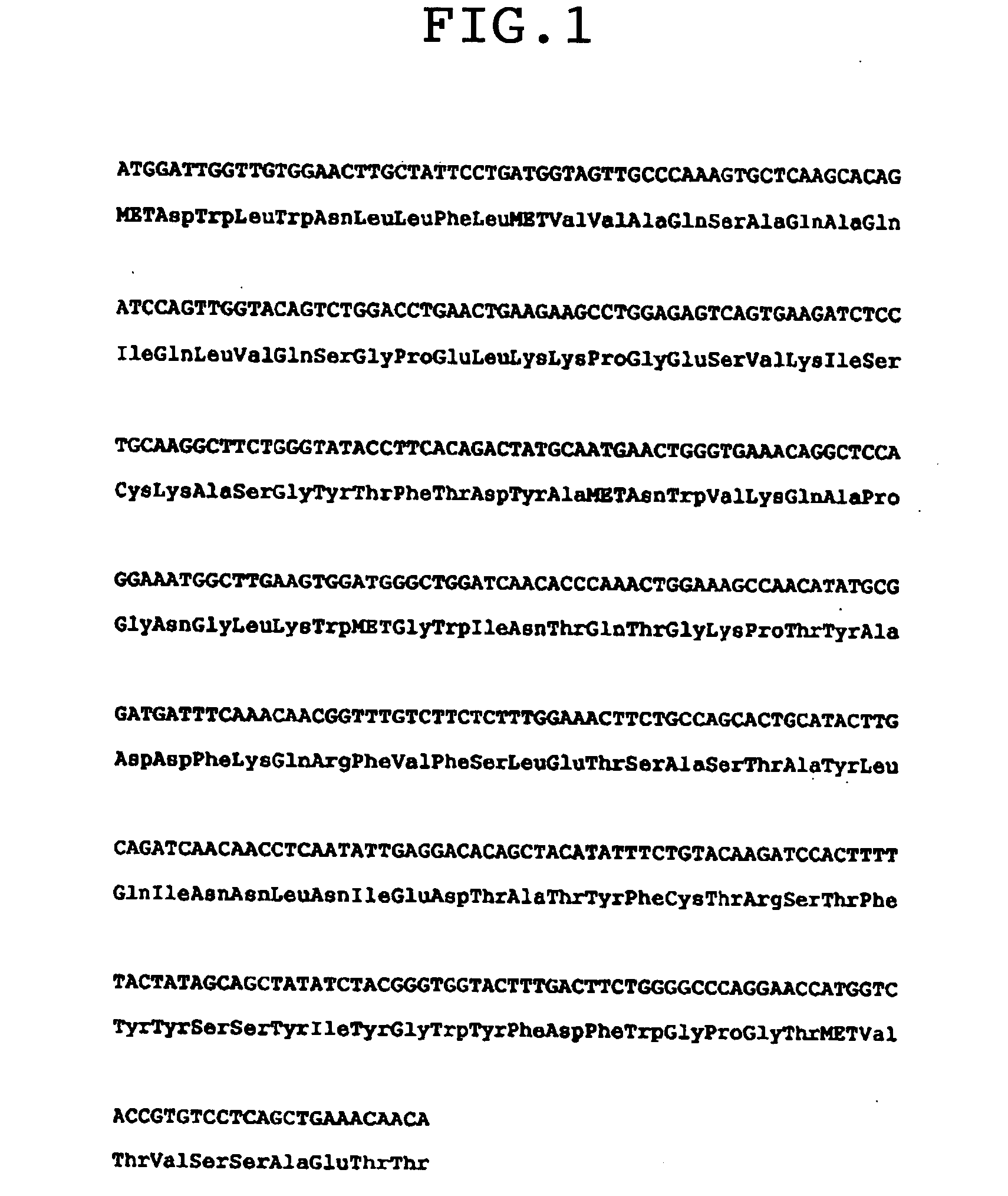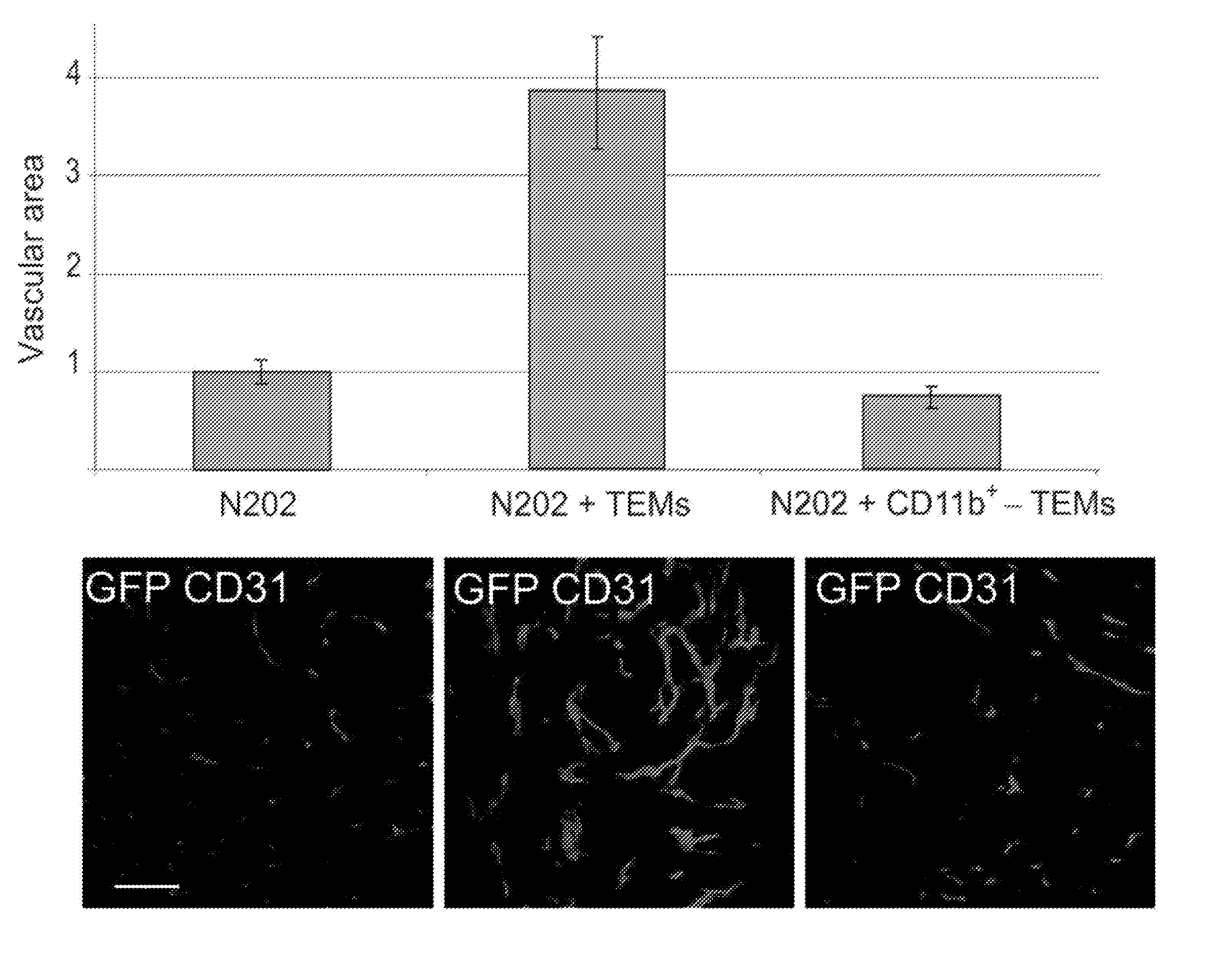Patents
Literature
204 results about "CD14" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
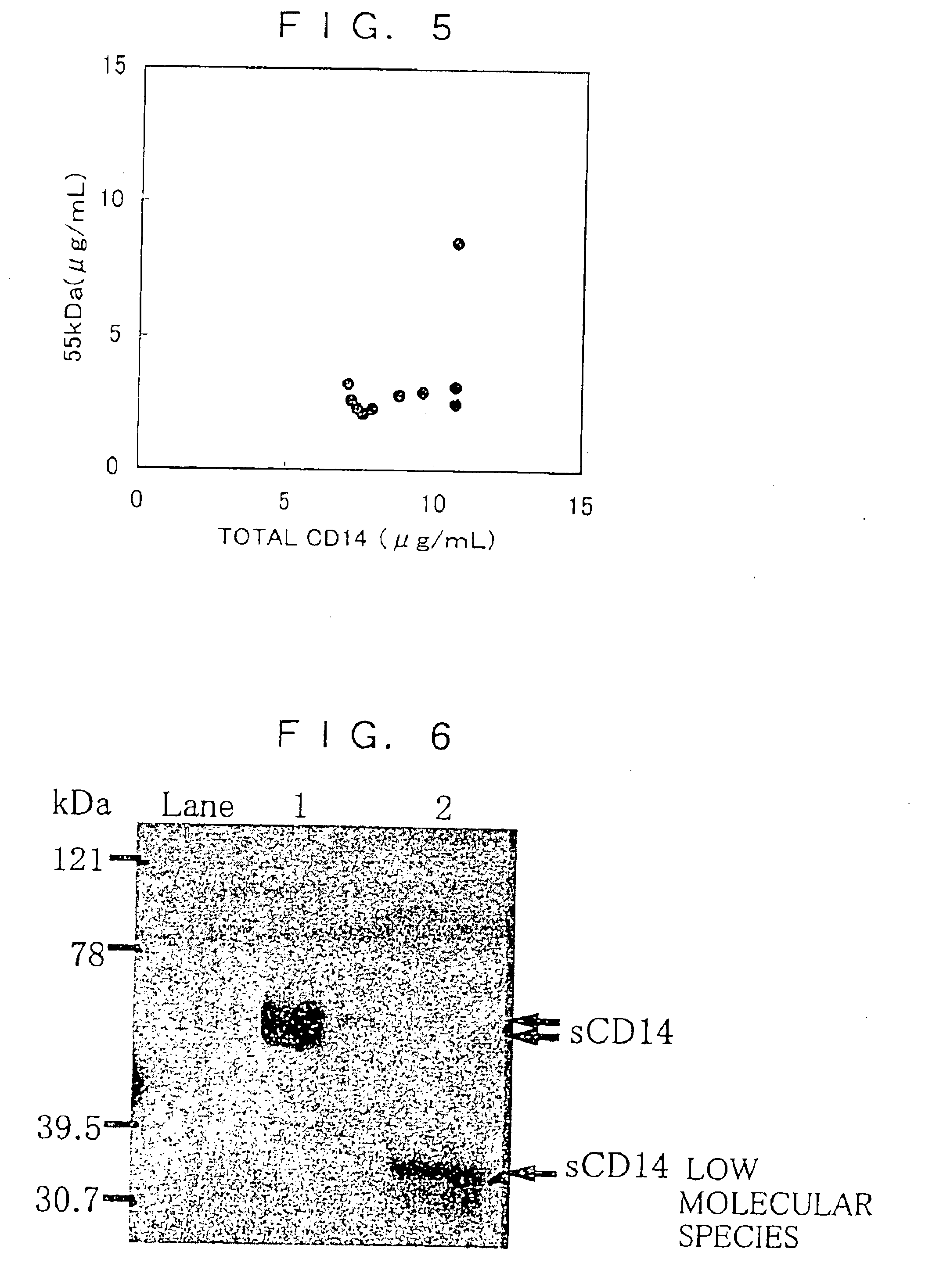
CD14 (cluster of differentiation 14) is a human gene. The protein encoded by this gene is a component of the innate immune system. CD14 exists in two forms, one anchored to the membrane by a glycosylphosphatidylinositol tail (mCD14), the other a soluble form (sCD14). Soluble CD14 either appears after shedding of mCD14 (48 kDa) or is directly secreted from intracellular vesicles (56 kDa).
Methods and compositions for the prevention and treatment of sepsis
The present invention includes compositions comprising one or more complement inhibitors and one or more CD14 pathway inhibitors for the prevention or treatment of sepsis. The complement inhibitors may be antibodies that bind to and inhibit complement proteins such as C5a and the CD14 pathway inhibitors may be antibodies that bind to and inhibit CD14 pathway components, such as CD14 and LPS. The invention also relates to methods of treating subjects suffering from sepsis comprising administering these compositions, as well as kits for supplying the compositions for treatment.
Owner:GENENTECH INC
Anti-Cd14 Antibody Fusion Protein
InactiveUS20080286290A1Prevention and therapyGuaranteed stable actionAntibacterial agentsBiocideProteinase activityCD14
Owner:MOCHIDA PHARM CO LTD
Identification and Isolation of Multipotent Cells From Non-Osteochondral Mesenchymal Tissue
Identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue. This invention relates to the identification and isolation of multipotent cells from non-osteochondral mesenchymal tissue. Specifically, it relates to an adult multipotent cell or a cell population or composition comprising said cell, isolated from non-osteochondral mesenchymal tissue, characterized in that it is positive for the following markers: CD9, CD10, CD13, CD29, CD44, CD49A, CD51, CD54, CD55, CD58, CD59, CD90 and CD105 and because it lacks expression of the following markers: CD11b, CD14, CD15, CD16, CD31, CD34, CD45, CD49f, CD102, CD104, CD106 and CD133.
Owner:AUTONOMOUS UNIVERSITY OF MADRID +1
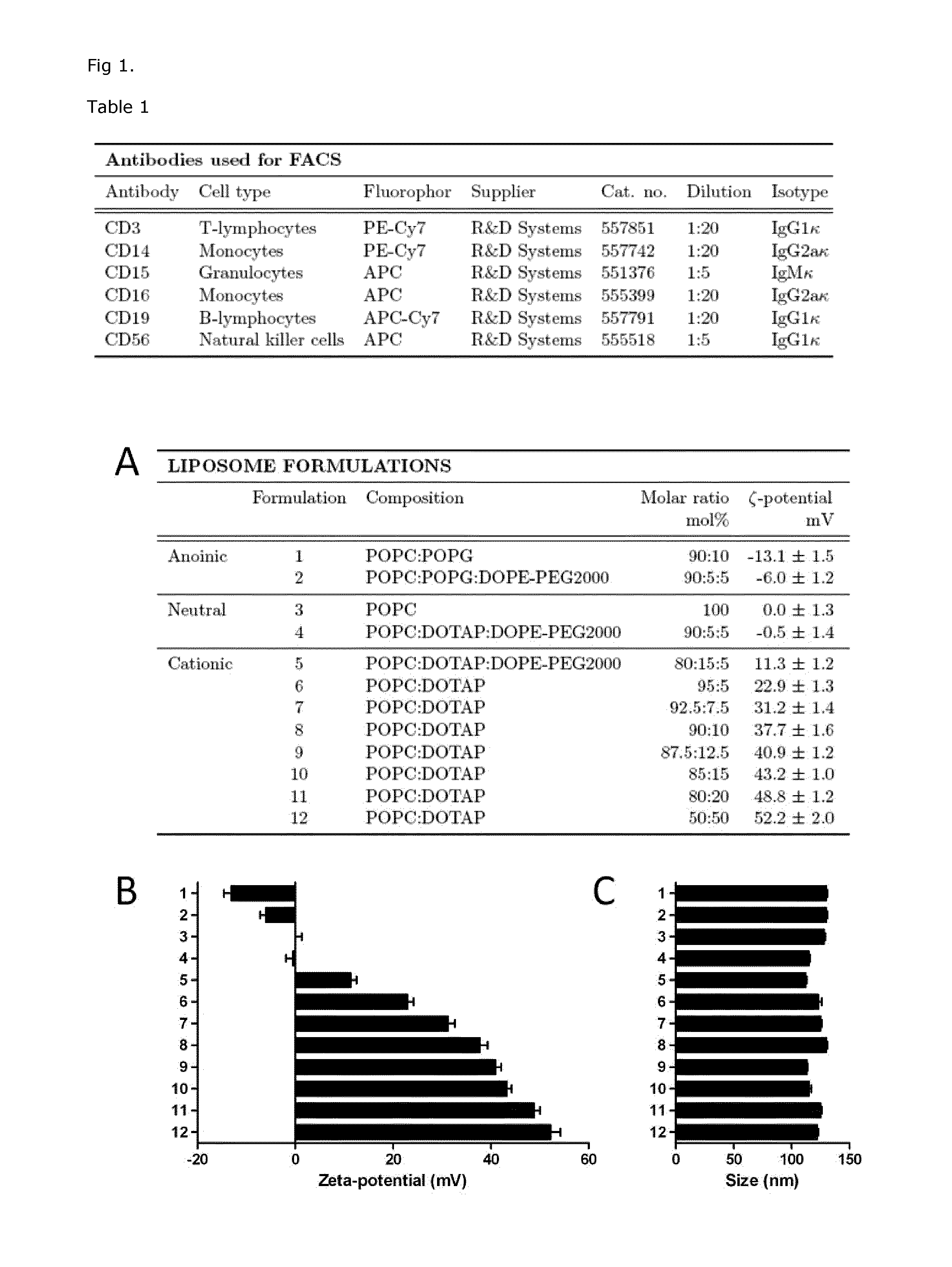
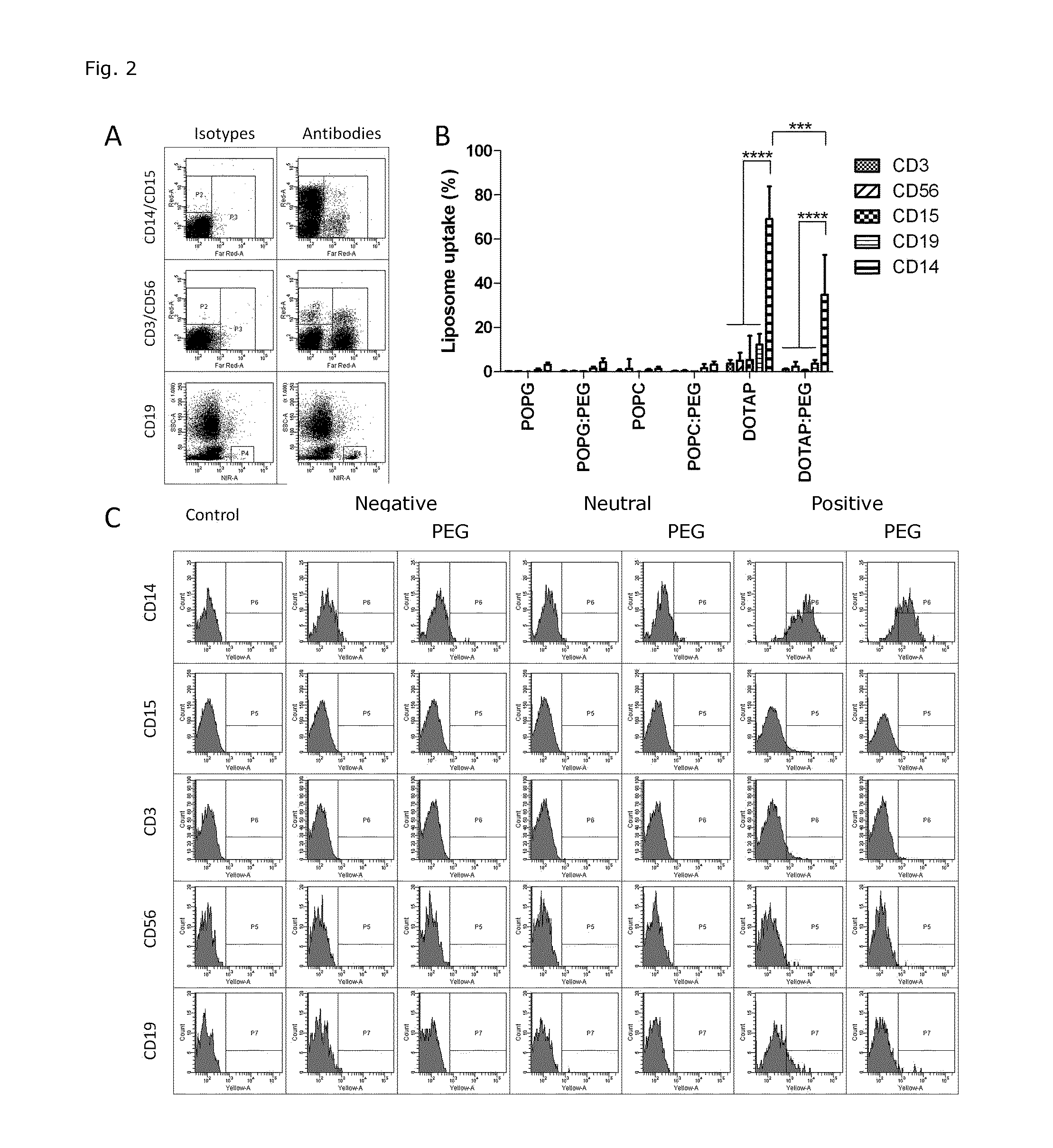
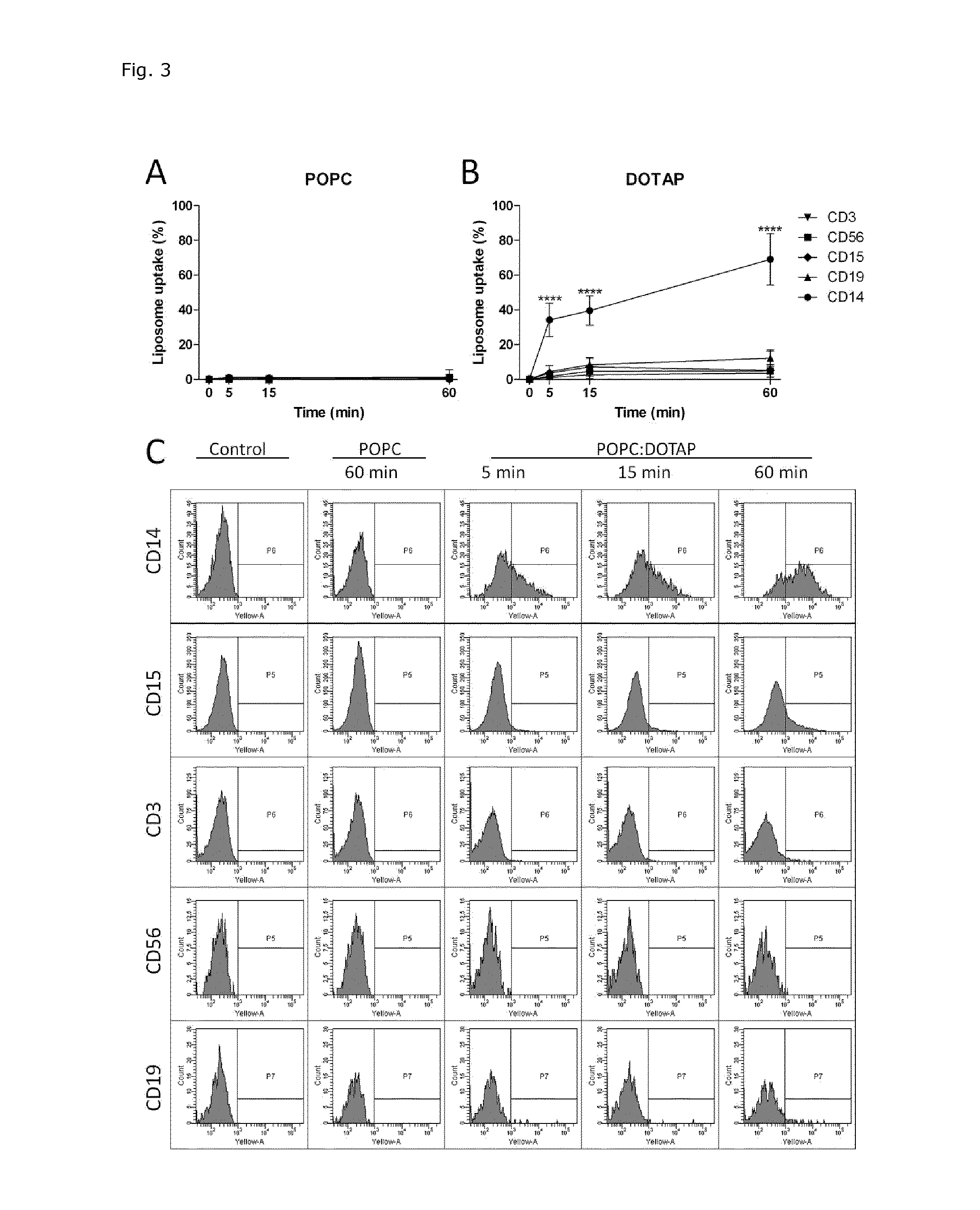
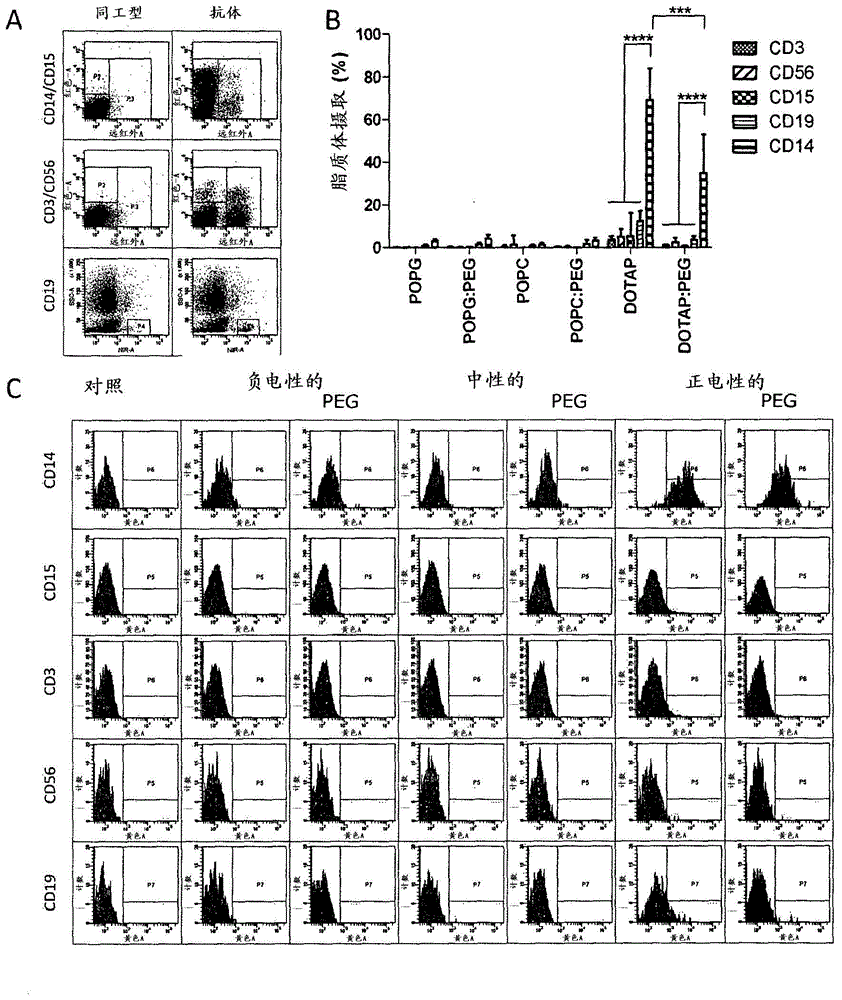
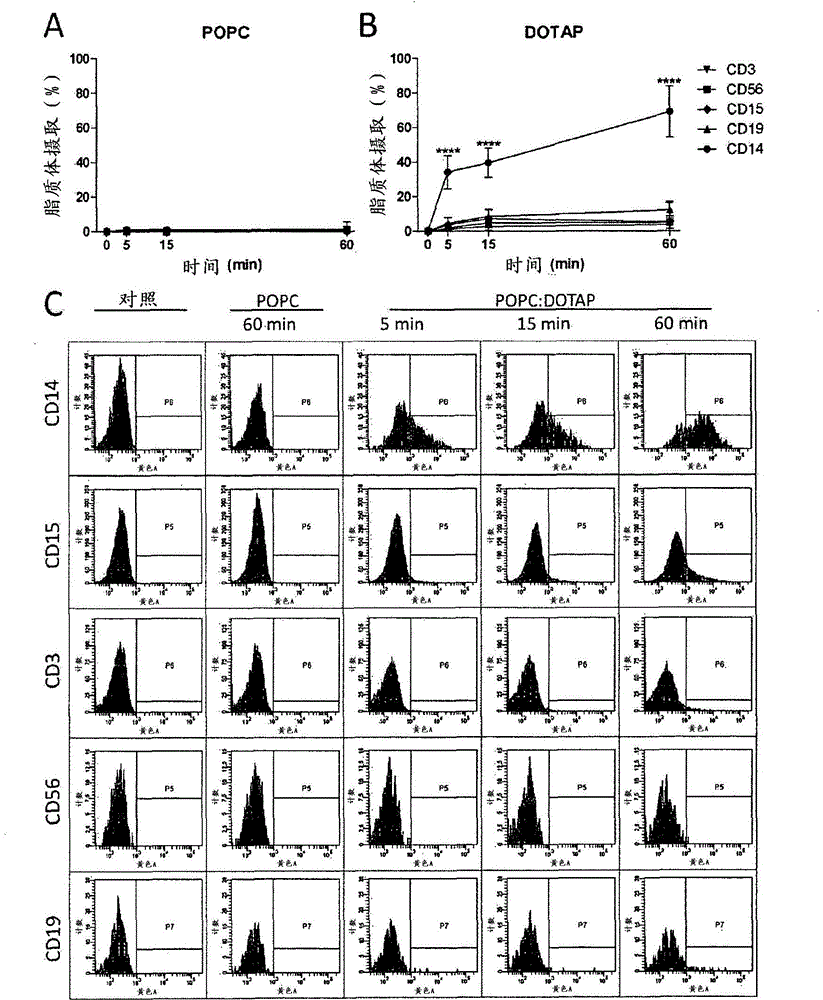
Cationic Liposomal Drug Delivery System for Specific Targeting of Human CD14+ Monocytes in Whole Blood
This invention concerns a liposome comprising lipids and at least one active ingredient, wherein at least one of the lipids is a cationic lipid; said liposome exhibiting a net positive charge at physiological conditions at which said liposome preferentially adheres to monocytes in freshly drawn blood when compared to adherence to granulocytes, T-lymphocytes, B-lymphocytes and / or NK cells in freshly drawn blood, to a lipid-based pharmaceutical composition comprising said liposomes and their use in monocytic associated prophylaxis, treatment or amelioration of a condition such as cancer, an infectious disease, an inflammatory disease, an autoimmune disease or allergy.
Owner:BIONEER +1
Construction method of cell model for detecting pyrogens, cell model and pyrogen detection kit
ActiveCN106148286AImprove stabilityIncreased sensitivityCell receptors/surface-antigens/surface-determinantsCulture processWestern blotCytokine
The invention provides a construction method of a cell model for detecting pyrogens, the cell model and a pyrogen detection kit. The cell model utilizes specific locations of CRISPR / CAS9 induced genomes to form double-bond fission, TLR4 and CD14-MD2 are knocked into two chromosomes of a cell line respectively by the aid of the homologous recombination repair principle, green fluorescence GFP and red fluorescence RFP are respectively used for tracing finally successfully constructed TLR4 / CD14 / MD2 fixed-point knocked-in fluorescent tracer cell models, and the LPS stimulating cell model can detect release of IL-6 and TNF-a cytokines by means of ELISA, Western Blot, mass spectrum and immunomagnetic beads. The cell model is good in stability and high in sensitivity, and the lowest detectable limit can reach 0.005EU / mL and is far lower than 0.025EU / mL of the Tachypleus Amebocyte Lysate method.
Owner:牛刚
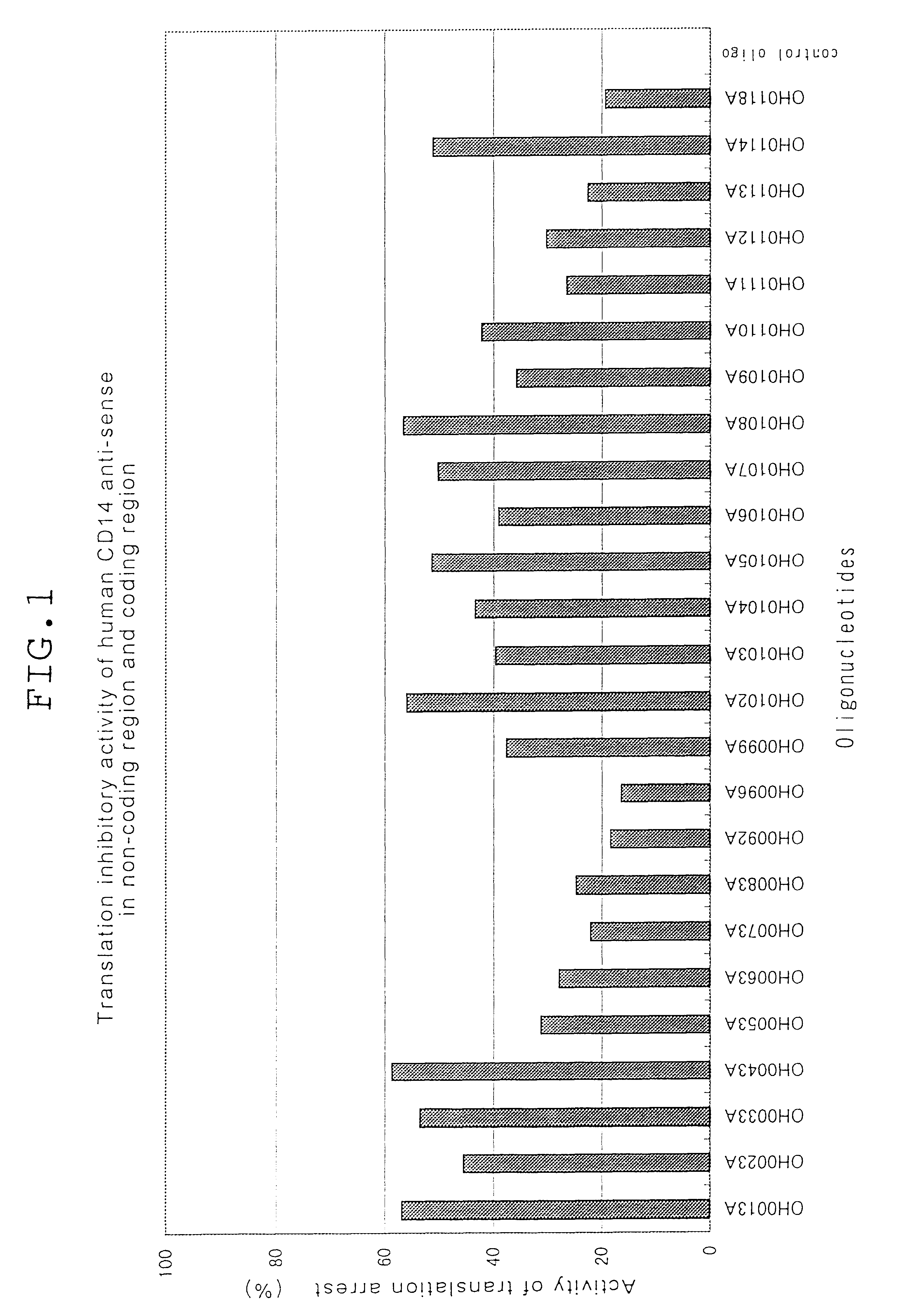
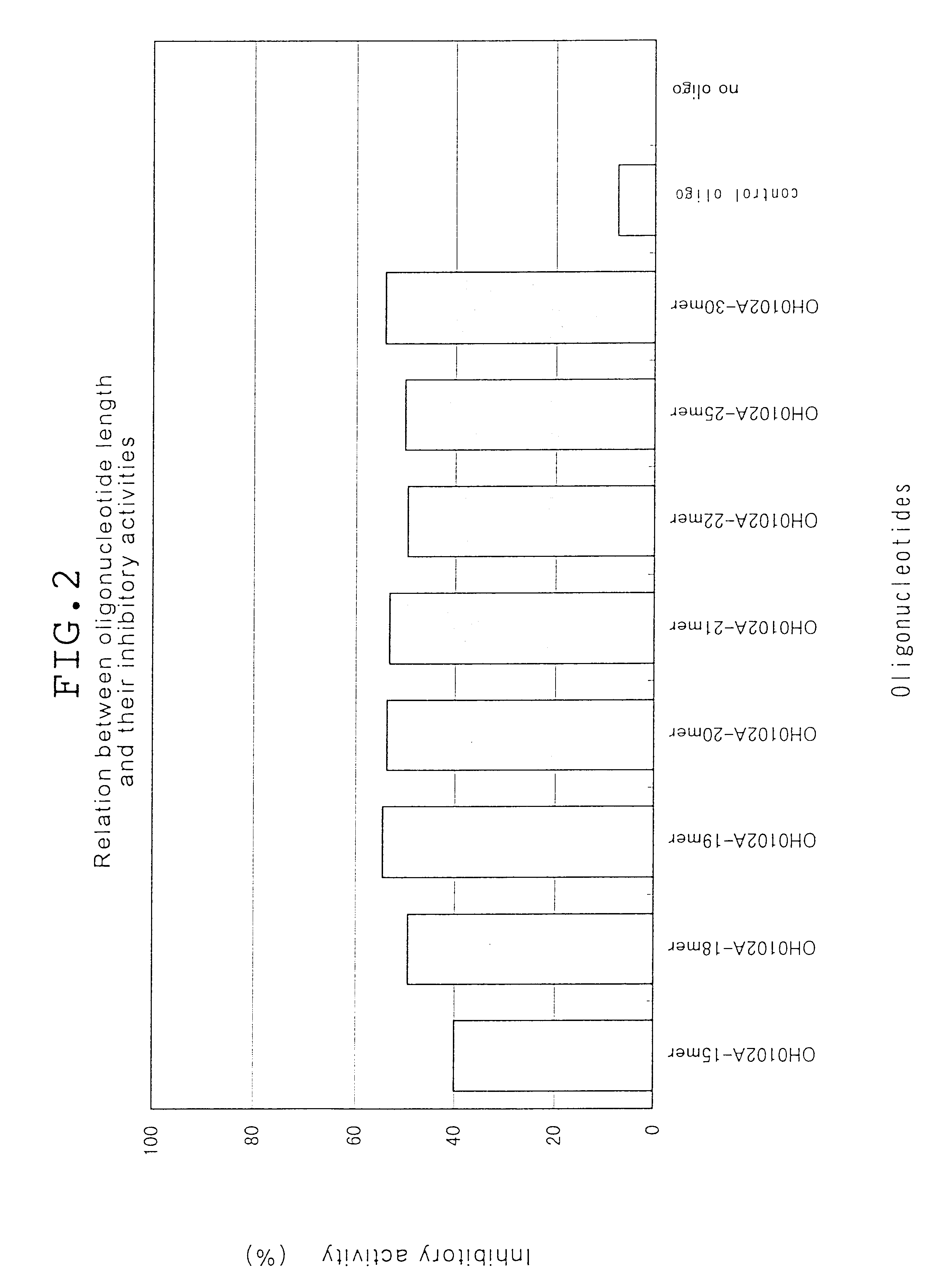
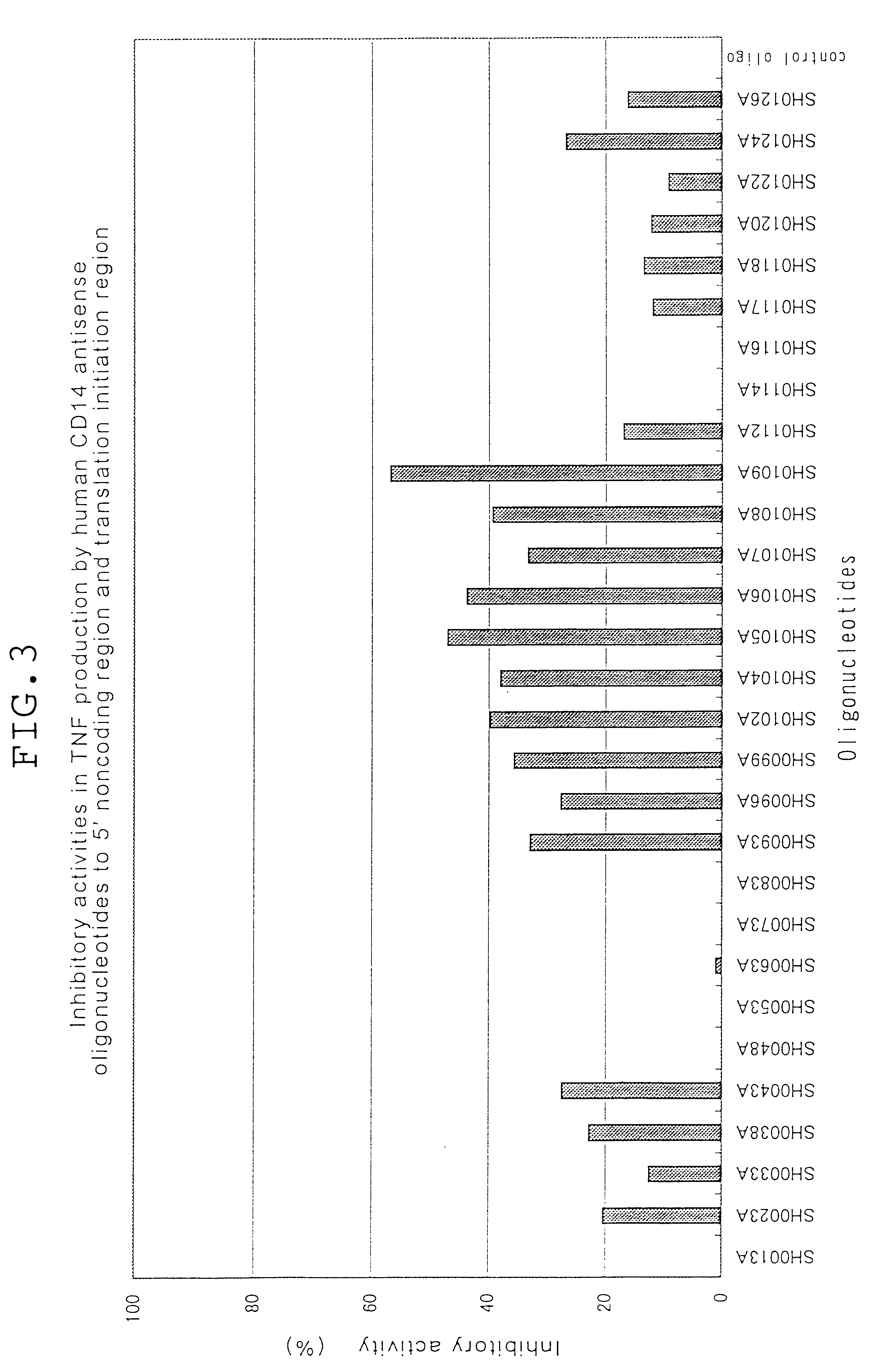
Antisense compounds to CD14
InactiveUS6251873B1Cell receptors/surface-antigens/surface-determinantsSugar derivativesAdditive ingredientWhole body
The present invention relates to an oligonucleotide and derivatives, hybridizable with or being complementary to at least a part of a gene encoding human CD14; and to pharmaceutical compositions, comprising the oligonucleotide or derivatives thereof as effective ingredient; and is utilisable of cure of systemic inflammatory response sydorome, etc., by the use of the pharmaceutical composition.
Owner:MOCHIDA PHARM CO LTD
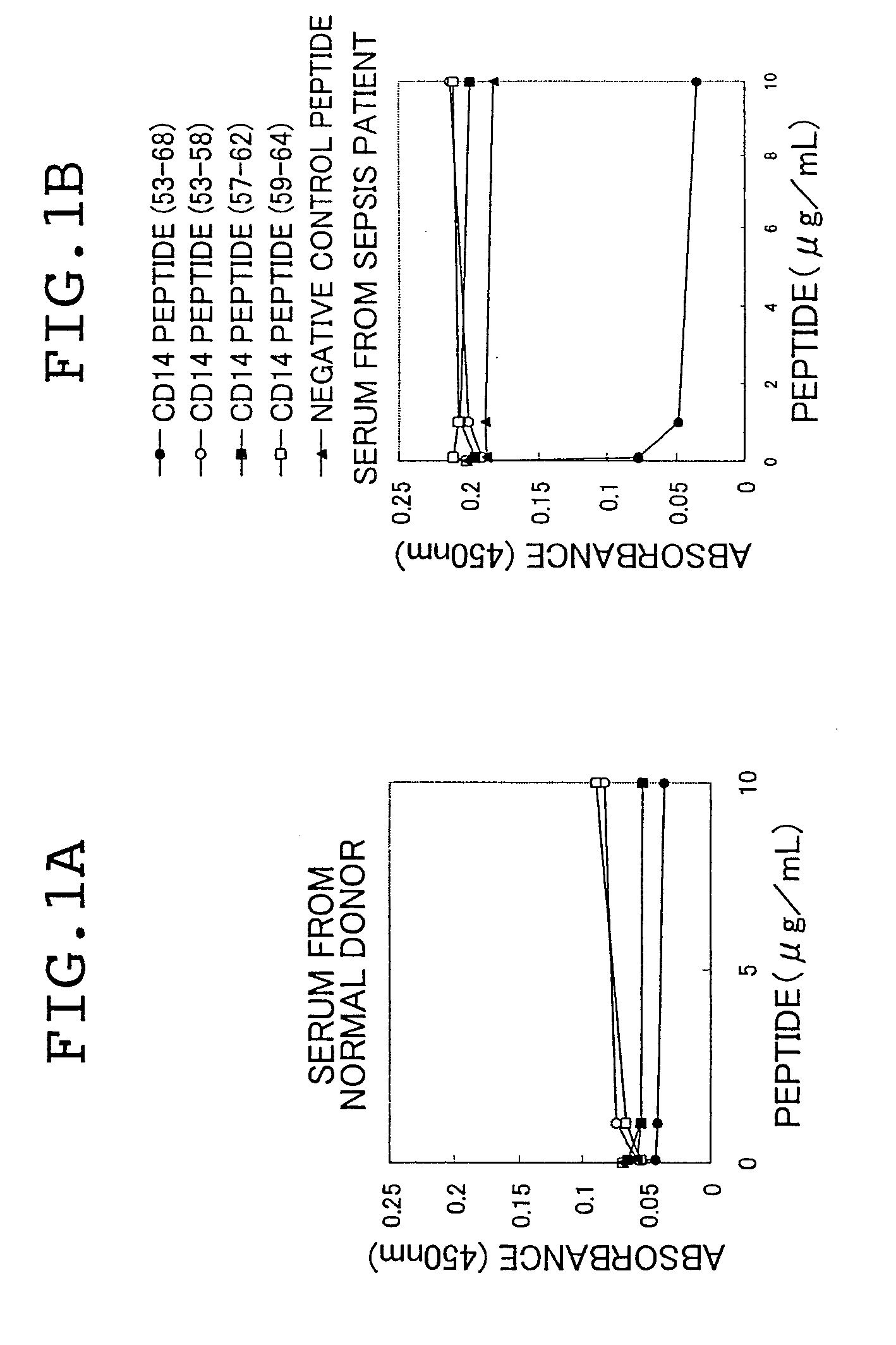
Novel soluble cd14 antigen
ActiveUS20090203052A1Enable productionEfficient use ofPeptide/protein ingredientsPeptide sourcesAntigenIn vivo
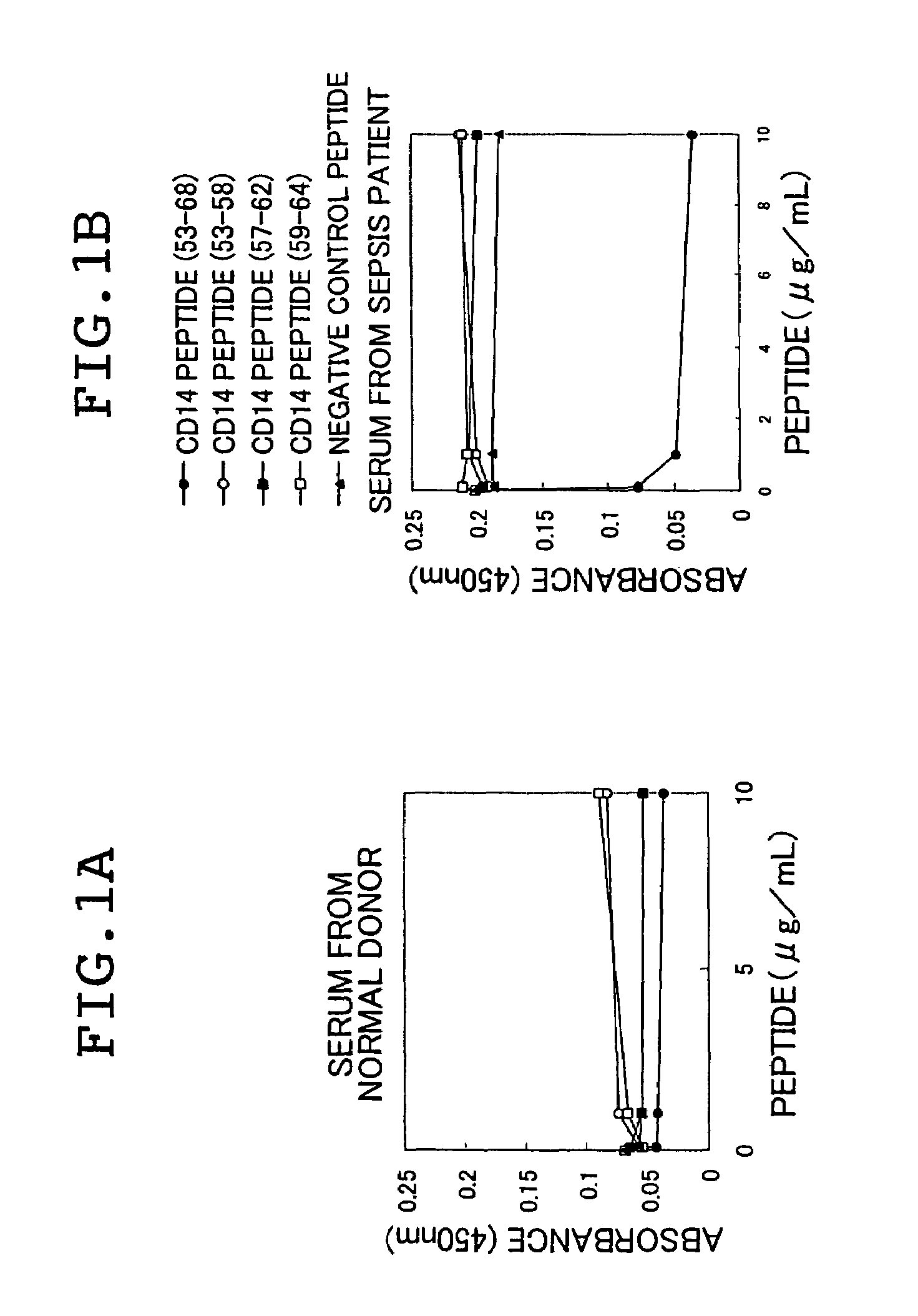
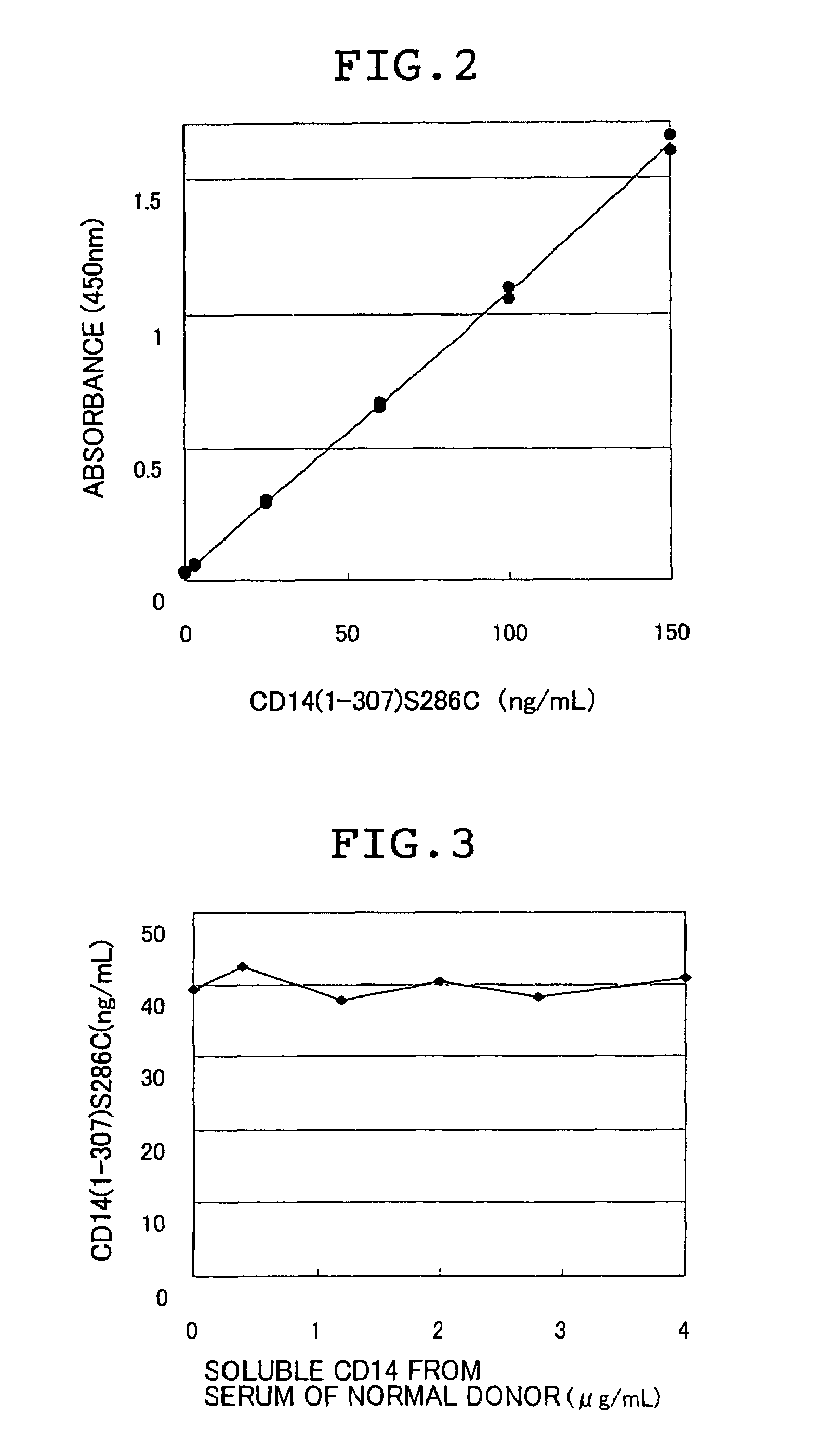
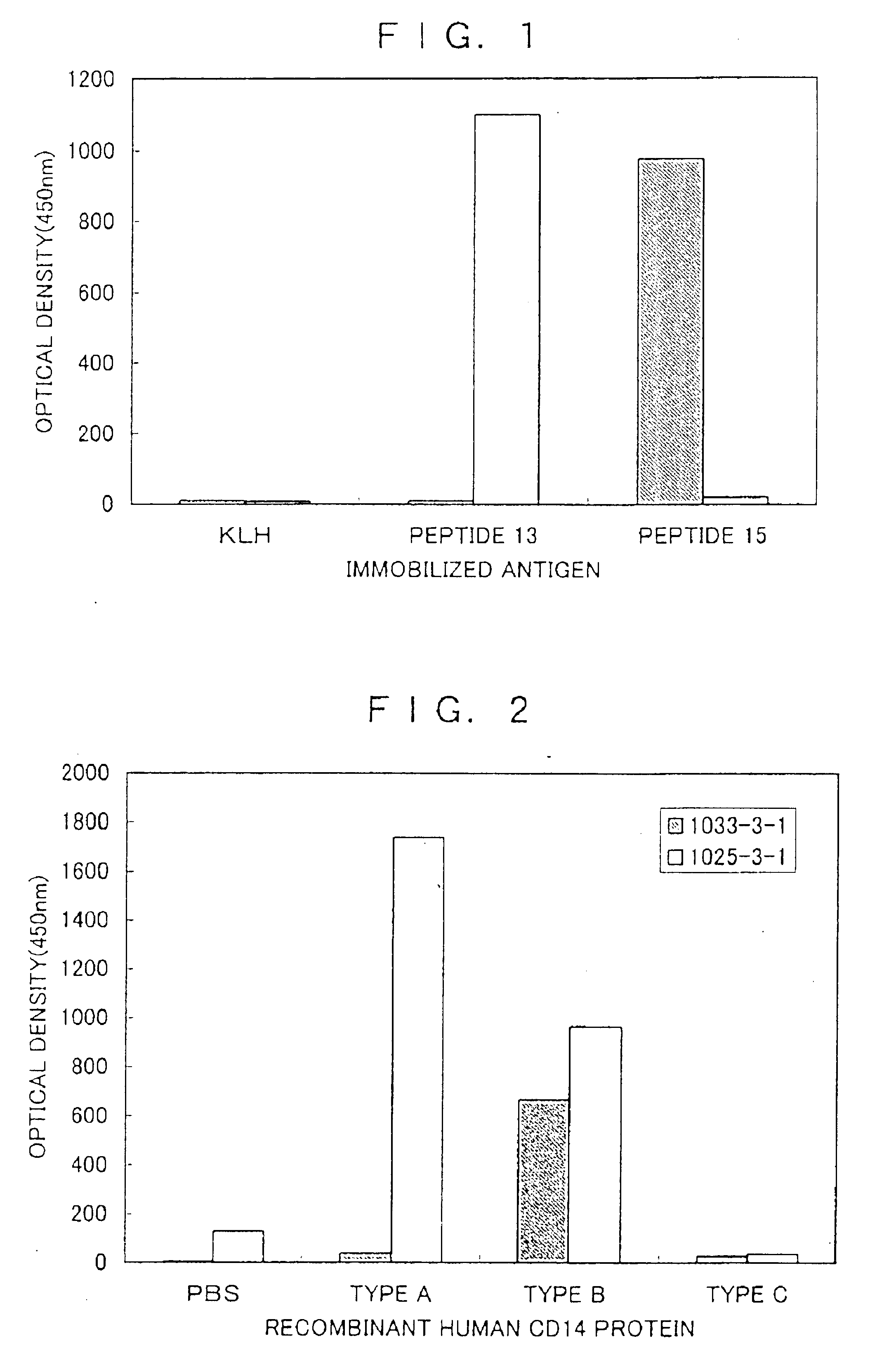
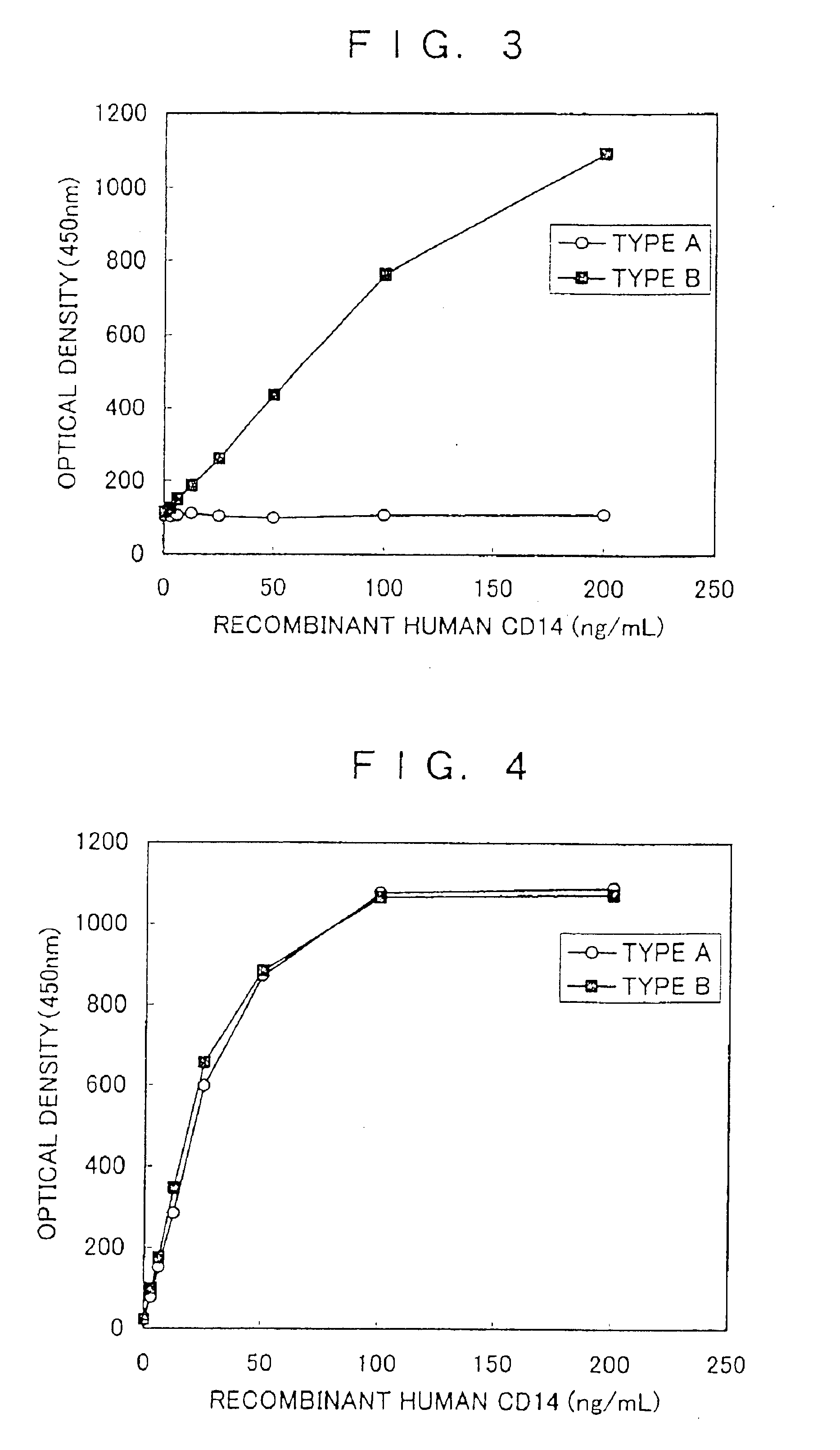
There are provided a soluble CD14 antigen which is a novel in vivo protein useful as a marker for diagnosing sepsis and has the following characteristic features 1) to 3):1) a molecular weight of 13±2 kDa when measured by SDS-PAGE under non-reducing conditions;2) an amino acid sequence in which the amino acid sequence of SEQ ID NO:1 is present on its N terminal; and3) ability to specifically bind to an antibody prepared by using a peptide comprising 16 amino acid residues described in SEQ ID NO:2 for the antigen; and a recombinant soluble CD14 fragment.
Owner:MOCHIDA PHARM CO LTD
Method for amplification and activation of NK cells by K562 cells
ActiveCN103232973AHelp identify and killHelp activate recognition and killMammal material medical ingredientsBlood/immune system cellsNatural Killer Cell Inhibitory ReceptorsCD86
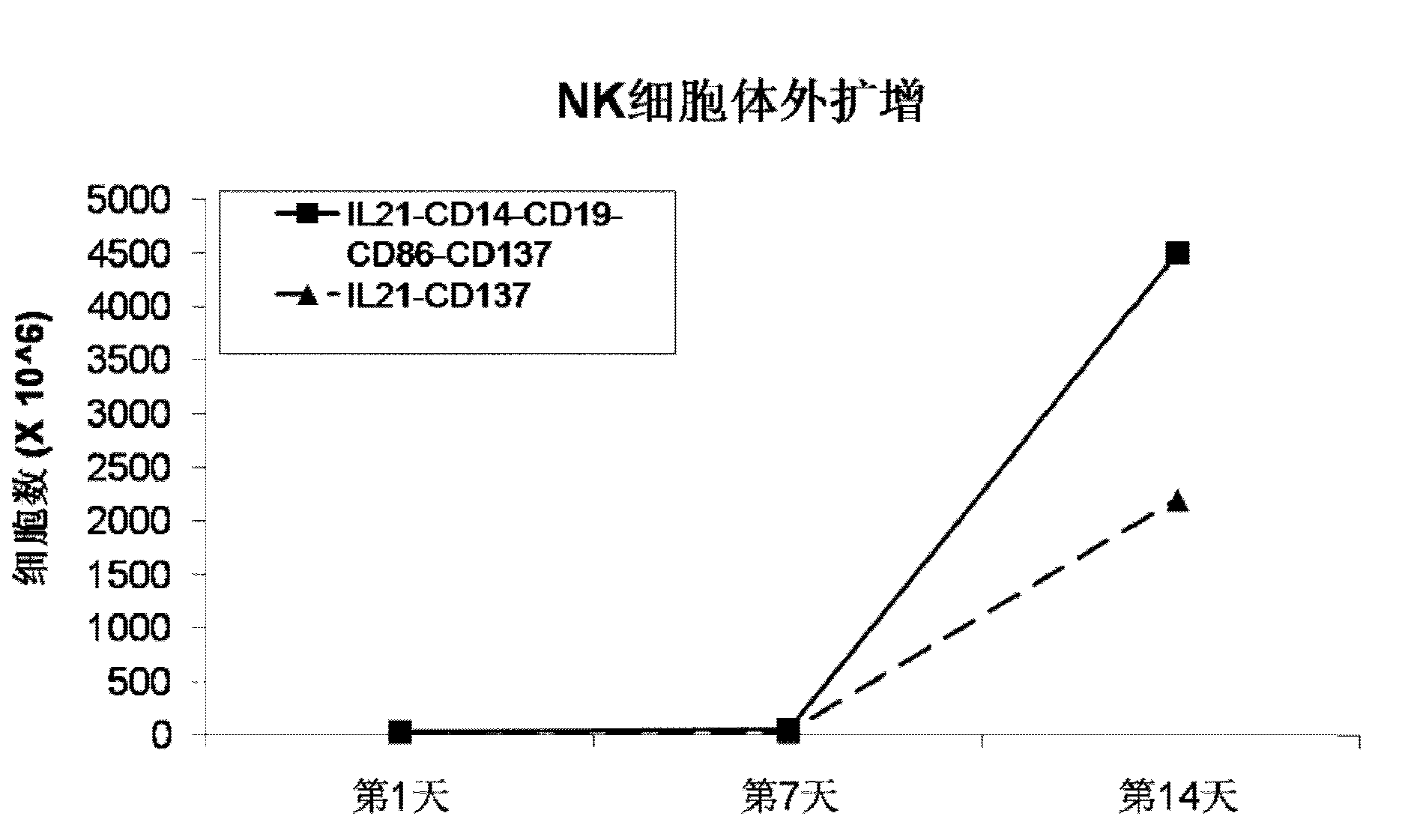
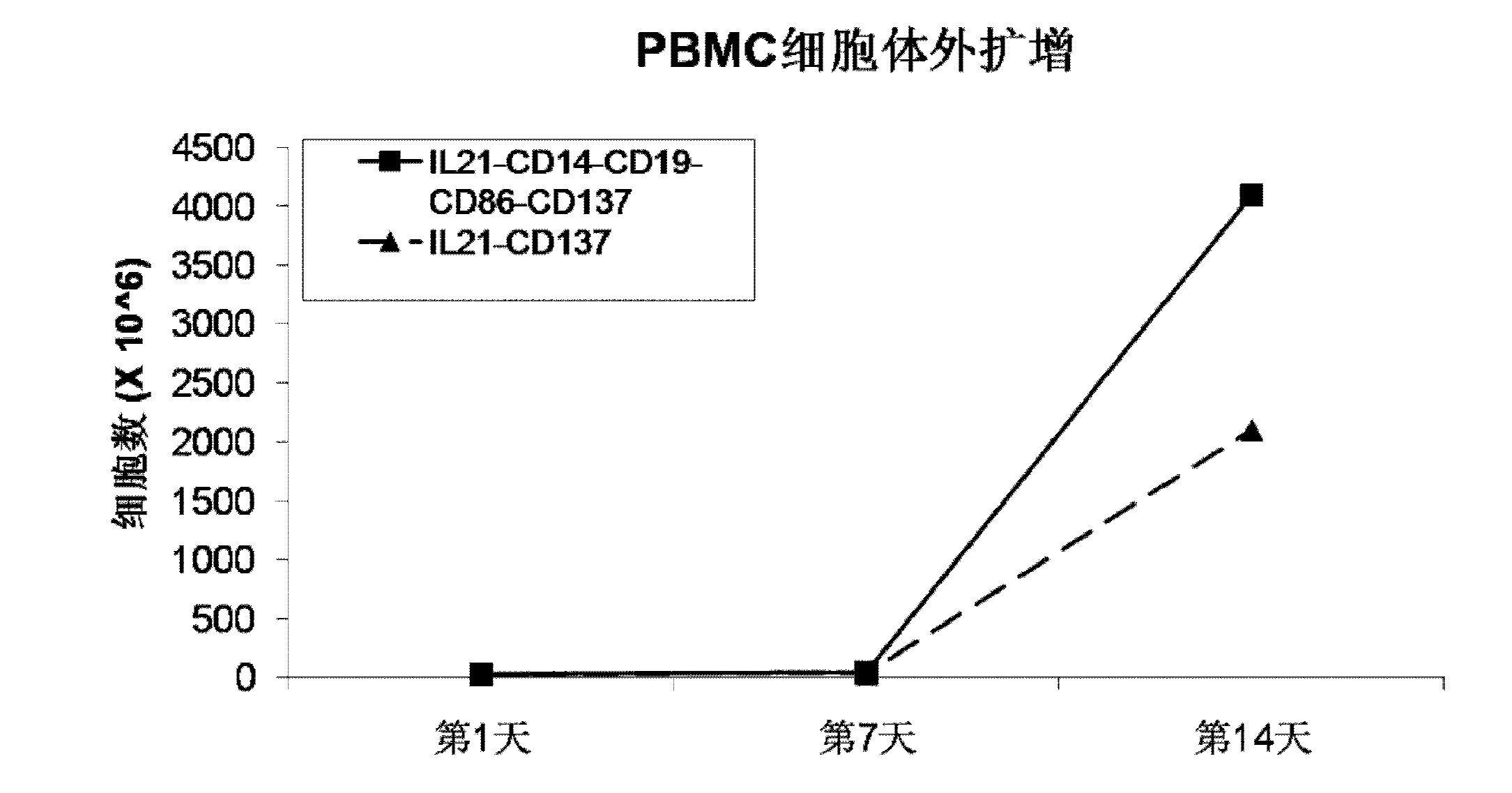
The invention discloses a method for amplification and activation of NK cells by K562 cells. The method comprises that through synergism of K562 cells transfected by transmembrane interleukin 21, CD14, CD19, CD86 and CD137, and low-concentration interleukin 2, NK cells are subjected to directed amplification and activation. Compared with the existing similar compounds, the compound provided by the invention has a stronger lymphocyte amplification and activation capability and higher efficiency. The method has wide prospects in immunological therapy.
Owner:杭州中赢生物医疗科技有限公司
Methods and Compositions for the Prevention and Treatment of Sepsis
The present invention includes compositions comprising one or more complement inhibitors and one or more CD14 pathway inhibitors for the prevention or treatment of sepsis. The complement inhibitors may be antibodies that bind to and inhibit complement proteins such as C5a and the CD14 pathway inhibitors may be antibodies that bind to and inhibit CD14 pathway components, such as CD14 and LPS. The invention also relates to methods of treating subjects suffering from sepsis comprising administering these compositions, as well as kits for supplying the compositions for treatment.
Owner:GENENTECH INC
Monocyte cell
ActiveUS20080057043A1Inhibit angiogenesisSimple procedureBiocideGenetic material ingredientsCD16CD14
Owner:OSPEDALE SAN RAFFAELE SRL +1
Genetic markers of food allergy
ActiveUS7732135B2Increase propensityIncreased propensity to food allergyMicrobiological testing/measurementFermentationIl 4 receptorGenetics
A genetic marker of food allergy is disclosed. The marker comprises variants of IL-4 receptor alpha, IL13 and CD14.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Methods and compositions for inhibiting CD14 mediated cell activation
This invention provides hybridoma cell lines producing monoclonal antibodies which inhibit CD14 mediated cell activation. Monoclonal antibodies produced by these cell lines also are provided. The antibodies are useful for the detection of the presence of cell surface and soluble CD14 in a sample. Chimeric and CDR grafted antibodies generated from the above monoclonal antibodies are further provided. Pharmaceutical compositions containing the above biological compositions are provided. These are useful to treat and prevent disorders with CD14 mediated cell activation, such as sepsis.
Owner:THE SCRIPPS RES INST
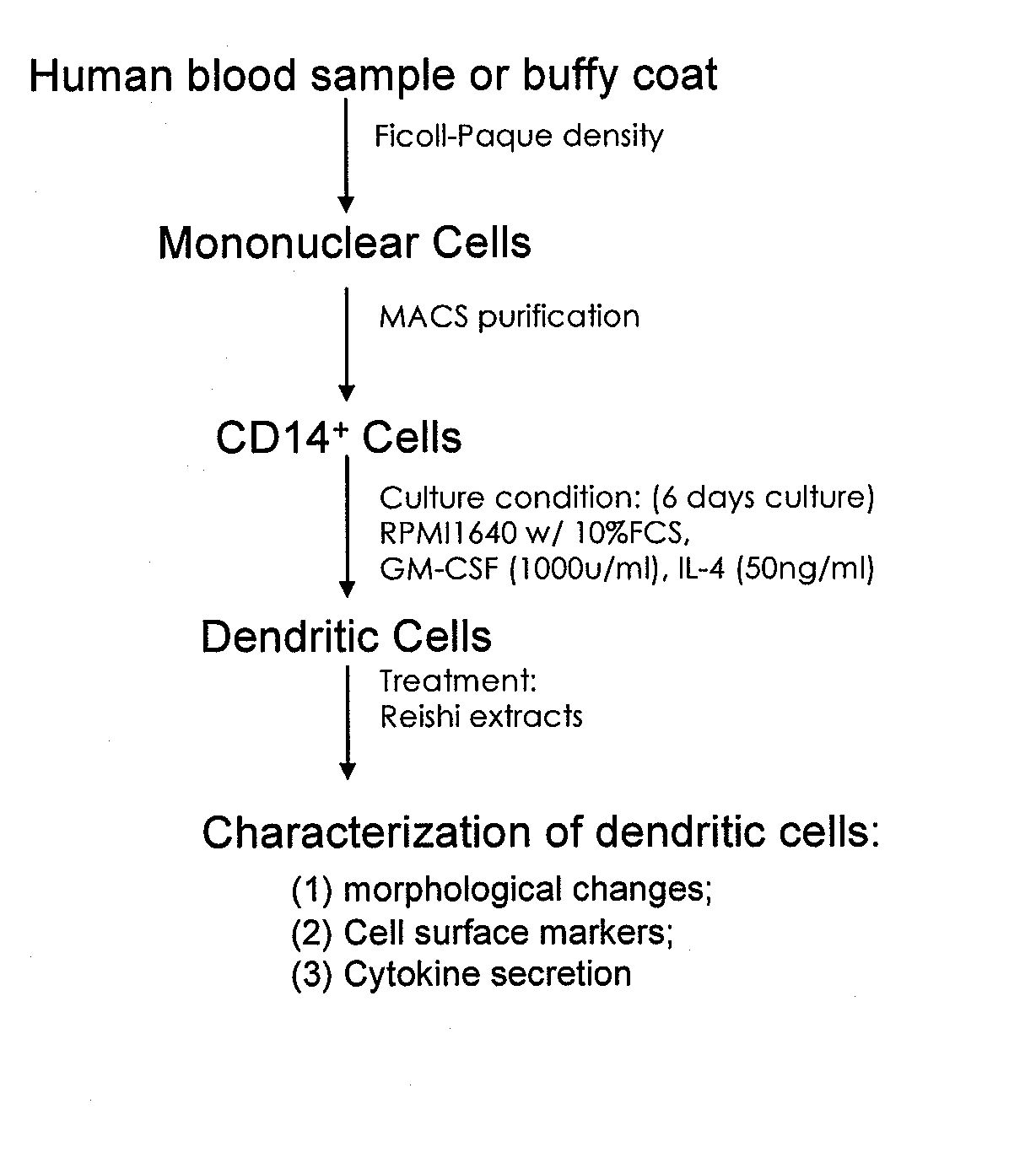
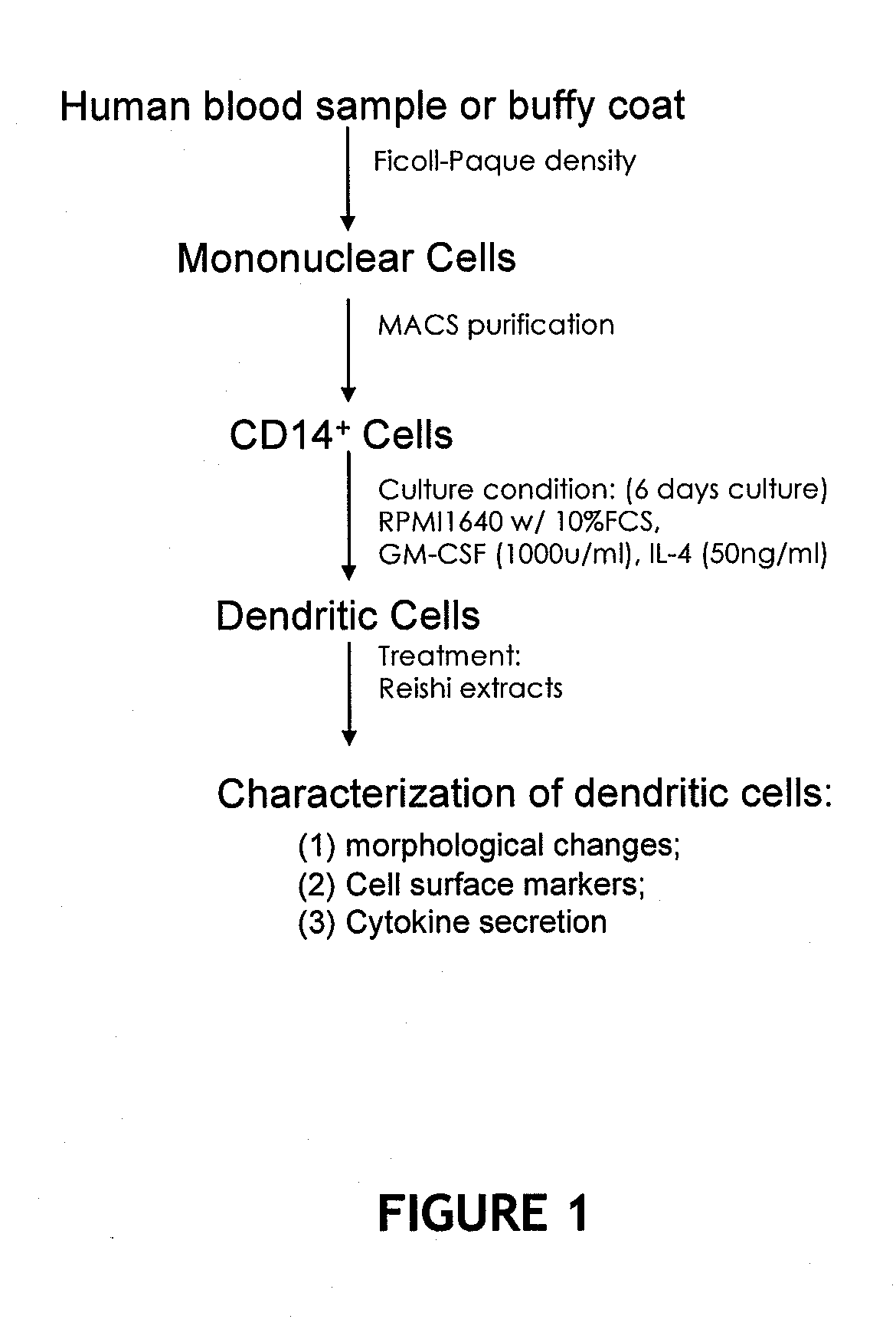
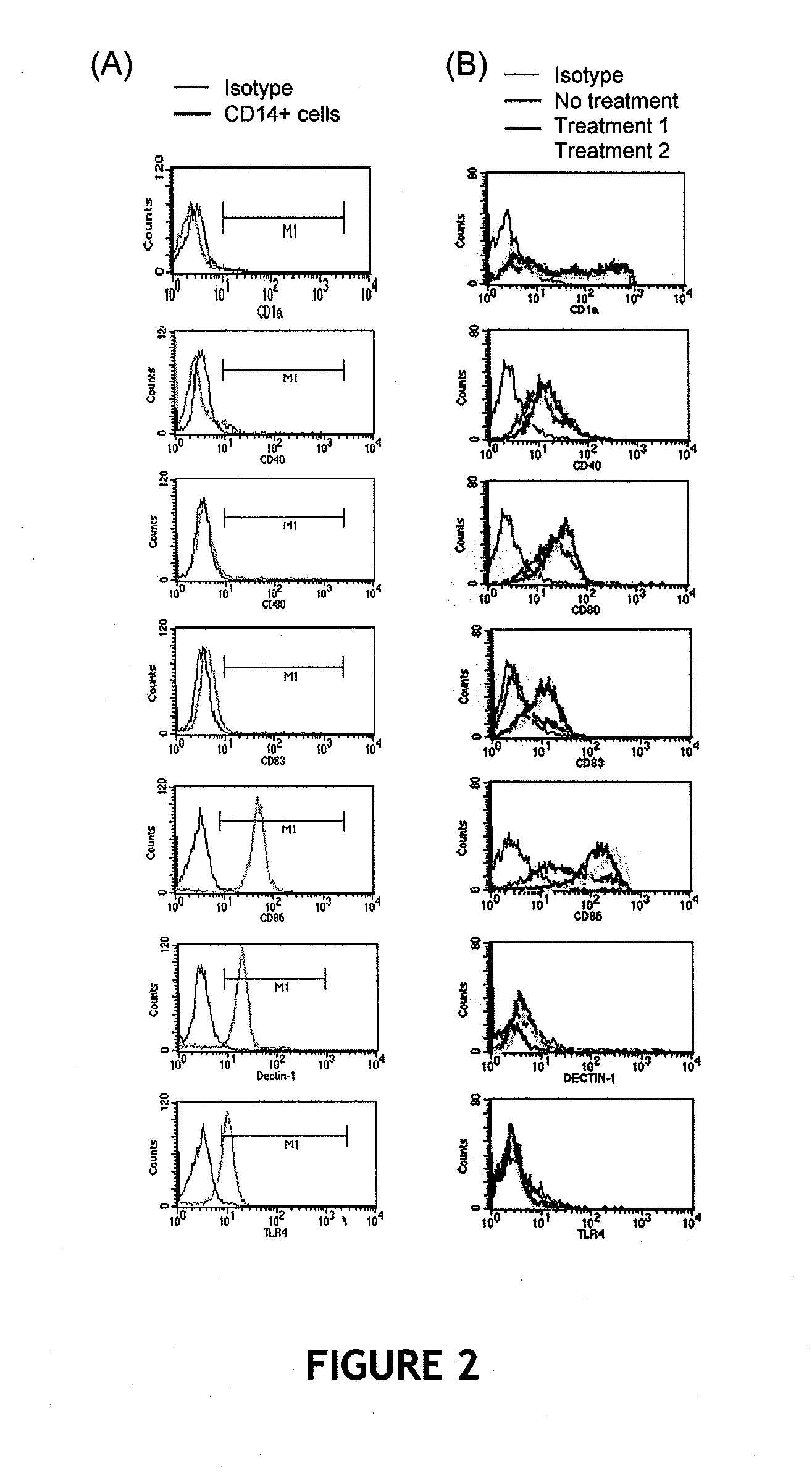
In vitro production of dendritic cells from CD14+ monocytes
InactiveUS20050008623A1High feasibilityHigh degree of reproducibilityBiocideCosmetic preparationsLangerhan cellMonolayer
The invention relates to the use of CD14+ monocytes for the production of dendritic cells. The invention comprises the use of CD14+ monocytes isolated from peripheral circulating blood for obtaining, by differentiation, at least one mixed population of Langerhans cells and interstitial dendritic cells, both Langerhans cells and interstitial dendritic cells being preconditioned and undifferentiated, and / or differentiated and immature, and / or mature, and / or interdigitated. The invention comprises their use in suspension, monolayer and three-dimensional cell and tissue models. The invention comprises the use of these cells and of these models as study models for the assessment of immunotoxicity / immunotolerance, for the development of cosmetic and pharmaceutical active principles and for the development and implementation of methods of cell and tissue therapy.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
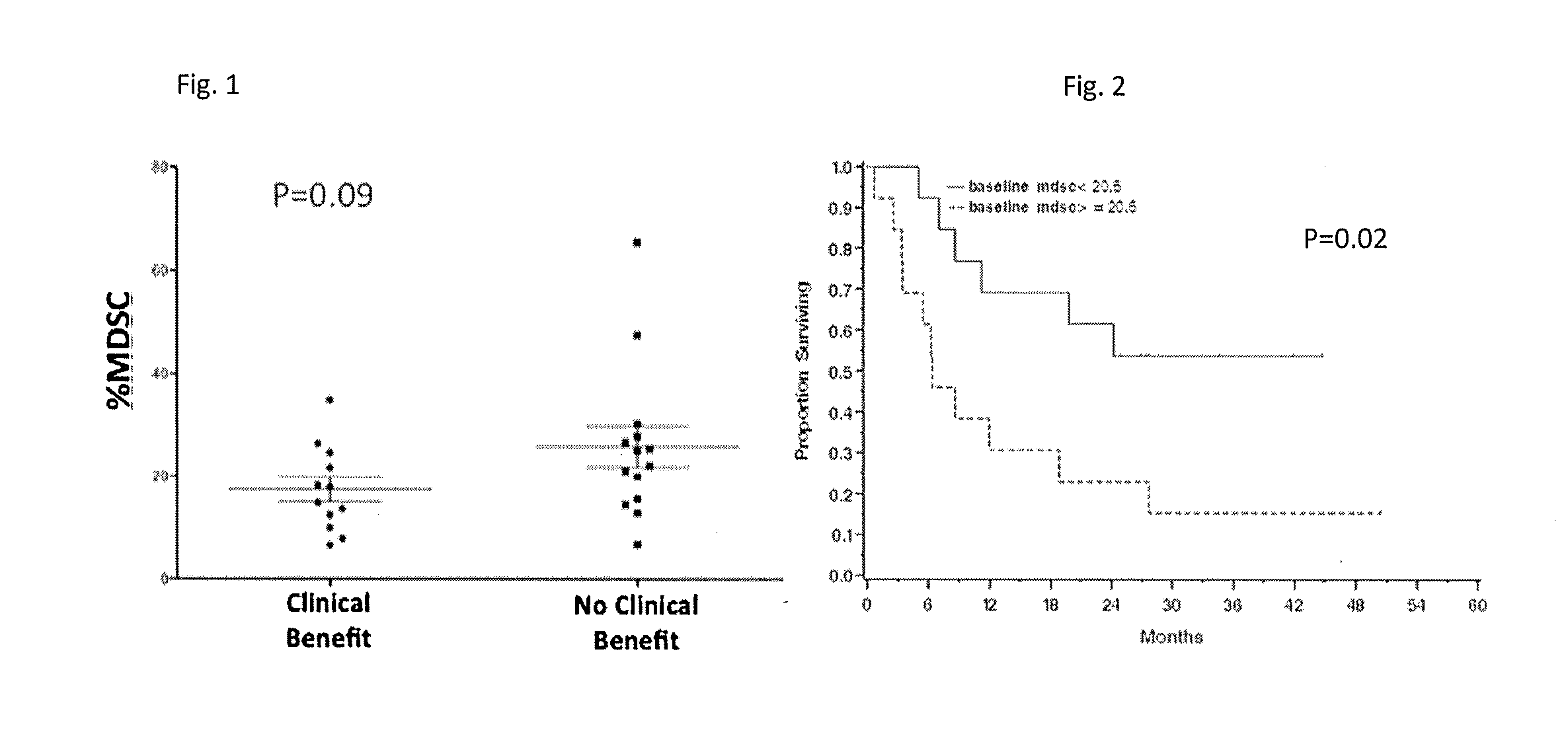
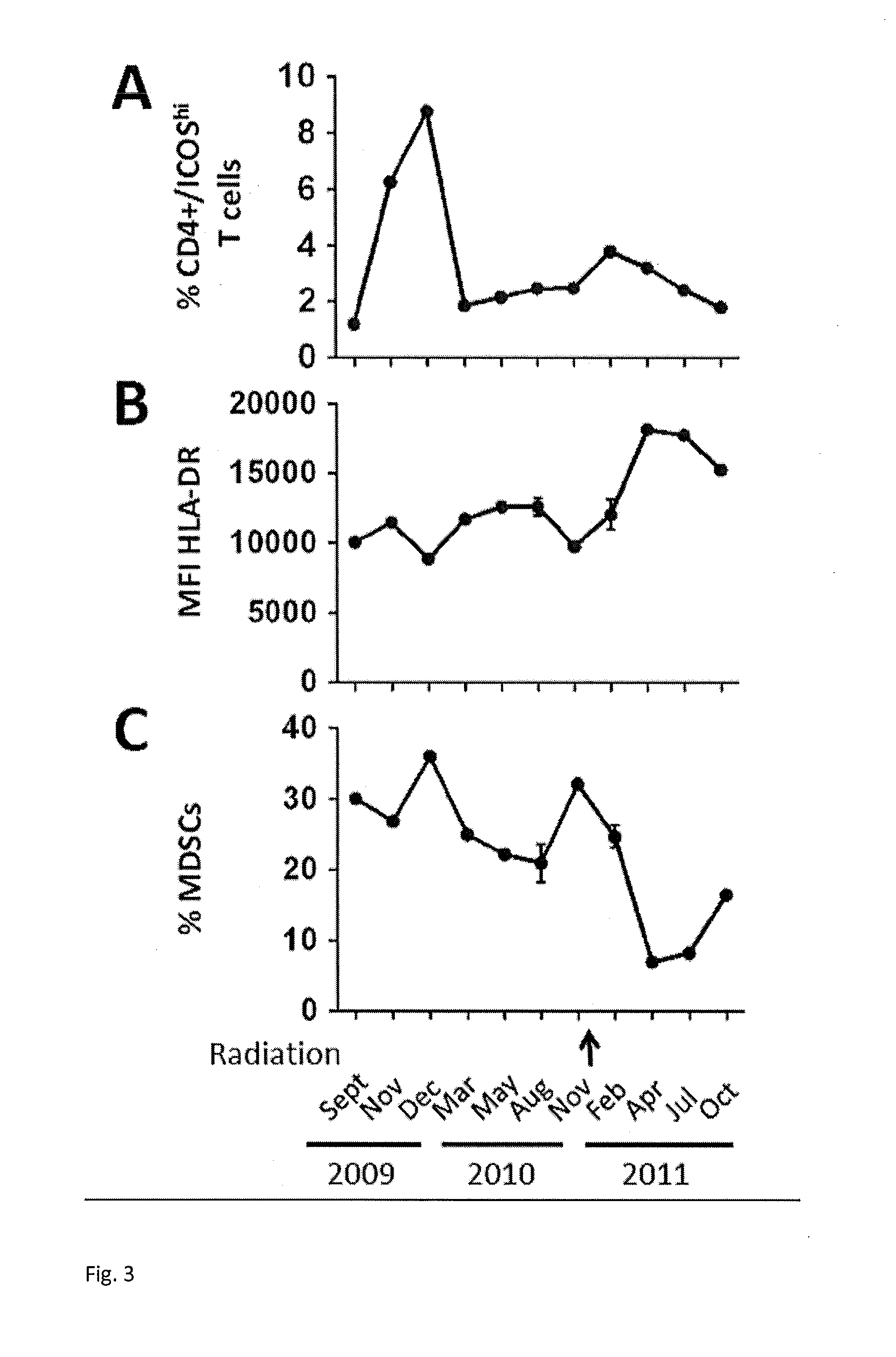
Prediction of Responsiveness to Treatment with Immunomodulatory Therapeutics and Method of Monitoring Abscopal Effects During Such Treatment
Efficacy of a therapeutic to enhance antitumor immunity in a patient is predicted, where the therapeutic is one that targets an immunomodulatory leukocyte membrane protein (ILMP) to enhance immune activity. Peripheral blood sample from the patient is tested for levels of monocytes having specific cell surface markers (CD14+, HLA-DRlow) prior to treatment. Low levels of monocytes of this type (PBM14+HLA-DRlow) indicate a greater likelihood of therapeutic efficacy. In specific exemplary embodiments of the invention, the therapeutic is an antibody to CTLA4, such as ipilimumab or tremelimumab.
Owner:SLOAN KETTERING INST FOR CANCER RES
Anti-CD14 antibody fusion protein
InactiveUS8252905B2Guaranteed stable actionPrevention and therapyAntibacterial agentsBiocideProteinase activityCD14
Owner:MOCHIDA PHARM CO LTD
Combined reagent for detecting acute myelocytic leukemia cells and system thereof
ActiveCN109655616AWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD33CD15
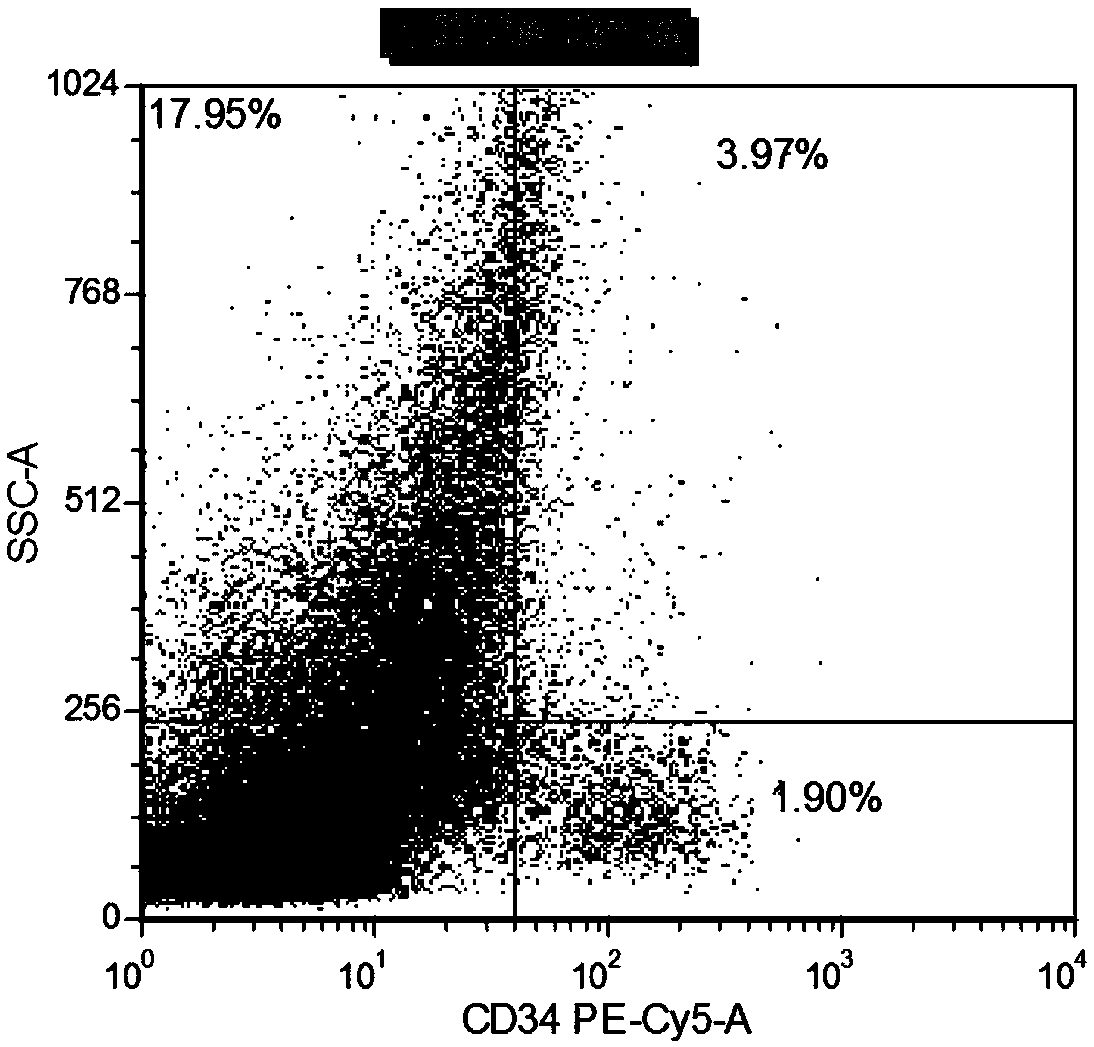
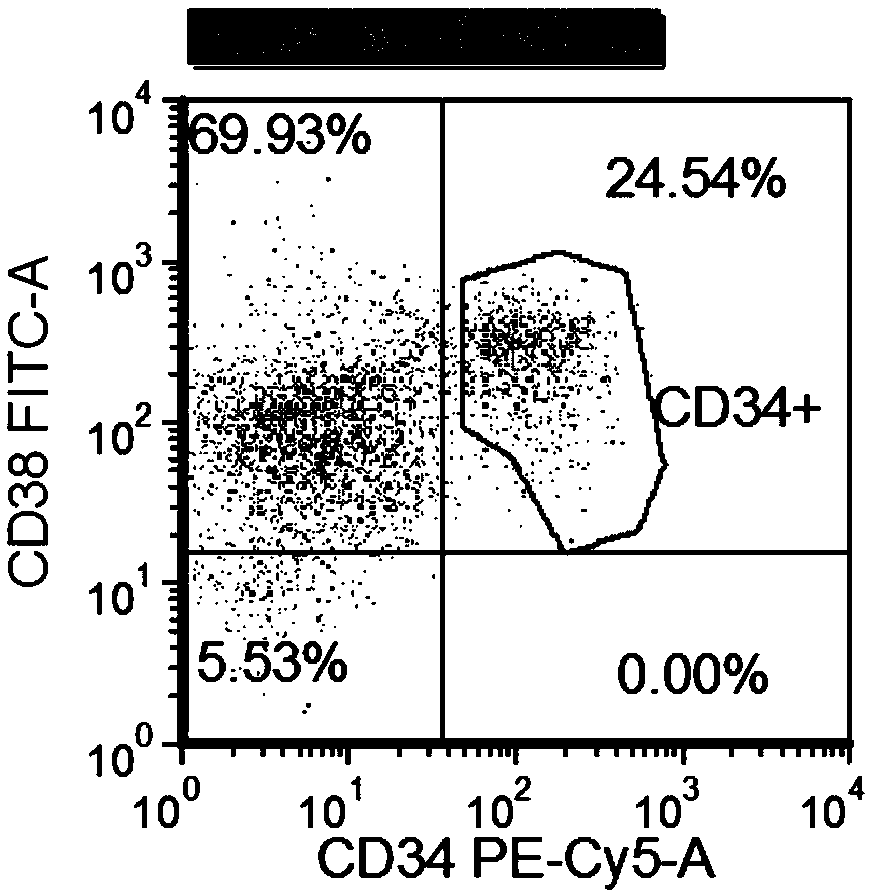
The invention relates to a combined reagent for detecting acute myelocytic leukemia cells and a system thereof, wherein the combined reagent and the system thereof belong to the field of medical technology. The combined reagent comprises at least one selected from the following antibody combinations: a first antibody combination which comprises CD38, CD13, CD34, CD117, CD33, CD19, HLA-DR and CD45antibodies; a second antibody combination which comprises CD38, CD64, CD34, CD123, CD56, CD14, HLA-DR and CD45 antibodies; and a third antibody combination which comprises CD38, CD7, CD34, CD5, CD11b,CD15 and CD45 antibodies. The antibody combinations of the invention cover the expression marks of three systems of granulocyte, single cell and lymphocyte. A normal antibody expression mode is established. Tumor cells can be identified maximally. Furthermore, through a large number of experiment data, the antibodies in each combination have no problem of mutual expression inhibition. FurthermoreAML-MRD can be comprehensively and quickly detected with high sensitivity through multi-parameter flow type cell analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
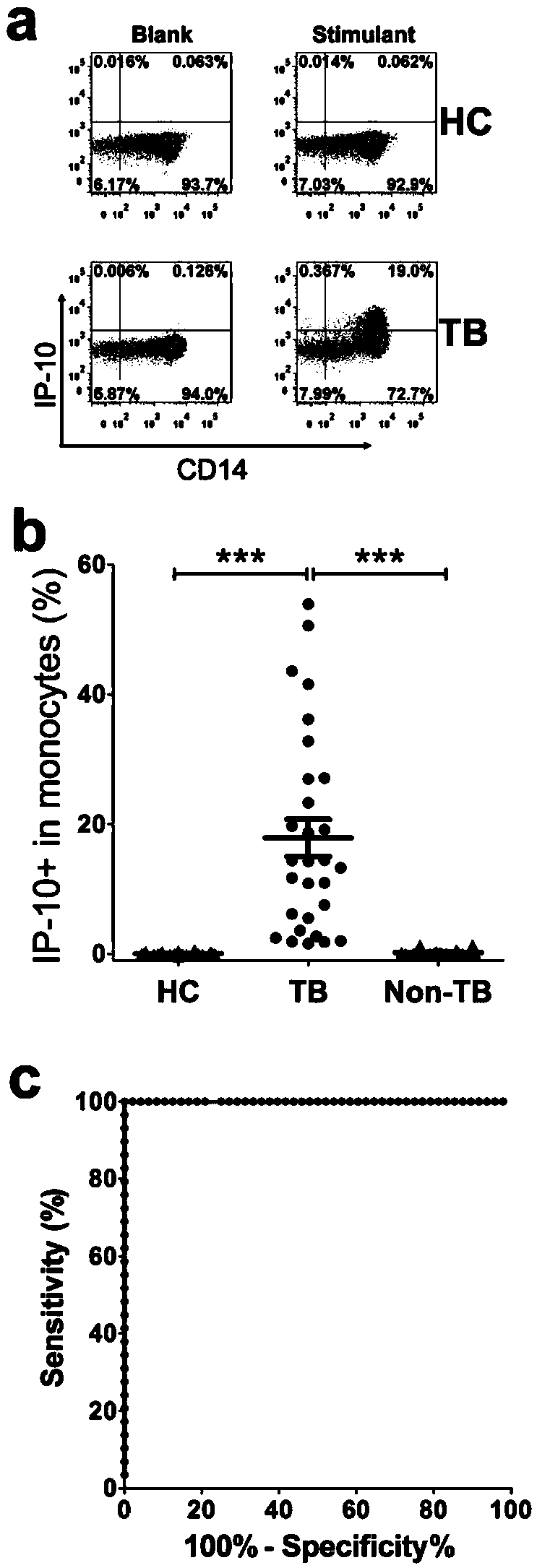
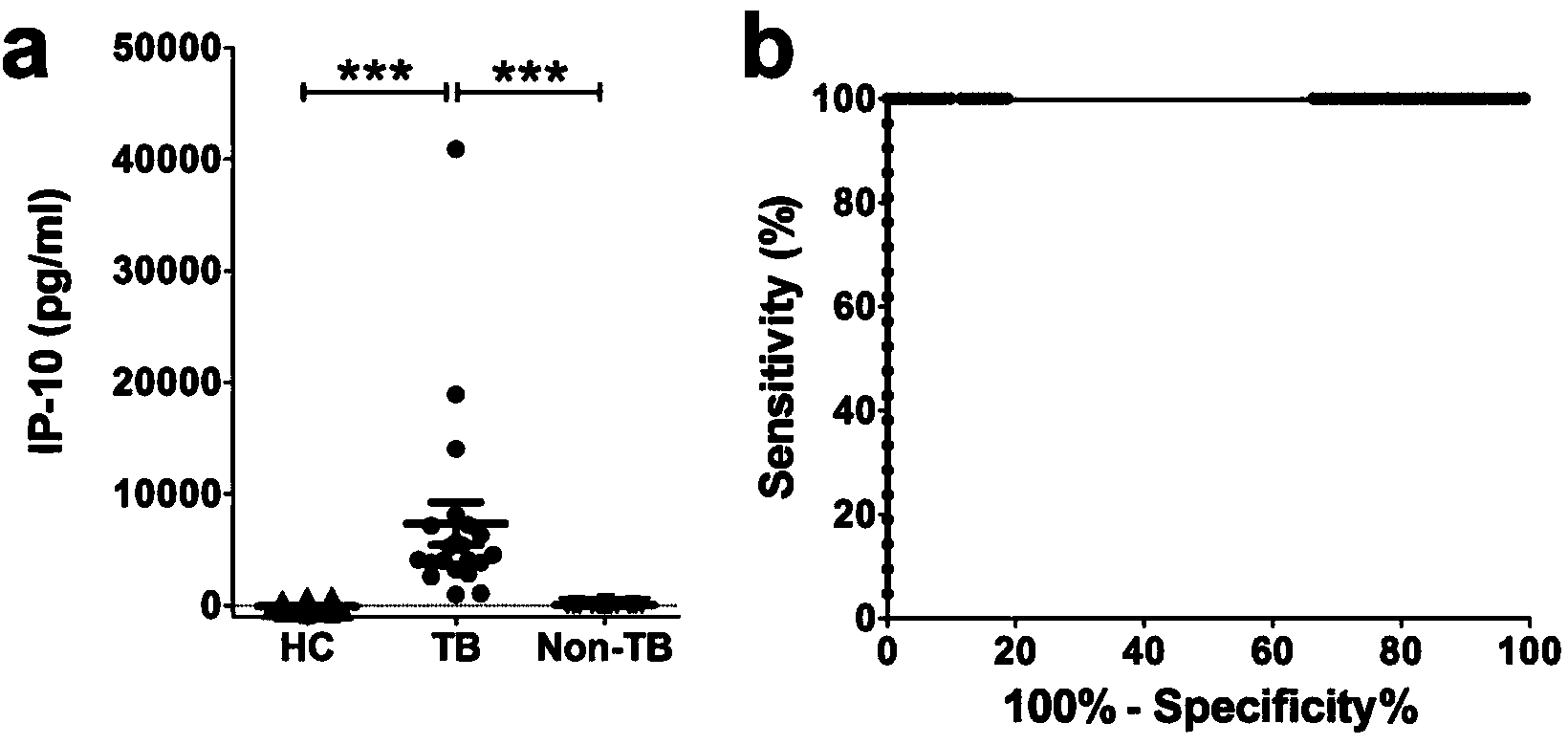
Kit for detecting mycobacterium tuberculosis infection and monitoring clinical treatment effect and application of kit
ActiveCN104020297AIncreased sensitivityImprove featuresDisease diagnosisBiological testingTherapeutic effectSpecific antibody
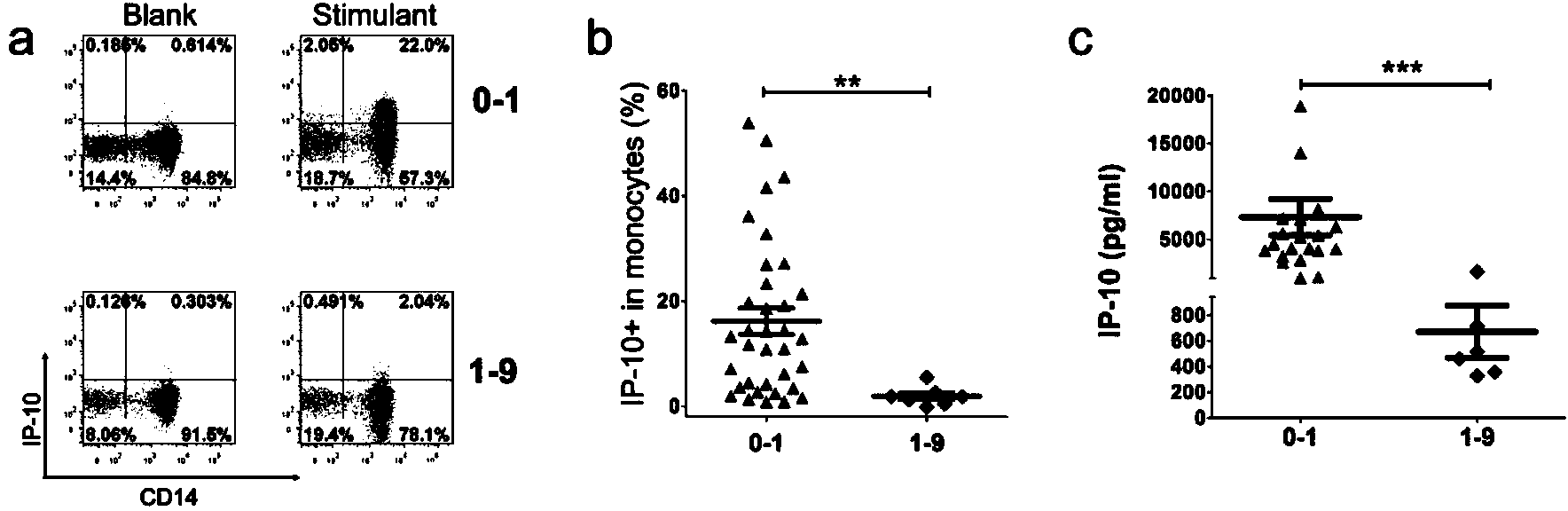
The invention discloses a kit for detecting the mycobacterium tuberculosis infection and monitoring a clinical treatment effect. The kit provided by the invention comprises a specific antibody, namely an IP-10 antibody and / or CD14 antibody, an antigen irritant and a positive contrast irritant. The kit disclosed by the invention can be used for diagnosing an active tuberculosis patient or a tuberculosis latent infection patient and is not affected by BCG (bacillus calmette-guerin) inoculation. The sensitivity and the specificity of the kit for diagnosing the active tuberculosis patient are higher than those of a commercial T-SPOT.TB kit and can be up to 100 percent. After the antituberculosis therapy is performed for 1 month, the detection rates of over 80 percent of clinical tuberculosis patients are converted to be negative, so that the kit can be used for detecting the clinical antituberculosis treatment effect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Fungal immunostimulatory compositions
Methods are disclosed which are useful in increasing maturation of dendritic cells from CD14+ mononuclear cells, by contact with a composition comprising a fucose-containing glycoprotein fraction from Ganoderma lucidum. The extract can also be used for increasing production of a cytokine or a chemokine in a dendritic cell or CD19+ B cell. In addition, a fucose-containing glycoprotein fraction from Ganoderma lucidum can be administered to a subject identified as needing increased immunoglobulin, cytokine, or chemokine production.
Owner:ACAD SINIC
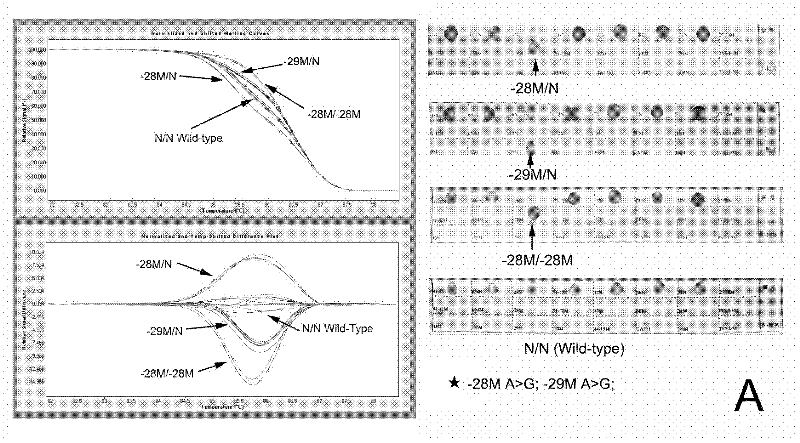
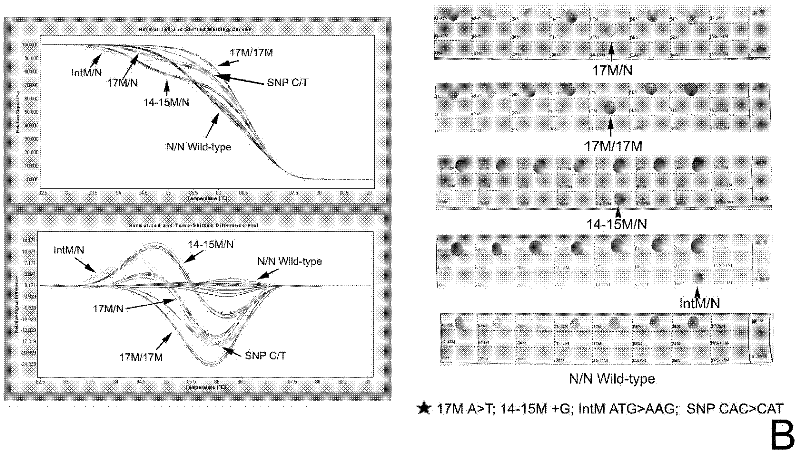
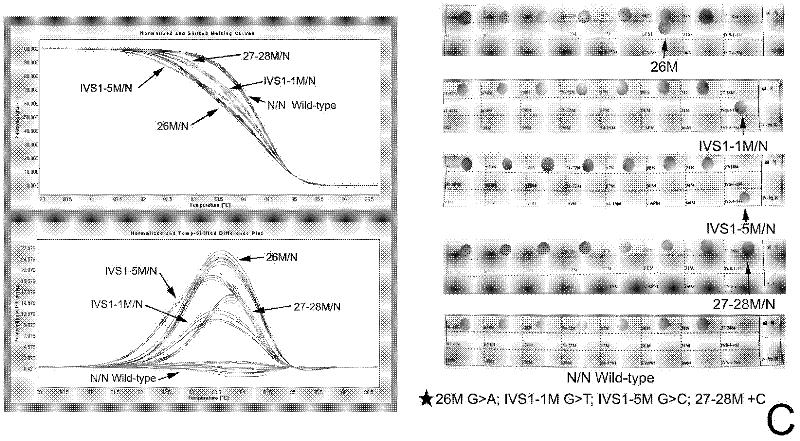
Real-time fluorescence quantitative PCR(Polymerase Chain Reaction) detection primers and kit for thalassemia
InactiveCN102409100AAvoid pollutionMeet the needs of rapid detectionMicrobiological testing/measurementDNA/RNA fragmentationStart codonCD43
The invention discloses a real-time fluorescence quantitative PCR(Polymerase Chain Reaction) detection kit and related primers for thalassemia genes. The kit comprises more than one pair of the following primers: SEQ ID NO.1 and SEQ ID NO.2 aiming at TATA kits nt-28 (A->G) (-28M), -29 (A->G) (-29M) and CAP-40-43 (AACC) (CAPM) genotypes; SEQ ID NO.3 and SEQ ID NO.4 aiming at CD14-15 (+G) (14-15M), CD17 (A->T) (17M) and the initiation codon mutant ATG->AGG(IntM) genotypes; SEQ ID NO.5 and SEQ ID NO.6 aiming at CD26 (G->A)(betaEM) CDs27-28+C(27-28M), IVS1-1(G->T)(IVS1-1M), IVS1-5(G->C)(IVS1-5M) genotypes; SEQ ID NO.7 and SEQ ID NO.8 aiming at CDs41-42-TCTT (41-42M), CD43G->T(43M), 41-42M / 41-42M genotypes; SEQ ID NO.9 and SEQ ID NO.10 aiming at CD71-72(+A) (71-72M) genotypes; SEQ ID NO.11 and SEQ ID NO.12 aiming at IVS2-654C->T(654M) and 654M / 654M genotypes; and SEQ ID NO.13 and SEQ ID NO.14 aiming at constant spring (CS), Quong Sze (QS) and Westmead (WS) genotypes. The kit has the advantages of good detection specificity and high detection sensitivity.
Owner:潮州市中心医院
Cationic liposomal drug delivery system for specific targeting of human cd14+ monocytes in whole blood
InactiveCN104703588ASenses disorderNervous disorderLipid formationNatural Killer Cell Inhibitory Receptors
This invention concerns a liposome comprising lipids and at least one active ingredient, wherein at least one of the lipids is a cationic lipid; said liposome exhibiting a net positive charge at physiological conditions at which said liposome preferentially adheres to monocytes in freshly drawn blood when compared to adherence to granulocytes, T-lymphocytes, B-lymphocytes and / or NK cells in freshly drawn blood, to a lipid-based pharmaceutical composition comprising said liposomes and their use in monocytic associated prophylaxis, treatment or amelioration of a condition such as cancer, an infectious disease, an inflammatory disease, an autoimmune disease or allergy.
Owner:碧奥尼尔股份公司 +1
Isolated myeloid-like cell populations and methods of treatment therewith
The present invention provides an isolated myeloid-like cell population comprising a majority of cells that are lineage negative, and which express both CD44 antigen, CD11b antigen, and hypoxia inducible factor 1α (HIF-1α). These cells have beneficial vasculotrophic and neurotrophic activity when intraocularly administered to the eye of a mammal, particularly a mammal suffering from an ocular degenerative disease. The myeloid-like cells are isolated by treating bone marrow cells, peripheral blood cells or umbilical cord cells with an antibody against CD44 (hyaluronic acid receptor), against CD11b, CD14, CD33, or against a combination thereof and using flow cytometry to positively select CD44 and / or CD11b expressing cells therefrom. The isolated myeloid-like bone marrow cells of the invention can be transfected with a gene encoding a therapeutically useful protein, for delivering the gene to the retina.
Owner:THE SCRIPPS RES INST
Purified Ethyl Ester Sophorolipid for the Treatment of Sepsis
InactiveUS20120142621A1High purityEfficient productionBiocideCarbohydrate active ingredients[Candida] apicolaChemical transformation
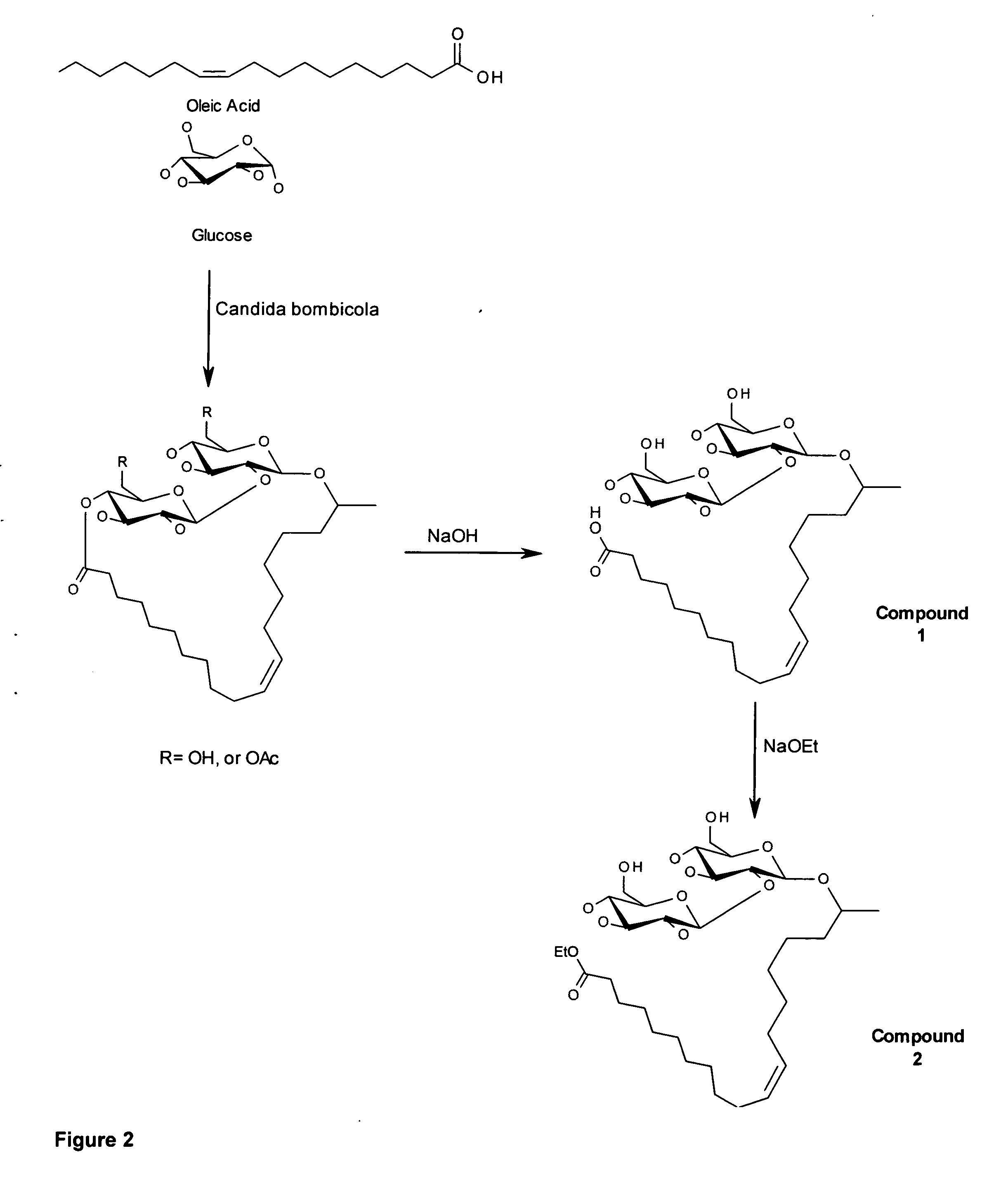
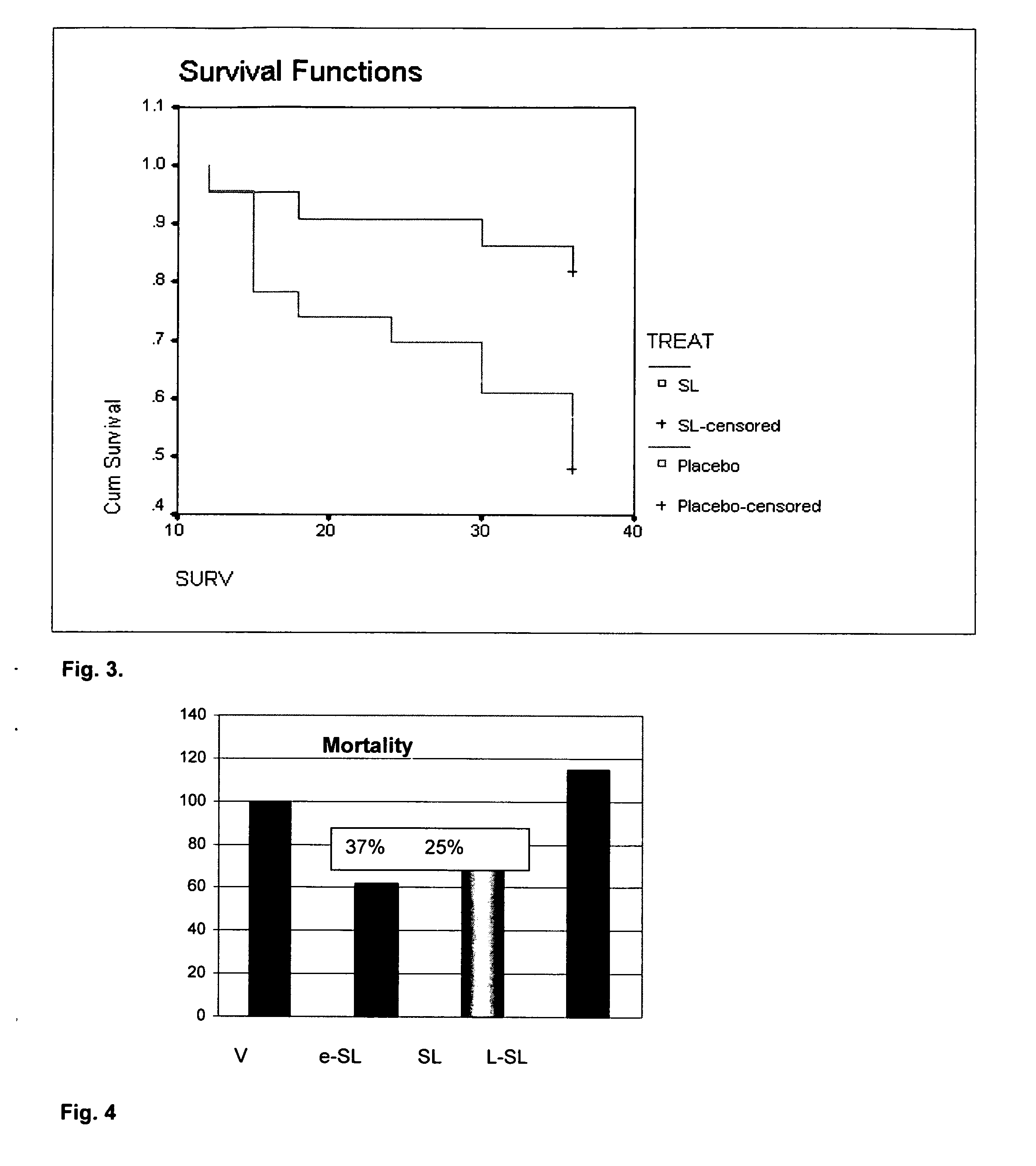
A microbial ethyl esther sophorolipid derivative with no acetylated groups produced by Candida species, for treating and preventing sepsis / septic shock. The method of producing sophorolipids is through microbial resting cells of Candida bombicola. The sophorolipids obtained from resting state cultures are isolated as a complex mixture of compounds and then decanted as a dense oil from the culture broth, subsequently washed to remove free fatty acids. Secondary chemical transformation via base catalyzed hydrolysis is used to reduce the 8 possible structural sophorolipid species to a single moiety, the 17-L-[(2′-O-b-D-glucopyranosyl-b-D-glucopyranosyl)-oxy]-cis-9-octadecenoate de-acetylated free acid. The compound acts primarily through decreasing inflammatory cytokines and eliciting other synergistic anti-inflammatory mechanisms by blocking TLR4-CD14 upstream of the inflammatory signaling cascade. The compound can be administered either intraperitoneally or intravenously at single or multiple doses of 5-30 mg / kg of weight in solvent media or in capped nanoparticles, preferably within 48 hours of sepsis inception.
Owner:STREETCAREC SLOAN
Methods and reagents for efficient and targeted gene transfer to monocytes and macrophages
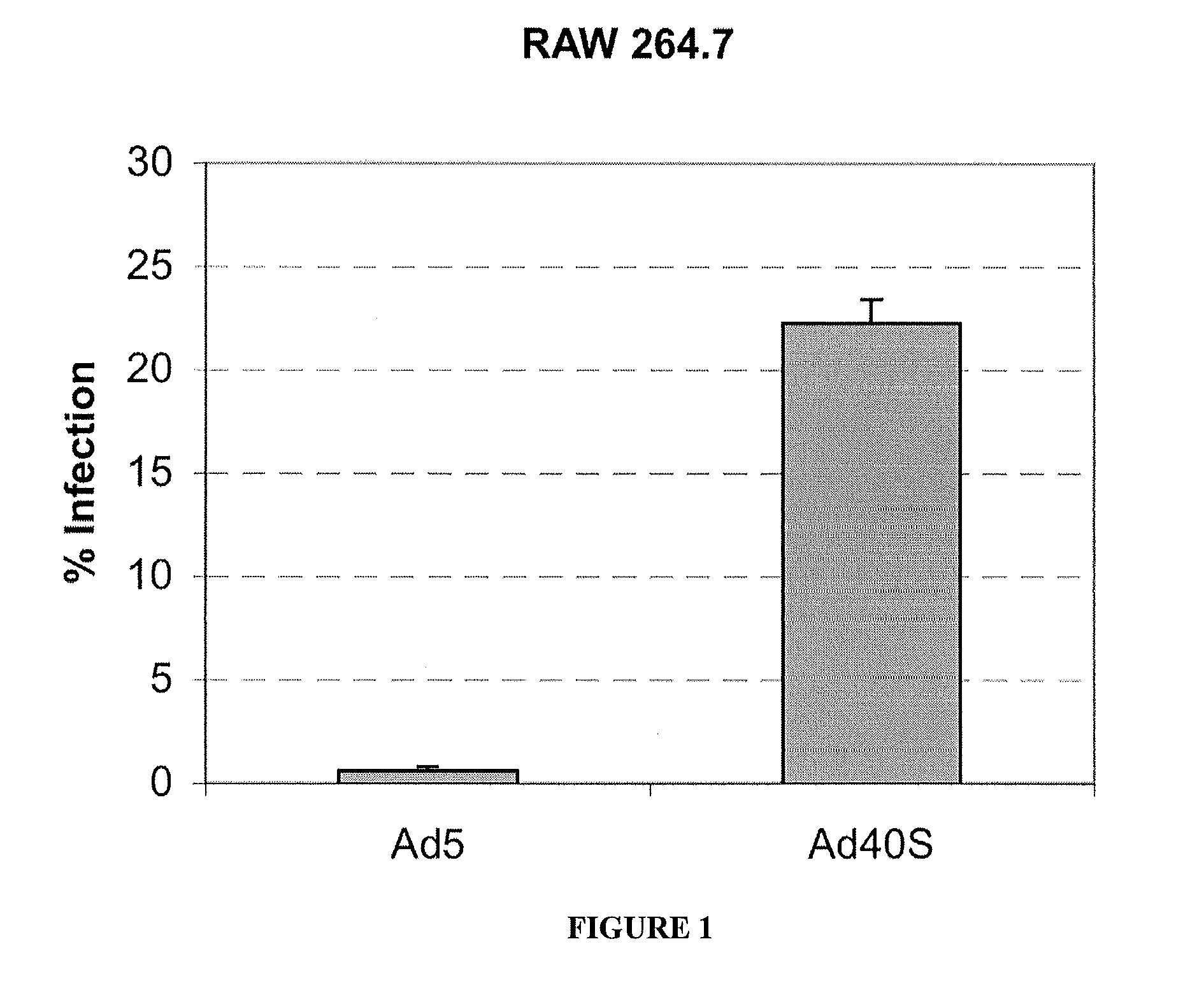
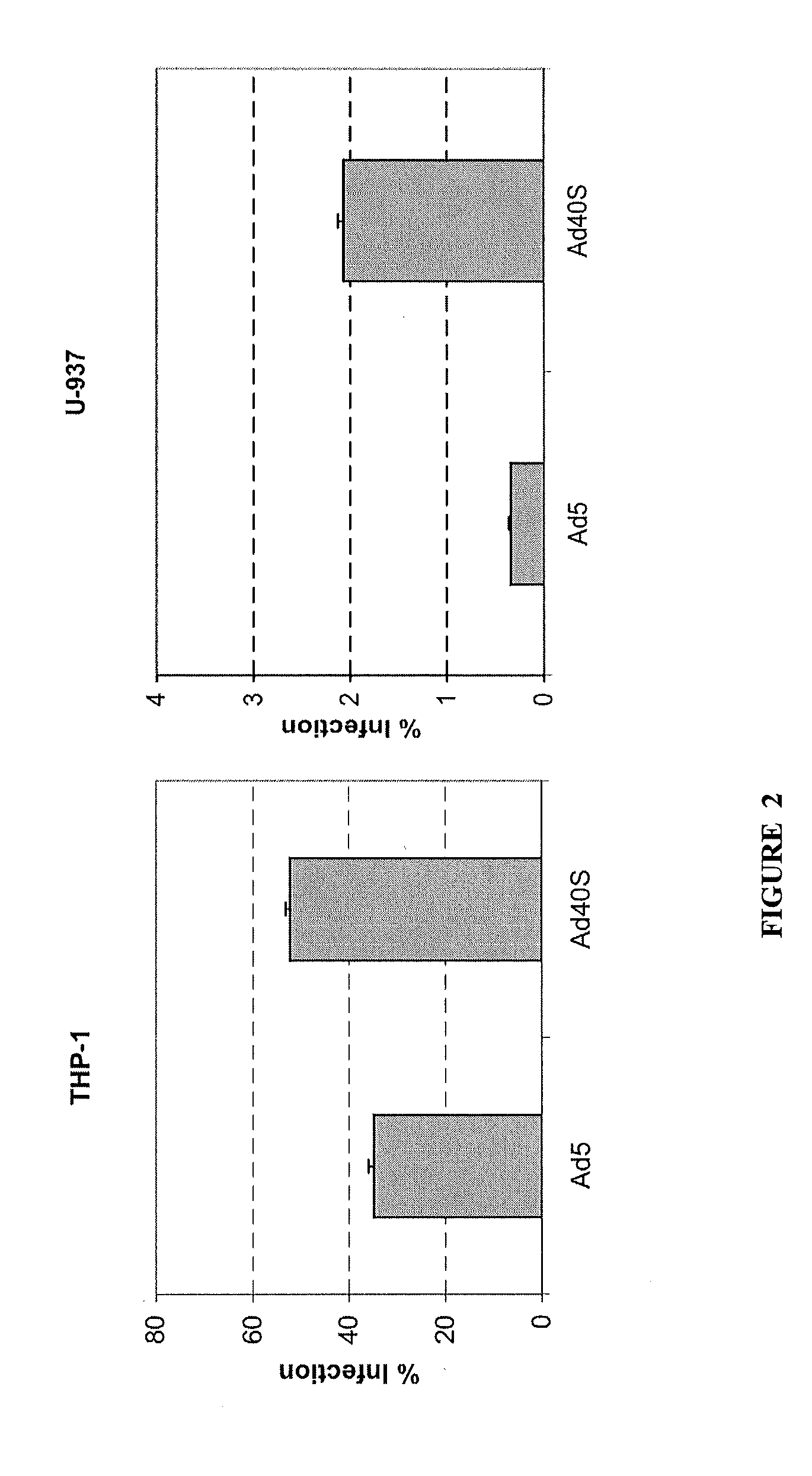
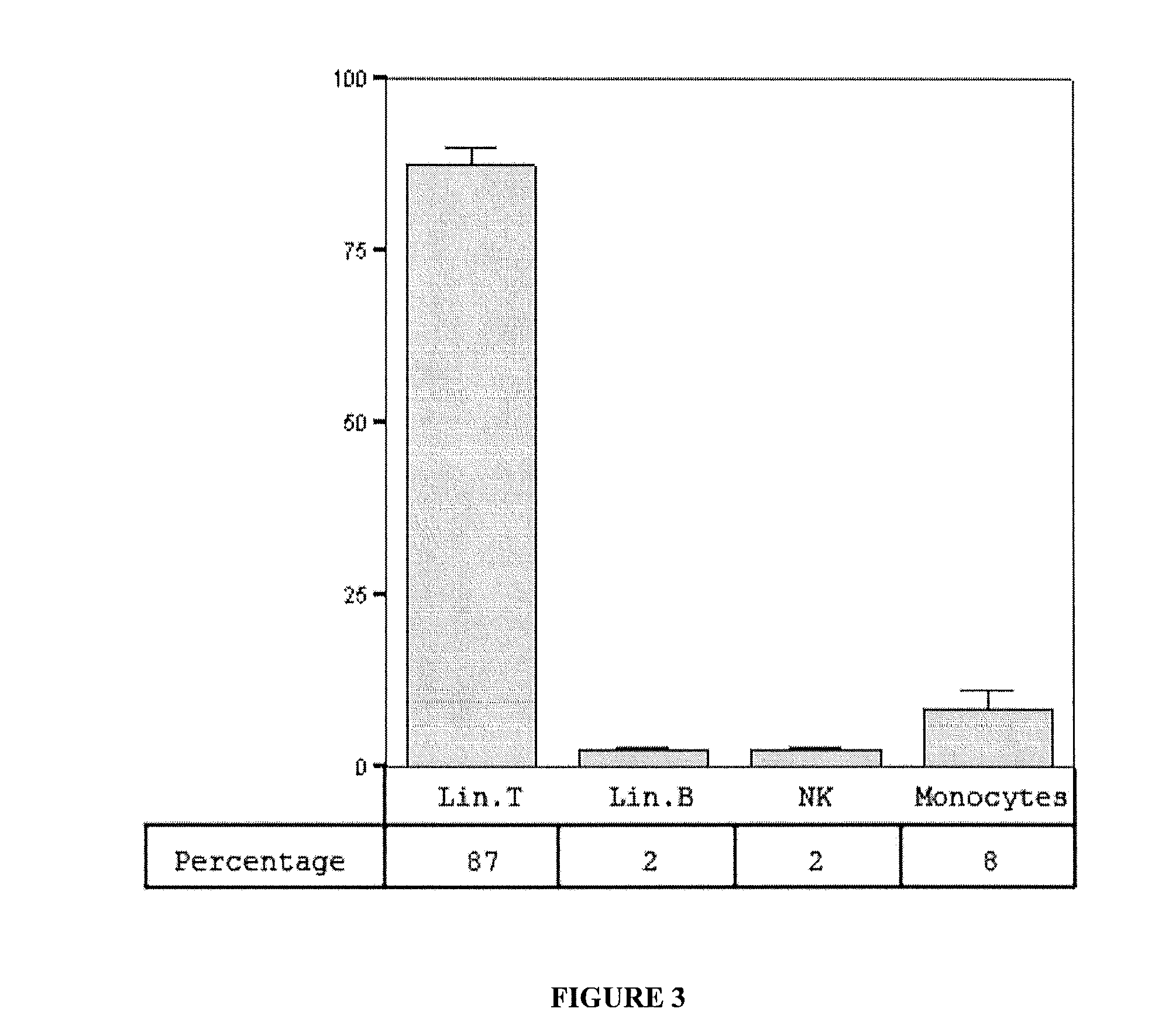
The present invention provides a biosafe and useful vector to transfer genetic material to CD14+ mononuclear cells (monocytes and monocyte-derived macrophages) in an efficient and specific manner. The embodiment of the invention makes use of the chimeric human adenovirus vectors 5 carrying the short fiber of enterotropic Ad40 to transfer genetic material to the target CD14+ mononuclear cells.
Owner:AUTONOMOUS UNIVERSITY OF BARCELONA +3
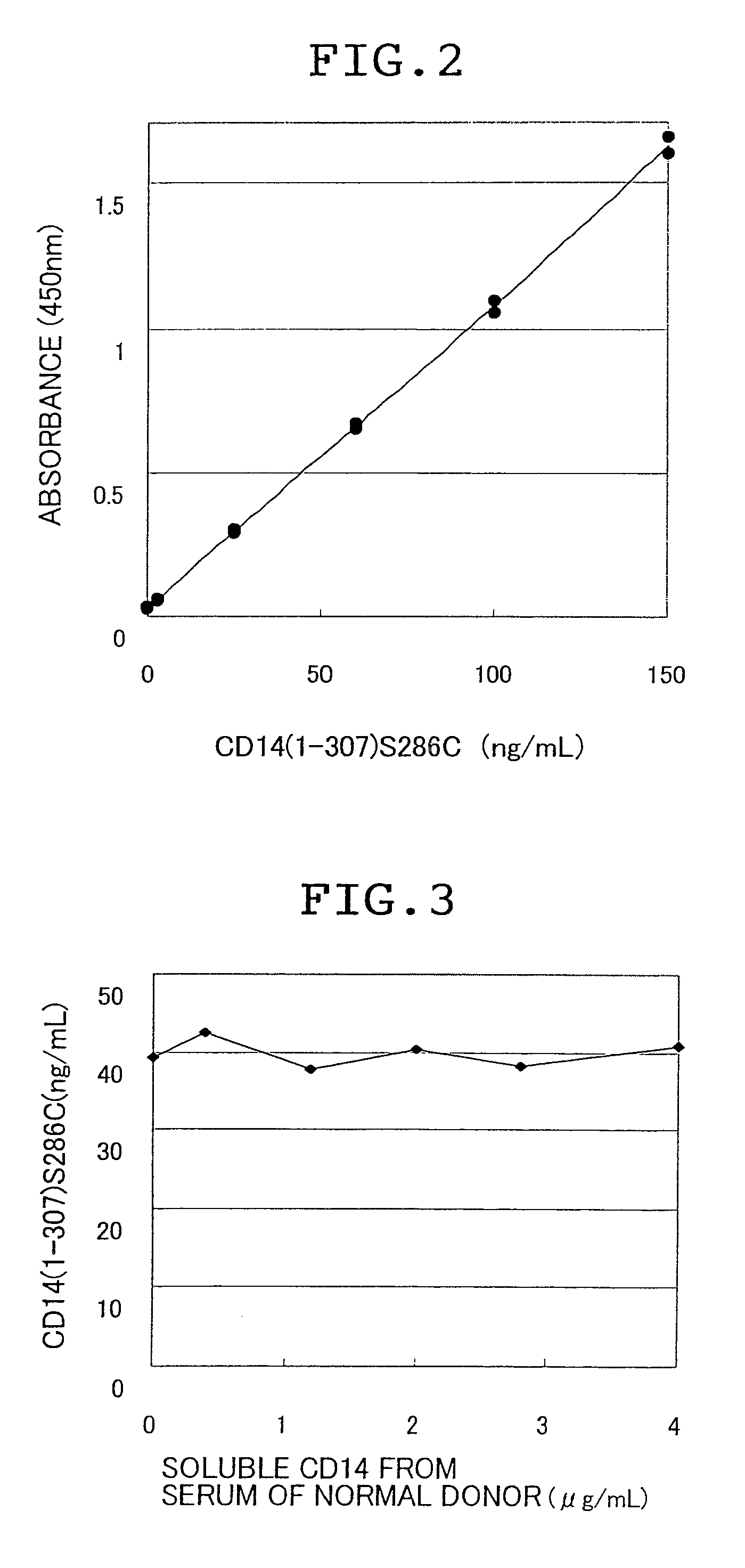
Soluble CD14 antigen
InactiveUS7608684B2Efficient use ofHigh sensitivityPeptide/protein ingredientsDisease diagnosisAntigenIn vivo
There are provided a soluble CD14 antigen which is a novel in vivo protein useful as a marker for diagnosing sepsis and has the following characteristic features 1) to 3):1) a molecular weight of 13±2 kDa when measured by SDS-PAGE under non-reducing conditions;2) an amino acid sequence in which the amino acid sequence of SEQ ID NO:1 is present on its N terminal; and3) ability to specifically bind to an antibody prepared by using a peptide comprising 16 amino acid residues described in SEQ ID NO:2 for the antigen; and a recombinant soluble CD14 fragment.
Owner:MOCHIDA PHARM CO LTD
Human liver progenitors
InactiveUS20050148072A1Low densityReduce in quantityHepatocytesGenetic material ingredientsProgenitorGlycophorin
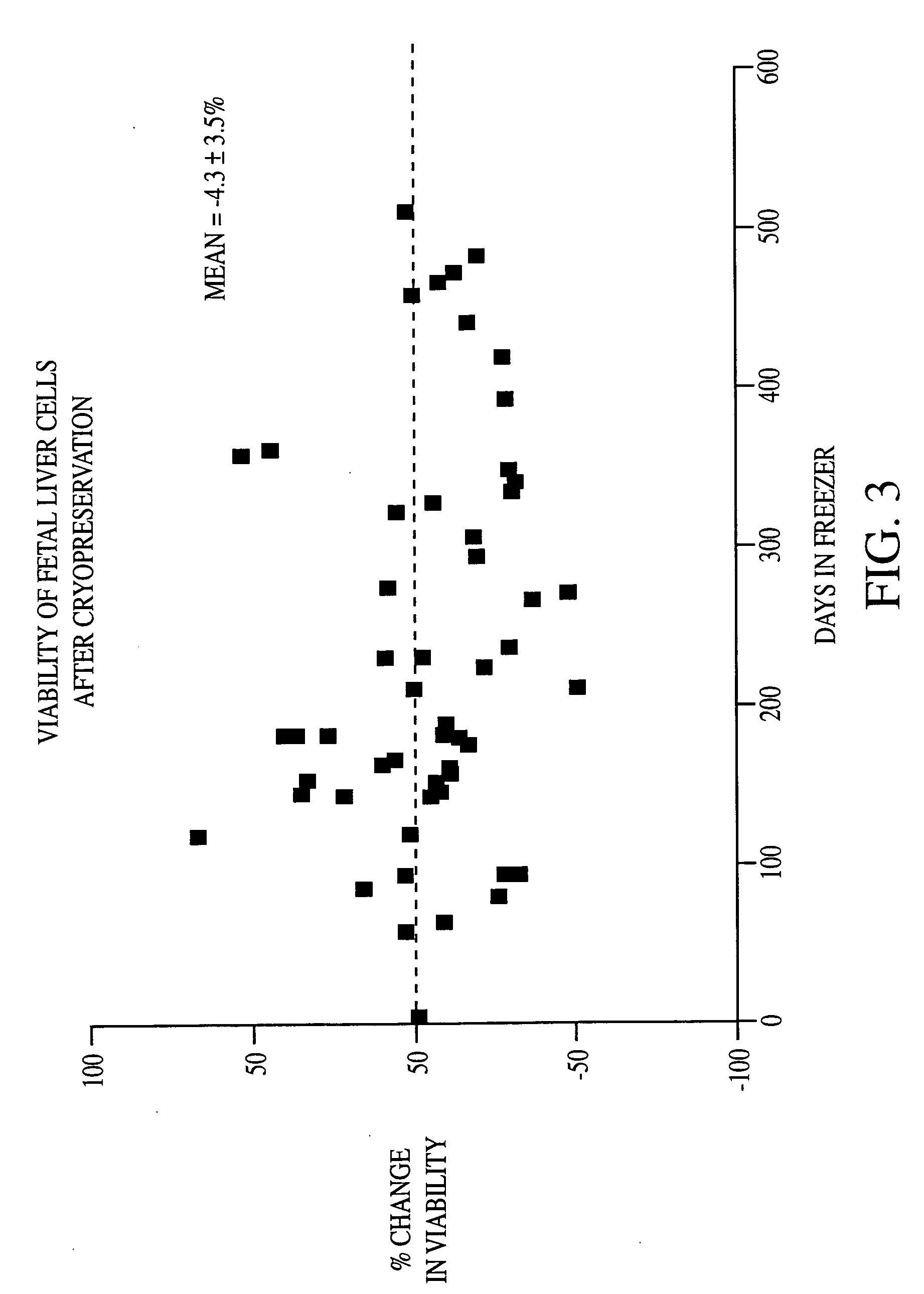
Methods of isolating and cryopreserving progenitors from human liver are disclosed which include processing human liver tissue to provide a substantially single cell suspension comprising progenitors and non-progenitors of one or more cell lineages found in human liver; subjecting the suspension to a debulking step, which reduces substantially the number of non-progenitors in the suspension, and which provides a debulked suspension enriched in progenitors exhibiting one or more markers associated with at least one of the one or more cell lineages; and selecting from said debulked suspension those cells, which themselves, their progeny, or more mature forms thereof express one or more markers associated with at least one of the one or more cell lineages. Among these markers are CD14, CD34, CD38, CD45, and ICAM. Hepatic progenitors are characterized as being 6-15μ in diameter, diploid, glycophorin A−, CD45−, AFP+++, ALB+, ICAM+, and with subpopulations varying in expression of CD14+. CD34++, CD38++, CD117+. These progenitor subpopulations have characteristics expected for cells that are particularly useful in liver cell and gene therapies and for establishing bioartificial organs.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Monocyte-origin multipotent cell momc
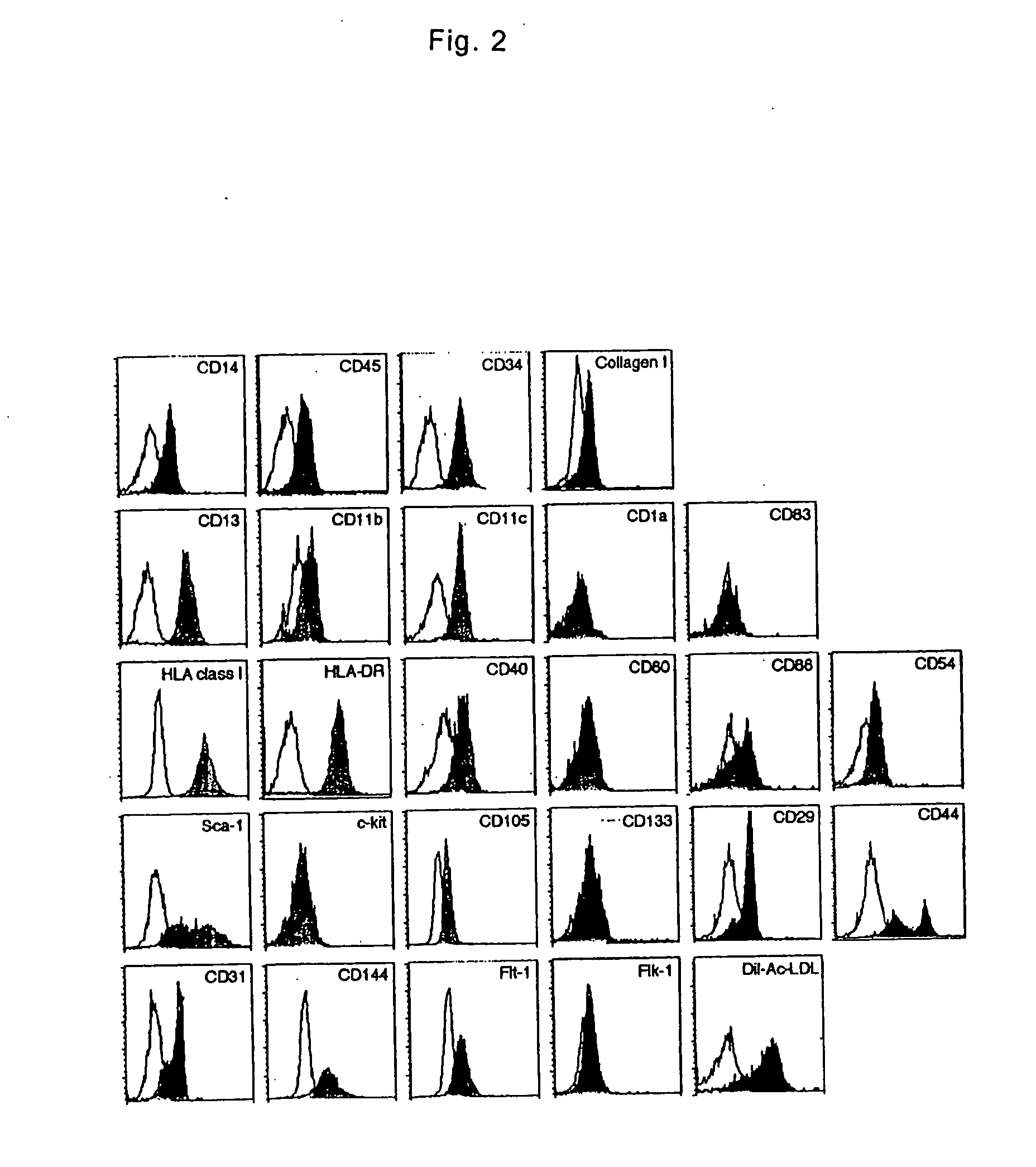
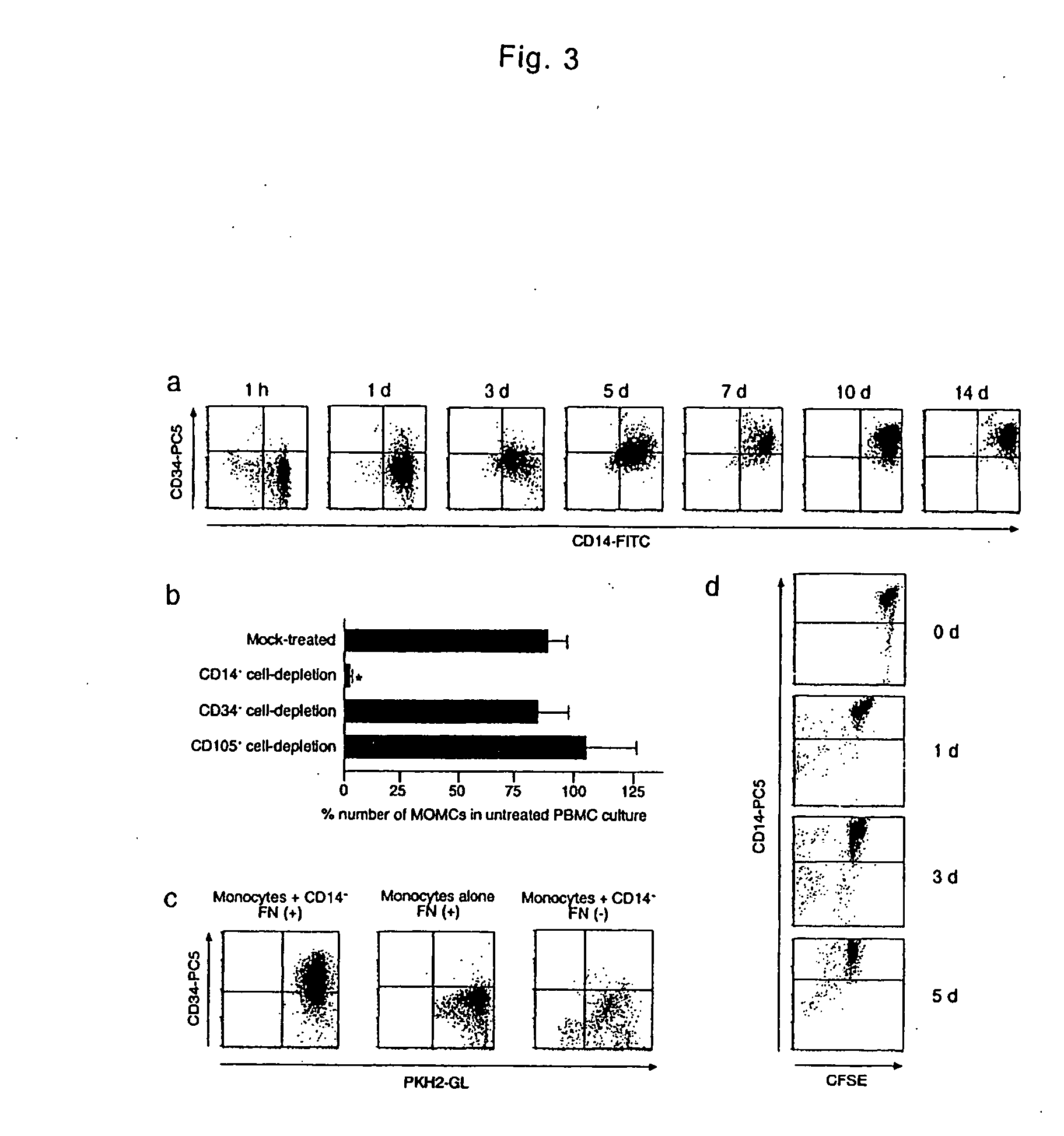
The present invention is to provide a multipotent cell wherein the sufficient amount necessary can be stably and conveniently supplied with a minimum invasion, that will not cause rejection at the time of cell transplantation, that has a potential to differentiate into various cells such as mesenchymal cells including bone, cartilage, skeletal muscle and fat, endothelial cells, myocardial cells, neurons, mesenchymal cells, myocardial cells, endothelial cells, neurons induced to differentiate from the multipotent cell, and a therapeutic agent / treating method comprising these as active ingredient. Peripheral blood mononuclear cells (PMBC) are cultured on fibronectin-coated plastic plates for 7 to 10 days. The generating cell population with a fibroblast-like morphology is derived from circulating CD14+ monocyte, with a unique phenotype of CD14+CD45+CD34+ type I collagen+. These cells have a potential to differentiate into mesenchymal cells including bone, cartilage, skeletal muscle and fat, endothelial cells, myocardial cells, and neurons under particular culture conditions.
Owner:KEIO UNIV
Determination of Exosomel Biomarkers for Predicting Cardiovascular Events
InactiveUS20120309041A1Reduce stepsComplicate to detectMicrobiological testing/measurementImmunoglobulins against animals/humansAmniotic fluidNGAL Protein
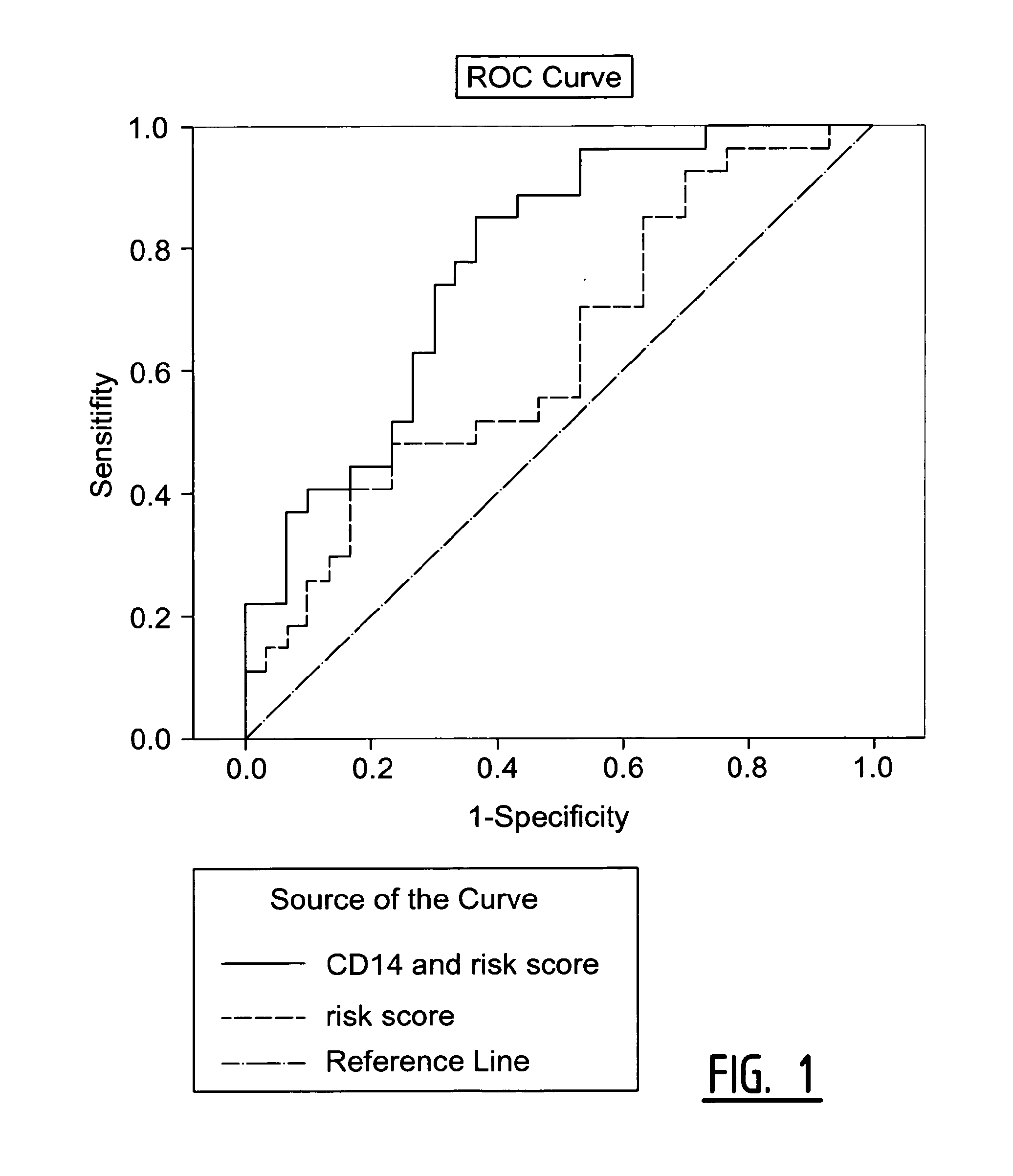
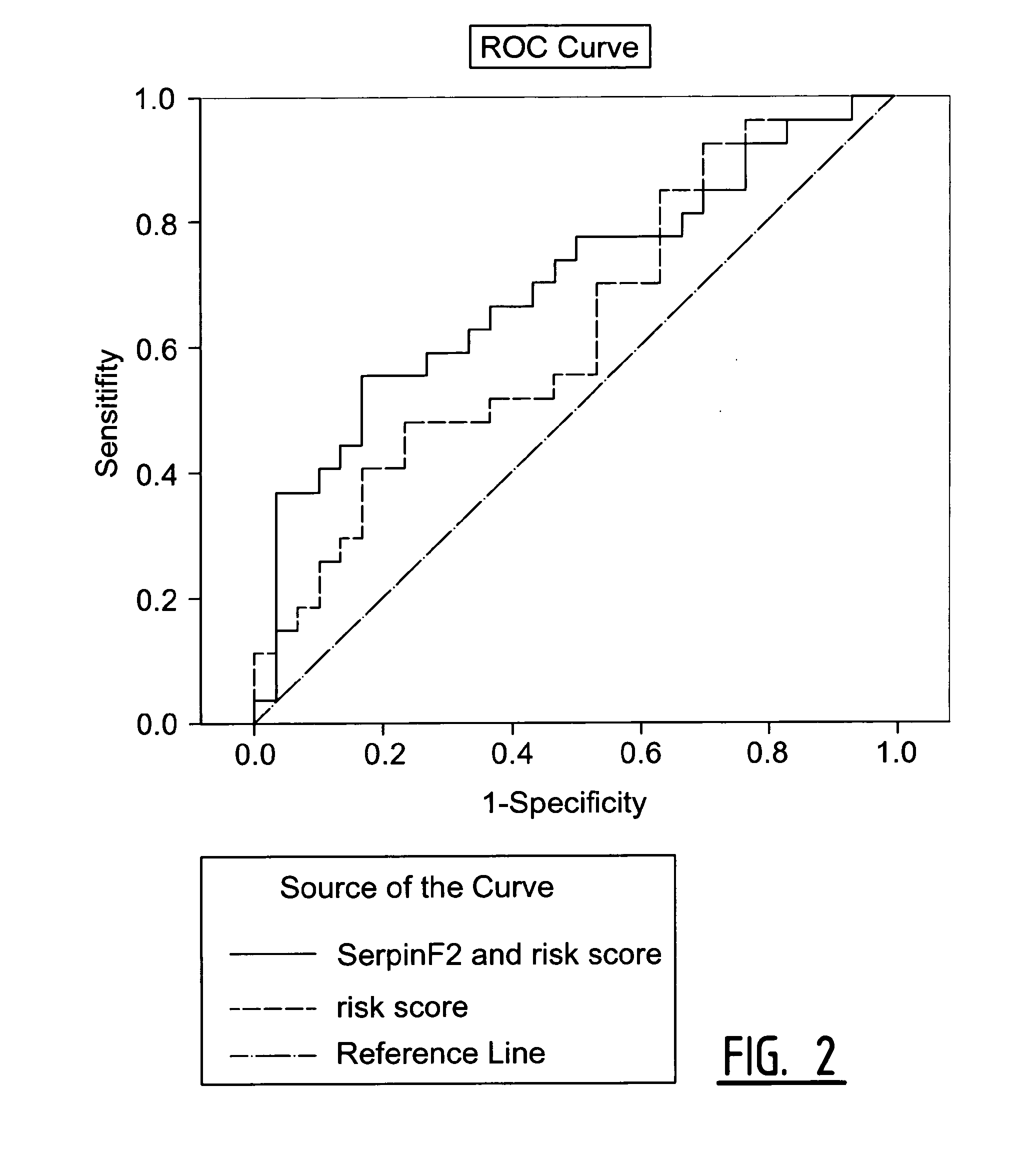
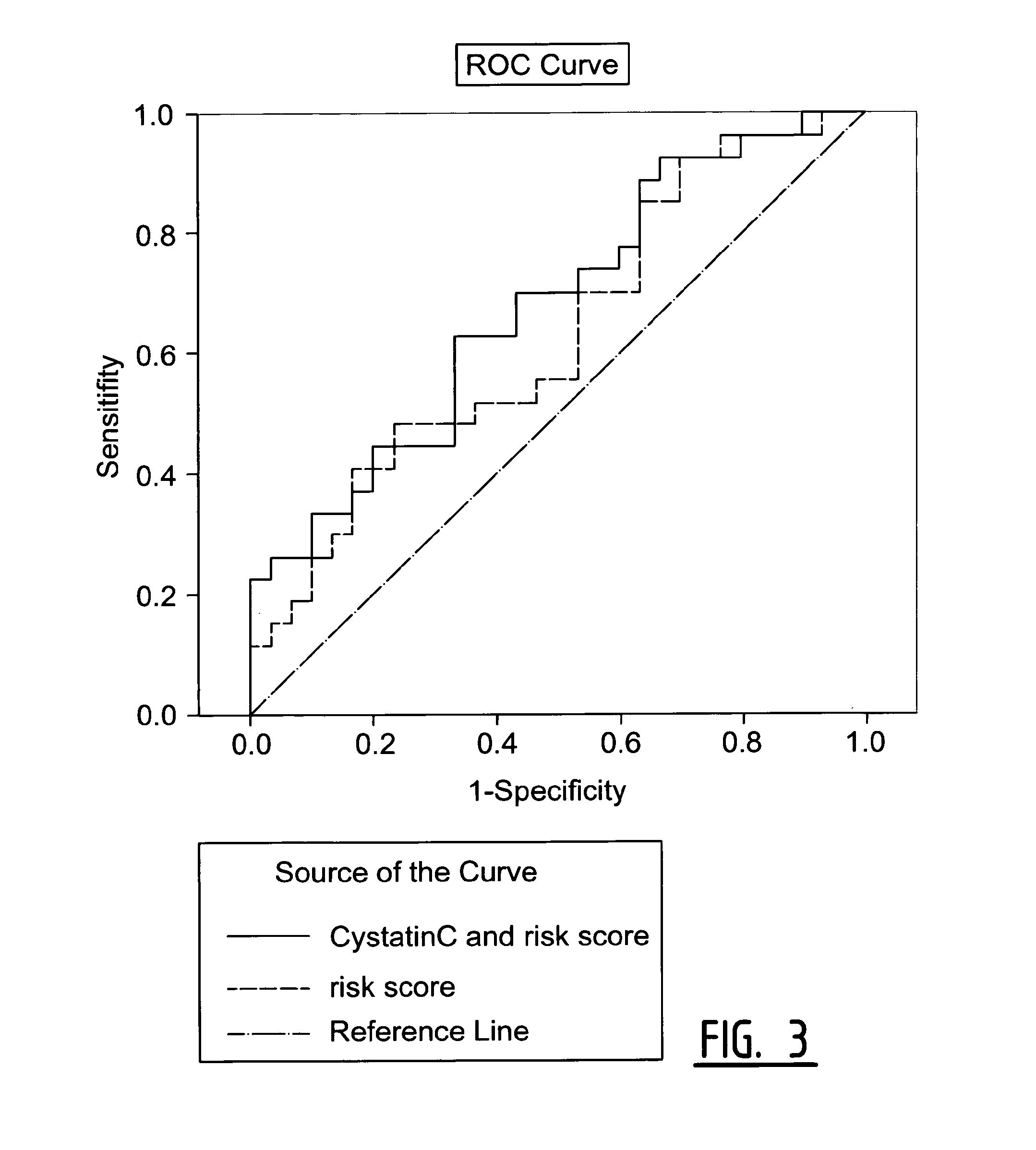
The present invention relates to a method of predicting the risk of a subject developing a cardiovascular event, comprising determining the presence of a biomarker that is indicative of the risk of developing a cardiovascular event in exosomes from the subject. The exosomes are suitably isolated from a body fluid selected from serum, plasma, blood, urine, amniotic fluid, malignant ascites, bronchoalveolar lavage fluid, synovial fluid, breast milk, saliva, in particular serum. The biomarker is selected from the proteins Vitronectin, Serpin F2, CD14, Cystatin C, Plasminogen, Nidogen 2 or any combination of two or more of these proteins.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Methods and reagents for quantitation of cell-surface molecule expression on peripheral blood cells
InactiveUS6951727B2Stable expressionRapid and reliable quantitative measurement of HLA-DREnzymologyImmunoglobulins against cell receptors/antigens/surface-determinantsImmunologic CompetenceLysosome
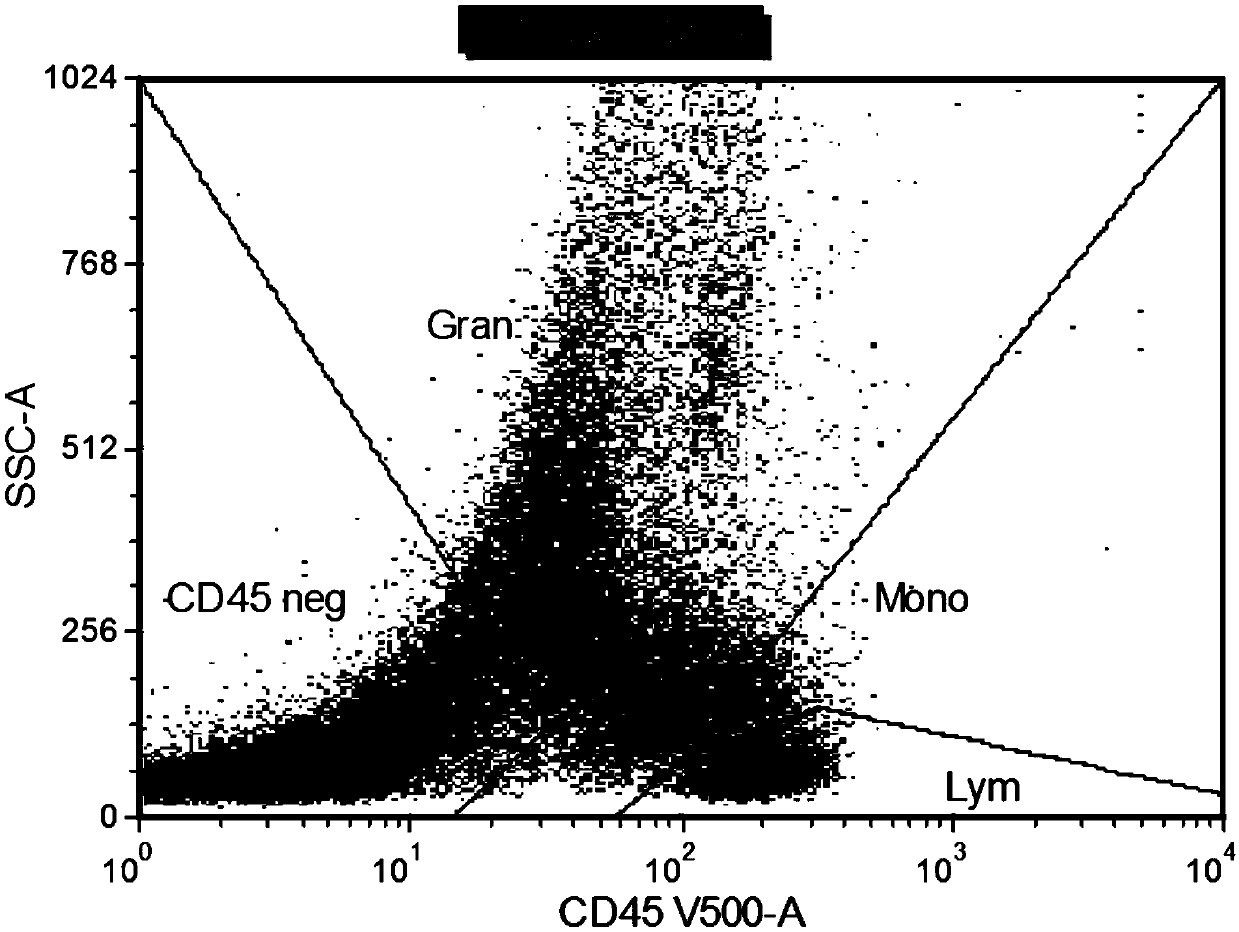
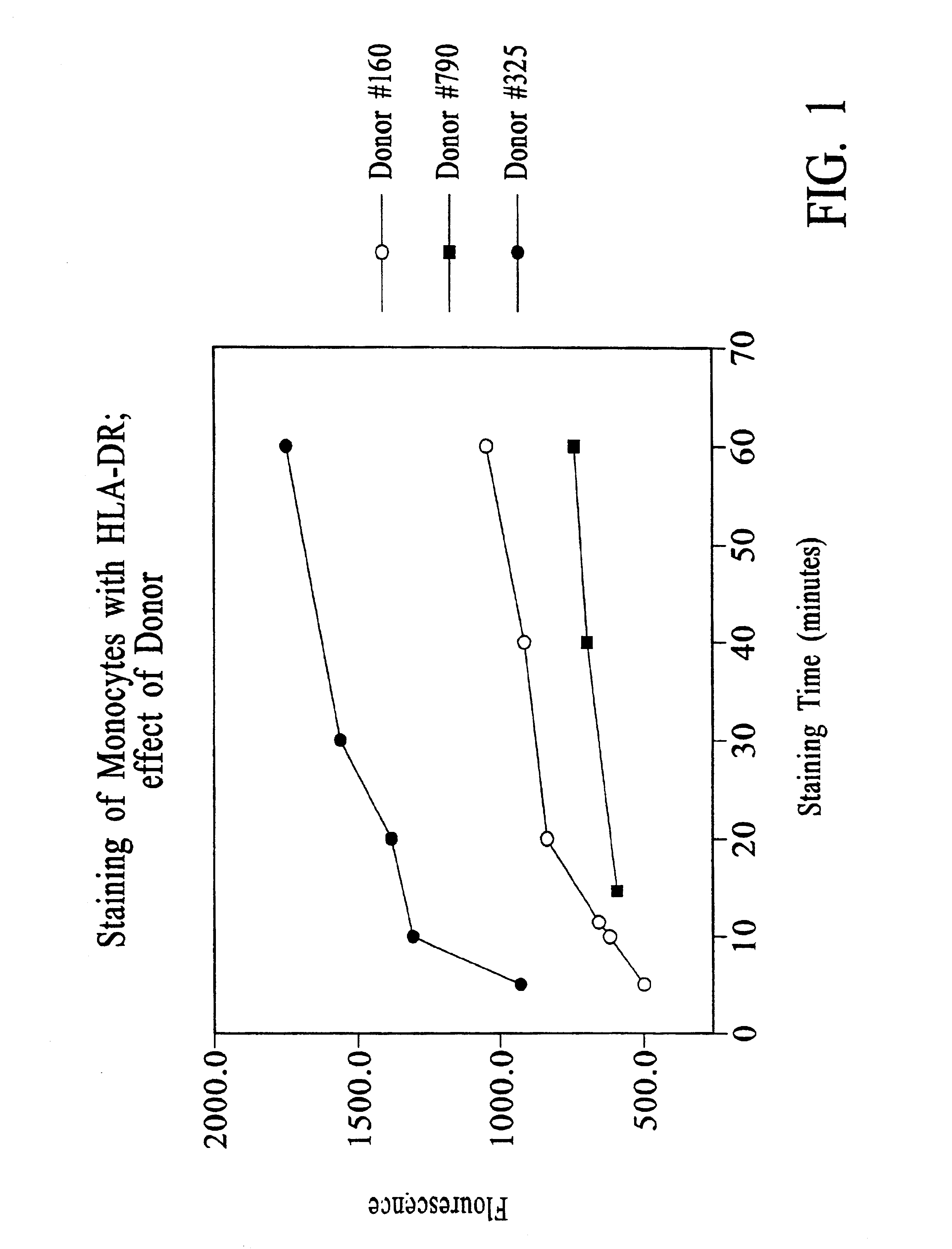
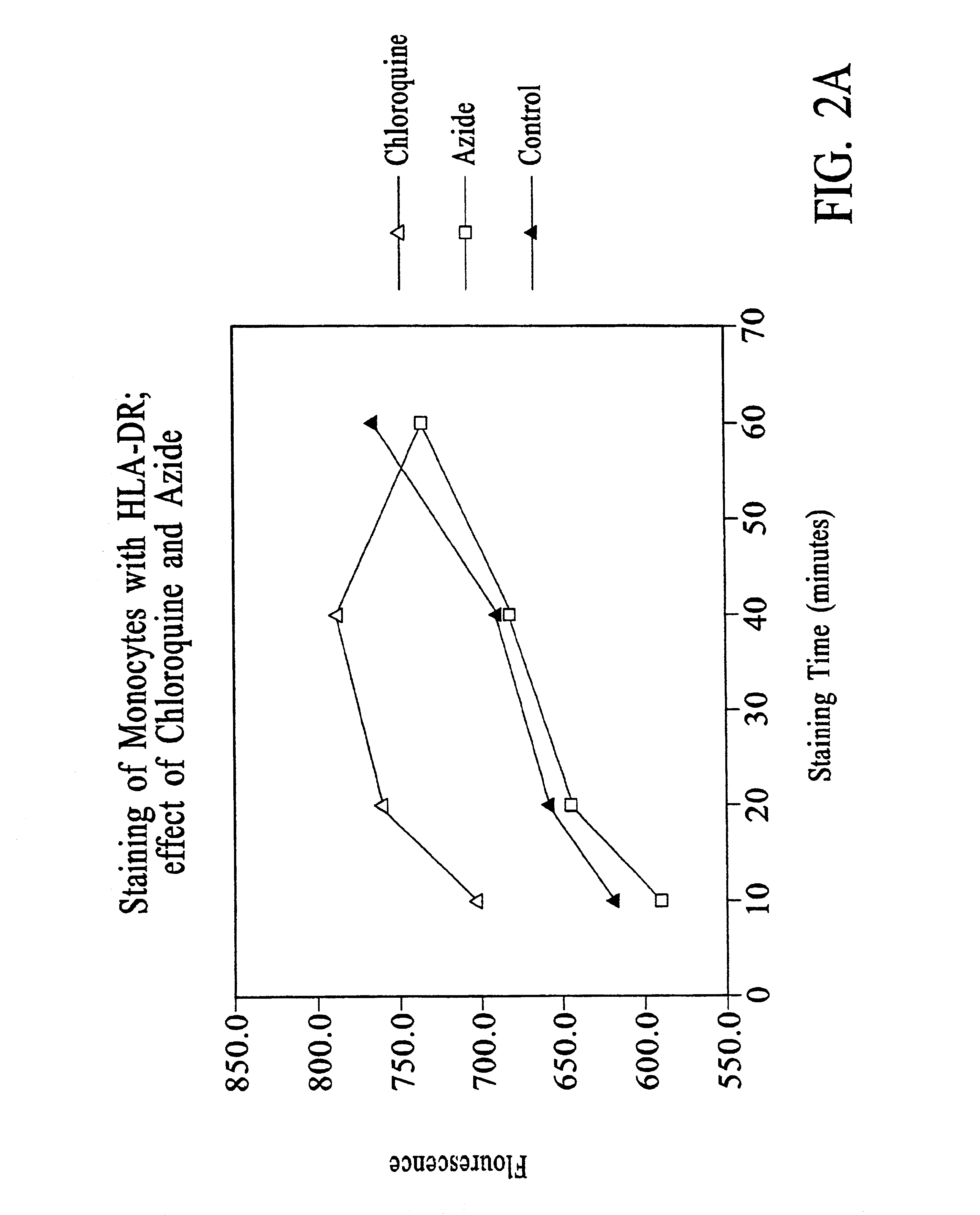
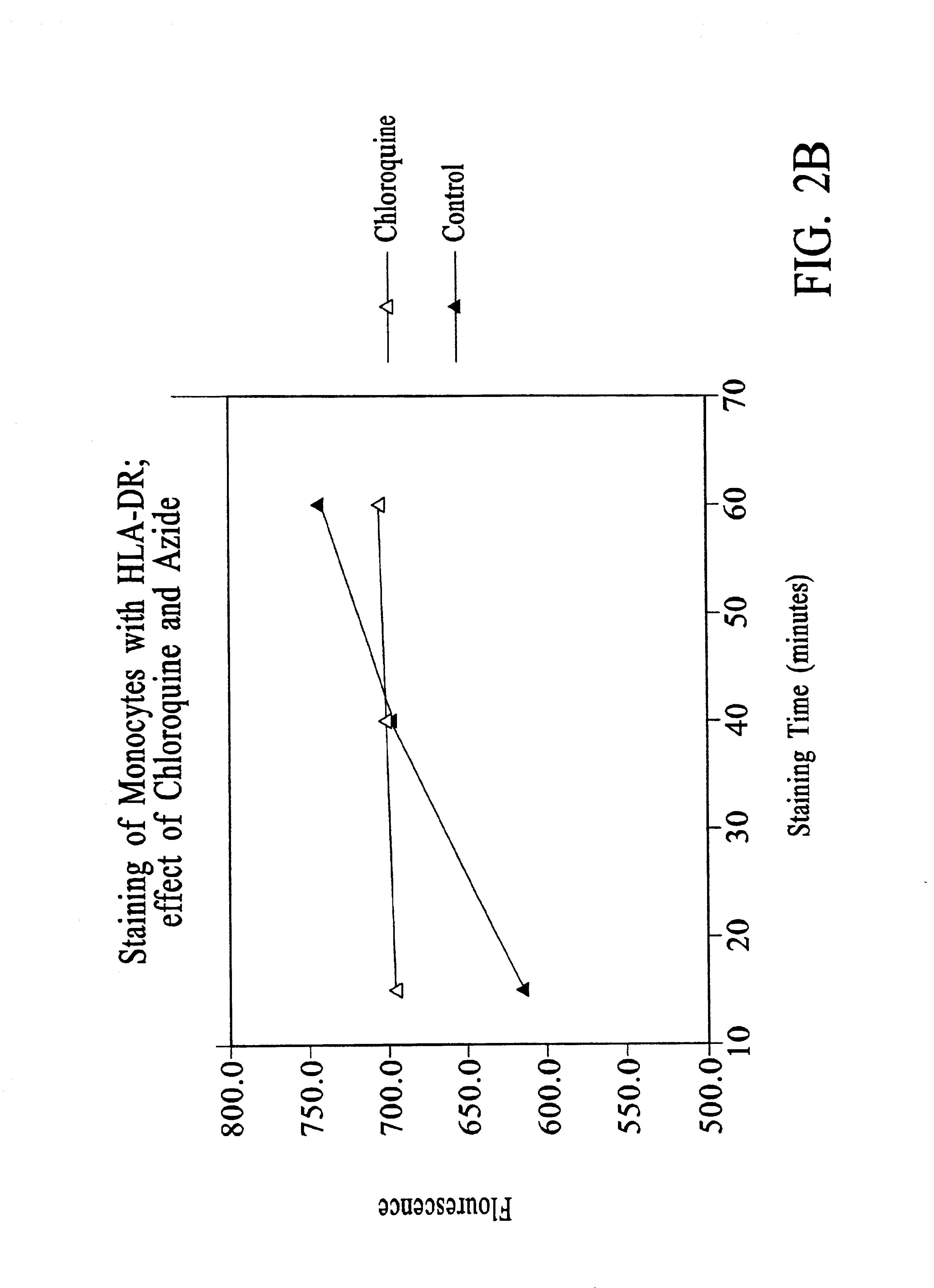
Improved methods, reagents, and kits for quantitation of HLA-DR and / or CD11b expression on peripheral blood cells are presented. Inclusion of a lysosomotropic amine, such as chloroquine, during staining stabilizes HLA-DR and CD11b expression. Use of a novel anti-CD14 conjugate, anti-CD14-PerCP / CY5.5, permits the ready discrimination of monocytes. The improved methods, reagents, and kits may be used to assess immune competence, and to direct and monitor immunostimulatory therapies in septic patients exhibiting monocyte deactivation.
Owner:BECTON DICKINSON & CO
Compositions and methods for modulating cells via CD14 and toII-like receptor 4 signaling pathway
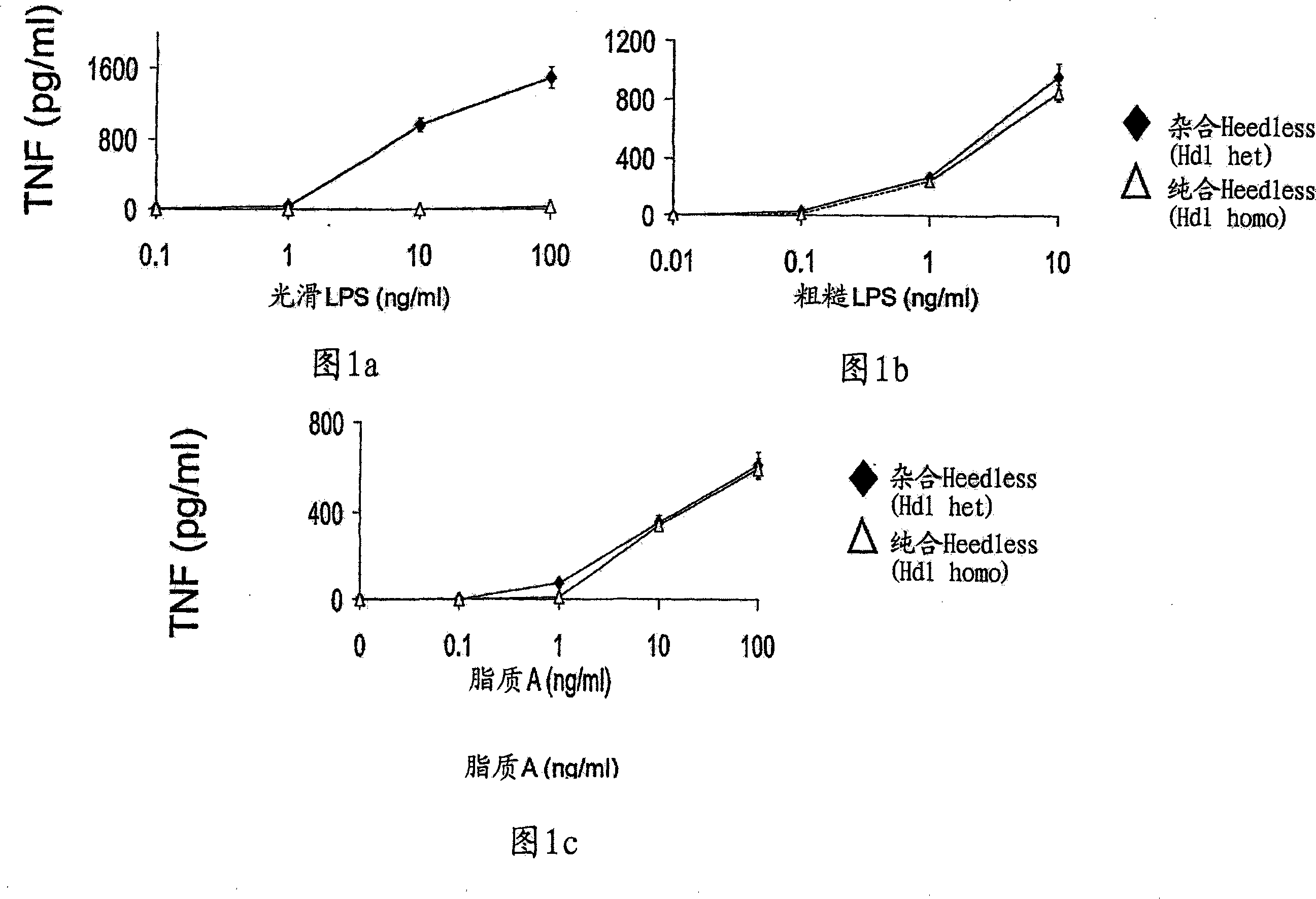
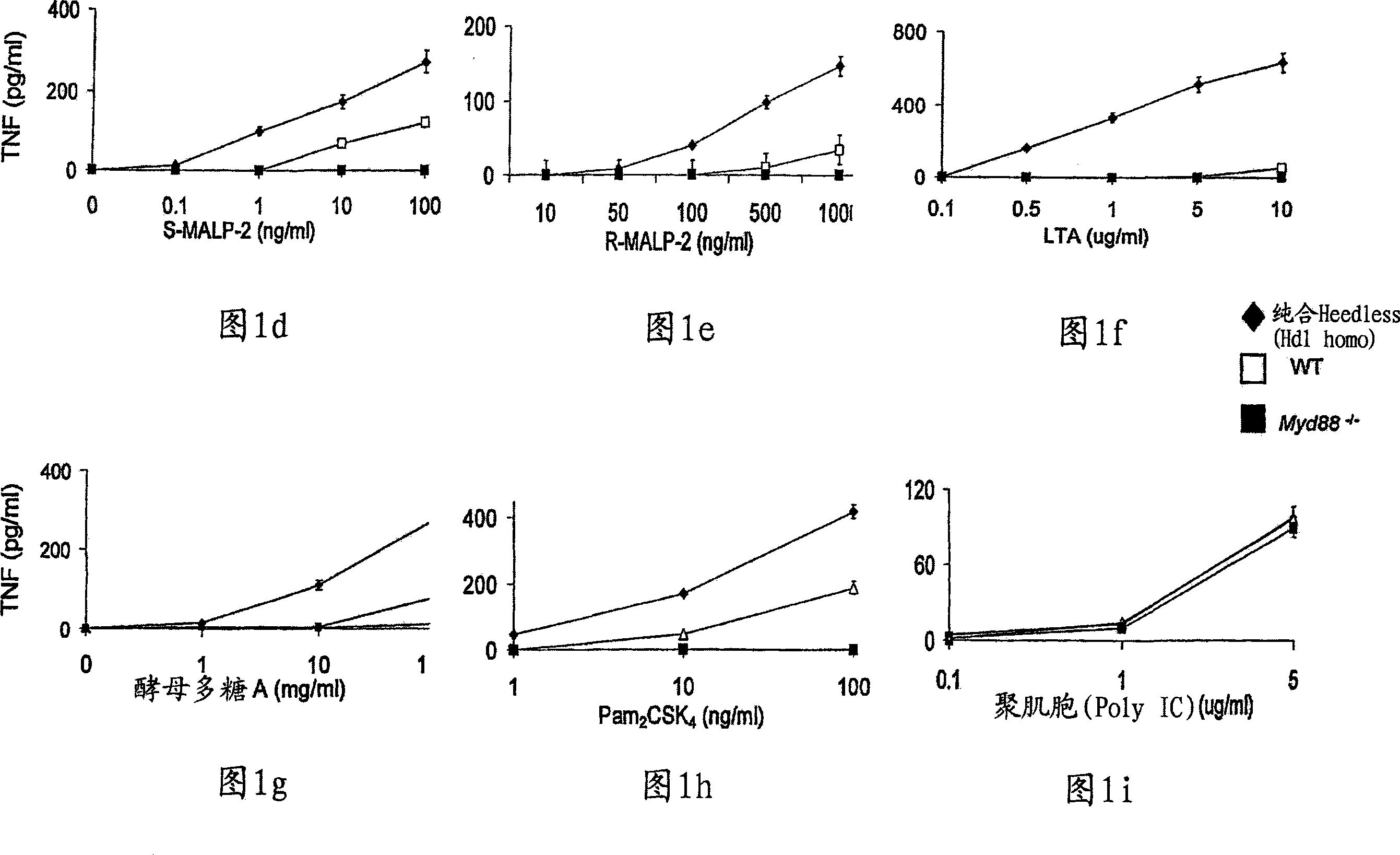
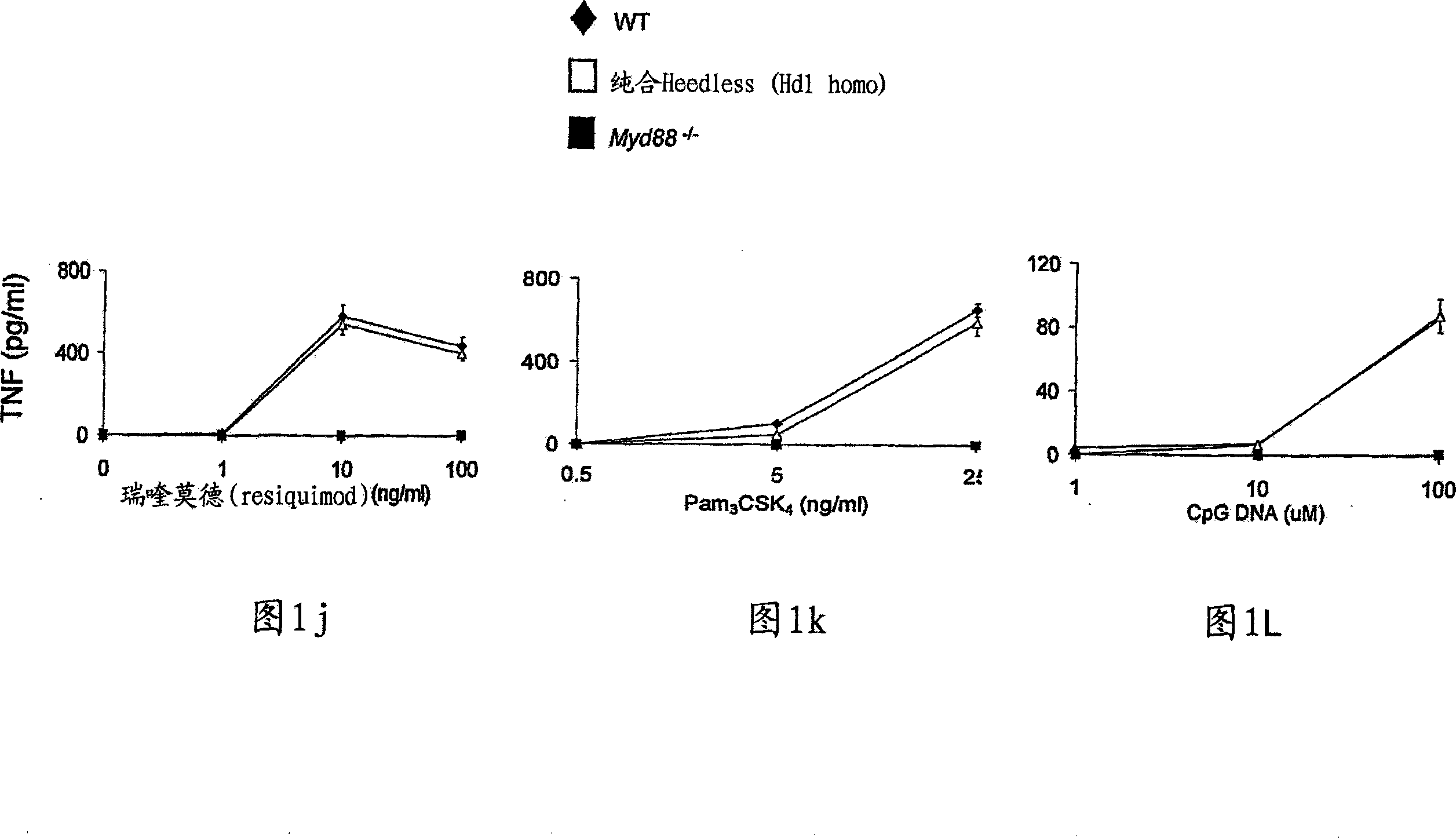
Compositions and methods for screening and identifying compounds that modulate Toll-like receptor 4 (TLR4) pathway signaling via CD14 and ligands are provided. Provided are methods of treating various disease states, such as inflammatory or autoimmune diseases, in mammalian subjects by modulating Toll-like receptor 4 (TLR4) pathway signaling through CD14 and ligands. Transgenic non-human animals and methods of developing transgenic non-human animals are provided, wherein the transgenic non-human animals contain a loss-of-function mutation in the CD14 gene.
Owner:THE SCRIPPS RES INST
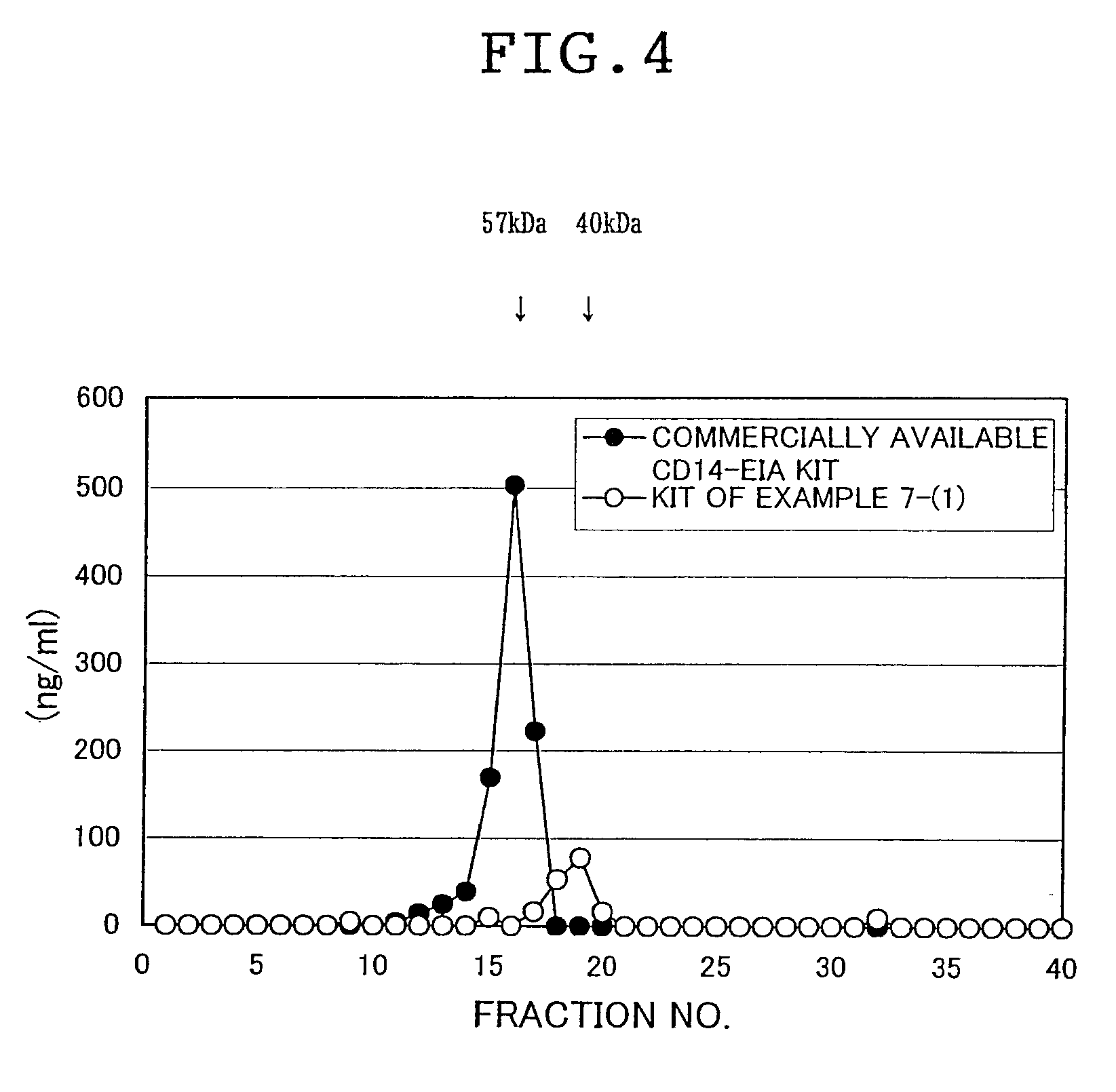
Method for the measurement of soluble CD14 proteins separately
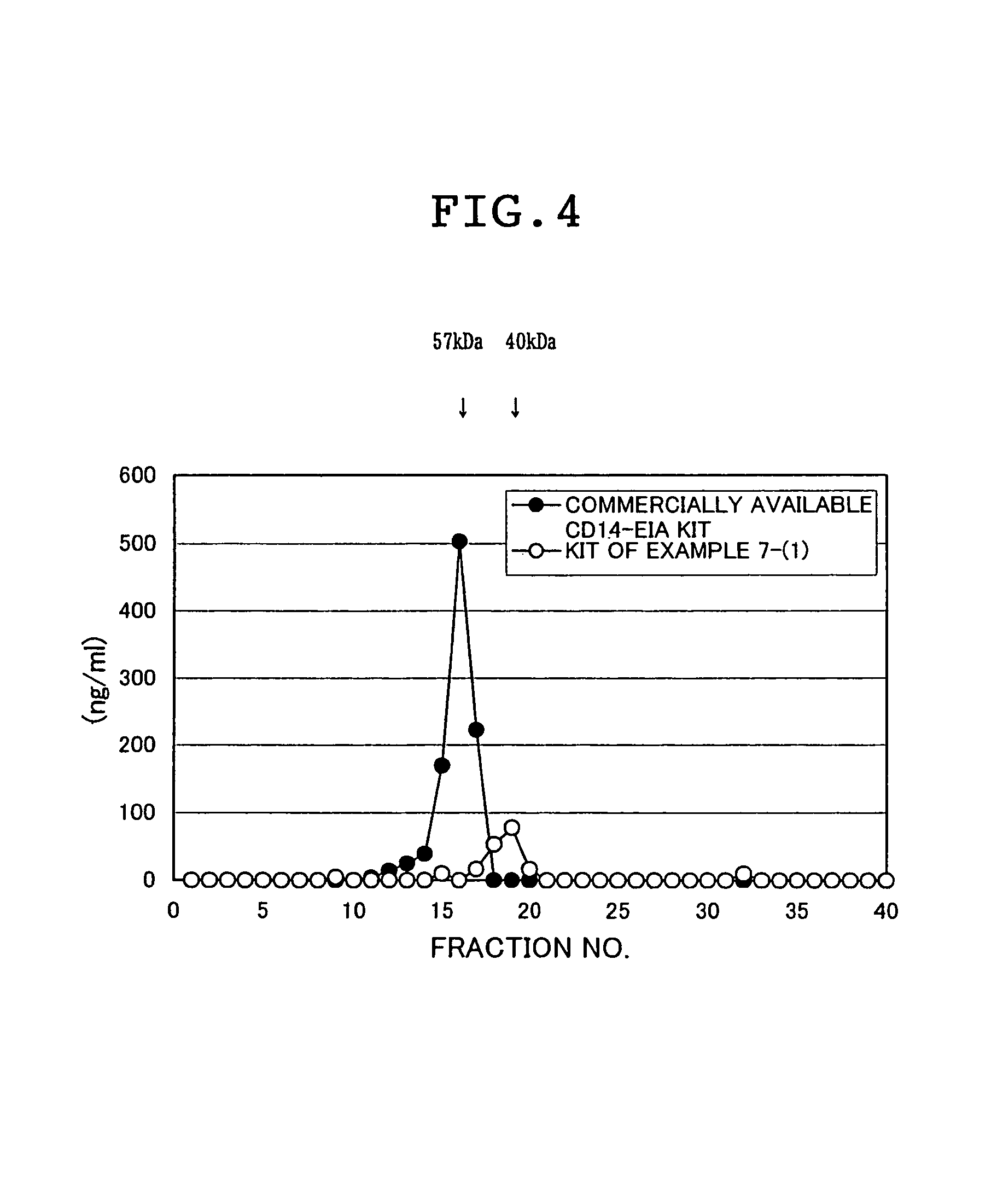
InactiveUS6916628B1High sensitivityAnimal cellsCell receptors/surface-antigens/surface-determinantsBiochemistryCD14
Owner:MOCHIDA PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com