Application of membrane protein CD81 in early forecasting, parting, diagnosis and treatment of eclampsia
A pre-eclampsia and membrane protein technology, applied in medical preparations containing active ingredients, disease diagnosis, gene therapy, etc., can solve problems such as CD81 that has not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
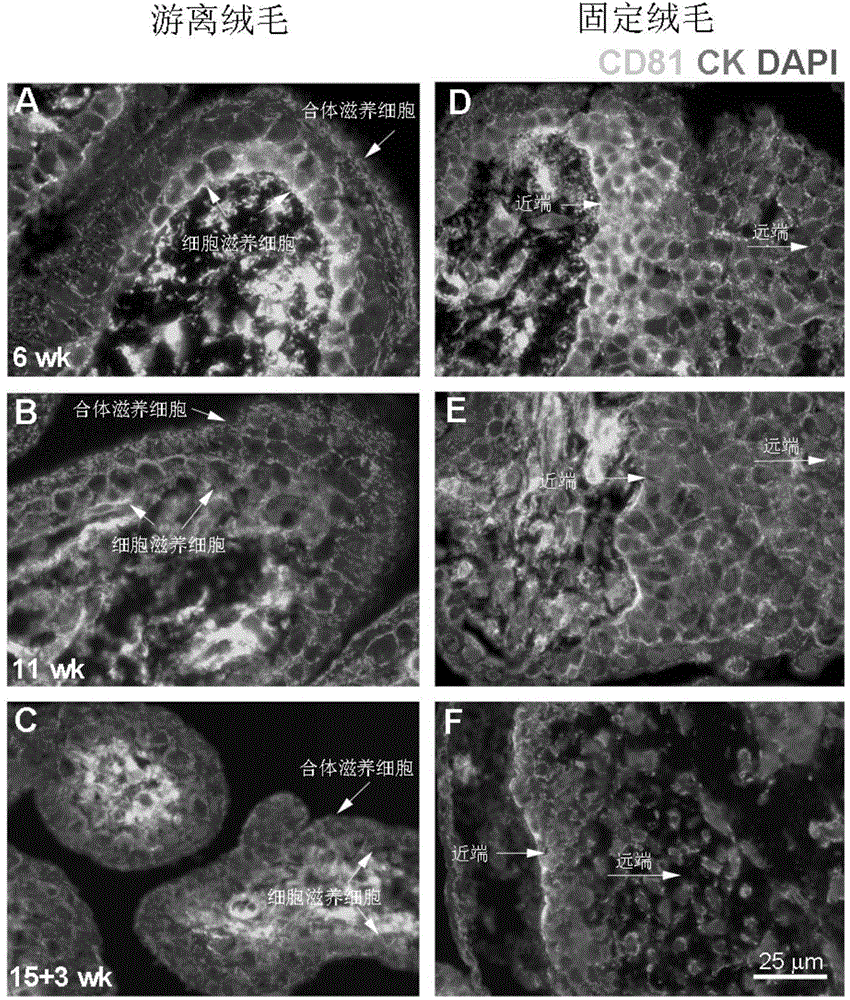
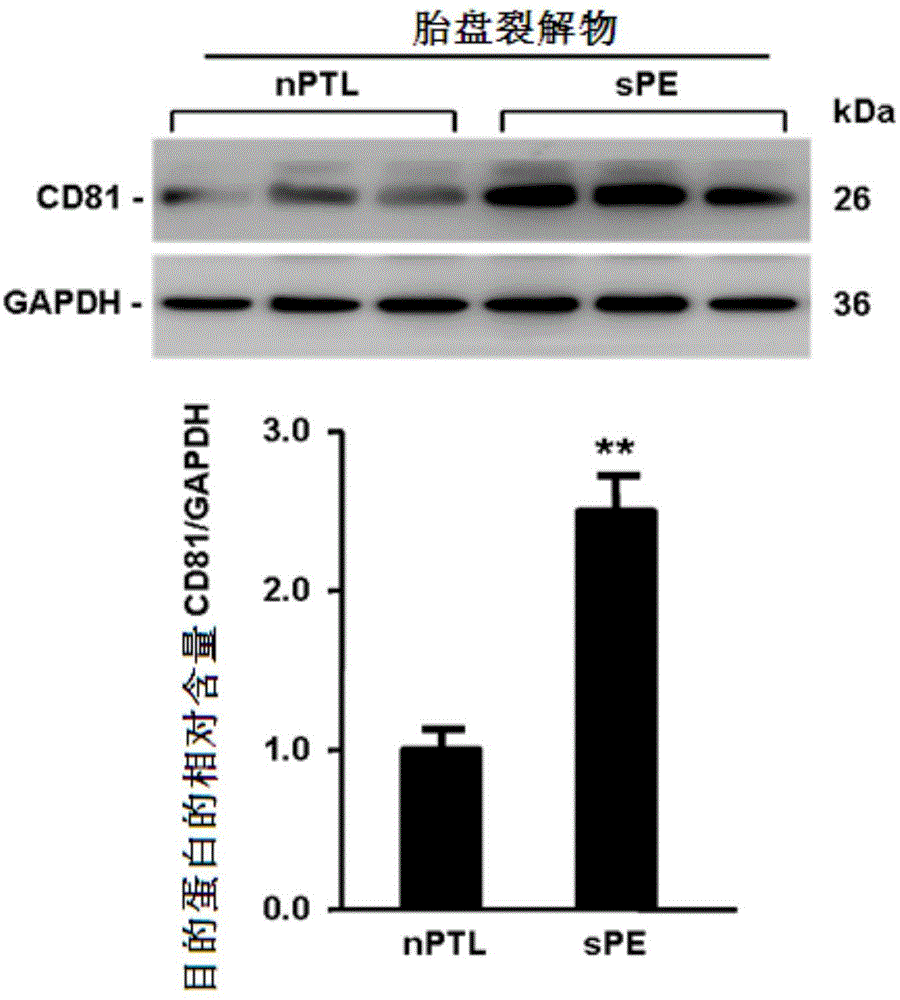
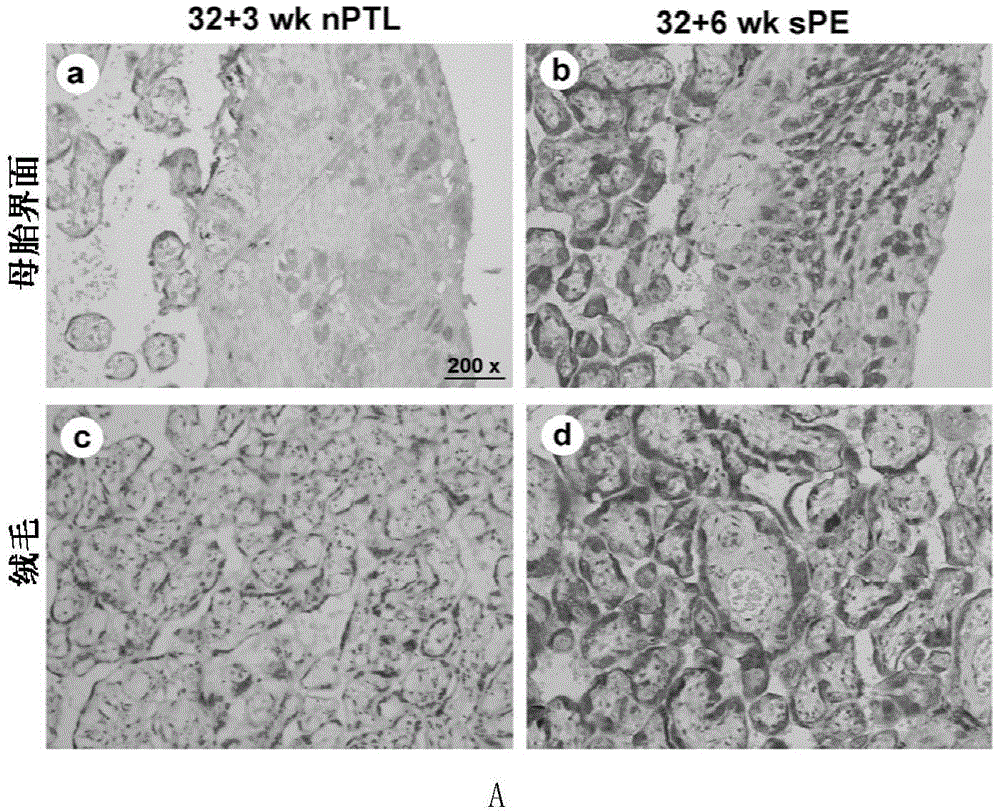
[0036] Example 1: CD81 is expressed in the placenta and serum of patients with early-onset severe PE
[0037] 1. Materials, reagents, equipment
[0038] 1.1 Human placenta tissue and serum source
[0039] Singleton primiparas who had elective cesarean section delivery in the Drum Tower Hospital Affiliated to Nanjing University School of Medicine between October 2011 and October 2014 were selected and signed informed consent. Chronic hypertension, pre-pregnancy diabetes, kidney disease, premature rupture of membranes, Infectious diseases and other pregnancy complications and complications. The diagnostic criteria for severe PE were according to the 23rd edition of Williams Obstetrics. Venous blood was drawn immediately after the pregnant woman was admitted to the hospital, and the serum was kept in a -80°C refrigerator after centrifugation. Within 10 minutes after the delivery of the placenta, several pieces of tissue with a size of about 1.0cm×1.0cm×1.0cm in the middle of t...
Embodiment 2
[0057] Example 2: The effect of CD81 on the invasion function of placental primary cytotrophoblasts (CTBs)
[0058] 1. Materials, reagents, equipment
[0059] 1.1 Collection of human villi in the first trimester
[0060] The fresh villi of pregnant women who requested artificial abortion due to early pregnancy and underwent abortion in the Family Planning Department of Nanjing Drum Tower Hospital were collected. Pregnant women without any disease, gestational weeks in 6-8w.
[0061] 1.2 Main reagents and equipment
[0062] Collagenase, hyaluronidase, DNA inhibitory enzyme, sodium pyruvate, glutamine, Hepes balance solution, BSA (Sigma); Nutridoma-SP (Roche); Percoll (GE Healthcare); Matrigel (BD); DME H medium, 0.05% trypsin, fetal bovine serum (FBS), penicillin and streptomycin, gentamicin (Invitrogen); Ad-CTL adenovirus and Ad-Flag-CD81 adenovirus (GENECHEM, Shanghai).
[0063] Ultra-clean table (Sujing Group Antai Company), high-speed centrifuge (Germany Heraeus Company...
Embodiment 3
[0074] Example 3: CD81 adenovirus-induced PE model in rats
[0075] 1.1 Materials and grouping
[0076] SPF grade SD rats were randomly divided into two groups: control group (Ad-CTL group) and Ad-Flag-CD81 group (n=8). According to 1:1 female and male cages, observe the vaginal plug, see the vaginal plug and record it as the 0th day of pregnancy. The amount of virus injected into each mouse was 3×10 9 PFU. The administration method is tail vein injection, and the injection speed is controlled by an intravenous injection pump. The administration time was the 5th day of pregnancy, and the mice were killed on the 15th day of pregnancy. At the same time, non-pregnant rats were randomly divided into two groups: control group (Ad-CTL group) and Ad-Flag-CD81 group (n=5), and were sacrificed on the 10th day after injection.
[0077] 1.2 Blood pressure monitoring
[0078] The systolic blood pressure of the tail artery was measured every day from one week before the experiment to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com