Patents
Literature
51 results about "Late onset" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Late onset diabetes is a medical condition characterized by difficulty processing dietary sugar as a result of the development of resistance in the body to insulin, the hormone involved in glucose metabolism. It is one of a family of conditions known colloquially as “diabetes” and is the most common form of diabetes,...
Compositions for Preventing and Reducing Delayed Onset Muscle Soreness
InactiveUS20080317886A1Inhibit inflammationSpeed up recoveryBiocideSugar food ingredientsSucroseL glutamate
The present invention relates to the compositions that enhance post-exercise recovery processes to increase both strength and muscle mass, replace glycogen stores, and prevent inflammation, resulting in the prevention and / or reduction of delayed onset muscle soreness. Additionally, it provides a feeling of muscle relaxation as well as a feeling of mental tranquility immediately following exercise. The composition consists of any or all high-glycemic sugars and / or polysaccharides (e.g., sucrose, glucose, maltodextrin), all essential amino acids and beta-hydroxy-beta-methylbutyrate and can include other amino acids sources (e.g. whey protein), performance enhancing agents (e.g., caffeine, L-glutamate), anti-inflammatory agents (e.g., ginger, boswellia, curcumen), antioxidants (vitamin C, vitamin E, selenium, polyphenols,), insulin-mimicking agents (cinnamon, Banaba), analgesics (e.g. aspirin, ibuprofen, naproxen, acetaminophen), and to methods of treating humans and animals by administration of these novel compositions to humans and animals in need thereof.
Owner:SOUTHWEST IMMUNOLOGY
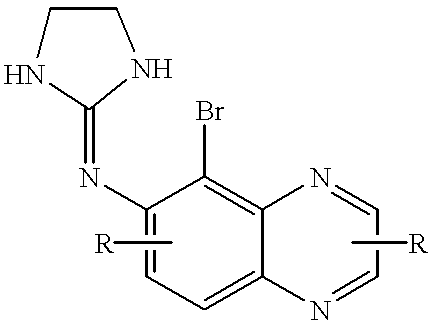
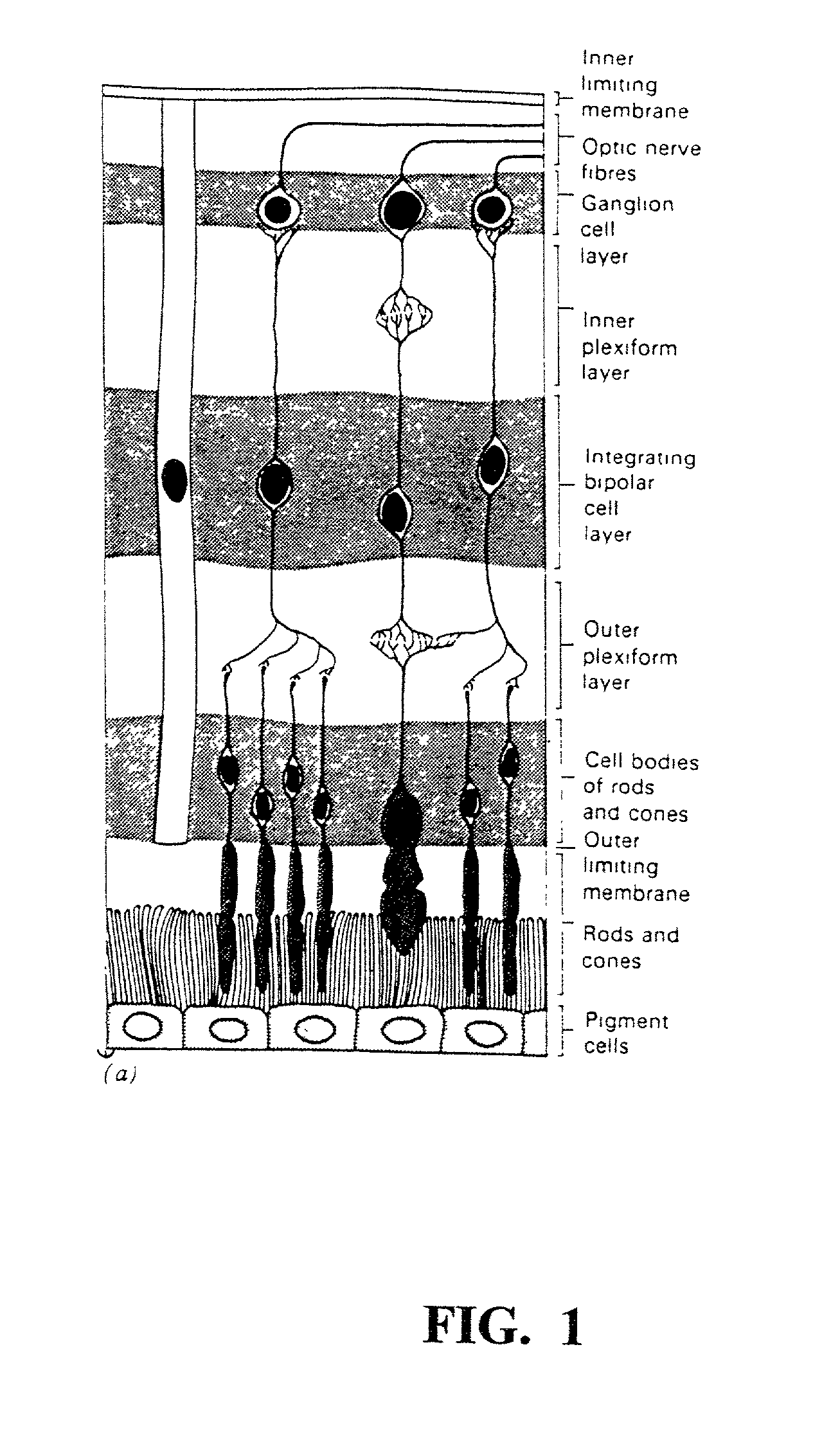
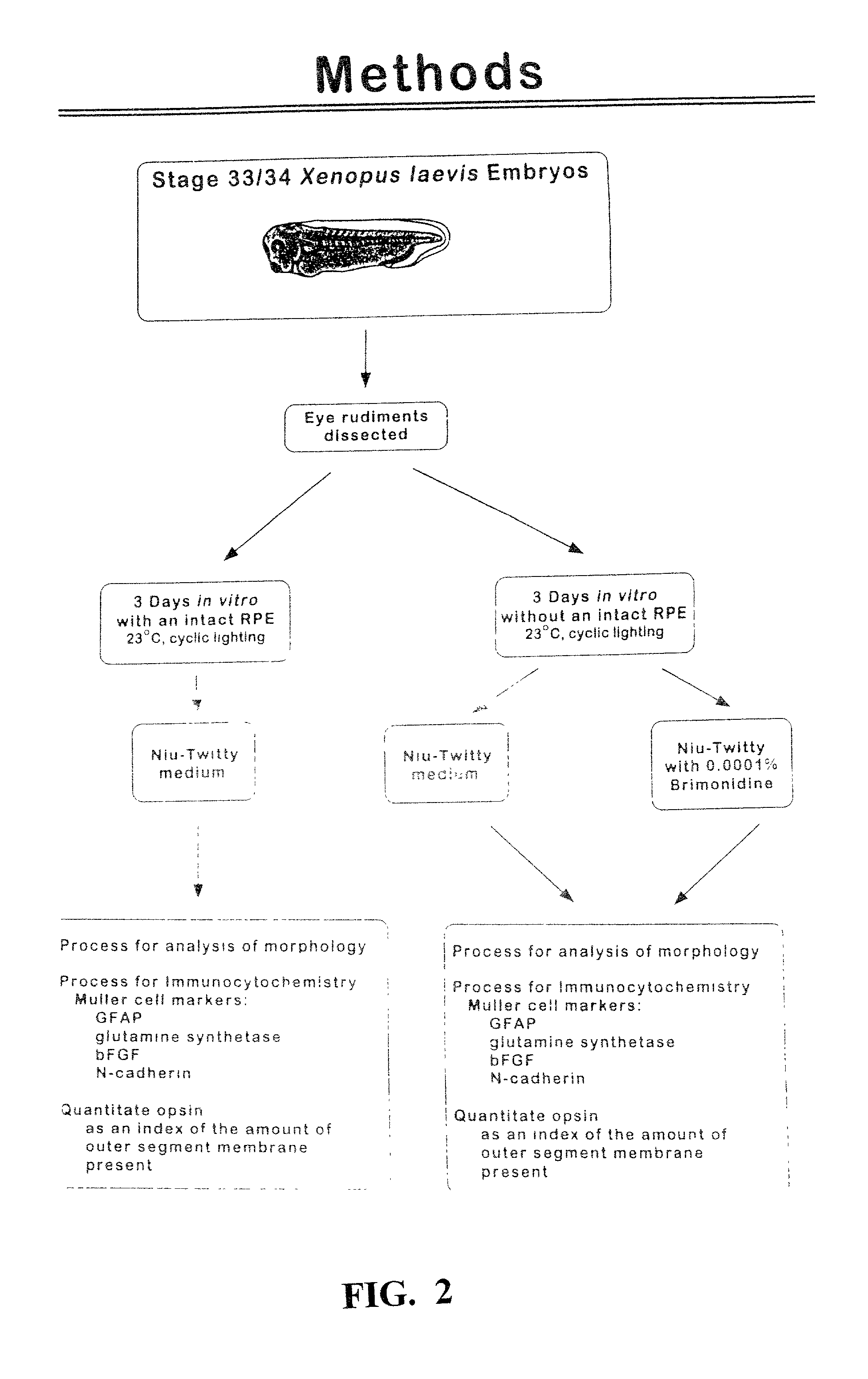
Brimonidine compositions and methods for retinal degeneration
InactiveUS20010049369A1Patient compliance is goodReduce incidenceBiocideAnimal repellantsAdjuvantStress marker
The present study demonstrates that brimonidine tartrate, an alpha-2 adrenergic receptor agonist, can prevent photoreceptor cell degeneration and the associated Muller cell degenerative signs in an in vitro model of retinal degeneration and retinal detachment (separation of the neuroretina from the retinal pigment epithelium). Similar to control conditions, brimonidine allowed for the formation of highly structured photoreceptor outer segments, prevented the expression of stress markers in Müller cells and preserved the expression patterns of Muller cell markers of proper cell-cell contact and differentiation. Ultrastructural studies also indicated that brimonidine favored the formation of cell-cell junctions between photoreceptor cells and Müller cells, indicating that this phenomenon is associated with the exertion of the neuroprotective effect. The results suggest that brimonidine compounds may be utilized as an effective therapeutic agent for early and late onset retinal degenerations caused by defects in photoreceptor cells, Müller cells or both, and as an adjuvant to therapeutic success in retinal detachment surgery or macular translocation surgery for age-related macular degeneration.
Owner:UNIV OF TENNESSEE RES FOUND
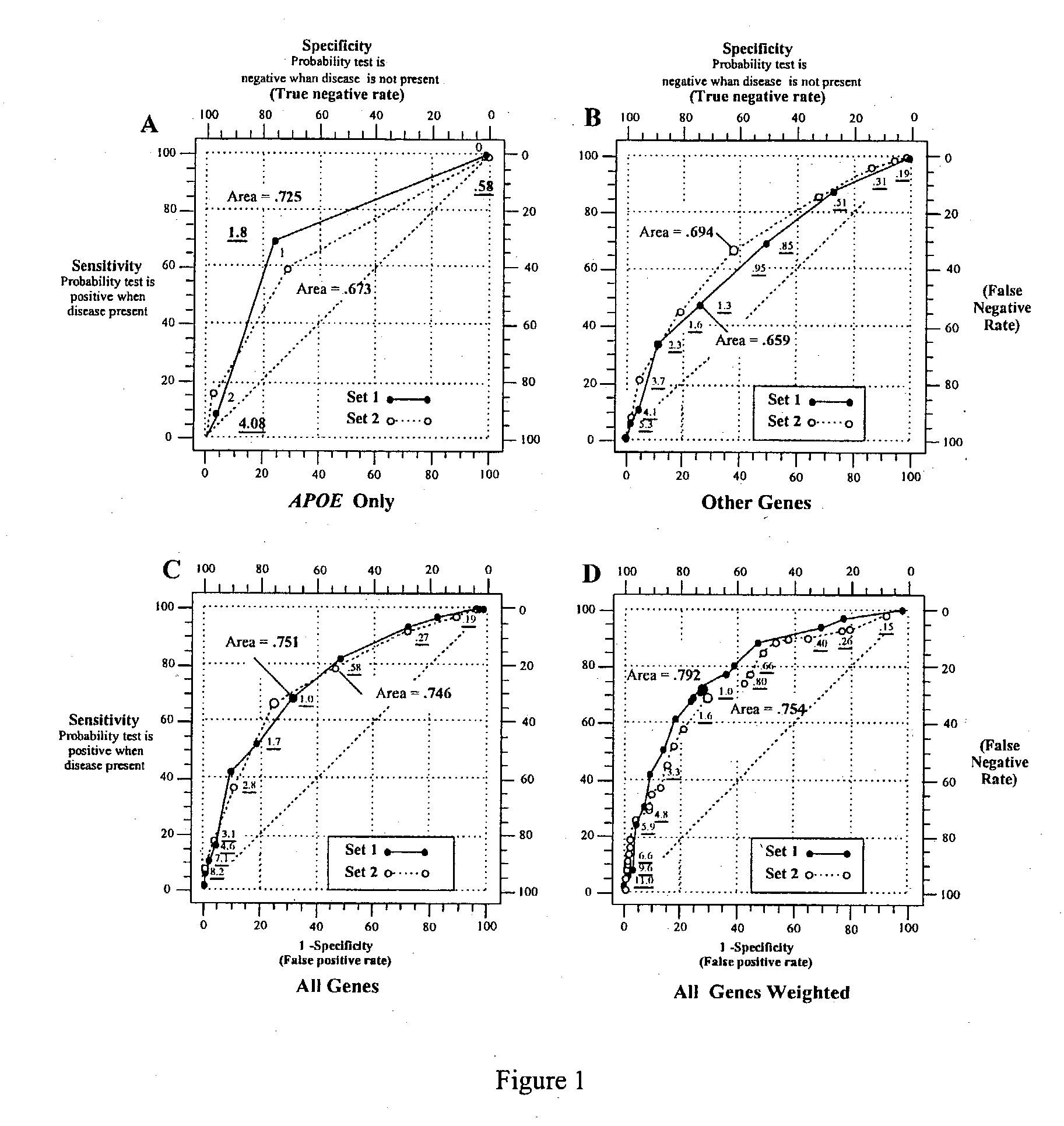
Multi-gene tests with ROC plots for the assessment of risk for polygenic disorders
InactiveUS20030162207A1Microbiological testing/measurementSpecial data processing applicationsDiagnostic testPolygenic disease
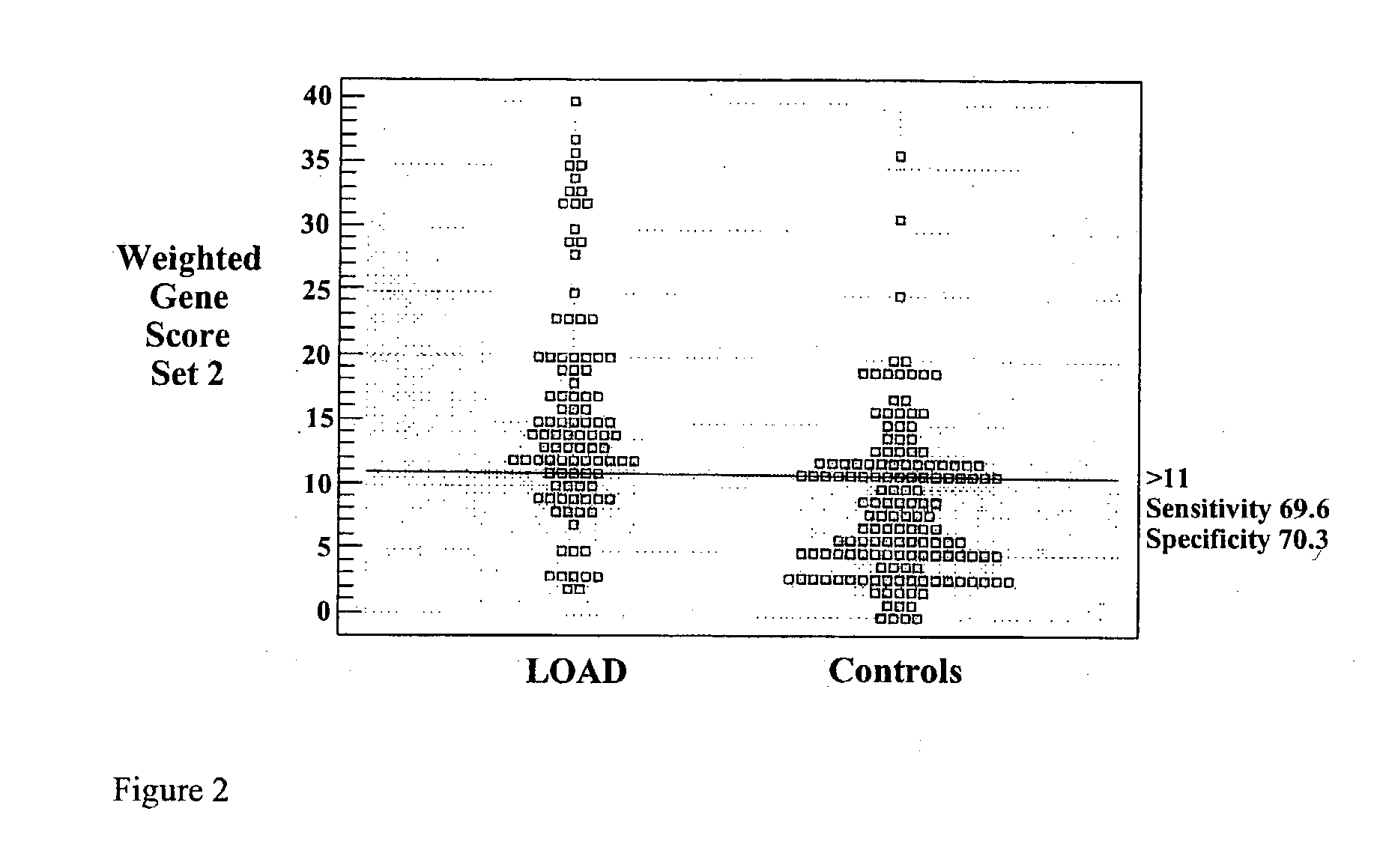
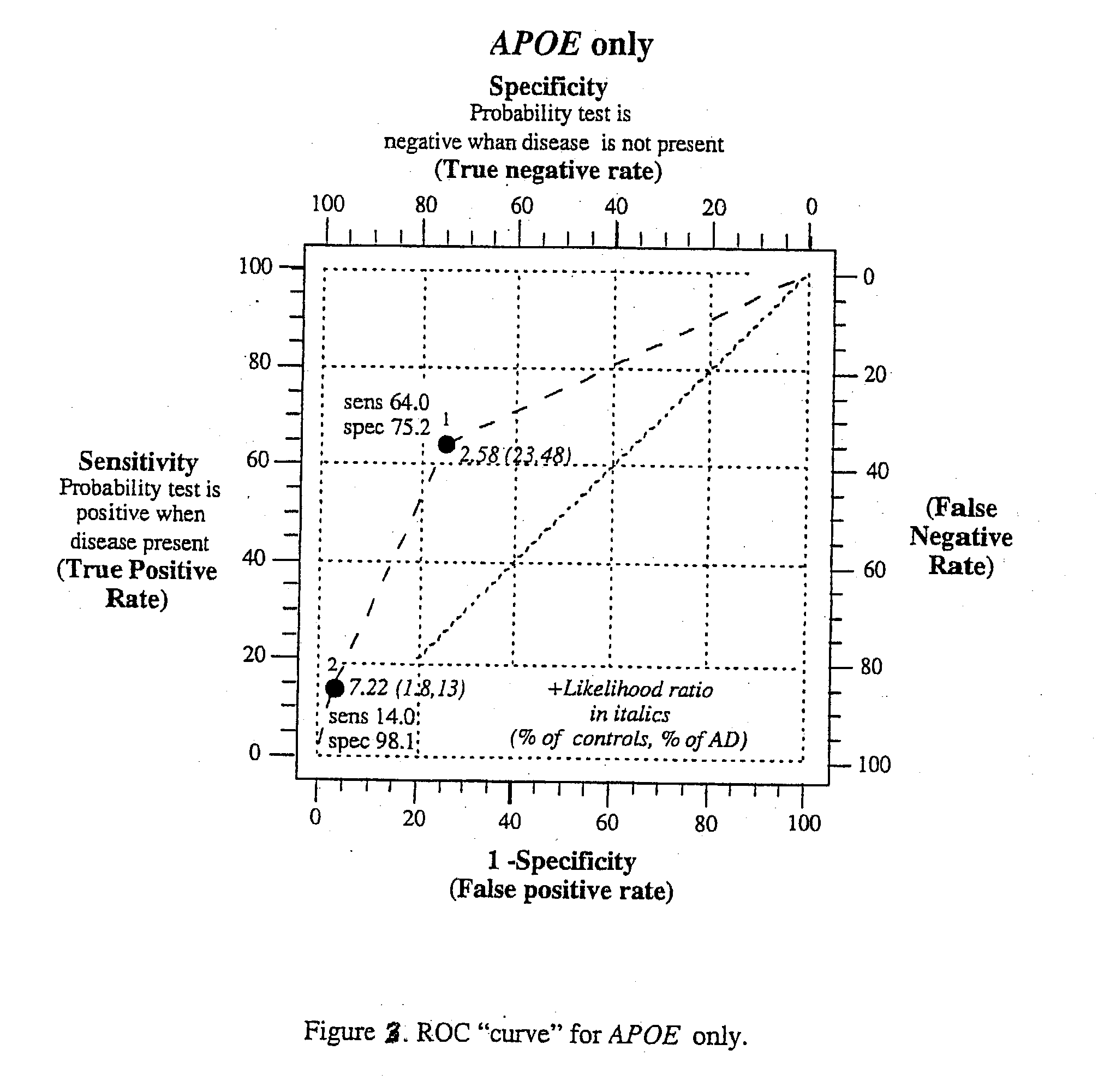
Polygenic disorders are due to the additive effect of multiple genes interacting with the environment. Because of the small effect size of each gene and considerable genetic heterogeneity, when single genes are examined, the outcome of association and linkage analyses are variable from study to study. Techniques are needed that take these unique characteristics of polygenic disorders into consideration. The present invention discloses that the formation of a polygenic score, consisting of the additive effect of multiple candidate genes, and its assessment using receiver operating characteristic (ROC) plots, provides such a technique. Six genes previously shown to be associated with Alzheimer's disease were examined, APOE, ACE, ACP1, ESR1, PNMT and SLC6A4. The total fraction of the variance, the area under the ROC plots, and the range of risks were similar for both groups indicating that despite genetic heterogeneity and the small effect size of most genes, consistent risk analyses could be obtained by examining the additive effect of these multiple genes. The present invention also discloses diagnostic tests for determining a subject's risk of developing Alzheimer's Disease or specifically Late Onset Alzheimer's Disease.
Owner:CITY OF HOPE
Treatment of hyperkinetic movement disorders
Methods for treating hyperkinetic diseases and disorders, such as tardive dyskinesia, are provided. In a certain embodiment, the potent VMAT2 inhibitor (+)α-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol ((+)α-HTBZ) is used in the methods described herein for treating a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
Methods to treat alzheimer's disease using apoe inhibitors
InactiveUS20150337030A1Avoid defectsTreating and preventing and delaying onsetNervous disorderPolymorphism usesAntibody inhibitorLate onset
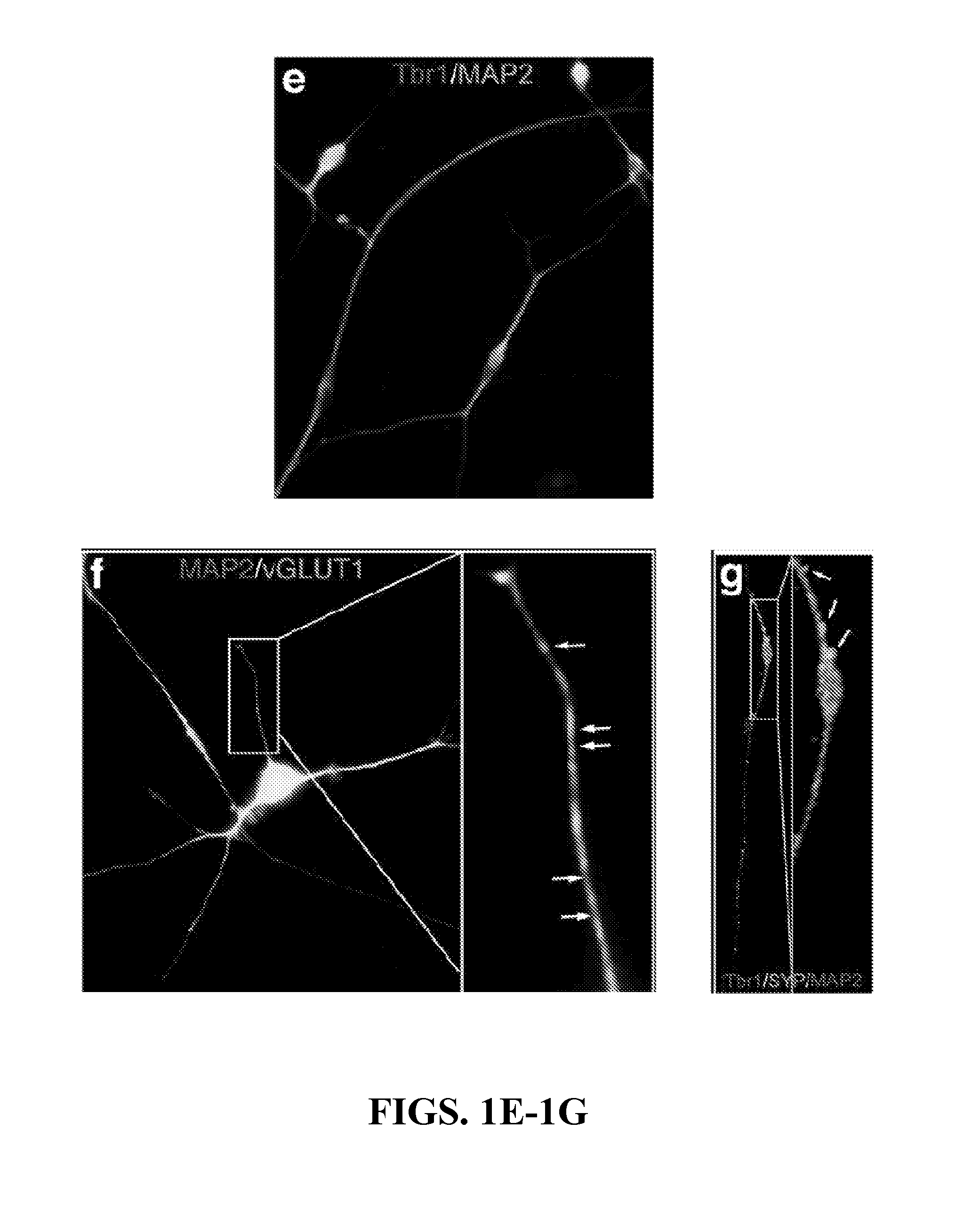
Non-familial late-onset Alzheimer's disease (LOAD), a condition associated with the accumulation of the amyloid precursor protein-derived (APP) Abeta fragment in the brain, can be the consequence of combined genetic and environmental risk factors. One of these risk factors is the presence of the apolipoprotein E4 (APOE4) allele. This invention provides for a neuron model that can be used to screen and identify compounds that can prevent the APOE4-induced pre-LOAD state. This invention provides for methods for the treatment and / or prevention of a neurodegenerative disorder by using an inhibitor of APOE4, such as an antibody inhibitor, or by using an excess of APOE3 protein.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Acute hepatic insufficiency depressant and method for evaluating drug efficacy thereof
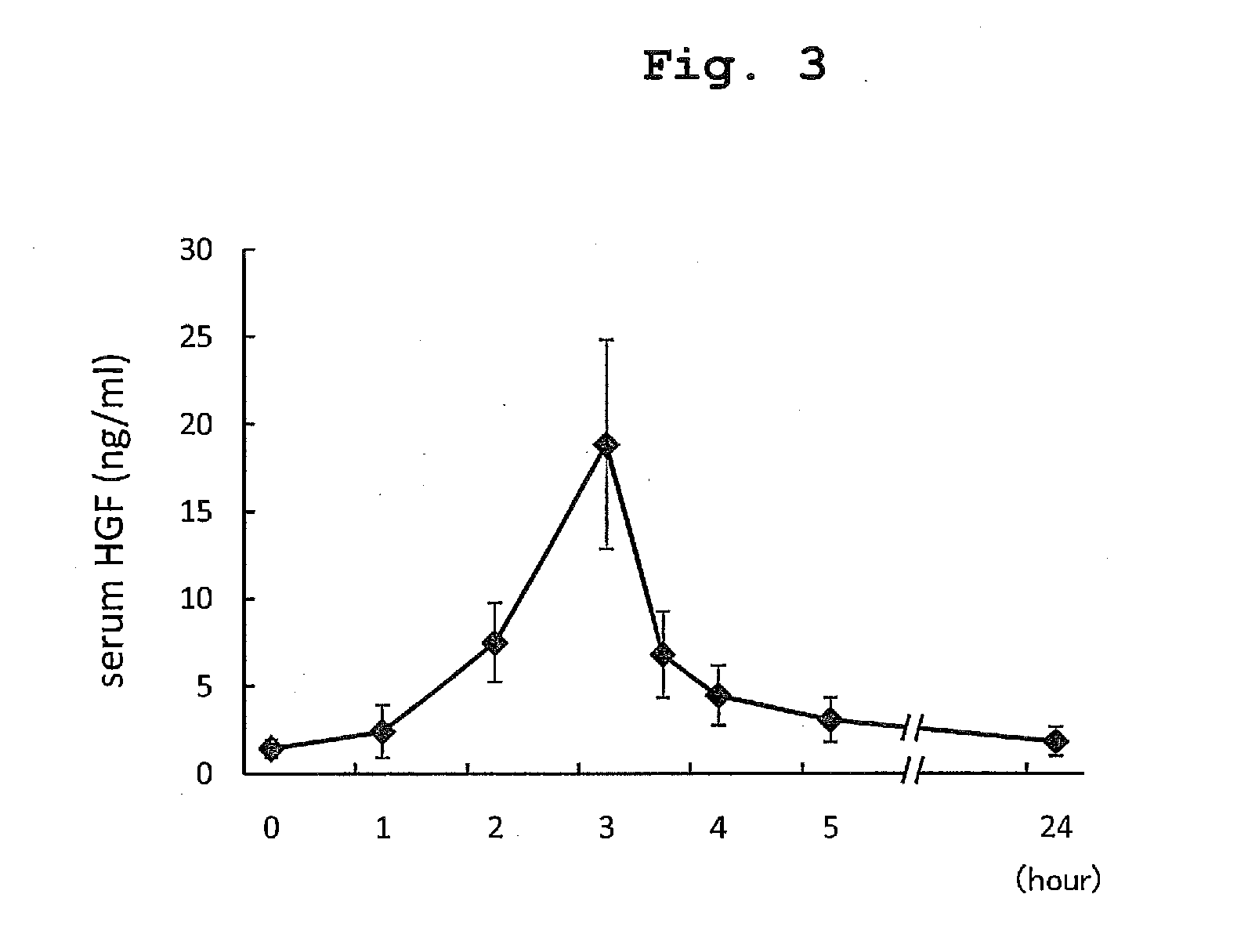
ActiveUS20140234341A1Constant effectPoor prognosisPeptide/protein ingredientsHepatocyte-growth/scatter/tumor-cytotoxic factorHepatic comaDepressant
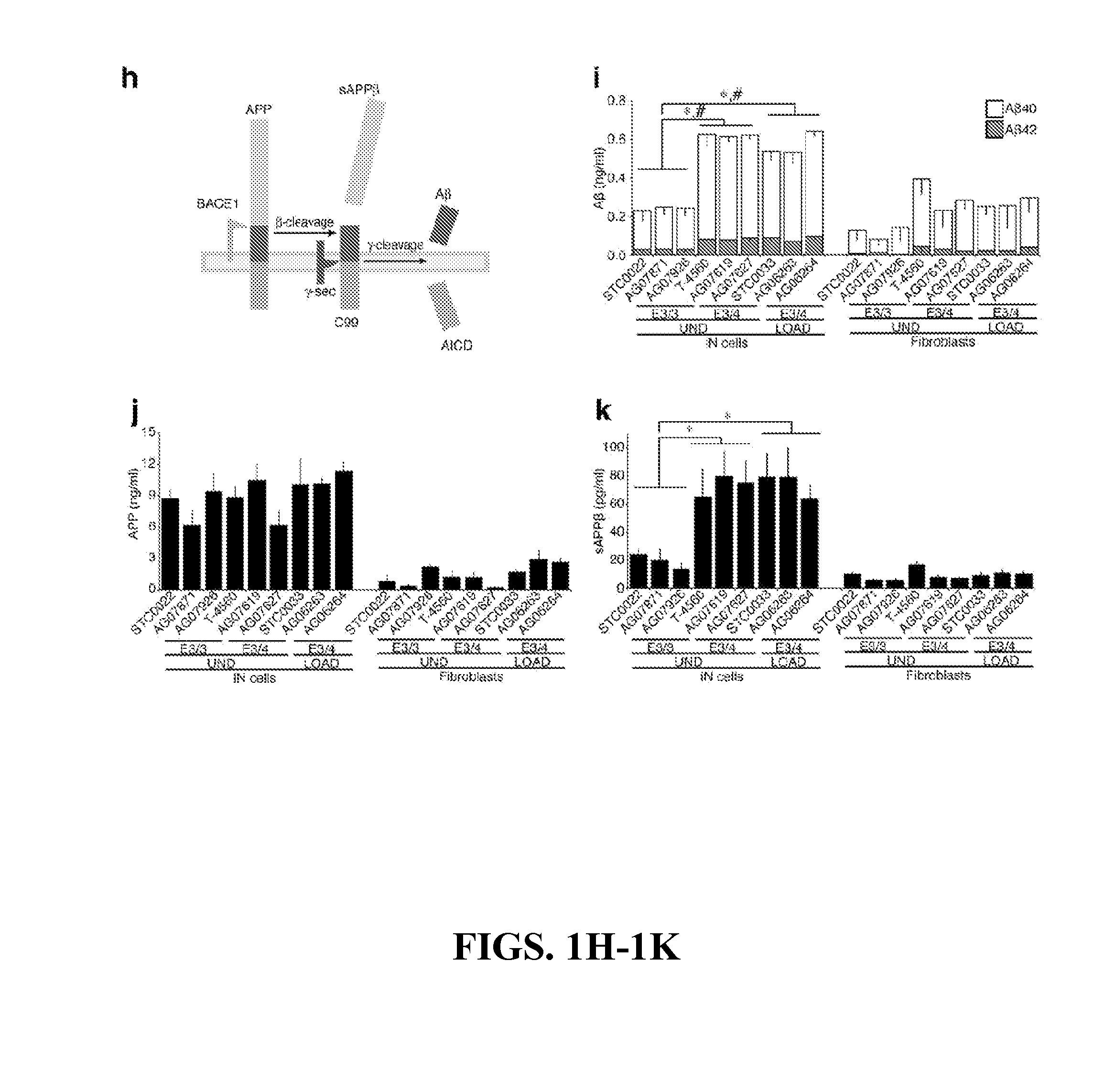
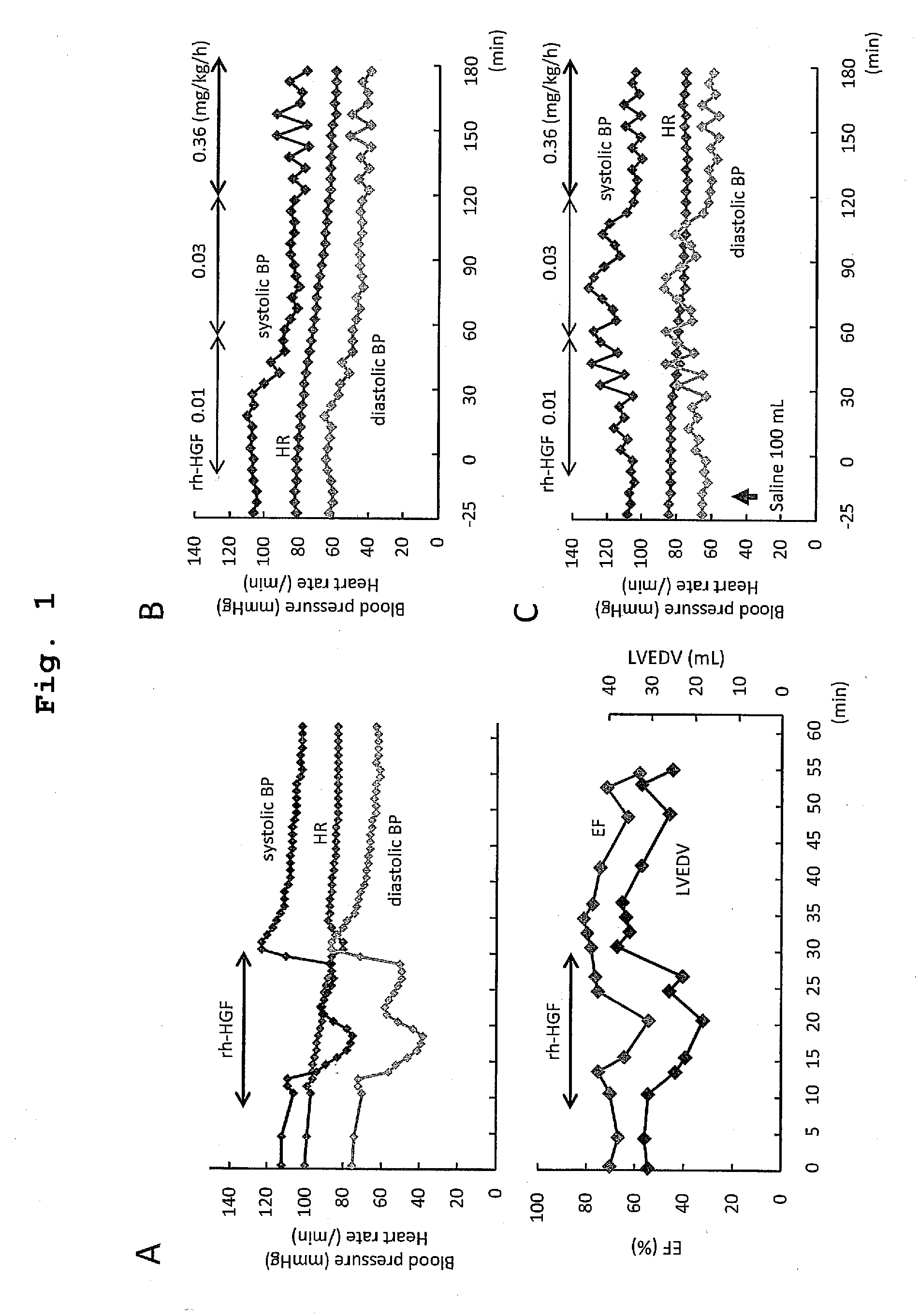
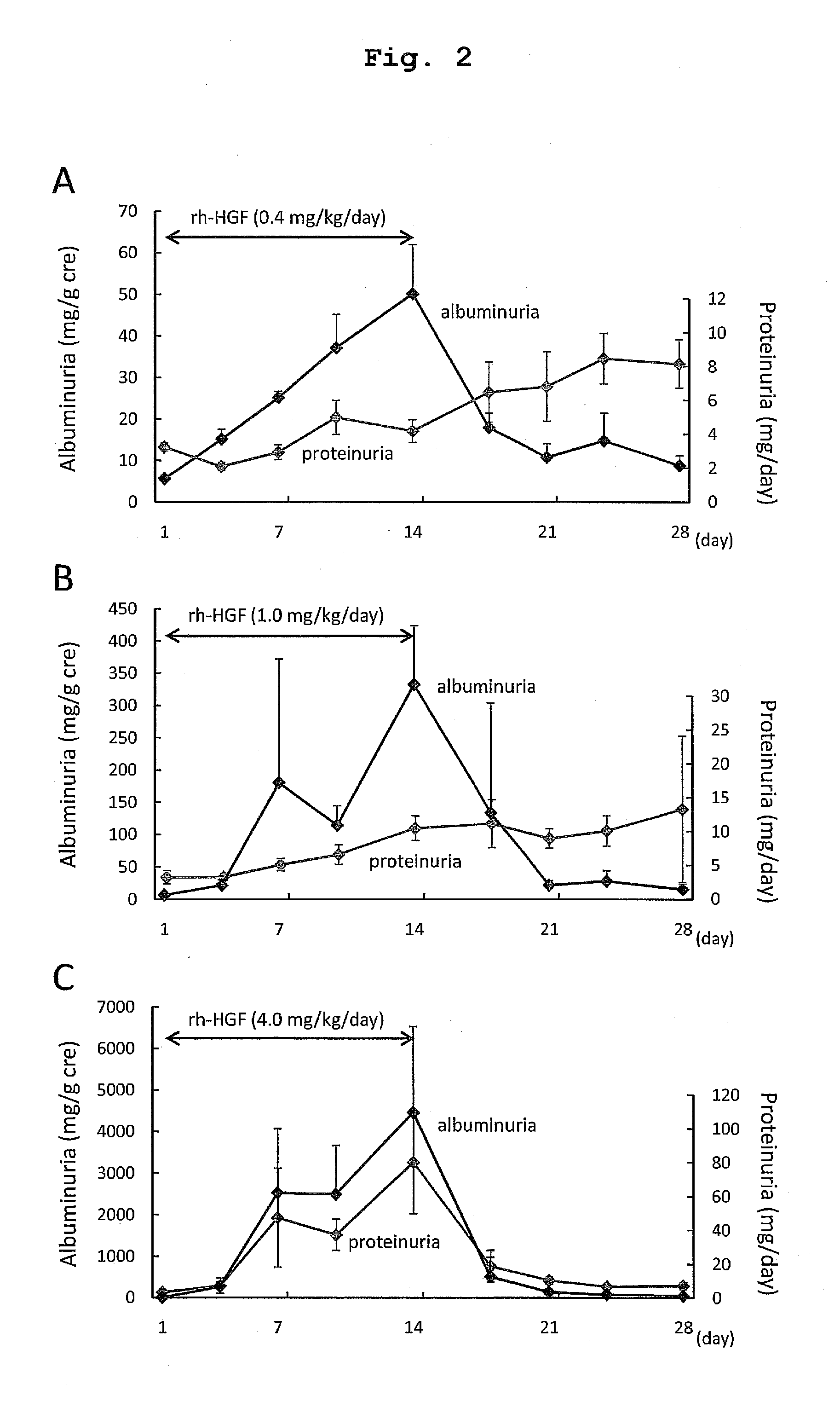
The present invention provides a therapeutic agent for acute liver failure containing a hepatocyte growth factor (HGF), particularly an agent for treating fulminant hepatitis or late onset hepatic failure or suppression of progression of acute liver failure without hepatic coma to fulminant hepatitis or late onset hepatic failure. The present invention also provides a method for evaluating the efficacy of HGF including measuring the amount of α-fetoprotein (liver regeneration biomarker) and / or soluble Fas (anti-apoptotic biomarker) in a sample obtained from a liver injury patient administered with HGF.
Owner:KYOTO UNIV
Leucine-rich repeat kinase (LRRK2) drosophila model for parkinson's disease: wildtype1 (WT1) and G2019S mutant flies
InactiveUS20100175140A1Effective preventionEffective treatmentCompounds screening/testingGenetic engineeringNeuronal degenerationWild type
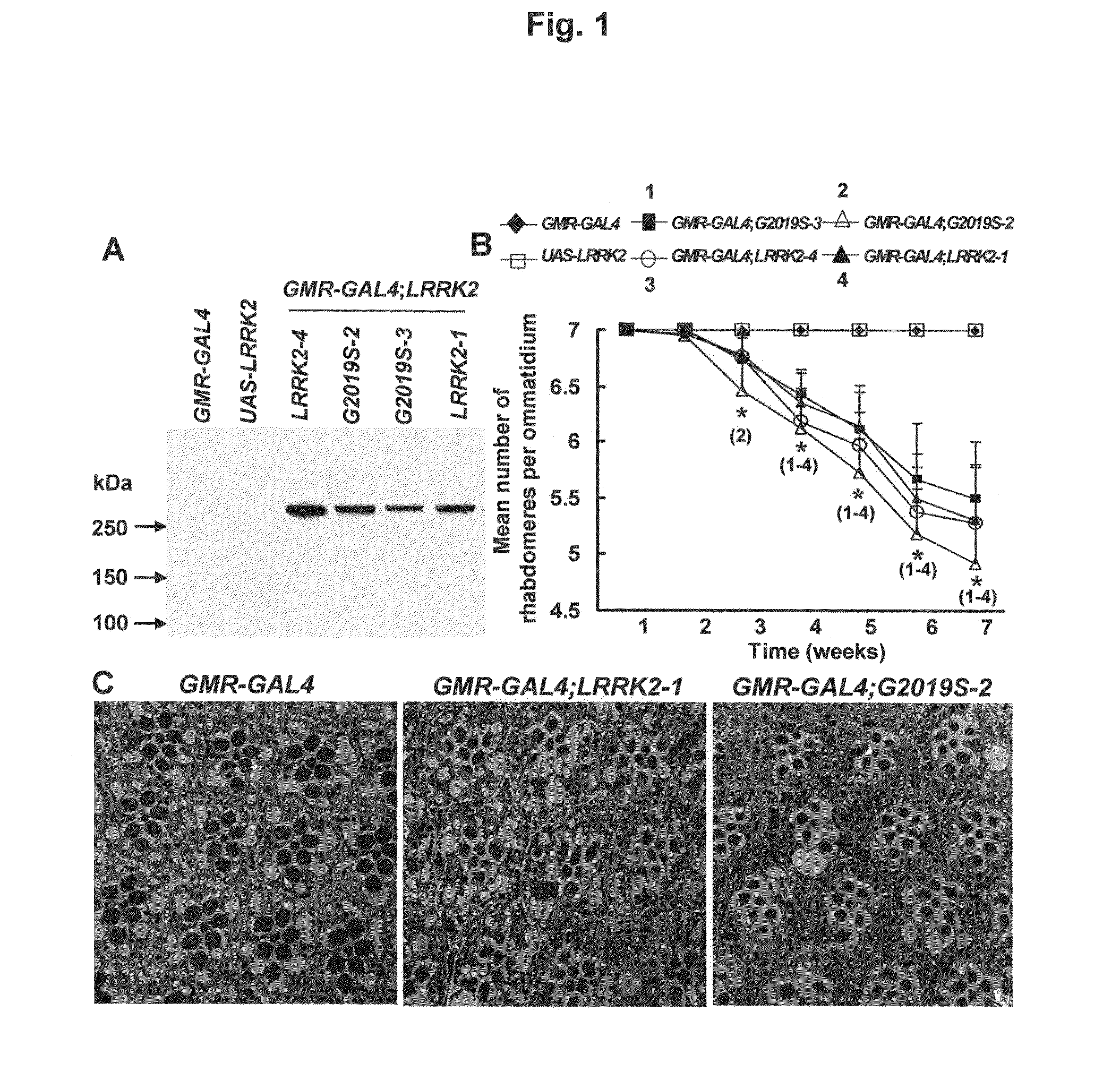
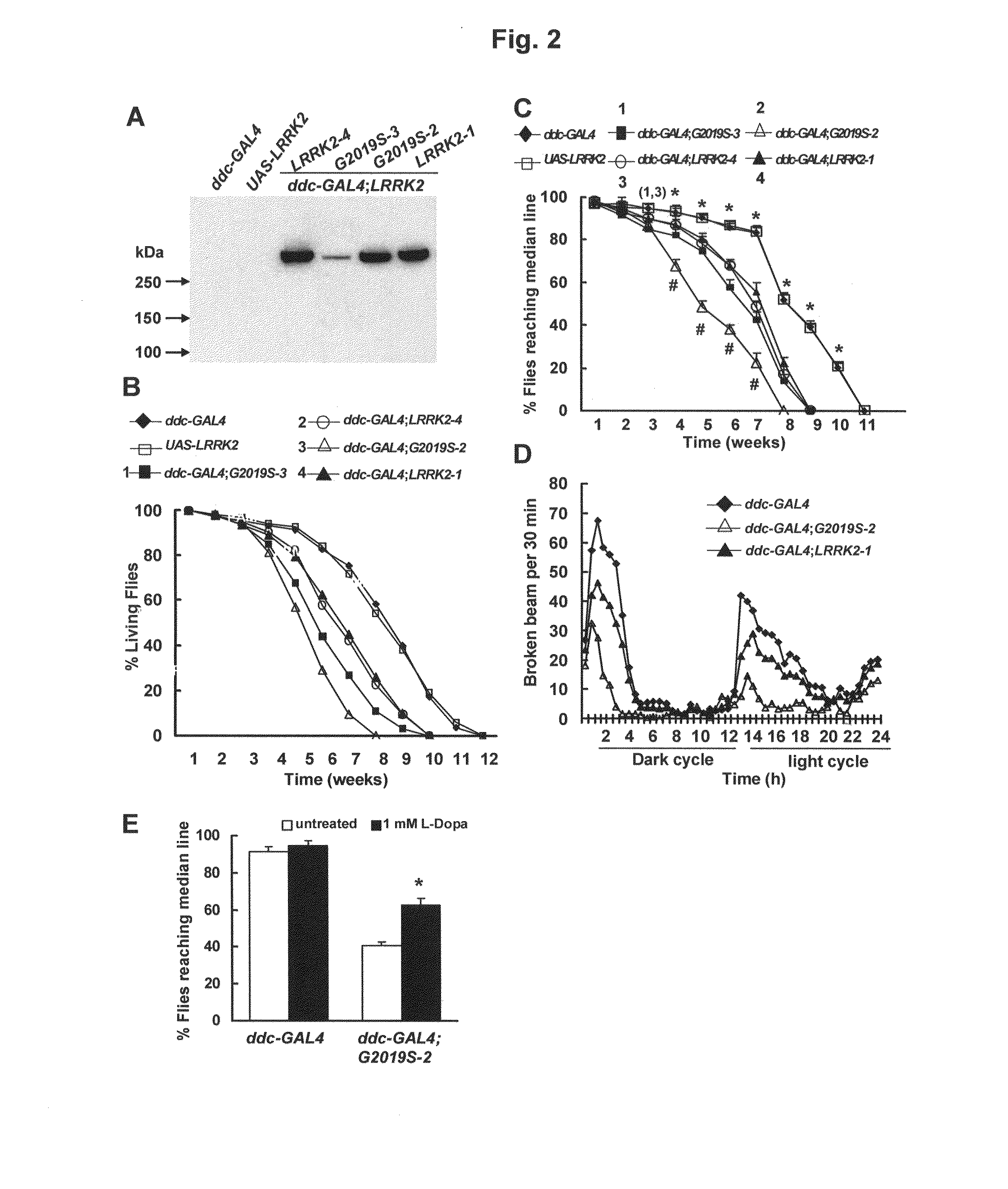
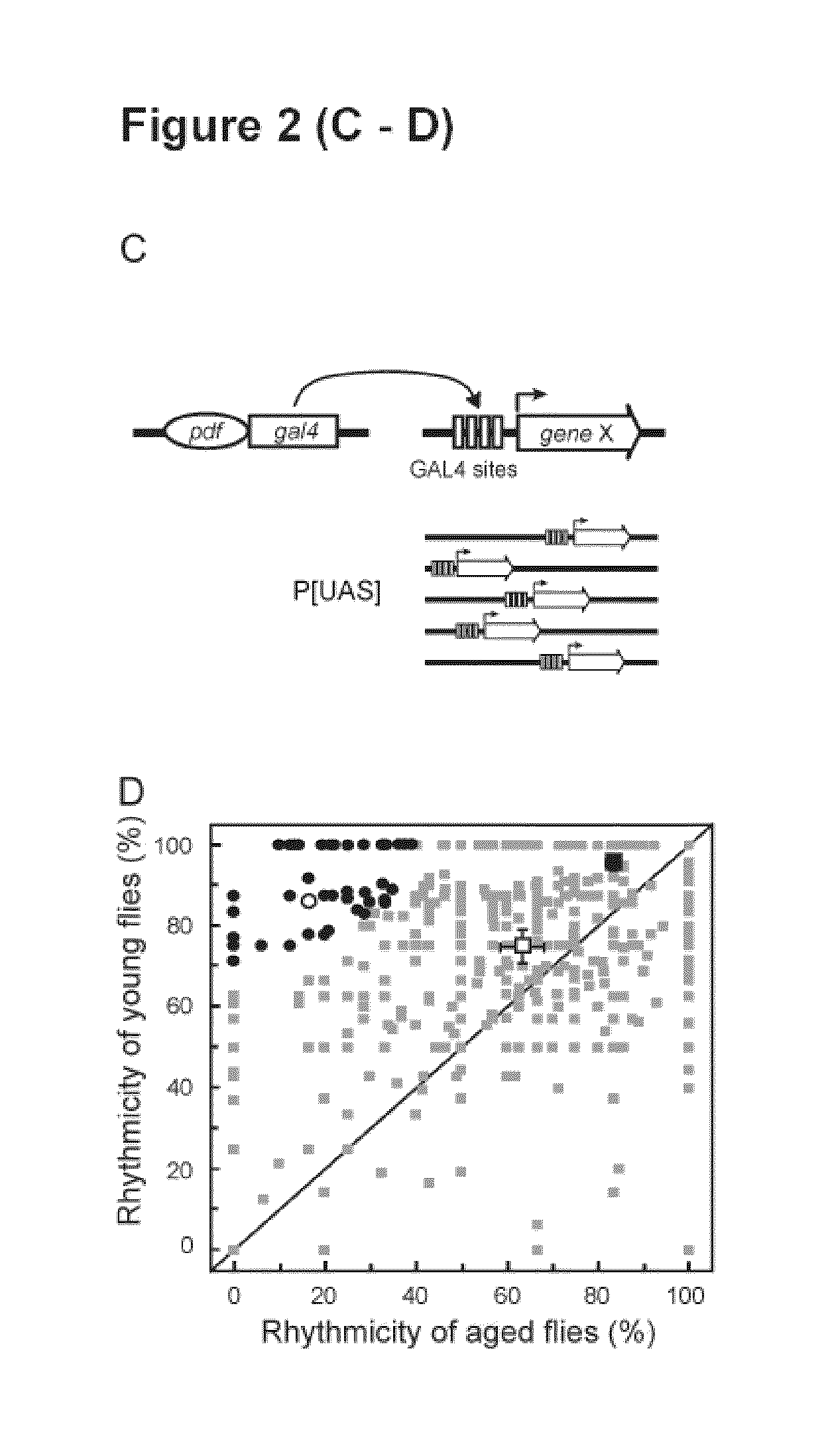
Mutations in the leucine-rich repeat kinase (LRRK2) gene cause late-onset autosomal dominant Parkinson's disease (PD) with pleiomorphic pathology. Previously, we and others found that expression of mutant LRRK2 causes neuronal degeneration in cell culture. Here we used the GAL4 / UAS system to generate transgenic Drosophila expressing either wild-type (WT1) human LRRK2 or LRRK2-G2019S, the most common mutation associated with PD. Expression of either WT1 human LRRK2 or LRRK2-G2019S in the photoreceptor cells caused retinal degeneration. Expression of WT1 LRRK2 or LRRK2-G2019S in neurons produced adult-onset selective loss of dopaminergic neurons, locomotor dysfunction, and early mortality. Expression of mutant G2019S-LRRK2 caused a more severe parkinsonism-like phenotype than expression of equivalent levels of WT1 LRRK2. Treatment with L-DOPA improved mutant LRRK2-induced locomotor impairment but did not prevent the loss of tyrosine hydroxylase (TH)-positive neurons. To our knowledge, this is the first in vivo “gain-of-function” model which recapitulates several key features of LRRK2-linked human parkinsonism. These flies may provide a useful model for studying LRRK2-linked pathogenesis and for future therapeutic screens for PD intervention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
GJB2 gene p.V37I mutation diagnostic kit for diagnosing late-onset deafness
InactiveCN102304584AEstablish priority areas of applicationMicrobiological testing/measurementGenes mutationFull Term Neonate
The invention relates to application of GJB2 gene p.V37I mutation to the preparation of products for diagnosing late-onset deafness. The GJB2 gene p.V37I mutation is p.V37I exclusive mutation. The invention also provides a GJB2 gene p.V37I mutation diagnostic kit for diagnosing the late-onset deafness and application of the kit. The GJB2 gene p.V37I mutation diagnostic kit has the advantages that: a common molecular identifier, namely the GJB2 gene p.V37I exclusive mutation related to the late-onset deafness highly is discovered at home and abroad for the first time; a molecular identifier-based gene screening method and the preferential application range of the molecular identifier in the screening of neonates are determined; and the alarm time of the late-onset deafness caused by the GJB2 gene p.V37I exclusive mutation is advanced to a neonate period, and the GJB2 gene p.V37I mutation diagnostic kit is widely applied to the early detection, intervention and rehabilitation of genetic susceptible late-onset deafness, the screening and diagnosis of deafness genes, late-onset deafness hearing-gene combined screening and other relevant study fields.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Breast cancer prognostication and screening kits and methods of using same
ActiveUS20160333413A1Improved and more accurate screening and prognostic testsHigh riskMicrobiological testing/measurementSpecial data processing applicationsBiomarker panelRegimen
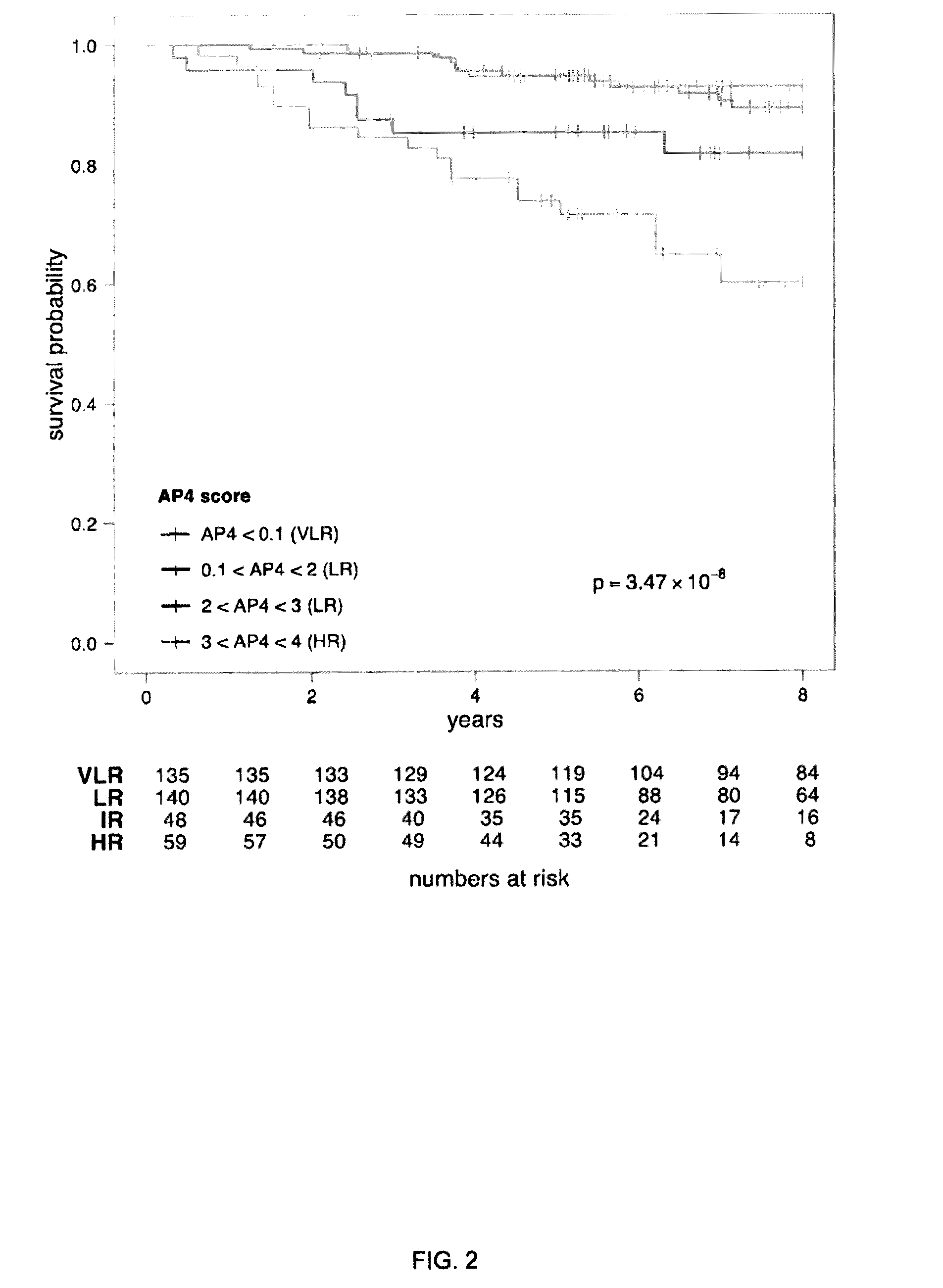
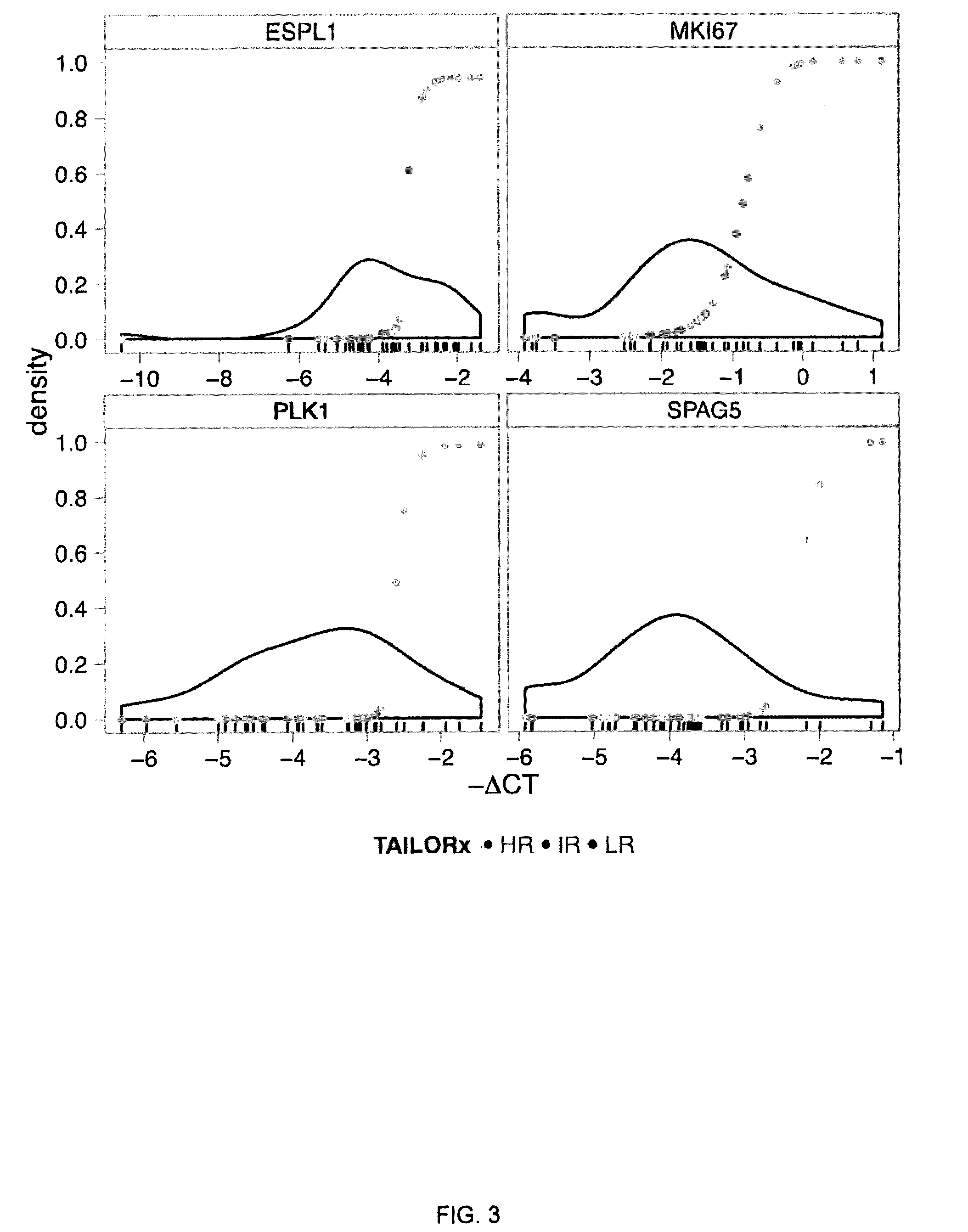
A genetic biomarker panel is provided for prognosing late onset ER+ breast cancer relapse, in a breast cancer survivor patient. Kits are also provided for measuring levels or the presence of an identified panel of genetic biomarkers. Methods are also provided for identifying a breast cancer survivor patient at a relatively high risk of suffering a breast cancer relapse within 8 years of diagnosis, and therefore suitable for treatment with an aggressive chemotherapeutic regimen. The method may also be used for identifying a breast cancer survivor patient not at high risk of suffering a breast cancer relapse within 8 years of diagnosis, and thus not suitable for treatment with an aggressive chemotherapeutic regimen. The genetic biomarker panel includes an oligonucleotide / nucleic acid sequence specific for the following genes: MKI67, SPAG5, ESPL1, PLK1, or a genetic panel for MKI67, SPAG5, ESPL1, PLK1 and PGR.
Owner:INDIANA UNIV RES & TECH CORP +1
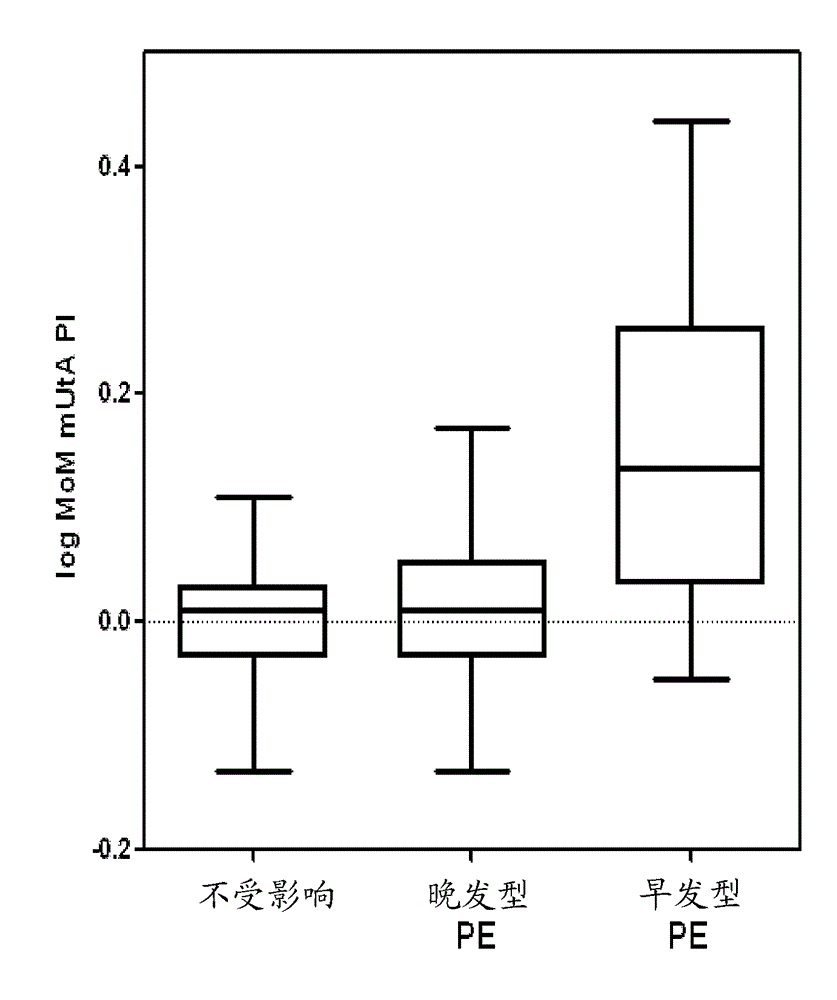
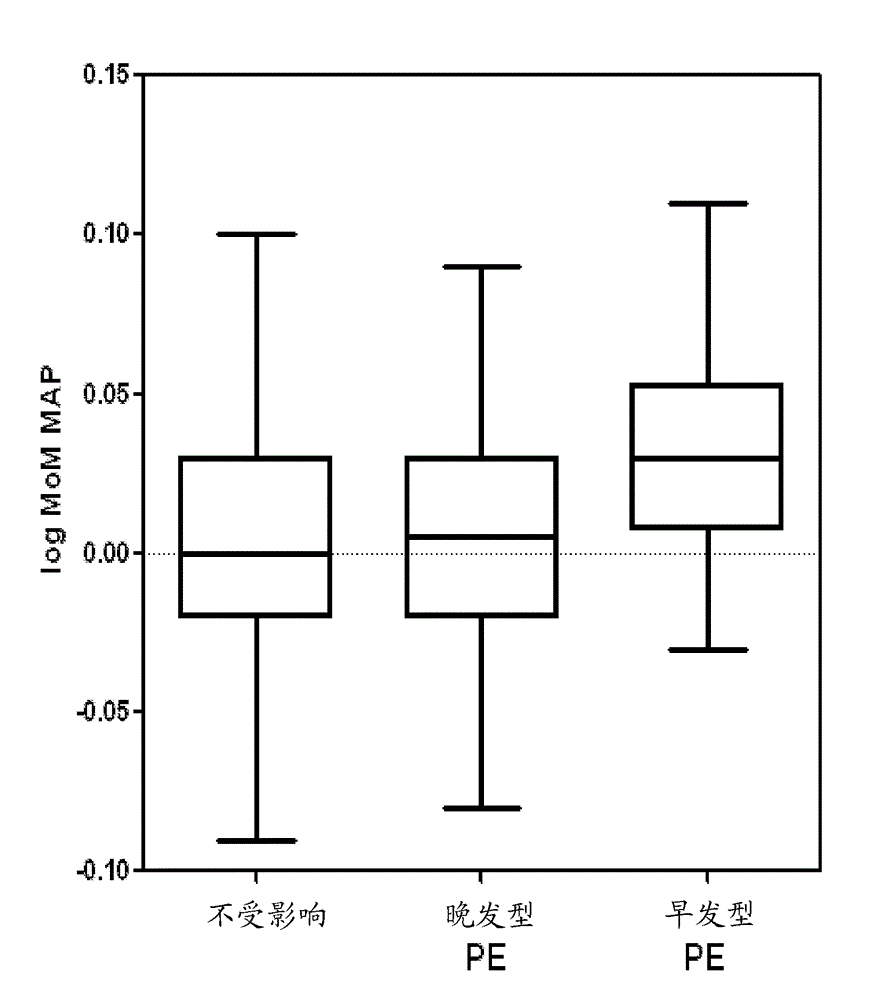
Pre-eclampsia screening methods
The present invention relates generally to methods for treating early and late onset pre-eclampsia, as well as to methods of screening for and predicting the likelihood that a pregnant female patient will develop early and / or late pre-eclampsia, by assessing specific combinations of factors. In the methods of the invention, the a priori risk of developing eariy preeclampsia may be calculated utilizing coefficients for each of the maternal factors (binary variables), the coefficients being generated utilizing logistic regression analysis. The a posteriori risk of developing eariy pre-eclampsia may be calculated utilizing coefficients for each of the patient-specific factors, the coefficients being generated utilizing logistic regression analysis.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Gaba biomarkers for depression
InactiveUS20110091381A1Useful in diagnosisReduce usageNervous disorderMicrobiological testing/measurementScreening methodLate onset
Differential expression of nucleic acids in the brains of subjects suffering from late-onset depression has been demonstrated. The invention provides methods useful in the determination of late-onset depression. Also provided by the present invention is a screening method for the identification of compounds for treatment, prevention or diagnosis of late-onset depression.
Owner:GE HEALTHCARE LTD
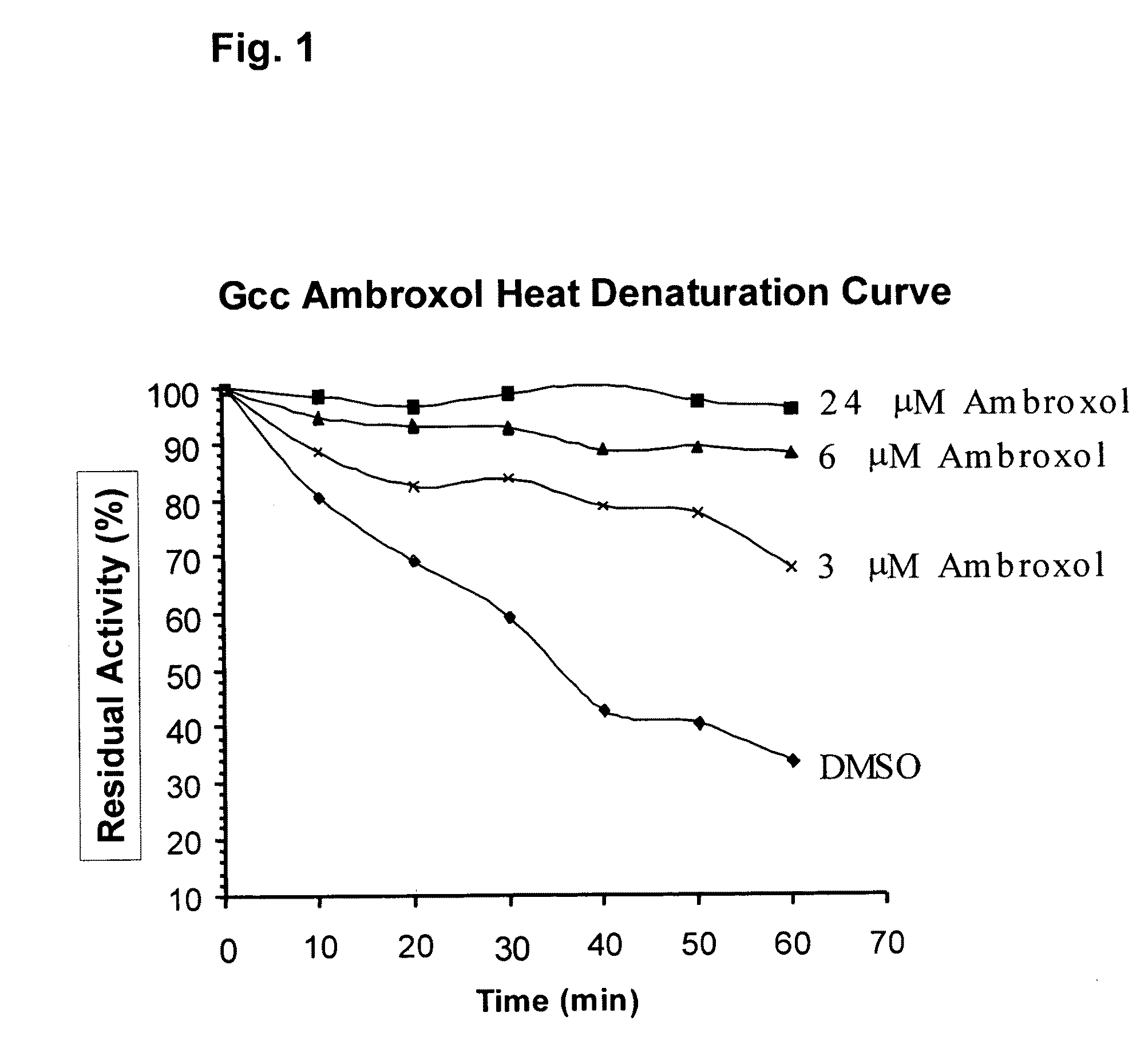
Method of treating gaucher disease
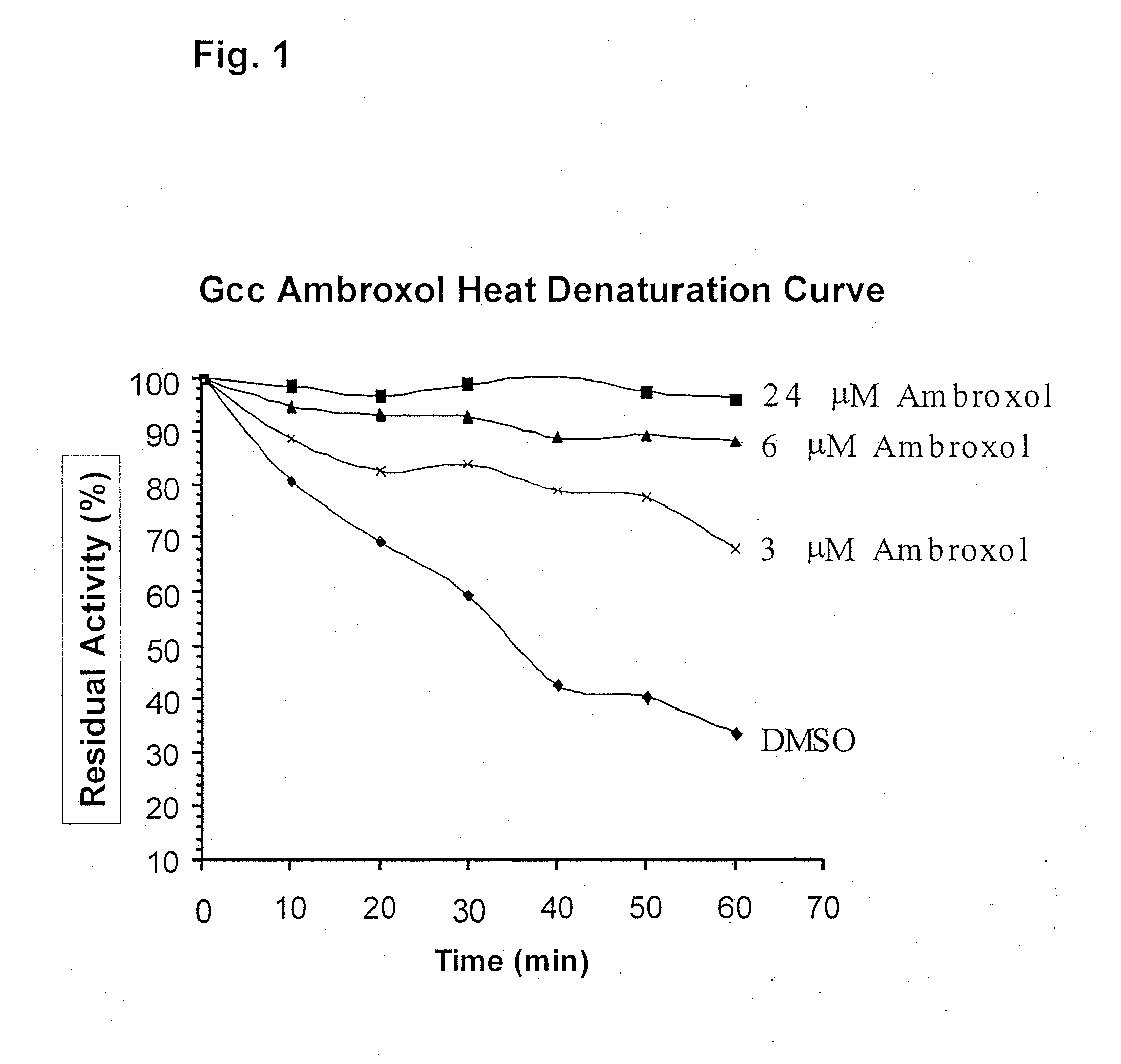
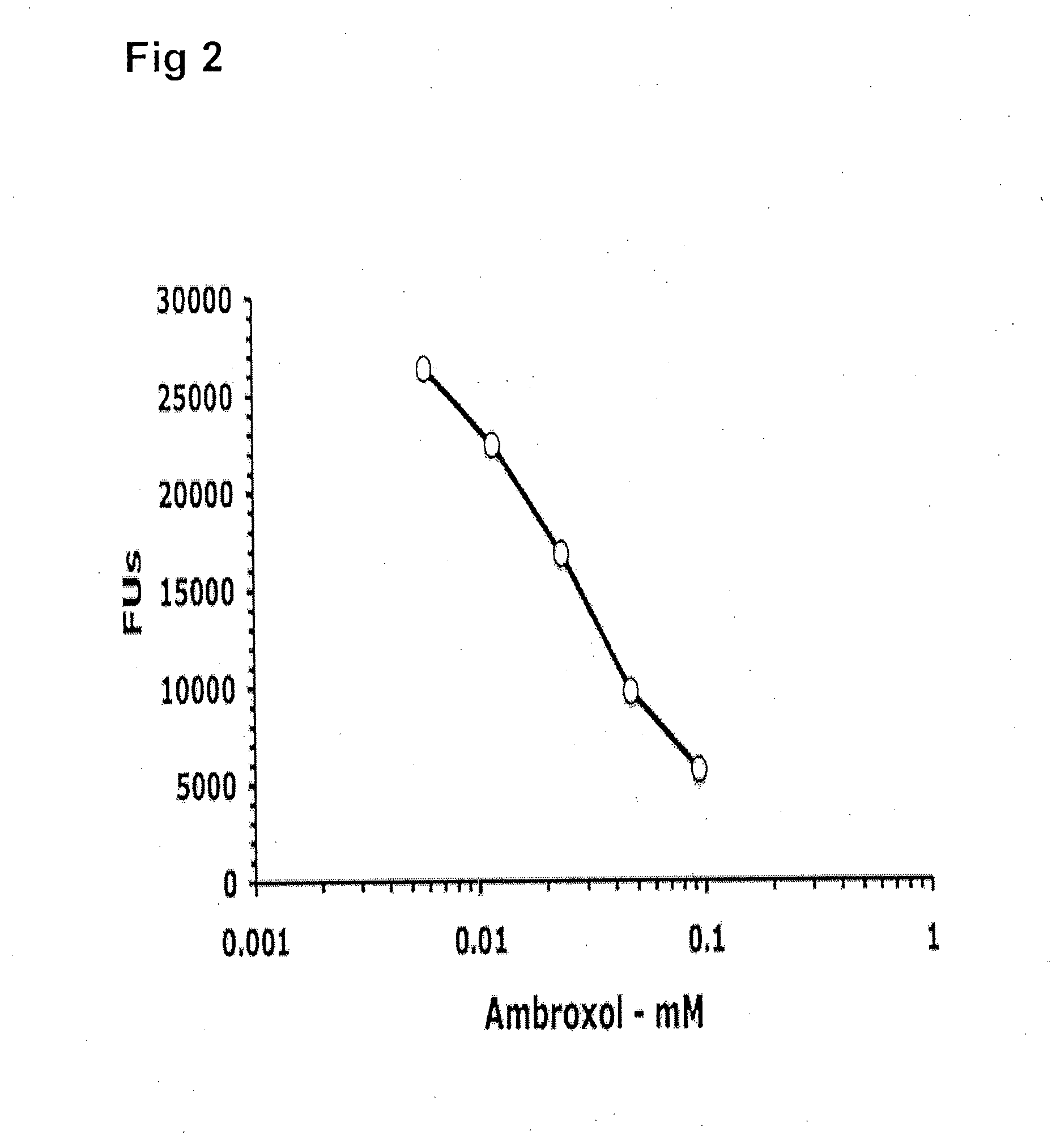
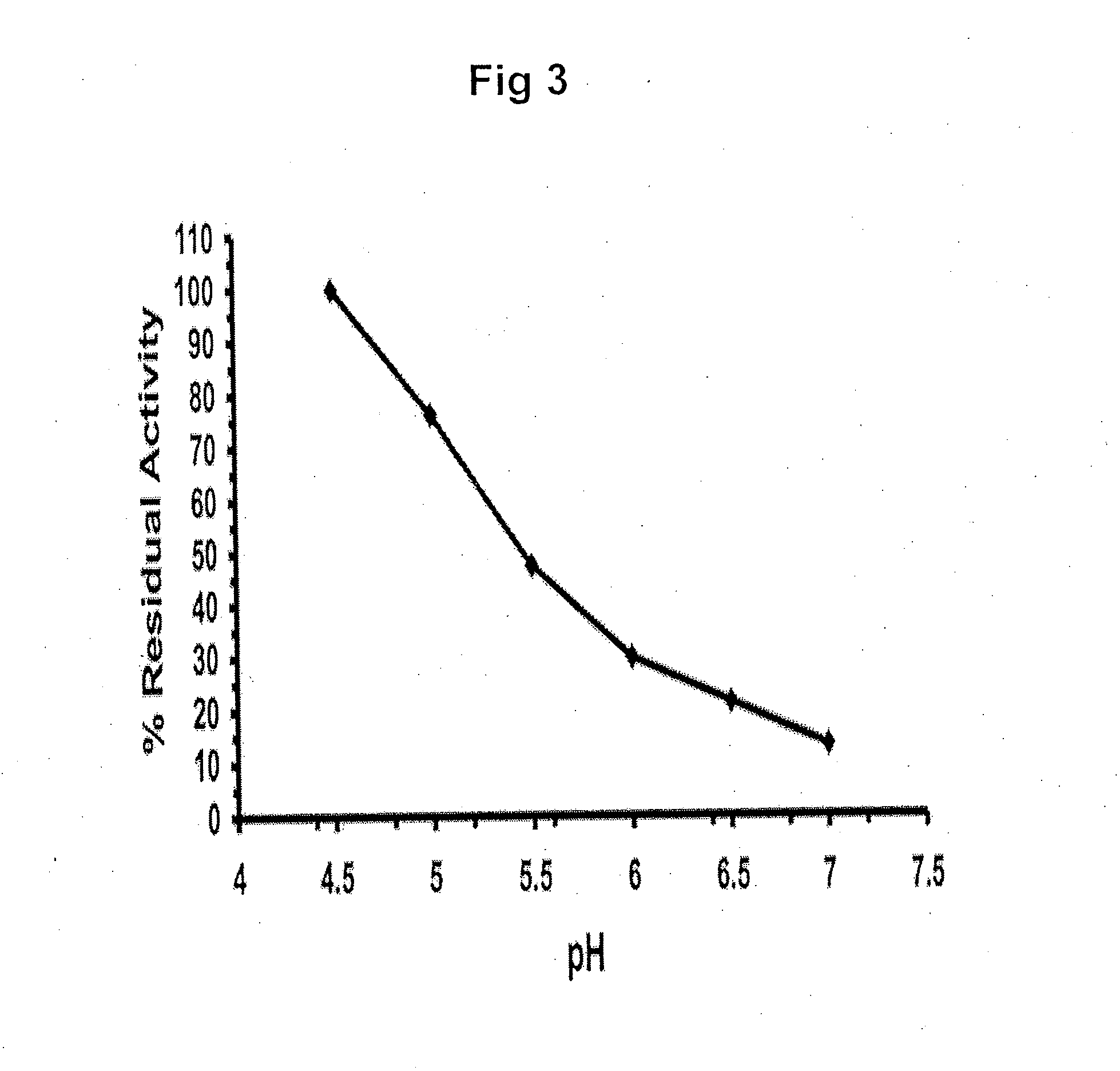
ActiveUS20090075960A1Optimal pharmacological chaperoningOptimal enzyme enhancementBiocidePeptide/protein ingredientsLate onsetPharmacological chaperone
Therapeutic compositions and methods for treatment of late-onset Gaucher disease are described herein. The compositions comprise compounds having activity as pharmacological chaperones for mutant forms of the beta-glucocerebrosidase. Methods of treatment involve providing therapeutically effective amounts of such compositions to subjects in need thereof.
Owner:HOSPITAL FOR SICK CHILDREN +1
Combined medicine for treating colorectal cancer
ActiveCN108159160ADefinite curative effectOvercome side effectsOrganic active ingredientsDigestive systemSide effectIrinotecan
The invention provides a combined medicine for treating colorectal cancer. The combined medicine comprises medicine compositions for simultaneous or respective administration of preparations with thesame or different specifications and units and irinotecan, as well as a pharmaceutically acceptable carrier. The combined medicine can effectively treat colorectal cancer, can reduce side effects, particularly delayed-onset diarrhea, caused by irinotecan, and provides a new choice for clinical medication.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
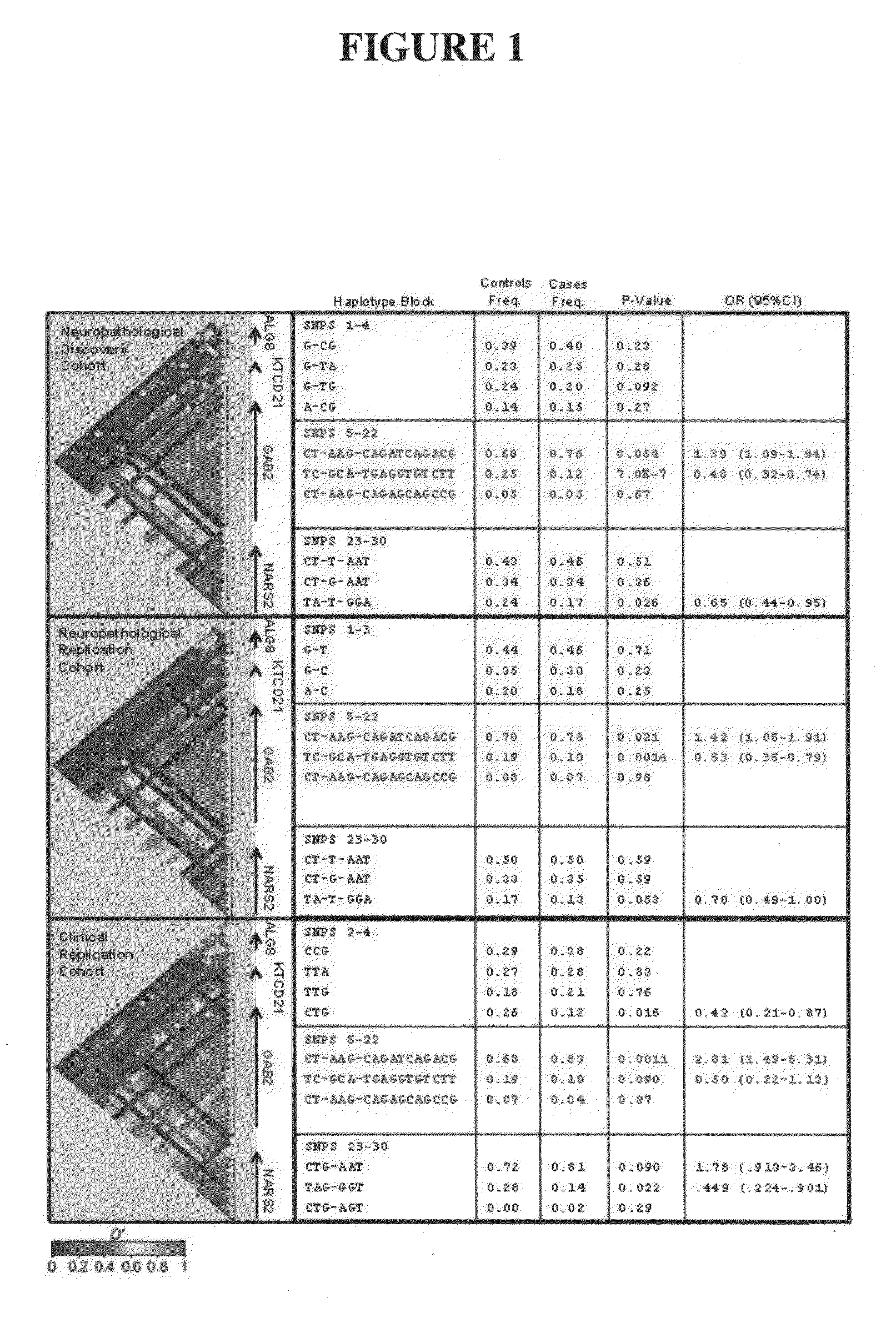
Methods of diagnosing alzheimer's disease and markers identified by set association
InactiveUS20090260092A1Reduce riskReduce the possibilityOrganic active ingredientsNervous disorderWhole Genome Association AnalysisLate onset
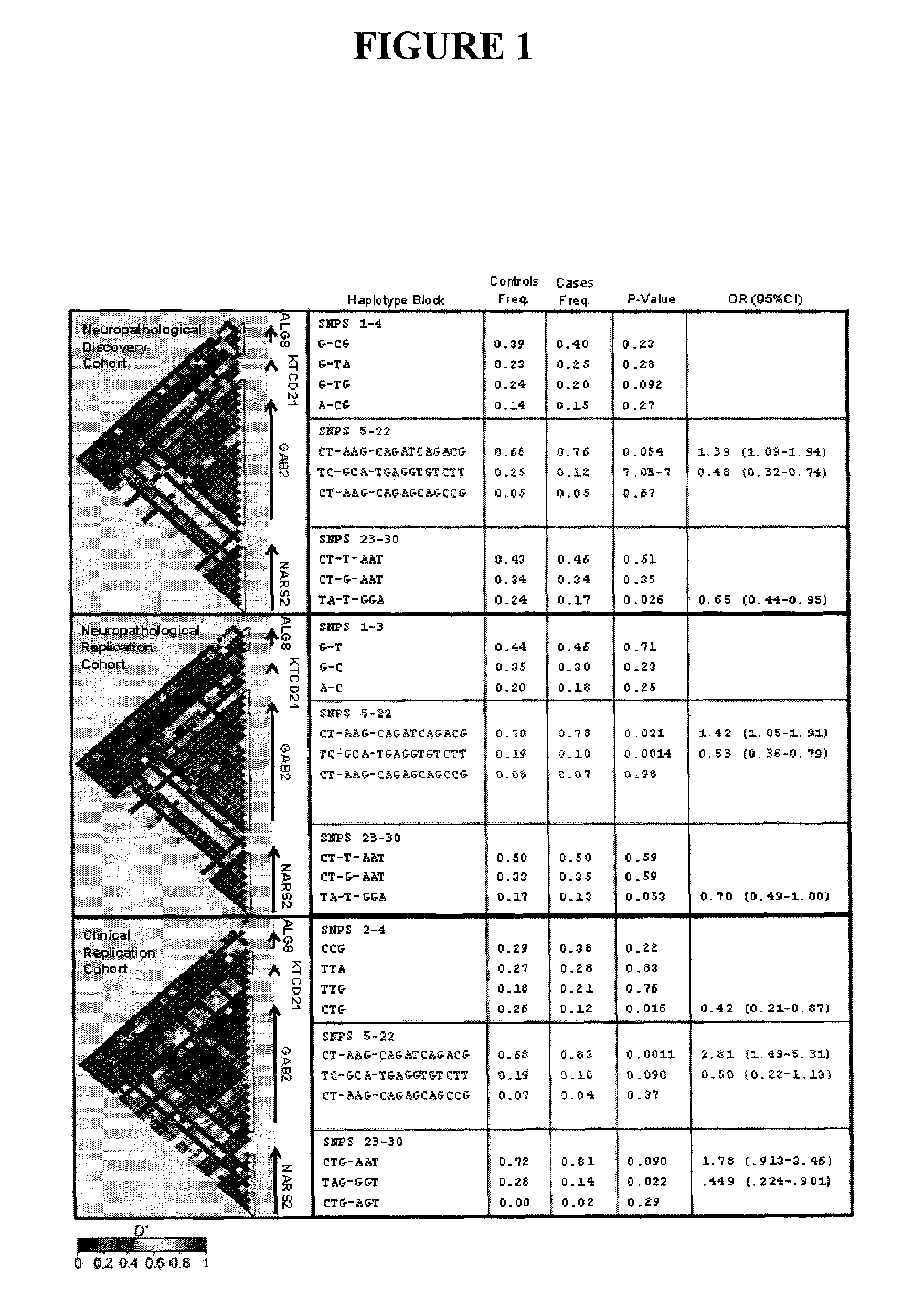
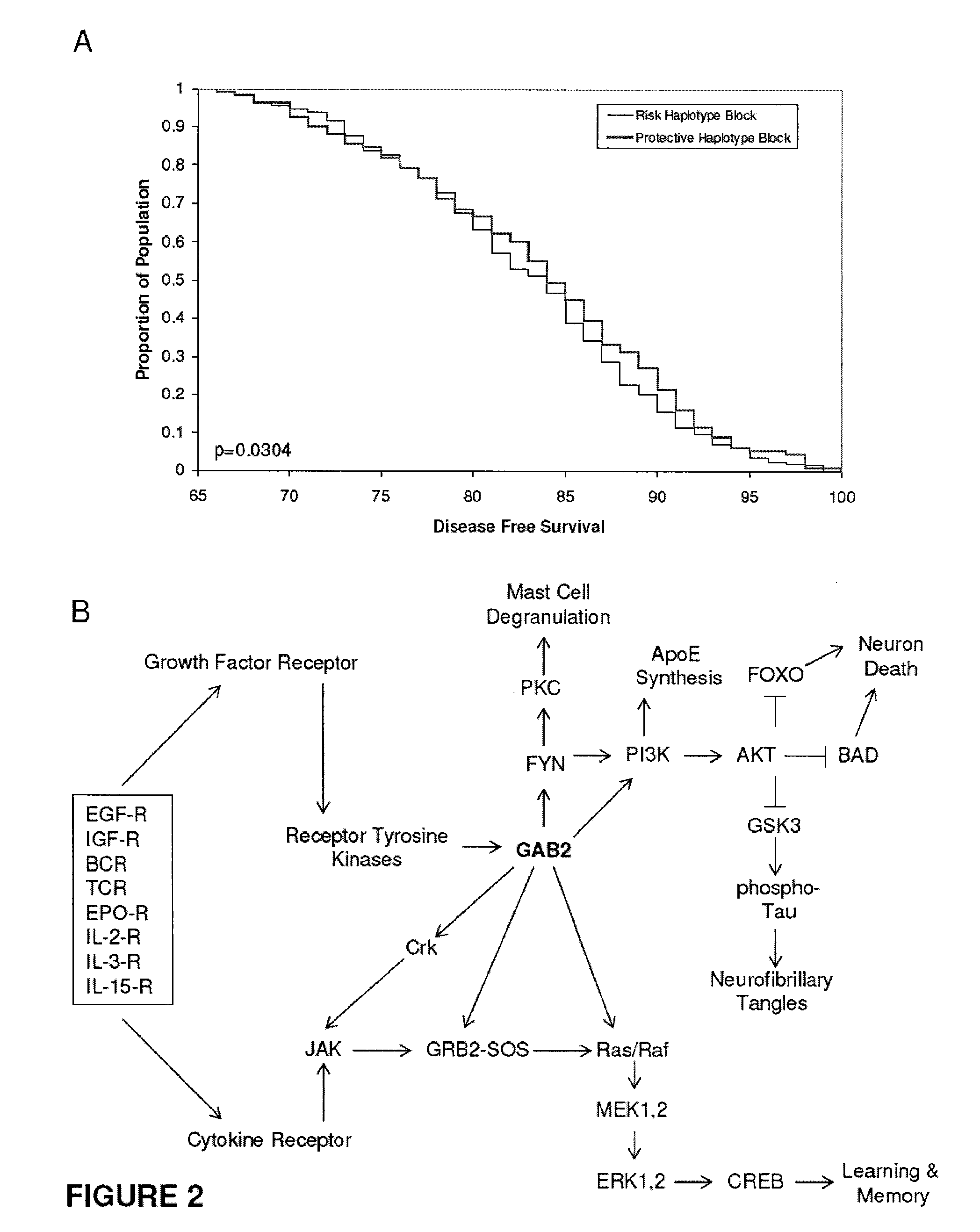
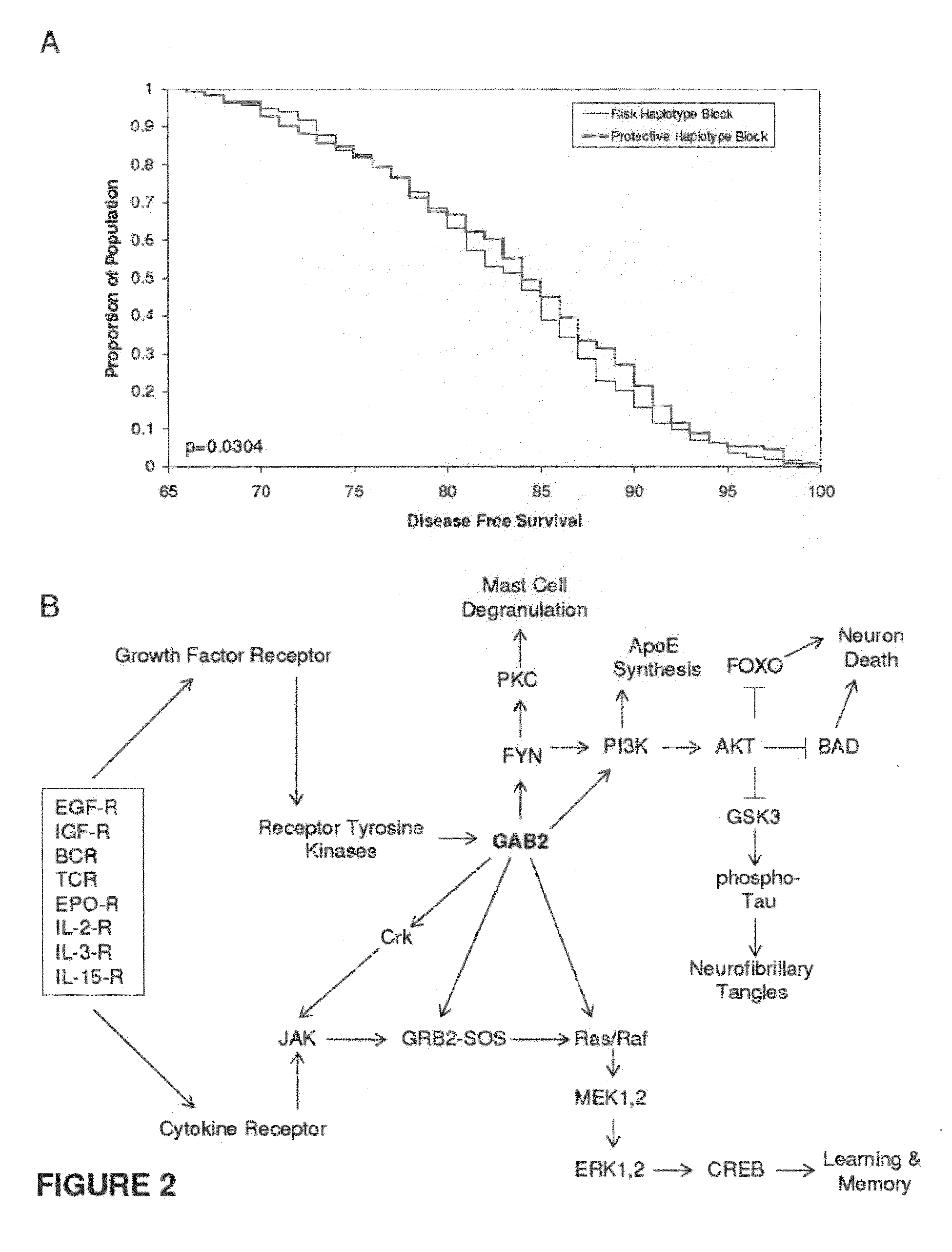
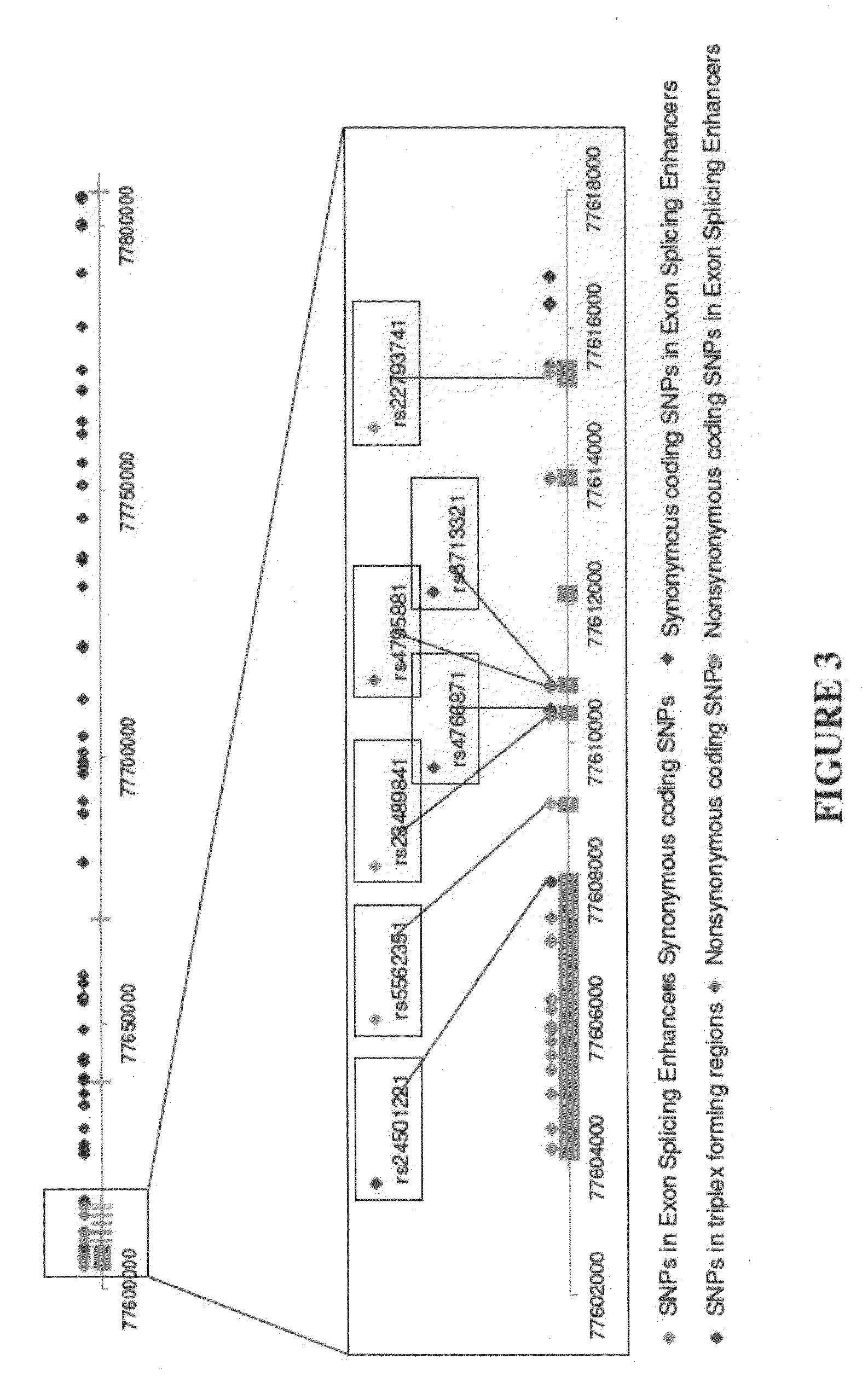
The present disclosure relates to genetic markers and methods of diagnosing and screening for late-onset Alzheimer's disease (LOAD). As such, the disclosure encompasses a whole-genome association analysis of single nucleotide polymorphisms (SNPs) of which a number are located within the GRB2-associated binding protein 2 (GAB2) gene as well as other markers associated with other genes. The disclosure identifies two novel haplotypes within the GAB2 gene, i.e., a LOAD risk-enhancing and a LOAD risk-decreasing haplotype. These haplotypes modify LOAD risk differentially in combination with APOE alleles. Further encompassed are therapeutic methods and agents of decreasing the deterioration of cells associated with LOAD.
Owner:TRANSLATIONAL GENOMICS RESEARCH INSTITUTE +1
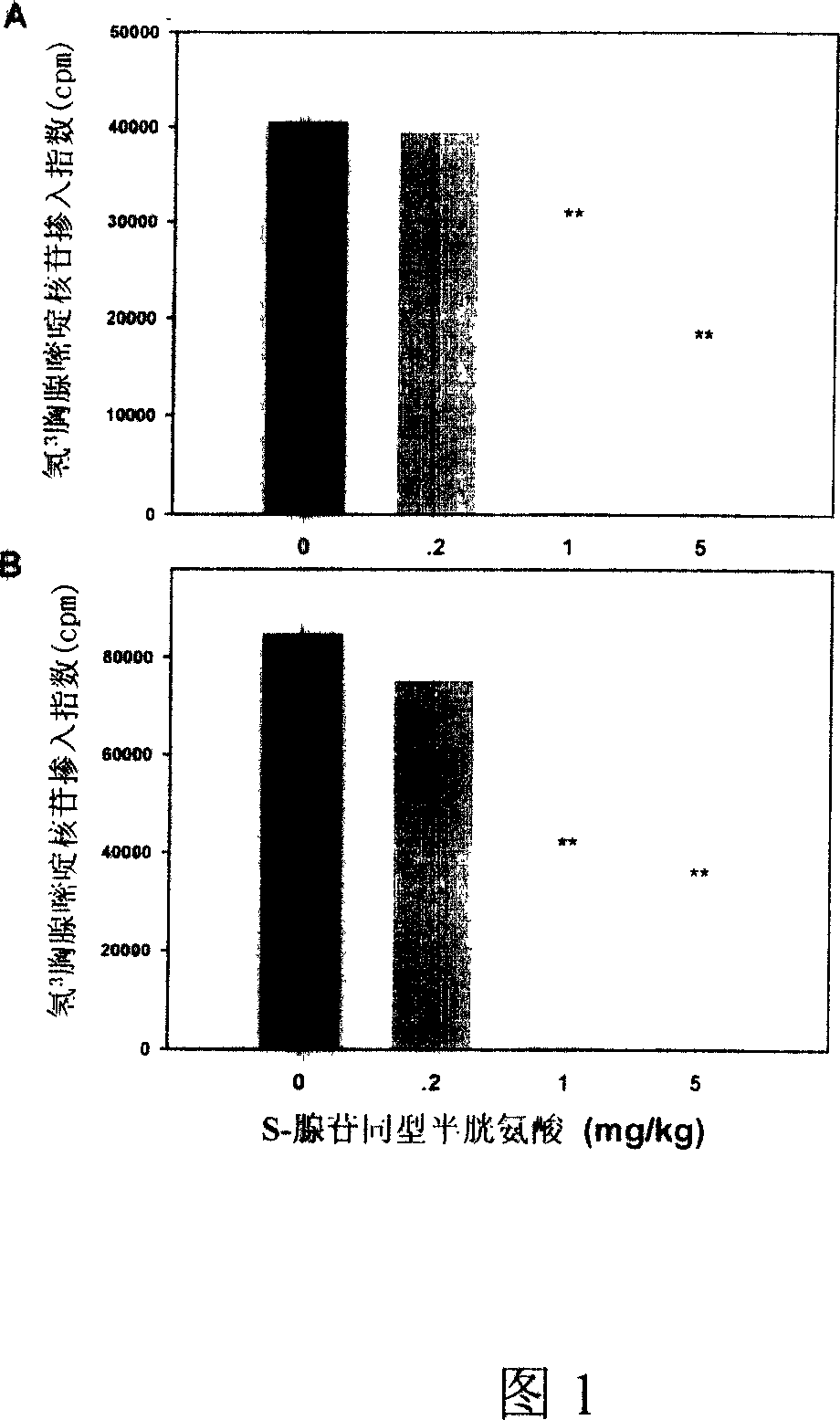
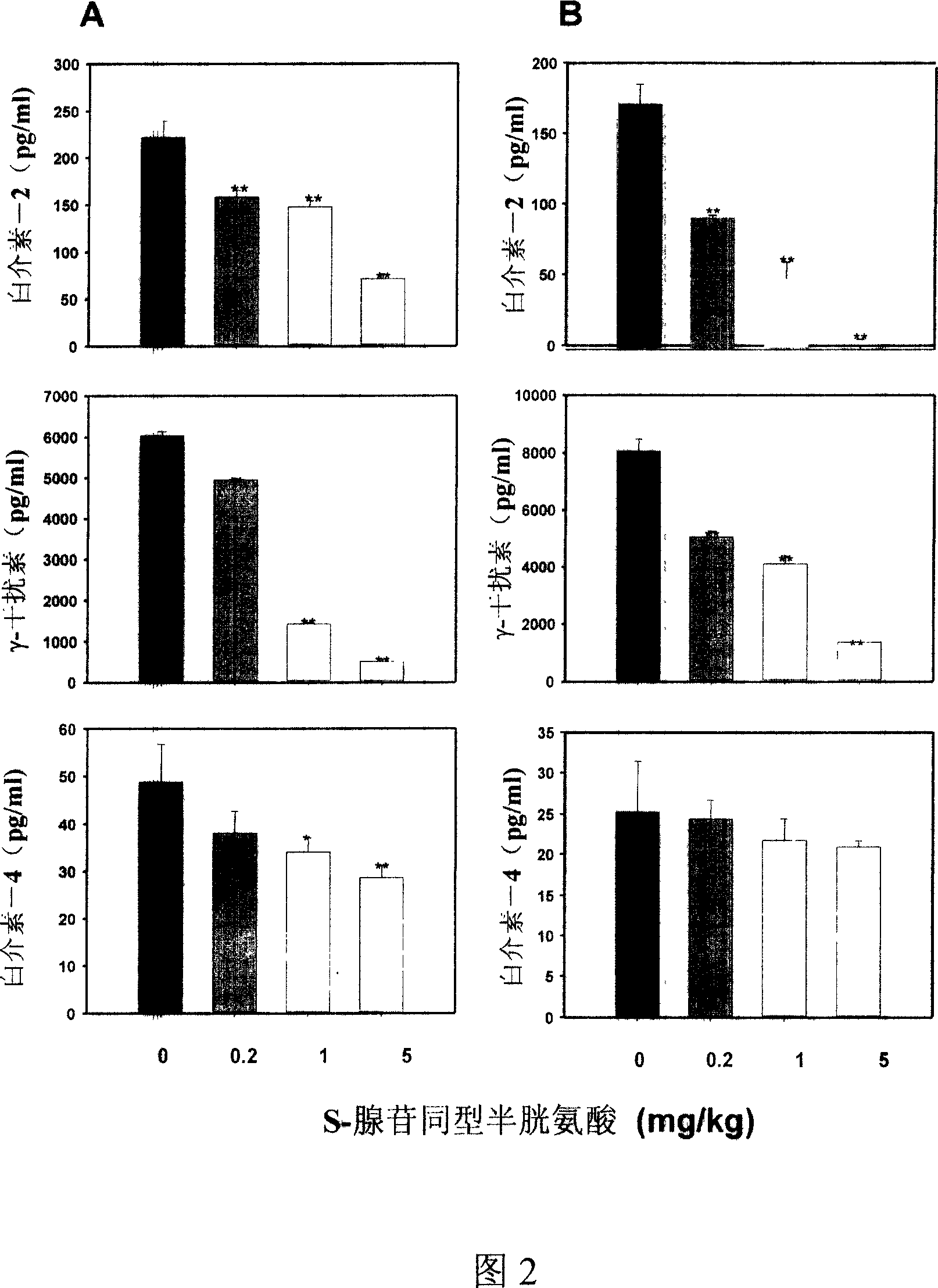
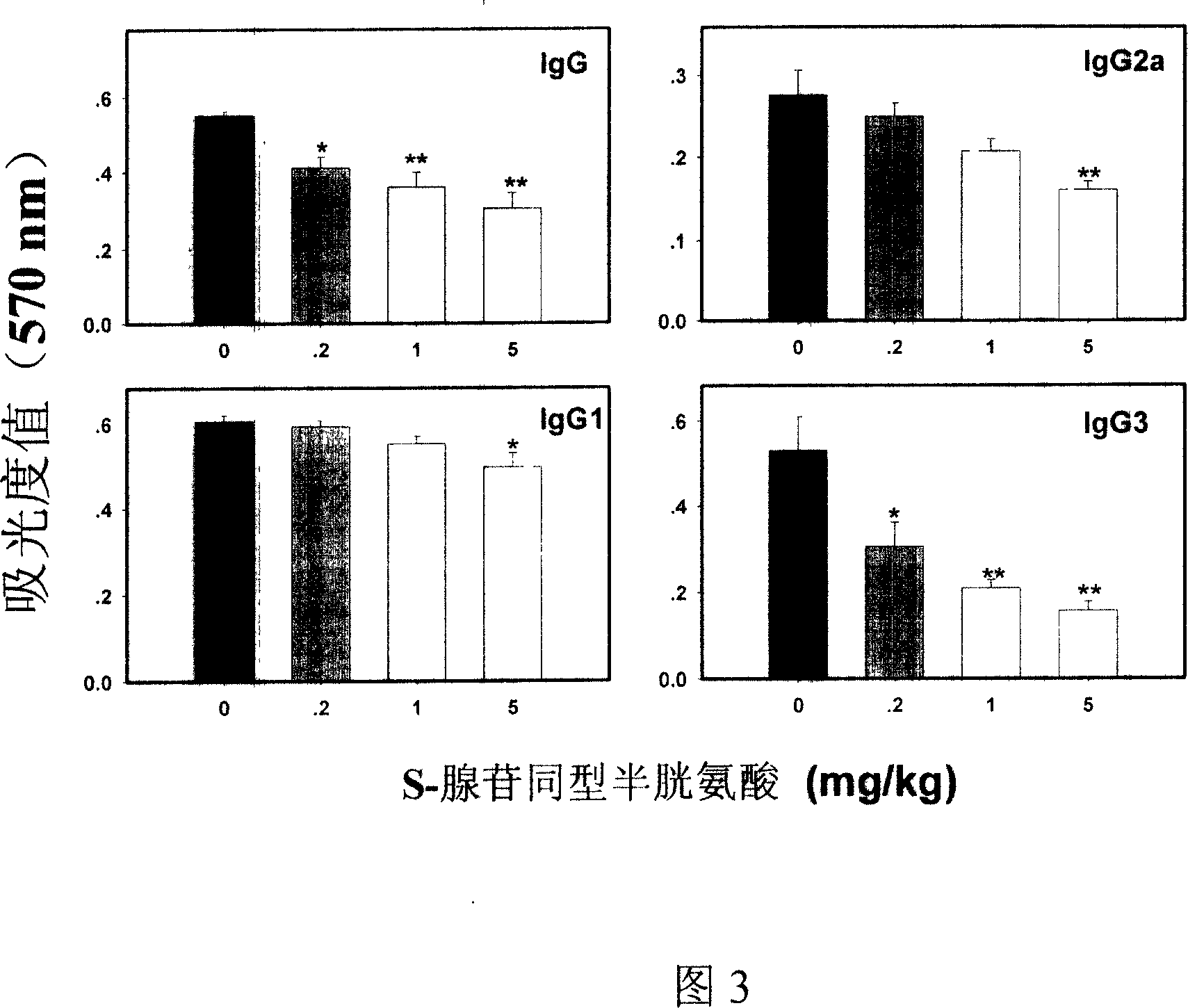
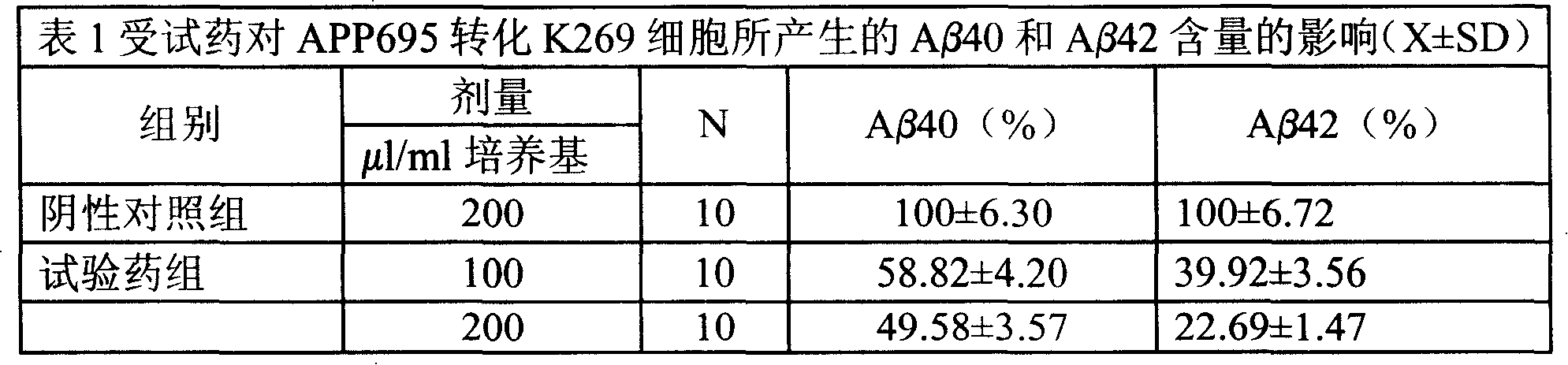
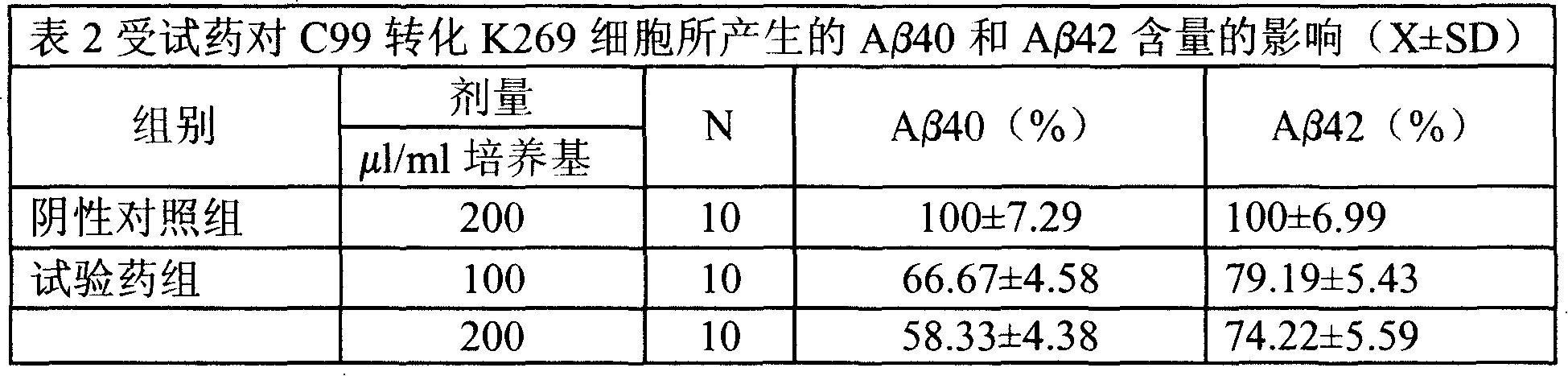
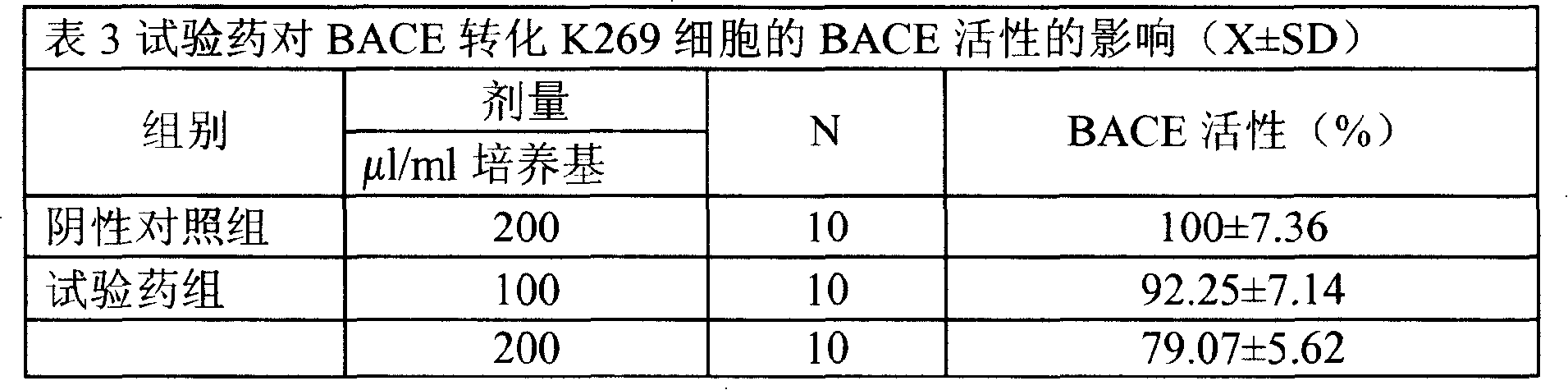
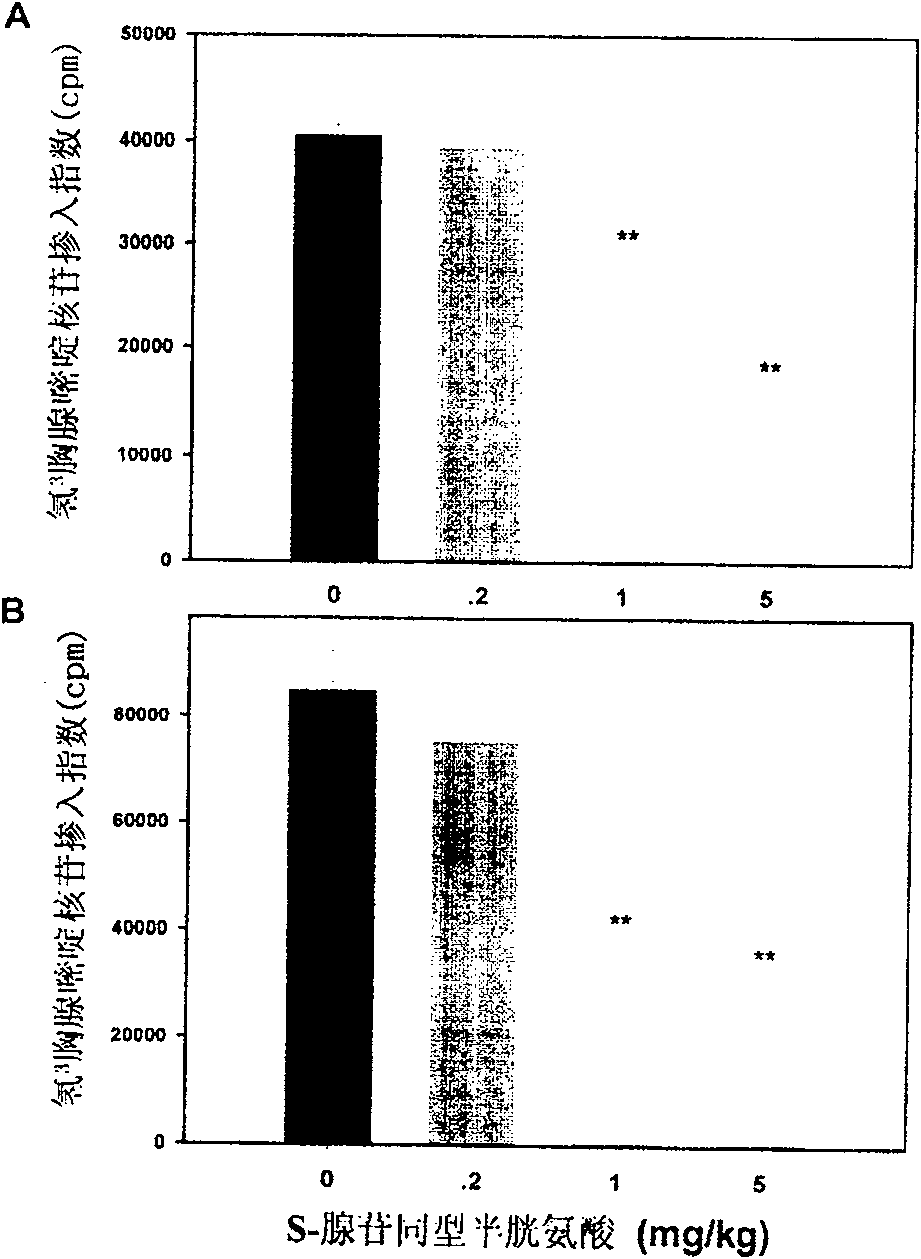
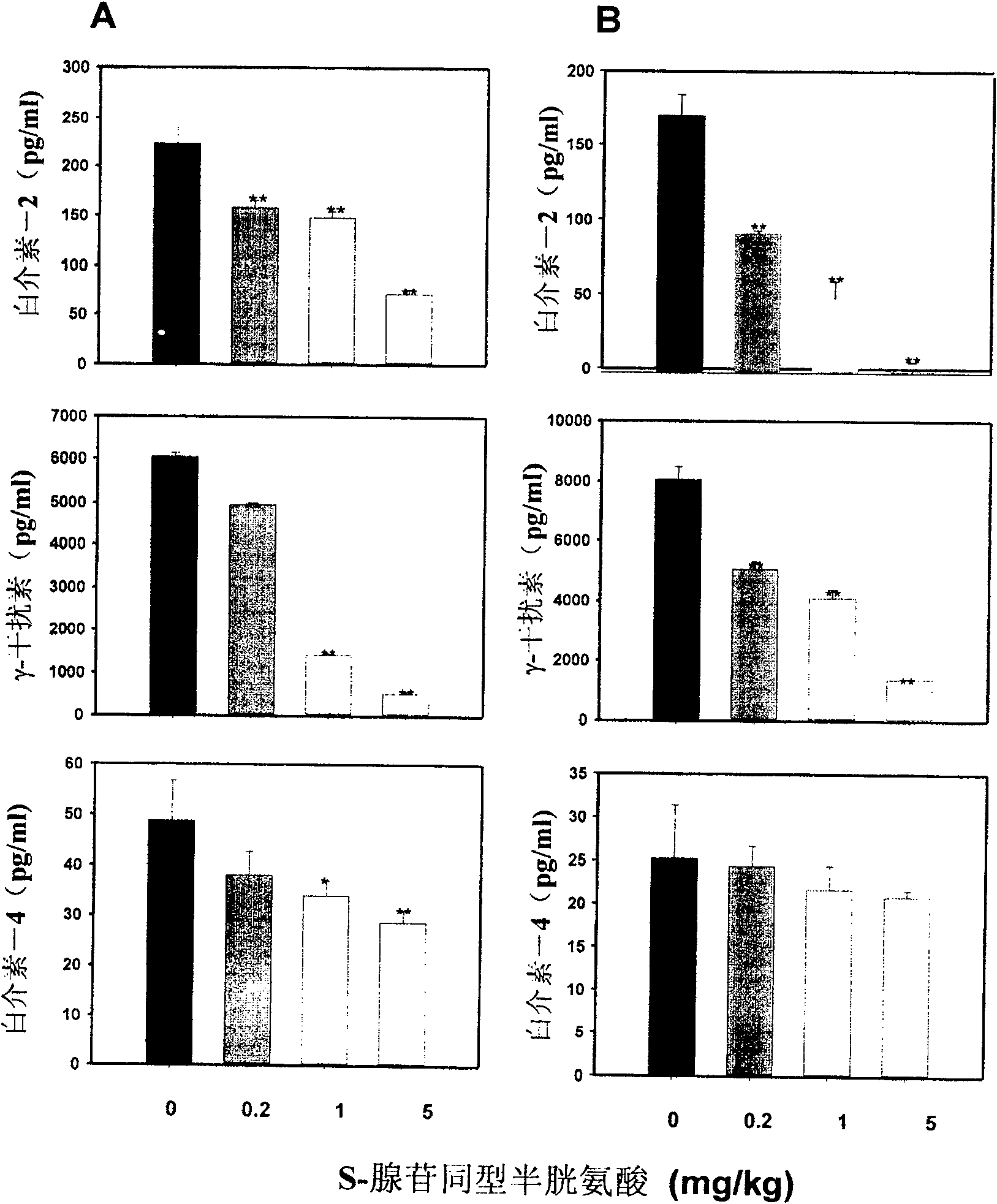
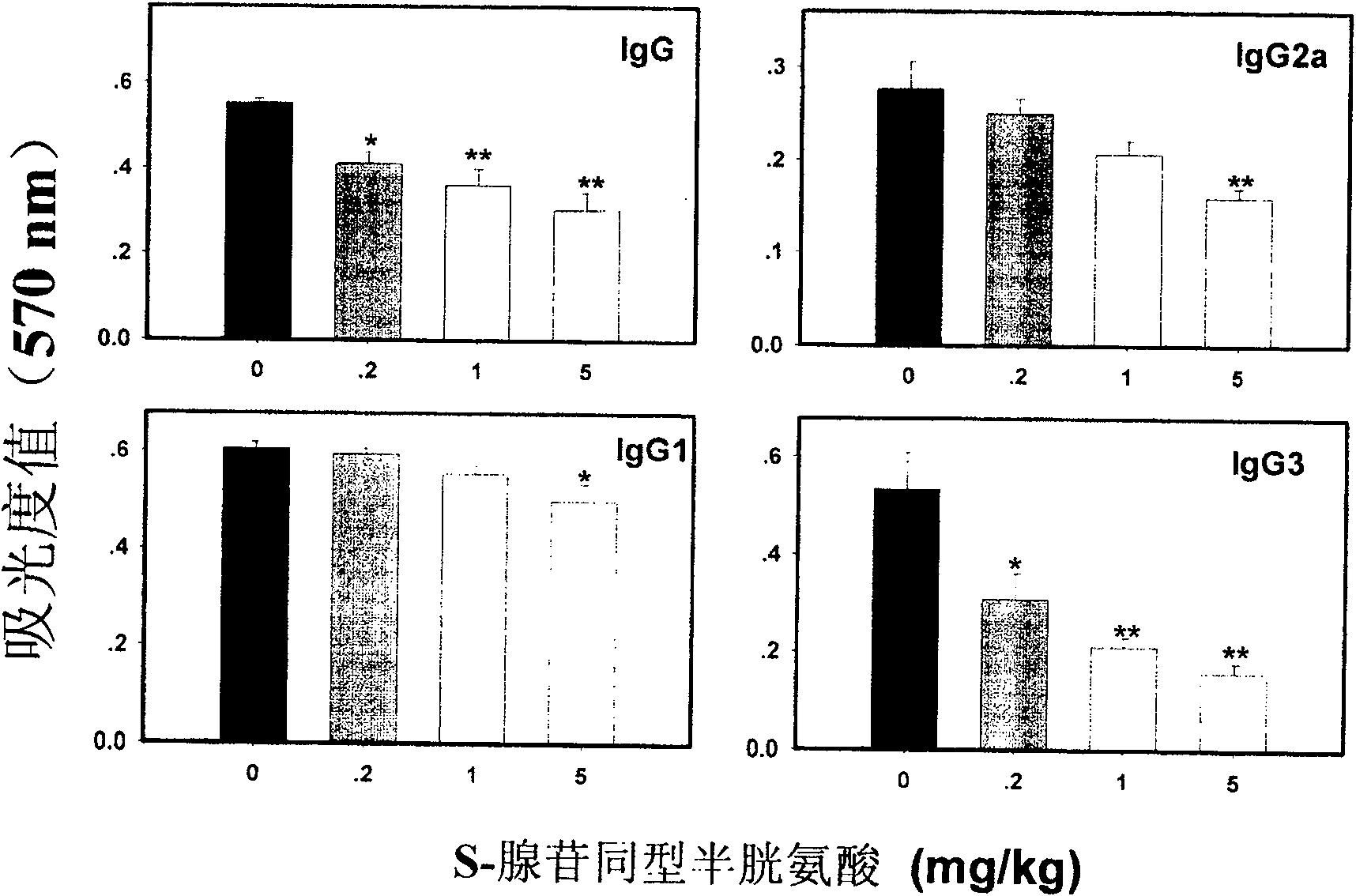
Medical usage of S-adenyhomotype cysteine
A medical application of S-adenosine homocysteine in preparing immunodepressant with high effect and low poison is disclosed.
Owner:NINGBO ZIYUAN PHARMA INC
Chinese traditional medicine compounds for treating Alzheimer's disease and preparing method thereof
InactiveCN101224247ASuppress secondary damageBlock depositionOrganic active ingredientsNervous disorderTreatment effectLate onset
The invention discloses a traditional Chinese medicine composition used for curing alzheimer disease and a preparation method thereof. The traditional Chinese medicine composition is made from the following crude medicines by weight portion: 150-200 portions of gardenia and 4-6 portions of panax notoginseng saponins. The traditional Chinese medicine composition of the invention can effectively inhibit the overproduction of A beta 40 and A beta 42, interdict the sedimentation of amyloid protein, keep stable adjustment of calcium ions and depress oxidative damage and non-specific inflammatory reaction caused by amyloid plaques as well secondary lesion on neurons resulted from metabolic toxins, thus removing the occurrence and development of lesions, relieving delayed neuronal death and cortical atrophy and having obvious treatment effect on alzheimer disease.
Owner:BEIJING UNIV OF CHINESE MEDICINE
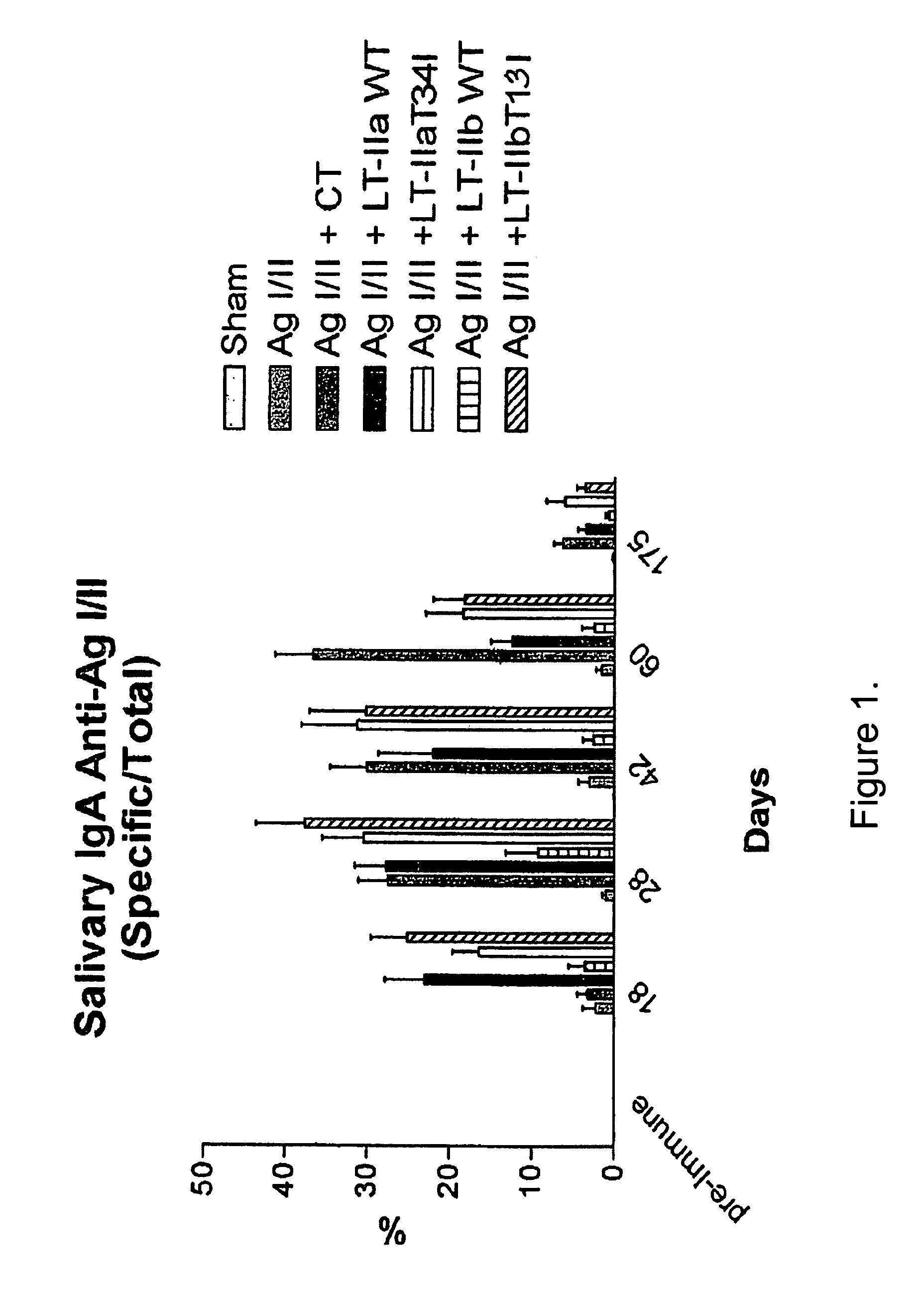
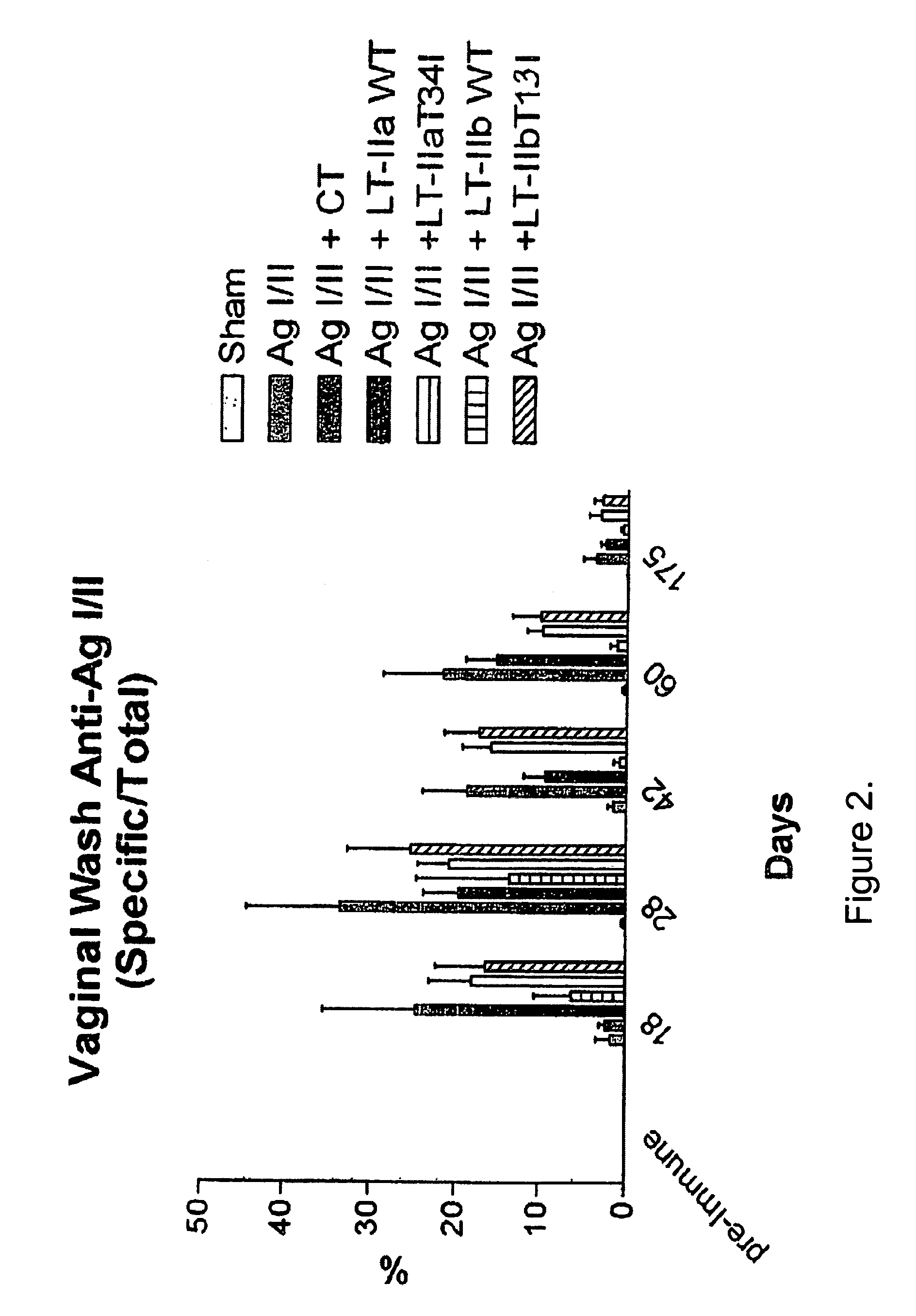
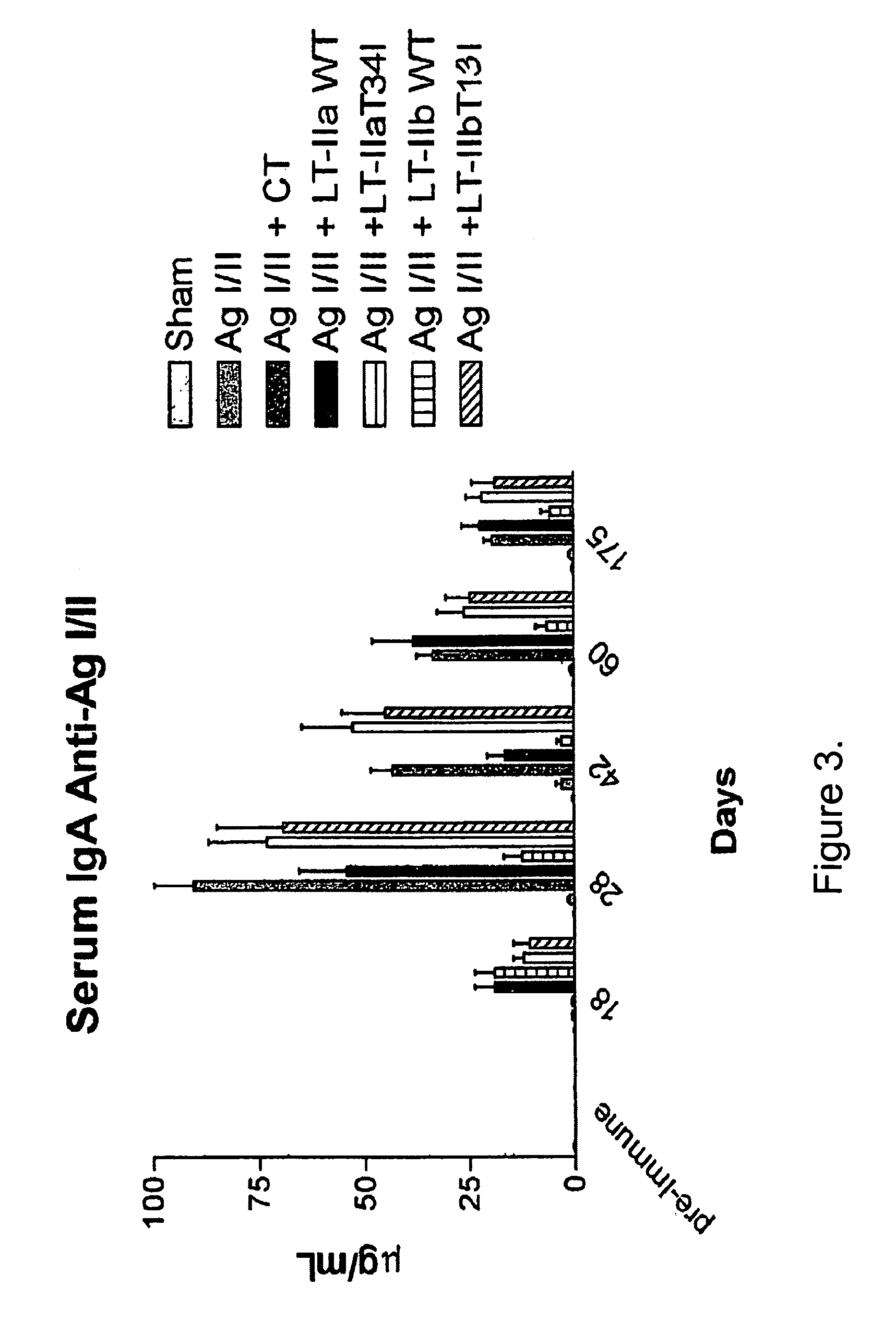
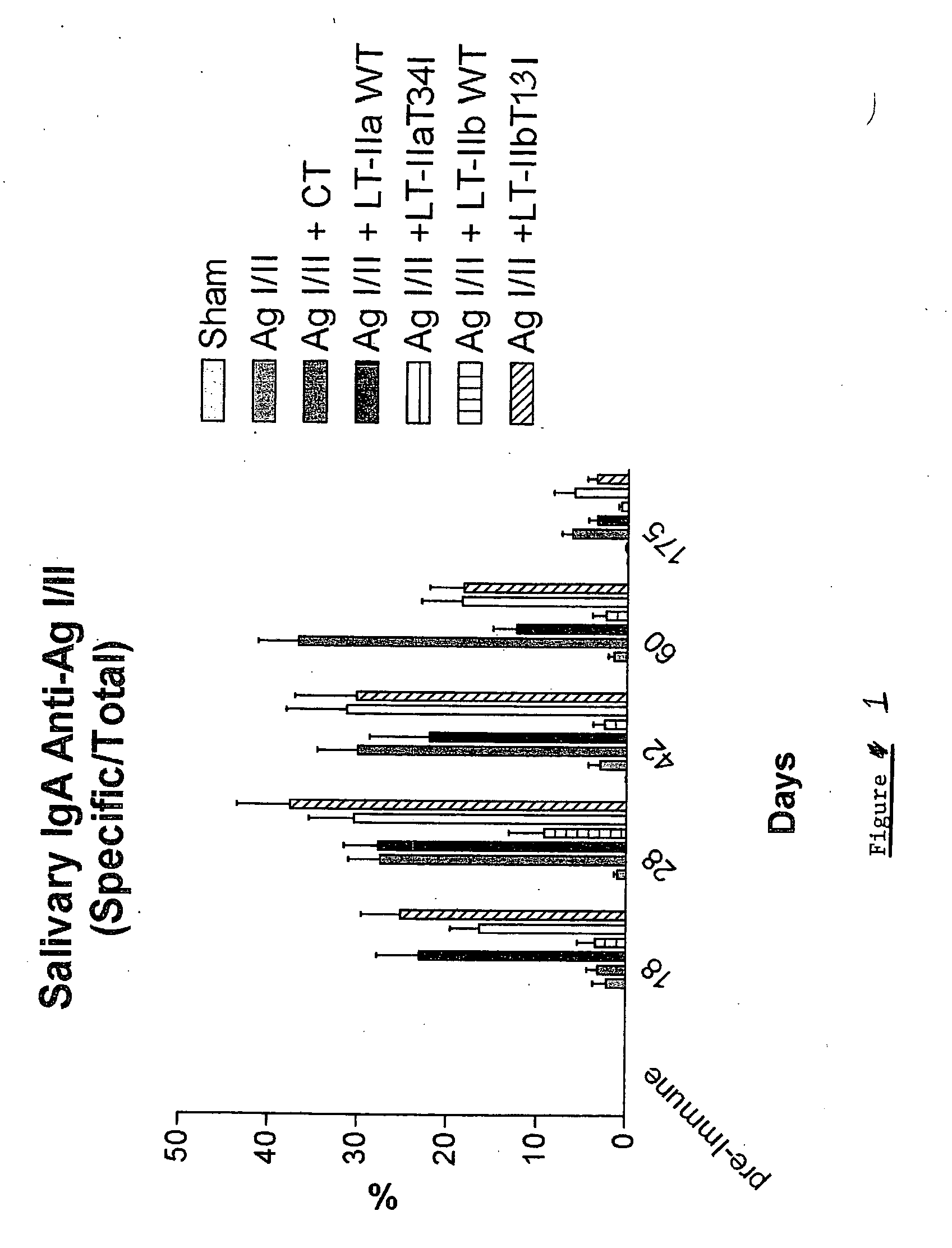
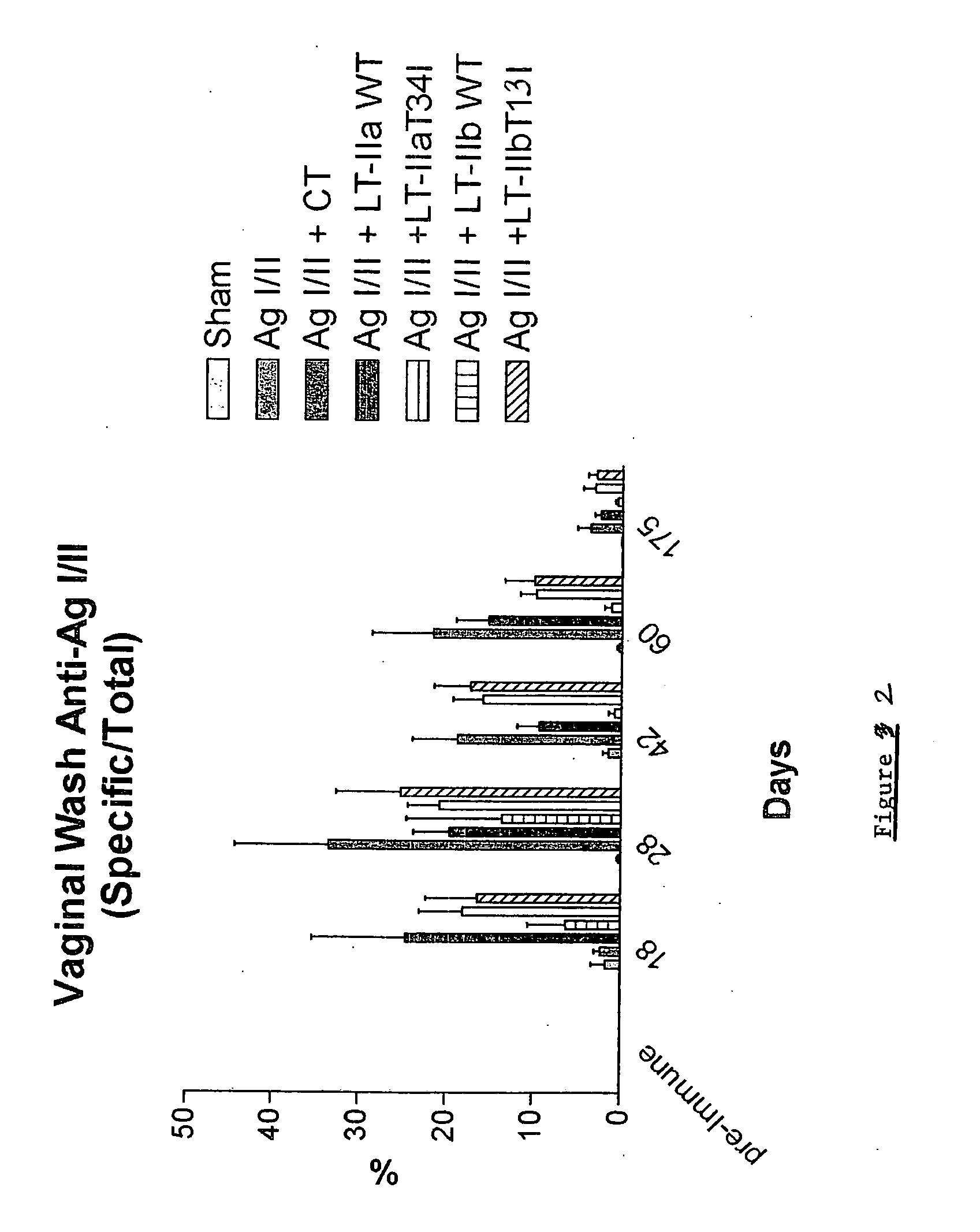
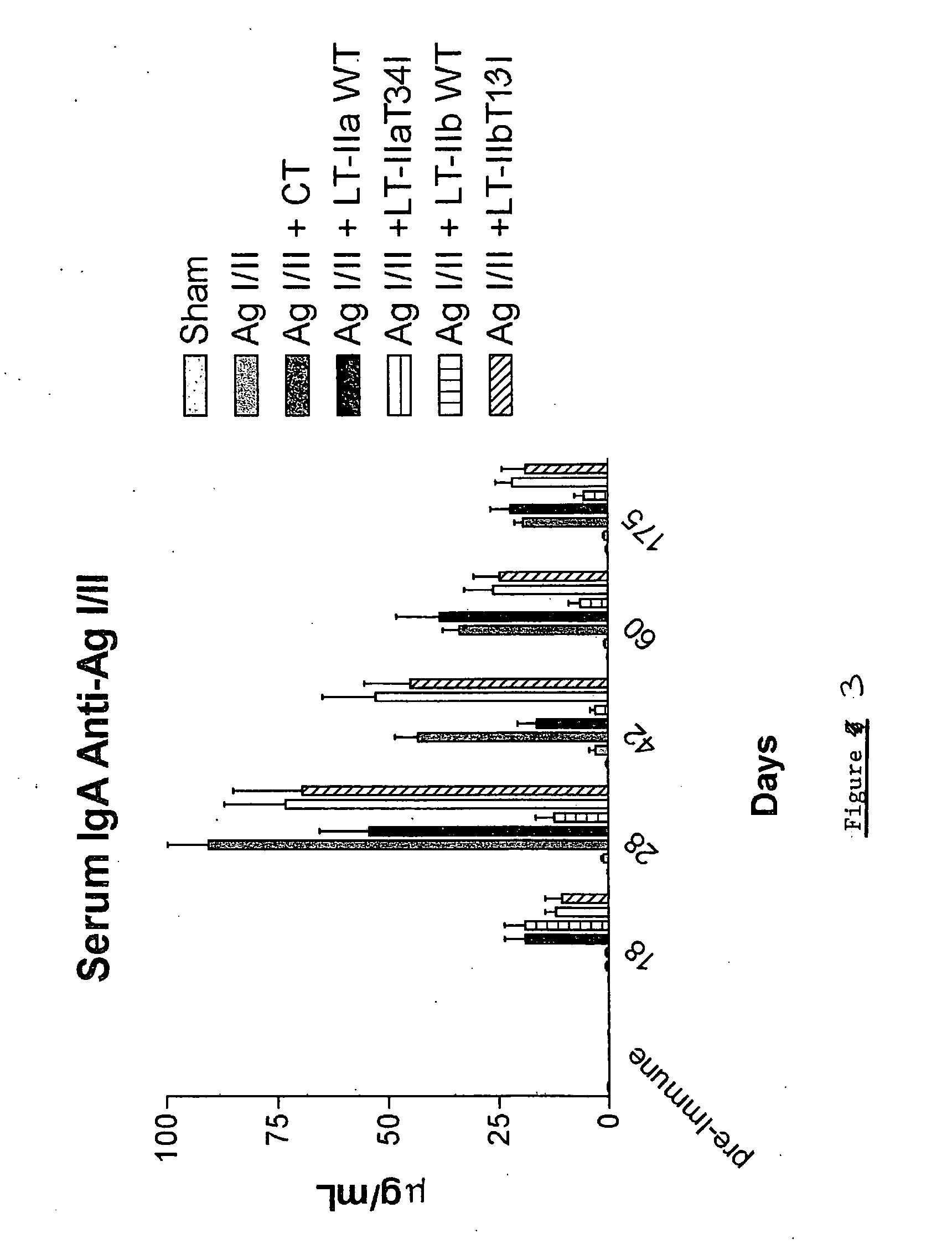
Adjuvant activities of mutants of LT-IIa and LT-IIb enterotoxin lacking binding to ganglioside
InactiveUS7455843B2Strong initial immune responseDelayed “memory” responseBiocideOrganic active ingredientsAntigenAdjuvant
The present invention describes the adjuvant activity of mutants of LT-IIa and LT-IIb enterotoxin which lack ganglioside binding activity. The adjuvant activity of the LT-IIb(T13I) mutant is comparable to that of the wild type LT-IIb. The adjuvant activity of LT-IIa(T34I) mutant is also described which exhibits a late onset adjuvant activity. These mutants are useful for enhancing immune response to antigens.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
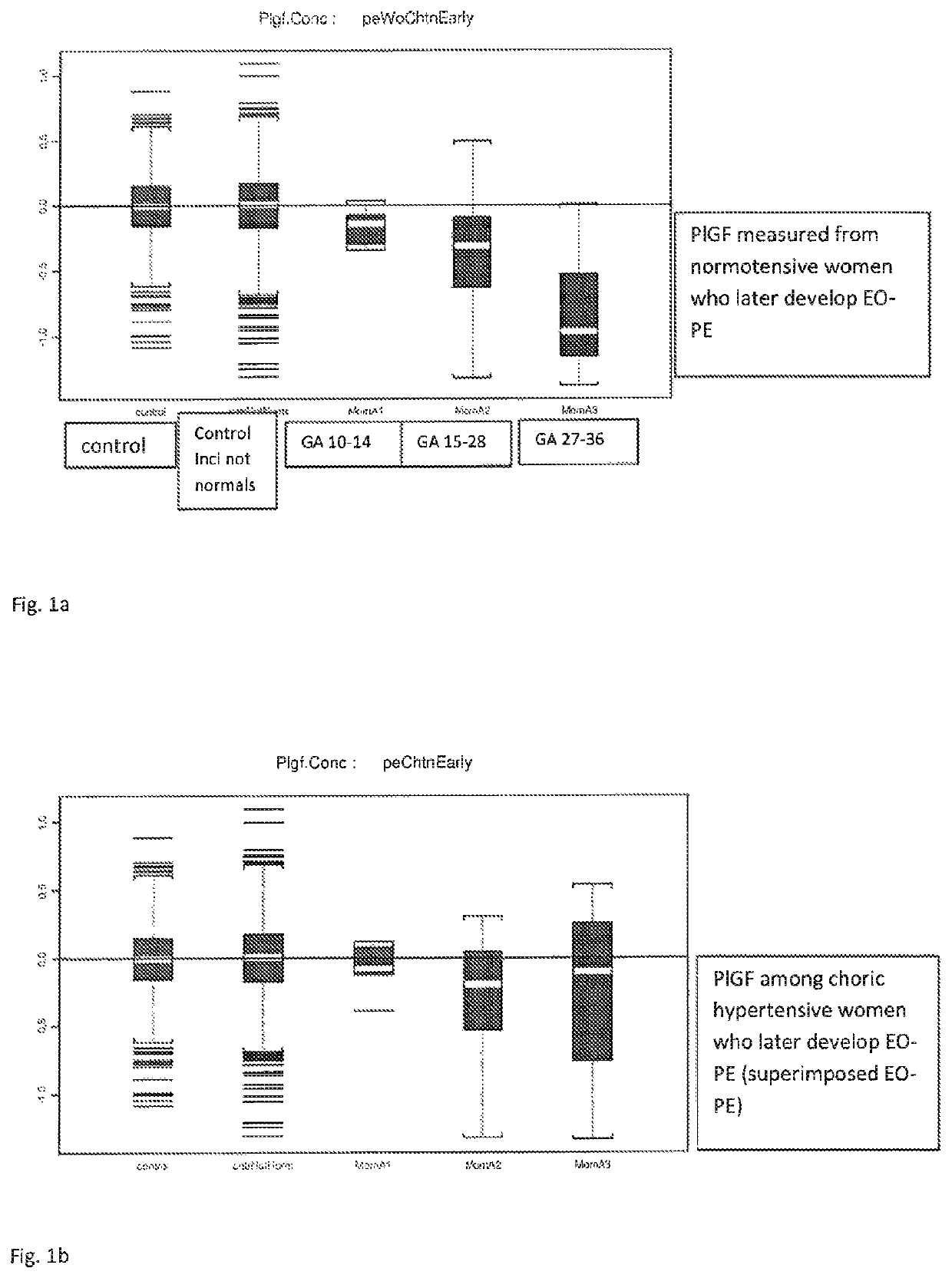
Method for determining risk of pre-eclampsia
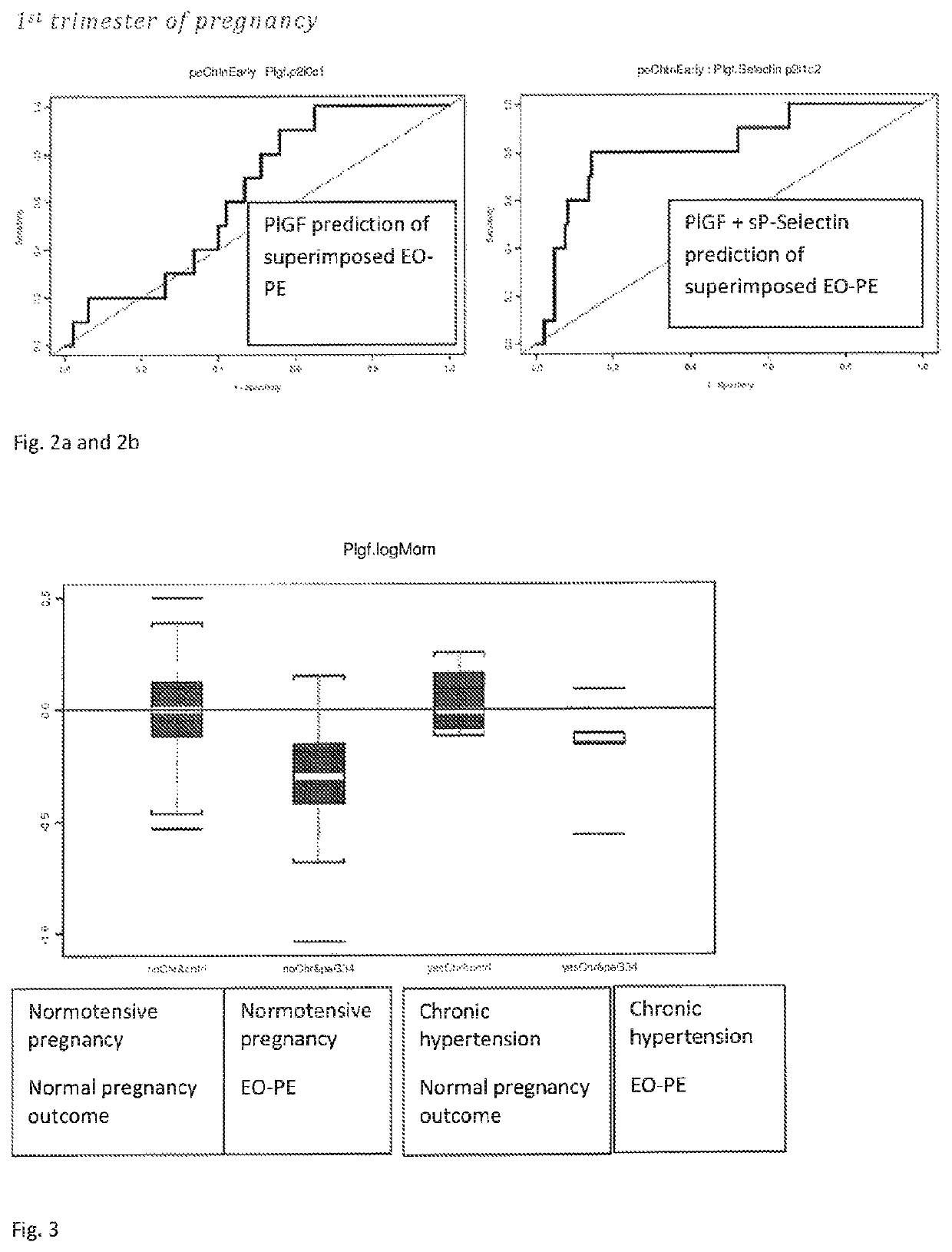
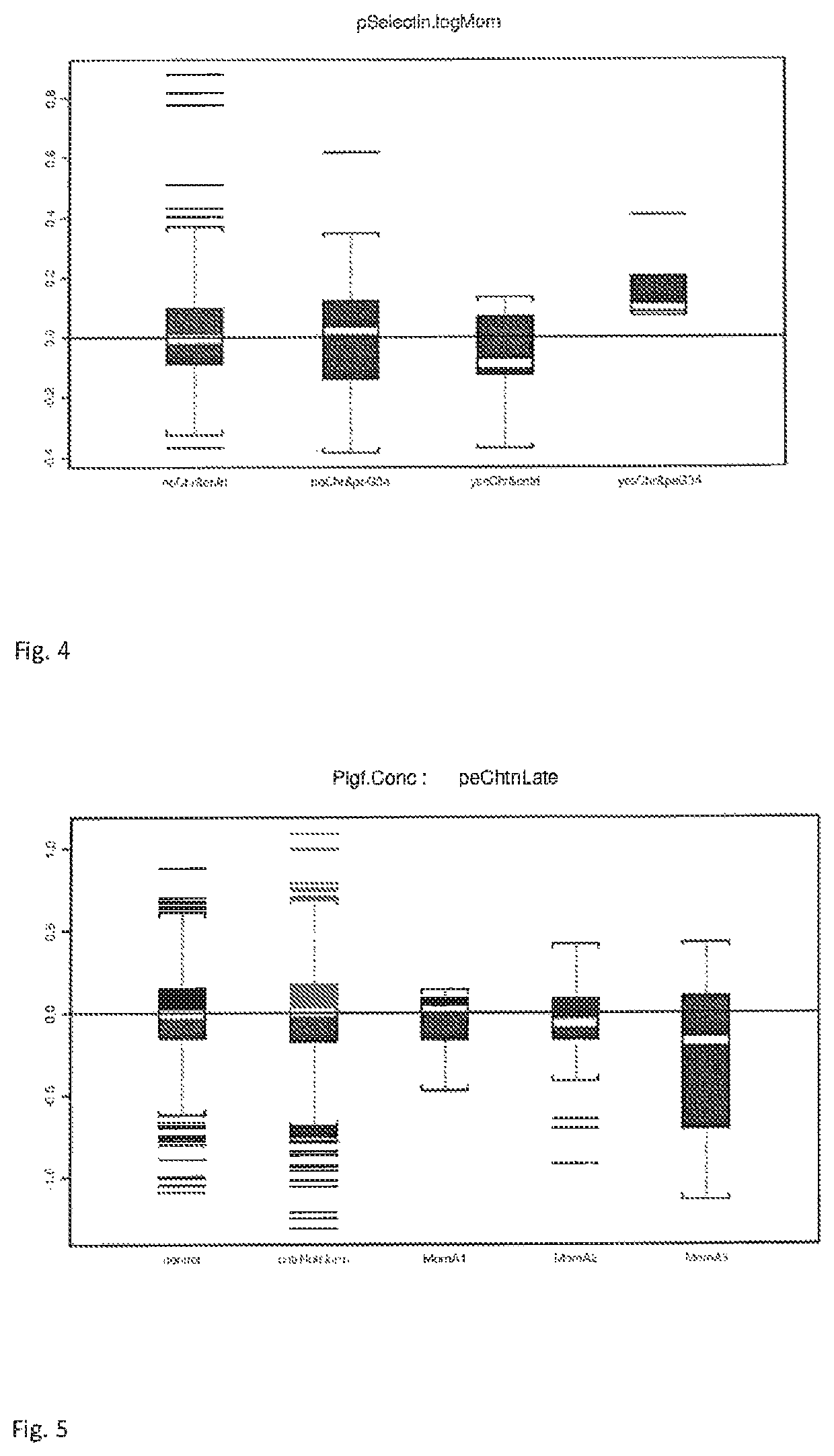
The present description relates to a method for determining the risk of a pregnant woman with chronic hypertension developing early or late onset pre-eclampsia. The present description provides methods useful for determining risk that a pregnant individual with chronic hypertension will develop an early pre-eclampsia or late pre-eclampsia. Useful combination of biochemical markers including PlGF and sP-Selectin and related clinical population studies are described herein.
Owner:WALLAC
Biomarkers for depression
Differential expression of nucleic acids in the brains of subjects suffering from late-onset depression has been demonstrated. The invention provides methods useful in the determination of late-onset depression. Also provided by the present invention is a screening method for the identification of compounds for treatment, prevention or diagnosis of late-onset depression.
Owner:GE HEALTHCARE LTD
Adjuvant activities of mutants of LT-Ila and LT-IIb enterotoxin lacking binding to ganglioside
InactiveUS20050169848A1Strong initial immune responseDelayed “memory” responsePowder deliveryOrganic active ingredientsAntigenAdjuvant
The present invention describes the adjuvant activity of mutants of LT-IIa and LT-IIb enterotoxin which lack ganglioside binding activity. The adjuvant activity of the LT-IIb(T13I) mutant is comparable to that of the wild type LT-IIb. The adjuvant activity of LT-IIa(T34I) mutant is also described which exhibits a late onset adjuvant activity. These mutants are useful for enhancing immune response to antigens.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Methods of diagnosing alzheimer's disease and markers identified by set association
ActiveUS20090249498A1Decreasing and preventing deteriorationAvoid developmentOrganic active ingredientsNervous disorderWhole Genome Association AnalysisLate onset
The present disclosure relates to genetic markers and methods of diagnosing and screening for late-onset Alzheimer's disease (LOAD). As such, the disclosure encompasses a whole-genome association analysis of single nucleotide polymorphisms (SNPs) of which a number are located within the GRB2-associated binding protein 2 (GAB2) gene as well as other markers associated with other genes. The disclosure identifies two novel haplotypes within the GAB2 gene, i.e., a LOAD risk-enhancing and a LOAD risk-decreasing haplotype. These haplotypes modify LOAD risk differentially in combination with APOE alleles. Further encompassed are therapeutic methods and agents of decreasing the deterioration of cells associated with LOAD.
Owner:TRANSLATIONAL GENOMICS RESEARCH INSTITUTE +1
Method for the identification of genes involved in neurodegenerative processes
InactiveUS20110214191A1Progressive arrhythmicityReduced expression levelNervous disorderMicrobiological testing/measurementLate onsetSleep wake
A method for the identification of genes involved in neurodegenerative processes, detectable by the late onset of a phenotype associated with neurodegeneration, by means of a genetic screen of deregulated genes, which comprises the measurement of sleep-wake cycle activity schemes in different stages of life, young and adult, of individuals of an animal model, such as Drosophila. A mutant fly whose genome comprises a disruption in its enabled gene, with decrease of the enabled gene expression, and exhibiting a late onset neurodegenerative phenotype in adulthood.
Owner:INIS BIOTEC LLC +2
Method for predicting the risk of late-onset alzheimer's diseases
InactiveUS20210230696A1Easy diagnosisImproved prognosisMicrobiological testing/measurementLate onsetOncology
A method for prognosis, risk assessment, risk stratification and / or diagnosis of a subject of developing late-onset Alzheimer's disease (LOAD), includes providing at least one sample isolated from the subject, and determining in the presence of ApoE4 and either rs1799931(G) or rs8192506(A), rs7653308(C), rs968529(C) and rs9658265(A). The presence or absence of these markers is indicative of a prognosis, a risk and / or a diagnosis of developing LOAD.
Owner:BIOTX AI GMBH
Method of treating gaucher disease
Therapeutic compositions and methods for treatment of late-onset Gaucher disease are described herein. The compositions comprise compounds having activity as pharmacological chaperones for mutant forms of the beta-glucocerebrosidase. Methods of treatment involve providing therapeutically effective amounts of such compositions to subjects in need thereof.
Owner:HOSPITAL FOR SICK CHILDREN +1
Application of S-adenyhomotype cysteine in medical preparation
Owner:NINGBO ZIYUAN PHARMA INC
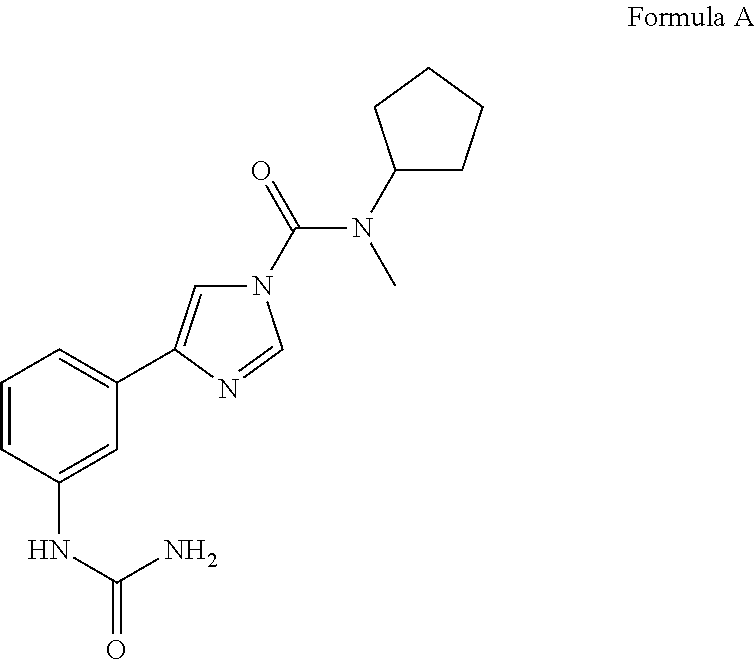
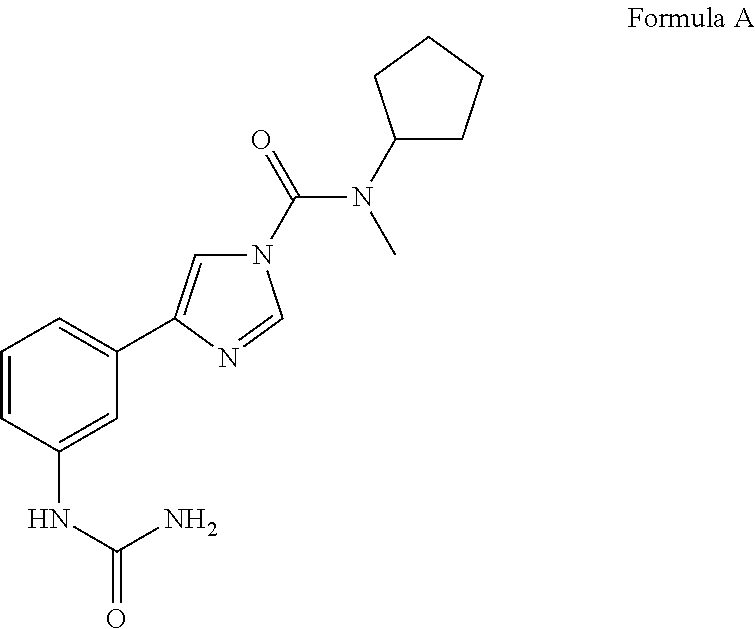
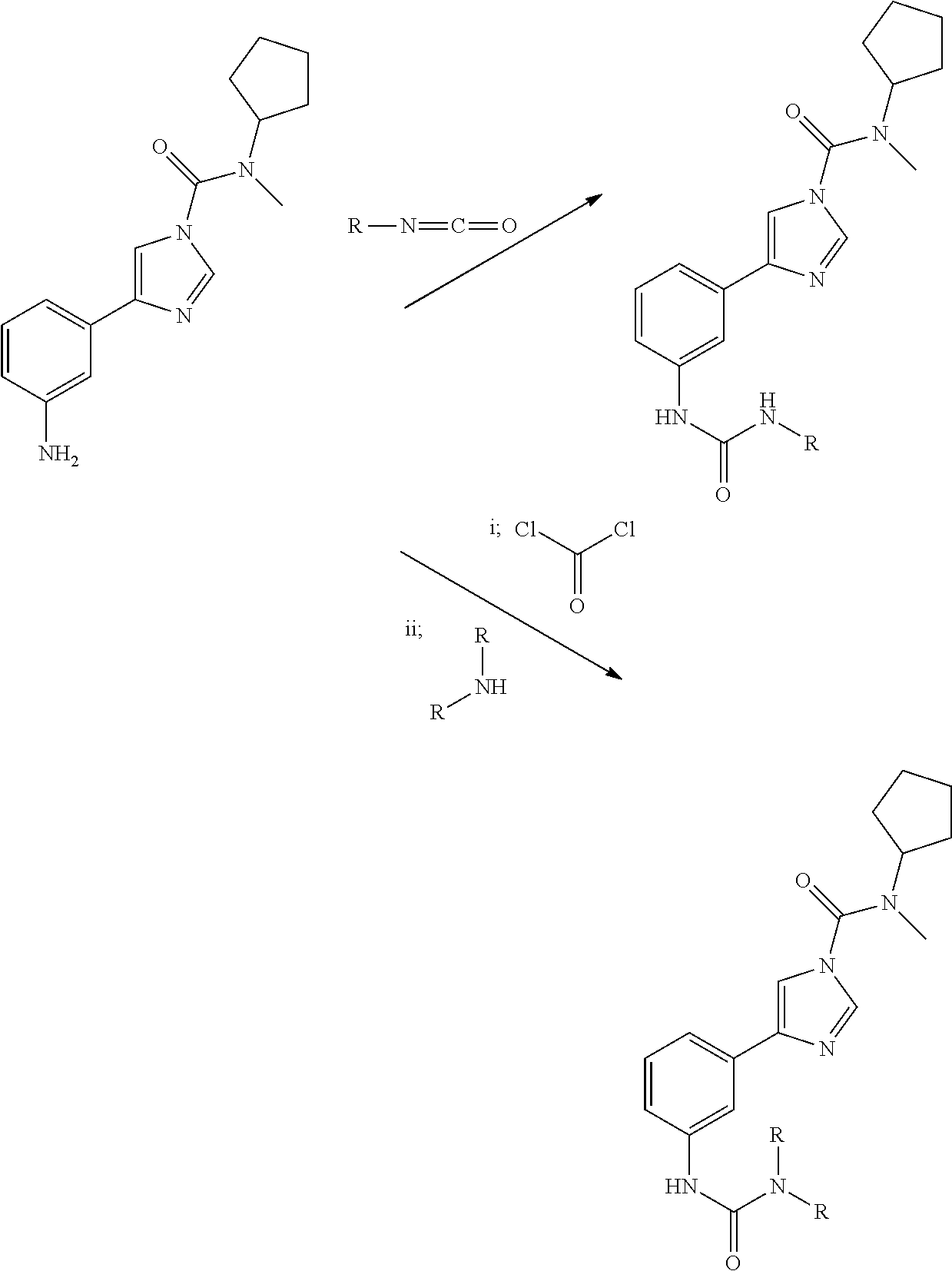
Urea compounds and their use as enzyme inhibitors
ActiveUS20150174103A1High yieldGood qualityBiocideOrganic active ingredientsLevodopa-induced dyskinesiaAsthma
A compound having the following structure:or a pharmaceutically acceptable salt or derivative thereof. The compound may be used in the treatment or prevention of a disorder selected from appetite regulation, obesity, metabolic disorders, cachexia, anorexia, pain, inflammation, neurotoxicity, neurotrauma, stroke, multiple sclerosis, spinal cord injury, Parkinson's disease, levodopa-induced dyskinesia, Huntington's disease, Gilles de la Tourette's syndrome, tardive dyskinesia, dystonia, amyotrophic lateral sclerosis, Alzheimer's disease, epilepsy, schizophrenia, anxiety, depression, insomnia, nausea, emesis, alcohol disorders, drug addictions such as opiates, nicotine, cocaine, alcohol and psychostimulants, hypertension, circulatory shock, myocardial reperfusion injury, atherosclerosis, asthma, glaucoma, retinopathy, cancer, inflammatory bowel disease, acute and chronic liver disease such as hepatitis and liver cirrhosis, arthritis and osteoporosis.
Owner:BIAL PORTELA & CA SA
Tricyclic diterpene-2-methylpyrimidine analogues, preparation method and application thereof
ActiveCN111362882AHigh activityMild reaction conditionsOrganic chemistrySexual disorderHaloform reactionLate onset
The invention discloses tricyclic diterpene-2-methylpyrimidine analogues represented by formulas (X) and (I)-(IV), and a preparation method thereof, wherein a target product is prepared through IBX oxidation, Aldol condensation, substitution, cyclization, haloform reaction, hydrolysis, condensation and other reactions. The invention also provides an application of the tricyclic diterpene-2-methylpyrimidine analogue in resisting late onset hypogonadism (LOH). The invention also relates to a new mechanism, namely an autophagy mechanism, of the tricyclic diterpene-2-methylpyrimidine analogue in LOH treatment so as to provide a beneficial reference for research in resisting late onset hypogonadism, wherein the good application prospect is achieved.
Owner:EAST CHINA NORMAL UNIVERSITY
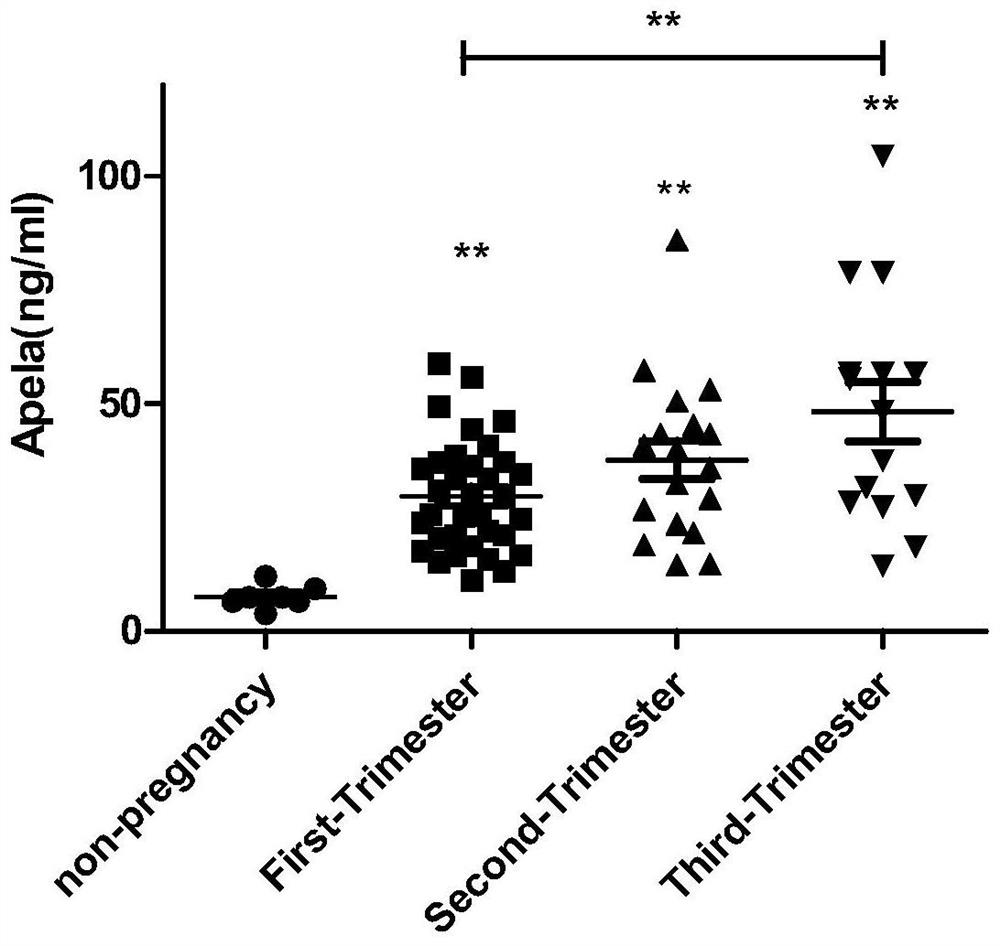
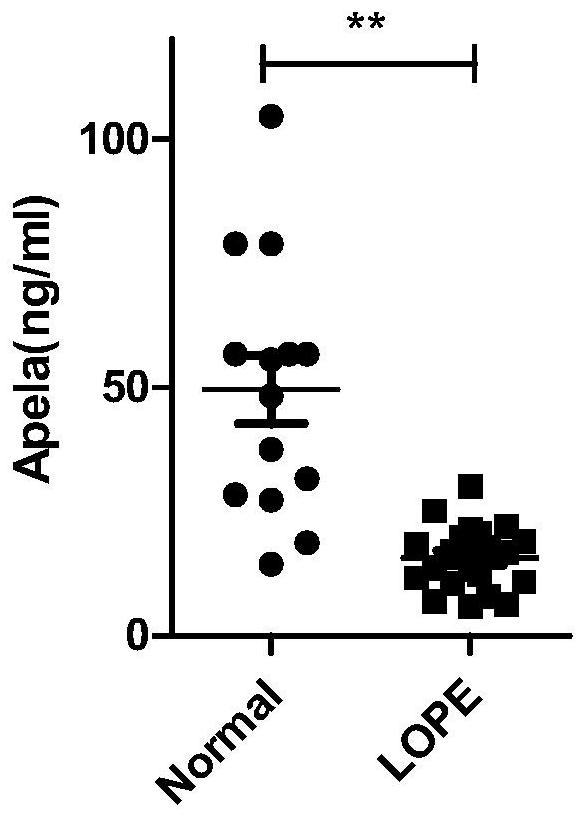
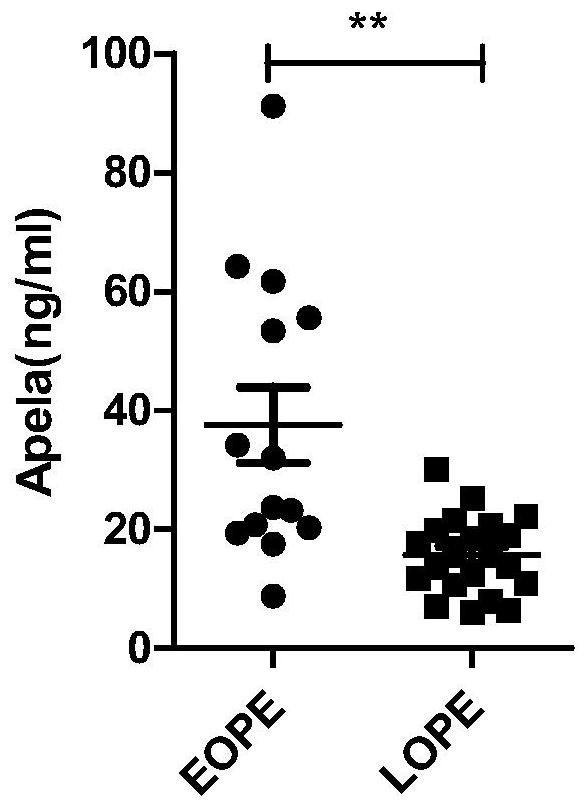
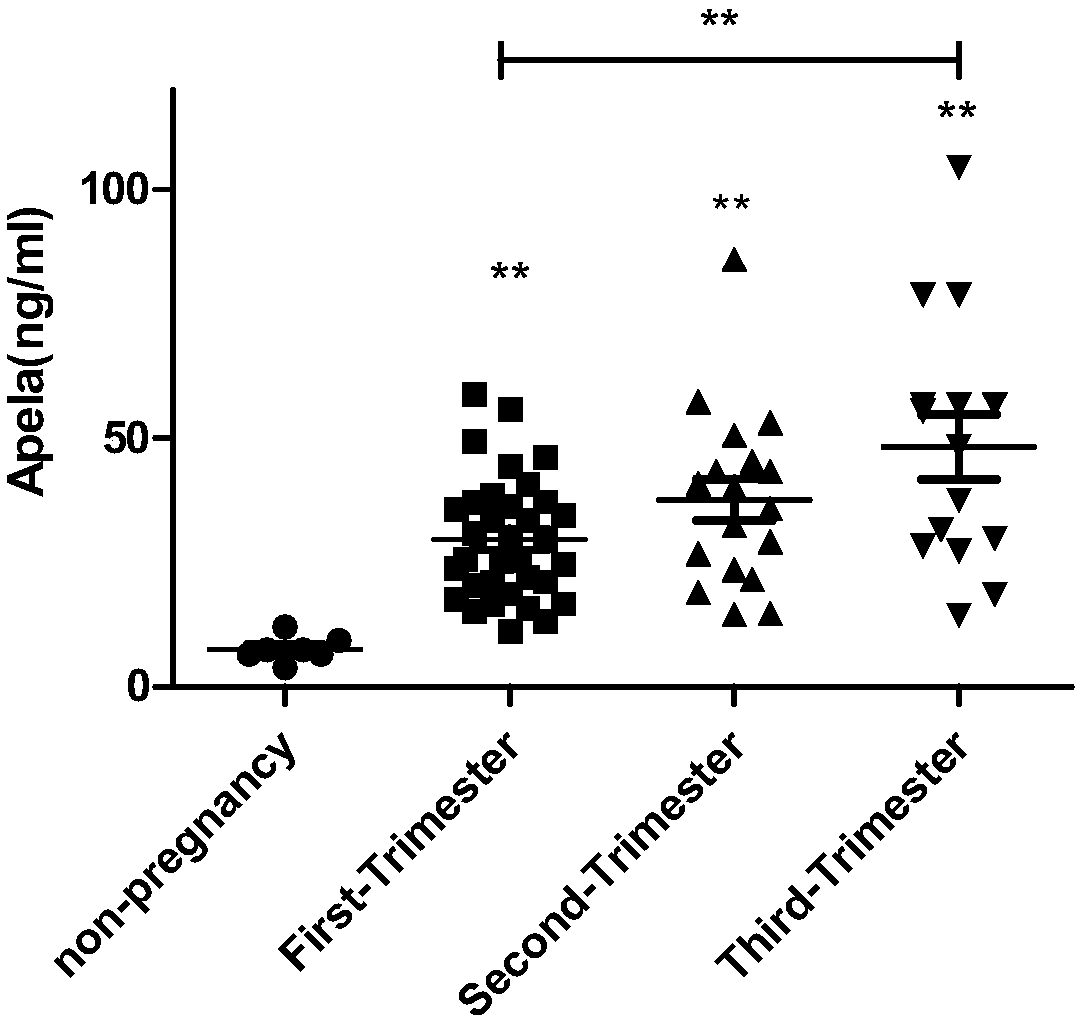
Test strips and kits for rapid screening of late-onset preeclampsia
ActiveCN108776226BReduce concentrationQuick screeningDisease diagnosisBiological testingObstetricsAids diagnostics
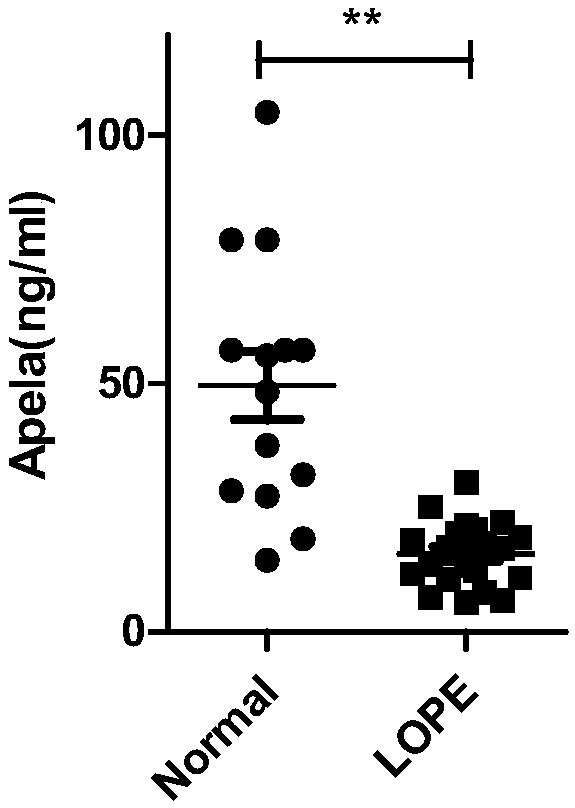
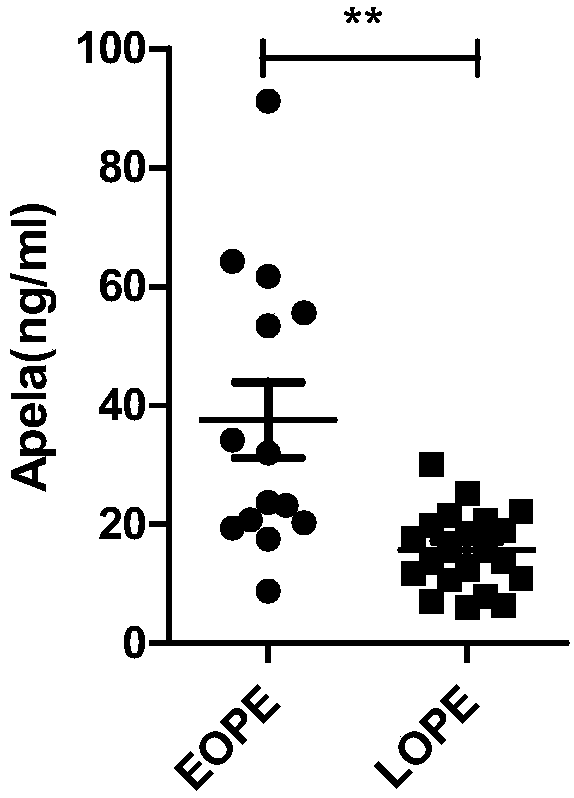
The invention discloses test paper for quickly screening late onset severepre-eclampsia. The test paper for quickly screening late onset severepre-eclampsia comprises optional test paper for detectingthe APELA concentration in blood or urine. The invention also discloses a kit for screening late onset severepre-eclampsia. The kit comprises an optional reagent for detecting the APELA concentrationin blood or urine. The invention also discloses application of the test paper for detecting the APELA concentration in blood or urine in preparing the test paper for screening late onset severepre-eclampsia. The invention also discloses application of the reagent for detecting the APELA concentration in blood or urine in preparing a reagent for screening late onset severepre-eclampsia. Accordingto the test paper and the kit for quickly screening late onset severepre-eclampsia disclosed by the invention, only blood and urine of a patient need to be acquired, and whether the patient is suffered from late onset severepre-eclampsia or not can be screened, so that the test paper and the kit can be used for noninvasively, effectively and quickly examining and screening late onset severepre-eclampsia, and are convenient to use and favorable in clinic application prospect.
Owner:邓成
Test paper and kit for quickly screening late onset severepre-eclampsia
ActiveCN108776226AReduce concentrationQuick screeningDisease diagnosisBiological testingPatient needBacteriuria
The invention discloses test paper for quickly screening late onset severepre-eclampsia. The test paper for quickly screening late onset severepre-eclampsia comprises optional test paper for detectingthe APELA concentration in blood or urine. The invention also discloses a kit for screening late onset severepre-eclampsia. The kit comprises an optional reagent for detecting the APELA concentrationin blood or urine. The invention also discloses application of the test paper for detecting the APELA concentration in blood or urine in preparing the test paper for screening late onset severepre-eclampsia. The invention also discloses application of the reagent for detecting the APELA concentration in blood or urine in preparing a reagent for screening late onset severepre-eclampsia. Accordingto the test paper and the kit for quickly screening late onset severepre-eclampsia disclosed by the invention, only blood and urine of a patient need to be acquired, and whether the patient is suffered from late onset severepre-eclampsia or not can be screened, so that the test paper and the kit can be used for noninvasively, effectively and quickly examining and screening late onset severepre-eclampsia, and are convenient to use and favorable in clinic application prospect.
Owner:邓成
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com