Test strips and kits for rapid screening of late-onset preeclampsia
A preeclampsia, late-onset technology, applied in biological testing, instrumentation, biomaterial analysis, etc., can solve problems such as non-occurrence, and achieve the effect of convenient use, good clinical application prospects, and rapid screening.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
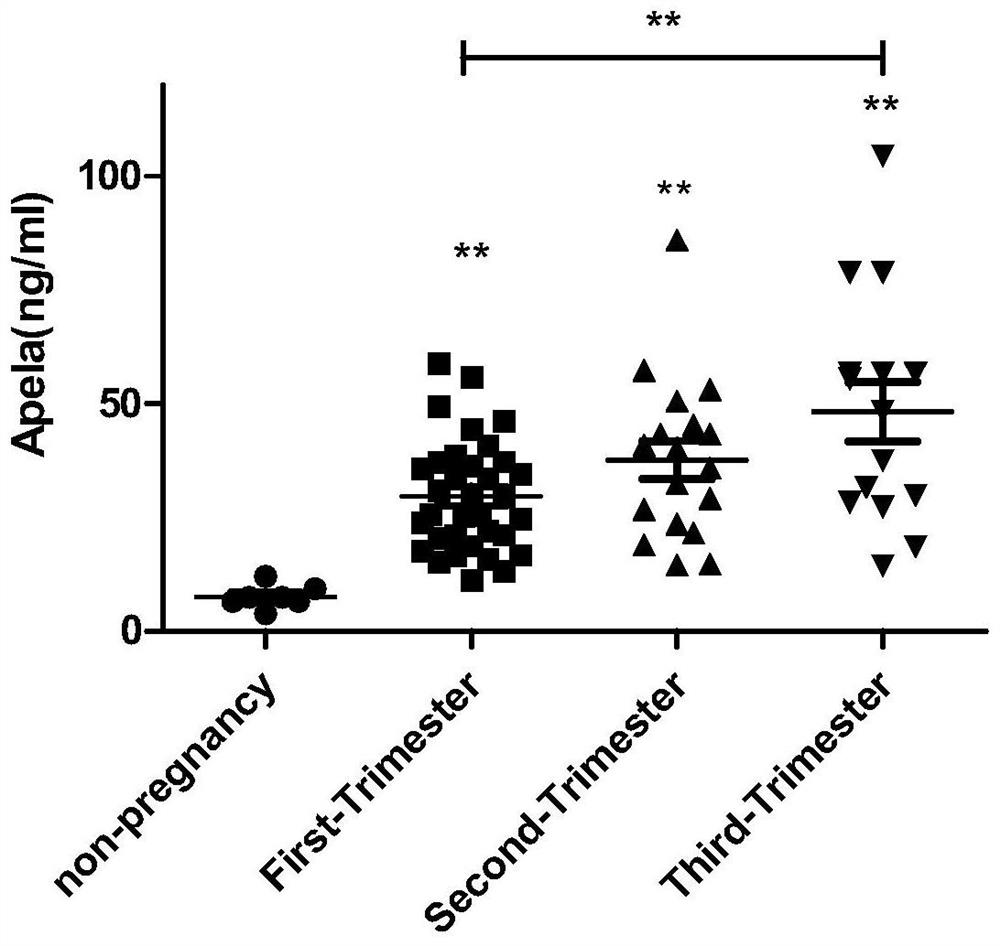
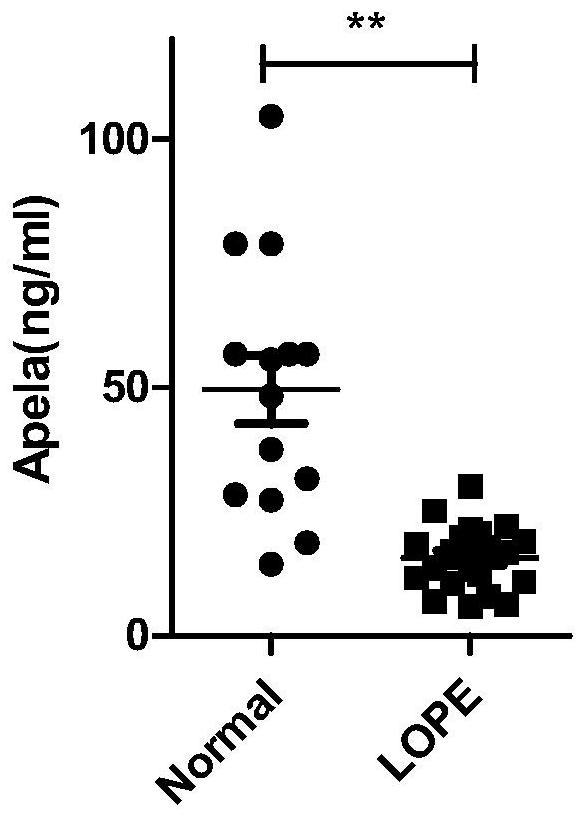
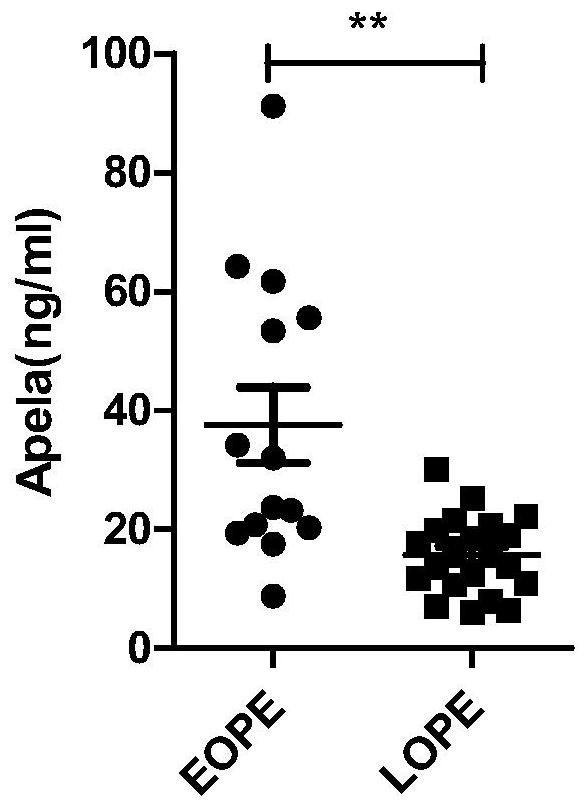
[0028] Example 1 Comparison of APELA concentration in the blood of non-pregnant women, normal pregnant women, early PE pregnant women and late PE pregnant women
[0029] 1. Samples: 7 non-pregnant women, 34 normal pregnant women in the early pregnancy, 18 normal pregnant women in the second trimester, 15 normal pregnant women in the late pregnancy, 21 pregnant women with late PE, and 14 pregnant women with early PE; the above-mentioned pregnant women with late PE and early PE All pregnant women were diagnosed as late PE or early PE by chief obstetricians and gynecologists with more than 15 years of clinical experience, with high reliability.
[0030] 2. The detection method of APELA concentration in the blood of the subject:
[0031] (1) Serum extraction
[0032] Take 2-3mL of blood from the vein, without adding anticoagulant, place at room temperature for 30min, centrifuge at 2500rpm for 5min, transfer the serum to a new EP tube, and store at -80°C after aliquoting.
[0033...
Embodiment 2
[0064] Example 2 Contrast of APELA concentration in urine of non-pregnant women, normal pregnant women and late PE pregnant women
[0065] 1. Samples: 2 non-pregnant women, 7 normal pregnant women in the early pregnancy, 7 normal pregnant women in the second trimester, 8 normal pregnant women in the late pregnancy, and 4 pregnant women in the late PE; The chief physician of obstetrics and gynecology with clinical experience diagnosed late PE or early PE with high reliability.
[0066] 2. The detection method of APELA concentration in the subject's urine:
[0067] Fresh urine samples were centrifuged at 2500rpm at 4°C for 15-20min, and the supernatant was kept at -20 or -80.
[0068] APELA in urine was detected with Human ELA ELISA kit (Phoenix Pharmaceuticals, Inc. Cat. No. EK-007-19). Specific steps are as follows:
[0069] ① Add 50 μL of standard, urine sample and positive control to the 96 wells of the pre-coated secondary binding protein, and then add 25uL of primary bi...
Embodiment 3
[0085] Embodiment 3 The composition and method of use of the kit for detecting the concentration of APELA in the blood or urine of pregnant women according to the present invention
[0086] 1. The composition of the kit
[0087] Detection kit (50 persons)
[0088] components volume secondary binding protein APELA standard 2500uL primary binding protein 1250uL Biotin-labeled peptides 1250uL Assay Buffer 52500uL DE SA-HRP solution 5000uL Substrate solution 5000uL Stop solution (2N HCl) 5000uL
[0089] 2. How to use the kit
[0090] (1) Serum extraction
[0091] Venous blood or urine 2-3mL, without anticoagulant, placed at room temperature for 30min, centrifuged at 2500rpm for 5min, then transferred serum or urine to a new EP tube, aliquoted and stored at -80°C.
[0092] (2) ELISA detection
[0093] Specific steps are as follows:
[0094] ① Add 50uL of standard, blood or urine sample and positive contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com