Patents
Literature
65 results about "CD81" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CD81 molecule, also known as CD81 (Cluster of Differentiation 81), is a protein which in humans is encoded by the CD81 gene. It is also known as 26 kDa cell surface protein, TAPA-1 (Target of the Antiproliferative Antibody 1), and Tetraspanin-28 (Tspan-28).
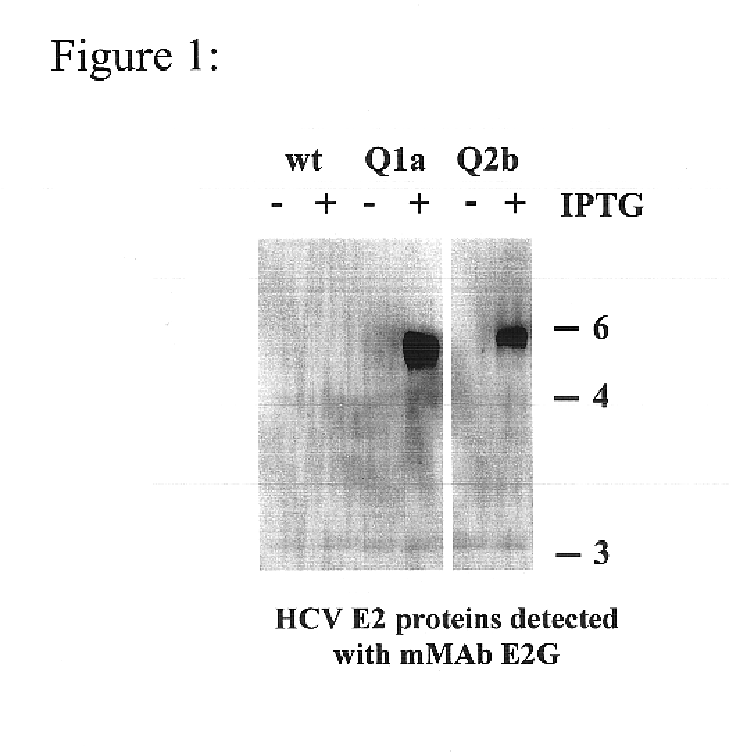
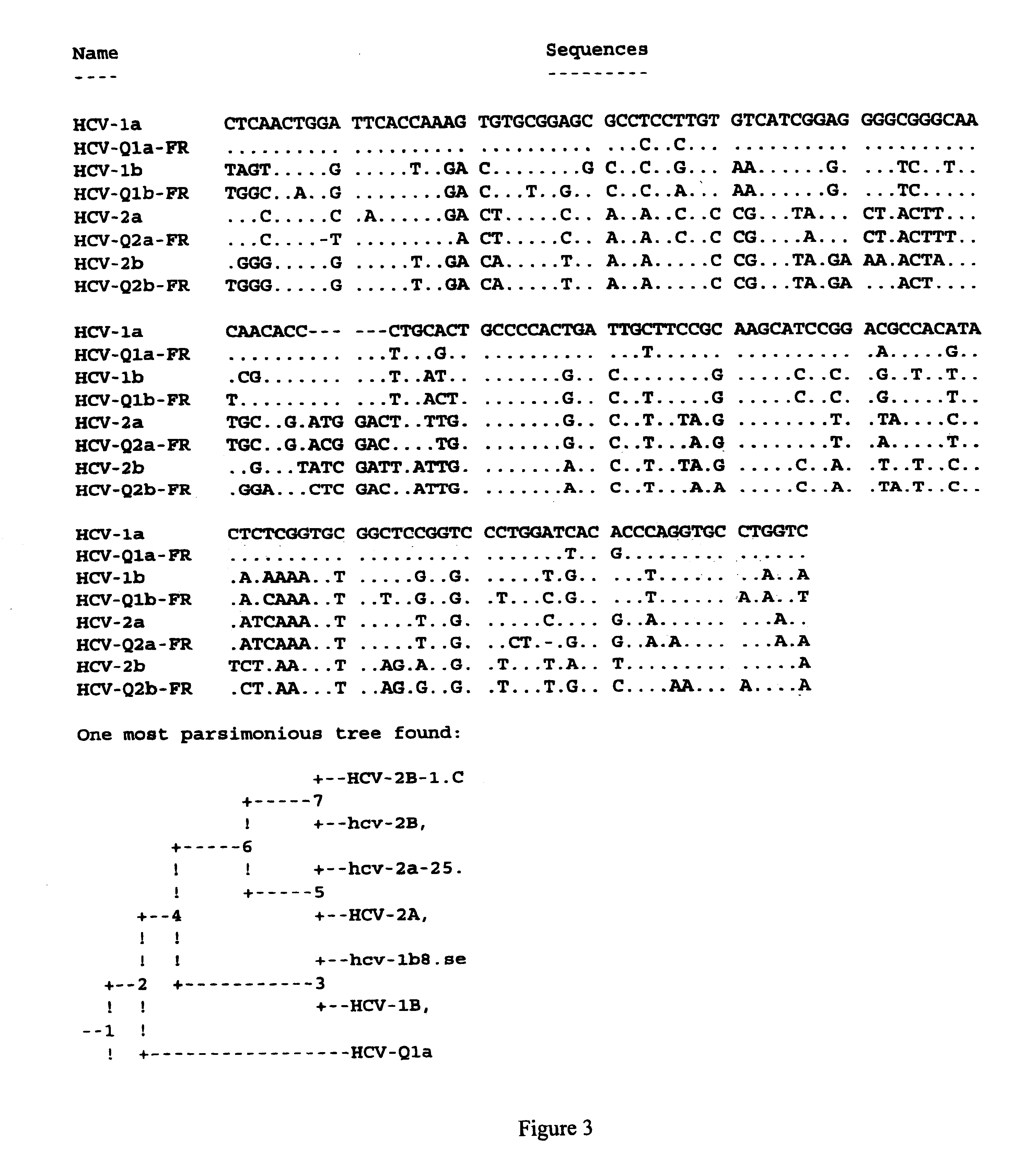
Prevention and treatment of HCV infection employing antibodies that inhibit the interaction of HCV virions with their receptor
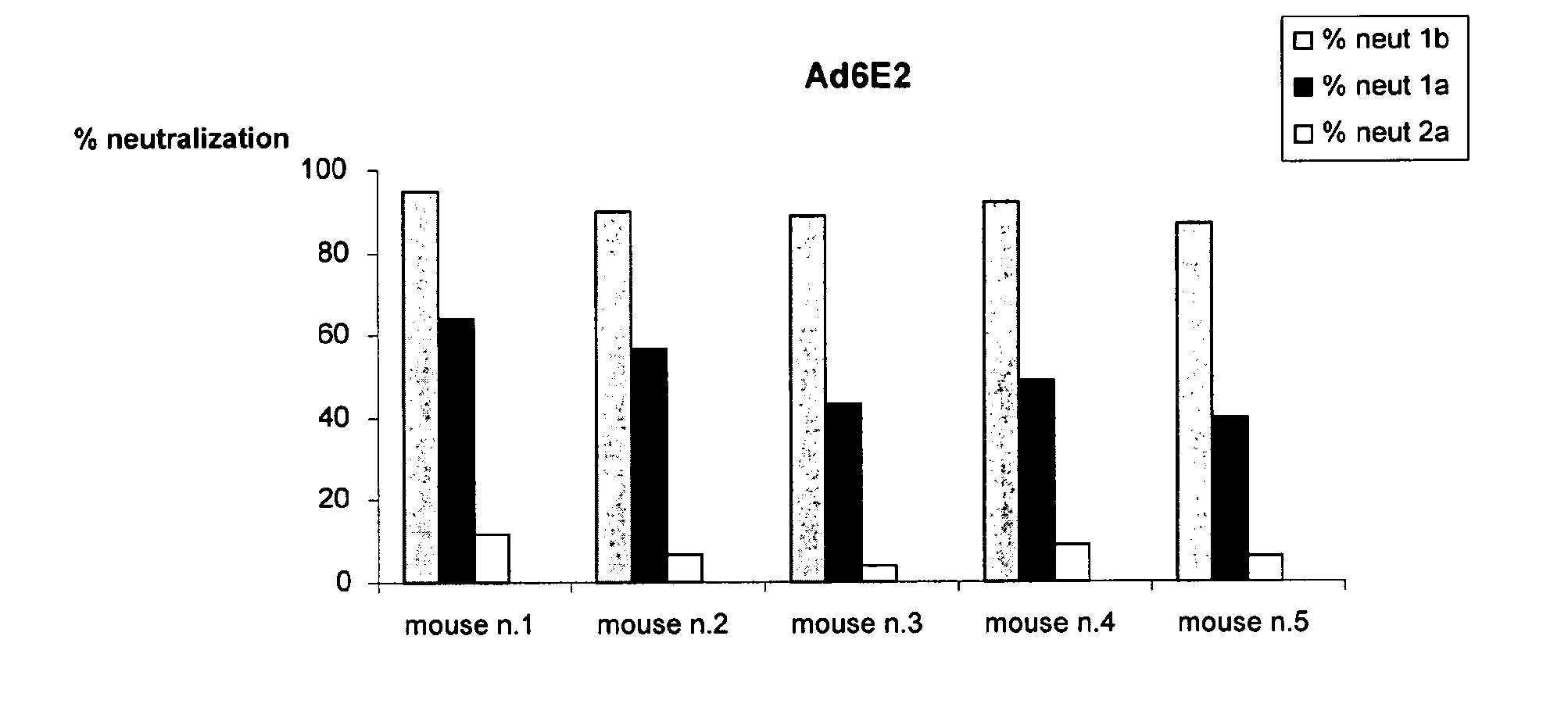
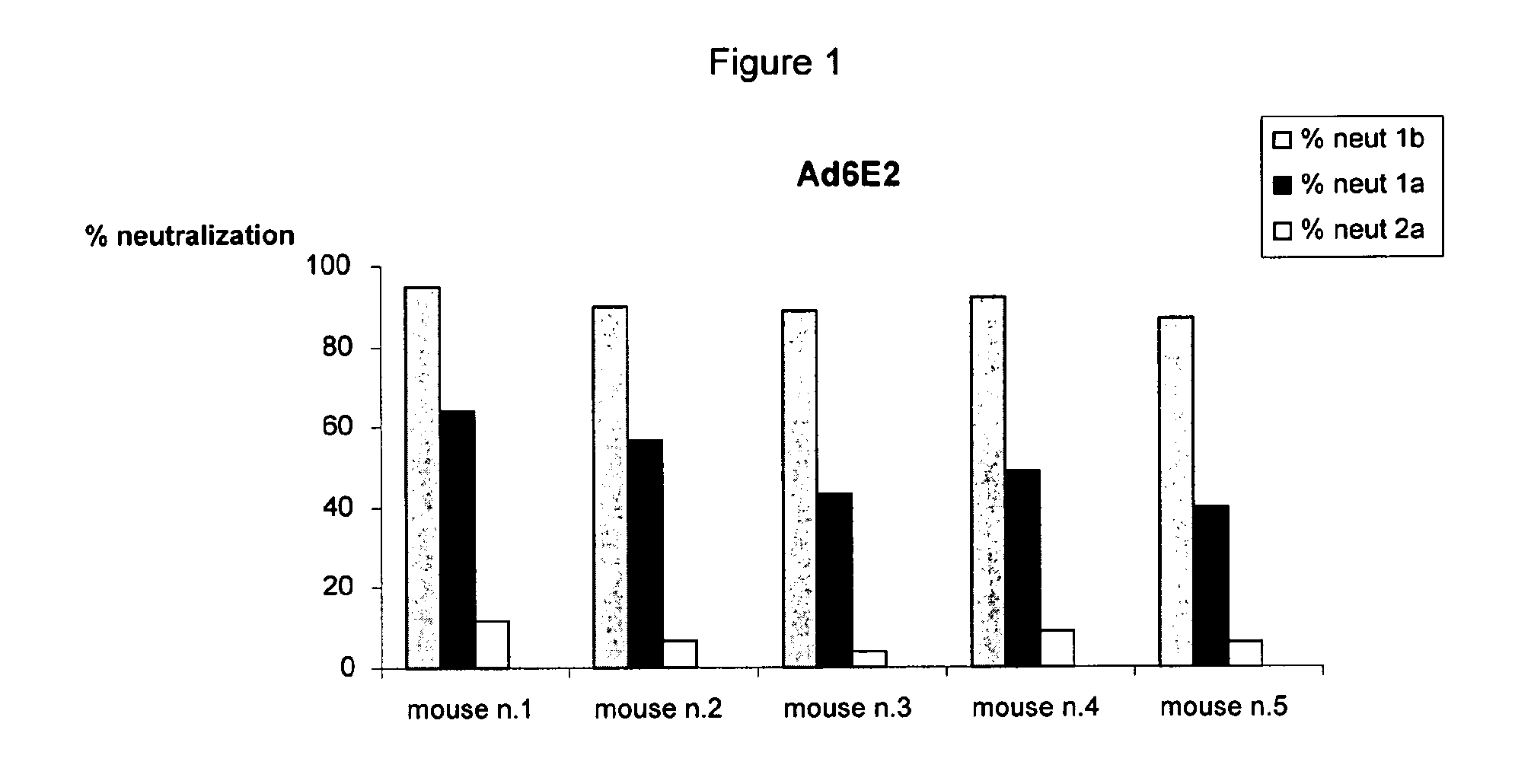
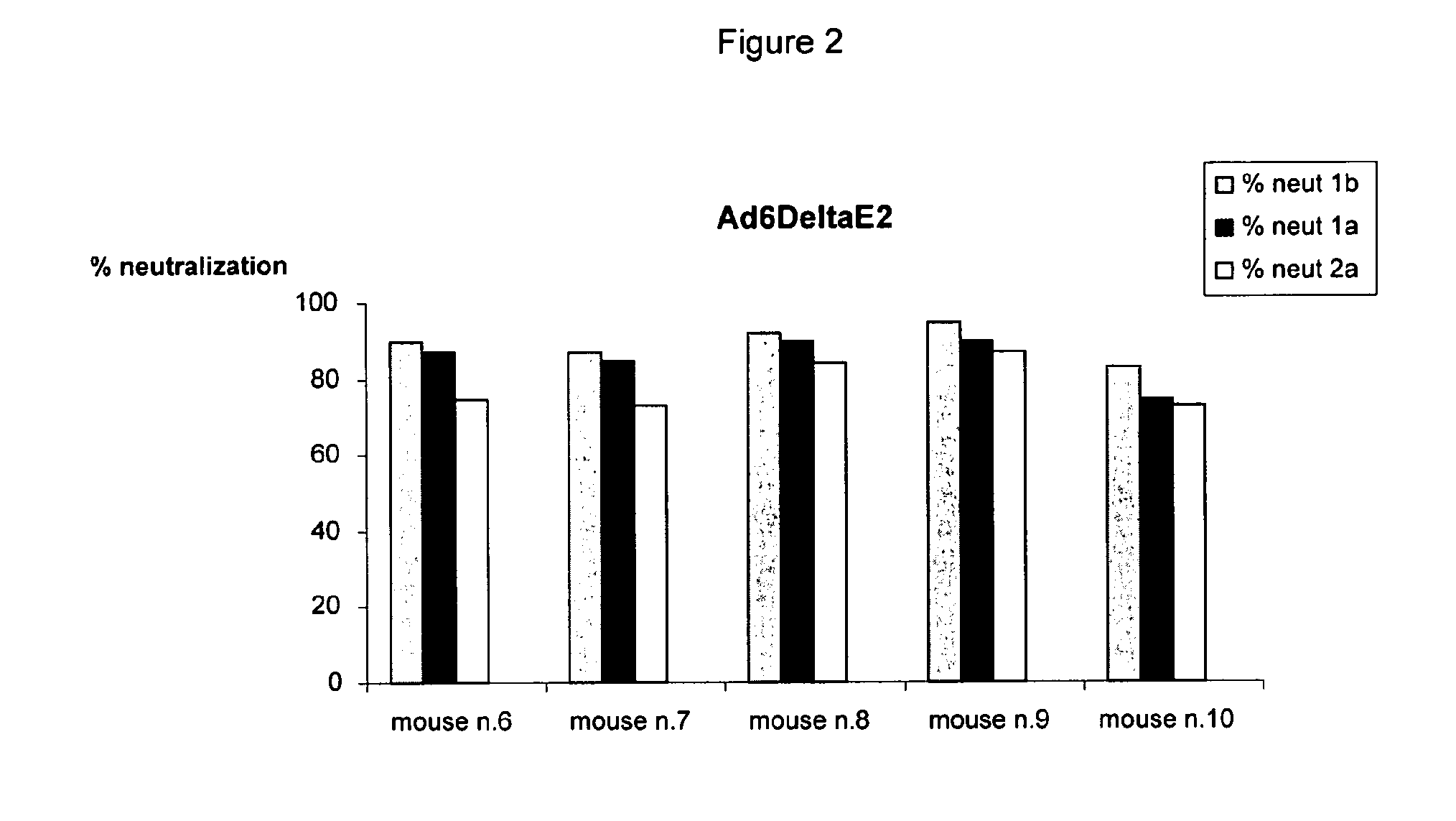
Human monoclonal antibodies binding to epitopes common to type 1 and 2 HCV are provided, as well as conformationally conserved HCV E2 2a and 2b proteins. Compositions comprising the antibodies find use in diagnosis and therapy. The antibodies recognize conformational epitopes that are conserved across multiple genotypes of HCV. Thus the antibodies have the potential to be useful in the prevention and treatment of the majority of HCV infections. A subset of the antibodies (CBH-2, CBH-5, CBH-7, CBH-8C, CBH-8E, and CBH-11) have the ability to prevent the binding of HCV E2 proteins of multiple genotypes to human CD81, a possible co-receptor for HCV infection. A subset of the antibodies (CBH-2 and CBH-5) have been shown to inhibit the binding of HCV virions (as opposed to purified E2 protein) to human CD81. A further subset of the antibodies (CBH-4D, CBH4B, CBH-8C, and CBH-9) have been shown to prevent HCV envelope mediated fusion using an HCV psuedotype system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
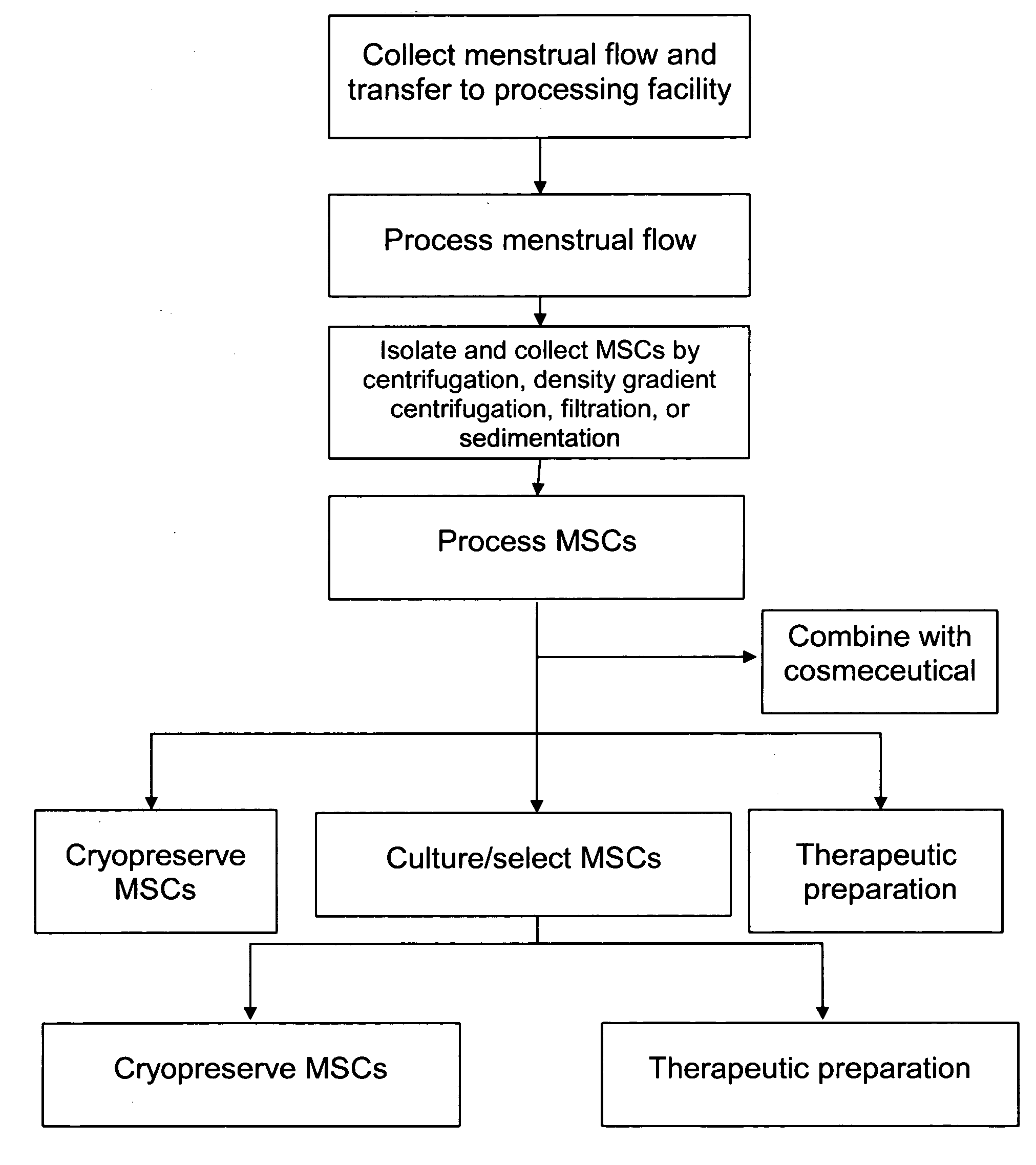
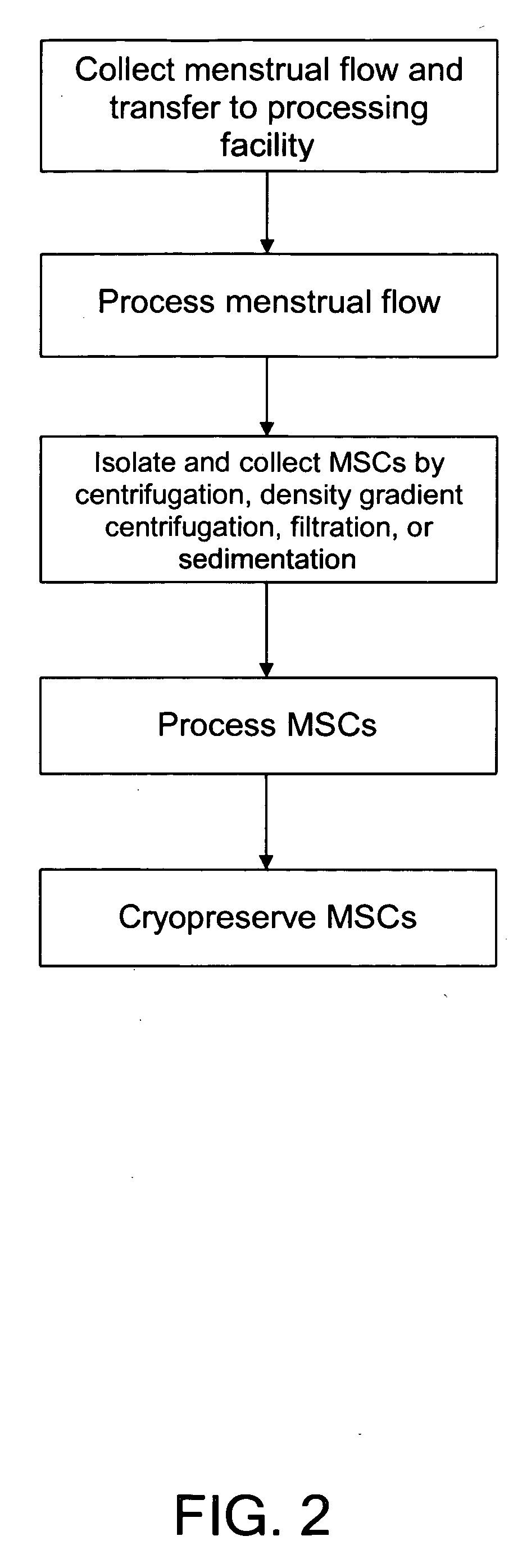
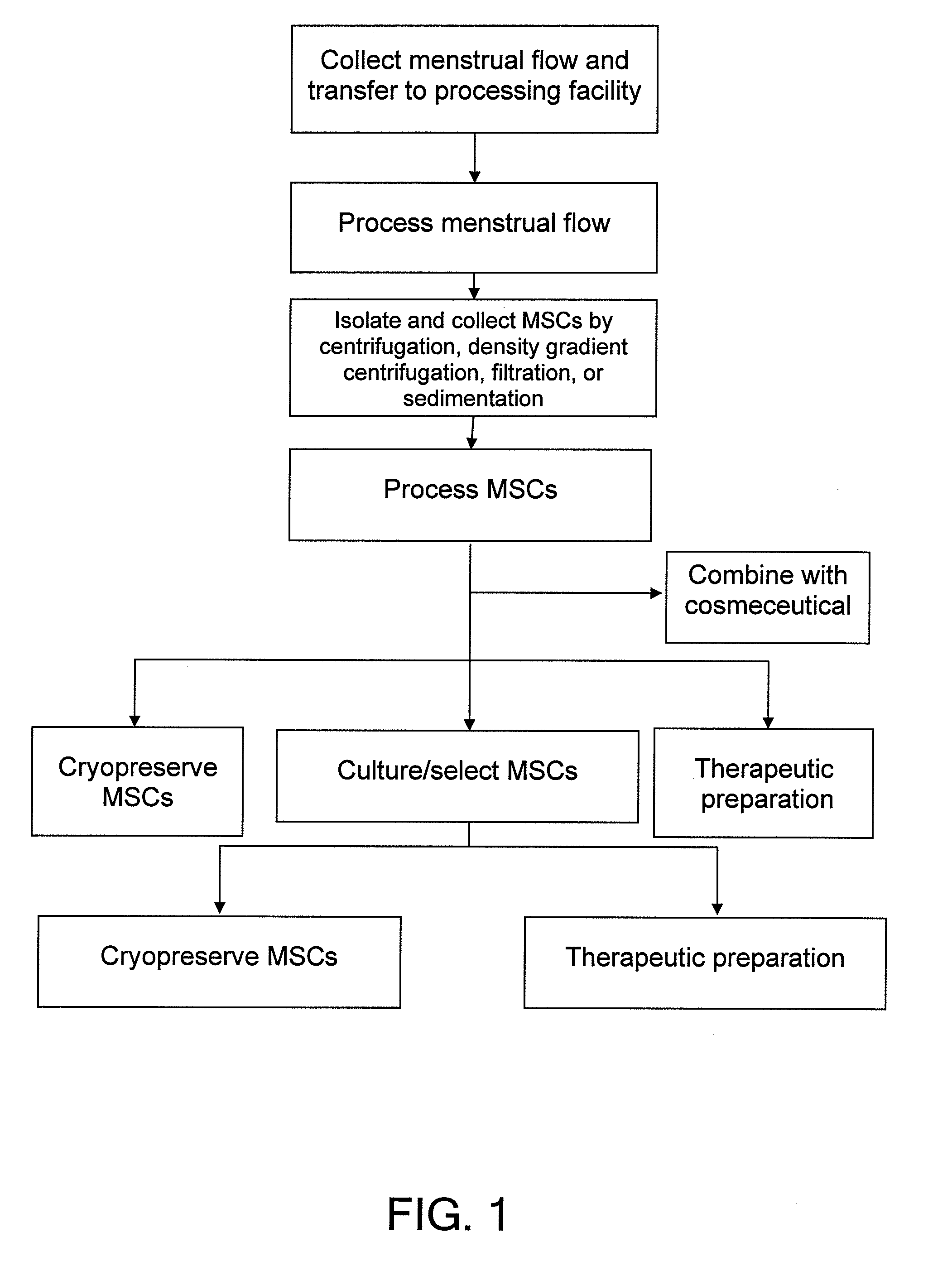
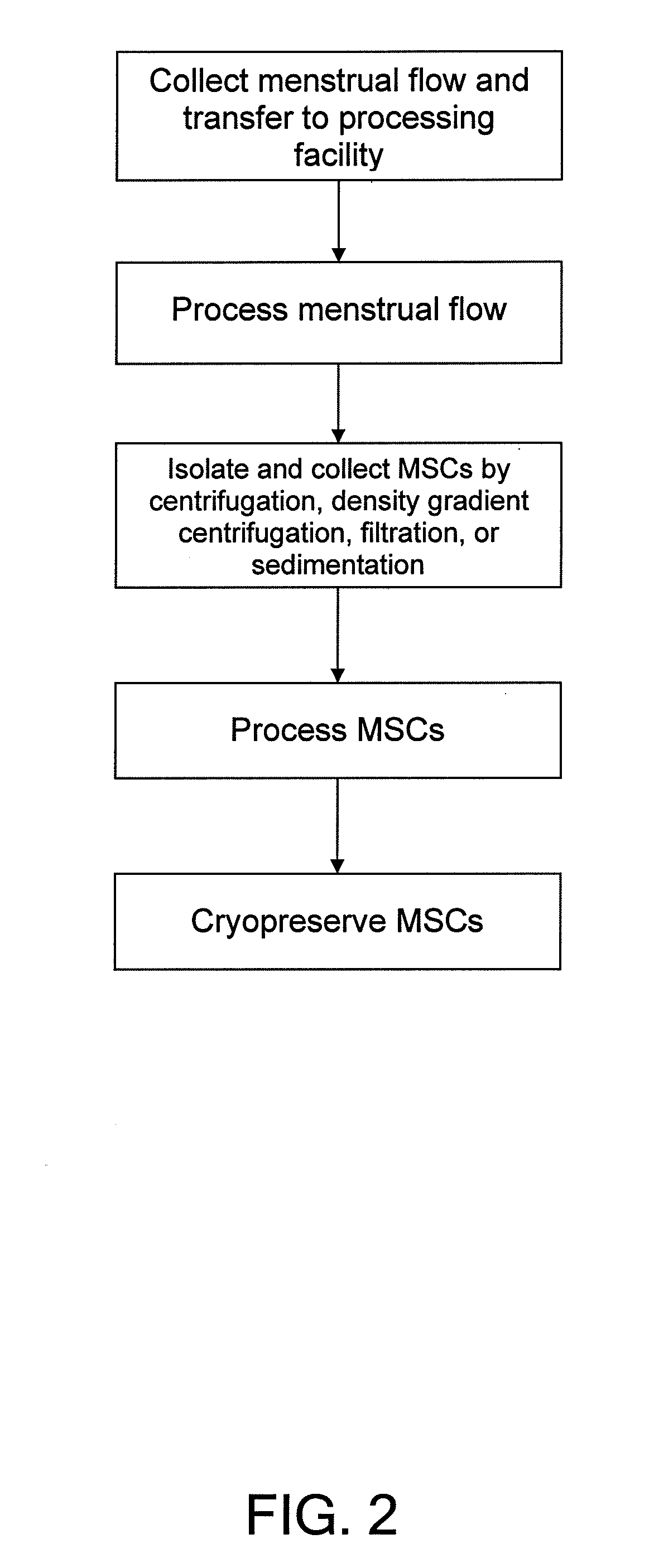
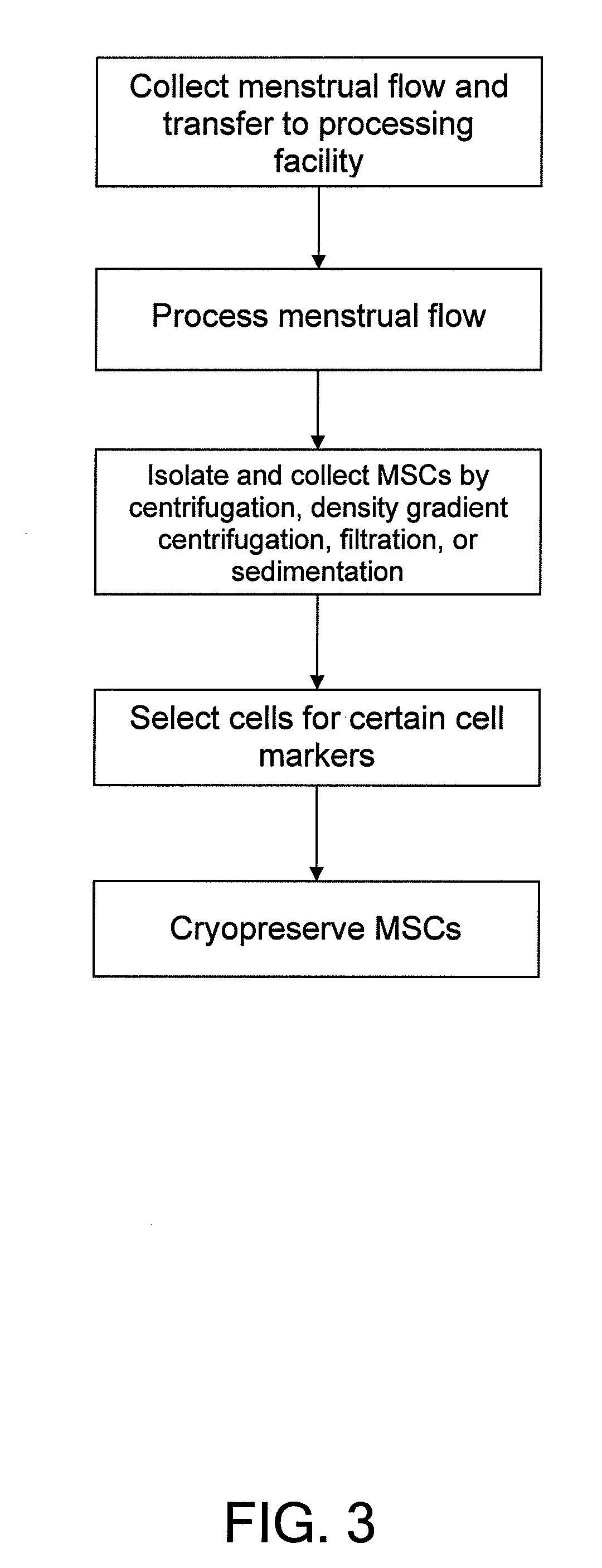
Procurement, isolation and cryopreservation of endometrial/menstrual cells
Compositions comprising menstrual stem cells (MSCs) and methods, processes, and system therefor are provided by the invention. MSCs are processed from menstrual flow collected during menses. MSCs may be cryopreserved, processed through various culturing and selection steps in preparation for cryopreservation, or processed for therapeutic or cosmeceutical use. Cryopreserved MSCs may be thawed in preparation for therapeutic and cosmeceutical use. MSCs express CD9, CD10, CD13, CD29, CD44, CD49e, CD49f, CD59, CD81, CD105, CD166, and HLA class I, and have low or no expression of CD3 and HLA class II.
Owner:CRYO CELL INTERNATIONAL INC
Efficient cell culture system for hepatitis C virus genotype 5A
Owner:HVIDOVRE HOSPITAL
Vaccine for the prevention and therapy of hcv infections
InactiveUS20110129498A1Enhance immune responseGenerate efficientlySsRNA viruses positive-sensePeptide/protein ingredientsAdjuvantMammal
The present invention relates to CD81-binding peptides of the hepatitis virus C(HCV) E2 glycoprotein, which are devoid of or mutated within the amino-terminal 27 amino acids of the mature E2 envelope glycoprotein, or variant thereof which retains the ability to bind to CD81. Furthermore, the present invention provides polypeptides comprising said CD81-binding peptide, polynucleotides encoding the CD81-binding peptide, and expression cassettes and vectors comprising the polynucleotide of the invention. Moreover, the present invention relates to compositions comprising the CD81-binding peptide, the polynucleotide encoding the CD81-binding peptide, the expression cassette, or the vector, and an adjuvant. Furthermore, the present invention provides a pharmaceutical composition comprising the CD81-binding peptide, the polynucleotide, the expression cassette, the vector, or the composition of the invention, and a pharmaceutically acceptable excipient, carrier, or diluent. Moreover, the present invention provides the CD81-binding peptide, the polynucleotide, the expression cassette, the vector, the composition, or the pharmaceutical composition of the invention for induction of an immune response, preferably a broad specificity immune response, against HCV in a mammal, and methods for inducing a therapeutic and / or prophylactic immune response against HCV in a mammal, preferably against HCV of various genotypes.
Owner:OKAIROS AG
Taqman fluorescence probe quantitative PCR method adopting plasmid constructed by tree shrew CD81 gene as standard substance
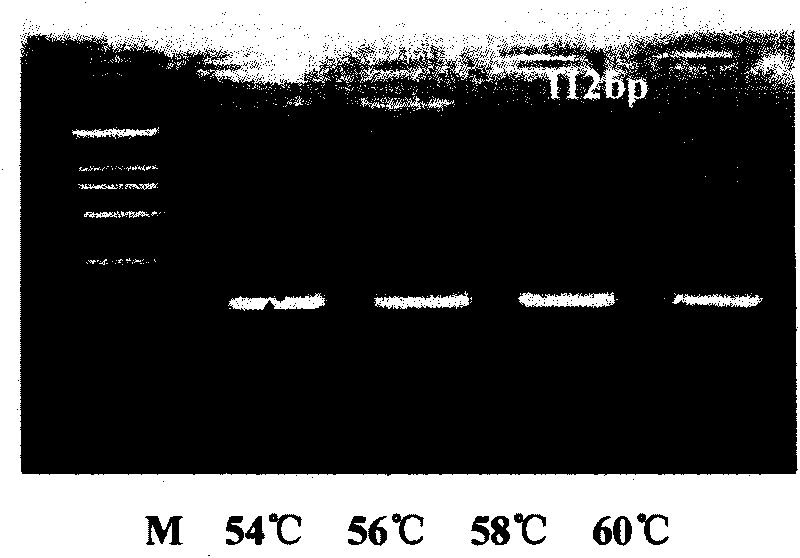
The invention relates to a method for detecting and estimating the change situation of CD81 molecule which is infected by hepatitis C virus (HCV) in vivo and in vitro, in particular to a Taqman fluorescence probe quantitative PCR detection method on the basis of using cloned tree shrew CD81 plasmid as standard substance, belongs to the molecular biology field. The method comprises the following steps: cloning tree shrew CD81 gene segment; designing reasonable probe and primer with Primers Express2.0 software on the cloned tree shrew CD81 gene segment; designing experiment to select annealing temperature (Tm value), primer concentration and probe concentration under optimal conditions; further adopting tree shrew CD81 plasmid as standard substance to perform 10 times concentration gradient dilution and building standard curve; verifying the repeatability, stability and sensitivity of the method of the invention; and finally detecting the change of CD81 molecule which is infected by hepatitis C virus (HCV) in vitro by the method of the invention. The method can detect the change situation of tree shrew CD81 accurately, fast and conveniently.
Owner:KUNMING UNIV OF SCI & TECH
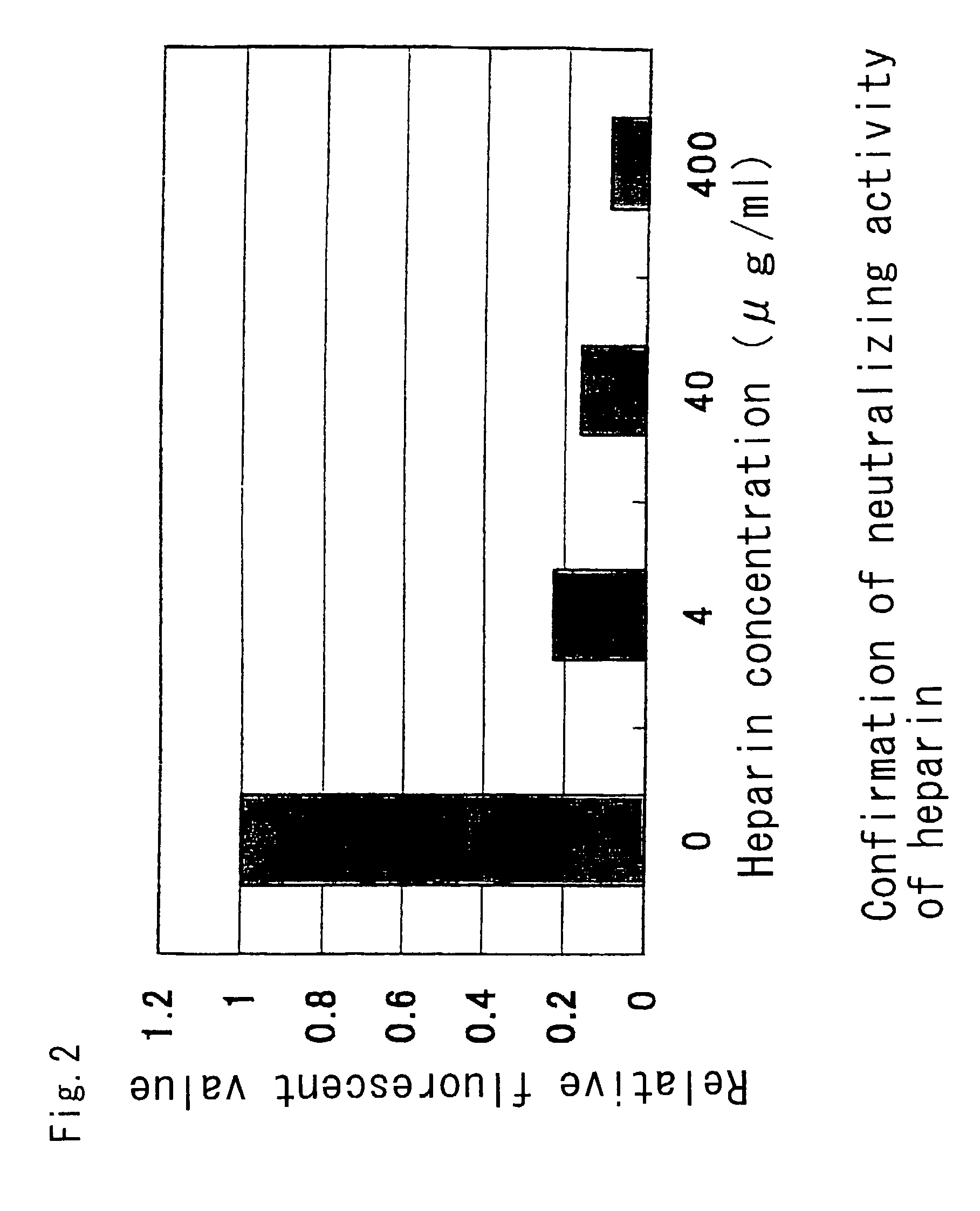
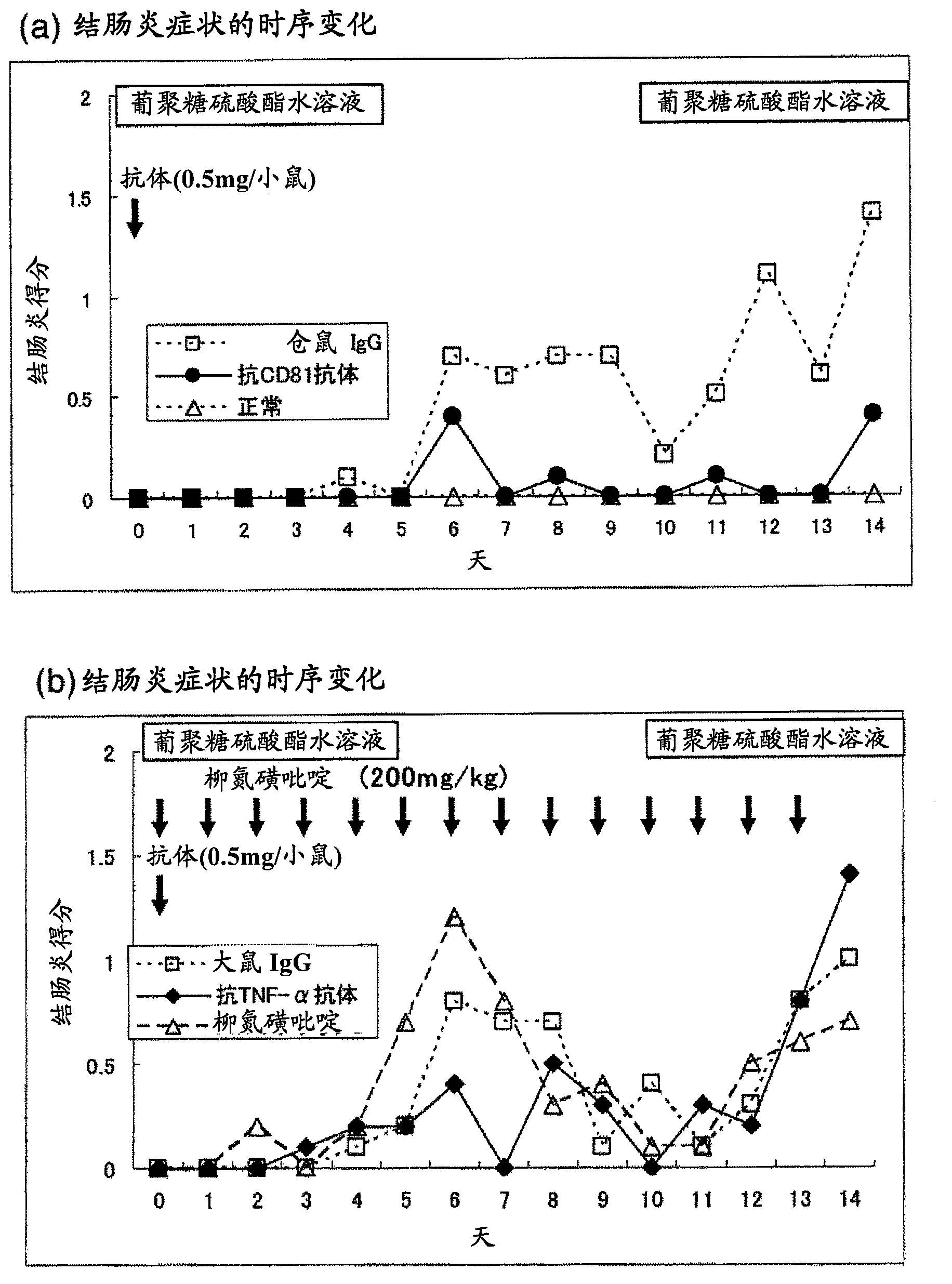
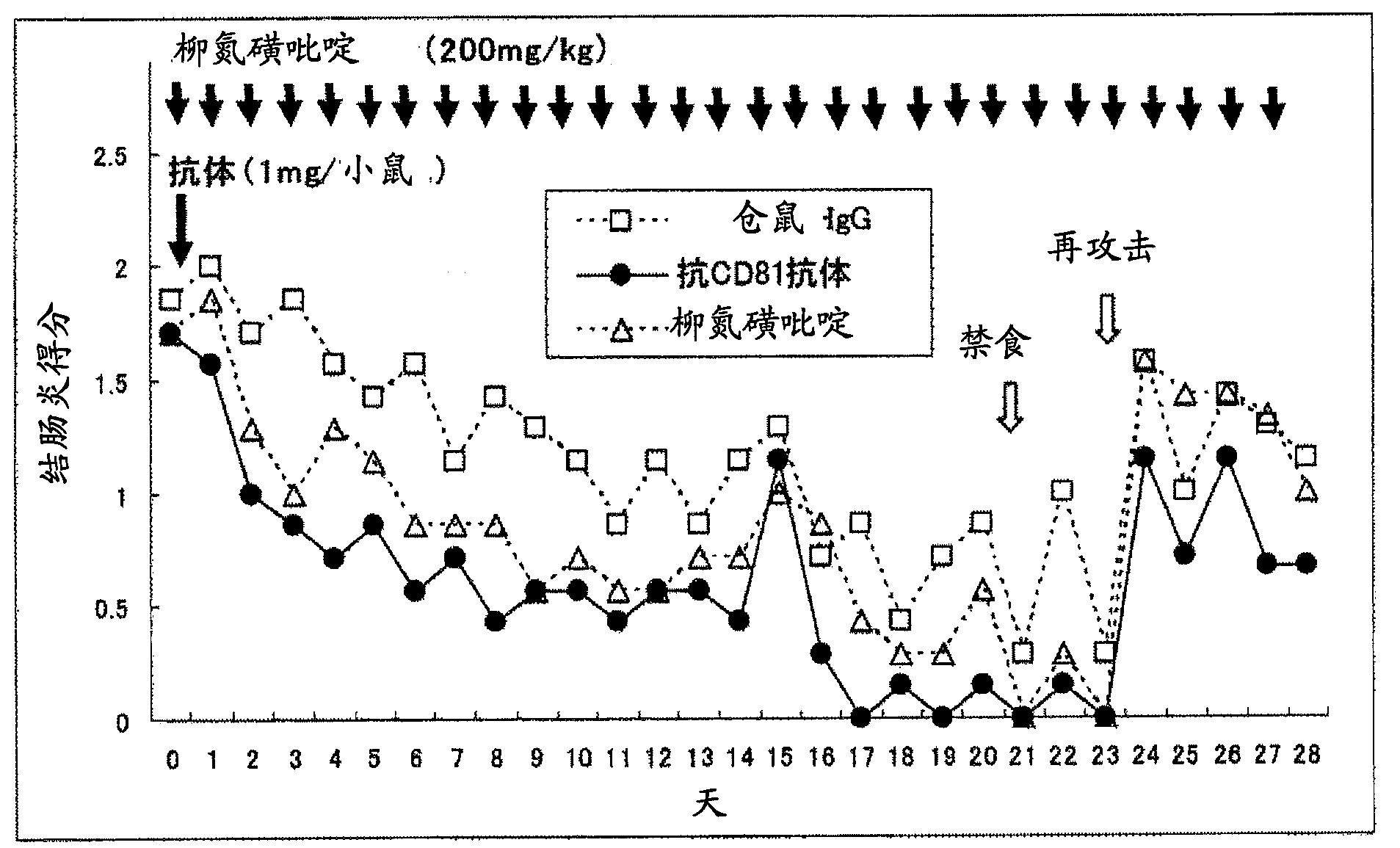
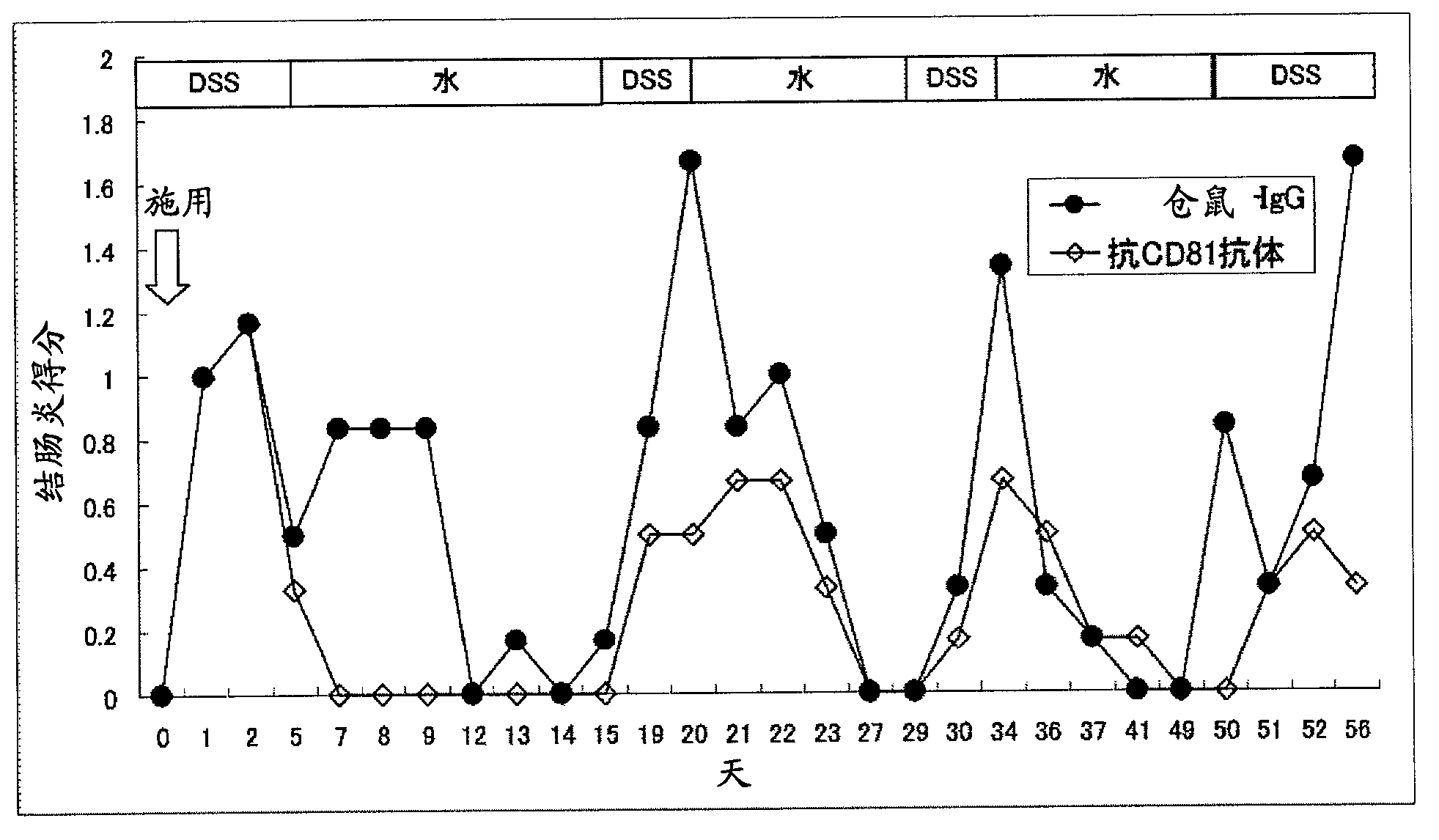
Preventive or remedy for inflammatory bowel diseases containing anti-cd81 antibody as the active ingredient
InactiveUS20060275289A1Easy to usePreventing, improving or treating inflammatory bowel diseasesAntipyreticMicrobiological testing/measurementNucleotideBULK ACTIVE INGREDIENT
It is possible to use a screening system using the inhibition of CD81 gene expression, the inhibition of CD81 expression or the inhibition of its function (activity) as an indication in searching for a drug for preventing, ameliorating or treating IBD. Also, anti-CD81 antibody is highly efficacious as the active ingredient of a drug for preventing, ameliorating or treating IBD. Further, it is possible to use a polynucleotide having at least 15 consecutive bases in the base sequence of CD81 gene and / or a polynucleotide complementary to the above polynucleotide as a disease marker for IBD.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Human monoclonal antibody
InactiveUS8440797B2Suppressed T cell migrationGood effectNervous disorderBacteriaMonoclonal antibodyCD81
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Methods of selecting retinal pigmented epithelial cells
ActiveUS20150010922A1Cell dissociation methodsBiological material analysisFluorescenceMixed Cellular Population
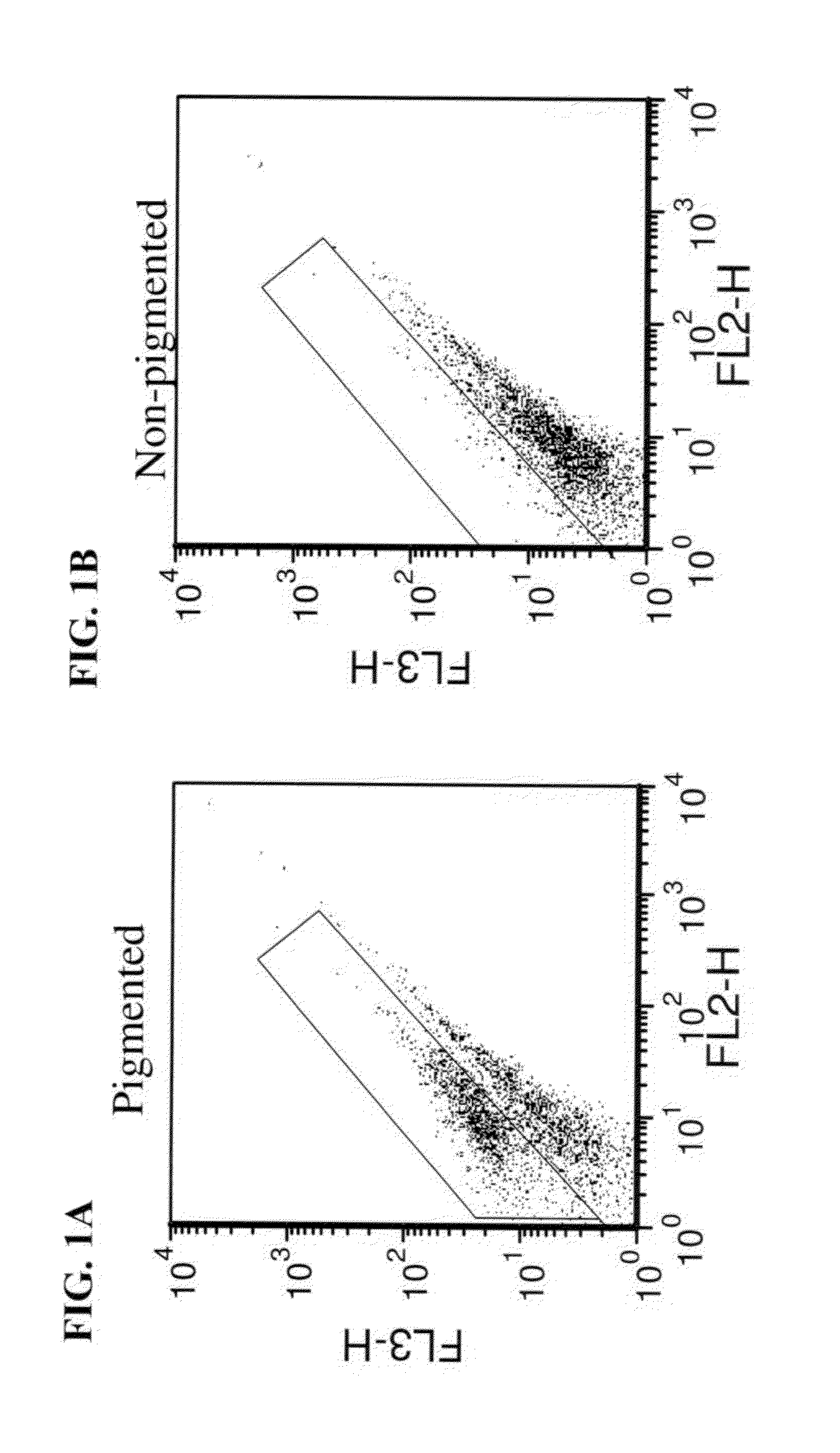
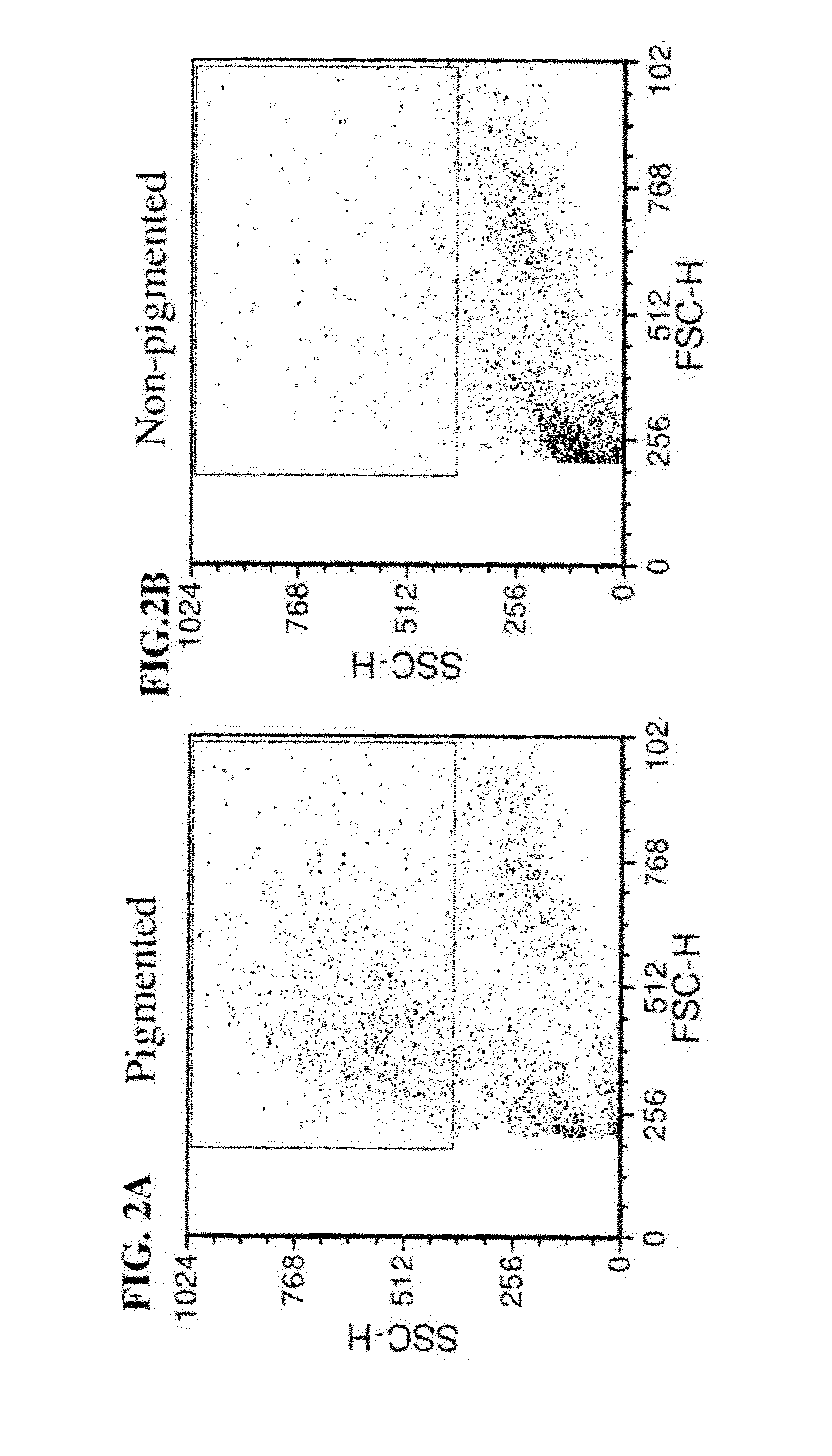
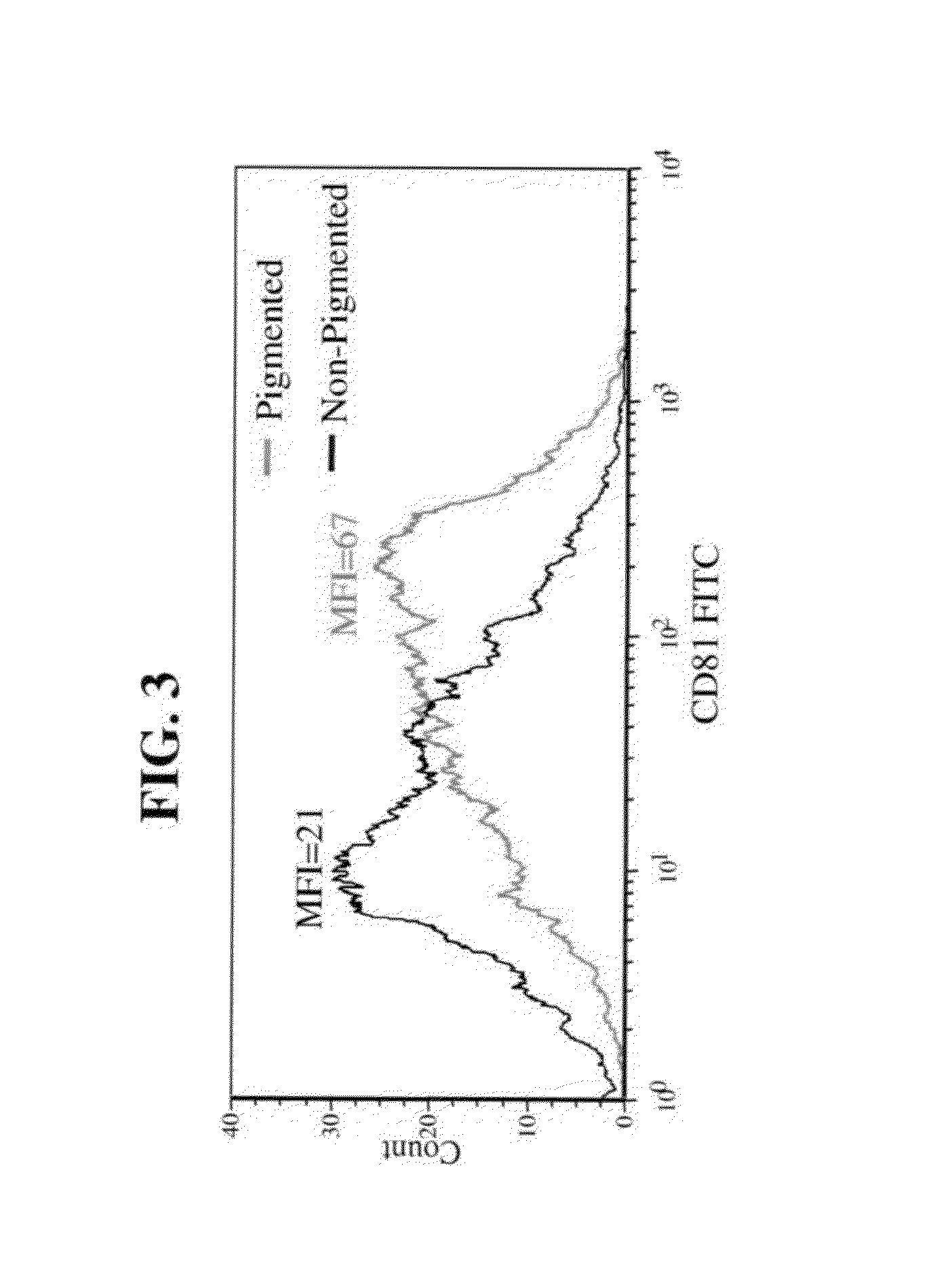
A method of selecting retinal pigmented epithelial (RPE) cells from a mixed population of cells is disclosed. The method comprises:(a) analyzing the cells of the mixed population of cells for at least one of the following parameters:(i) cells which autofluorescence above a predetermined threshold;(ii) cells which express CD81 above a predetermined threshold; and(iii) cells which scatter light perpendicular to a laser beam above a predetermined threshold; and(b) selecting cells which are positive for at least one of the parameters, thereby sorting RPE cells from a mixed population of cells.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
Efficient cell culture system for hepatitis c virus genotype 5a
InactiveUS20110021611A1Organic active ingredientsSsRNA viruses positive-senseSequence analysisSerial passage
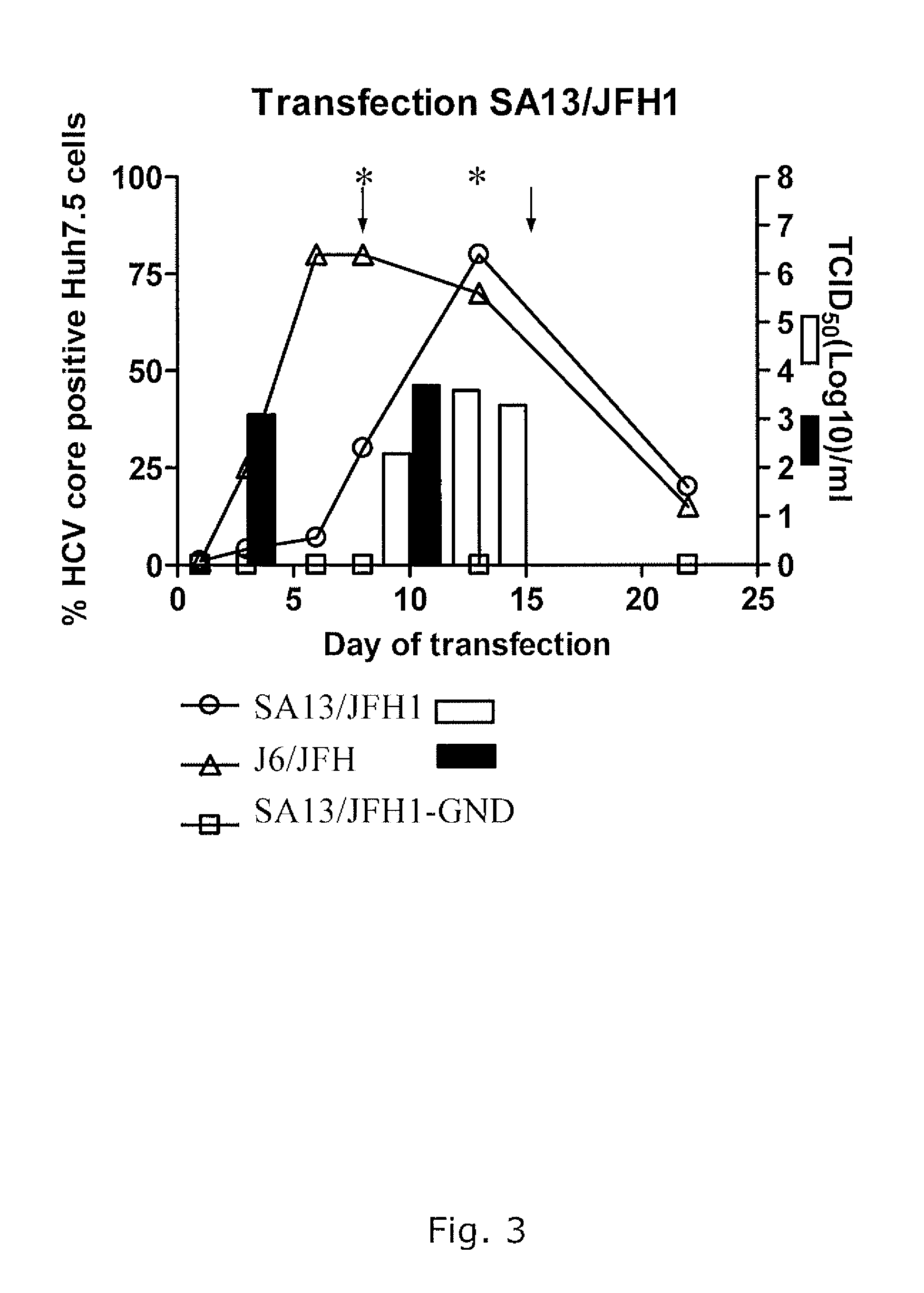
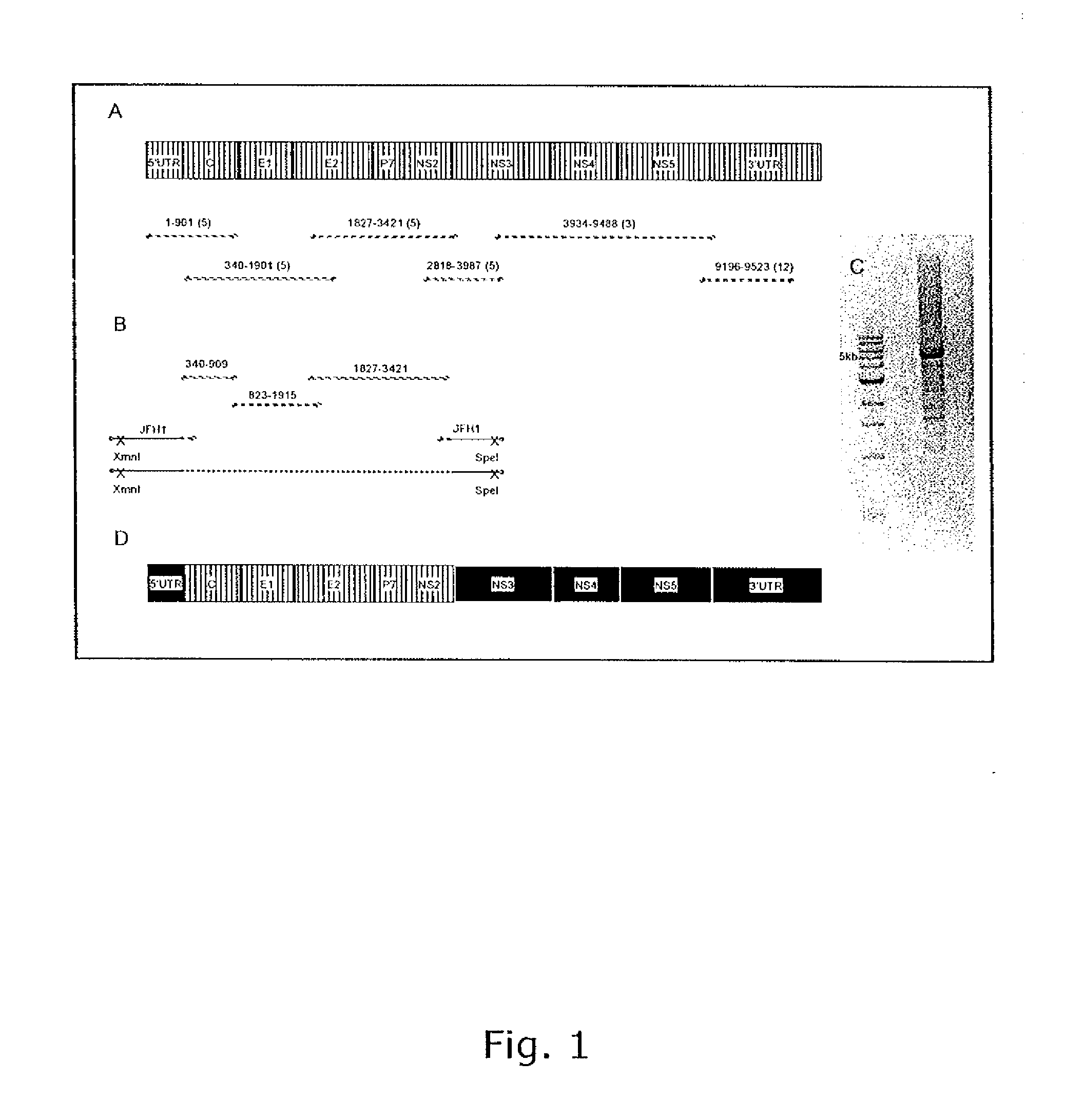
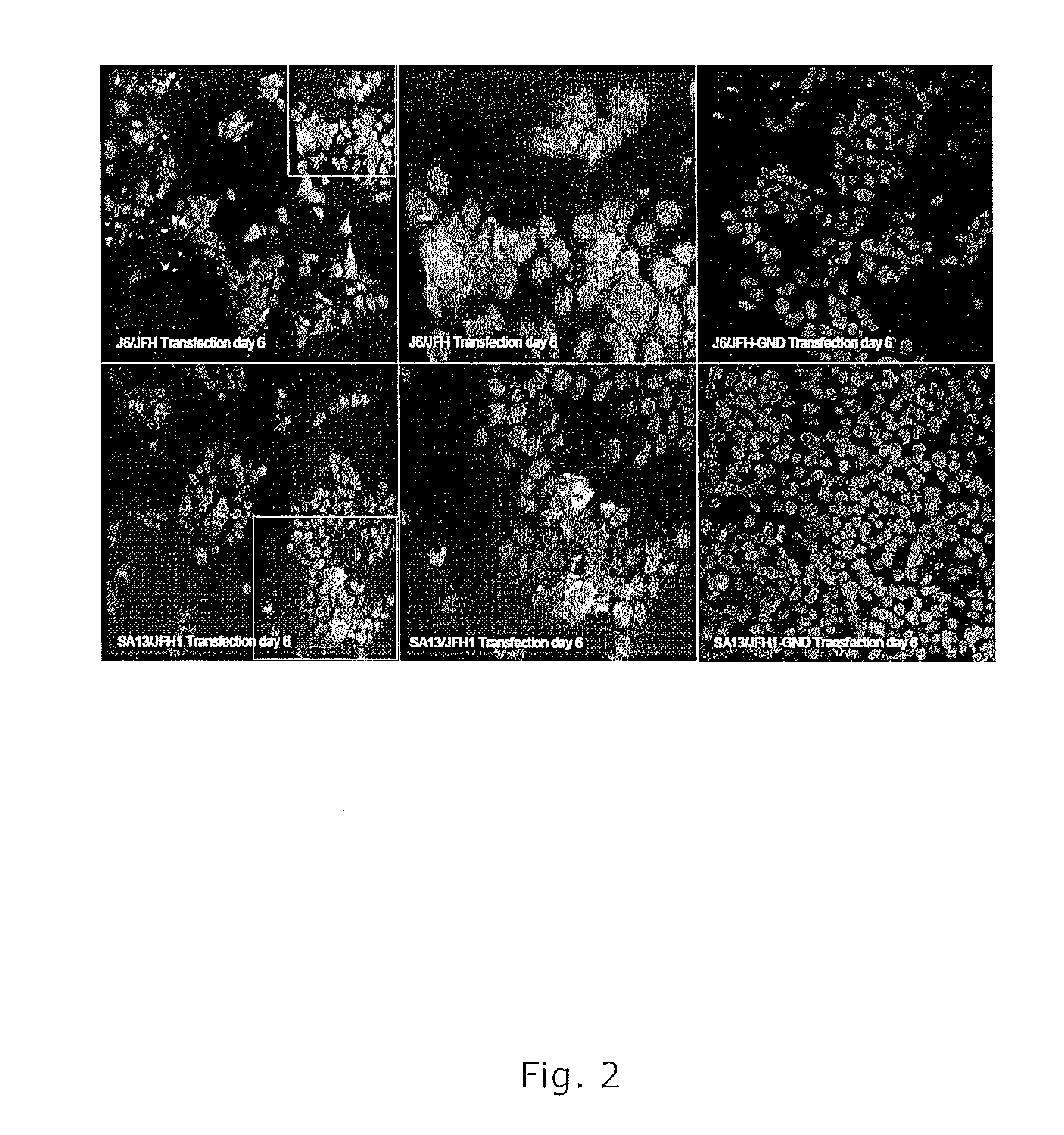
The present inventors developed 5a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and all of or part of NS2 were replaced by the corresponding genes of the genotype 5a reference strain SA13. Compared to the J6 / JFH control virus, after transfection of in vitro transcripts in Huh7.5 cells, production of infectious viruses was delayed. However, in subsequent viral passages efficient spread of infection and HCV RNA titers as high as for J6 / JFH were obtained. Infectivity titers were at all time points analyzed comparable to J6 / JFH control virus. Sequence analysis of recovered 5a / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in p7, NS2 and / or NS3. Infectivity of the 5a / 2a viruses was CD81 and SR-BI dependant, and the recombinant viruses could be neutralized by chronic phase sera from patients infected with genotype 5a. Conclusion: The developed 5a / 2a viruses provide a robust in vitro tool for research in HCV genotype 5, including vaccine studies and functional analyses of an increasingly important genotype in South Africa and Europe.
Owner:HVIDOVRE HOSPITAL
Exosome detection and typing micro-fluidic chip and exosome detection and typing method
ActiveCN111796104AAppropriate amountEffectively fixedLaboratory glasswaresBiological testingAntiendomysial antibodiesCD63
The invention relates to an exosome detection and typing micro-fluidic chip and an exosome detection and typing method. The micro-fluidic chip comprises an antibody strip chip and a sample injection chip, CD63 and CD81 antibodies are coupled to the antibody strip chip, and the coupling method comprises the following steps of: firstly, preparing a glass slide with a zinc oxide nano array, then carrying out MPS modification, GMBS modification and G protein modification, and finally connecting the CD63 and CD81 antibodies. A fishbone-shaped microstructure is arranged above a main channel of the sample injection chip, and the height of the channel is 20 microns. The exosome detection and typing method is completed by adopting the micro-fluidic chip, and the sample injection speed is 1.8-2.2 [mu] L / min. The micro-fluidic chip provided by the invention can capture related exosomes to the greatest extent, and is very high in sensitivity.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Human monoclonal antibody
The present invention provides an anti-CD81 antibody usable as a pharmaceutical product for human. Specifically, the present invention provides an anti-human CD81 antibody capable of binding to a peptide region consisting of the amino acid sequence of the amino acid numbers 80 to 175 in the amino acid sequence shown in SEQ ID NO:22.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
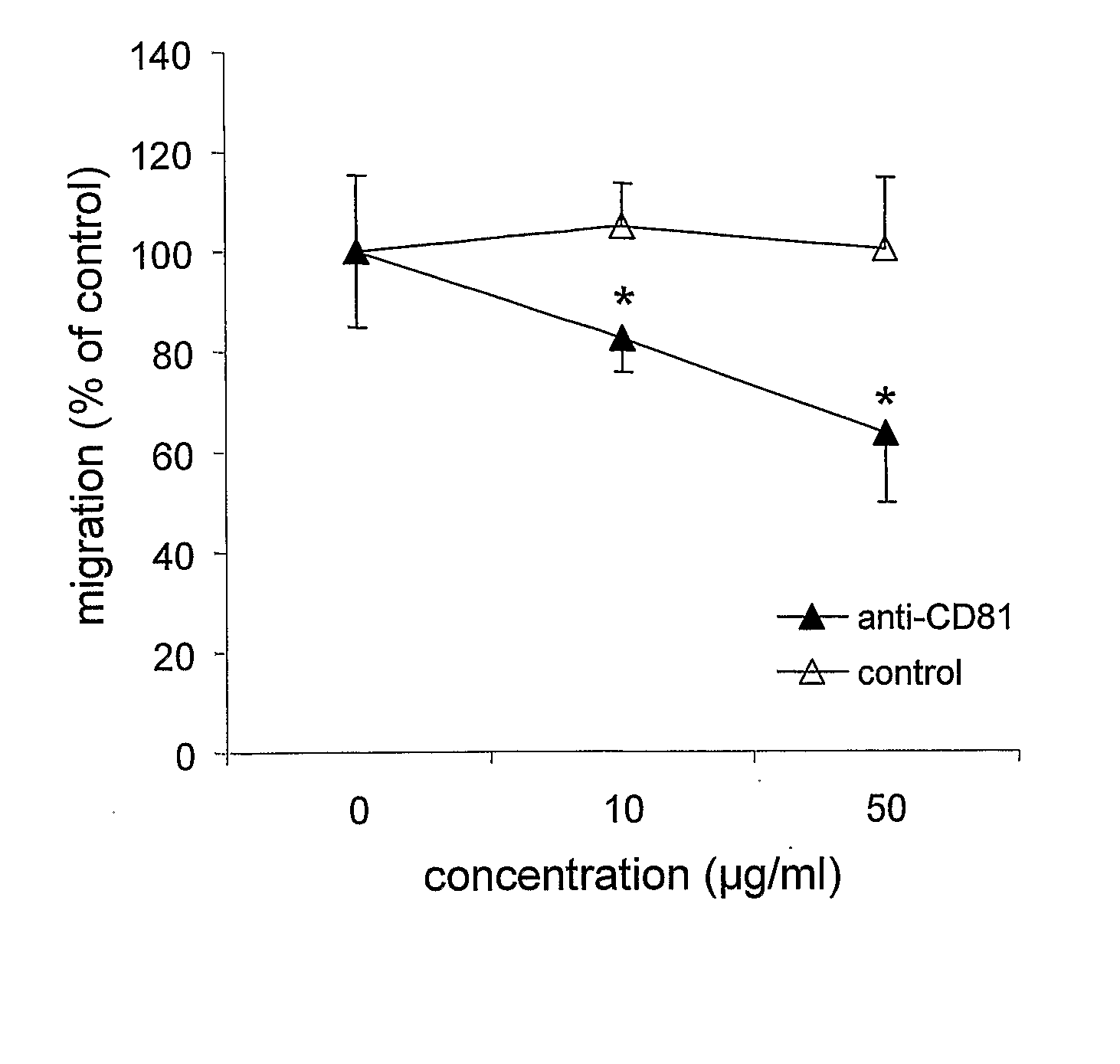
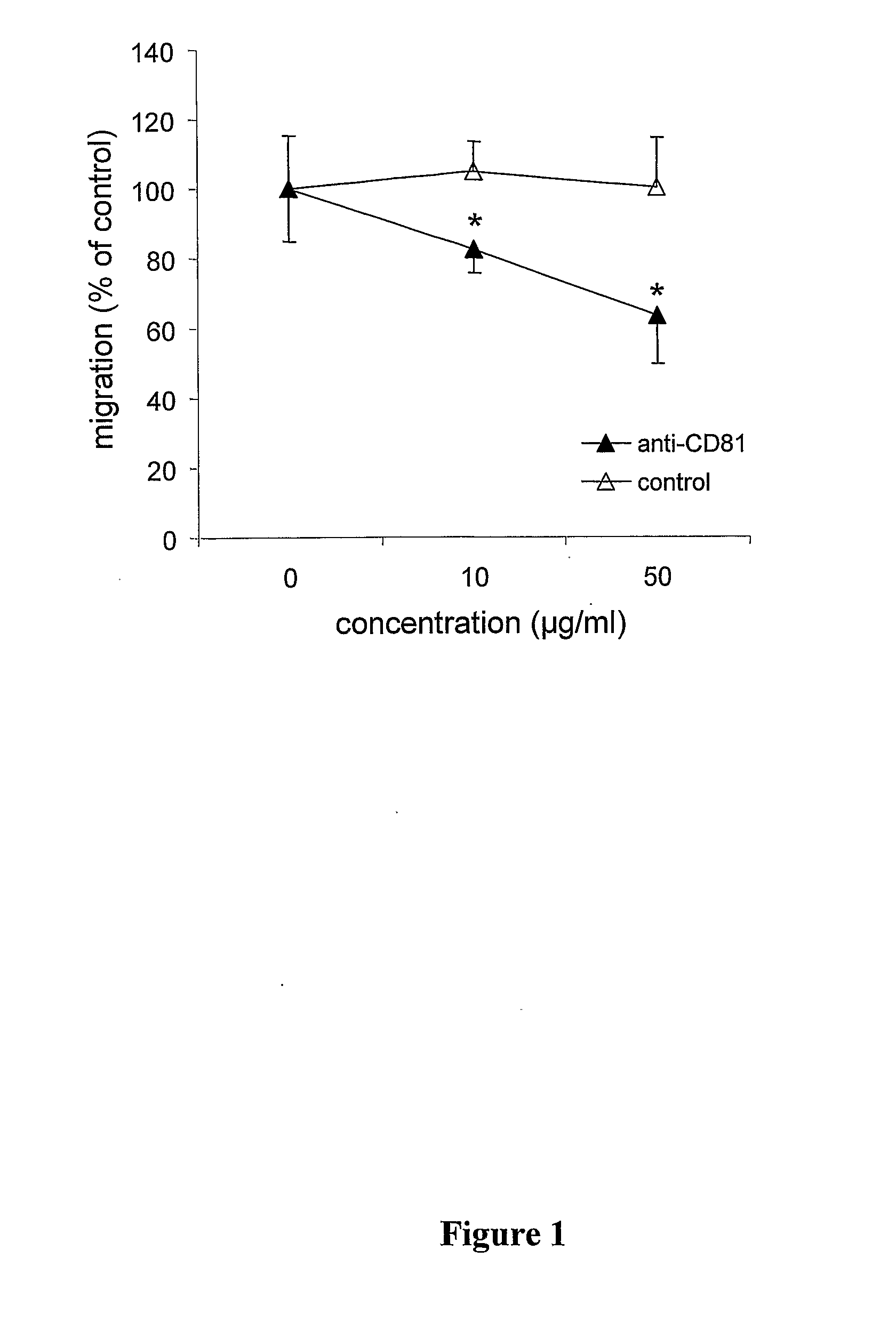
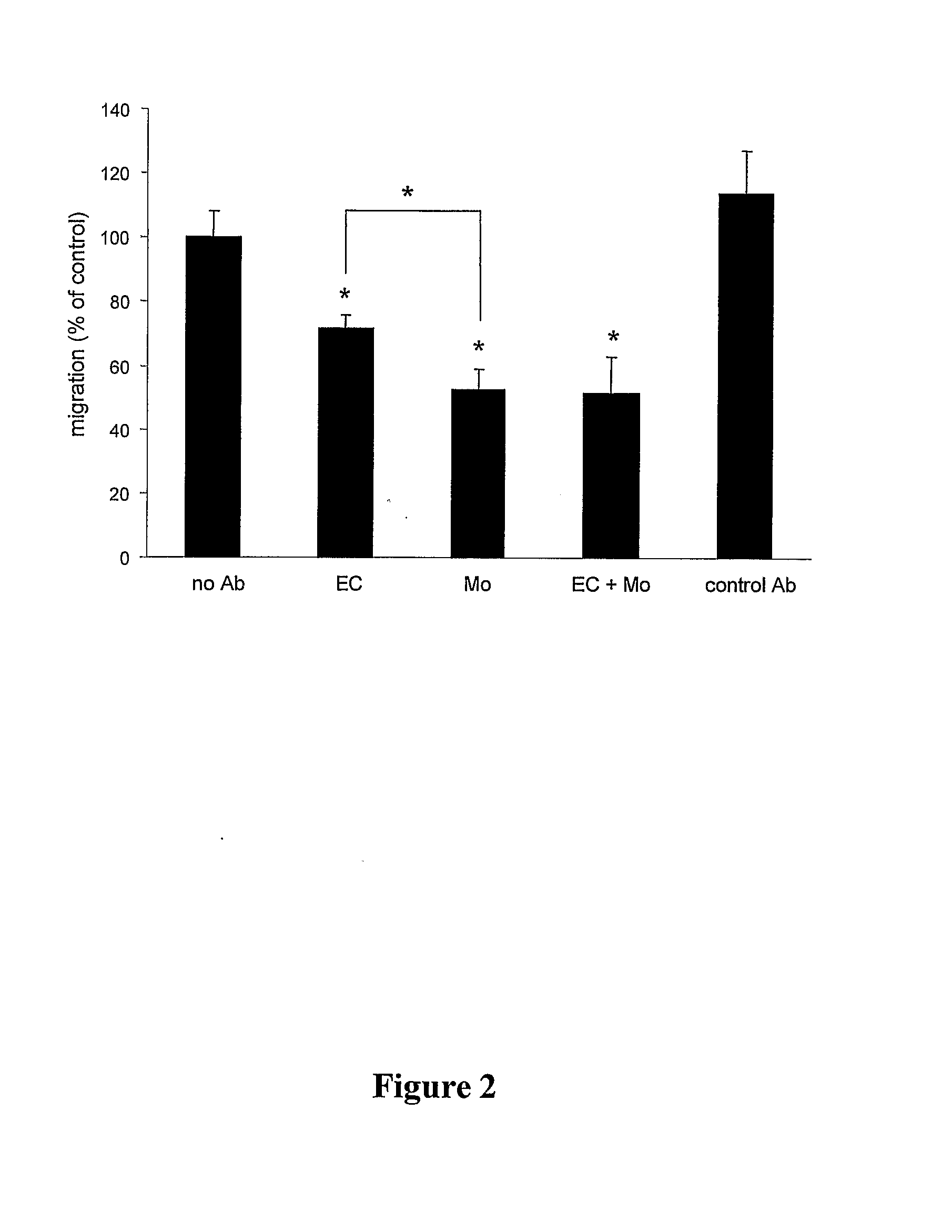
Method For Inhibiting The Transendothelial Migration Of Cells Such As Leukocytes Or Tumor Cells By A Cd-Binding Substance And Uses Thereof
InactiveUS20080199482A1Inhibition is effectiveInhibits transmigrationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAbnormal tissue growthLymphatic Spread
The invention provides a method for inhibiting the transmigration of cells, such as leukocytes or tumor cells, across endothelial cells, by contacting said cells with a CD81 binding agent. Furthermore, the invention provides a method for retarding or inhibiting tissue damage in a subject suffering from an inflammatory disorder, or tumor metastasis, comprising administering to said subject a pharmaceutical composition containing a CD81 binding agent capable of inhibiting transmigration of cells, such as leukocytes and tumor cells.
Owner:NEDERLANDSE ORG VOOR TOEGEPAST-NATUURWETENSCHAPPELIJK ONDERZOEK (TNO)
Adaptive mutations allow establishment of JFH1-based cell culture systems for hepatitis C virus genotype 4A
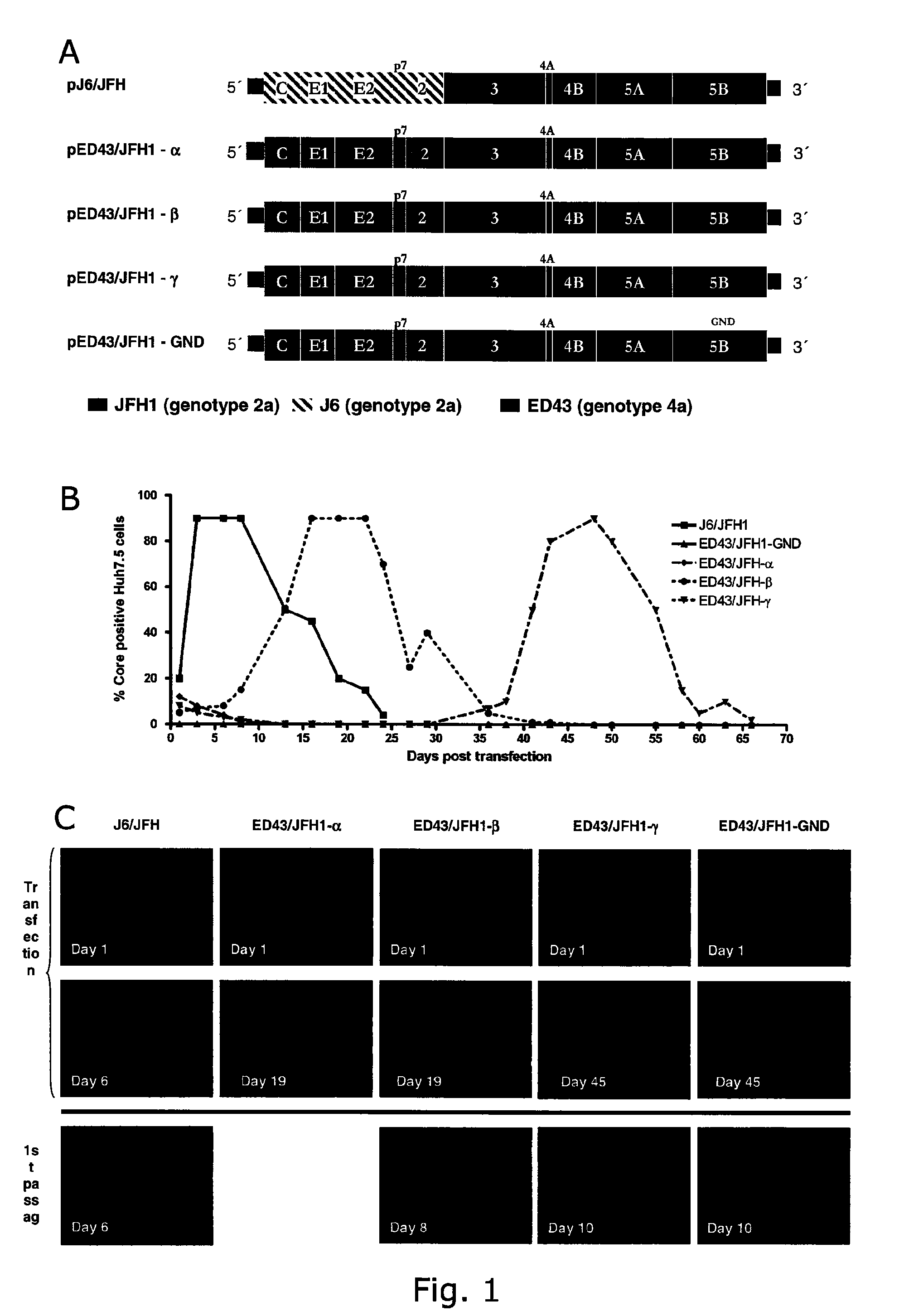
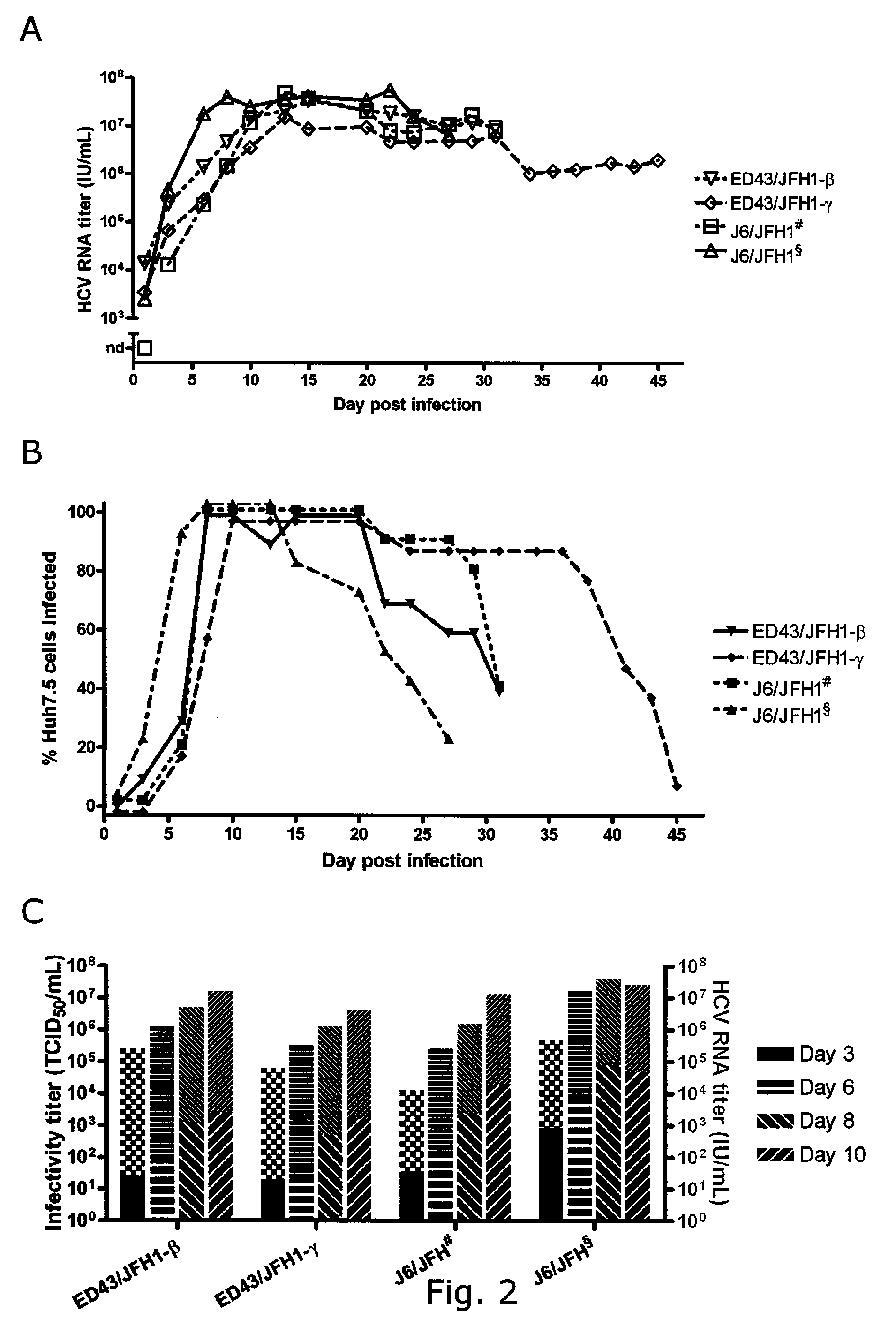
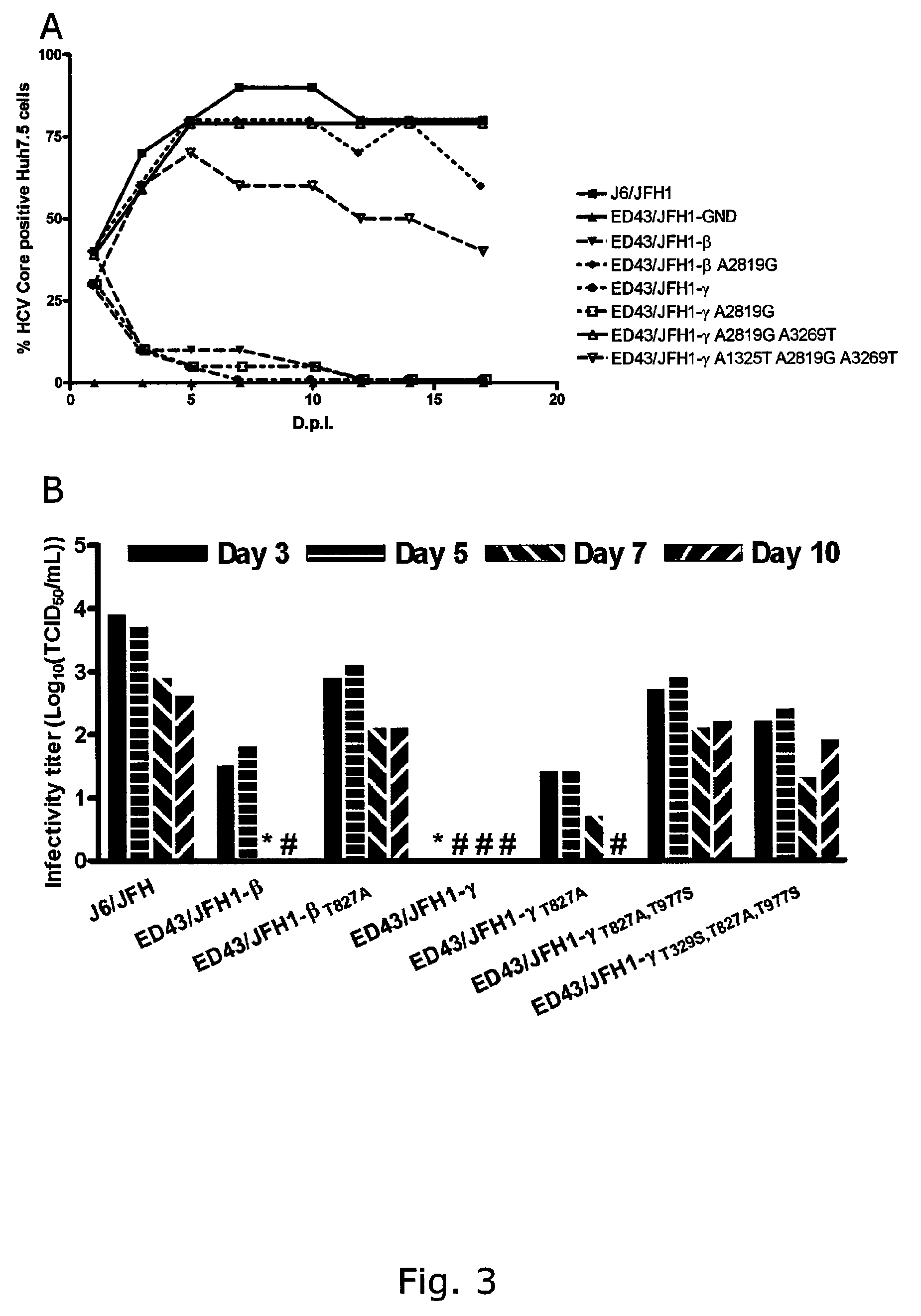
The present inventors developed three 4a / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and all of or part of NS2 were replaced by the corresponding genes of the genotype 4a reference strain ED43. The 4a / 2a junction in NS2 was placed after the first transmembrane domain (α), in the cytoplasmic part (β) or at the NS2 / NS3 cleavage site (y). Following transfection of Huh7.5 cells with RNA transcripts, infectious viruses were produced in the ED43 / JFH1-β and -y cultures only. Compared to the 2a control virus, production of infectious viruses was significantly delayed. However, in subsequent passages efficient spread of infection and high HCV RNA titers were obtained. Infectivity titers were approximately 10-fold lower than for the 2a control virus. Sequence analysis of recovered 4a / 2a recombinants from 3 serial passages and subsequent reverse genetic studies revealed a vital dependence on a mutation in the NS2 4a part. ED43 / JFH1-γ further depended on a second NS2 mutation. Infectivity of the 4a / 2a viruses was CD81 dependent. Conclusion: The developed 4a / 2a viruses provide a robust in vitro tool for research in HCV genotype 4, including vaccine studies and functional analyses of an increasingly important genotype in the Middle East and Europe.
Owner:HVIDOVRE HOSPITAL
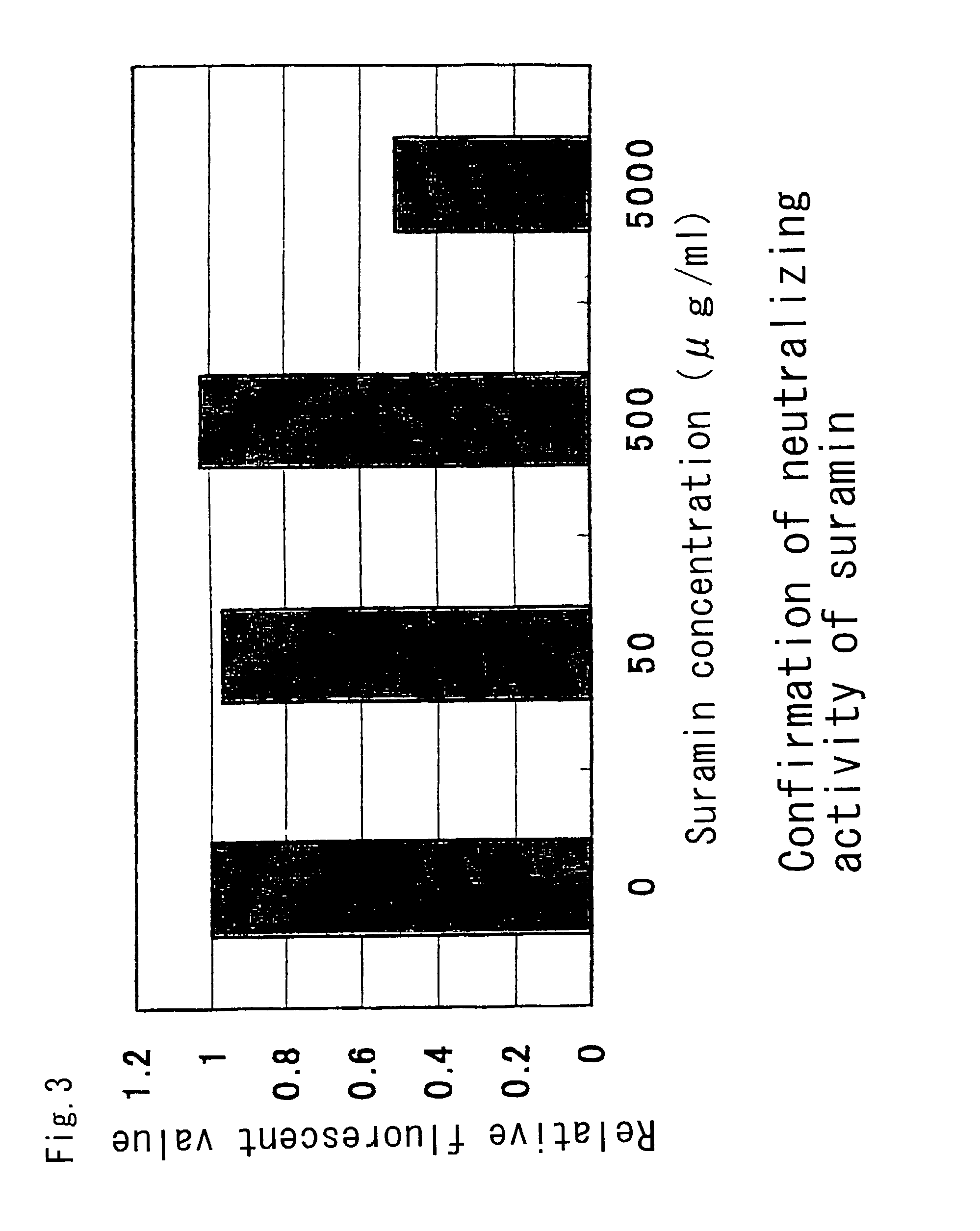
Remedies for hepatitis C
InactiveUS7101551B2Avoid infectionInhibit bindingOrganic active ingredientsPeptide/protein ingredientsCD81Inhibitory effect
The present invention provides a substance which inhibits the binding between E2 / NS1 protein of hepatitis C virus and a cell infectious with hepatitis C virus, a cell expressing CD81, or CD81. The present invention can provide a novel medicament which has an anti-viral effects such as an inhibitory action against HCV infection.
Owner:MITSUBISHI TANABE PHARMA CORP +1
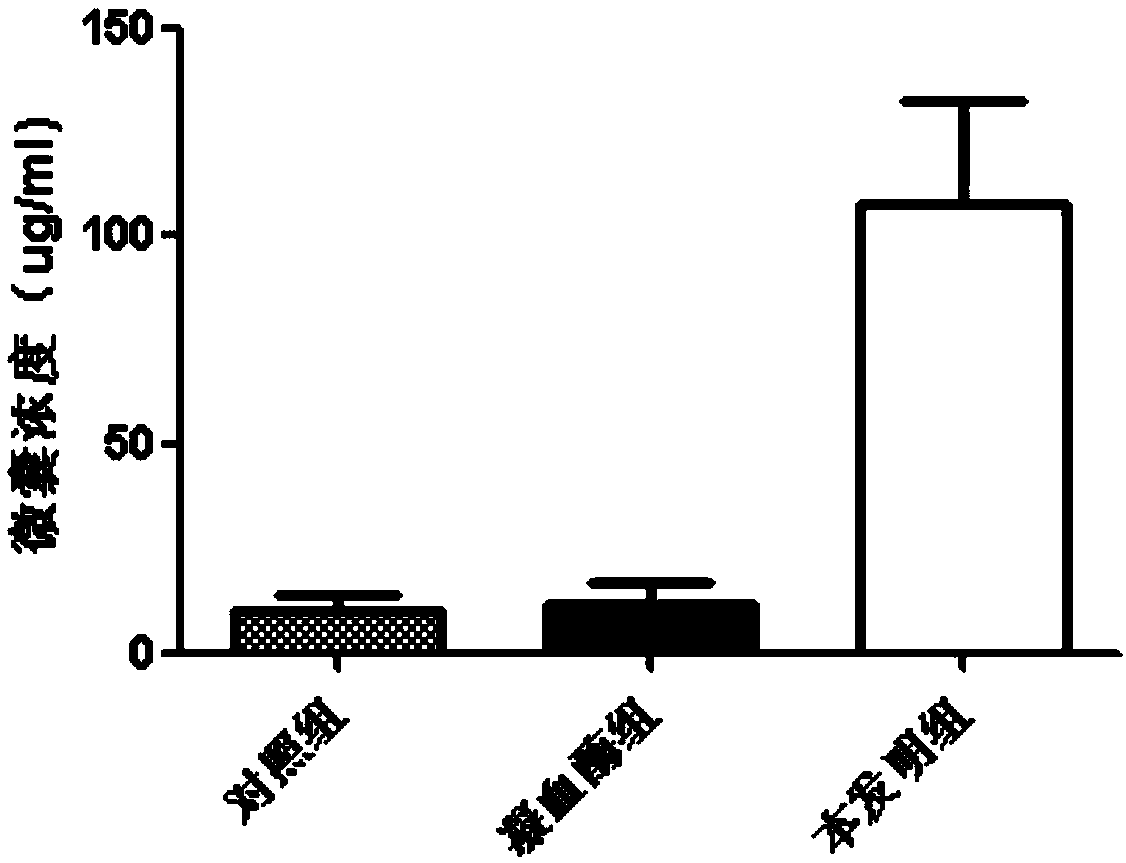
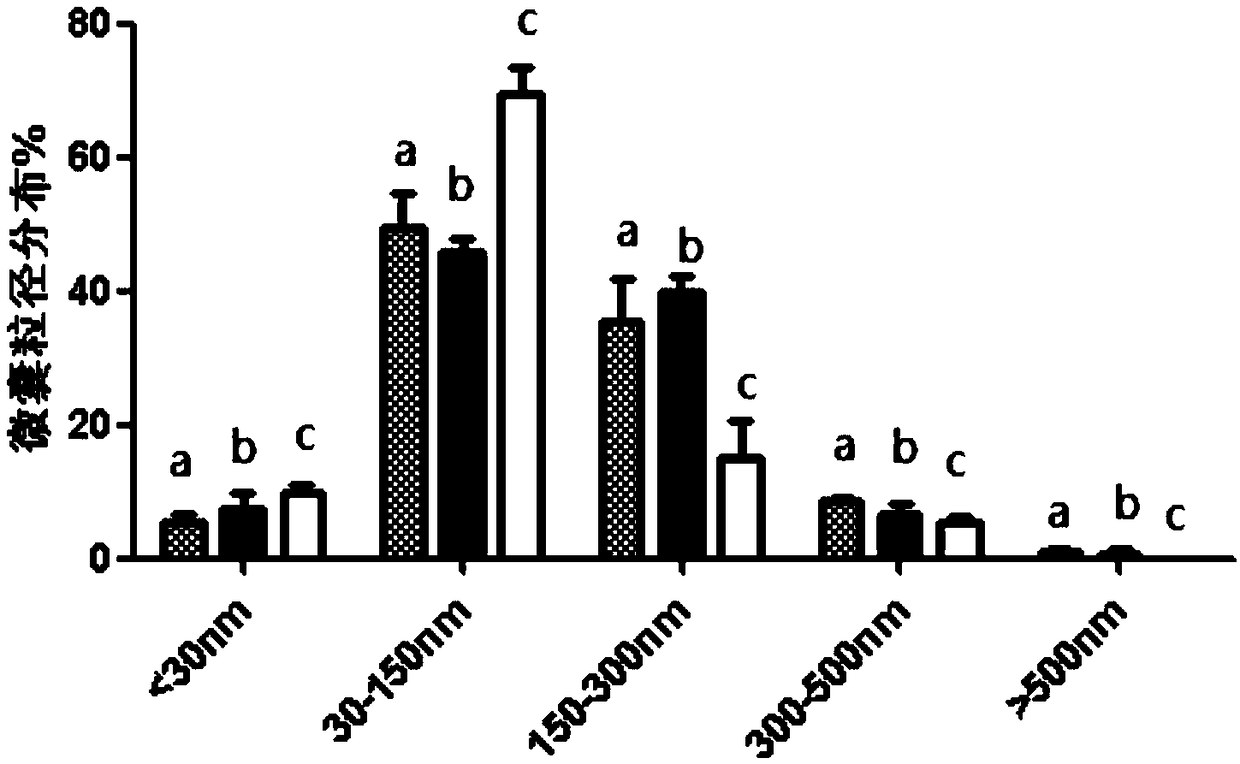
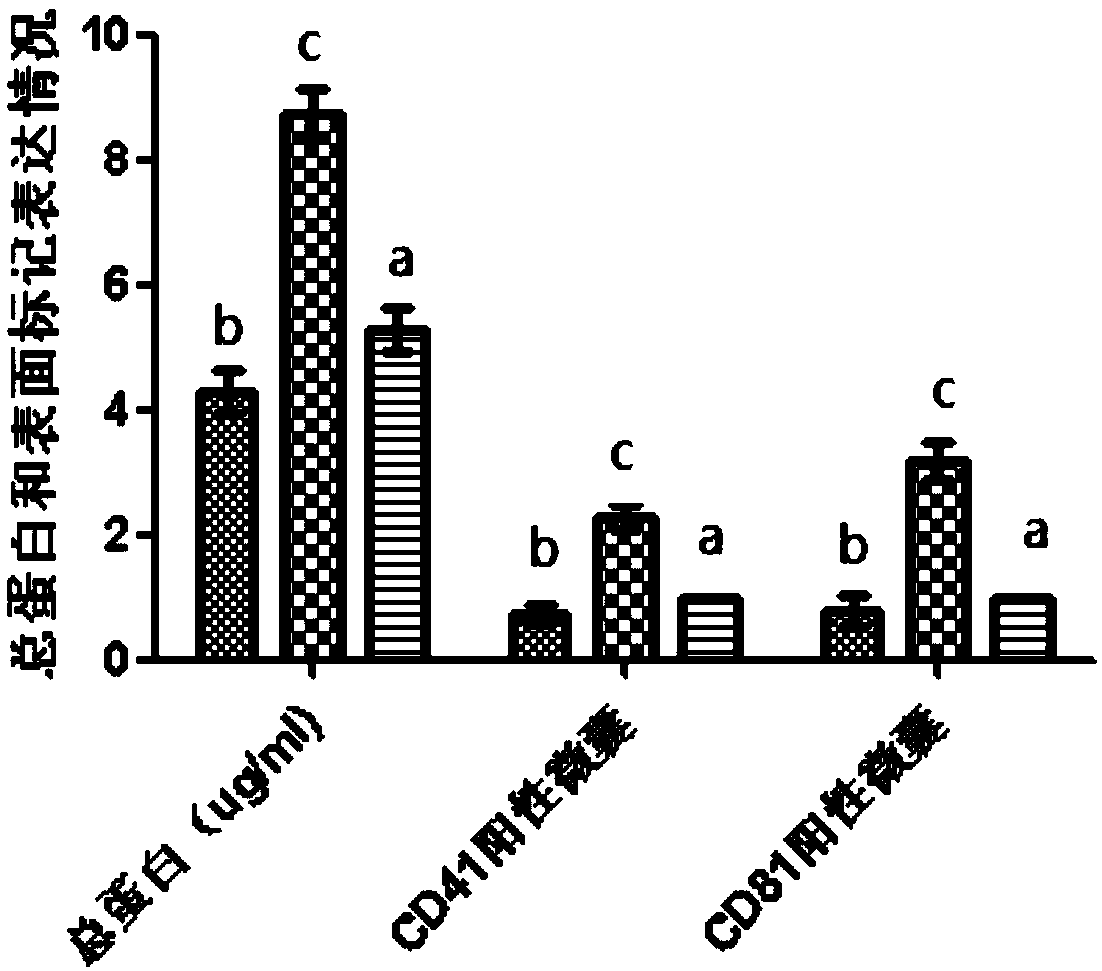
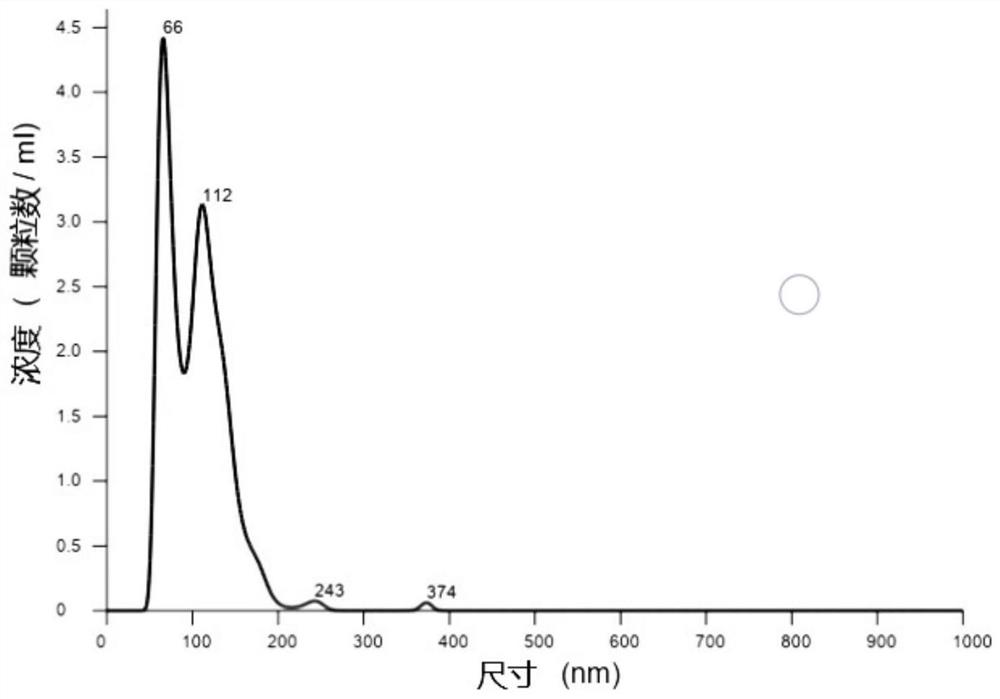
Preparation method of platelet lysate rich in CD41<+> and CD81<+> micro-capsules
ActiveCN108715834AStrong regeneration and repair functionSimple methodPeptide/protein ingredientsMammal material medical ingredientsTotal proteinCytokine
The invention relates to a preparation method of platelet lysate rich in CD41<+> and CD81<+> micro-capsules, and aims to improve the CD41<+> and CD81<+> micro-capsules in the platelet lysate. By meansof impact wave grading impact, the micro-capsules for forming and releasing cell factors and CD41<+> and CD81<+> in platelets are increased. The concentration of the micro-capsules in the platelet lysate is 120 microgram / mL, total protein of the micro-capsules is 7-9 microgram / mL, the particle size of 69.33+ / -2.33% of the micro-capsules is centralized within the range of 30-150nm, the particle size of 15.10+ / -3.20% of the micro-capsules is centralized within the range of 150-300nm, a large number of platelet cell factors can be released, and the preparation method is applied to the technicalfield of biology.
Owner:天晴干细胞股份有限公司
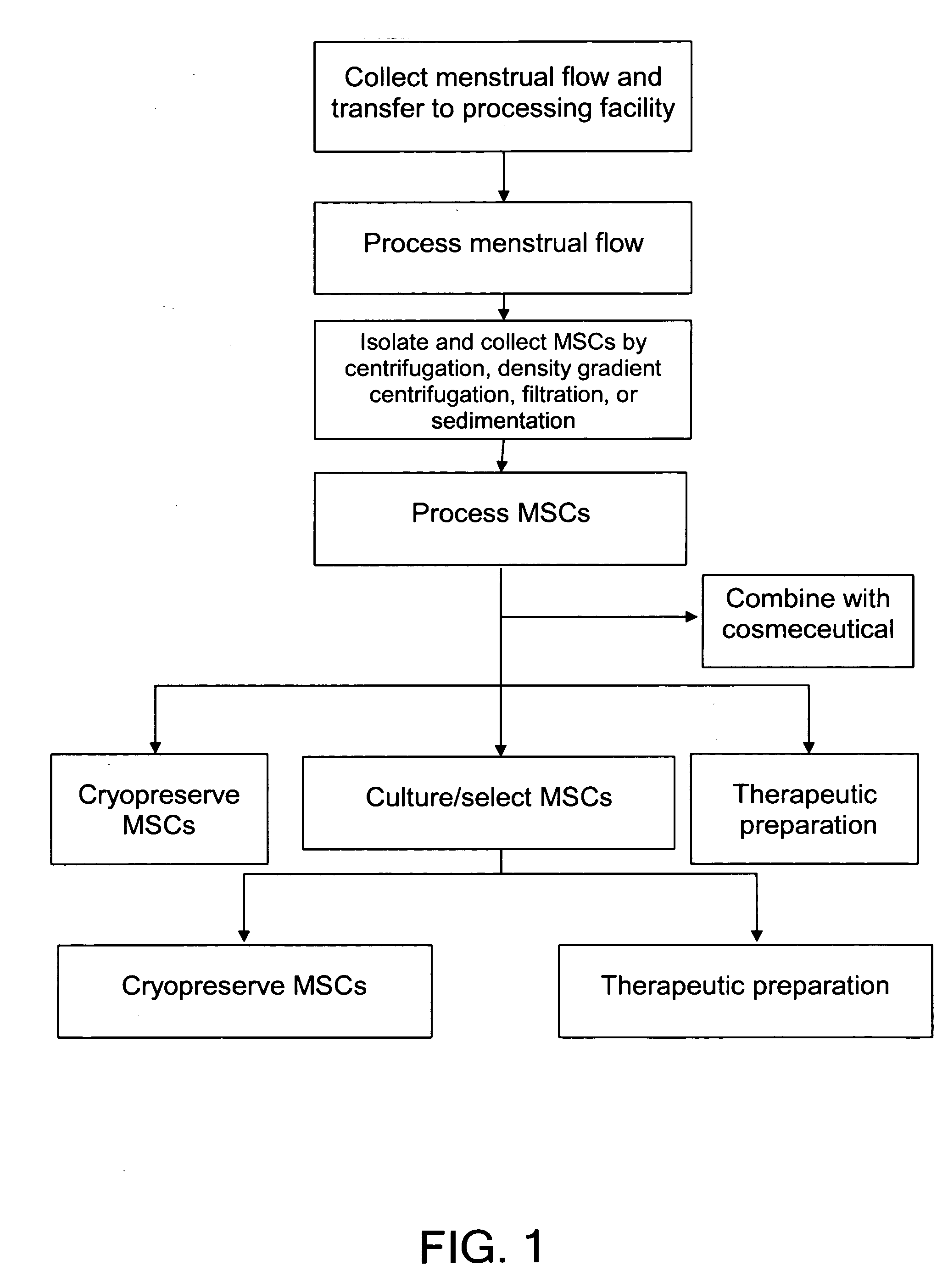
Procurement, Isolation, and Cryopreservation of Endometrial/Menstrual Cells
Compositions comprising menstrual stem cells (MSCs) and methods, processes, and system therefor are provided by the invention. MSCs are processed from menstrual flow collected during menses. MSCs may be cryopreserved, processed through various culturing and selection steps in preparation for cryopreservation, or processed for therapeutic or cosmeceutical use. Cryopreserved MSCs may be thawed in preparation for therapeutic and cosmeceutical use. MSCs express CD9, CD10, CD13, CD29, CD44, CD49e, CD49f, CD59, CD81, CD105, CD166, and HLA class I, and have low or no expression of CD3 and HLA class II.
Owner:CRYO CELL INTERNATIONAL INC
Scalable production of standardized extracellular vesicles, extracellular vesicle preparations and uses thereof
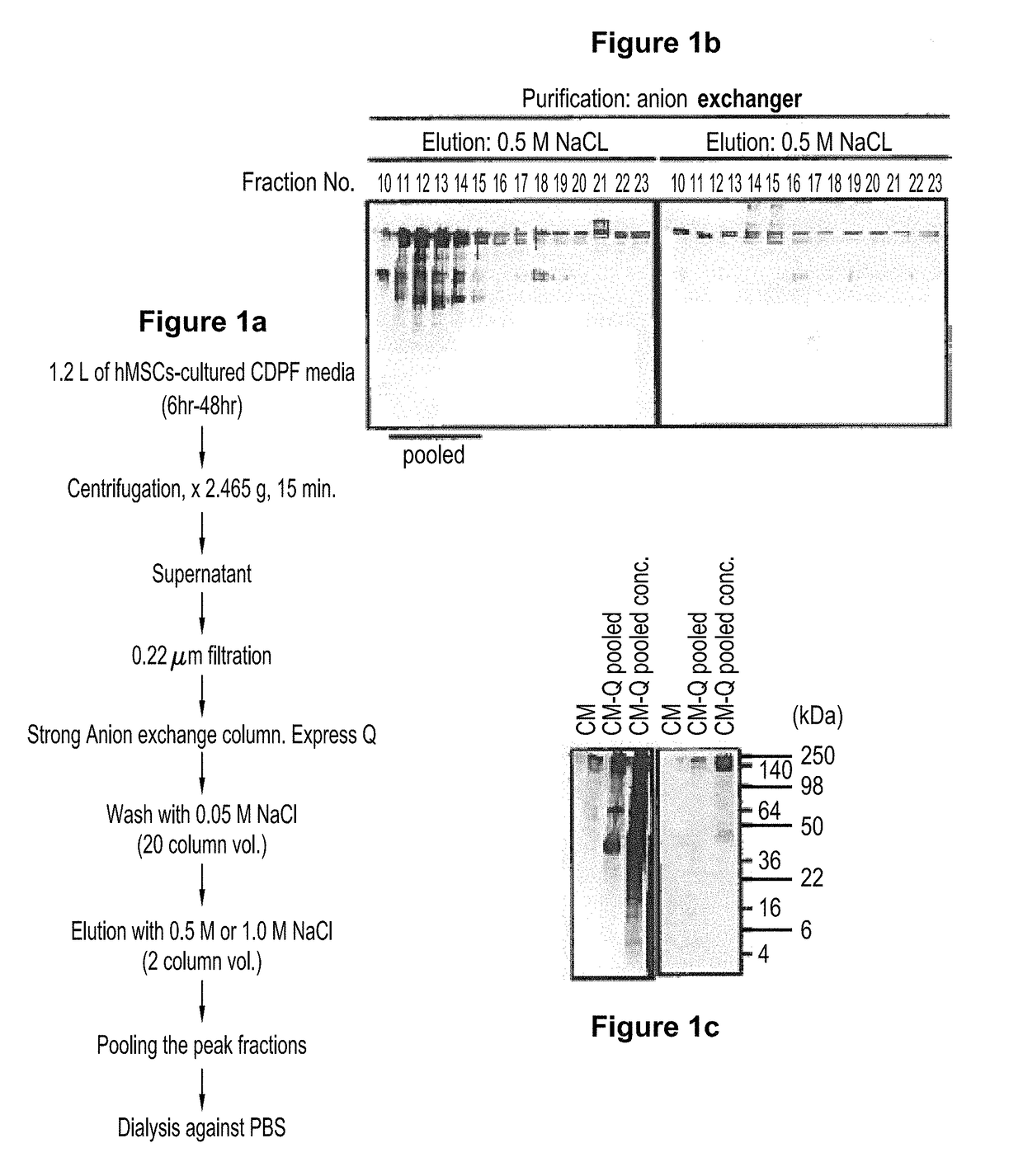
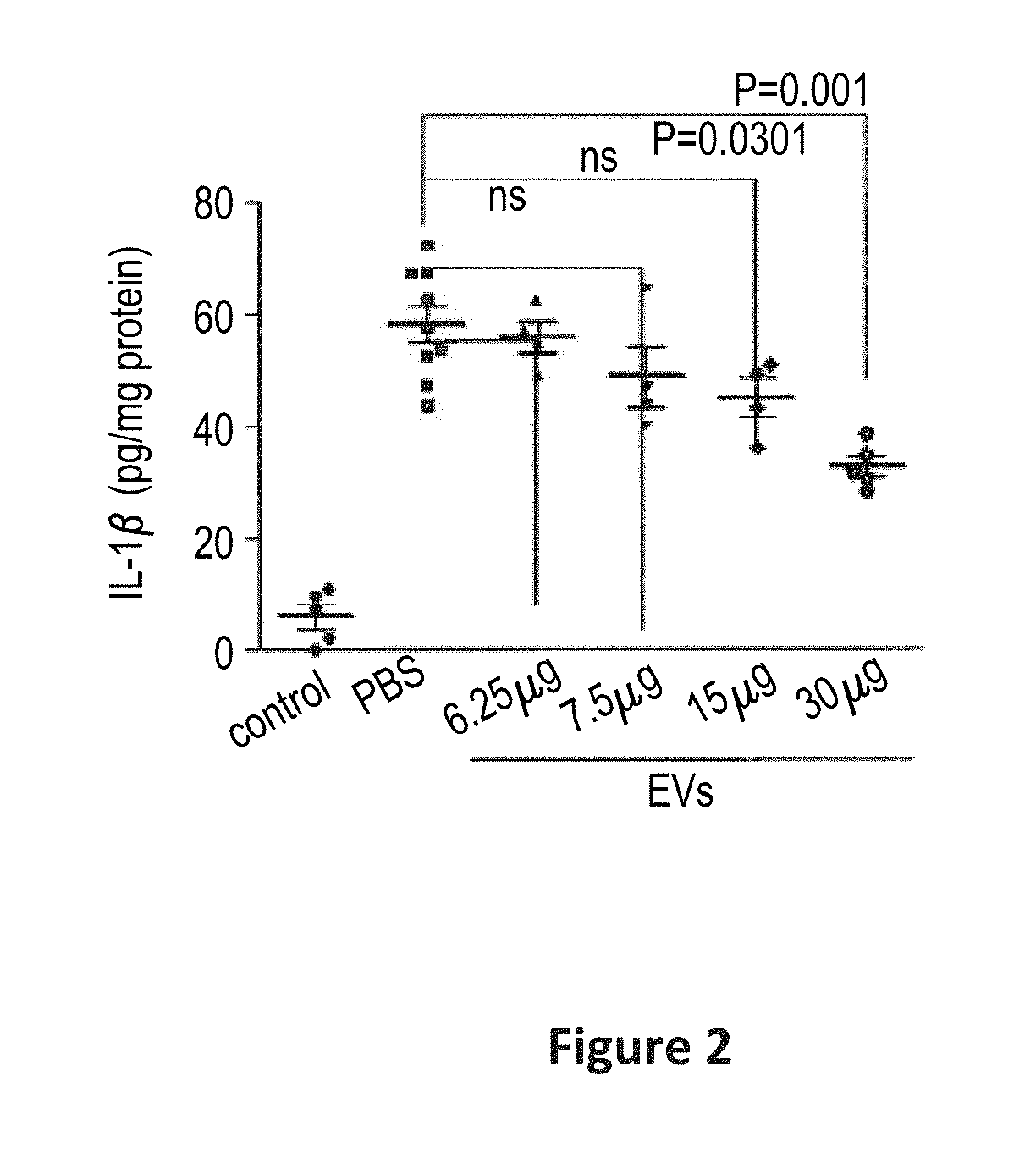
Preparations comprising an enriched population of extracellular vesicles (nEVs) having a negatively charged surface, and that are CD81+ and CD9−, are provided. Improved processes and methods for producing an enriched population of nEVs from non-murine cells, especially human origin cells and / or tissues, are disclosed. Therapeutic methods for using the preparations, including for reducing brain inflammation and treatment of various pathologies associated with brain inflammation, such as by intravenous or intranasal administration, are also described. Methods and preparations for reducing brain inflammation associated with traumatic brain injury (TBI) are also disclosed. A method for treating a patient having suffered a mild traumatic injury (mTMI), or concussion, such as a sports-related head injury, is also disclosed. The nEVs are also demonstrated to reduce the expression level of IL-Iβ in brain tissue of an animal having had traumatic brain injury. Methods for improving cognitive function and performance in animals after a traumatic brain injury is also demonstrated using the preparations of nEVs disclosed herein.
Owner:TEXAS A&M UNIVERSITY
ELISA (Enzyme-Linked Immunosorbent Assay) detection method of avian exosome
PendingCN114487386AHighly specificBiological material analysisBiological testingBiotechnologyPoultry disease
The invention belongs to the technical field of poultry disease detection, and particularly relates to a patent application of an ELISA (enzyme-linked immuno sorbent assay) detection method for poultry-derived exosomes. The ELISA detection method of the poultry-derived exosome comprises the steps of sample preparation, coating, sealing, sample adding, detection, result judgment and the like. In the prior art, human-derived exosome surface marker proteins CD9 and CD81 are taken as targets in an exosome-based ELISA detection method, but the marker proteins are not suitable for poultry, namely, the marker proteins are not suitable for detecting poultry-derived exosomes. Therefore, the poultry-derived exosome surface marker protein CD63 is used as a marker, poultry-derived CD63 protein is prepared, corresponding multi-antibody serum is prepared, and on the basis, an ELISA detection method of the poultry-derived exosome is further designed and developed, so that a certain technical foundation can be laid for poultry-related physiological work research.
Owner:JILIN UNIV
Methods of selecting retinal pigmented epithelial cells
ActiveUS9658216B2Cell dissociation methodsBiological material analysisFluorescenceMixed Cellular Population
A method of selecting retinal pigmented epithelial (RPE) cells from a mixed population of cells is disclosed. The method comprises:(a) analyzing the cells of the mixed population of cells for at least one of the following parameters:(i) cells which autofluorescence above a predetermined threshold;(ii) cells which express CD81 above a predetermined threshold; and(iii) cells which scatter light perpendicular to a laser beam above a predetermined threshold; and(b) selecting cells which are positive for at least one of the parameters, thereby sorting RPE cells from a mixed population of cells.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
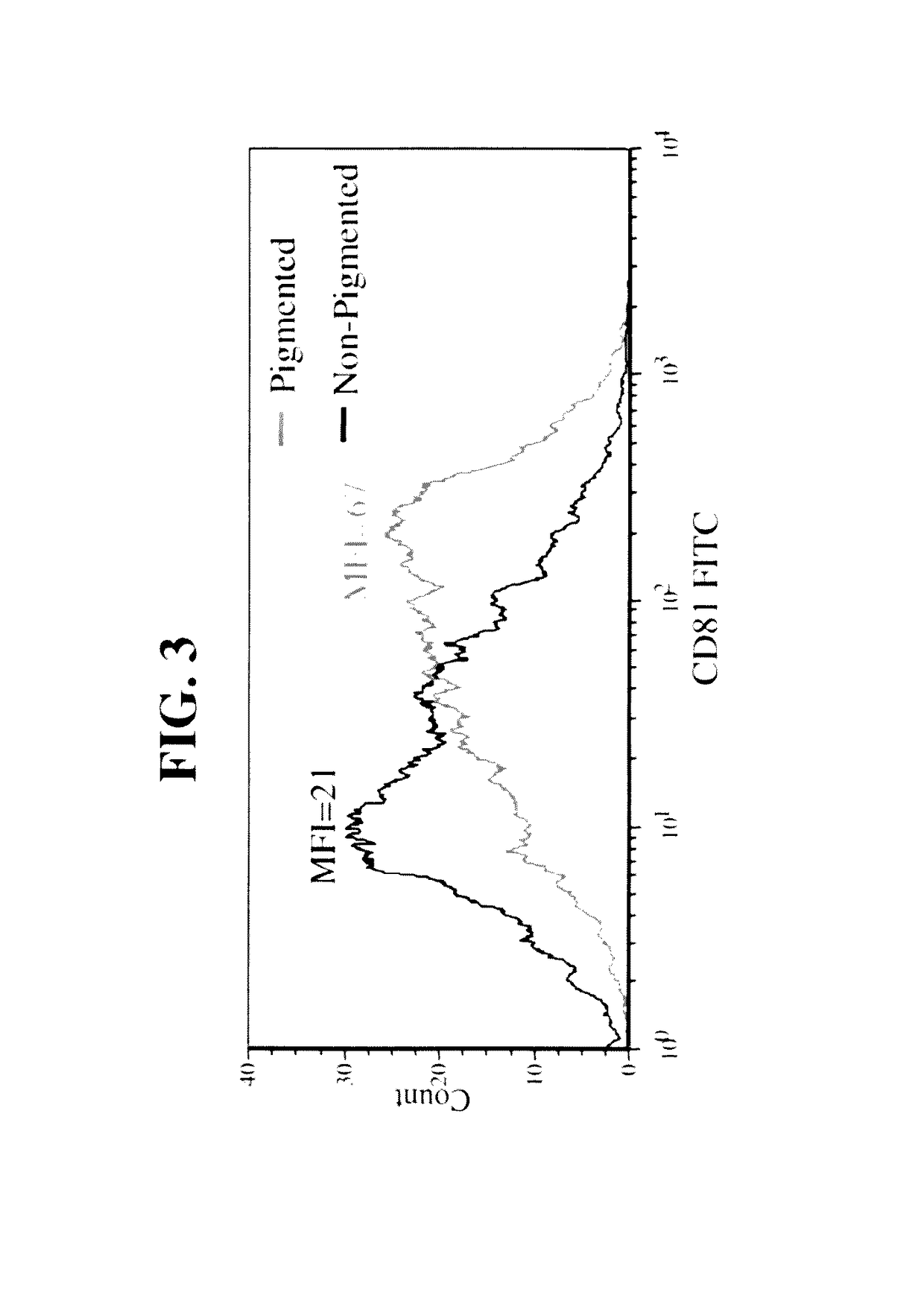
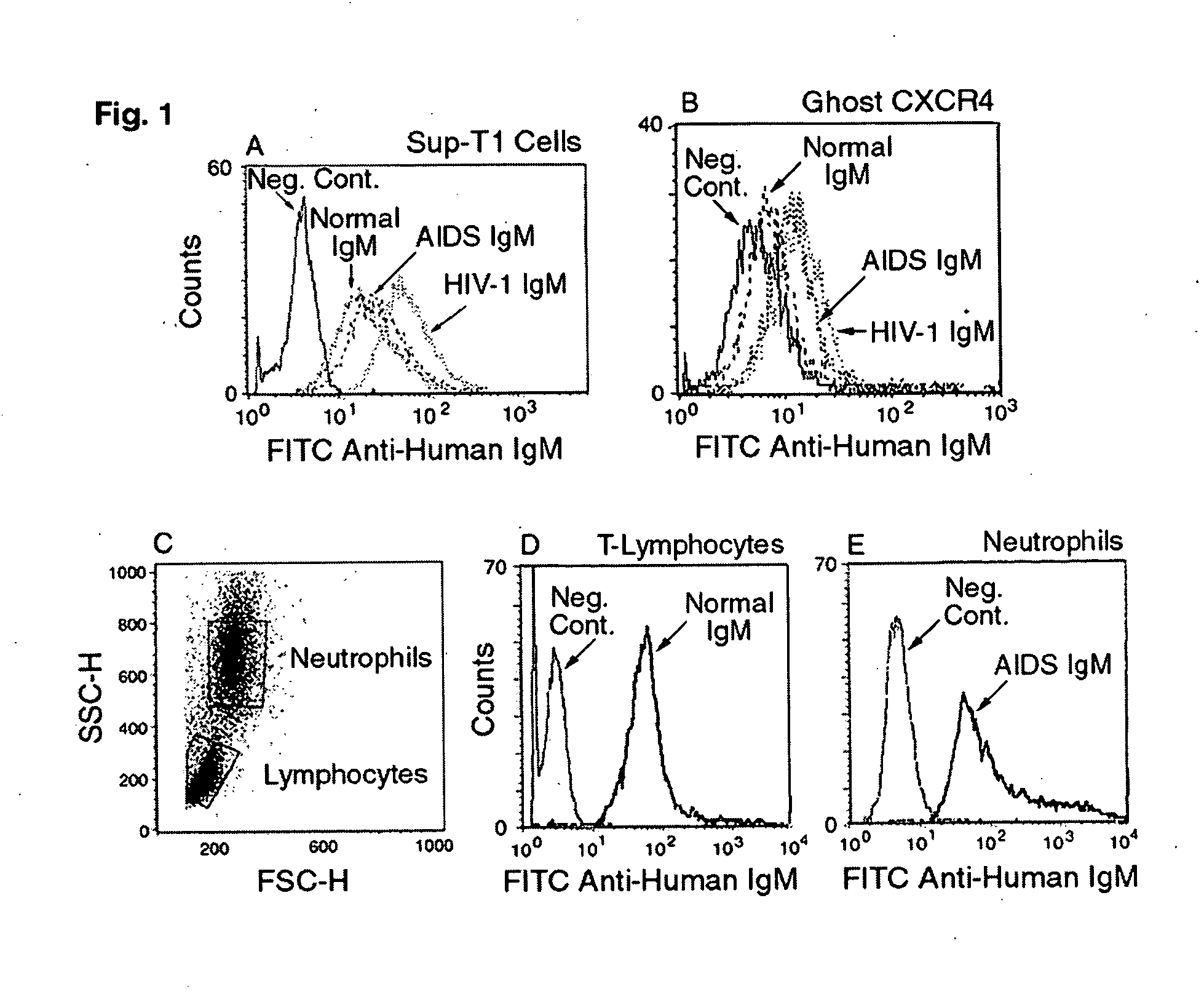
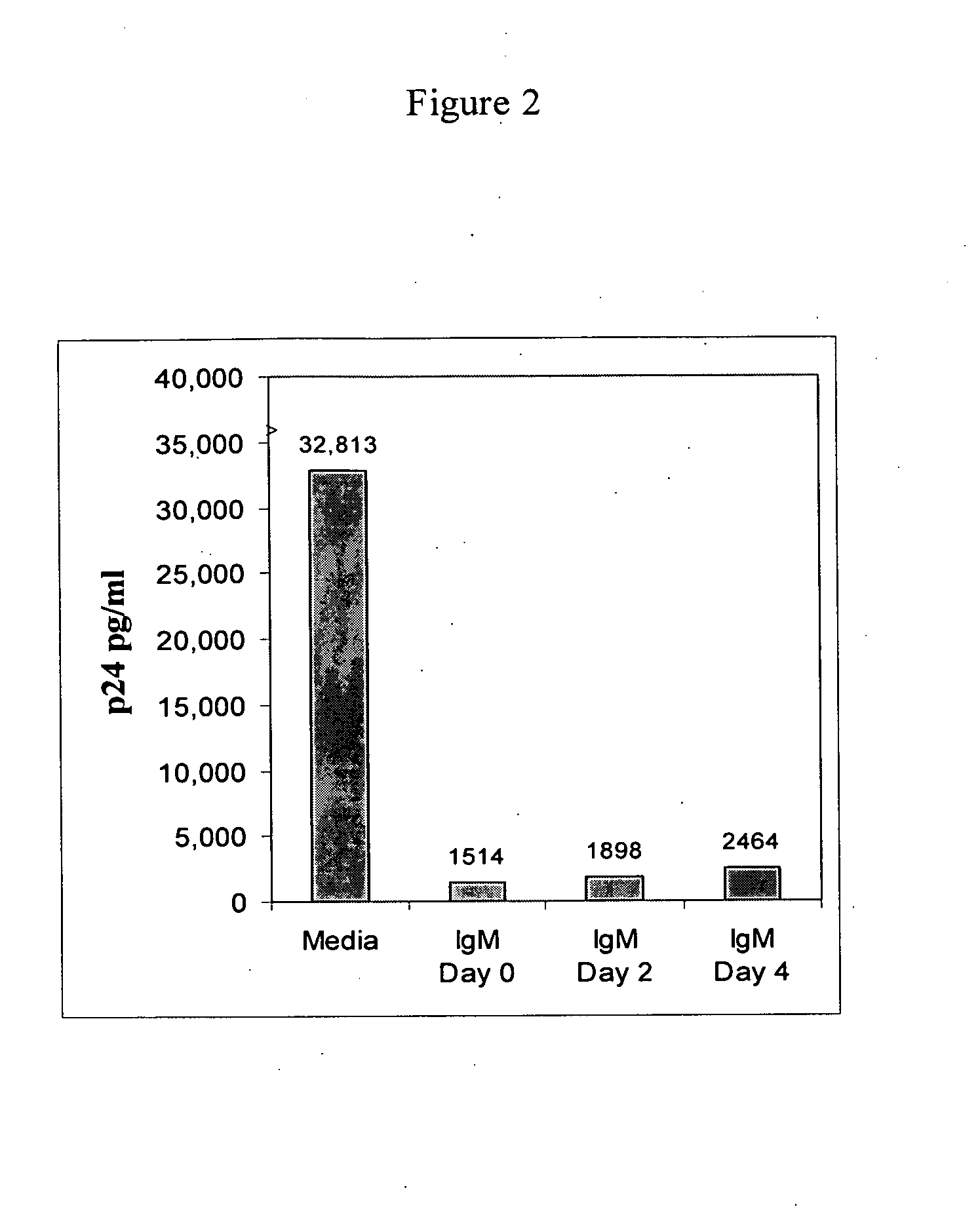
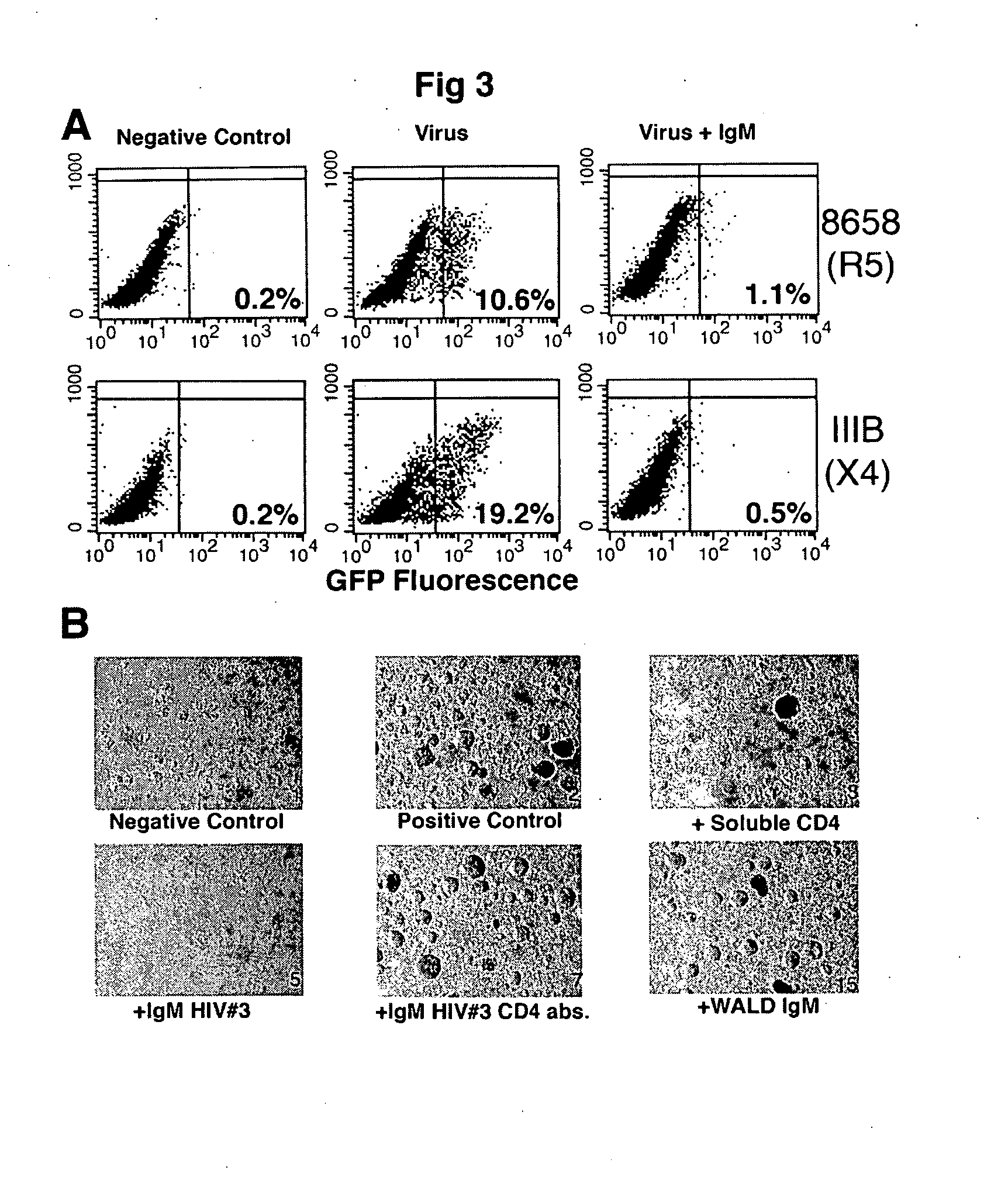
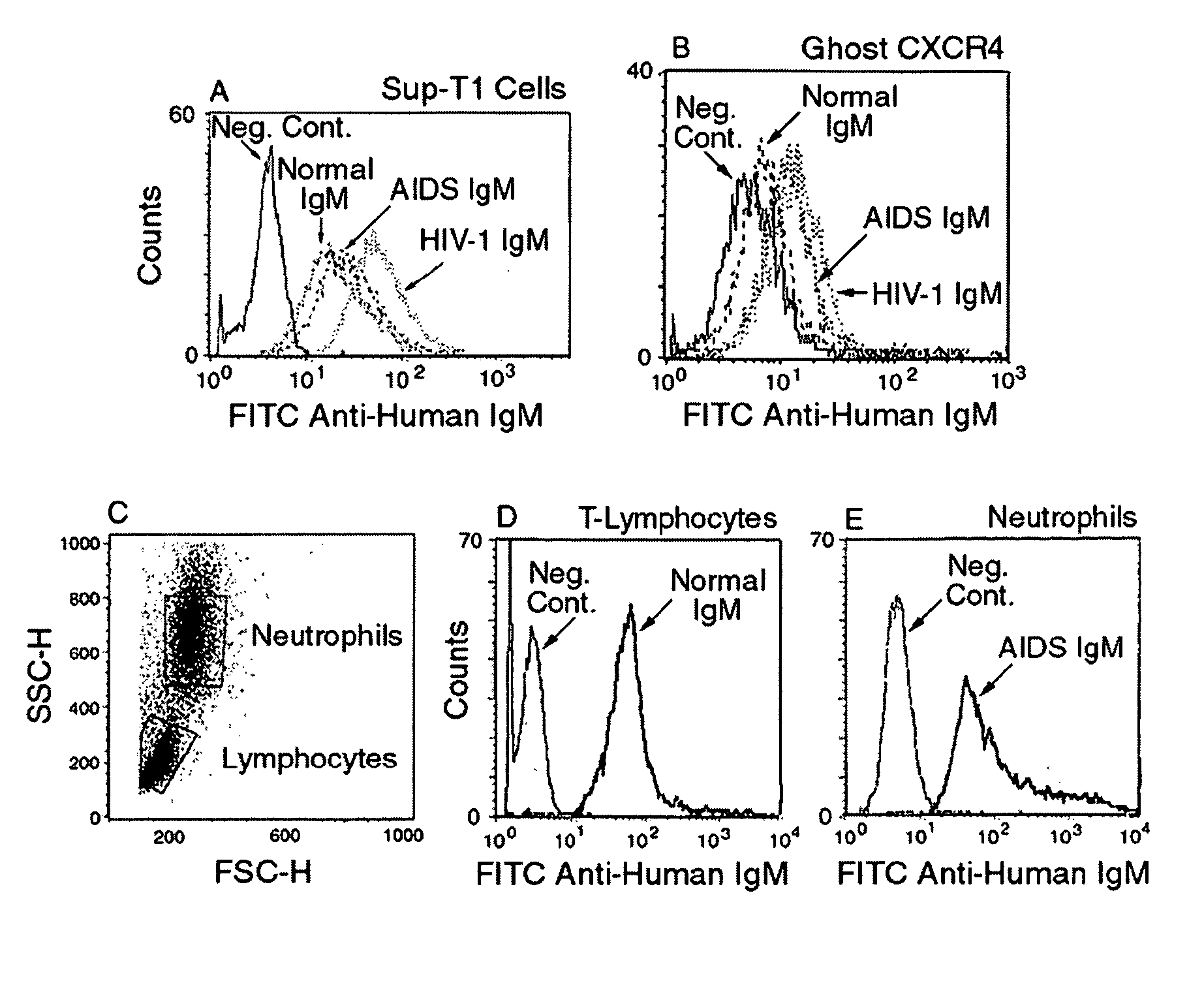
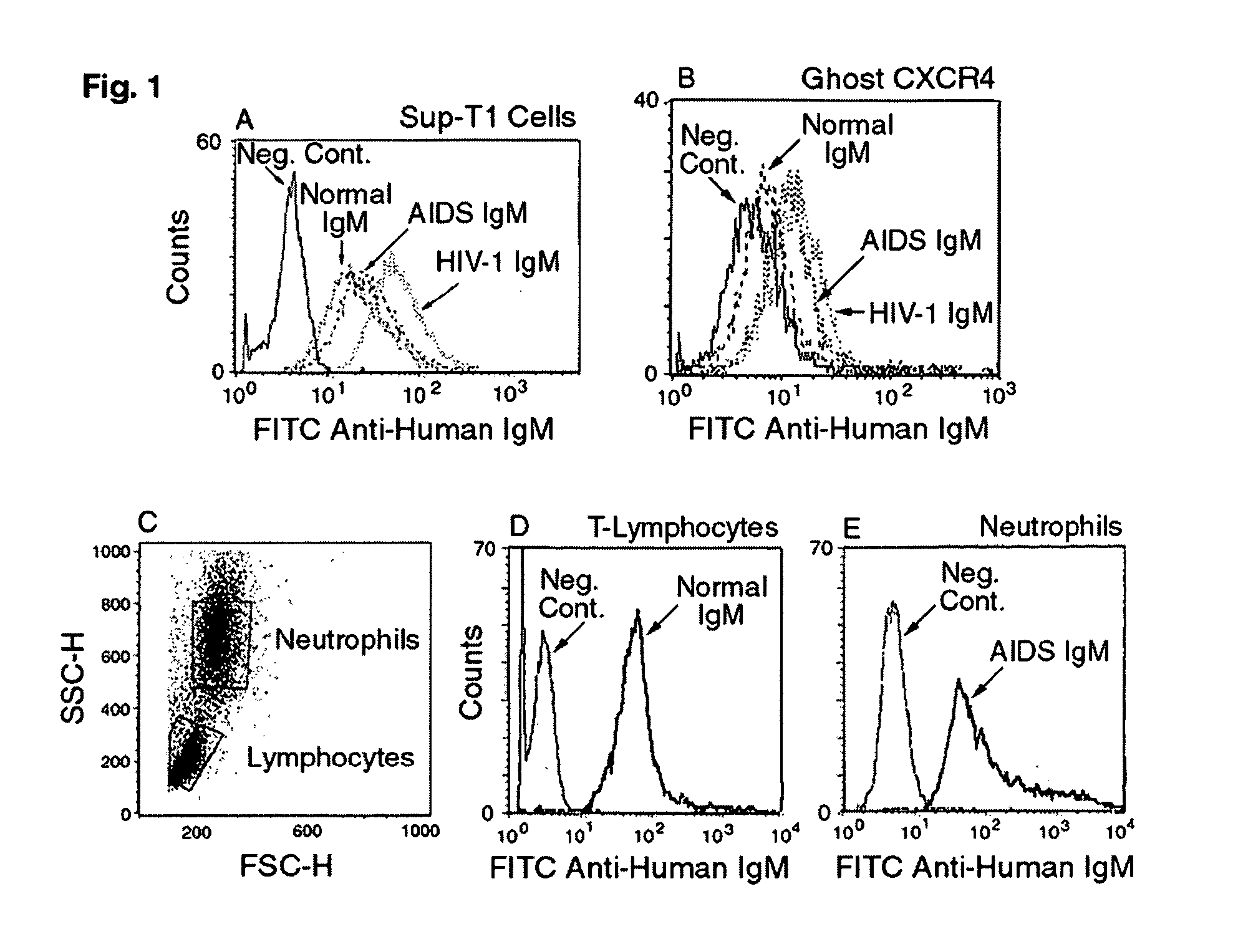
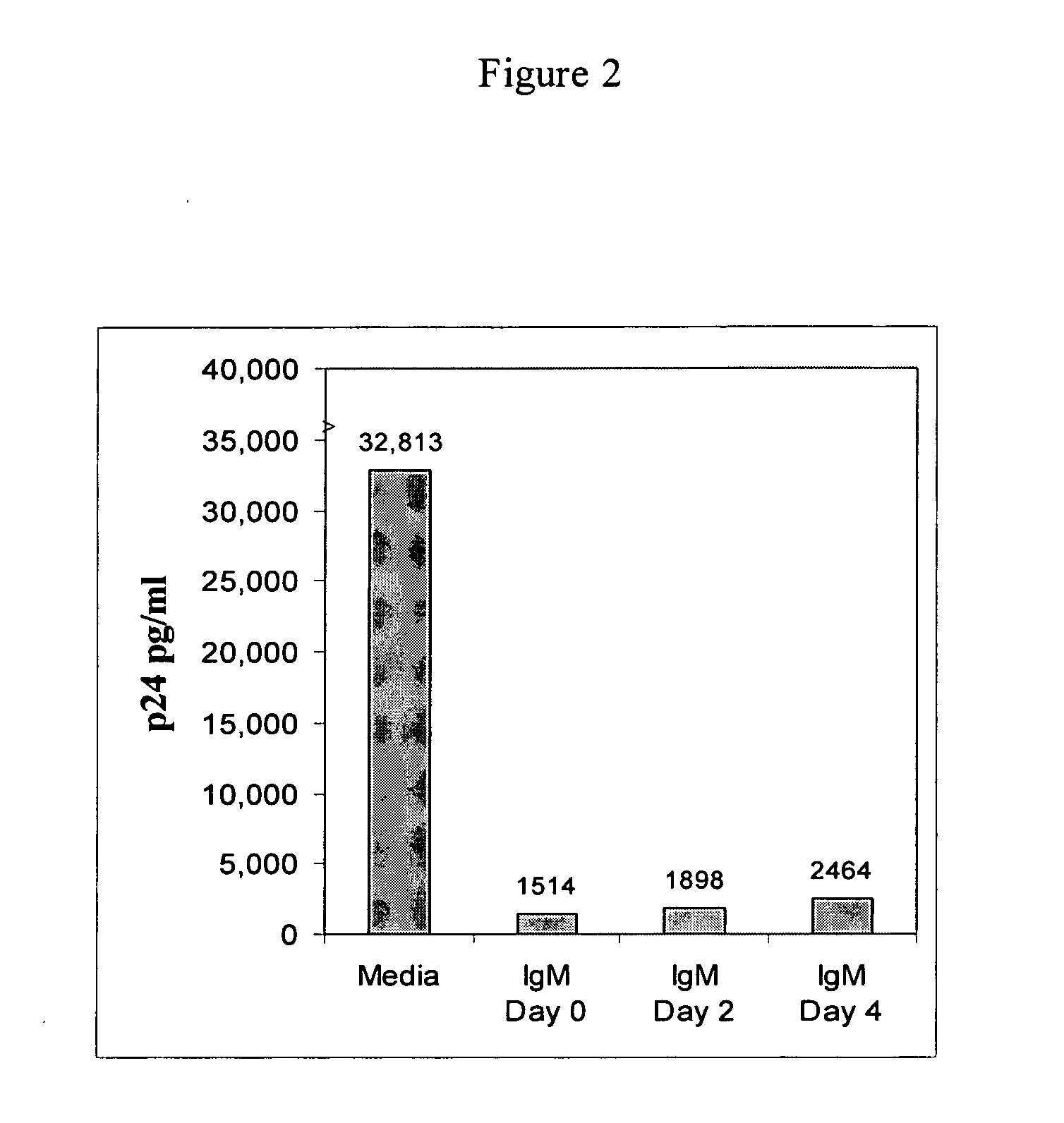
Naturally occurring IgM antibodies that bind lymphocytes
InactiveUS20080112950A1Inhibits Hepatitis CInhibit binding of chemokinesVertebrate antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHeterologousLymphocyte Antibody
Human and animal serum contains naturally occurring autoantibodies that develop at birth in absence of deliberate immunization. These antibodies are predominantly of IgM isotype but can include all immunoglobulin isotypes such as IgD, IgA and IgG. Here we describe IgM anti-lymphocyte autoantibodies (IgM-ALA) and show that these antibodies are heterogenous with some antibodies binding to chemokine receptors such as CCR5 and CXCR4 and others binding to other lymphocyte receptors including CD3, CD2, CD4 and CD81. These IgM-ALA, unlike IgG antibodies, are not cytolytic to cells at 37 C and hence function to alter lymphocyte function including cytokine production and act as “blocking antibodies to inhibit binding of chemokines and viruses including HIV-1 and Hepatitis C. IgM antibodies that bind to receptors on lymphocyte also bind to the same or similar class of receptors on other leucocytes and other cells such as cancer cells and endothelial cells. The inventor claims that naturally occurring anti-lymphocyte antibodies inhibit viral infections, cancer and several inflammatory states by binding to chemokine receptors and other cell membrane receptors that activate cells or promote viral entry and replication. Inventor also claims methods for quantitating levels of IgM-ALA with different receptor specificities to aid in preventing disease progression and also claims methods to enhance in-vivo or in-vitro production of IgM-ALA.
Owner:LOBO
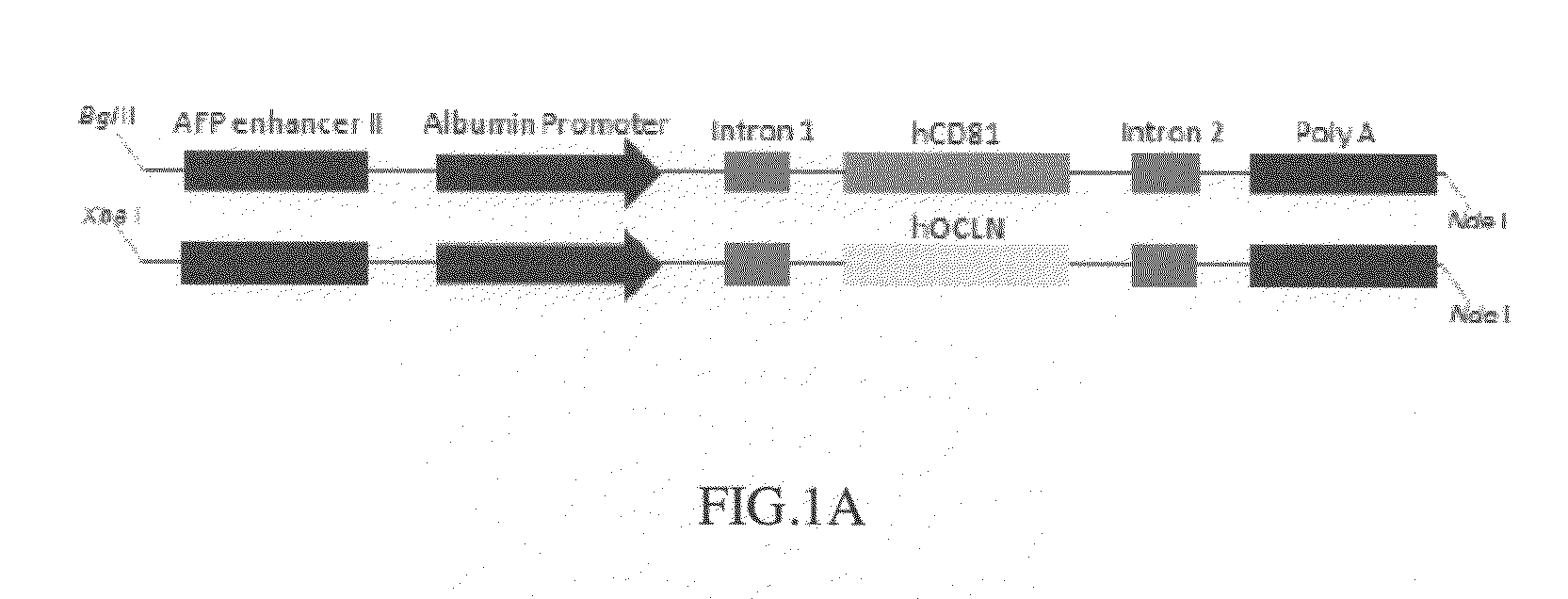
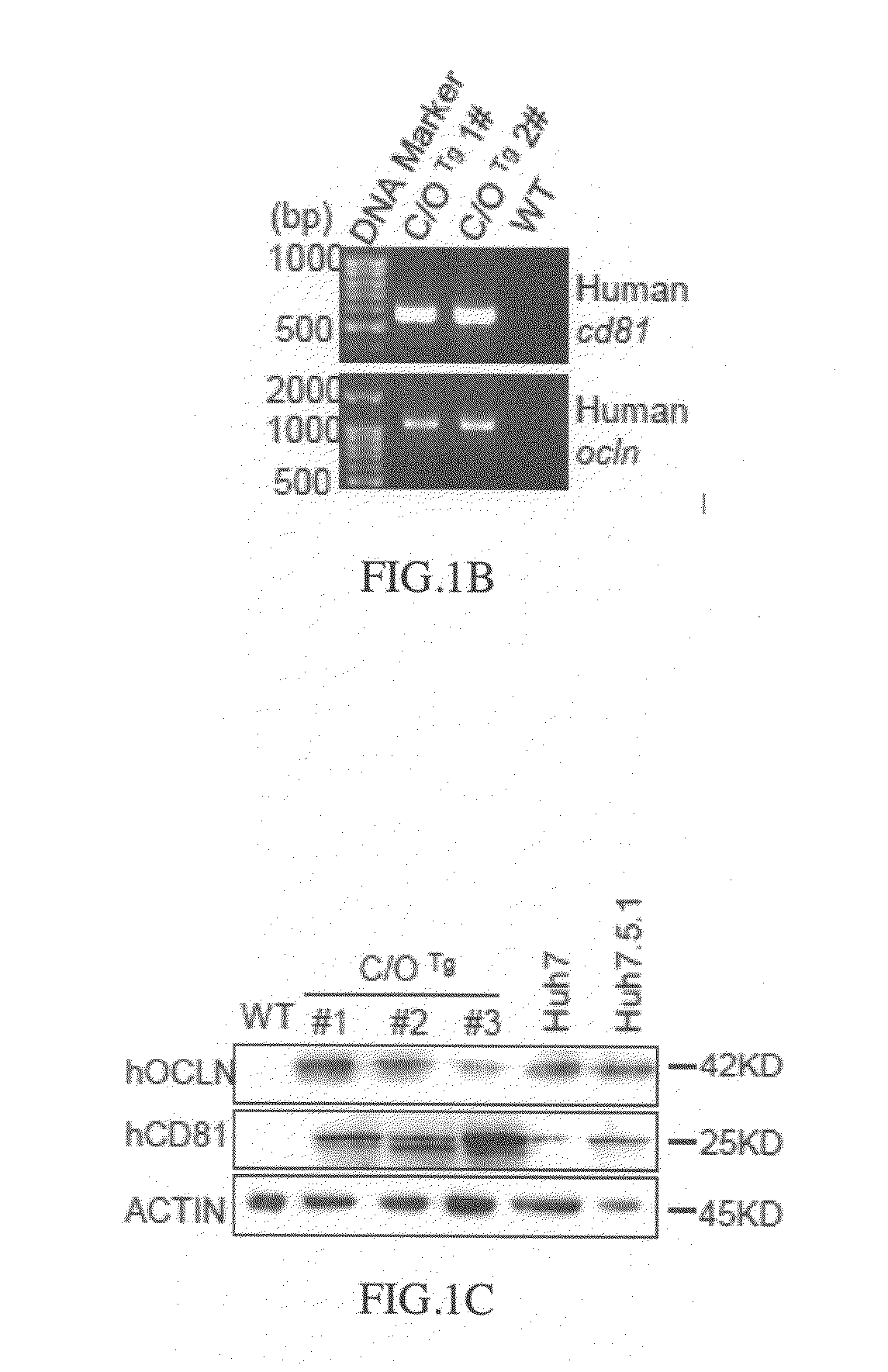
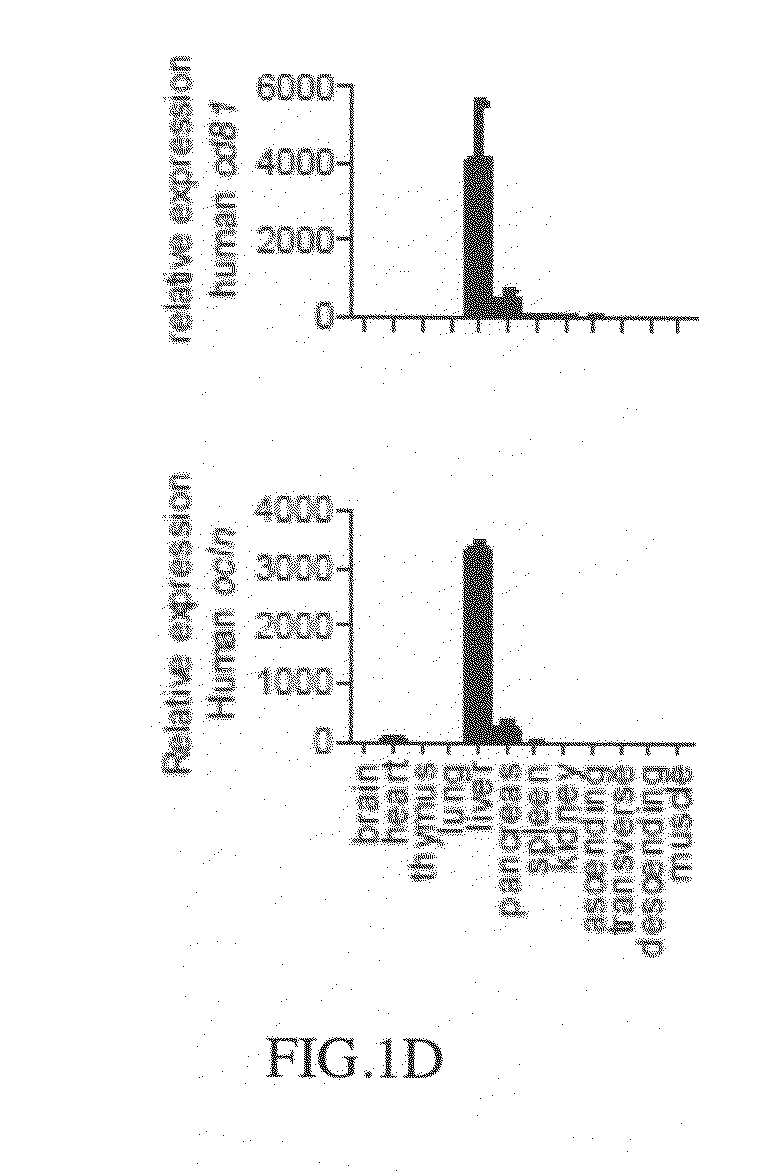
Cd81 and ocln double transgenic mouse and its construction methods
Owner:WUHAN INST OF VIROLOGY CAS
Naturally occuring IgM antibodies that bind lymphocytes
InactiveUS20080118522A1Eliminate side effectsInhibit inflammationBiocideAntipyreticLymphocyte AntibodyWhite blood cell
Human and animal serum contains naturally occurring autoantibodies that develop at birth in absence of deliberate immunization. These antibodies are predominantly of IgM isotype but can include all immunoglobulin isotypes such as IgD, IgA and IgG. Here we describe IgM anti-lymphocyte autoantibodies (IgM-ALA) and show that these antibodies are heterogenous with some antibodies binding to chemokine receptors such as CCR5 and CXCR4 and others binding to other lymphocyte receptors including CD3, CD2, CD4 and CD81. These IgM-ALA, unlike IgG antibodies, are not cytolytic to cells at 37 C and hence function to alter lymphocyte function including cytokine production and act as “blocking antibodies to inhibit binding of chemokines and viruses including HIV-1 and Hepatitis C. IgM antibodies that bind to receptors on lymphocyte also bind to the same or similar class of receptors on other leucocytes and other cells such as cancer cells and endothelial cells. The inventor claims that naturally occurring anti-lymphocyte antibodies inhibit viral infections, cancer and several inflammatory states by binding to chemokine receptors and other cell membrane receptors that activate cells or promote viral entry and replication. Inventor also claims methods for quantitating levels of IgM-ALA with different receptor specificities to aid in preventing disease progression and also claims methods to enhance in-vivo or in-vitro production of IgM-ALA.
Owner:LOBO
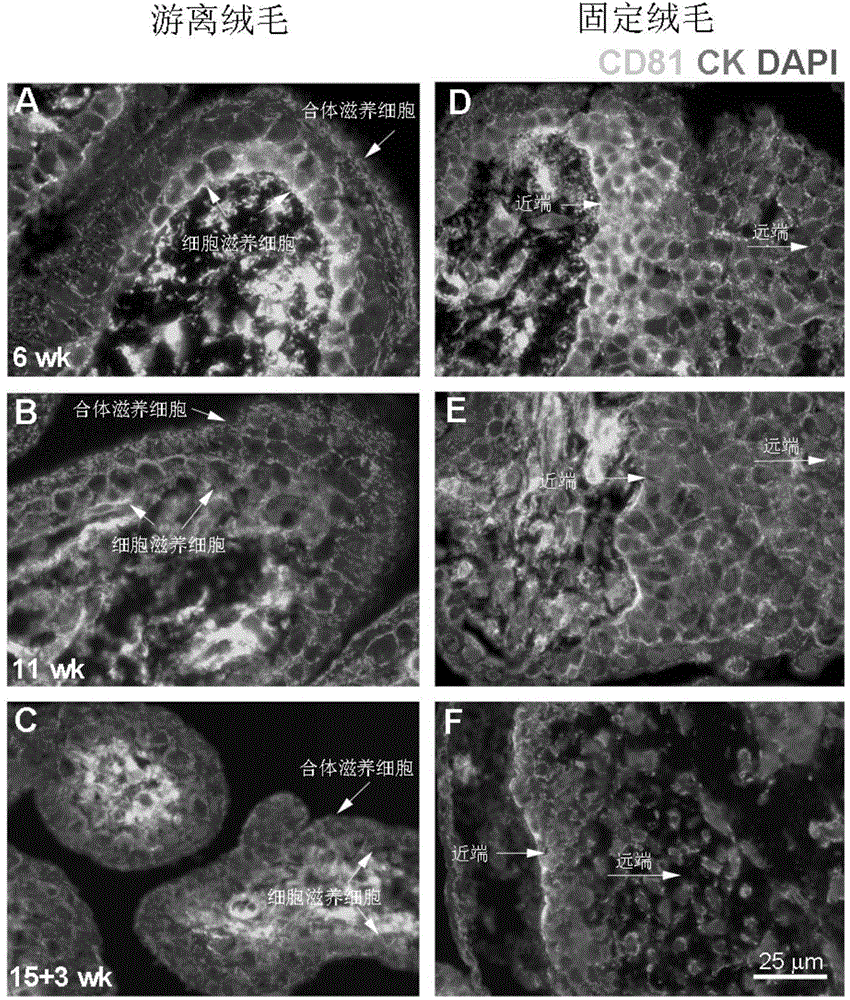
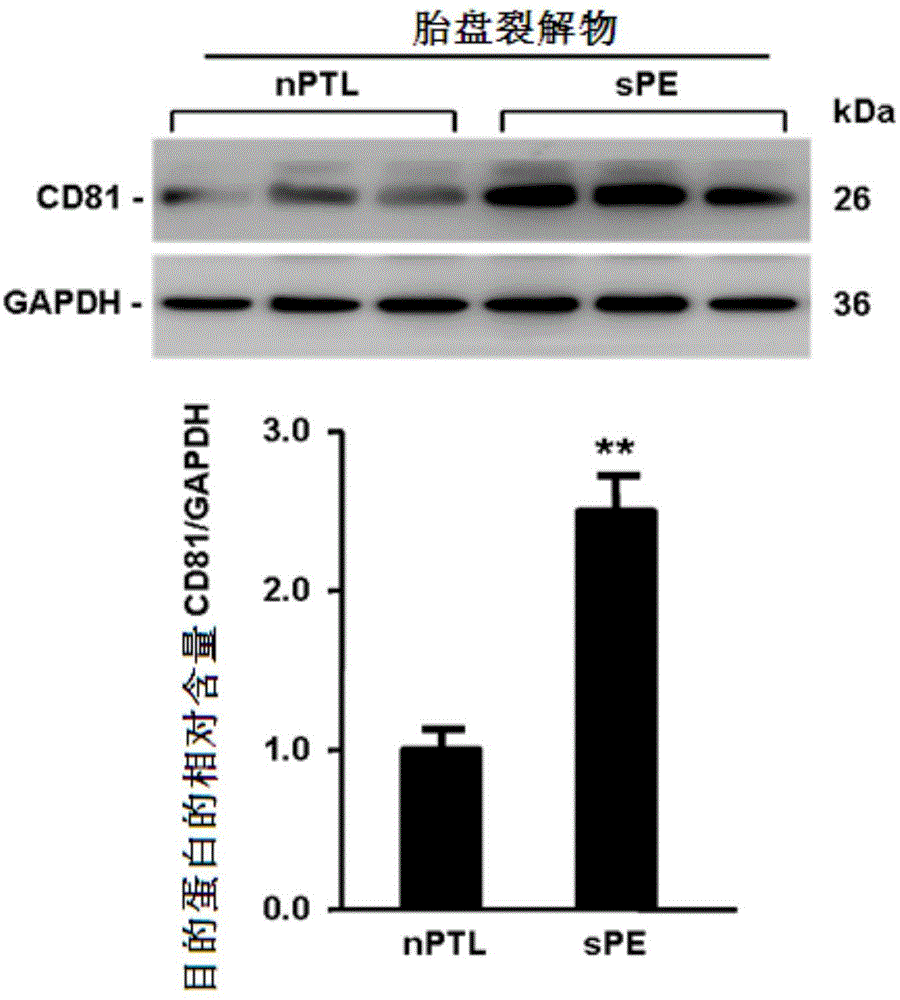
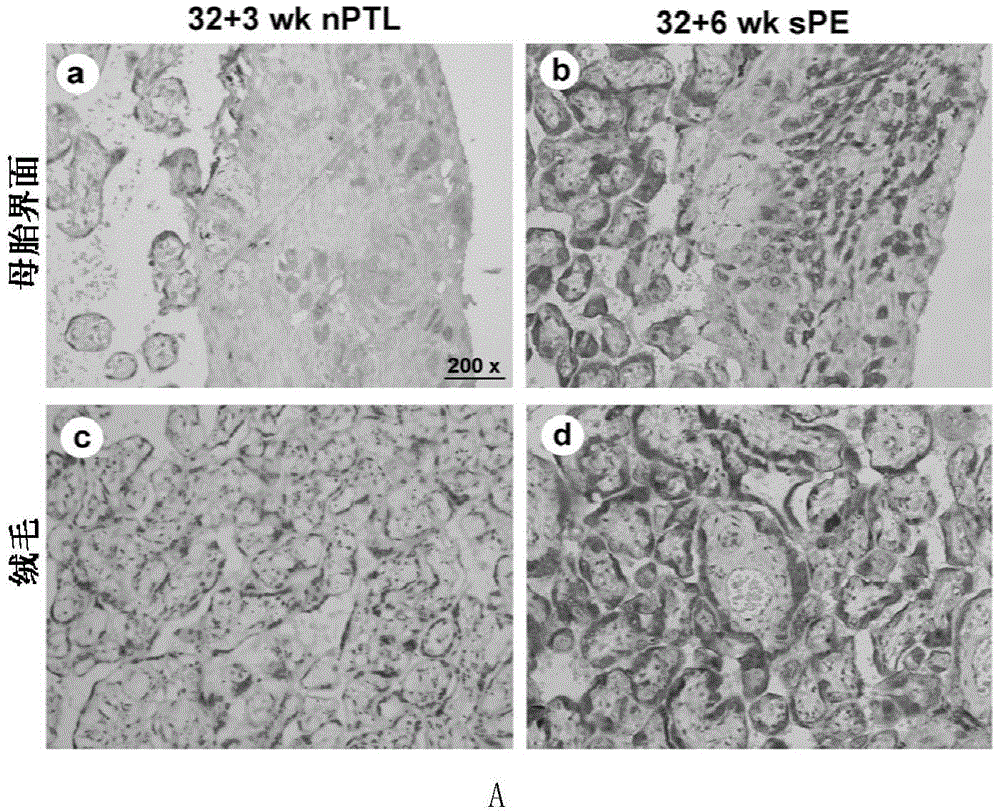
Application of membrane protein CD81 in early forecasting, parting, diagnosis and treatment of eclampsia
ActiveCN104698187APrevent invasionGenetic material ingredientsDisease diagnosisSpiral arteryMedicine
The invention discloses application of a membrane protein CD81 in early forecasting, parting, diagnosis and treatment of eclampsia. Clinical investigation, an in-vitro molecular experiment and an animal experiment show that through suppression of the invasion ability of nurse cells, CD81 is capable of leading the remodeling obstacle of the uterine spiral artery, so that PE illness is caused. Therefore, the CD81 can be used as a marking molecule for forecasting PE and is possibly used as a marking molecule for PE parting or a target for preventing and treating PE.
Owner:NANJING DRUM TOWER HOSPITAL
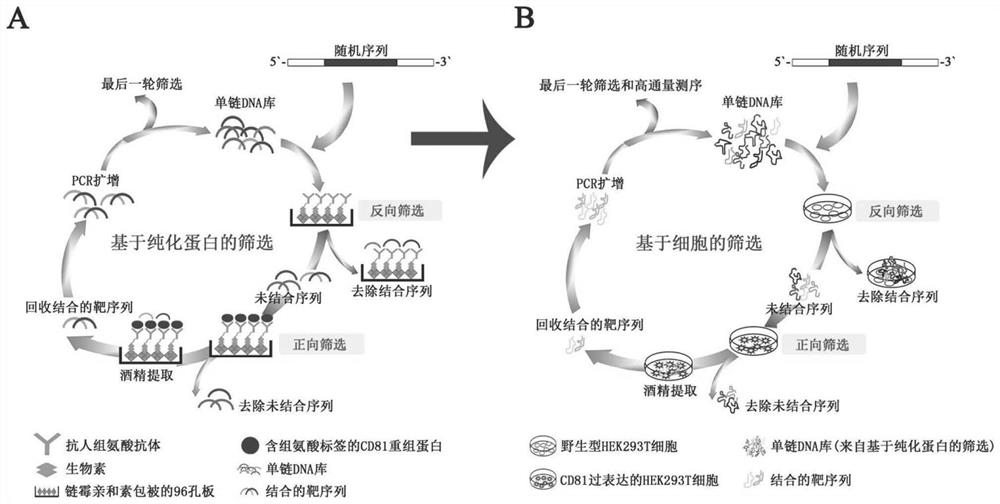
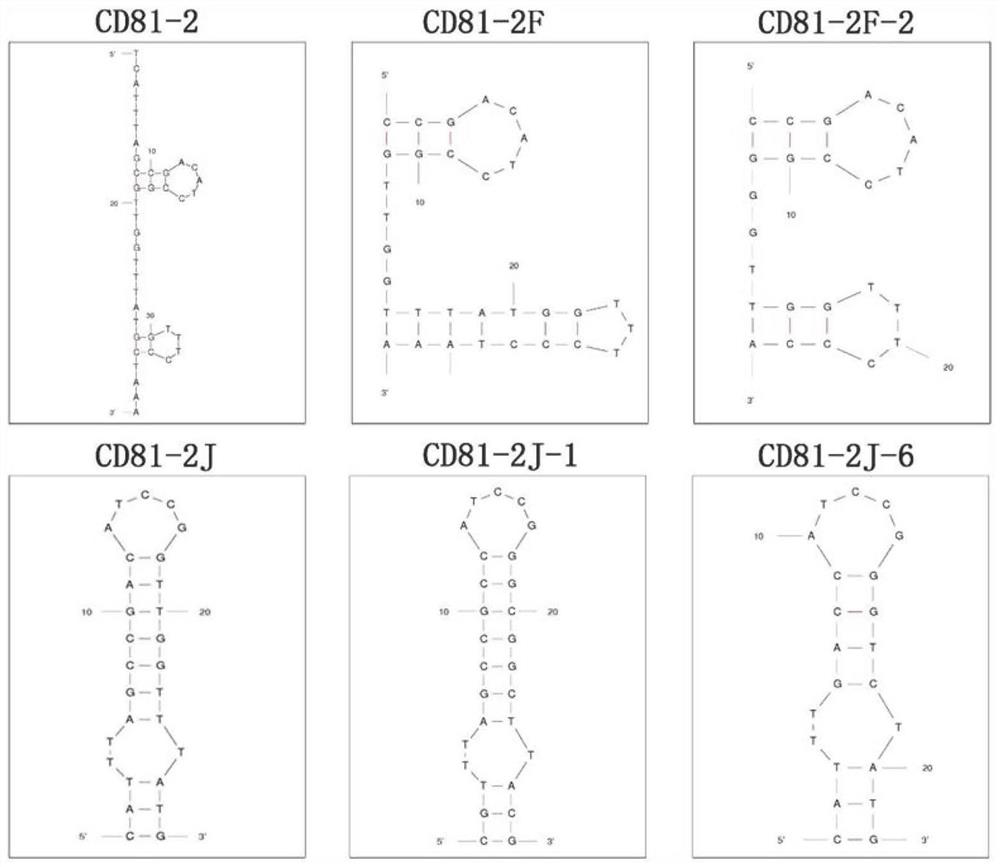
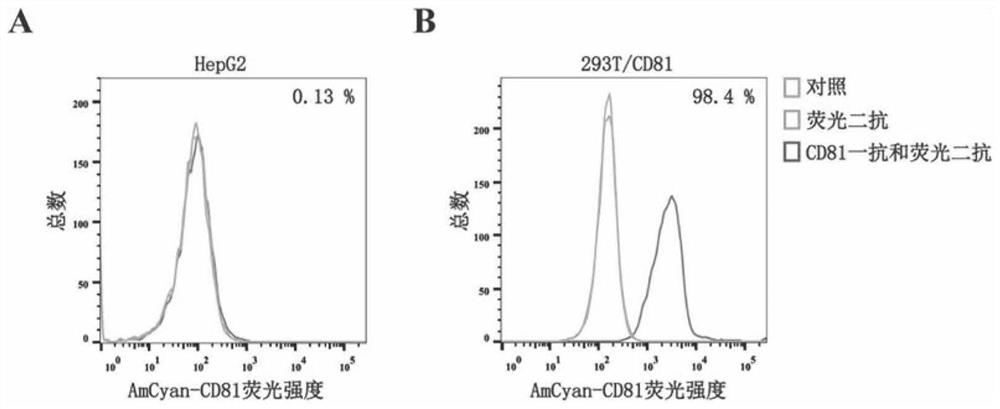
CD81 aptamer and application thereof
The invention relates to the technical field of biology, in particular to a CD81 aptamer and application thereof. The CD81 aptamer has (a) a nucleotide sequence as shown in a sequence 1 or (b) a sequence obtained by deleting or replacing one or more bases of the nucleotide sequence as shown in the sequence 1. The combination of the CD81 aptamer provided by the invention and the CD81 has extremely high selectivity and specificity.
Owner:SUZHOU GENEPHARMA +1
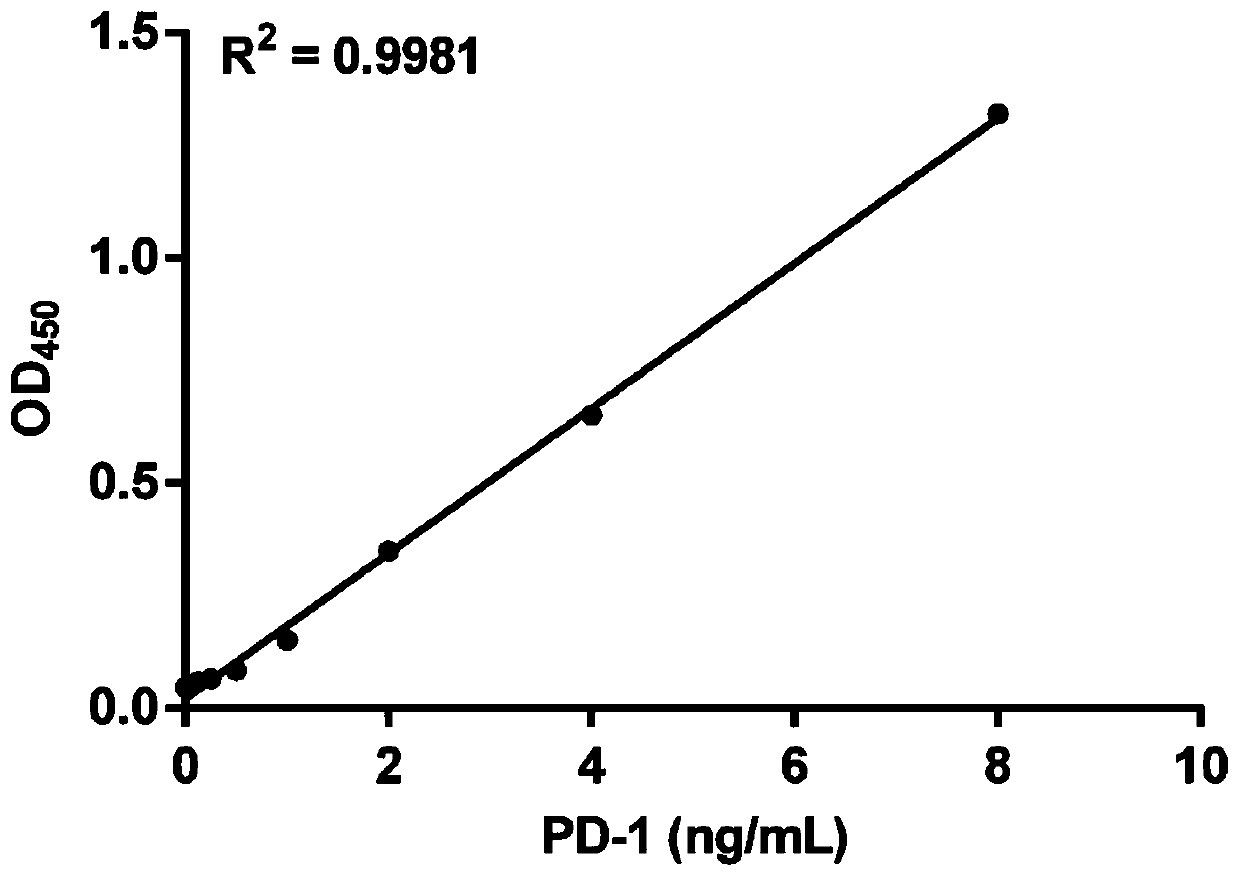
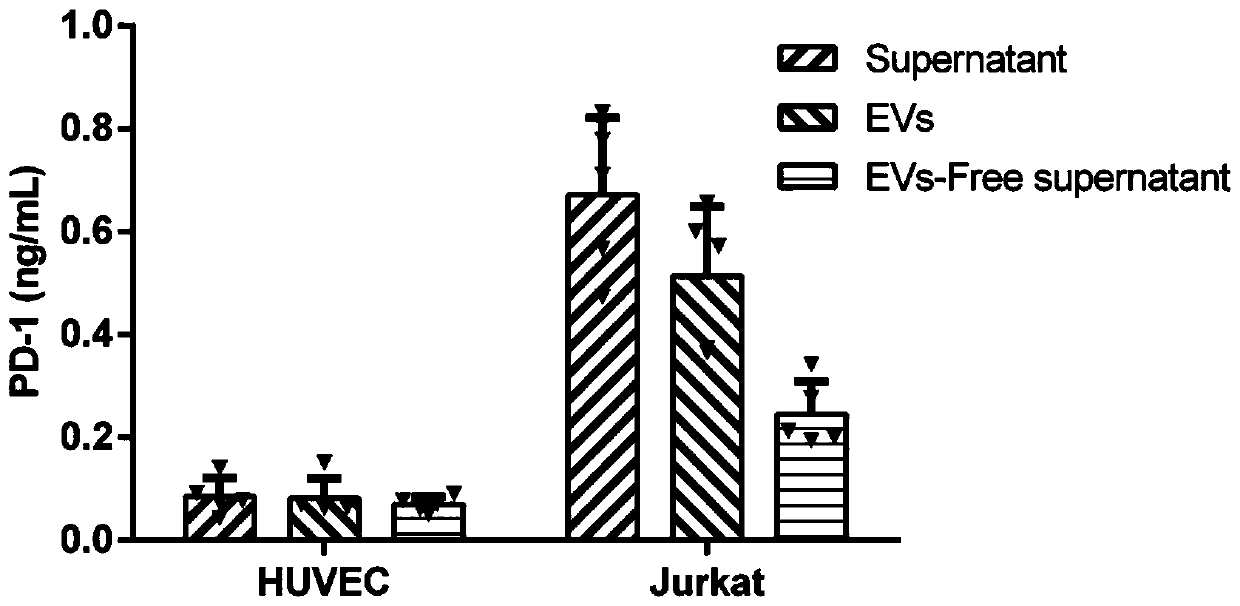
Method for efficiently and quantitatively detecting PD-1 level in extracellular vesicles, ELISA kit and use method
PendingCN111537725ARealize quantitative detectionAvoid interferenceBiological material analysisBiological testingExtracellular vesicleElisa kit
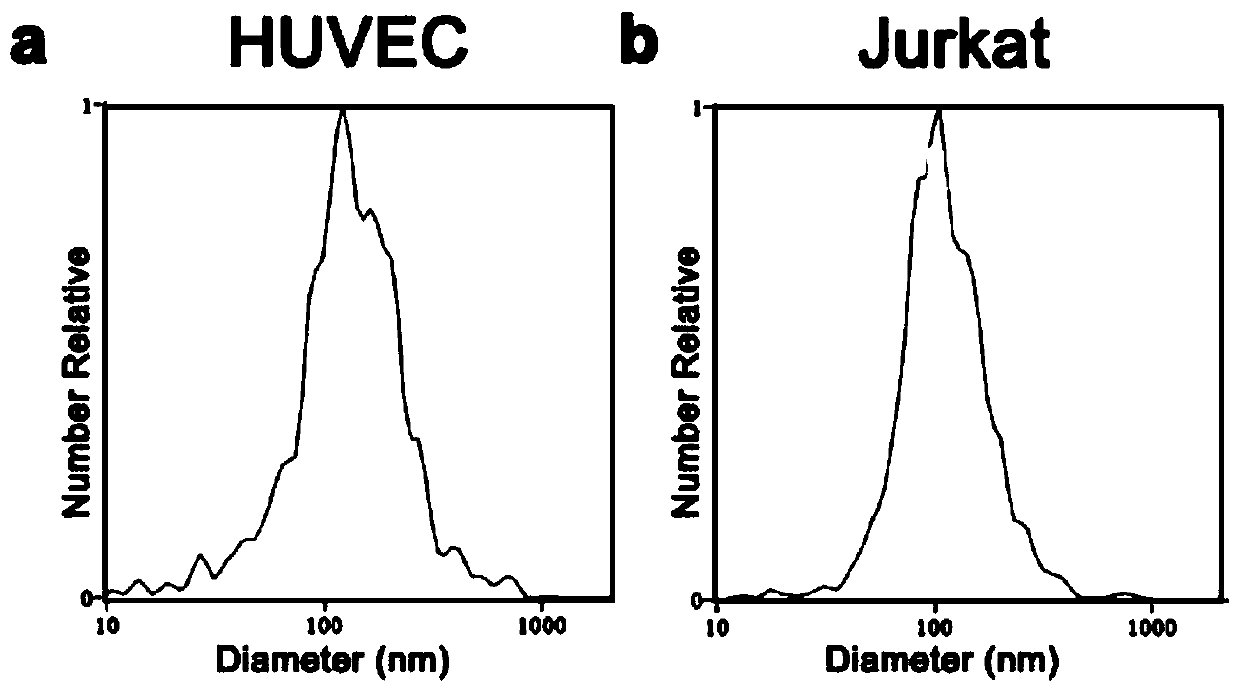
The invention relates to the field of molecular biology and biotechnology, and in particular relates to a method for efficiently and quantitatively detecting the PD-1 level in extracellular vesicles,an ELISA kit and a use method. The method comprises the steps of capturing extracellular vesicles of human body fluid by utilizing the specificity of an anti-CD63 antibody, an anti-CD9 antibody or ananti-CD81 antibody; using the anti-PD-1 antibody for detecting the PD-1 protein level in the extracellular vesicles; and using the constructed corresponding CD63-PD-1, CD9-PD-1 or CD81-PD-1 fusion protein as a standard substance to realize the quantitative detection of the PD-1 in the extracellular vesicles. According to the invention, quantitative detection of the extracellular vesicles of a complex structure is realized; and meanwhile, the fusion proteins CD63-PD-1, CD9-PD-1 and CD81-PD-1, which are independently constructed, are used as standard proteins, so that the detection result is characterized by the specific protein concentration.
Owner:珈泌生物科技(武汉)有限责任公司
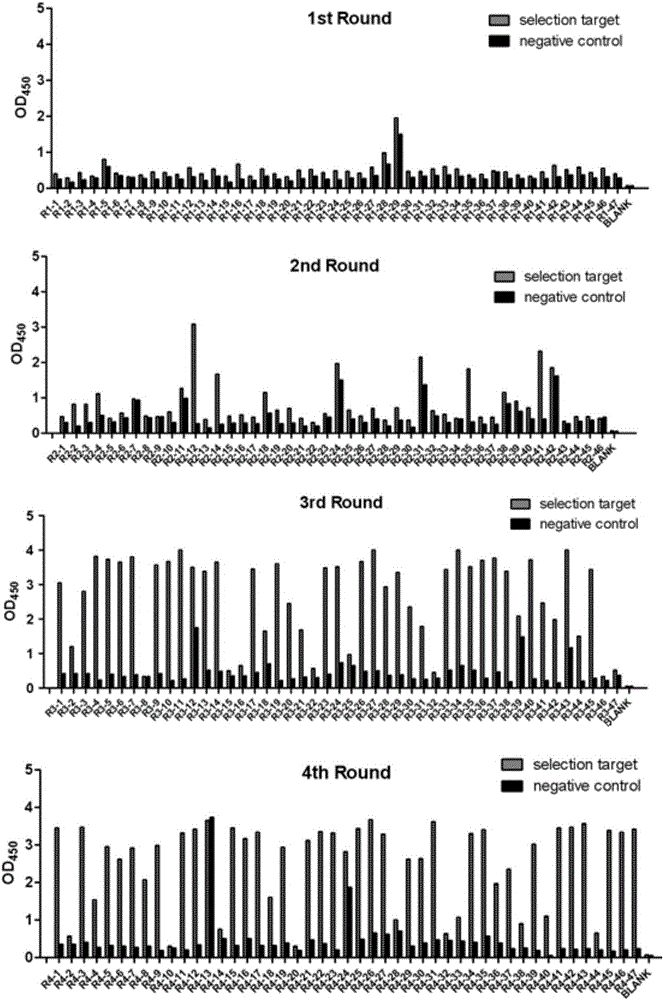
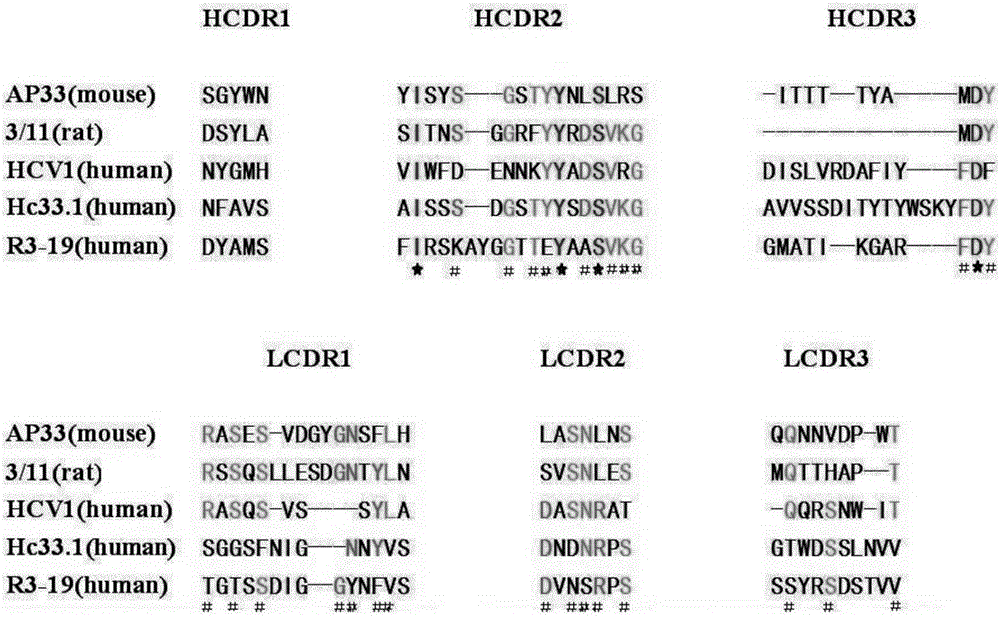
Multi sub-gene resistant type HCV (Hepatitis C Virus) antibody gene R3-19 and application thereof
InactiveCN105219780AExtensive featuresBroad neutralization capacityImmunoglobulins against virusesAntiviralsLinear epitopeScFv Antibodies
The invention discloses a multi sub-gene resistant type HCV (Hepatitis C Virus) antibody gene R3-19, wherein the heavy chain amino acid sequence thereof is shown by SEQ ID NO.38, and the light chain amino acid sequence is shown by SEQ ID NO.39, the antibody gene is obtained by vanning in an independently-constructed anti-HCV scFv antibody library through E2 region aa412-423 linear epitope (an HCV CD81 receptor binding epitope) synthetic peptide which is highly conservative in various subtype HCVs; the antibody library is formed by 39 parts of anti-HCV antibody positive peripheral blood samples of Chinese patients, the HCVs infected by the patients cover the main HCV popular subtypes in China, therefore, the R3-19 is an anti-HCV antibody which is typical in China and has wide binding characteristic, and has excellent application value in the fields of HCV diagnosis, HCV treatment, vaccine research and development and the like in China.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Liquid-phase chip kit for detection of acute coronary syndrome based on serum exosomes and preparation method
InactiveCN103376313AImprove accuracyImprove detection efficiencyMaterial analysisBiotin-streptavidin complexCD86
The invention relates to a kit used for auxiliary judgment of risk stratification of patients with acute coronary syndrome based on detection of serum exosomes and a preparation method. The kit mainly comprises exosomes extraction reagents, relevant microballoons capturing antibodies (the above antibodies are one or some selected from CD11c, CD81, MHCII, CD86, CD106, CD54 and CD3), biotin labeled detection antibodies and streptavidin phycoerythrin. After being optimized, the diagnostic kit has advantages of high detection efficiency, less amount of required samples, high specificity, high sensitivity, fast and accurate detection and the like, so the risk stratification conditions of patients can be evaluated comprehensively, and relevant references are provided for clinical intervention.
Owner:梁春 +2
Kit for separating exosome from cell supernatant and use method of kit
InactiveCN113215075AHigh purityEasy to operateCell dissociation methodsAnimal cellsCD63Antiendomysial antibodies
The invention provides a kit for separating exosome from cell supernate. The kit comprises a reagent A, a reagent B, a reagent C and a reagent D, wherein the reagent A is a PEG 6000 and / or PEG 8000 aqueous solution with the concentration of 35 to 45 g / mL; the reagent B is a magnetic particle solution with the concentration of 1-10%; the reagent C is an antibody solution with the concentration of 20-100 [mu] g / mL, wherein an antibody is selected from one or more than two of CD63, CD81 and CD9; and the reagent D is a Tween 20 phosphate buffer solution with the concentration of 0.05 to 1%. According to the kit, the use method and the application, the high-purity exosome can be efficiently obtained, the influence of substances such as impure protein and the like is eliminated, and the kit has the characteristics of simplicity, convenience, stability, high purity, easiness in operation, high reliability in analysis of protein components and the like.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Drug for inflammatory bowel disease
InactiveCN102869381ALong-term remission maintenanceAchieving remission inductionDigestive systemAntibody ingredientsInflammatory bowel diseaseCD81
Anti-CD81 antibody displays not only a remission induction effect, but also a long-term remission maintenance effect on inflammatory bowel disease when administered in a single dose, and is therefore effective as a drug for maintaining remission from inflammatory bowel disease. Moreover, anti-CD81 antibody is effective as a drug for the prevention, amelioration and treatment of inflammatory bowel disease because it has preventive, therapeutic and ameliorating effects on refractory inflammatory bowel disease.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Method for establishing HCV (hepatitis C virus) cell model by using tree shrew bone marrow mesenchymal stem cells
ActiveCN109680000AInfectiousSolving the limitations of non-passageMicrobiological testing/measurementFermentationViral vectorOrganism
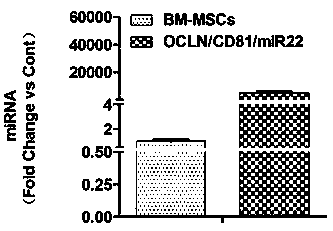
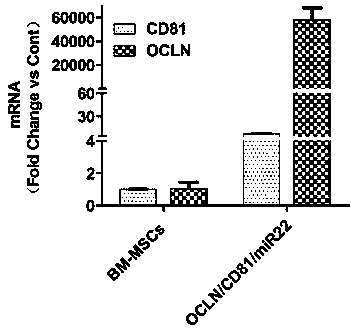
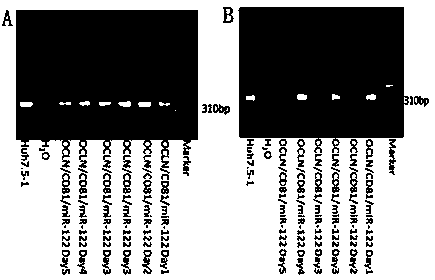
The invention relates to a method for establishing an HCV (hepatitis C virus) cell model by utilizing tree shrew bone marrow mesenchymal stem cells, and belongs to the technical field of biological medicines. The method comprises the steps that firstly, an OCLN (occludin) lentiviral vector is adopted to infect the tree shrew bone marrow mesenchymal stem cells, then a CD81 lentiviral vector is added for further infection, then a miR-122 lentiviral vector is added for infection for 4 hours, then a fresh culture medium containing 4 mcg / mL of polybrene with 1 / 2 volume of a current culture medium is added, and the used culture medium is removed the next day and is replaced with the fresh culture medium for continuous culture so as to obtain the HCV (hepatitis C virus) cell model. According to the method, the cell model has the basic characteristics and the differentiation potential of the bone marrow mesenchymal stem cells and is susceptible to HCV (hepatitis C virus), HCV (hepatitis C virus) endocytosis and copy are supported, infective virus particles are produced, and new cell model resources are provided for HCV (hepatitis c virus) pathogenesis researching and drug screening.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com