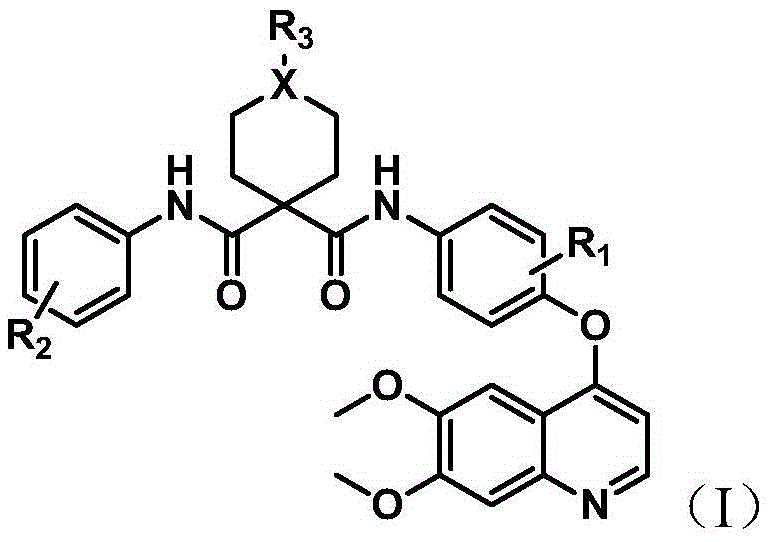
Quinoline multi-target kinase inhibitor with antitumor activity and preparation method thereof
A technology of anti-tumor activity, quinolines, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
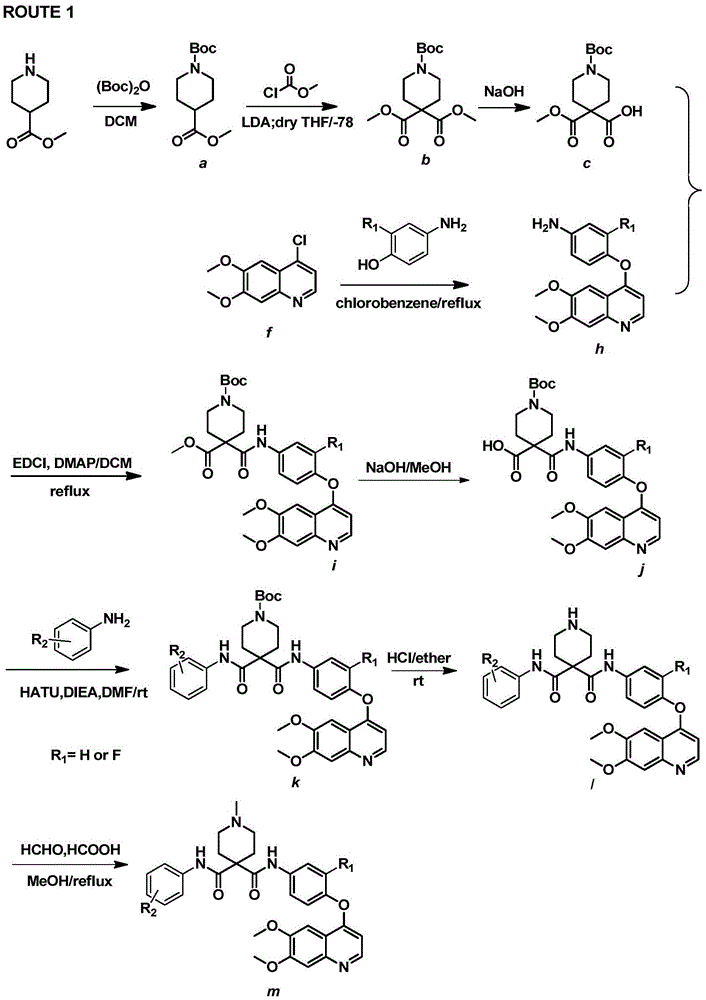
[0116] Embodiment 1: According to ROUTE1 synthetic intermediate quinoline aniline h
[0117] (1) Synthesis of compound 4-((6,7-dimethoxyquinolin-4-yl)oxy)-3-fluoroaniline compound (h)
[0118] Add NaH (3.3g, 82.5mmol) and anhydrous DMSO into a 100ml round-bottomed flask, stir well, add p-aminophenol (6g, 55mmol), stir at room temperature for 10min, then add 4-chloro-6,7-di Methoxyquinoline (12.3g, 55.1mmol) was then stirred at 100°C for 3h and monitored by TLC. After the reaction was complete, water was added, extracted with chloroform, and the extract was evaporated to dryness to obtain 12.2g of a gray solid with a yield of 74.6%. Spectral data is: 1 HNMR (300MHz, CDCl 3 )δ8.50(d,J=5.5Hz,1H),7.64(d,J=7.0Hz,1H),7.48(s,1H),7.09–6.97(m,2H),6.86–6.76(m,2H ), 6.48 (d, J=5.3Hz, 1H), 4.09 (s, 6H), 3.7 (s, 2H).
Embodiment 2
[0119] Embodiment 2: Synthesize target compound 1 and m according to ROUTE1
[0120] (1) Synthesis of compound 1-tert-butyl-4-methylpiperidine-1,4-dicarboxylate (a)
[0121] Add 4-methyl piperidine formate (30g, 0.21mol) into a 1L round bottom flask, dissolve in 500ml of DCM, add (Boc) dropwise with a constant pressure dropping funnel 2 O (45.72g, 0.21mol), triethylamine (42.3g, 0.42mol), stirred at room temperature, and after 8 hours, after the reaction was complete, an equal volume of water was added to wash the DCM layer 3 times, and the colorless oil in the organic phase was evaporated to dryness 97.5 g, yield 95.5%. Spectral data is: 1 HNMR (300MHz, CDCl 3 )δ4.05–3.83(m,2H)3.68–3.56(m,3H),2.76(t,J=11.6Hz,2H),2.45–2.30(m,1H),1.80(dd,J=13.4,2.9 Hz, 2H), 1.64–1.47 (m, 2H), 1.38 (s, 9H).
[0122] (2) Synthesis of compound 1-tert-butyl-4,4-dimethylpiperidine-1,4,4-tricarboxylate (b)
[0123] Under nitrogen protection, add 90ml of dry THF and compound a (10g, 41mmol) into...
Embodiment 3
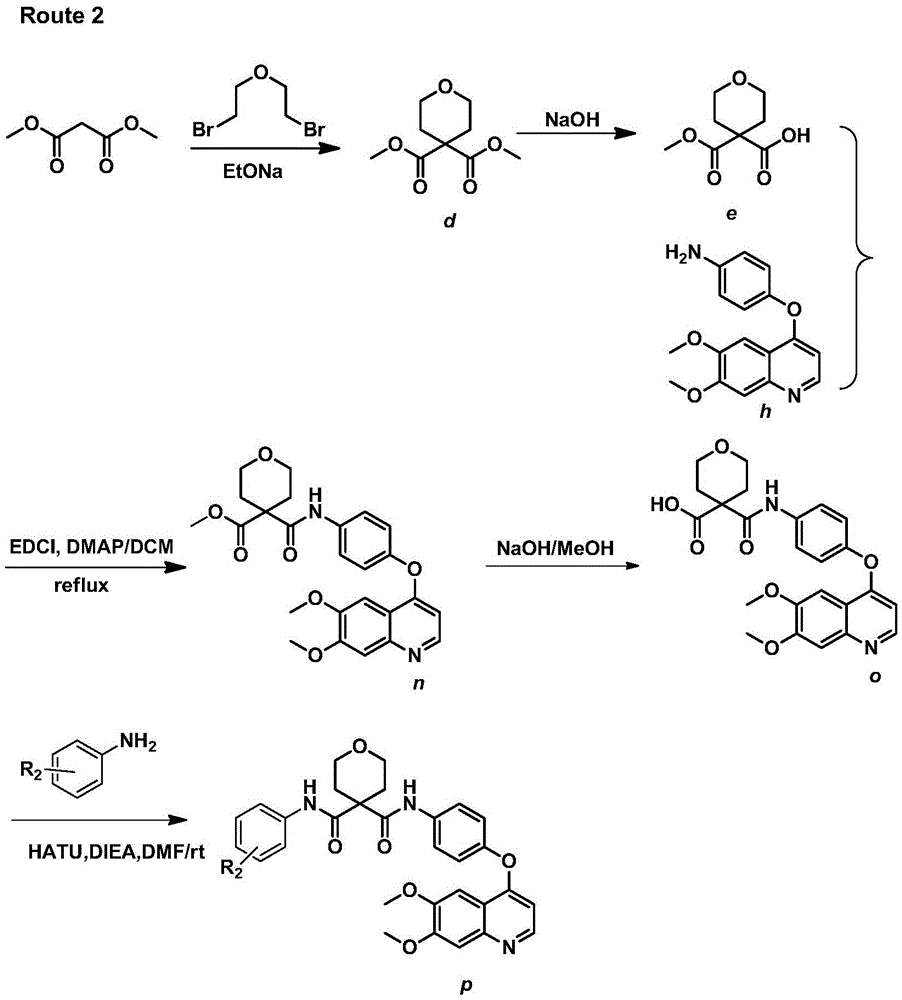
[0188] Embodiment 3: Synthesize target compound p according to ROUTE2
[0189] (1) Synthesis of compound dimethyl-2H-pyran-4,4(3H)-dicarboxylate (d)
[0190] Add absolute ethanol 400ml and sodium block (10.8g, 0.47mol) in 1L three-necked round bottom flask, stir in ice bath, after the sodium block dissolves completely, slowly add dimethyl malonate (30g, 0.23mol), stir After 20 minutes, dibromoethyl ether (57.9g, 0.25mol) was added dropwise and heated to reflux for 32 hours, monitored by TLC. After the reaction of the raw materials was completed, most of the ethanol was evaporated and water was added, and the pH of the aqueous phase was adjusted to 3 with dilute hydrochloric acid solution. Ethyl ester extraction, the organic phase was evaporated to dryness to a brownish-red oily substance, and a colorless oily substance was obtained by distillation under reduced pressure of an oil pump at 150°C, and then 21 g of a colorless oily substance was obtained by passing through a chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com