Identification and Isolation of Multipotent Cells From Non-Osteochondral Mesenchymal Tissue
a mesenchymal tissue and multipotent cell technology, applied in the field of isolated multipotent cells, can solve the problems of high ethical burden, low yield, pain in the process of obtaining bone marrow,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Multipotent Adult Cells from Soft Tissue and Characterization of Surface Markers
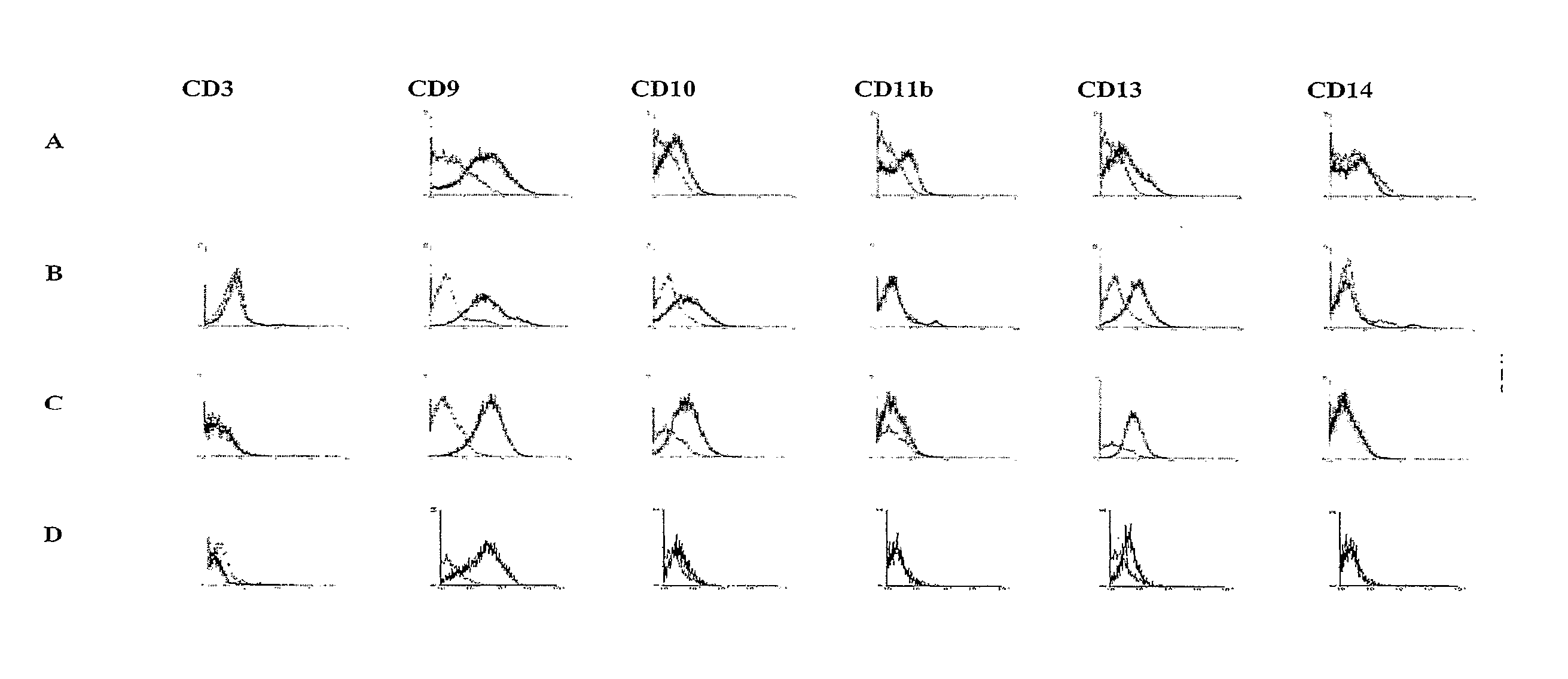
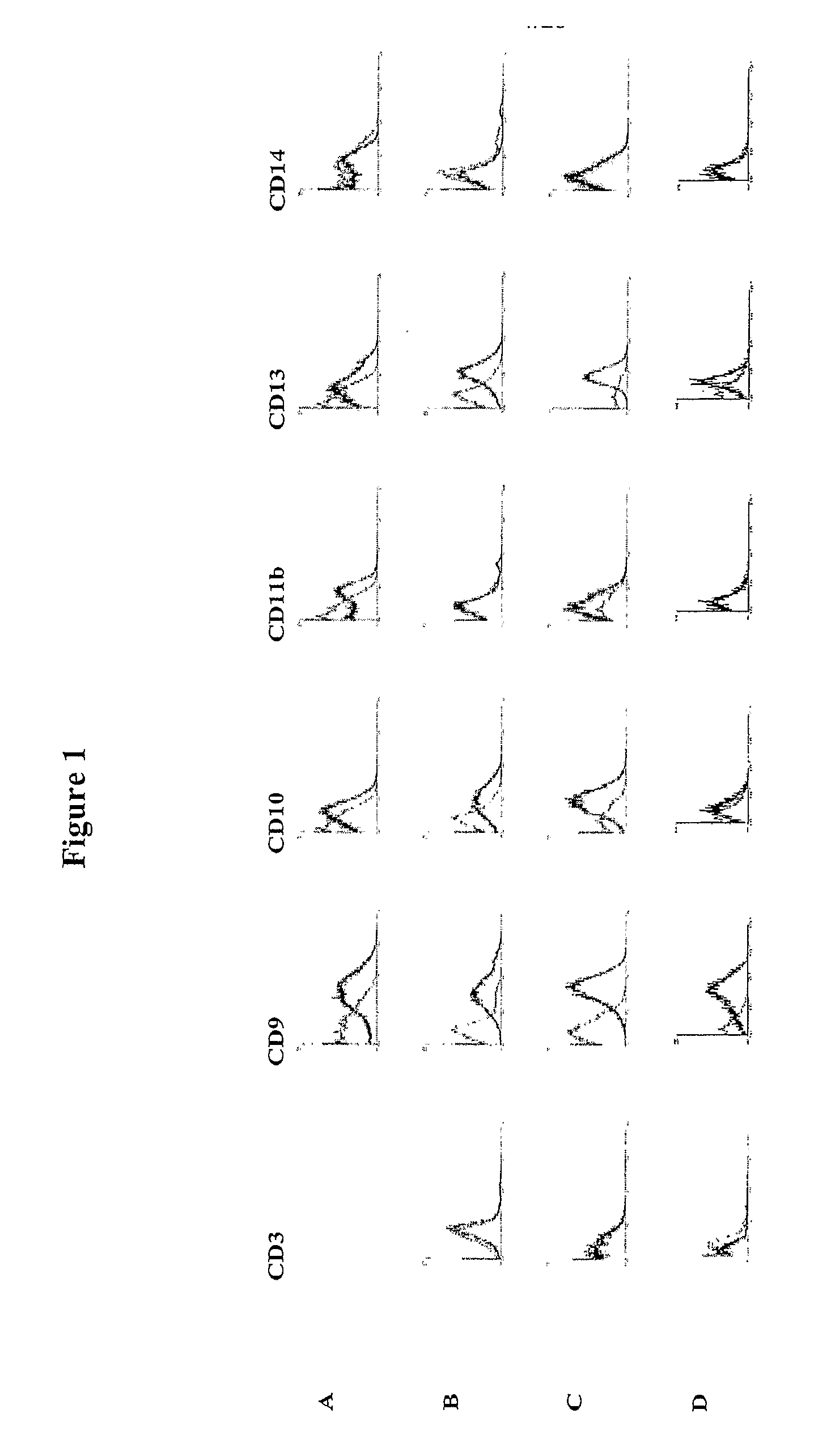
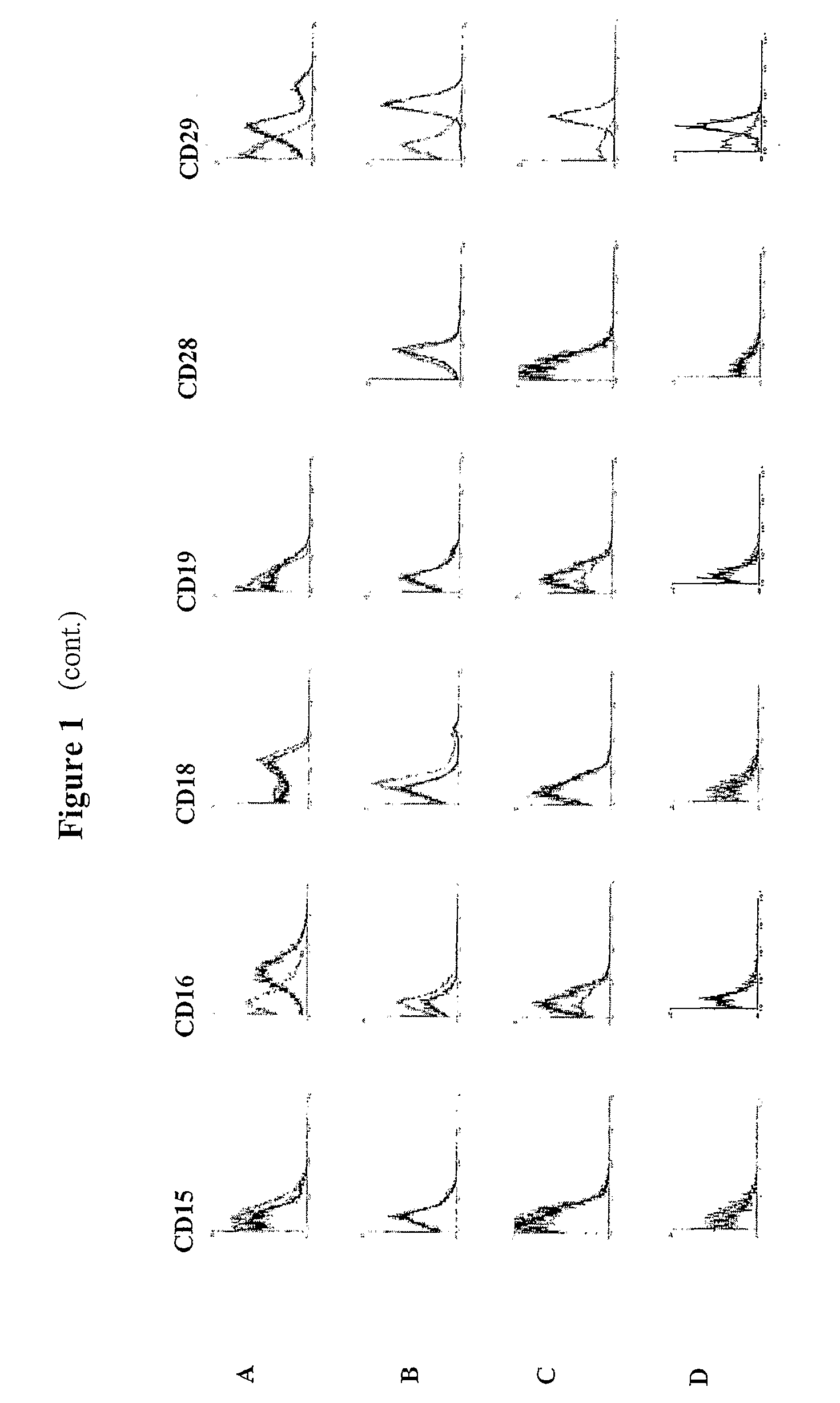
[0125] The isolation of multipotent adult cells from soft tissue was performed by selecting those cells with a capacity for proliferation and differentiation, characterized in that they show adhesion to the plastic container of the cell culture. Then, the cells were characterized by monitoring by flow cytometry the expression of a series of surface markers on the recently isolated cells and during the course of the culture development in vitro.
[0126] The isolation of the multipotent adult cells was carried out from sub-dermal adipose tissue, obtained by liposuction from 3 healthy human donors (donors 1, 2 and 3). First, the sample from the sub-dermal adipose tissue was washed with phosphate buffered saline solution (PBS). To achieve destruction of the extracellular matrix and the isolation of the cells, an enzymatic digestion was performed with type 11 collagenase in saline solution (5 mg / ...
example 2
In Vitro Differentiation of Multipotent Adult Cells from Human Non-Osteochondral Mesenchymal Tissue into Bone Phenotype Cells
[0144] In the differentiation assay, characterized human multipotent adult cells of the present invention were used. The cells were isolated from the 3 samples of analyzed lipoaspirates, each corresponding to a healthy donor (Example 1). The multipotent adult cells were isolated and characterized as mentioned in Example 1. A sample of mesenchymal stem cells (MSC) of human bone marrow was used as positive control.
[0145] The isolated cells were seeded at a density of 10,000 cells / cm2 onto 6-well plates (one plate per sample), and were incubated in standard culture medium (DMEM, 10% FBS, L-Glutamine 2 mM and antibiotic). After two days of culturing, the culture medium of one of the wells (control) is replaced with fresh medium, and the remaining wells with osteogenesis inducing medium, which contains the standard culture medium with the following added:
[0146] ...
example 3
In Vitro Differentiation of Multipotent Adult Cells from Human Non-Osteochondral Mesenchymal Tissue into Muscle Phenotype Cells
[0151] In the differentiation assay, characterized human multipotent adult cells of the present invention were used. The cells were isolated from the 3 samples of analyzed lipoaspirates, each corresponding to a healthy donor (Example 1). The multipotent adult cells were isolated and characterized as mentioned in Example 1. A sample of MSC of human bone marrow was used as positive control.
[0152] The isolated cells were seeded at a density of 10,000 cells / cm2 into standard culture medium (DMEM, 10% FBS, L-Glutamine 2 mM and antibiotic). After two days of culturing, the culture medium of one of the wells (control) was replaced with fresh medium, and the remaining wells with myogenesis inducing medium (Wakitani et al., 1995), which contains the standard culture medium with the following added:
[0153] Ascorbate-2-phosphate 0.1 mM
[0154] Dexamethasone 0.01 μM
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com