Stem cell preparation for treating primary liver cancer and preparation method thereof
A technology of stem cell preparation and primary liver cancer, which is applied to medical preparations containing active ingredients, medical raw materials derived from mammals, and pharmaceutical formulas, etc., can solve the problems of immunogenicity and limited quantity easily infected by viruses, and achieve Effects of normal karyotype and telomerase activity, high activity, and strong proliferative ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
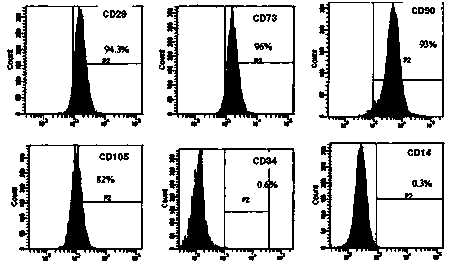
[0036] Embodiment 1: Preparation of human umbilical cord mesenchymal stem cell preparation
[0037]The umbilical cord that passed the test was fully washed with D-hank's balanced salt solution, the residual blood in the umbilical vein and umbilical artery was washed away, the adventitial tissue and vascular tissue of the umbilical cord were separated and removed, and cut into pieces of about 1 cubic millimeter , transferred to collagenase type II with a mass fraction of 0.1%, digested at 37°C for 20 hours, passed through a 100-mesh sieve, collected the filtrate containing cells, centrifuged at 1200 rpm for 10 minutes at room temperature, discarded the supernatant, and retained the precipitate; The cells were inoculated in culture flasks containing 10% fetal bovine serum and DMEM / F12 medium containing 100u / mL double-antibody (penicillin, streptomycin), and the culture flasks were placed at 37°C with a volume fraction of 5% carbon dioxide. Cultivate in a saturated humidity envir...
Embodiment 2
[0047] Embodiment 2: the preparation of PRP lysate
[0048] The whole process of making PRP lysate is strictly aseptic. Take 10ml of venous blood from the patient, centrifuge it at 200g for 20min, and collect the supernatant, which is platelet-rich plasma. Platelet Rich Plasma Pass-80 0 C refrigerator and 37 0 C water bath quick freezing - thawing, the supernatant after centrifugation is the platelet-rich plasma lysate.
Embodiment 3
[0049] Example 3: Detection of hepatocyte growth factor and fibroblast growth factor-4 to promote the differentiation of umbilical cord mesenchymal stem cells into hepatocyte-like cells
[0050] 1. Cell culture: Inoculate umbilical cord mesenchymal stem cells in a culture dish containing 10% fetal bovine serum and 100u / mL double-antibody (penicillin, streptomycin) in DMEM / F12 culture medium, change the medium every other day, and wait for the cells to fuse Realize when the speed reaches 50%;
[0051] 2. Experimental grouping: Group A (negative control group): no induction factor; Group B: HGF (20 ng / ml); Group C: FGF-4 (10 ng / m1); Group D: HGF (20 ng / ml). m1)+FGF-4 (10 ng / m1); E group (positive control group): L-02 human liver cell line;
[0052] 3. RT-PCR detection: RT-PCR detection of AFP and ALb mRNA was carried out in each group on the 14th and 21st day of induction, and groups A and E were respectively negative and positive control groups. Expression of AFP: AFP Sense 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com