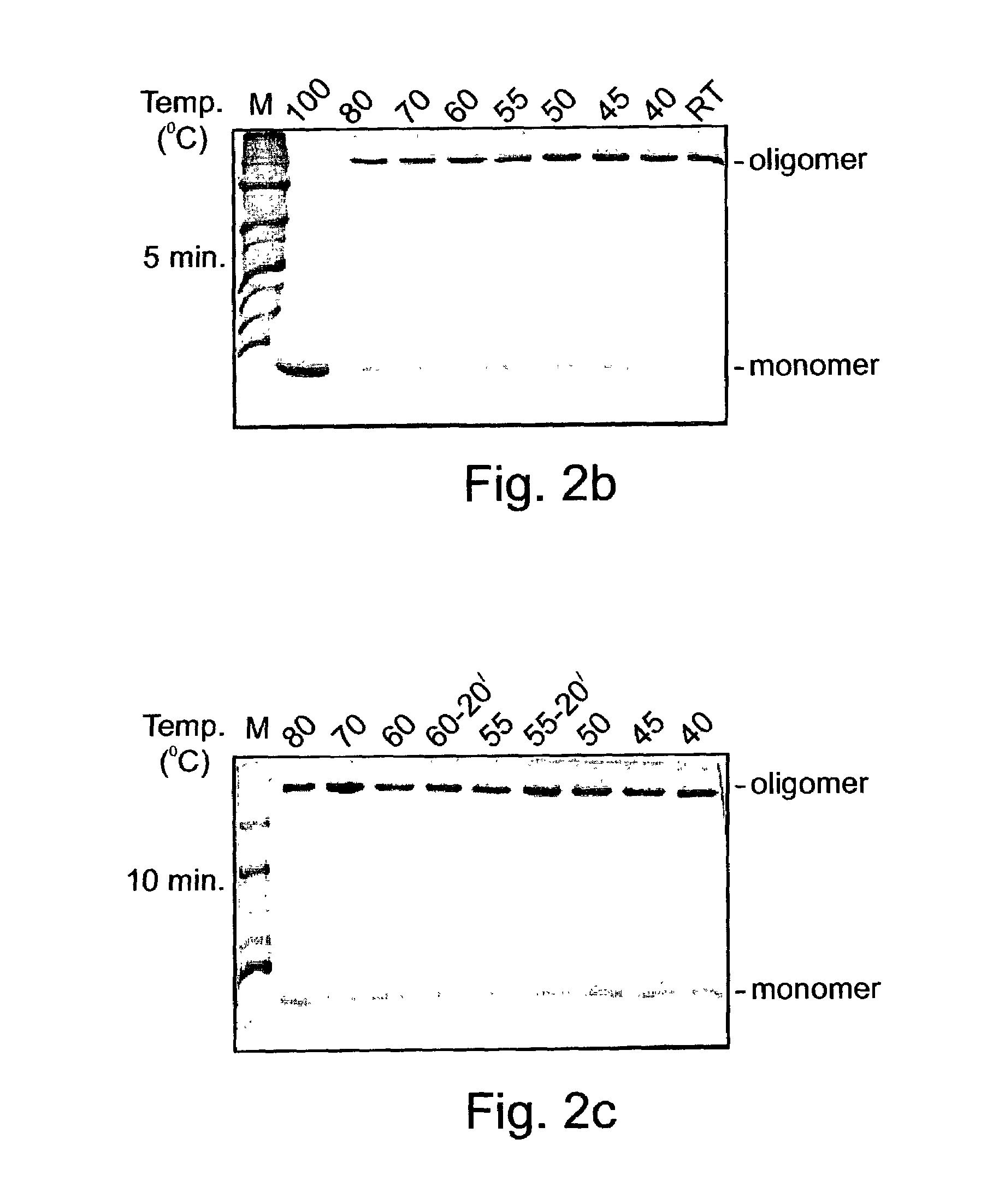
Novel denaturant-stable, protease resistant, homo-oligomeric proteins, also referred to herein as stable proteins (SPs), having chaperone-like activity; methods of production and purification of SPs; nucleic acids encoding SPs; methods of isolating nucleic acids encoding SPs; antibodies recognizing SPs; the use of SPs for stabilizing, refolding, repairing, preventing aggregation and de-aggregating macromolecules such as proteins; fusion proteins including SPs; nucleic acid constructs encoding the fusion proteins; and their uses in a variety of methods and applications.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
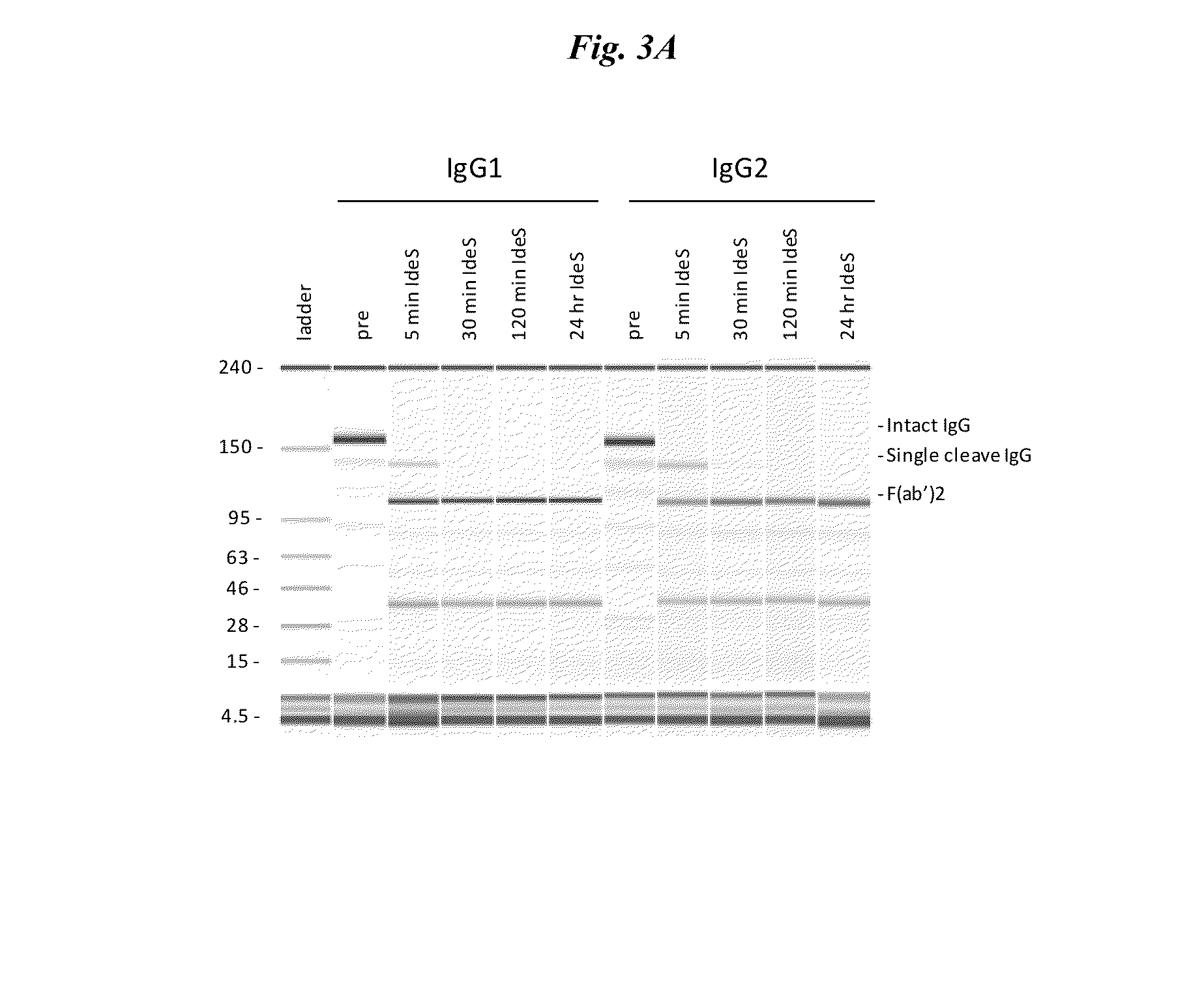
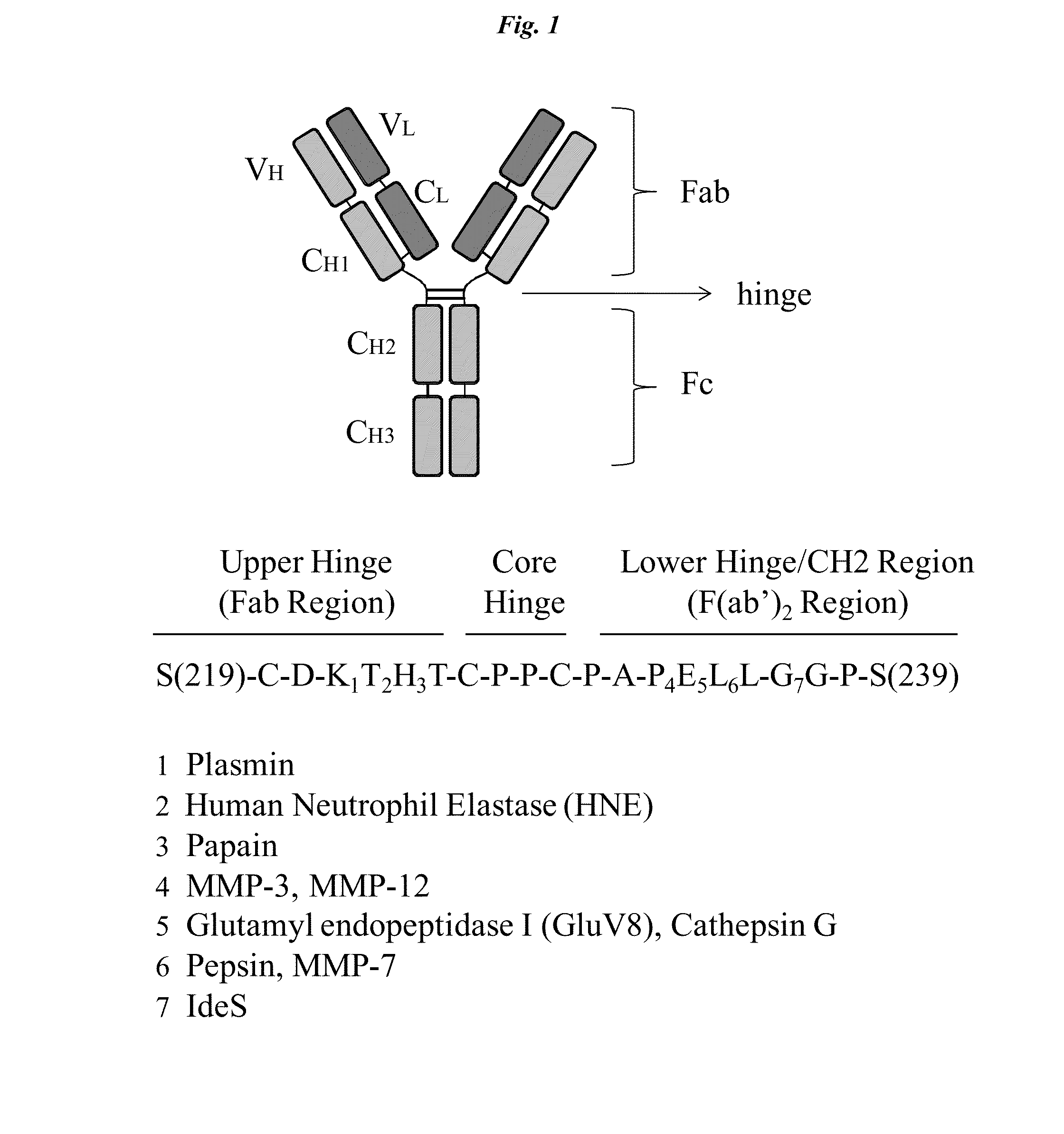
Active protease-resistant antibody fc mutants
InactiveUS20130011386A1Improve abilitiesHigh activityAntibacterial agentsHybrid immunoglobulinsProteinase activityProtease resistant
The present invention relates to modified Fc-containing molecules including modified antibodies characterized by increased resistance to host and pathogen-derived proteases, ability to interact with FcγR receptors except with FcγRI, and lack of induction of IL-10 secretion by macrophages, and methods of using and making them.
Owner:JANSSEN BIOTECH INC
Active protease-resistant antibody FC mutants
InactiveUS8871204B2Improve abilitiesHigh activityAntibacterial agentsPeptide/protein ingredientsProteinase activityProtease resistant
The present invention relates to modified Fc-containing molecules including modified antibodies characterized by increased resistance to host and pathogen-derived proteases, ability to interact with FcγR receptors except with FcγRI, and lack of induction of IL-10 secretion by macrophages, and methods of using and making them.
Owner:JANSSEN BIOTECH INC
Method for the early detection of renal injury
InactiveUS20090142774A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseSide effect
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Denaturat stable and/or protease resistant, chaperone-like oligomeric proteins, polynucleotides encoding same, their uses and methods of increasing a specific activity thereof
Novel denaturant-stable, protease resistant, homo-oligomeric proteins, also referred to herein as stable proteins (SPs), having chaperone-like activity; methods of production and purification of SPs; nucleic acids encoding SPs; methods of isolating nucleic acids encoding SPs; antibodies recognizing SPs; the use of SPs for stabilizing, refolding, repairing, preventing aggregation and de-aggregating macromolecules such as proteins; fusion proteins including SPs; nucleic acid constructs encoding the fusion proteins; and their uses in a variety of methods and applications.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Method and kit for detecting the early onset of renal tubular cell injury
InactiveUS20090181407A1Organic active ingredientsMicrobiological testing/measurementSide effectNGAL Protein
A method and kit for detecting the early onset of renal tubular cell injury, utilizing NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the urine is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Recombinant modified Bacillus anthracis protective antigen for use in vaccines
The invention relates to improved methods of producing and recovering sporulation-deficient B. anthracis mutant stains, and for producing and recovering recombinant B. anthracis protective antigen (PA), especially modified PA which is protease resistant, and to methods of using of these PAs or nucleic acids encoding these PAs for eliciting an immunogenic response in humans, including responses which provide protection against, or reduce the severity of, B. anthracis bacterial infections and which are useful to prevent and / or treat illnesses caused by B. anthracis, such as inhalation anthrax, cutaneous anthrax and gastrointestinal anthrax.
Owner:DEPT OF HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE +1
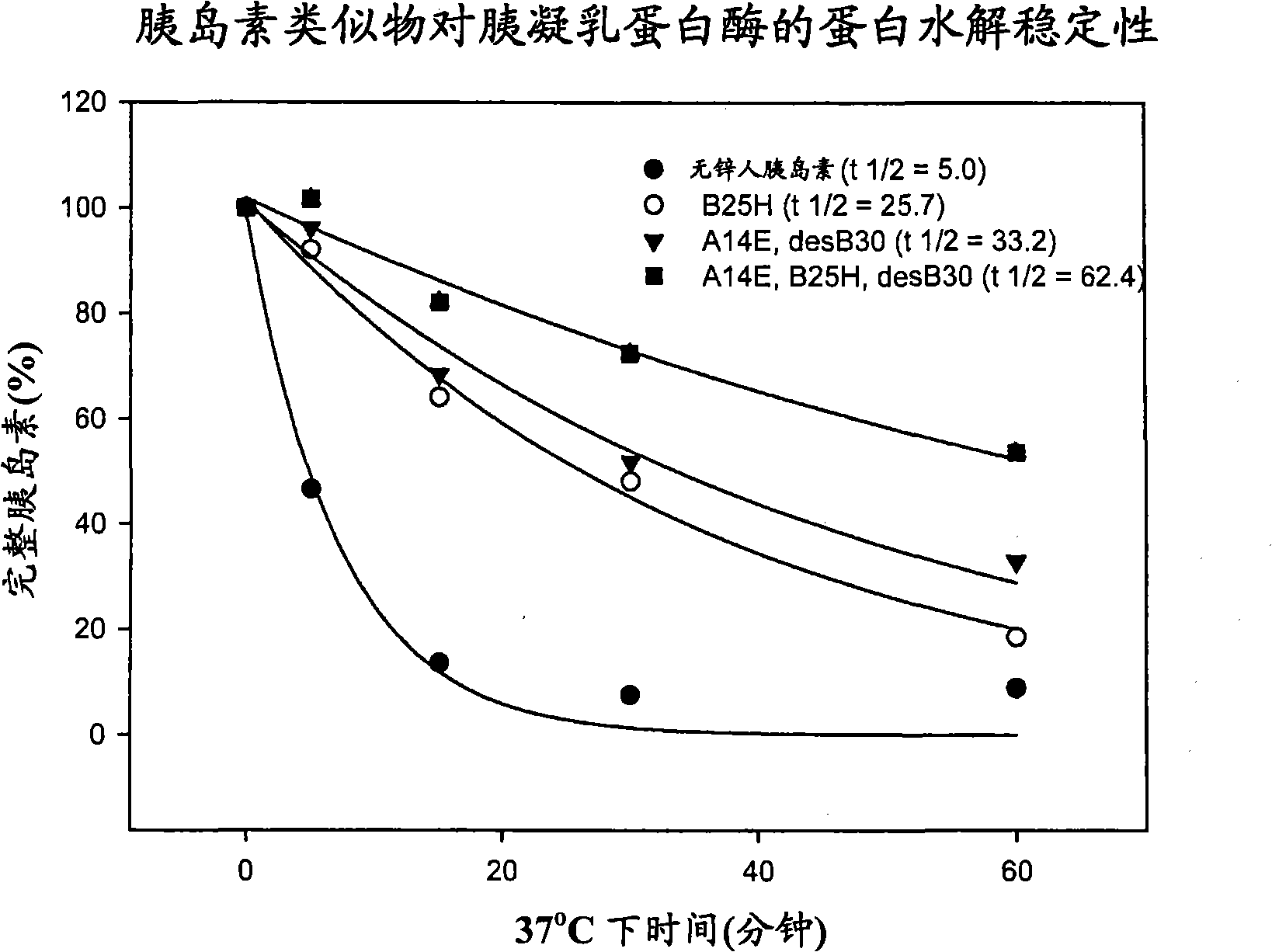
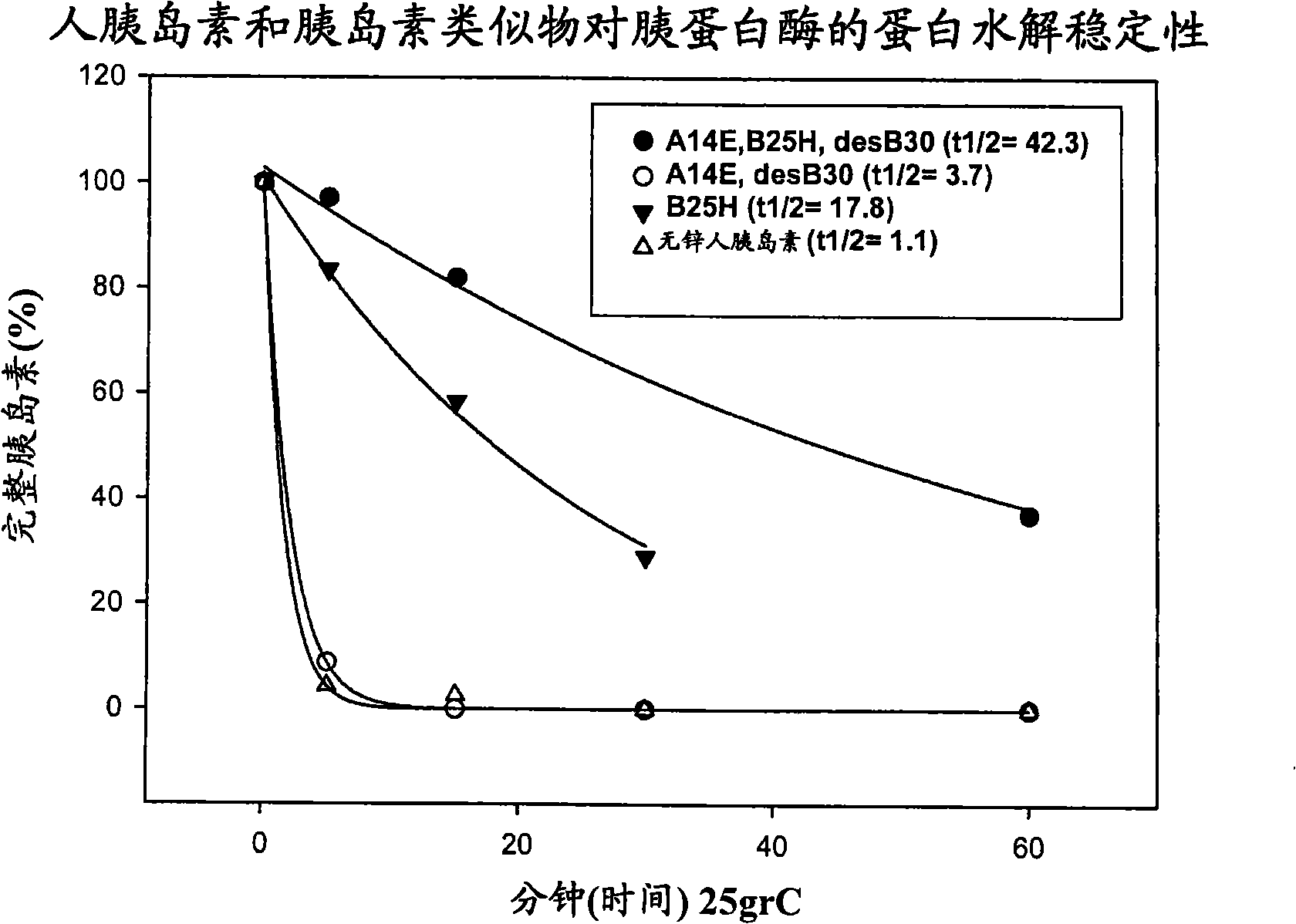
Protease resistant insulin analogues
PendingCN101541830APeptide/protein ingredientsMetabolism disorderPancreatic hormoneProtease resistant
Owner:NOVO NORDISK AS
Method and kit for detecting the early onset of renal tubular cell injury
InactiveUS20090123941A1Bioreactor/fermenter combinationsOrganic active ingredientsSide effectNGAL Protein
A method and kit for detecting the early onset of renal tubular cell injury, utilizing NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the urine is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Protease-resistant systems for polypeptide display and methods of making and using thereof
ActiveUS9309510B2Reduce capacityIncrease flexibilityPolypeptide with localisation/targeting motifPeptide-nucleic acidsProtease resistantBioinformatics
The present invention generally relates to bacterial polypeptide display systems, libraries using these bacterial display systems, and methods of making and using these systems, including methods for improved display of polypeptides on the extracellular surface of bacteria using circularly permuted transmembrane bacterial polypeptides that have been modified to increase resistance to protease degradation and to enhance polypeptide display characteristics.
Owner:CYTOMX THERAPEUTICS
Denaturant stable and/or protease resistant, chaperone-like oligomeric proteins, polynucleotides encoding same, their uses and methods of increasing a specific activity thereof
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Production of fmdv-resistant livestock by allele substitution
ActiveUS20140041066A1Native protein cleavage is reducedSugar derivativesTissue cultureBiotechnologyProtease resistant
Owner:RECOMBINETICS INC
Peptides and compositions for treatment of joint damage
ActiveUS20140256643A1Organic active ingredientsPeptide/protein ingredientsJoint damageProtease resistant
The present invention provides new protease resistant polypeptides, as well as compositions and methods for treating, ameliorating or preventing conditions related to joint damage, including acute joint injury and arthritis.
Owner:NOVARTIS AG
Denaturat stable and/or protease resistant, chaperone-like oligomeric proteins, polynucleotides encoding same, their uses and methods of increasing a specific activity thereof
InactiveUS20060172298A1Increasing cell migrationPromote wound healingCosmetic preparationsFungiPurification methodsNucleotide
Novel denaturant-stable, protease resistant, homo-oligomeric proteins, also referred to herein as stable proteins (SPs), having chaperone-like activity; methods of production and purification of SPs; nucleic acids encoding SPs; methods of isolating nucleic acids encoding SPs; antibodies recognizing SPs; the use of SPs for stabilizing, refolding, repairing, preventing aggregation and de-aggregating macromolecules such as proteins; fusion proteins including SPs; nucleic acid constructs encoding the fusion proteins; and their uses in a variety of methods and applications.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Protease resistant insulin analogues
ActiveUS20100009898A1Improve stabilityRetained biological insulin activitySugar derivativesPeptide/protein ingredientsPancreatic hormoneProtease resistant
The present invention relates to novel insulin analogues exhibiting resistance towards protease, wherein at least two amino acids are substituted and / or deleted relative to the parent insulin molecule. A method for the preparation of such insulin analogues, insulin preparations containing the insulin analogues of the invention and a method of treating diabetes mellitus using these insulin analogues is also provided.
Owner:NOVO NORDISK AS
Protease resistant compositions for wound healing
Owner:MICHIGAN UNIV OF RGT THE
Protease resistant recombinant bacterial collagenases
InactiveUS20100159564A1Long fermentation timeIncrease temperatureSugar derivativesHydrolasesClostridium histolyticum CollagenaseSpectroscopy
The identification of the most sensitive sites of Clostridium histolyticum collagenase Class 1 to proteolysis by proteases present during the fermentation and purification of the enzyme is described. Culture supernatant obtained after fermentation of C. histolyticum is used as the starting material for further purification of the enzyme. Native collagenase Class 1 and its proteolytic fragments are partially purified by a combination of hydrophobic interaction and strong anion exchange chromatographies. The pools containing enriched levels of the proteolytic fragments are further purified by high performance anion exchange chromatography. These polypeptides are then characterized by Q-TOF mass spectroscopy. A total of three sensitive bonds are identified along with substitution and deletion strategies that will result in resistance of the enzyme to proteolytic degradation.
Owner:DWULET FRANCIS E +2
Hyperglycosylated polypeptide variants and methods of use
The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites. The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites, as well as erythropoietin and darbepoetin alfa, each of which are linked to a penetrating peptide that facilitates translocation of a substance across a biological barrier as well as pharmaceutical compositions, including oral formulations, of the same. The present invention further provides oral formulations of hyperglycosylated or protease-resistant, hyperglycosylated polypeptide variants, which polypeptide variants lack at least one protease cleavage site found in a parent polypeptide, and thus exhibit increased protease resistance compared to the parent polypeptide, which polypeptide variants further include (1) a carbohydrate moiety covalently linked to at least one non-native glycosylation site not found in the parent protein therapeutic or (2) a carbohydrate moiety covalently linked to at least one native glycosylation site found but not glycosylated in the parent protein therapeutic. The present invention further provides compositions, including oral pharmaceutical compositions, comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides containers, devices, and kits comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides therapeutic methods involving administering an effective amount of an oral pharmaceutical composition comprising a synthetic Type I interferon receptor polypeptide agonist, a hyperglycosylated polypeptide variant, or a hyperglycosylated, protease-resistant polypeptide variant to an individual in need thereof.
Owner:ALIOS BIOPHARMA INC +1
Inhibitor-resistant HCV NS3 protease
An inhibitor-resistant HCV NS3 protease is provided which is useful to screen for compounds having therapeutic value against drug resistant HCV strains. In particular, the inhibitor-resistant HCV NS3 protease comprises an amino acid sequence which is mutated in the substrate binding pocket thereof rendering the protease resistant to inhibitor. In a specific aspect of the present invention, at least one of the amino acids at position 155, 156 and 168 of the HCV NS3 protease is mutated to yield an inhibitor-resistant protease.
Owner:BOEHRINGER INGELHEIM INT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com