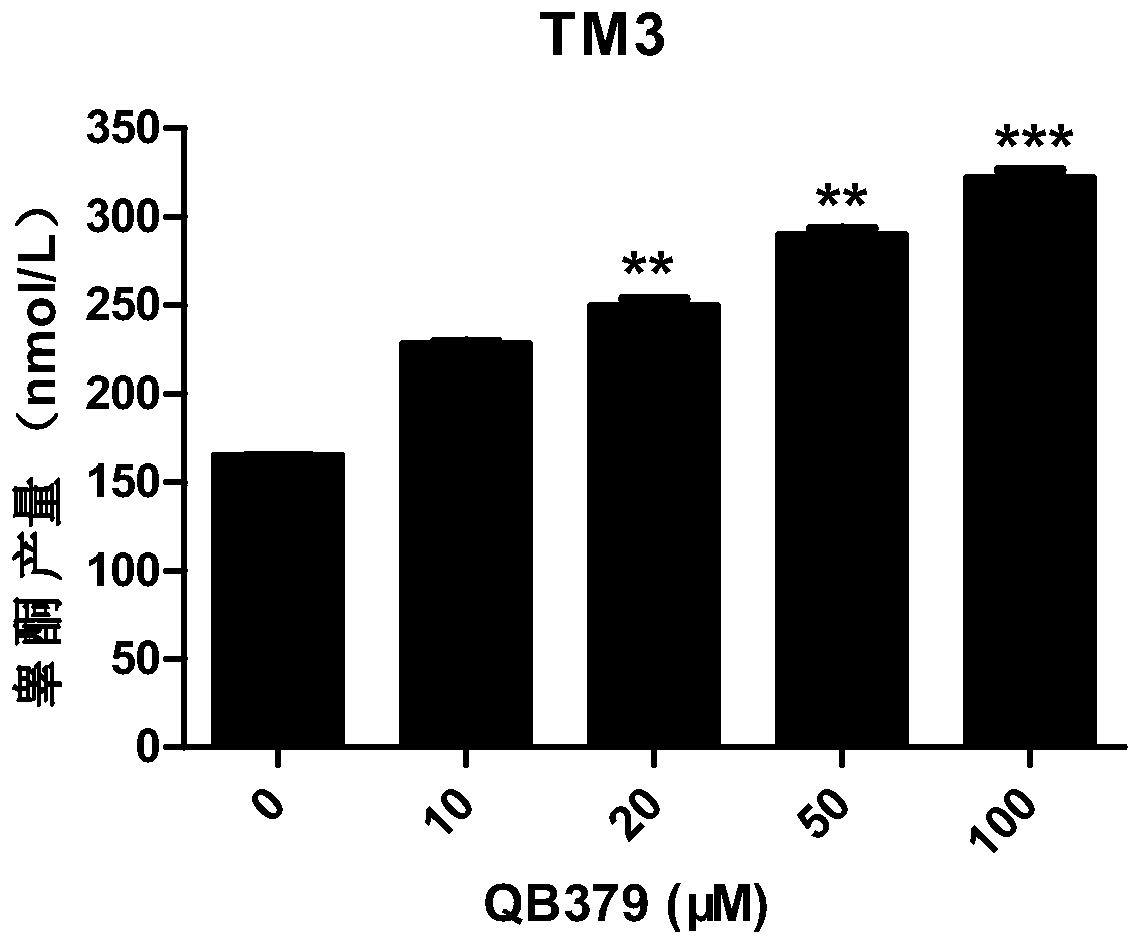
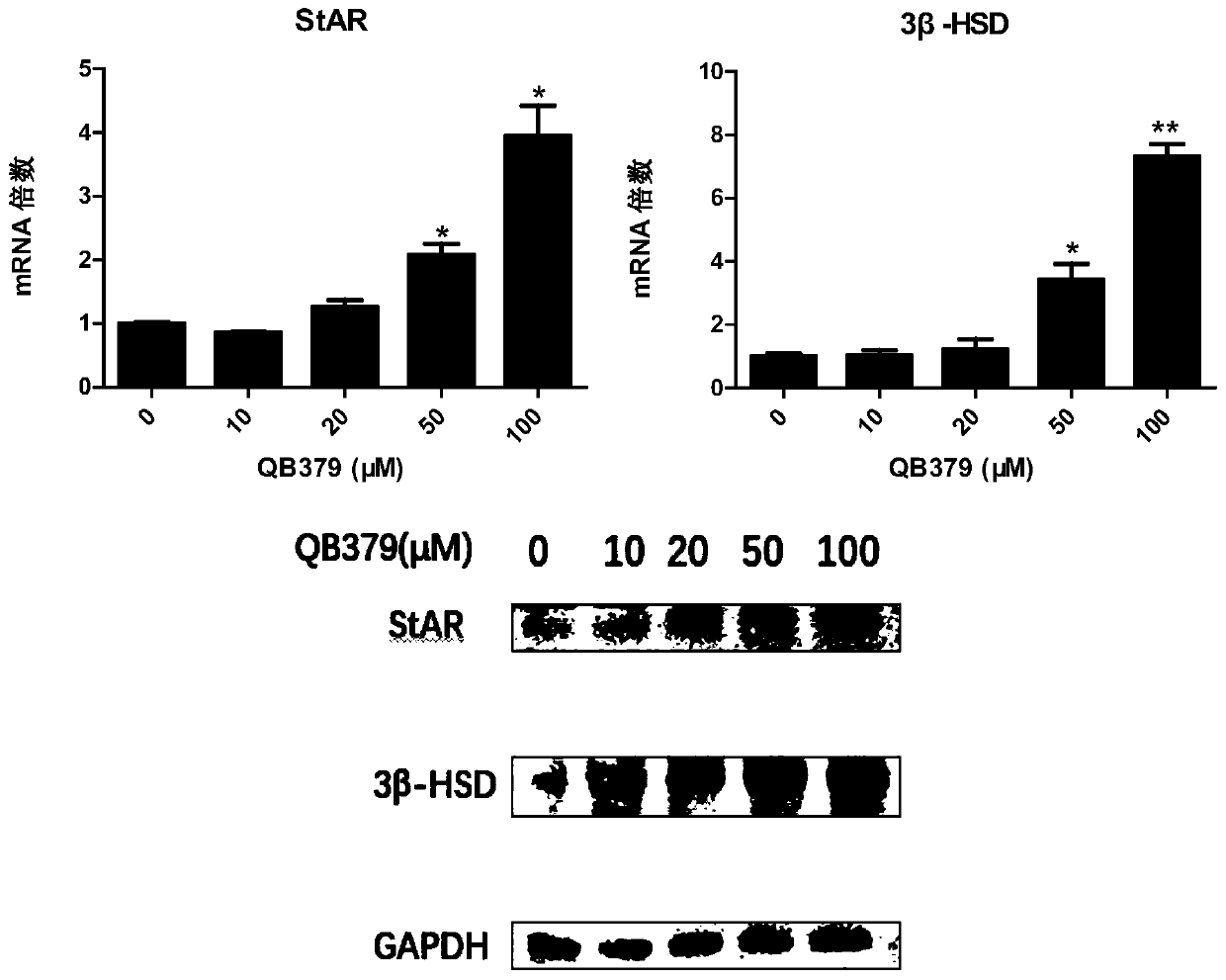
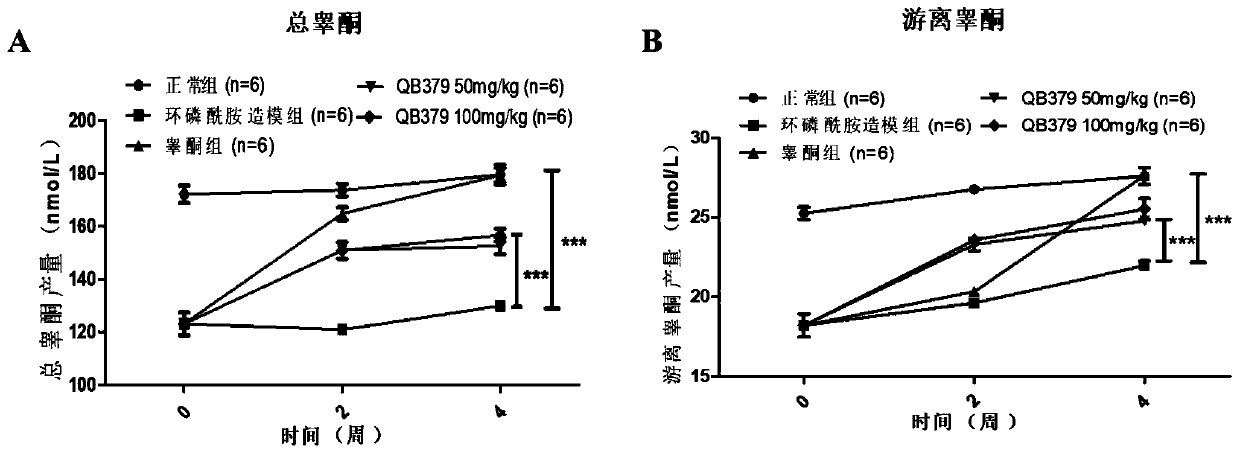
Tricyclic diterpene-2-methylpyrimidine analogues, preparation method and application thereof
A technology of methyl pyrimidine and tricyclic diterpene is applied in the field of tricyclic diterpene and 2-methylpyrimidine analogs and their preparation, and can solve the problems of low serum testosterone level, decreased sperm count, azoospermia and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0264] The preparation of embodiment 1 compound 9
[0265]
[0266] Compound 4 (10 g, 36.5 mmol) was dissolved in 100 mL of anhydrous THF, IBX (12.3 g, 43.8 mmol) was added, DMSO (50 mL) was added, and the reaction was carried out at 40° C. for 12 h. After the reaction is complete, add 200 mL of water to the system, add EA (50 mL) to stir, pad diatomaceous earth for suction filtration, wash the filter residue repeatedly with EA until no product remains, separate the filtrate, combine the EA phases, and wash with 1M sodium hydroxide solution. Anhydrous Na 2 SO 4 Drying and distillation under reduced pressure gave crude product 6 as a white solid, which was purified by column chromatography with PE:EA=5:1 to give pure compound 6 (8.9 g white solid, yield 90%).
[0267] Compound 6 (8.9g, 32.7mmol) was dissolved in 80mL of ethyl formate, placed in an ice-salt bath, sodium hydride (7.8g, 327.0mmol) was slowly added, a huge amount of heat was released, slowly added dropwise, vi...
Embodiment 2
[0270] The preparation of embodiment 2 compound 10-1-10-8
[0271]
[0272] Compound 9 (2g, 6.211mmol) was dissolved in anhydrous DCM. After cooling the system to 0°C, anhydrous aluminum chloride (2.5g, 18.634mmol) was added, and then the corresponding acid chloride (12.422mmol) was added dropwise. After the dropwise addition, the reaction was continued at 0° C. for 2 hours, and the TLC raw material was completely reacted. Slowly add ice water to quench (huge exotherm, slow dropwise, vigorous stirring). After liquid separation, the aqueous phase was extracted with DCM several times, and the organic phases were combined, washed with saturated NaCl, anhydrous NaCl 2 SO 4 After drying, the crude product was obtained by distillation under reduced pressure. The crude product was purified by silica gel column chromatography (PE:EA=10:1→5:1) to obtain compound 10-1-10-8 (white solid, 70-88%).
[0273] Compound 10-1, white solid, yield 70%. 1 H NMR (400MHz, Chloroform-d) δ8.39(s...
Embodiment 3
[0281] The preparation of embodiment 3 compound 11
[0282]
[0283]Compound 9 (258mg, 0.8mmol) was dissolved in dichloromethane (30mL), then liquid bromine (25mg, 0.4mmol) and p-toluenesulfonic acid (0.4mL, 8mmol) were added under ice-cooling, and the reaction was carried out under nitrogen protection for 2 hours. TLC detects that the reaction is complete, the reaction solution is poured into 100 ml of water, extracted with ethyl acetate (3 × 15mL), the organic phases are combined, washed with saturated brine, dried over anhydrous sodium sulfate, spin-dried in vacuo, and subjected to silica gel column chromatography (PE: EA=5:1) to obtain compound 11 (white solid, 244 mg, yield 76%). 1 HNMR (400MHz, CDCl 3 )δ8.39(s,1H),7.29(s,1H),6.89(s,1H),3.93(s,3H),3.15(d,J=15.5Hz,1H),2.94–2.73(m,3H ),2.71(s,3H),2.01–1.94(m,1H),1.88–1.82(m,1H),1.80–1.72(m,1H),1.37(s,3H),1.34(s,3H), 1.15(s,3H). 13 C NMR (101MHz, CDCl 3 )δ172.10,166.07,157.62,154.19,146.27,133.41,129.53,123.40,109.46...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com