Compositions and methods for the preparation of protease resistant human growth hormone glycosylation mutants
a technology of protease and growth hormone, which is applied in the field of compositions and methods for the preparation of protease resistant human growth hormone glycosylation mutants, can solve the problems of limited use of therapeutic peptides, the immunogenic nature of most peptides, and the addition of peg molecules, and achieves the effect of pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
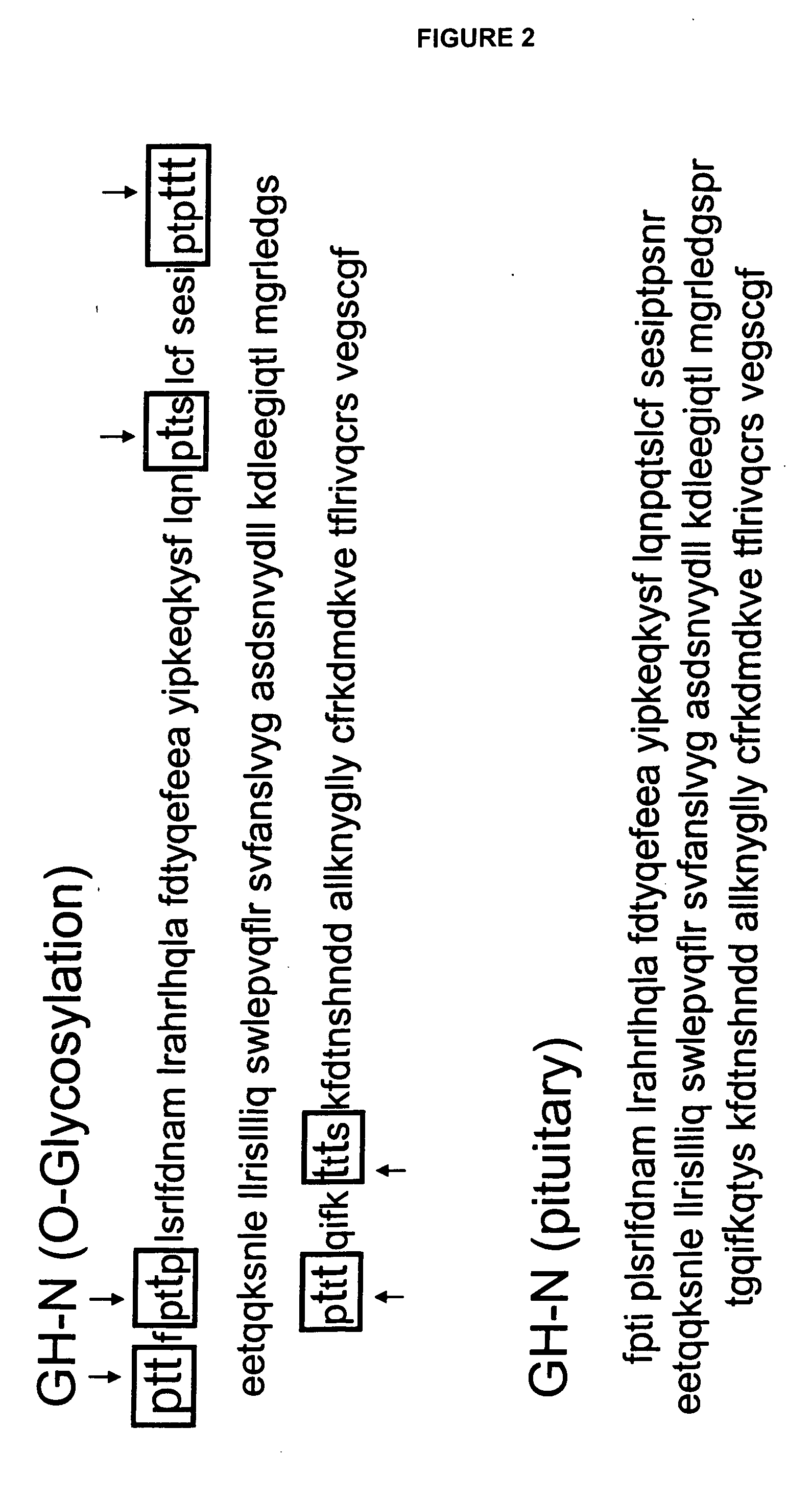
[0464] Human growth hormone occurs in a variety of different isoforms and different amino acid sequences. The two best characterized forms include placental derived hGH, which is also known as GH-V (PDB P01242) and pituitary derived hGH, which is also known as somatotropin or GH-N(P01241); see FIG. 1. The pituitary derived hGH is not glycosylated and is produced in Escherichia coli as a therapeutic. The placental derived hGH (GH-V) has one N-glycosylation site at amino acid 140 (see Table 4 and FIG. 1, see arrow).
TABLE 4Human Growth Hormone (GH-V), Placenta Derived; P01242 (SEQ ID NO:2)fptiplsrlfdnamlrarrlyqlaydtyqefeeayilkeqkysflqnpqtslcfsesiptpsnrvktqqksnlellrisllliqswlepvqllrsvfanslvygasdsnvyrhlkdleegiqtlmwrledgsprtgqifnqsyskfdtkshnddallknygllycfrkdmdkvetflrivqcrsvegscgf ↑
[0465] The pituitary derived hGH (GH-N) can be modified at amino acid position 140 to introduce an N-linked glycosylation site by mutating the nucleotide sequence encoding this polypeptide so that i...
example 2
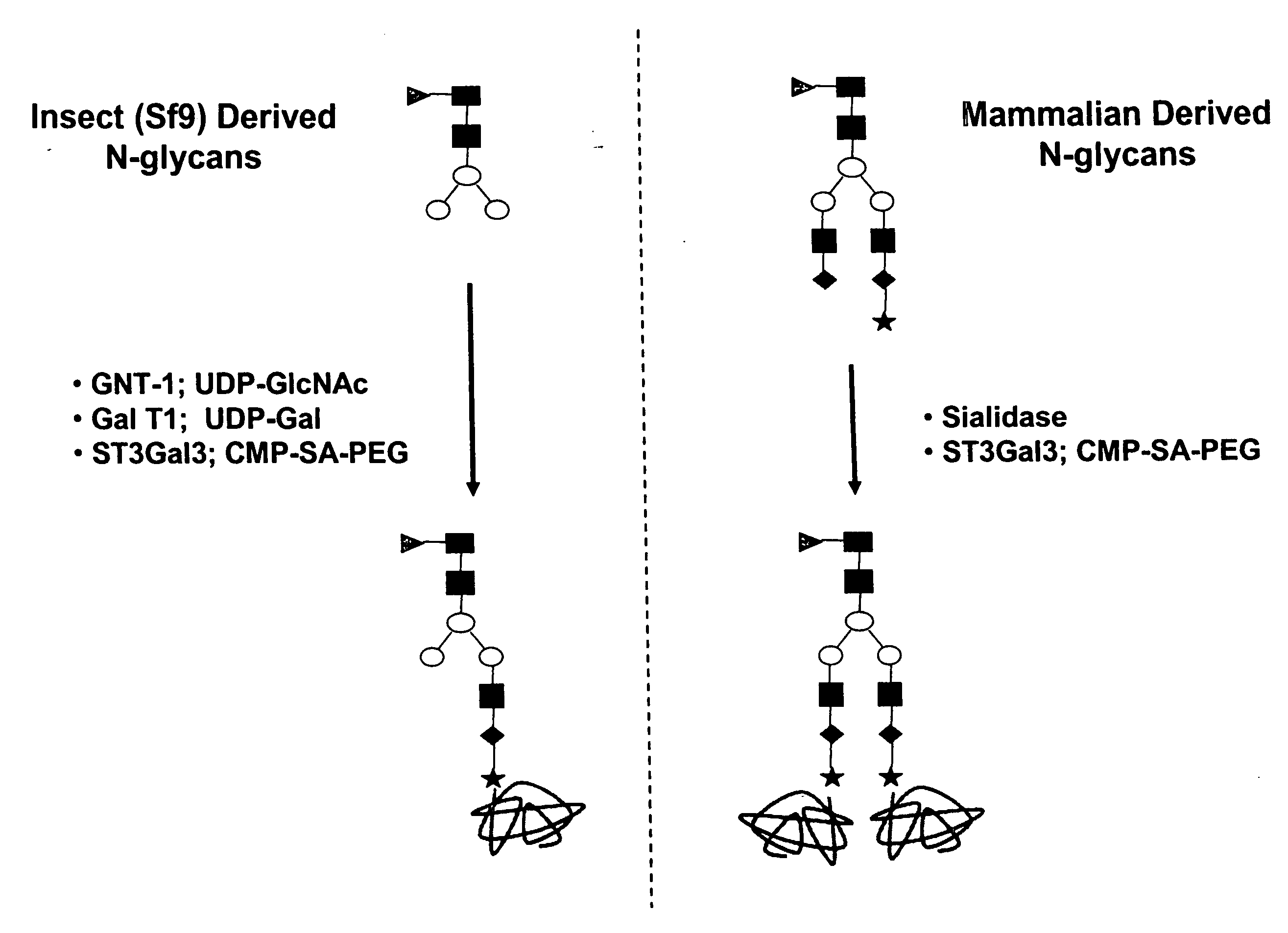
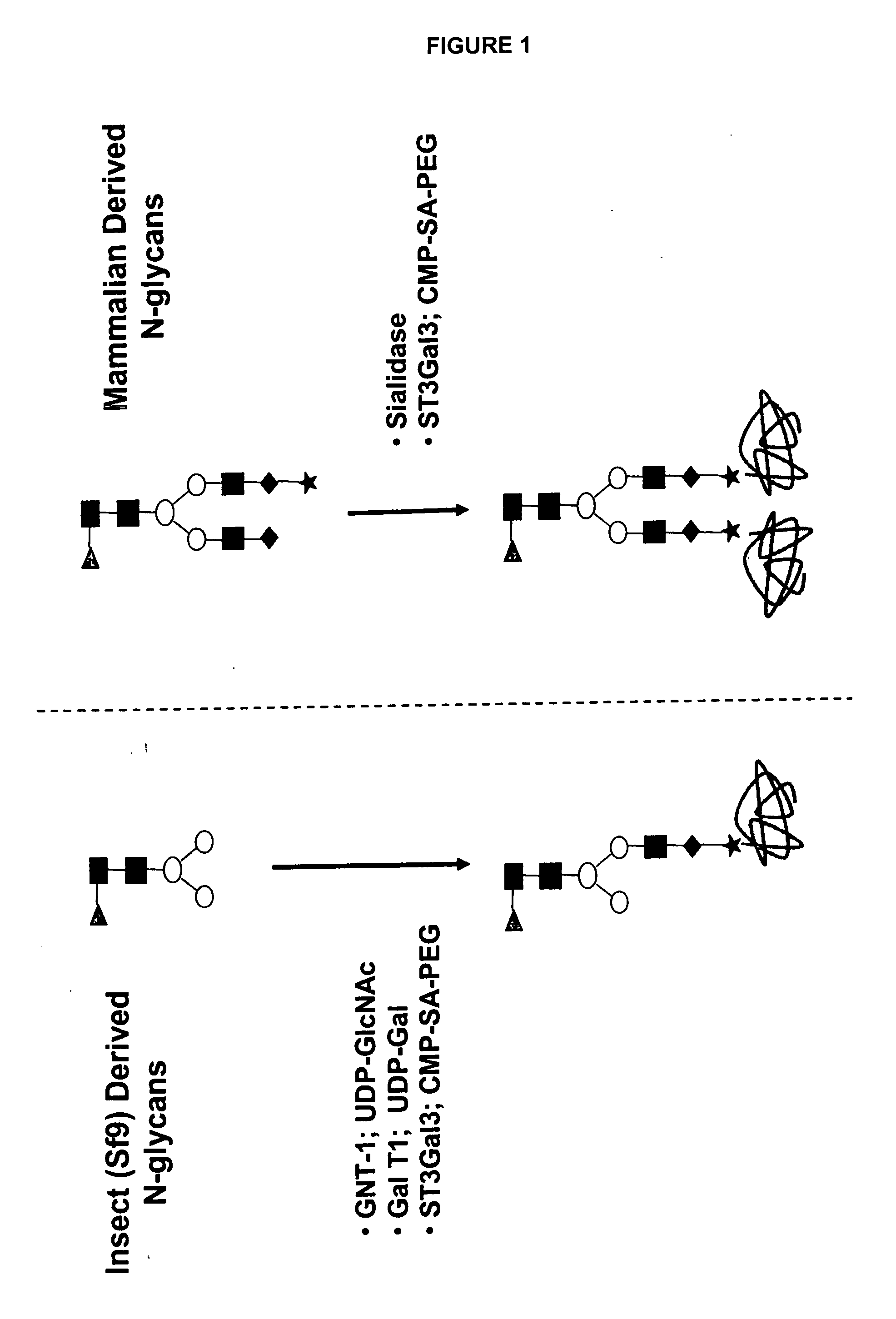
[0467] An alternative approach is to create an O-linked glycosylation site into the pituitary derived hGH polypeptide. This O-linked glycosylation site may then be used as a site on which the mutated hGH polypeptide can be glycoPEGylated using a GalNAcT2 enzyme or the like. One or more additional transferases may then be used to add glycans or glycoconjugates to that site. Preferably, the mutated pituitary derived hGH polypeptide is glycoPEGylated. FIG. 4 describes the glycoPEGylation of an hGH O-linked glycan mutant produced in Escherichia coli.
example 3
[0468] As identified by the crystal structure of hGH and its receptor, the protein loop regions on pituitary derived hGH are best suited for mutation to introduce a glycosylation site (FIG. 5). Specifically, the nucleotide sequence that encodes amino acids 1-6 (FPTIPL; SEQ ID NO:10), amino acids 48-52 (PQTSL; SEQ ID NO:11), amino acids 59-64 (PTPSNR; SEQ ID NO:12), amino acids 133-139 (PRTGQIF; SEQ ID NO:13), amino acids 133-145 (PRTGQIFKQTYSK; SEQ ID NO:14), or amino acids 139-142 (FKQT; SEQ ID NO:15) of the wild-type pituitary derived hGH amino acid sequence (see Table 5 and FIG. 1) can be mutated so that either an N-linked or an O-linked glycosylation site is introduced into the resulting mutated pituitary-derived hGH polypeptide.
[0469]FIG. 6 illustrates six (6) of these introduced O-linked glycosylation sites. The arrows in FIG. 6 each represent the threonine residue on which O-linked glycosylation will occur in the GH-N O-linked glycan hGH mutant.
[0470]FIG. 7 and FIG. 8 each ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com