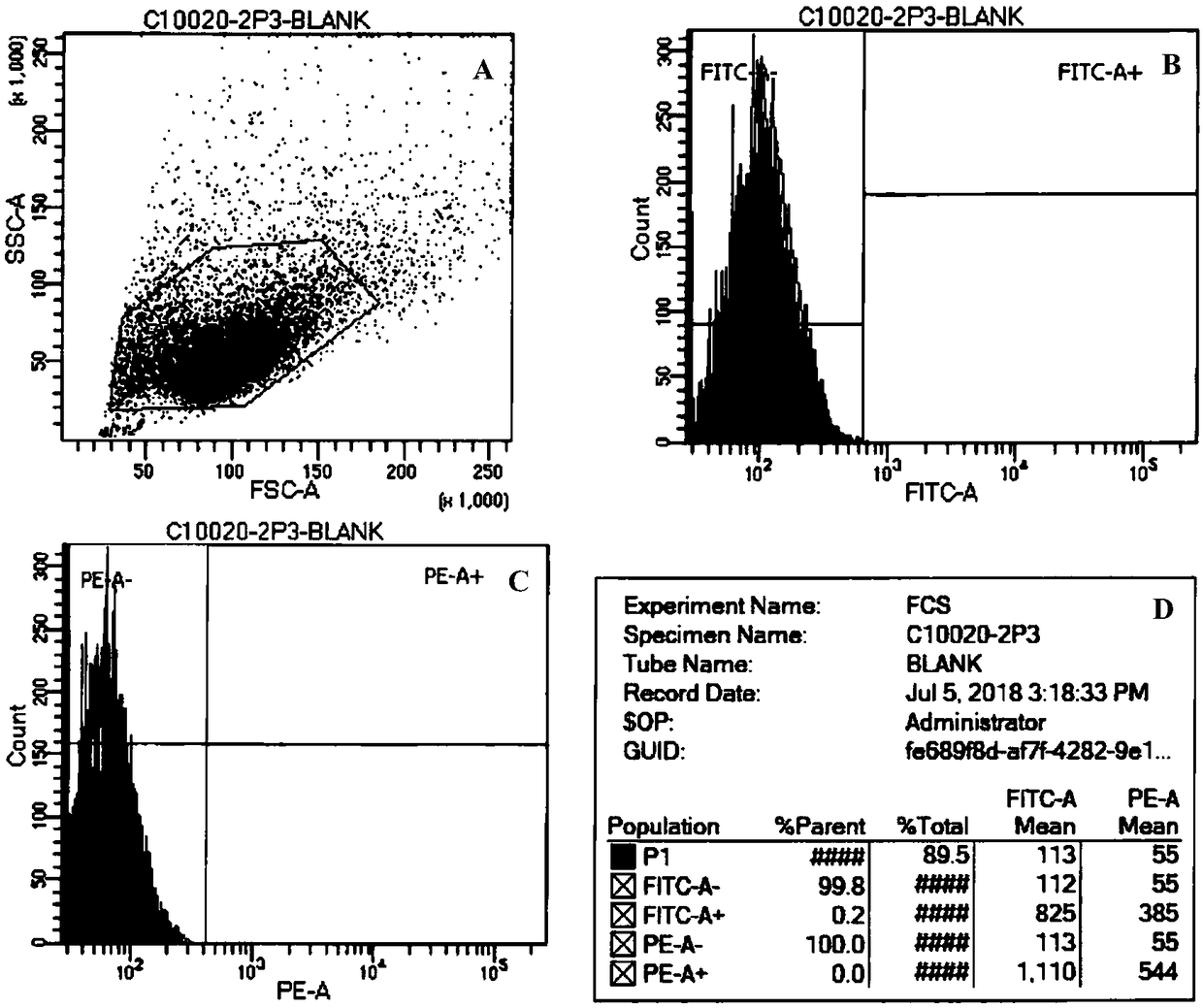
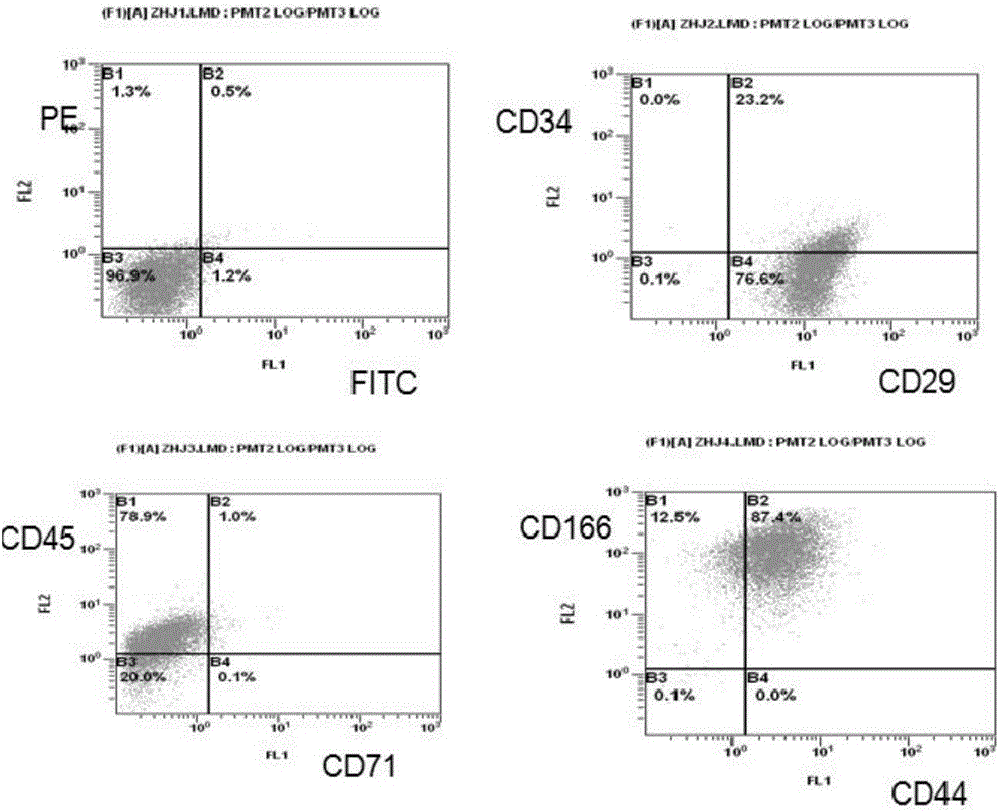
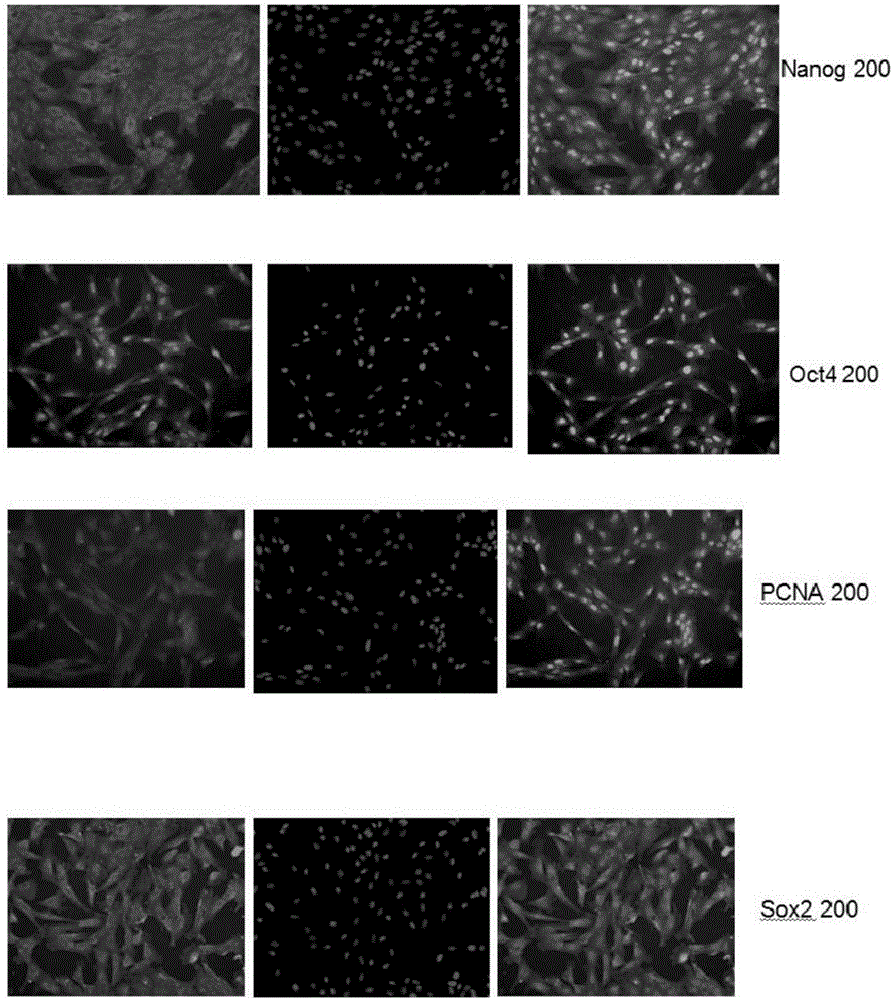
Patents
Literature
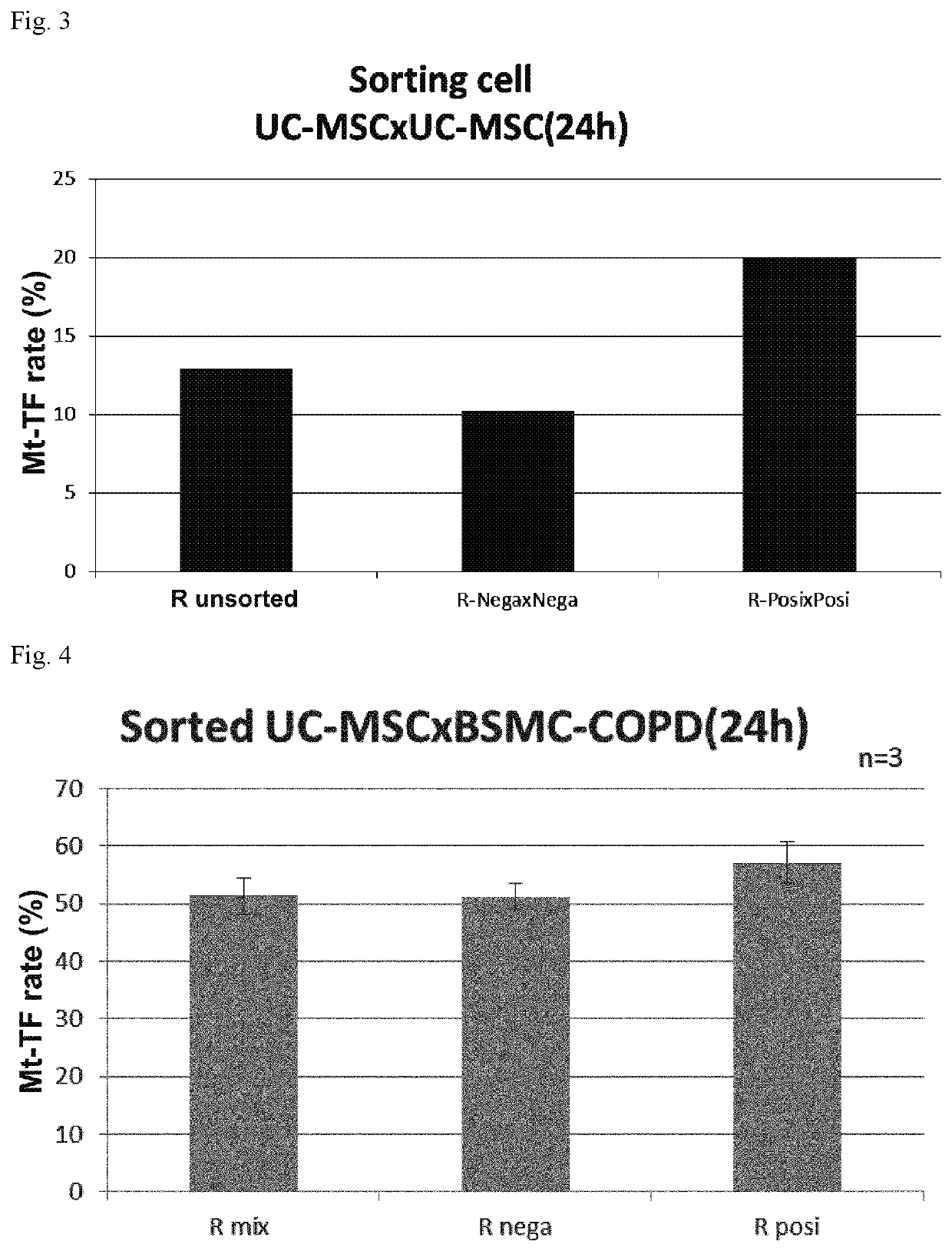
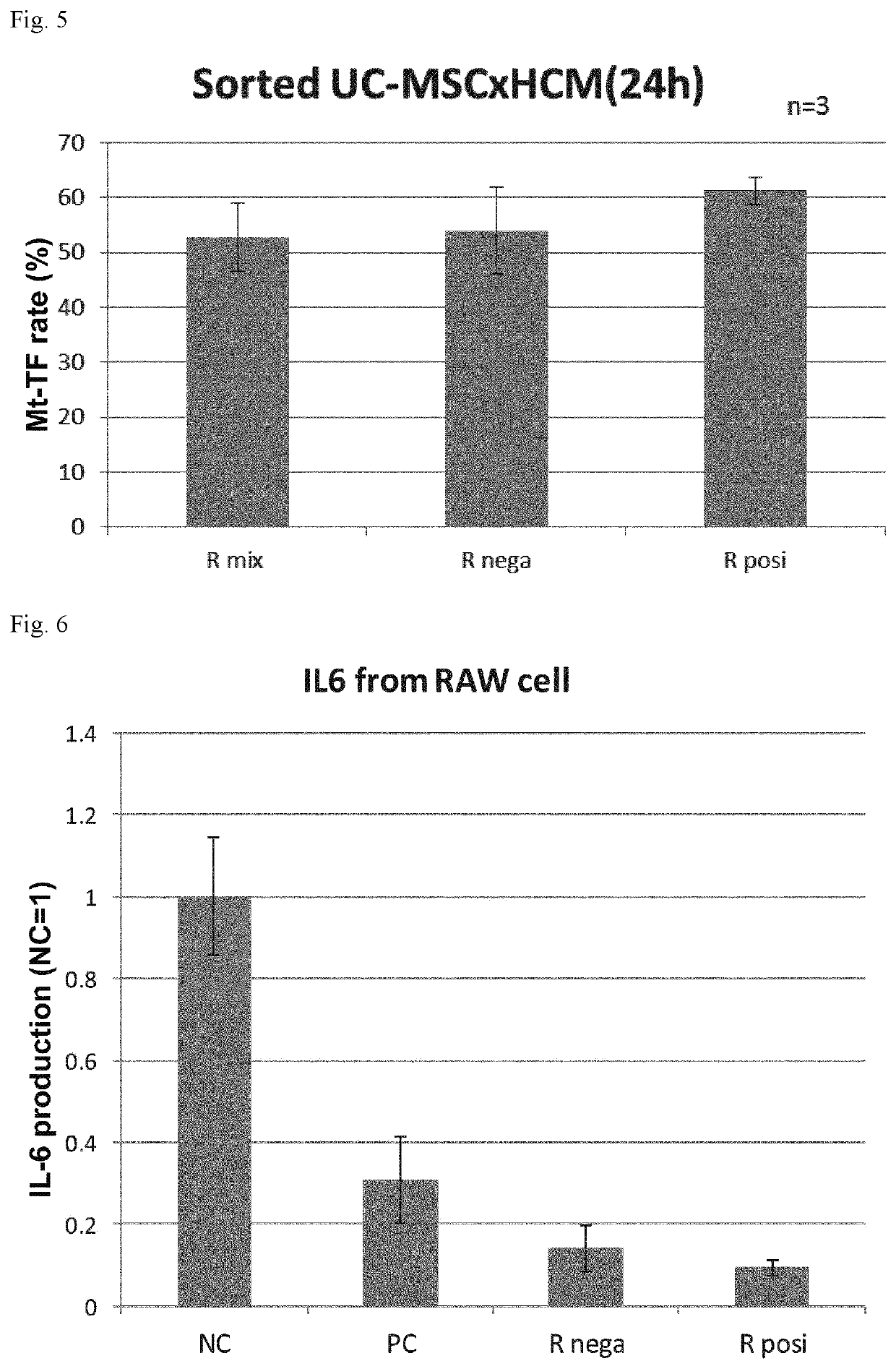
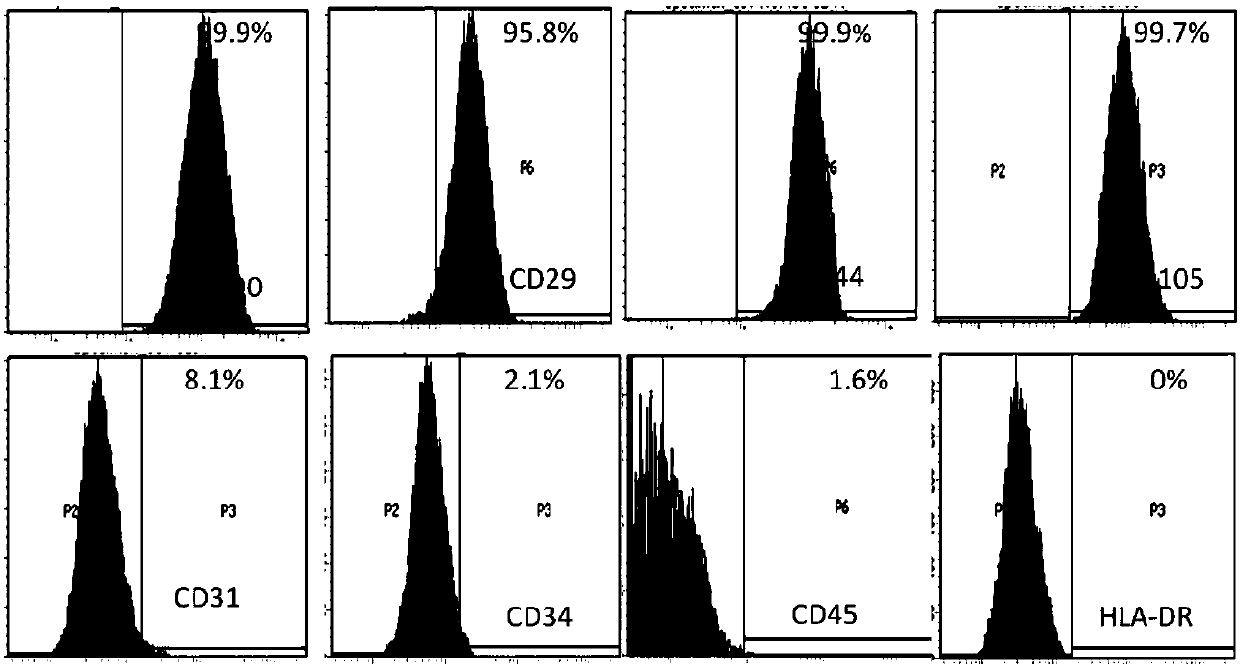
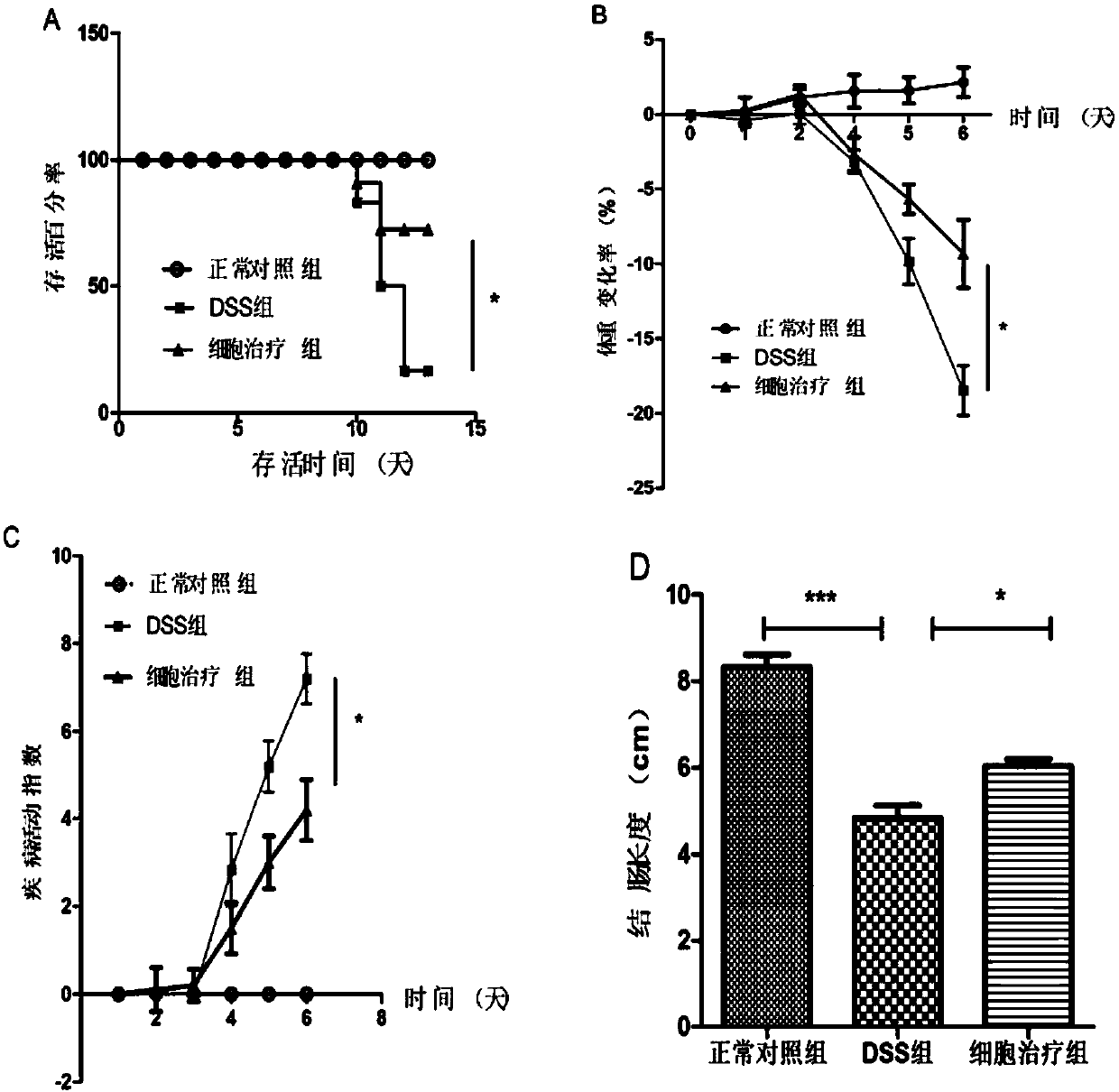
54 results about "CD29" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
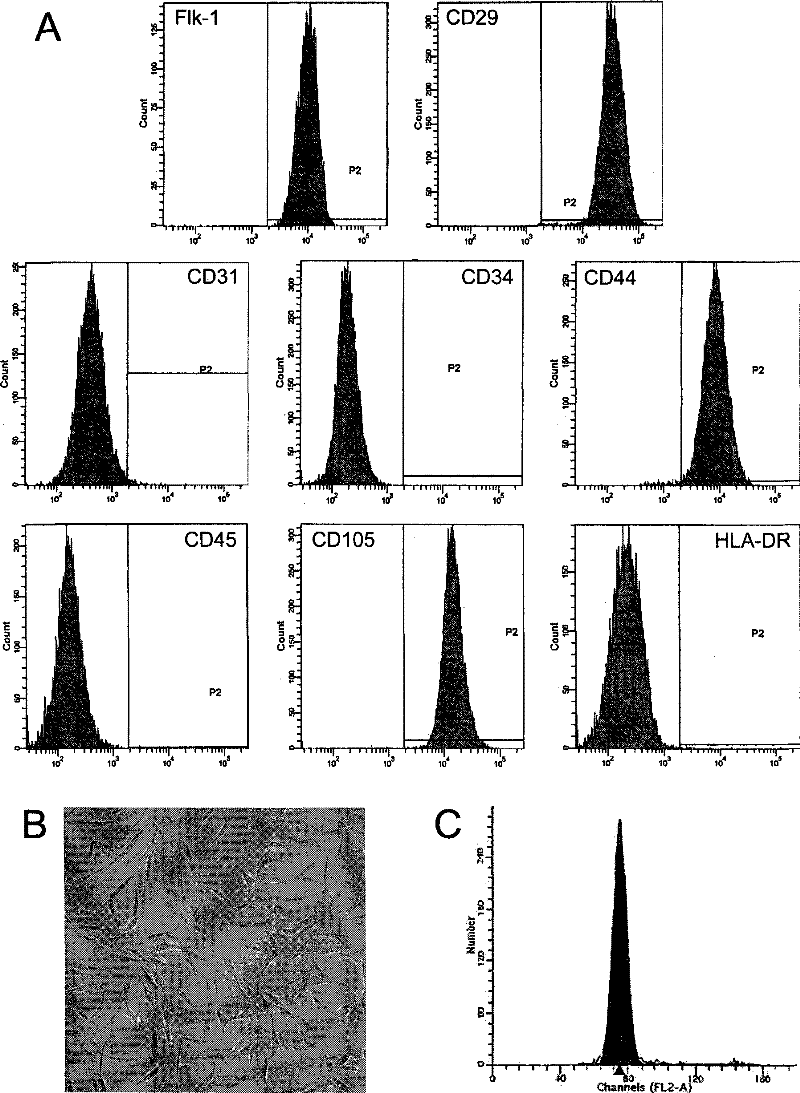
Integrin beta-1 (ITGB1), also known as CD29, is a cell surface receptor that in humans is encoded by the ITGB1 gene. This integrin associates with integrin alpha 1 and integrin alpha 2 to form integrin complexes which function as collagen receptors. It also forms dimers with integrin alpha 3 to form integrin receptors for netrin 1 and reelin. These and other integrin beta 1 complexes have been historically known as very late activation (VLA) antigens.
Identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue
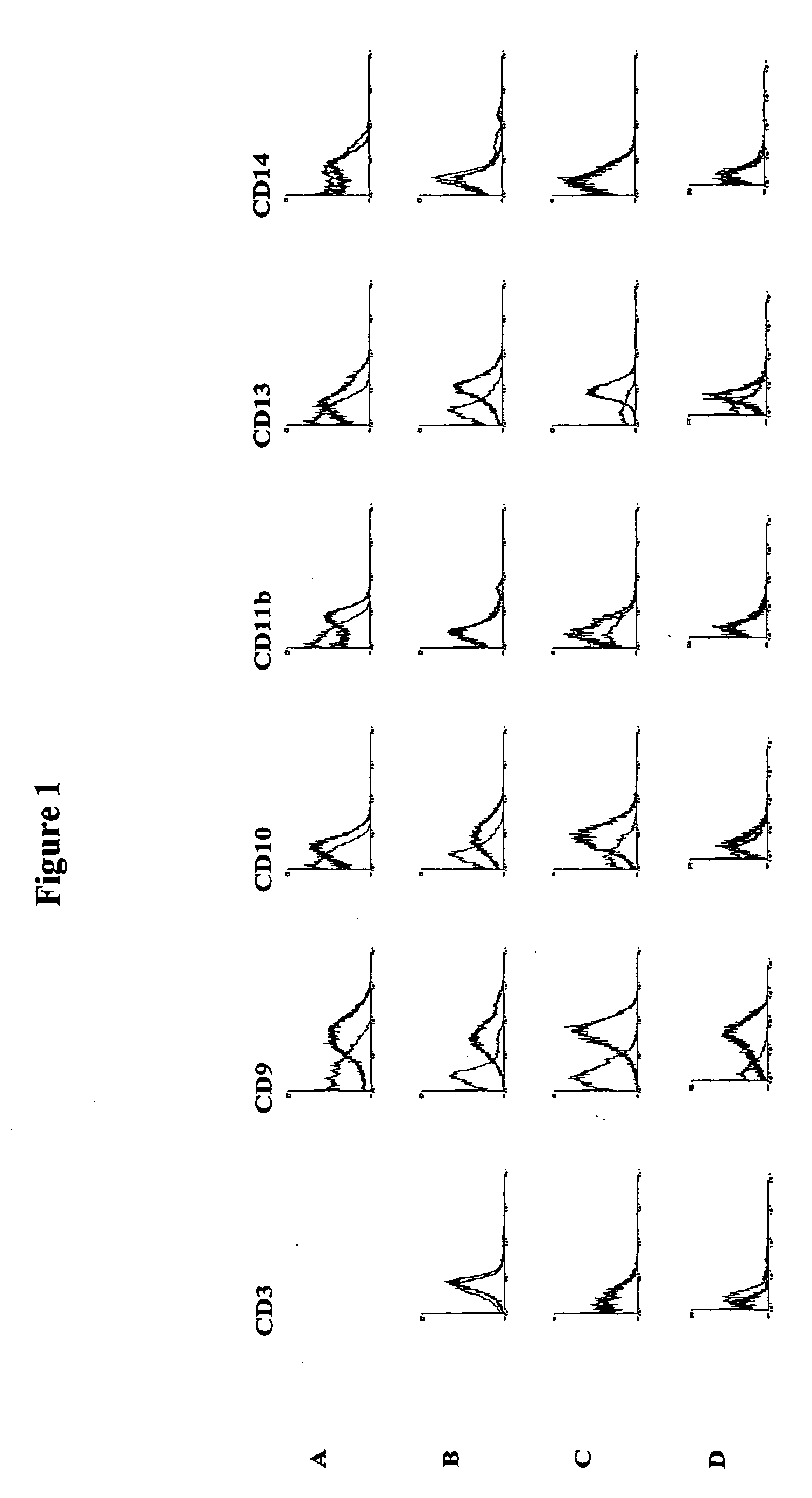
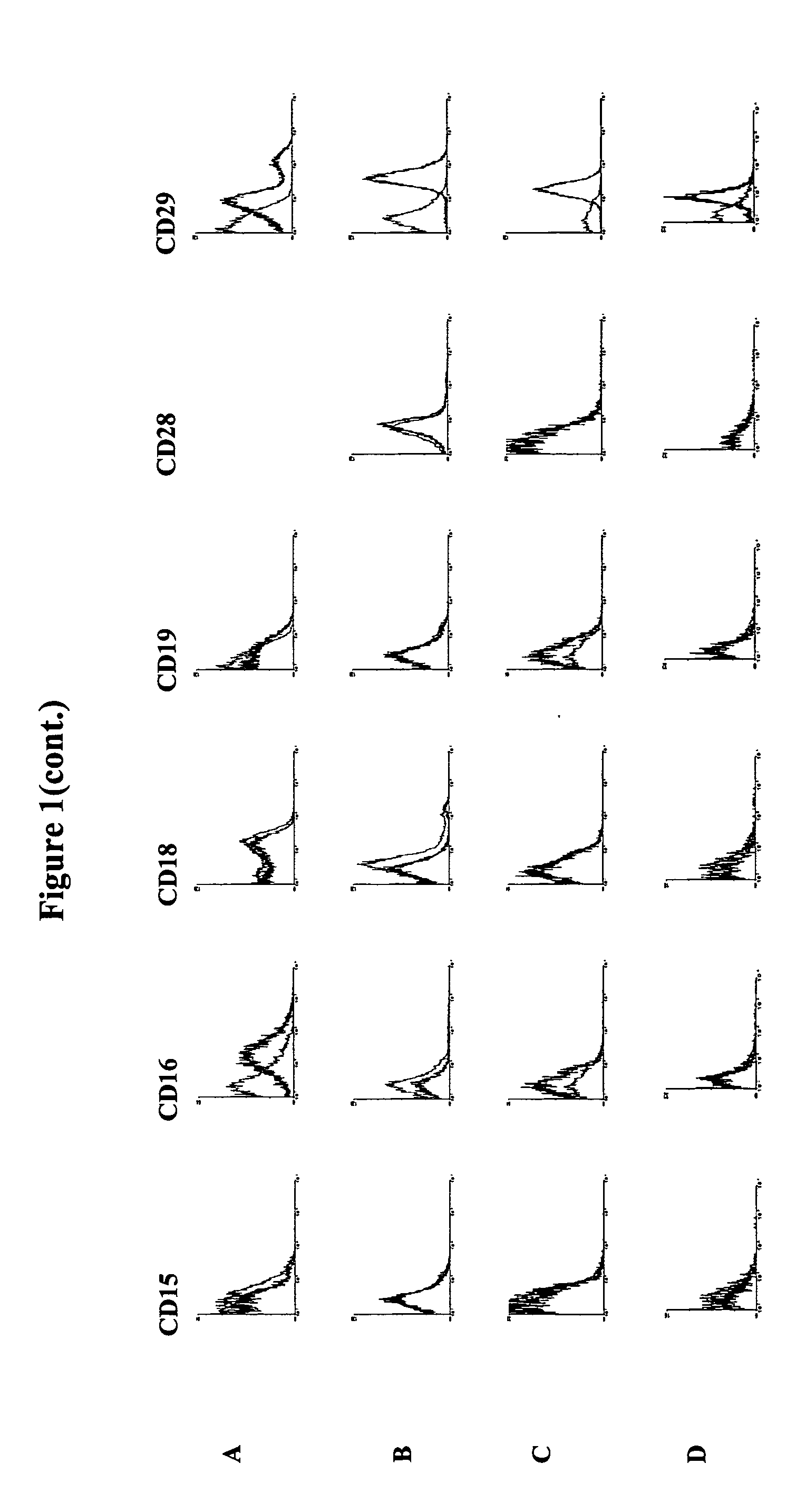
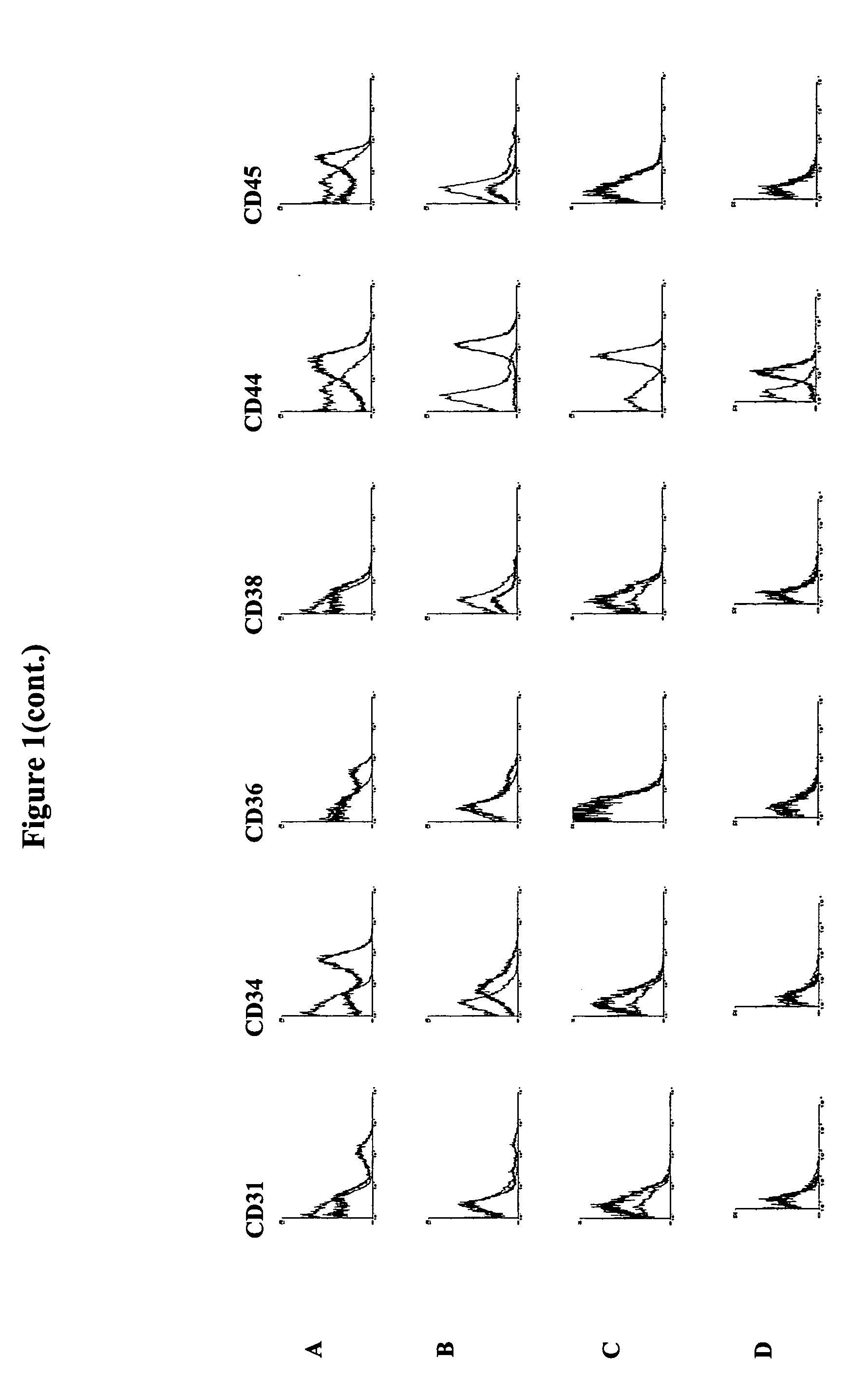
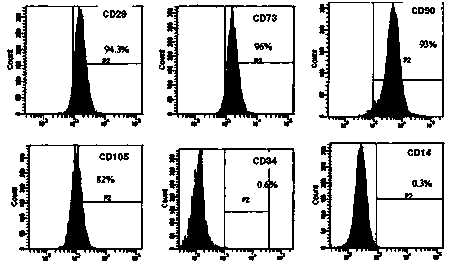
Methods for the identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue. Specifically, this invention relates to an adult multipotent cell or a cell population or composition comprising said cell, isolated from non-osteochondral mesenchymal tissue, characterized in that the cell is positive for the following markers: CD9, CD10, CD13, CD29, CD44, CD49A, CD51, CD54, CD55, CD58, CD59, CD90 and CD105 and because it lacks expression of the following markers: CD11b, CD14, CD15, CD16, CD31, CD34, CD45, CD49f, CD102, CD104, CD106 and CD133.
Owner:AUTONOMOUS UNIVERSITY OF MADRID +1
Multipotent stem cells derived from placenta tissue and cellular therapeutic agents comprising the same
InactiveUS20070243172A1Negative immunological responseBiocideArtificial cell constructsGerm layerDisease
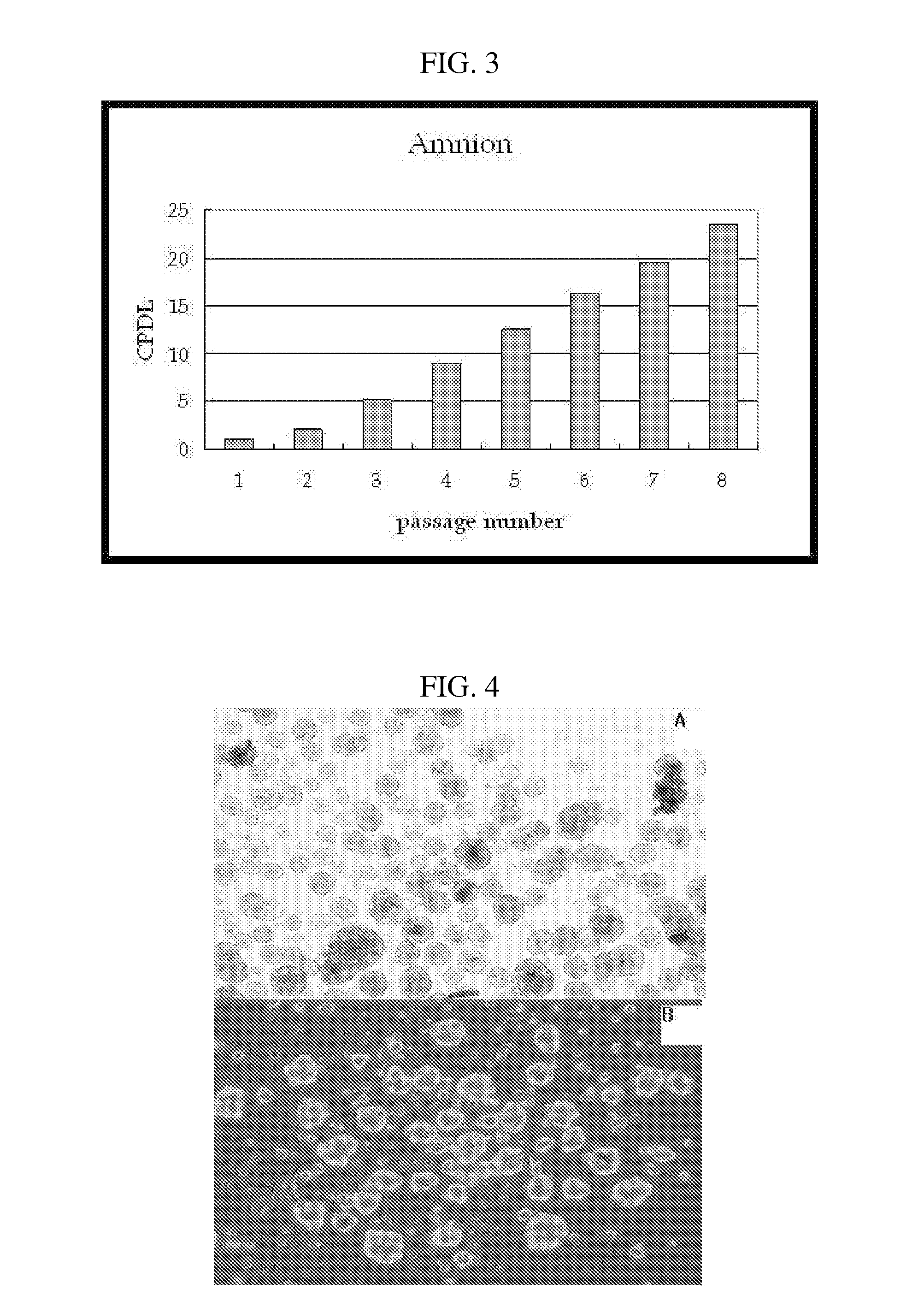
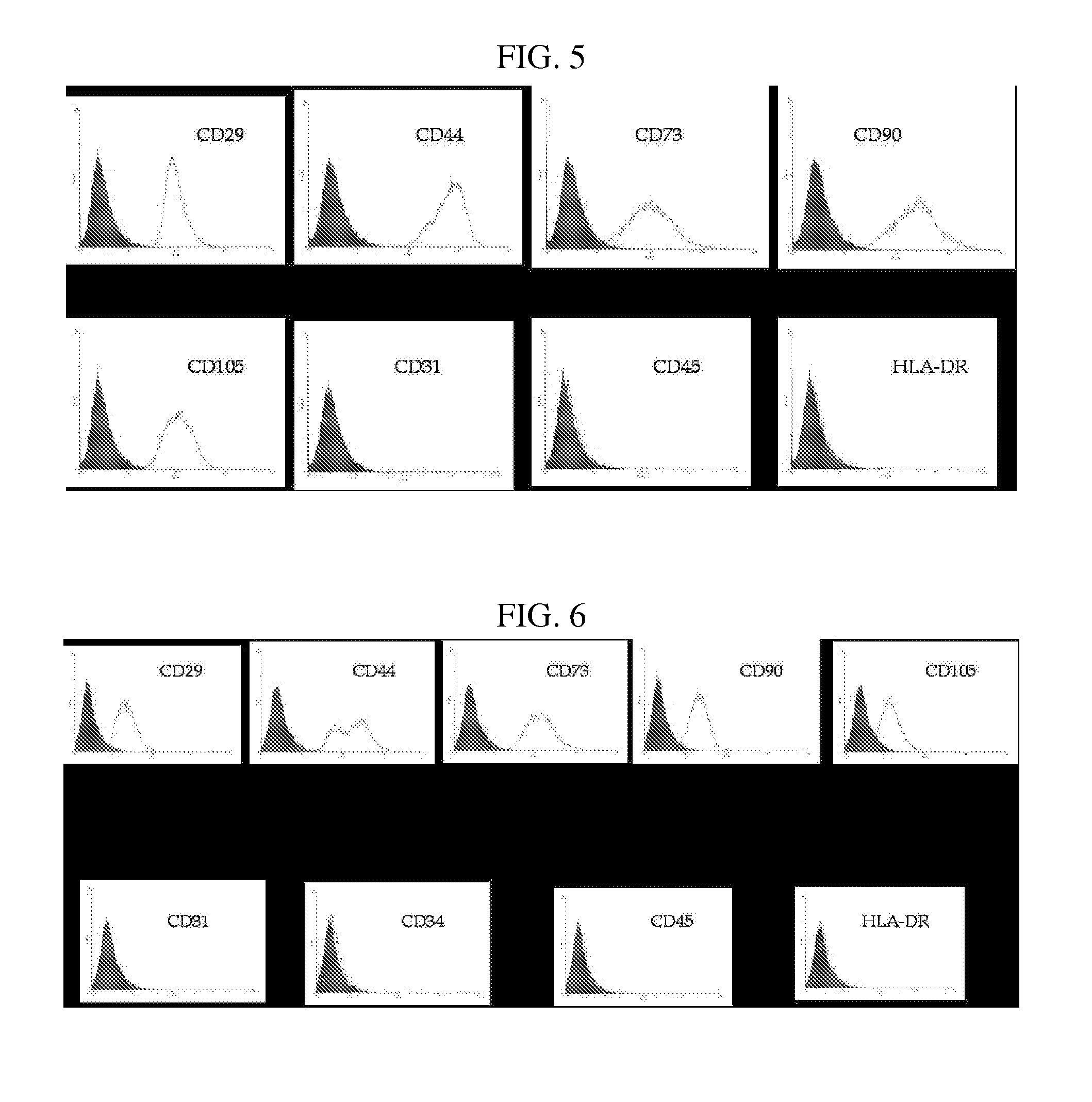
The present invention relates to placenta tissue-derived multipotent stem cells and cell therapeutic agents containing the same. More specifically, to a method for producing placenta stem cells having the following characteristics, the method comprising culturing amnion, chorion, decidua or placenta tissue in a medium containing collagenase and bFGF and collecting the cultured cells: (a) showing a positive immunological response to CD29, CD44, CD73, CD90 and CD105, and showing a negative immunological response to CD31, CD34, CD45 and HLA-DR; (b) showing a positive immunological response to Oct4 and SSEA4; (c) growing attached to plastic, showing a round-shaped or spindle-shaped morphology, and forming spheres in an SFM medium so as to be able to be maintained in an undifferentiated state for a long period of time; and (d) having the ability to differentiate into mesoderm-, endoderm- and ectoderm-derived cells. Also the present invention relates to placenta stem cells obtained using the production method. The inventive multipotent stem cells have the ability to differentiate into muscle cells, vascular endothelial cells, osteogenic cells, nerve cells, satellite cells, fat cells, cartilage-forming cells, osteogenic cells, or insuline-secreting pancreatic β-cells, and thus are effective for the treatment of muscular diseases, osteoporosis, osteoarthritis, nervous diseases, diabetes and the like, and are useful for the formation of breast tissue.
Owner:RNL BIO
Identification and Isolation of Multipotent Cells From Non-Osteochondral Mesenchymal Tissue
Identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue. This invention relates to the identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue. Specifically, it relates to an adult multipotent cell or a cell population or composition comprising said cell, isolated from non-osteochondral mesenchymal tissue, characterized in that it is positive for the following markers: CD9, CD10, CD13, CD29, CD44, CD49A, CD51, CD54, CD55, CD58, CD59, CD90 and CD105 and because it lacks expression of the following markers: CD11b, CD14, CD15, CD16, CD31, CD34, CD45, CD49f, CD102, CD104, CD106 and CD133.
Owner:AUTONOMOUS UNIVERSITY OF MADRID +1
Isolation and characterization of muscle regenerating cells
InactiveUS20060014287A1Useful for transplantationBiocideArtificial cell constructsSurface markerProgenitor
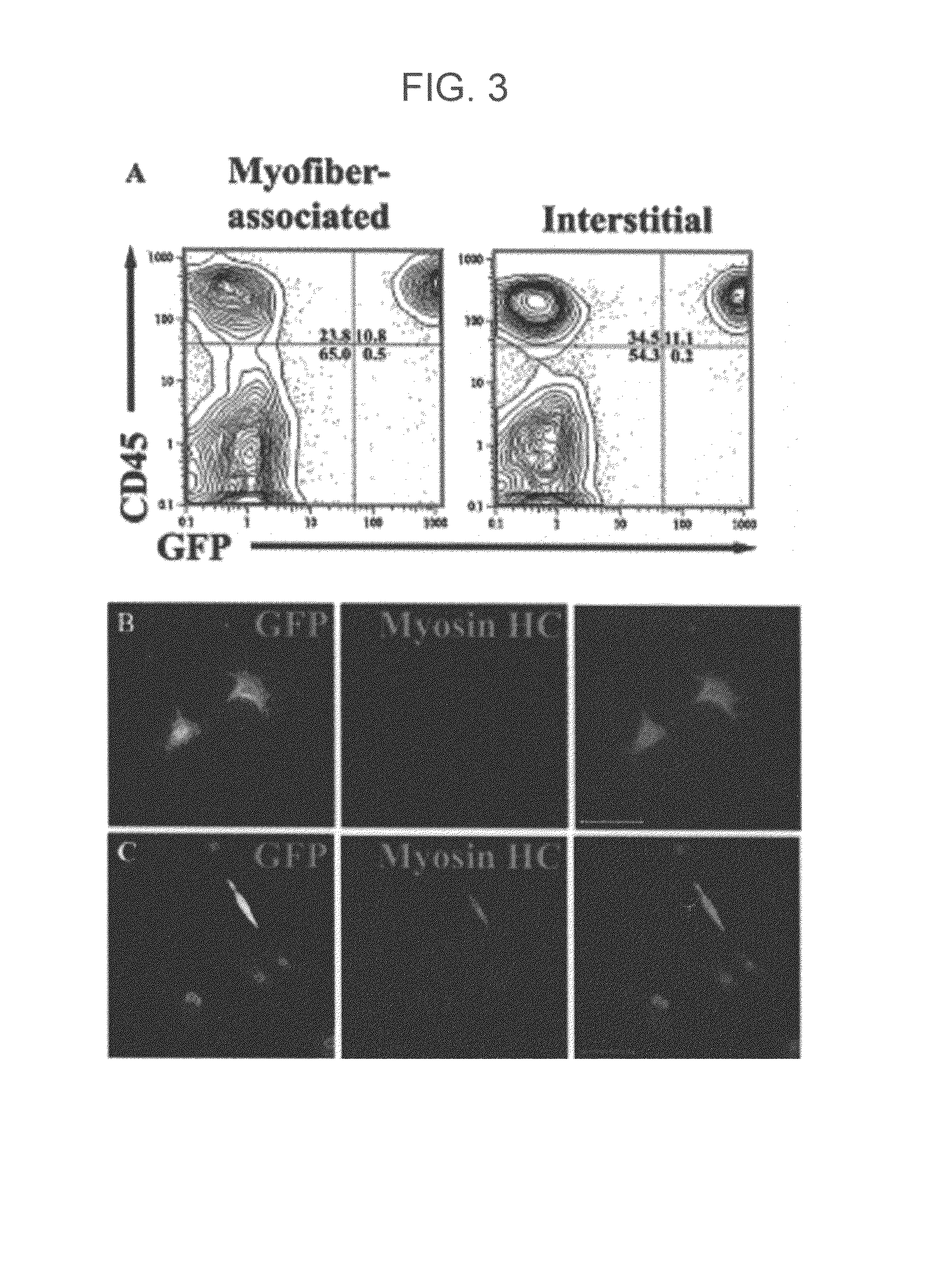
Populations enriched for myogenic progenitors are obtained by selection on the basis of expression of specific cell surface markers. The muscle progenitor cells are characterized as being CD45− and CD34+, and may further be characterized as lacking expression of Mac-1 (CD11b) and positive for expression of CXCR4 (CD184) and β1-integrin (CD29).
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
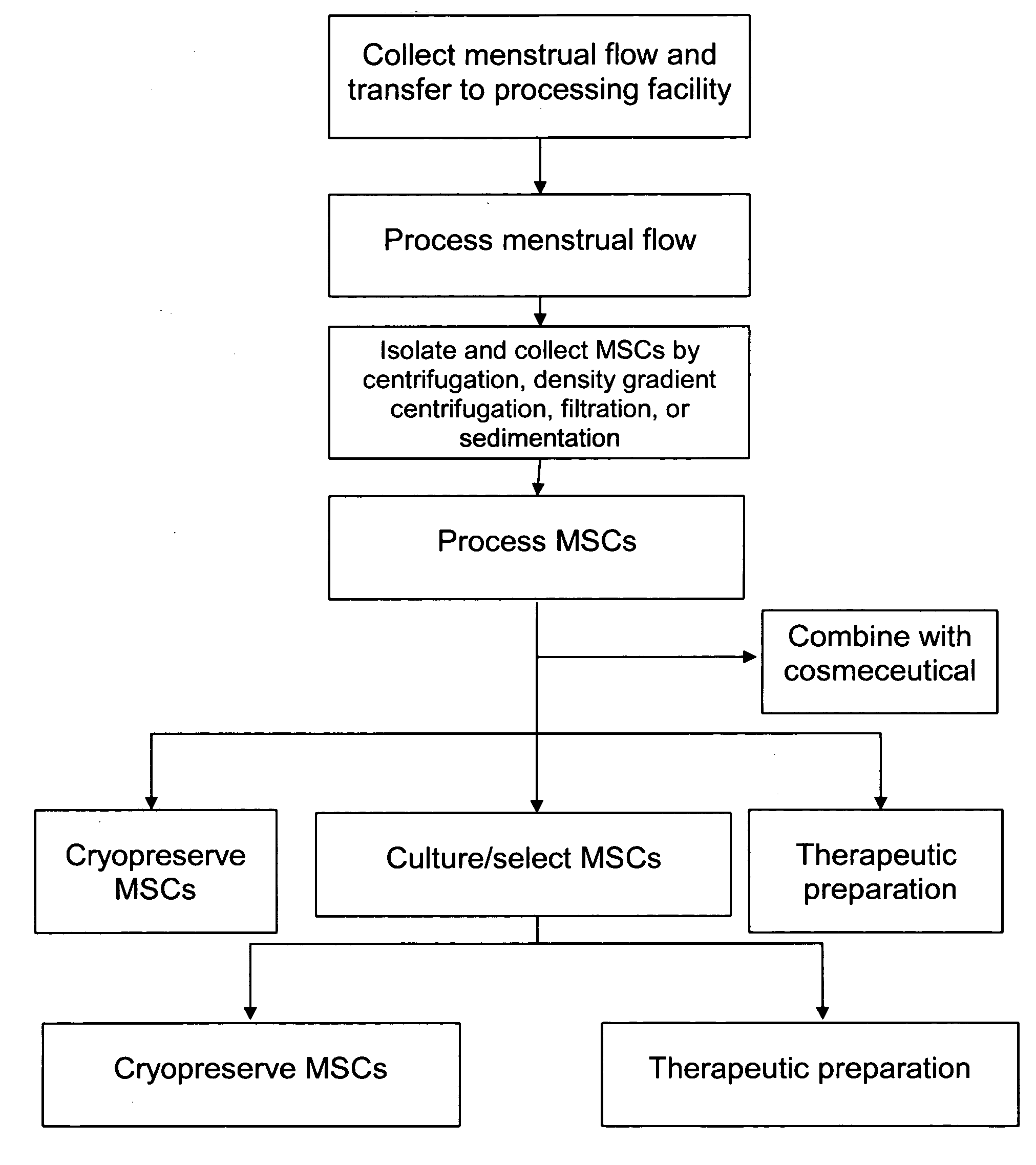
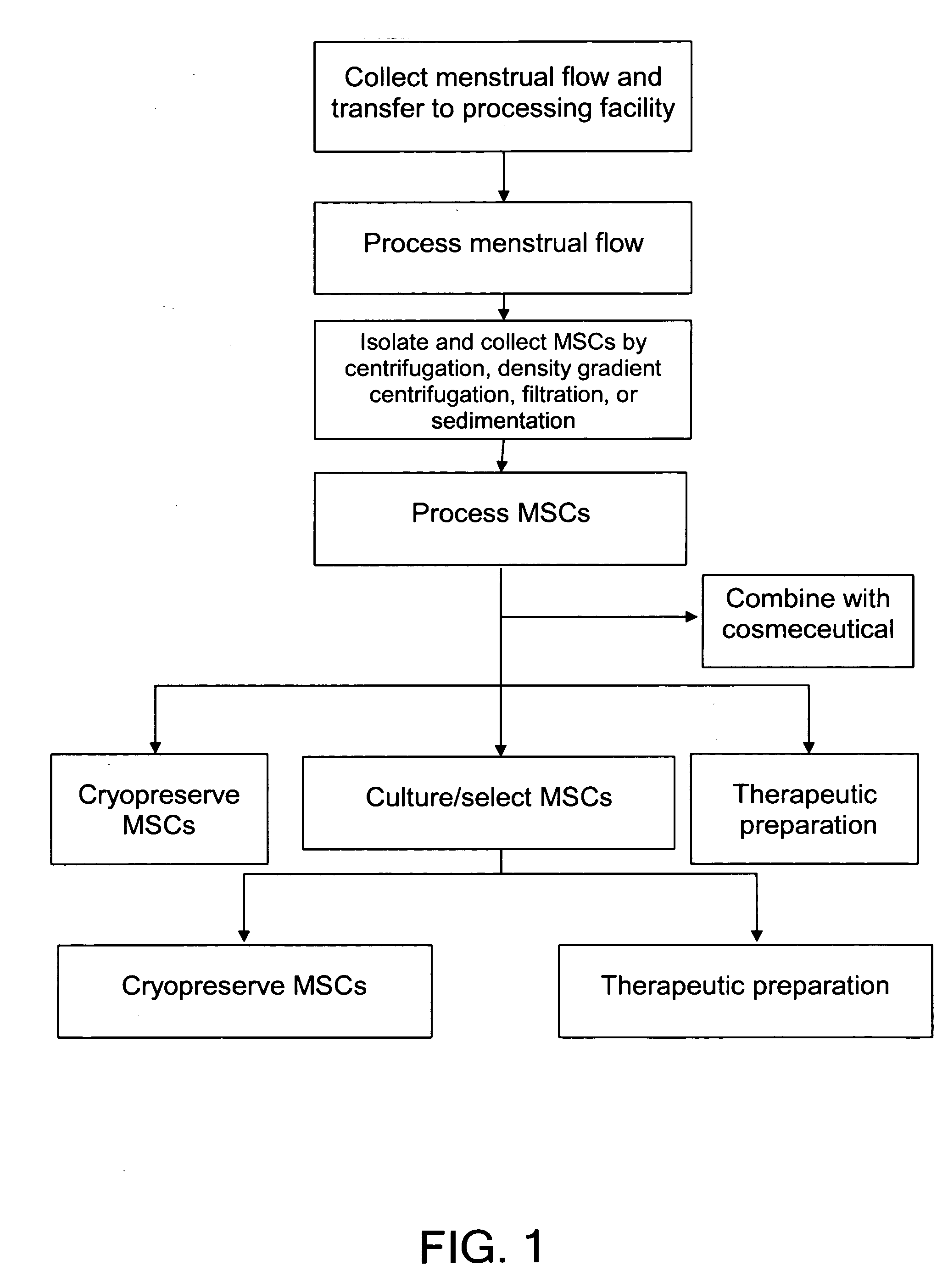
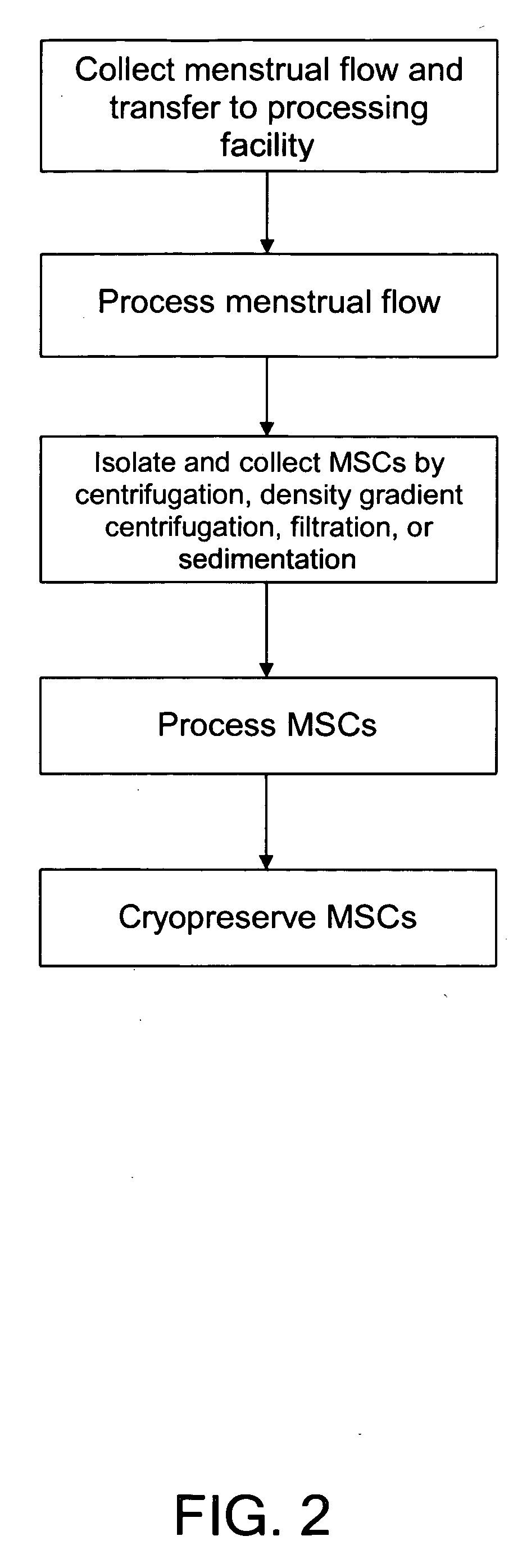
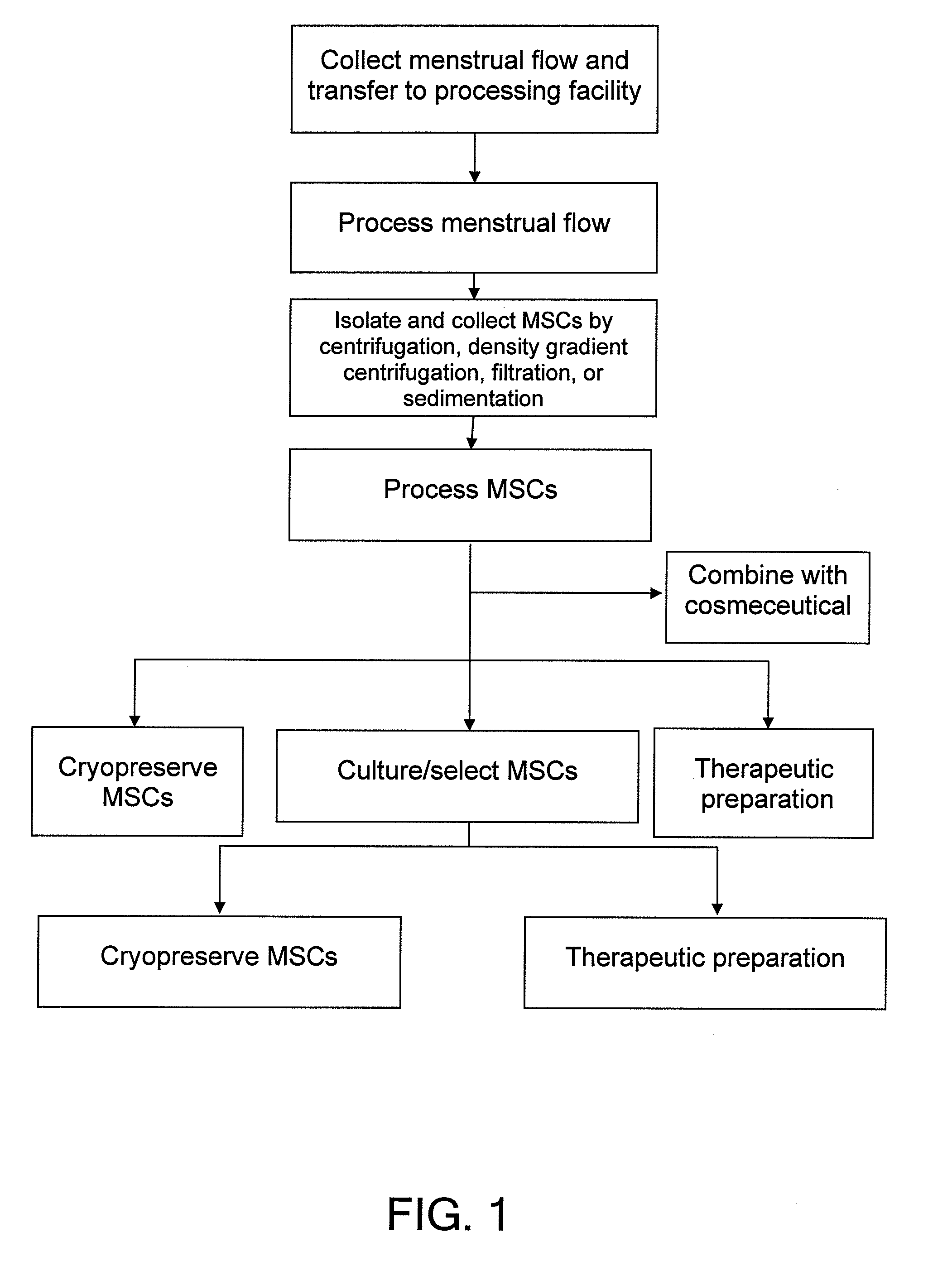
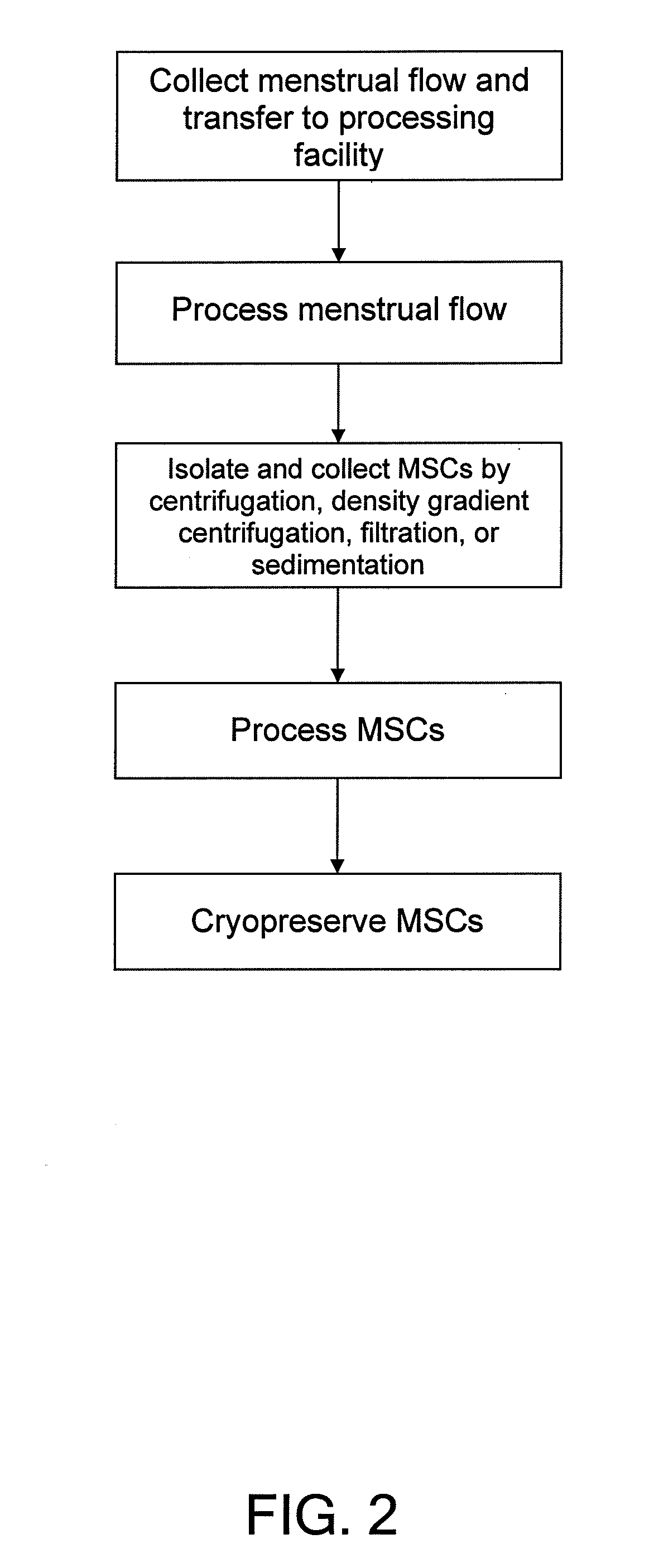
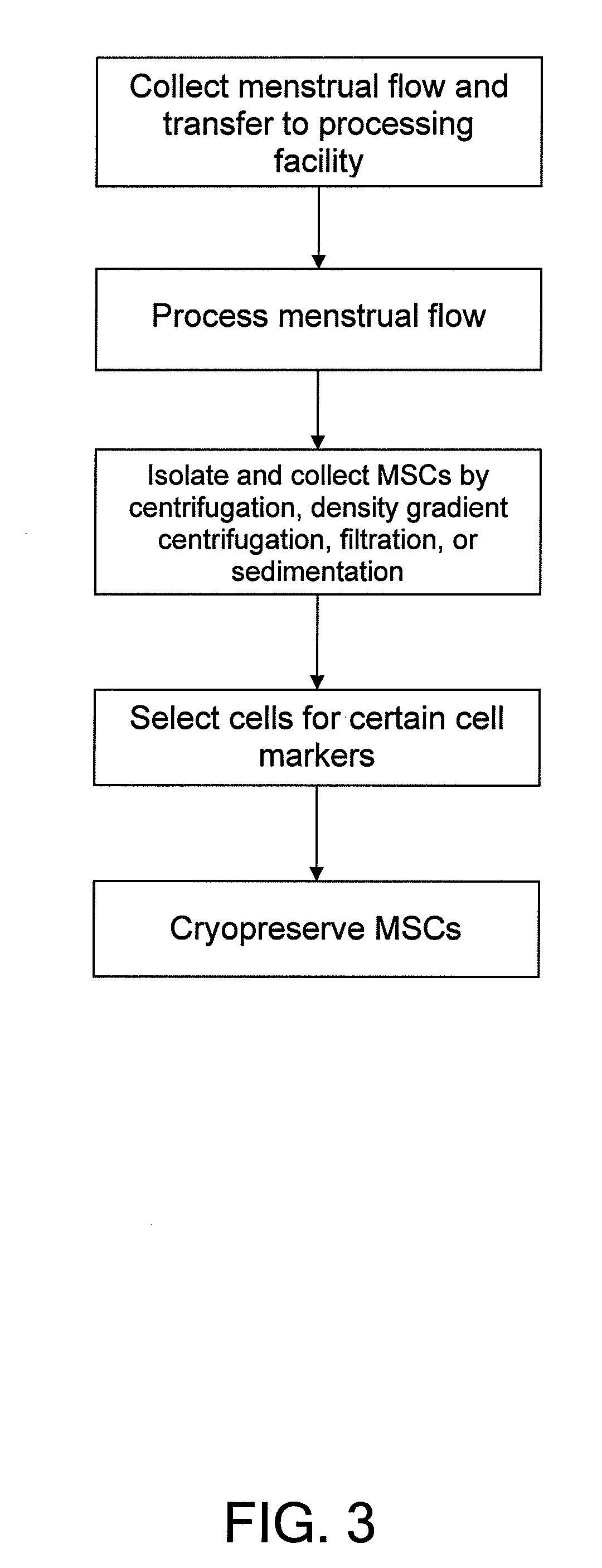
Procurement, isolation and cryopreservation of endometrial/menstrual cells
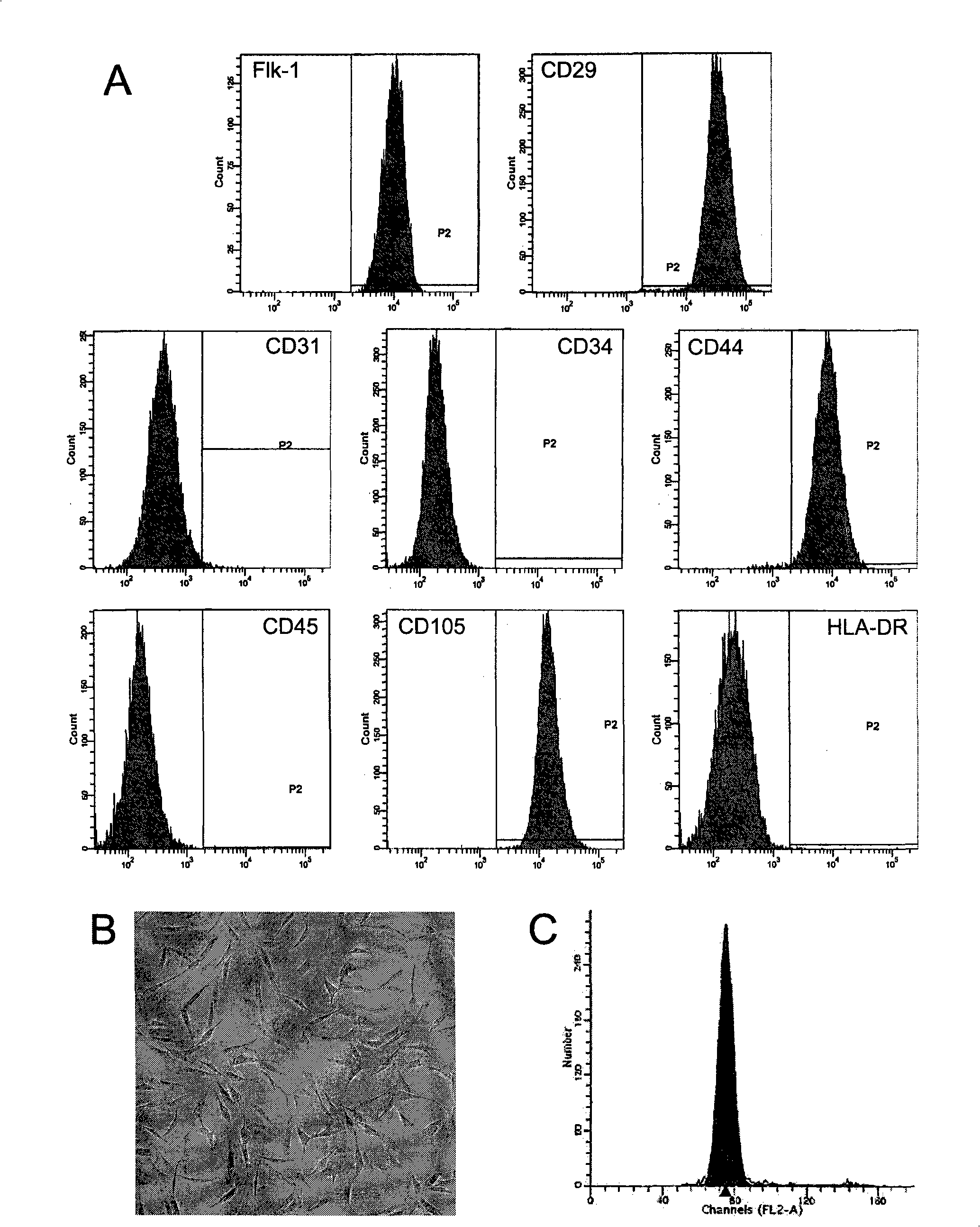
Compositions comprising menstrual stem cells (MSCs) and methods, processes, and system therefor are provided by the invention. MSCs are processed from menstrual flow collected during menses. MSCs may be cryopreserved, processed through various culturing and selection steps in preparation for cryopreservation, or processed for therapeutic or cosmeceutical use. Cryopreserved MSCs may be thawed in preparation for therapeutic and cosmeceutical use. MSCs express CD9, CD10, CD13, CD29, CD44, CD49e, CD49f, CD59, CD81, CD105, CD166, and HLA class I, and have low or no expression of CD3 and HLA class II.
Owner:CRYO CELL INTERNATIONAL INC
Method for isolated culture of human fat mesenchyma stem cell and special culture medium thereof
ActiveCN101314766AThe method of isolation and culture is simpleImprove efficiencySkeletal/connective tissue cellsAntigenMuscle injury
The invention discloses a method for separately culturing a human adipose mesenchymal stem cell and a dedicated culture medium thereof. The culture medium used for separately culturing the human adipose mesenchymal stem cell comprises an animal cell basic culture medium, fetal calf serum, an epidermal growth factor and a platelet-derived growth factor. The final concentration of the fetal calf serum is 1-200 mL / L, the final concentration of the epidermal growth factor is 1-100 ng / ml, and the final concentration of the platelet-derived growth factor is 1-100 ng / ml. The adipose mesenchymal stem cell of the invention has CD31-, CD34-, CD45- and HLA-DR-, as well as the phenotype of CD29+, CD44+, CD105+ and Flk-1+. The specificity cell surface marker and the relevant antihelion molecule of a skeletal muscle cell and a vascular endothelia cell can be expressed after inducement is performed in vitro. Muscle fiber, vascular endothelin and functional muscle satellite cells can be differentiated in a muscle injury model mouse body caused by medicine and the expression of dystrophin protein on the ducheme muscular dystrophy (DMD) model mouse (mdx) myolemma can be partially recovered, so as to release the pathological symptom of the model mouse.
Owner:微能生命科技集团有限公司
Separation, purification and identification methods of human amnion mesenchymal stem cells
InactiveCN102559586AMeet the treatment needsAccurate identification methodIndividual particle analysisEmbryonic cellsLow glucoseStaining
The invention discloses separation, purification and identification methods of human amnion mesenchymal stem cells. The separation method of hAMSCs (human amnion mesenchymal stem cells) comprises the following steps: fragmentating human amnion; and carrying out two-step rotating digestion with trypsin of EDTA (ethylene diamine tetra-acetic acid) and collagenase of DNaseI, filtering with a steel mesh and collecting cell filtrate namely separated original hAMSCs. The purification method of hAMSCs comprises the following steps: incubating original hAMSCs with an LG (low glucose)-DMEM (dulbecco modified eagle medium) culture medium in a CO2 incubator; removing amnion epithelial cells which do not perform complete adherence growth under an inverted microscope; replacing a new culture medium on the third day; digesting with a trypsin-EDTA solution after cell converge degree reaches 80-90%; and collecting cells so as to obtain high-purity hAMSCs. The identification method of hAMSCs comprises the following steps: identifying hAMSCs and the amnion epithelial cells by adopting immunocytochemical staining vimentin and CK19; and detecting expressions of CD29, CD44, CD166, CD34 and CD45 by adopting a flow cytometry. The separation and purification methods disclosed by the invention have the advantages of high yield, high activity and high purity of hAMSCs; and the identification method is simple, convenient and precise.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Isolation and characterization of muscle regenerating cells
Populations enriched for myogenic progenitors are obtained by selection on the basis of expression of specific cell surface markers. The muscle progenitor cells are characterized as being CD45− and CD34+, and may further be characterized as lacking expression of Mac-1 (CD11b) and positive for expression of CXCR4 (CD184) and β1-integrin (CD29).
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Stem cell preparation for treating primary liver cancer and preparation method thereof
ActiveCN103861088AImprove survivalGood conditionPeptide/protein ingredientsDigestive systemSolventImmunogenicity
The invention discloses a stem cell preparation for treating primary liver cancer and a preparation method thereof. The preparation contains (60-100)*10<6> pieces / ml of mesenchymal stem cells, 20ng / ml of HGF and 10ng / ml of FGF-4, and takes autologous platelet-rich plasma lysate as a solvent. The preparation method comprises the following steps of: (1) collecting an umbilical cord, and carrying out primary culture to obtain cells; (2) detecting by using a flow cytometry, wherein such cells express CD29, CD73, CD90 and CD105; (3) collecting cells, namely dissolving (60-100)*10<6> mesenchymal stem cells of the human umbilical cord in 1 ml of autologous platelet-rich plasma lysate, and adding 20ng of HGF and 10ng of FGF-4 to be prepared into 1 ml of a stem cell preparation. According to the stem cell preparation disclosed by the invention, the source of raw materials is rich, the raw materials are easy to obtain, the umbilical cord mesenchymal stem cells are weakly immunogenic, and platelet-rich plasma is taken from the patients, so no immunogenicity exists.
Owner:奥思达干细胞有限公司
Methods and compositions for promoting thermogenic potential
ActiveUS20180362623A1Decrease weightReduce weightOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsOn cellsUncoupling protein
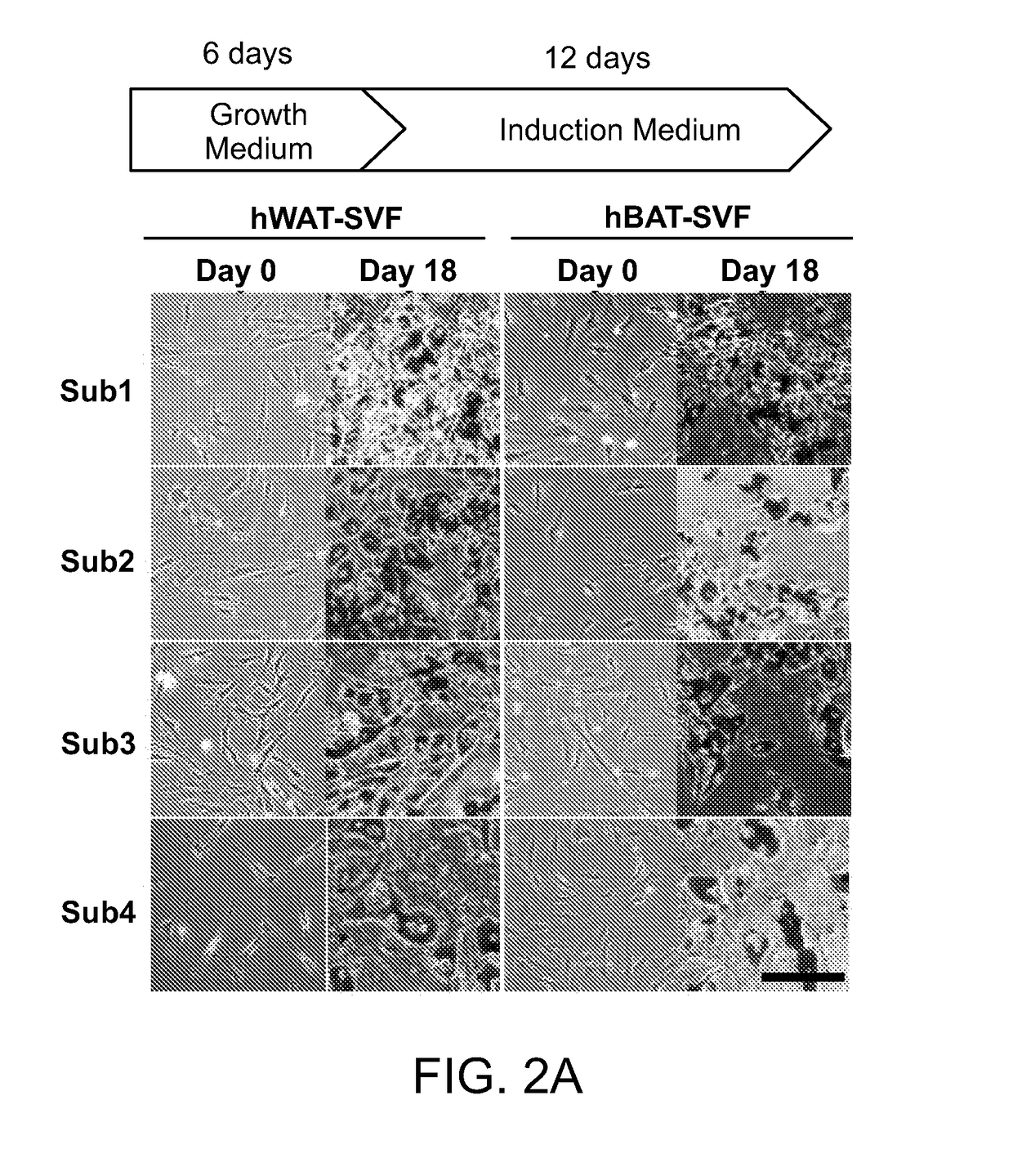
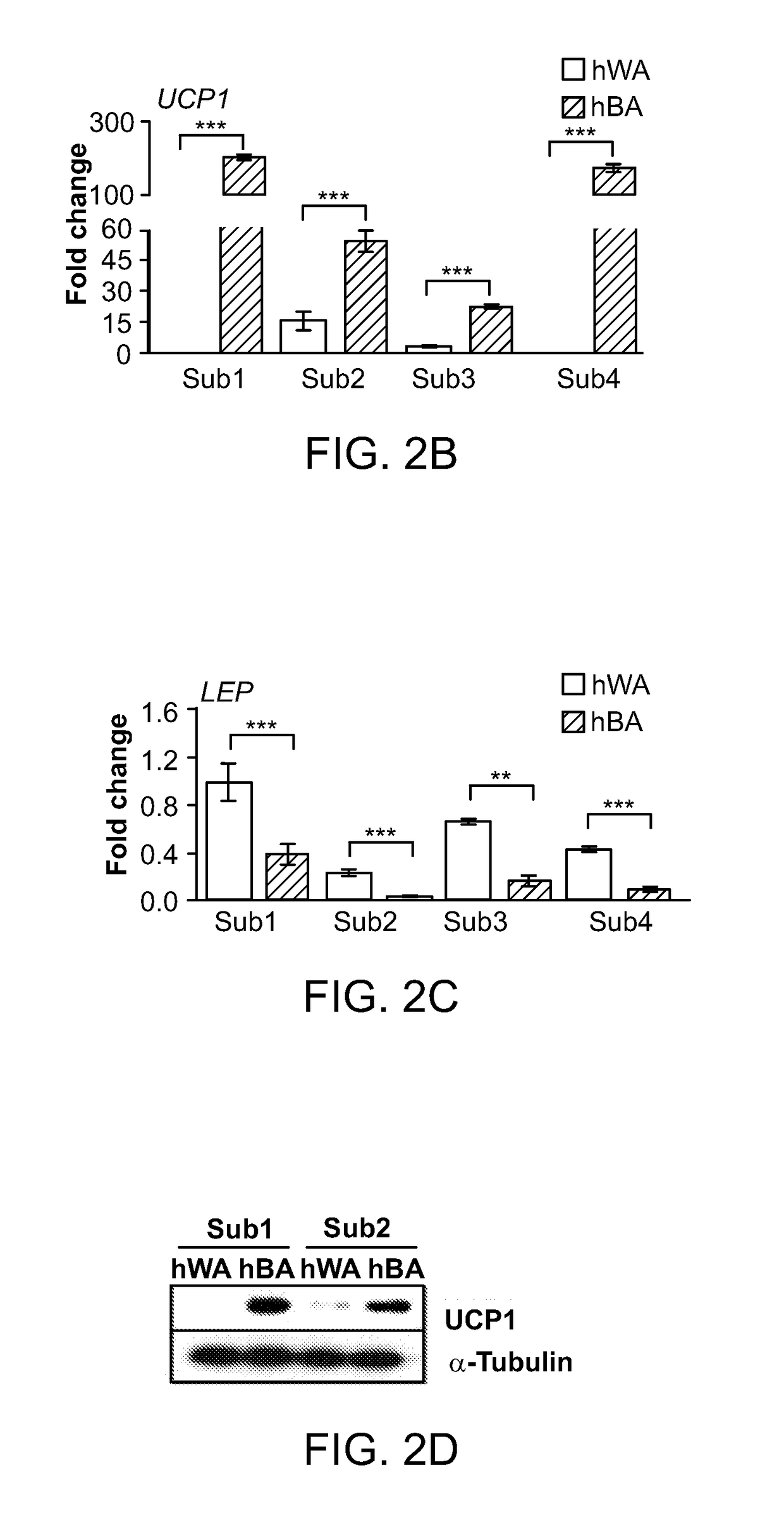
The invention provides methods and compositions relating to molecular targets identified as being capable of increasing or decreasing thermogenic potential in cells, including preadipocytes. Included in the invention are methods and compositions relating to inhibiting or suppressing the activity of an uncoupling protein 1 (UCP1) negative regulator, such as cardiac actin 1 (ACTC1), somatostatin receptor 1 (SSTR1), FAT atypical cadherin 1 (FAT1), and protein tyrosine phosphatase receptor type B (PT-PRB). Also included in the invention are methods and compositions relating to activating a UCP1 positive regulator, such as phosphatidylinositol-3,4,5-triphosphate-dependent Rac exchange factor 1 (PREX1), cortactin binding protein 2 (CTTNBP2), doublesex and mab-3-related transcription factor-like family A1 (DMRTA1), and endothelin receptor type B (ENDRB). The invention also provides methods and compositions relating to enrichment of cells having thermogenic potential based on cell surface markers, e.g., CD29, identified as being predictive of such.
Owner:JOSLIN D ABETES CENTER INC
Multipotent adult stem cells having an ability of oct4 expression derived from umbilical cord blood and method for preparing the same
InactiveUS20090305413A1Negative immunological responseBlood/immune system cellsCell culture active agentsGerm layerDisease
The present invention relates to multipotent adult stem cells expressing Oct4, derived from umbilical cord blood (UCB) and also these cell are expressing CD29, CD31, CD44, simultaneously, a method for preparing the same, and more specifically to multipotent adult stem cells which are obtained by culturing umbilical cord blood-derived monocytes in a medium containing bFGF (basic fibroblast growth factor) and human serum or plasma. In addition, multipotent adult stem cells expressing Oct-4 from UCB are morphologically spindle or round shaped cells Although the stem cells according to the present invention are adult stem cells, they are multipotent and capable of differentiating into ectodermal-, messodermal-, endodermal-originated tissue or cells including osteogenic cells or nerve cells etc, thus they can be effectively used in the treatment of intractable diseases and incurable diseases.
Owner:SEOUL NAT UNIV R&DB FOUND
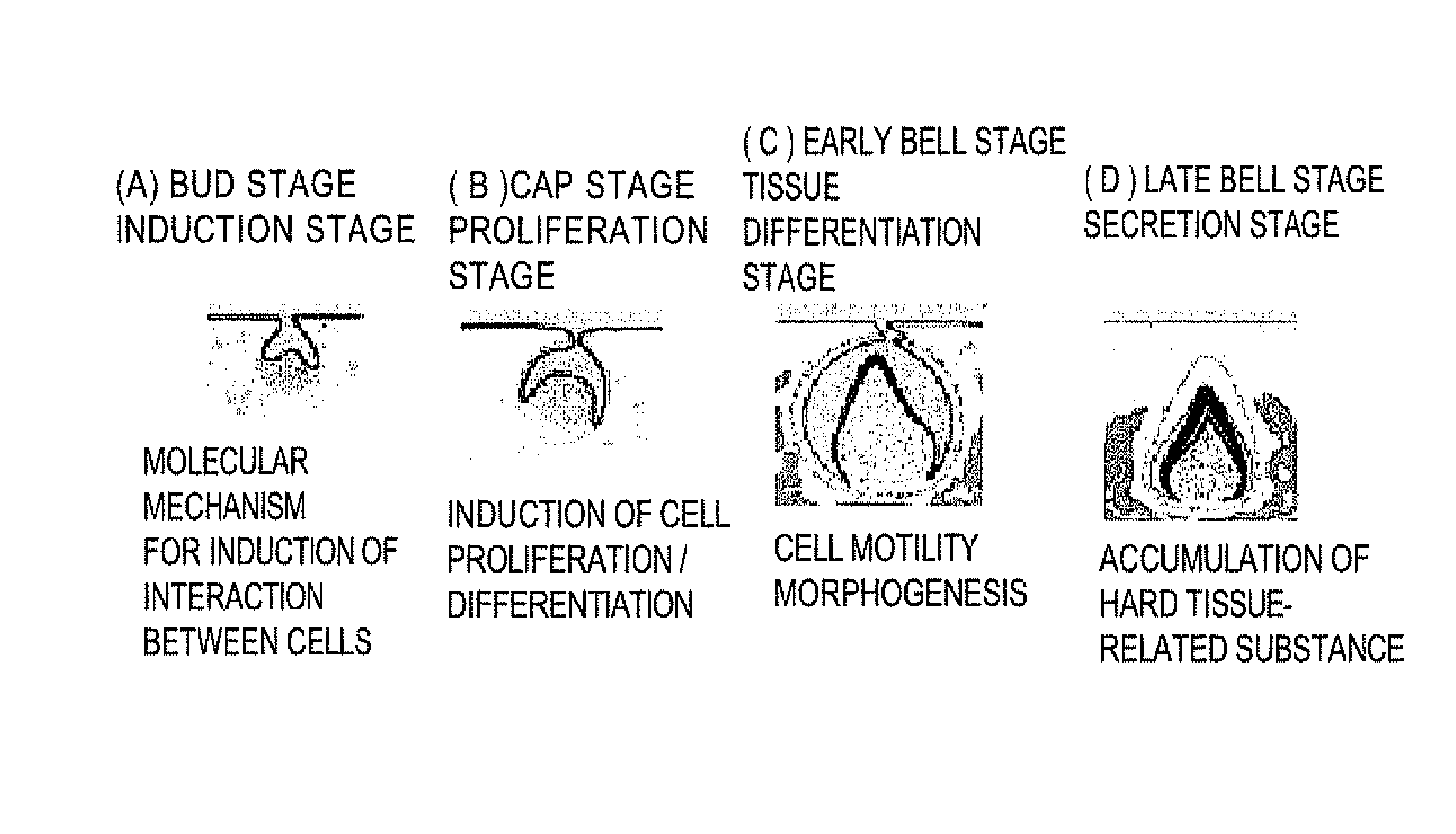
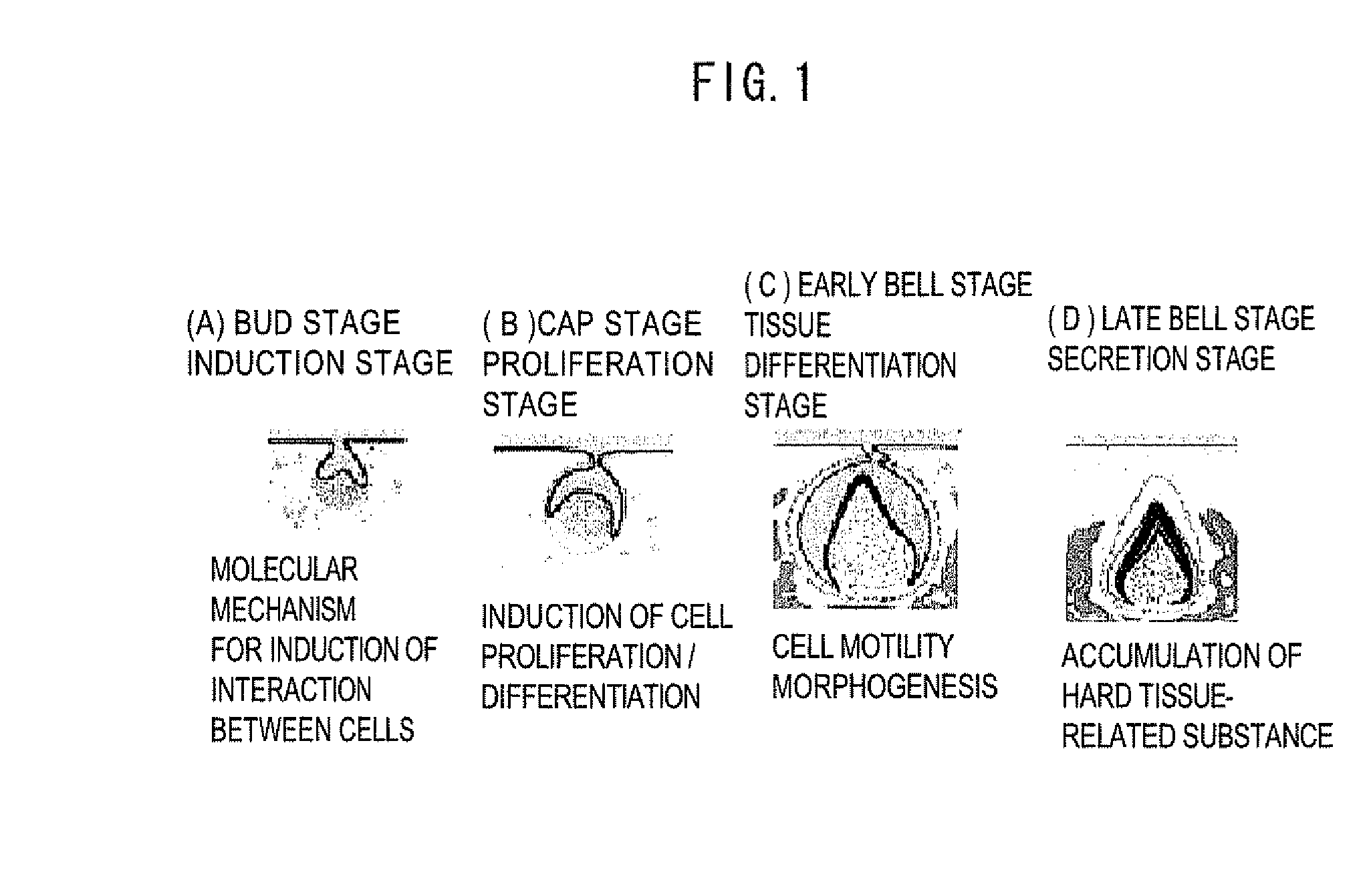
Method for production of mesenchymal cell, method for production of tooth, and mesenchymal cell for formation of tooth
InactiveUS20100119997A1Effectively in large amountEfficient productionTeeth fillingMammal material medical ingredientsCD29Cell mass
The present invention provides a method for producing mesenchymal cells for production of mesenchymal cells for formation of a tooth, the method comprising: culturing totipotent stem cells in the presence of a differentiation inducer to produce a cell population after differentiation induction treatment, the cell population containing CD44-positive and CD29-positive cells or CD44-positive and CD 106-positive cells; and selecting, from the cell population after the differentiation induction treatment, the CD44-positive and CD29-positive cells or CD44-positive and CD 106-positive cells as the mesenchymal cells for the formation of the tooth. The present invention also provides a method for producing a tooth comprising: positioning, in a support carrier capable of retaining cells in a state of contacting therewith, a first cell mass substantially consisting of only either one of mesenchymal cells and epithelial cells and a second cell mass substantially consisting of only the other one of the mesenchymal cells and epithelial cells, the first and second cell masses being not mixed with each other but made to closely contact with each other; and culturing the first and second cell masses; wherein the mesenchymal cells comprise the mesenchymal cells for the formation of the tooth.
Owner:ORGAN TECH
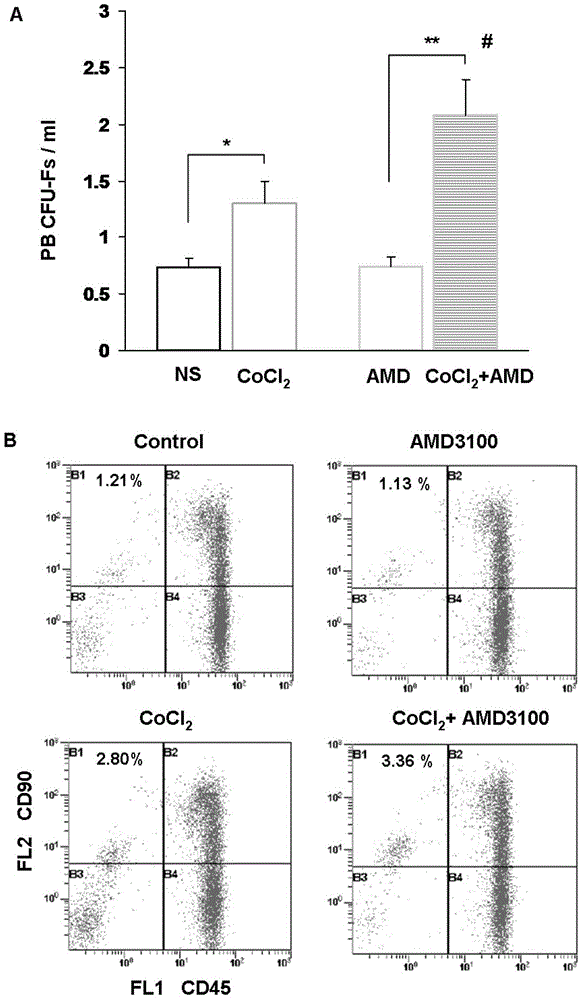
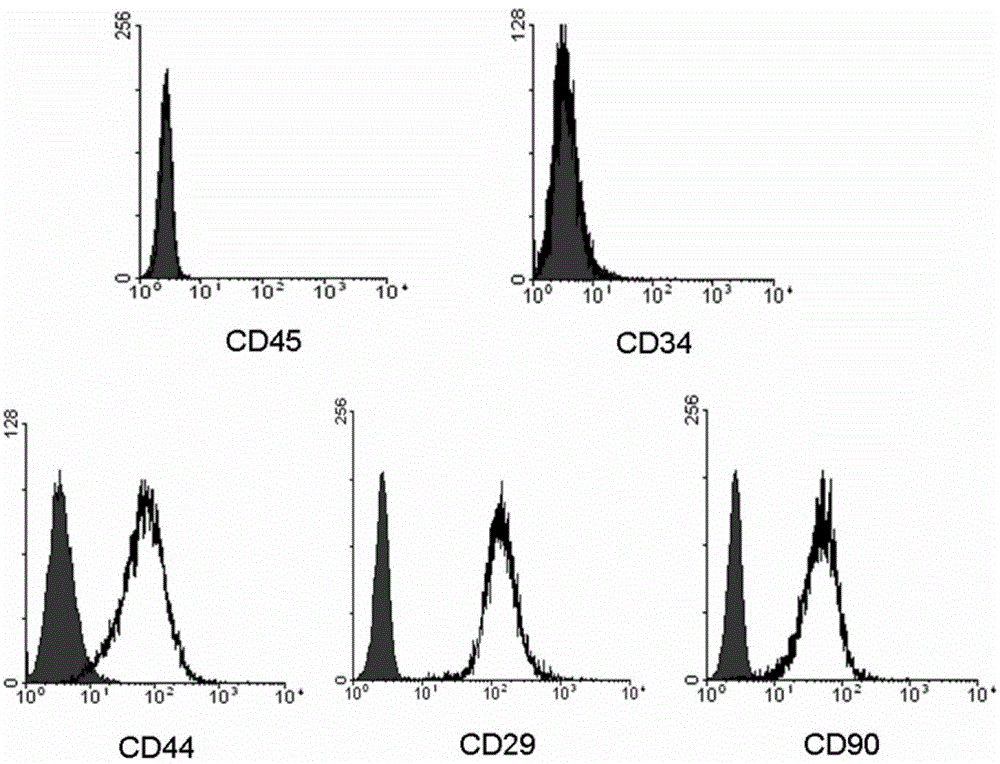
Preparation for mobilizing mesenchymal stem cells and method for separating mesenchymal stem cells
InactiveCN103146646AHigh mobilization efficiencySkeletal/connective tissue cellsLymphocytic cellRegenerative medicine
The invention provides a preparation for mobilizing mesenchymal stem cells and a method for separating the mesenchymal stem cells. The preparation for mobilizing the mesenchymal stem cells comprises CoCl2, wherein the CoCl2 is a hypoxia mimetic agent, and the dosage is in a range of 5-20mg / kg. The CoCl2 and AMD3100 are used in a combined manner. The dosage of the AMD3100 is 5mg / kg. According to the method for separating the mesenchymal stem cells by using the preparation, the preparation is used for actuating the mesenchymal stem cells to be mobilized, enter peripheral blood and then be separated. The method comprises the following separation steps of: sampling the periphery blood, separating mononuclear cells by using a lymphocyte separating medium, performing resuspension by using a DMEM (dulbeccos modified eagle medium) containing 20% of fetal bovine serum, inoculating to a culture flask, culturing for 7 days, and changing a culture solution, thus obtaining the mesenchymal stem cells of the peripheral blood on the 10th day. The mesenchymal stem cells of the peripheral blood highly express CD90, CD29 and CD44, do not express CD45 and CD34, and have the capacities of in vitro bone formation, fat formation and cartilage differentiation formation. The preparation is high in MSCs (mesenchymal stem cells) mobilization efficiency, and an effective preparation and an effective method are provided for tissue engineering and regenerative medicine.
Owner:ZHEJIANG UNIV
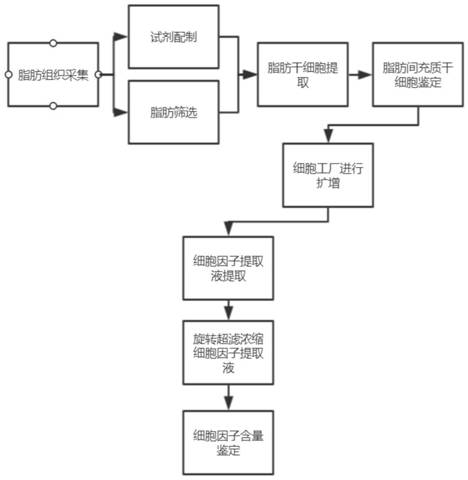
Method for extracting paracrine factor from adipose-derived stem cells
PendingCN112080465AStable karyotypeStable extraction efficiencyPeptide preparation methodsSkeletal/connective tissue cellsCD29Digestion
The invention discloses a method for extracting a paracrine factor from adipose-derived stem cells. The method comprises the following steps: taking a collected fat sample, and washing the fat samplewith normal saline for 1-3 times; adding a proper amount of adipose cell digestive juice for digestion; performing centrifugation, resuspending cell precipitate, and performing primary culture with aserum-free medium; performing subculture; performing enlarged culture; and performing extraction with a paracrine factor extracting solution, and performing purification to obtain the paracrine factor, wherein the paracrine factor extracting solution is prepared from a PBS buffer solution, L-glutamine, D-glucose and L-ascorbic acid, and the concentrations of the L-glutamine, the D-glucose and theL-ascorbic acid are 1-3mmol / L, 10-20 micromoles / L and 10-100 micromoles / L respectively. The method disclosed by the invention can stably extract the adipose-derived stem cells from adipose tissues, and extract the paracrine factor of the adipose-derived stem cells by utilizing the adipose-derived stem cells, so that the cell quality and the factor extraction efficiency are stable; and the adipose-derived stem cells are separated and cultured for a long time in a GMP environment, do not contact any animal-derived component, and can efficiently express adipose-derived stem cell markers such as CD29, CD44 and CD105.
Owner:北京银丰鼎诚生物工程技术有限公司
Mesenchymal stem cell expressing at least one cell surface marker selected from the group consisting of cd201, cd46, cd56, cd147, and cd165, method for preparing the same, pharmaceutical composition containing the mesenchymal stem cells, and method for preparing the same
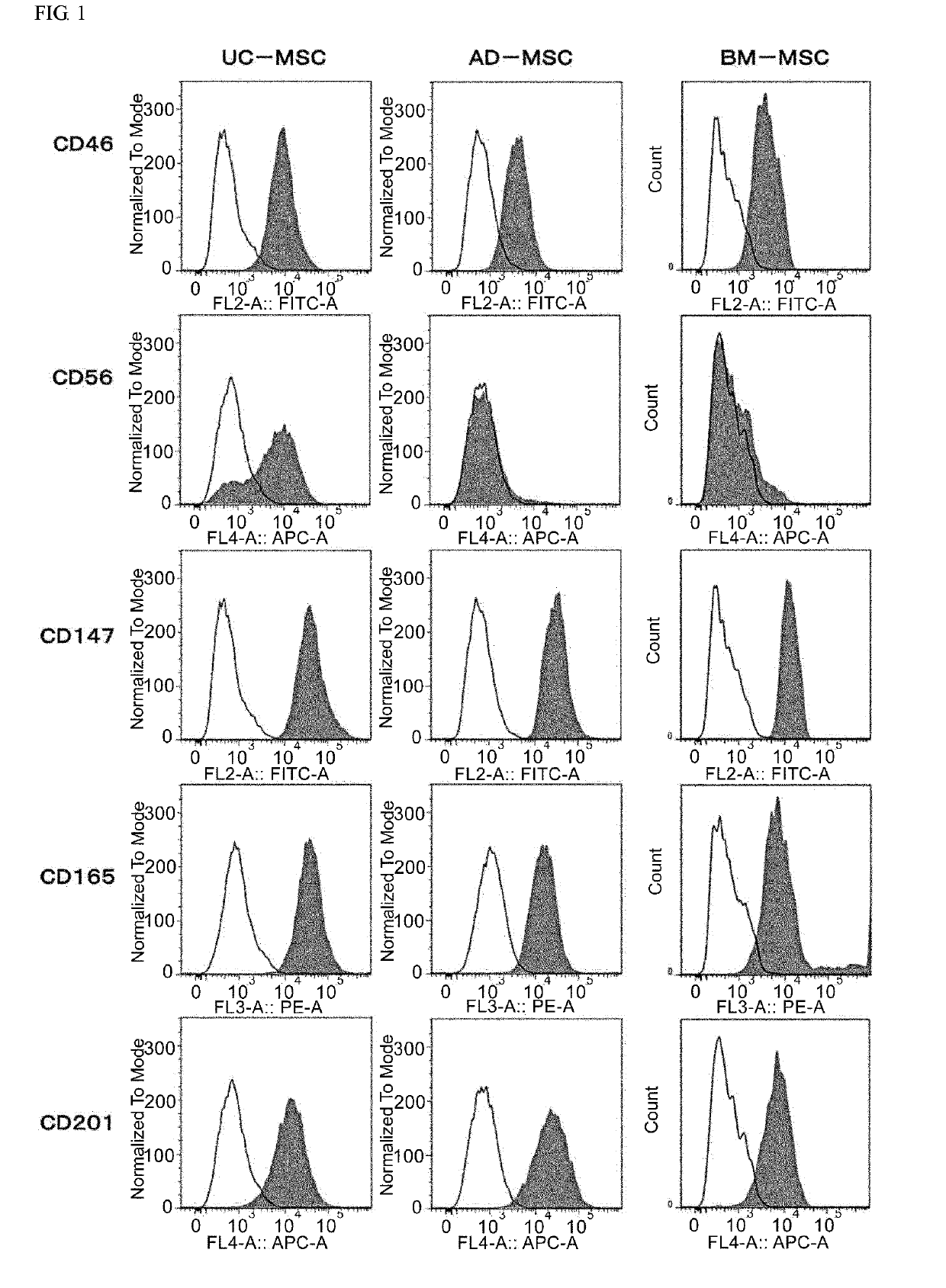
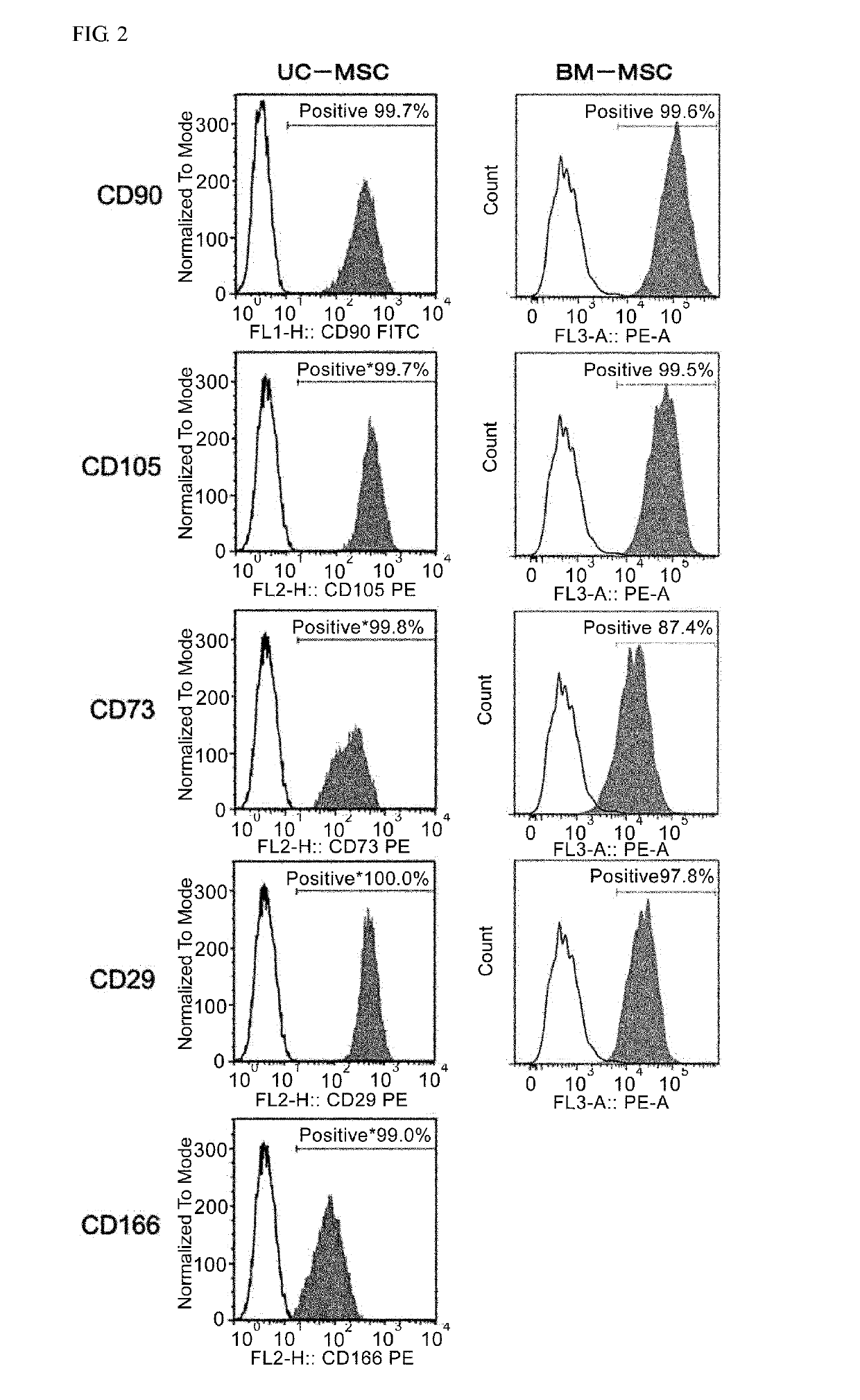
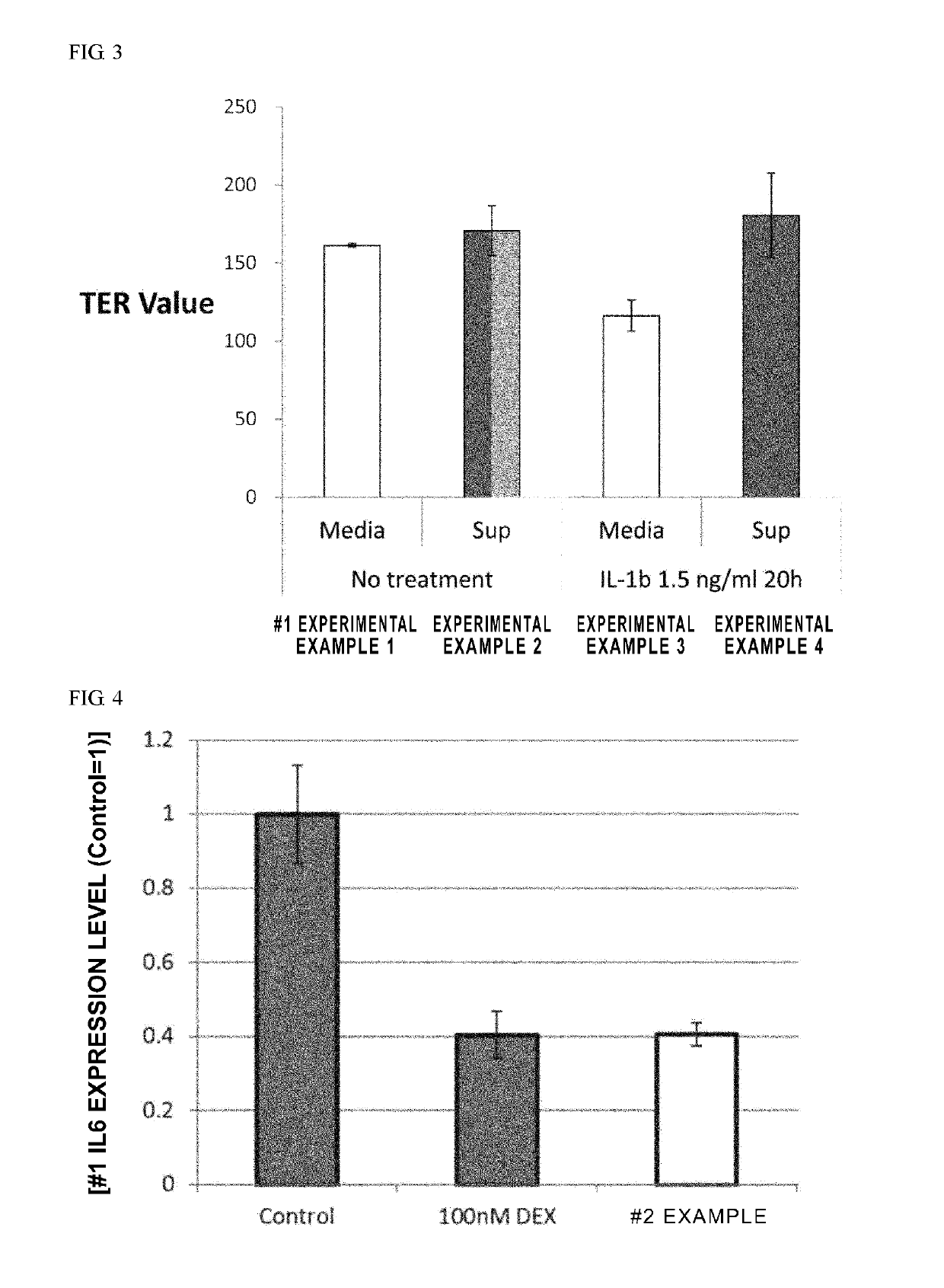
An object of the present invention is to provide novel mesenchymal stem cells demonstrating superior therapeutic effects for various diseases, a novel pharmaceutical composition containing the mesenchymal stem cells, and methods for preparing these. The present invention provides mesenchymal stem cells expressing at least one cell surface marker selected from the group consisting of CD201, CD46, CD56, CD147 and CD165. The mesenchymal stem cells expressing such a specific marker are positive for CD29, CD73, CD90, CD105 and CD166, and maintain an undifferentiated state.
Owner:ROHTO PHARM CO LTD
Ror1-positive mesenchymal stem cells and method for preparing same, pharmaceutical composition containing ror1-positive mesenchymal stem cells and method for preparing same, and method for preventing or treating diseases by using ror1-positive mesenchymal stem cells
ActiveCN108350427ANot easy to damageImprove mobilityMetabolism disorderGenetically modified cellsDiseaseMedicine
The purpose of the present invention is to provide: new mesenchymal stem cells having excellent therapeutic effect on various diseases, a new pharmaceutical composition containing the mesenchymal stemcells, and a method for preparing the same. The present invention is ROR1-positive mesenchymal stem cells. Preferably, the ROR1-positive mesenchymal stem cells are CD29-, CD73-, CD90-, CD105-, and CD166-positive, and are derived from an umbilical cord or fat.
Owner:ROHTO PHARM CO LTD
Subpopulations of spore-like cells and uses thereof
Subpopulations of spore-like cells expressing specific cell surface and gene expression markers are provided. In one embodiment, the cells express at least one cell surface or gene expression marker selected from the group consisting of Oct4, nanog, Zfp296, cripto, Gdf3, UtF1, Ecat1, Esg1, Sox2, Pax6, nestin, SCA-1, CD29, CD34, CD90, B1 integrin, cKit, SP-C, CC10, SF1, DAX1, and SCG10. Also provided are methods for purifying a subpopulation of spore-like cells of interest from a population of spore-like cells, and methods for inducing differentiation of the isolated spore-like cells into cells of endodermal, mesodermal or ectodermal origin. The spore-like cells can be used to generate cells originating from all three germ layers and can be used to treat a patient who has a deficiency of functional cells in any of a wide variety of tissues, including the retina, intestine, bladder, kidney, liver, lung, nervous system, or endocrine system.
Owner:VCELL THERAPEUTICS
Method for isolating stem cells and their use in cell therapy
The invention relates to a method for isolating muscle-derived stem cells that can be used in cell therapy, said method comprising the steps of (i) dissociating cells from at least one muscle sample, (ii) plating the cells obtained at the end of step (i) on a non-coated cell container, (iii) isolating the cells present in the supernatant of the non-coated cell container obtained at the end of step (ii), (iv) plating the cells obtained at the end of step (iii) on a coated cell container, (v) isolating the cells present in the supernatant of the coated cell container obtained at the end of step (iv), (vi) repeating, or not, the steps (iii) and (iv) at least one or two times, (vii) plating and culturing the cells isolated from the supernatant of the coated cell container obtained at the end of step (vi) until said cells have reached a confluence level of at least 50%, (viii) isolating, at the end of step (vii), the stem cells which can be used in cell therapy, wherein after expansion (a) at least 95% of said cells express CD44, CD73, (b) at least 95% of said cells express CD29, (c) at least 70% of said cells express CD90, and (d) said cells do not express CD4, CD8, CD34, CD45, CD31, CD1 17, CD144 and CD133. The invention also relates to said isolated stem cells and pharmaceutical compositions containing them.
Owner:INSTITUT NATIONAL DE LA RECHERCHE AGRONOMIQUE +1
Ror1-positive mesenchymal stem cell-containing pharmaceutical composition for preventing or treating disease associated with fibrosis, method for preparing same, and method for preventing or treating disease associated with fibrosis using ror1-positive mesenchymal stem cells
InactiveCN110418645AImprove mobilityGood treatment effectDigestive systemCulture processDiseaseFibrosis
The purpose of the present invention is to provide the following: novel mesenchymal stem cells having an excellent therapeutic effect on various diseases, particularly diseases associated with fibrosis; and a pharmaceutical composition containing such mesenchymal stem cells. The present invention is a therapeutic agent for fibrosis that contains ROR1-positive mesenchymal stem cells and / or a culture supernatant thereof and that is for preventing or treating a disease associated with fibrosis. The mesenchymal stem cells are CD29-, CD73-, CD90-, CD105-, and CD166-positive and are preferably derived from the umbilical cord or fat. In addition, the abovementioned disease associated with fibrosis is preferably a liver disease, a lung disease, a kidney disease, or a heart disease.
Owner:ROHTO PHARM CO LTD
Procurement, Isolation, and Cryopreservation of Endometrial/Menstrual Cells
Compositions comprising menstrual stem cells (MSCs) and methods, processes, and system therefor are provided by the invention. MSCs are processed from menstrual flow collected during menses. MSCs may be cryopreserved, processed through various culturing and selection steps in preparation for cryopreservation, or processed for therapeutic or cosmeceutical use. Cryopreserved MSCs may be thawed in preparation for therapeutic and cosmeceutical use. MSCs express CD9, CD10, CD13, CD29, CD44, CD49e, CD49f, CD59, CD81, CD105, CD166, and HLA class I, and have low or no expression of CD3 and HLA class II.
Owner:CRYO CELL INTERNATIONAL INC
Ror1-positive mesenchymal stem cell-containing pharmaceutical composition for preventing or treating disease associated with fibrosis, method for preparing same, and method for preventing or treating disease associated with fibrosis using ror1-positive mesenchymal stem cells
The purpose of the present invention is to provide the following: novel mesenchymal stem cells having an excellent therapeutic effect on various diseases, particularly diseases associated with fibrosis; and a pharmaceutical composition containing such mesenchymal stem cells. The present invention is a therapeutic agent for fibrosis that contains ROR1-positive mesenchymal stem cells and / or a culture supernatant thereof and that is for preventing or treating a disease associated with fibrosis. The mesenchymal stem cells are CD29-, CD73-, CD90-, CD105-, and CD166-positive and are preferably derived from the umbilical cord or fat. In addition, the abovementioned disease associated with fibrosis is preferably a liver disease, a lung disease, a kidney disease, or a heart disease.
Owner:ROHTO PHARM CO LTD
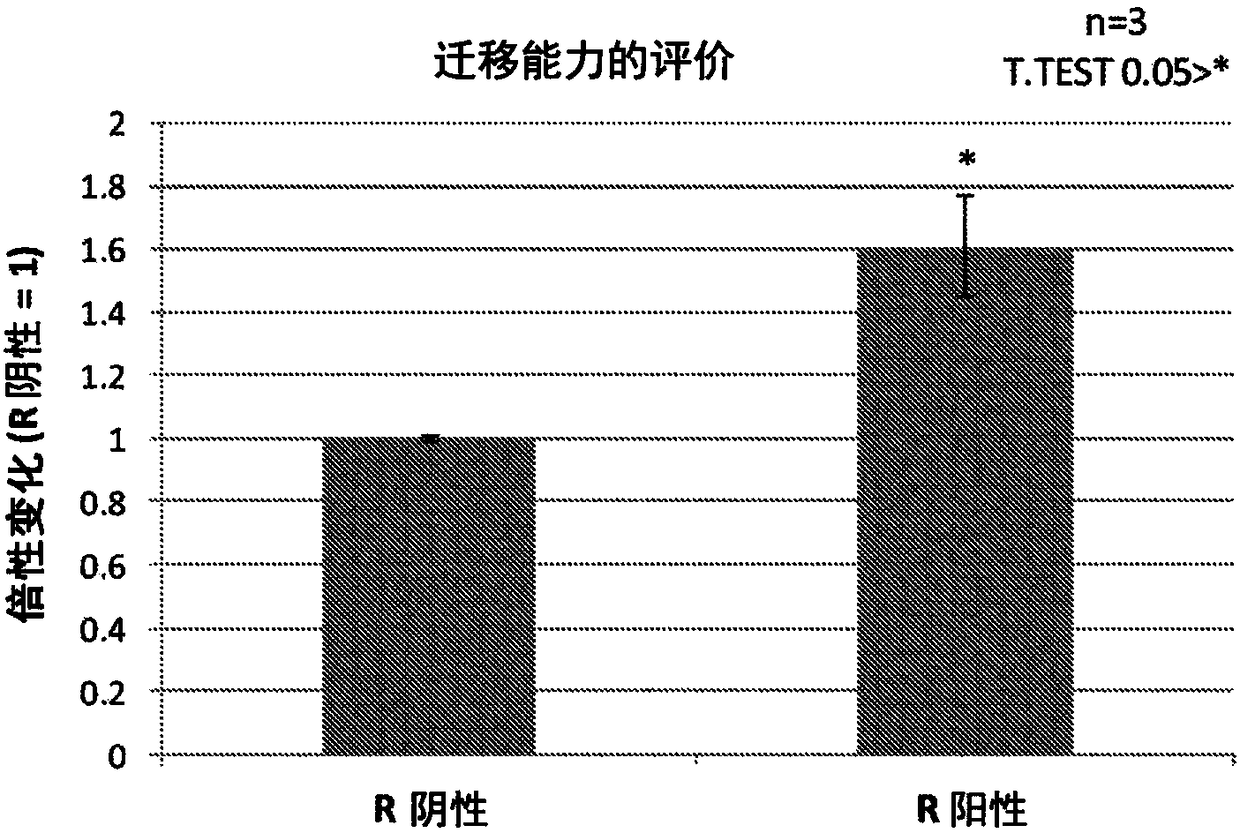
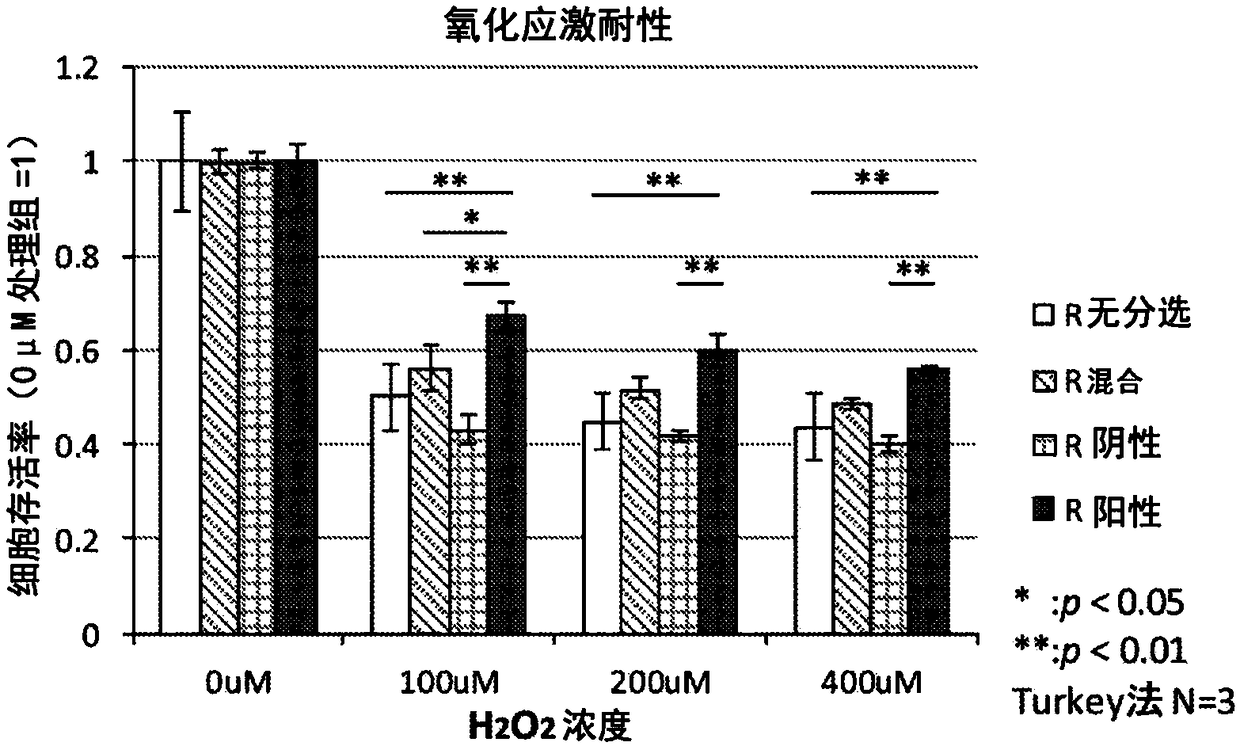
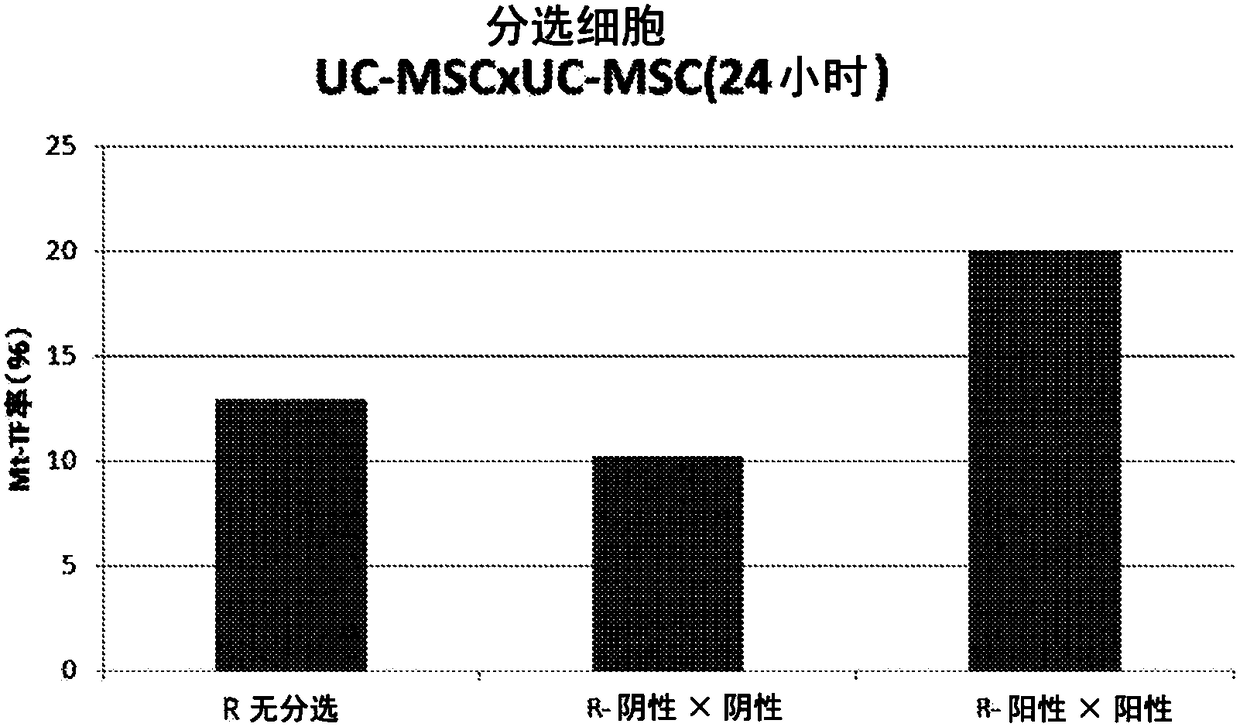
CD29<+> human umbilical cord source mesenchymal stem cell and use thereof in preparation of drug for treating skeletal muscle atrophy in high-sugar and high-fat environments
ActiveCN107557332AImprove hyperlipidemiaImprove symptoms of skeletal muscle atrophyMetabolism disorderMuscular disorderAcute hyperglycaemiaAntigen
The invention discloses a CD29<+> human umbilical cord source mesenchymal stem cell and use thereof in the preparation of a drug for treating skeletal muscle atrophy in high-sugar and high-fat environments. The CD29<+> human umbilical cord source mesenchymal stem cell represents the following mesenchymal stem cell cytomembrane molecules: a human leukocyte differentiation antigen CD73, a human leukocyte differentiation antigen CD90, a human leukocyte differentiation antigen CD105 and a human leukocyte differentiation antigen CD29. According to the CD29<+> human umbilical cord source mesenchymalstem cell, the hyperlipidemia and hyperglycemia of a db<- / -> mouse can be obviously improved, muscle fiber cross sections of soleus and gastrocnemius muscle of the db<- / -> mouse can be increased, andthe contents and cell number of each myotube are increased, so that the symptoms of the skeletal muscle atrophy of the db<- / -> mouse are improved. According to the CD29<+> human umbilical cord sourcemesenchymal stem cell, the theoretical foundation and experiment basis are laid for the subsequent research and development of the drug for treating skeletal muscle atrophy in the high-sugar and high-fat environments, and the CD29<+> human umbilical cord source mesenchymal stem cell has wide application prospects.
Owner:JILIN TUO HUA BIOTECH
Cell preparation for treating inflammatory enteritis
The invention relates to a cell preparation for treating inflammatory enteritis. The cell preparation is a mesenchymal stem cell preparation of Wharton's jelly of human umbilical cord; the cell preparation is derived from mesenchymal stem cells from Wharton's jelly of fetal umbilical cord; and the positive rates of CD29, CD44, CD90 and CD105 of the cell preparation detected and identified througha flow cytometry are all more than 90%, and preferably, more than 95%.
Owner:TSINGHUA UNIV
A cellular therapy for ocular degeneration
Compositions and methods applicable to cell-based or regenerative therapy for ophthalmic diseases and disorders comprising mesenchymal stem cells, particularly those characterized by the expression of at least one of the following surface markers: CD29, CD44, CD105 or CD166, and the lack of expression of at least one of CD14, CD34 or CD45.
Owner:CENTOCOR
Method for isolated culture of human fat mesenchyma stem cell and special culture medium thereof
ActiveCN101314766BThe method of isolation and culture is simpleImprove efficiencySkeletal/connective tissue cellsAntigenMuscle injury
Owner:微能生命科技集团有限公司
Extracting method of dog adipose-derived stem cells, and preparation and application of dog adipose-derived stem cells
PendingCN109370986AVigorousThe separation method is simpleCell dissociation methodsSkeletal/connective tissue cellsRed blood cellAdipogenesis
The invention discloses an extracting method of dog adipose-derived stem cells, and a preparation and application of the dog adipose-derived stem cells. The preparation for treating dog chronic nephrosis is prepared from the dog allogeneic adipose-derived stem cells. The extracting method of the adipose-derived stem cells comprises the steps: the dog abdominal adipose tissue is obtained, digesting, filtering and centrifuging are conducted, red blood cell lysis buffer resuspending is conducted, and the cells of P0-P3 generations are cultured; and the obtained adipose-derived stem cells of the P3 generation are subjected to surface antigen testing, the adipose-derived stem cells which have more than 70% of CD29 and MHC-1 expressions, less than 2% of CD34 and CD45 expressions, and the high differentiative potential, achieve adipogenesis, osteogenesis and chondrogenesis, and have normal chromosomes, feminine endotoxin and nont-detected mycoplasma are screened out and resuspended with the dosage of 1*10<6> cells / kg-5*10<6> cells / kg through normal saline of 0.5-1.0 mL to prepare the application of the dog allogeneic adipose-derived stem cells, and the preparation is used for treating dogchronic nephrosis. The stem cell preparation is weak in immunogenicity and is not involved in argument in the aspects of society, ethic and law; a transplanted person does not need to provide autologous stem cells, and nearly no damage is caused; and a separating method is simple, and the cells are very high in activity and easy to increase massively.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Dental pulp marrow similar cells (DPMSC) and methods of isolating and using
ActiveUS20160244724A1Improve isolationEasy to expand in cultureMetabolism disorderDigestive systemCD29Isolated population
Owner:UNIV DEGLI STUDI DI UDINE UNIV OF UDINE +1
Isolated culture and differentiation method for testicular mesenchymal stem cells
InactiveCN106754684AEfficiently obtainedSimple methodCell dissociation methodsSkeletal/connective tissue cellsCulture cellCD29
The invention discloses an isolated culture and differentiation method for testicular mesenchymal stem cells. The method comprises the following steps: 1, separating, culturing and amplifying an obtained testicular tissue in vitro; 2, culturing cells of the generation P4, and forming an embryoid body; 3, carrying out inoculation differentiation on the embryoid body. A research shows that the isolated culture and differentiation method can be used for effectively obtaining the testicular mesenchymal stem cells; the obtained stem cells have the typical form of mesenchymal stem cells; effective labels for expressing the mesenchymal stem cells include CD29, CD166 and the like; labels for expressing stem cells include OCT4, NANOG and the like; EB can be formed; after induction is completed, differentiation of fat cells, osteogenesis cells and other cells can be realized. Therefore, the method can be used for effectively obtain the testicular mesenchymal stem cells for performing induced differentiation.
Owner:泊迈生物医学检测(苏州)有限公司
Application of antibody CD29 in identification and sorting of human-placenta-chorion-avillosum-derived mesenchymal stem cells
InactiveCN112063581AFast and accurate identificationImprove detection stabilityCell dissociation methodsBiological material analysisSurface markerCD29
The invention discloses an application method of CD29 in identification and sorting of human-placenta-chorion-avillosum-derived mesenchymal stem cells. According to the application method disclosed bythe invention, the human-placenta-chorion-avillosum-derived mesenchymal stem cells (PMSCs) are firstly marked with a variety of cell surface markers separately, then, detection is carried out by using a flow cytometer to survey qualified rate and stability of flow-type detection marked with all the markers, and it is discovered that the expression of a marker CD90 in a universal standard is unstable, and detections are all qualified after the PMSCs are marked with the CD29; and through repeated test, it is discovered that the CD29 has good detection stability, the qualified rate of detectionis 100%, thus, the CD29 has a good and stable identification effect on the PMSCs and can be applied to rapid and accurate identification on the PMSCs instead of the CD90.
Owner:CHENGDU QINGKE BIOTECH
CD29<+> human umbilical cord-derived mesenchymal stem cell and application thereof in preparation of bone tissue engineering seed cell used for treating bone injury
InactiveCN107502587ALow immunogenicityGood treatment effectSkeletal disorderUnknown materialsAntigenTreatment effect
The invention discloses a CD29<+> human umbilical cord-derived mesenchymal stem cell and an application thereof in preparation of a bone tissue engineering seed cell used for treating bone injury. The CD29<+> human umbilical cord-derived mesenchymal stem cell expresses the following four mesenchymal stem cell membrane molecules: a human leukocyte differentiation antigen CD73, a human leukocyte differentiation antigen CD90, a human leukocyte differentiation antigen CD105 and a human leukocyte differentiation antigen CD29. The CD29<+> human umbilical cord-derived mesenchymal stem cell disclosed by the invention can be converted into a bone cell in a mouse to participate in fracture healing, has low immunogenicity and can be taken as a cell source of tissue-engineered bone, then the traditional surgical therapy scheme is combined, and a good therapeutic effect can be achieved, so that the CD29<+> human umbilical cord-derived mesenchymal stem cell disclosed by the invention has a broad application prospect.
Owner:JILIN TUO HUA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com