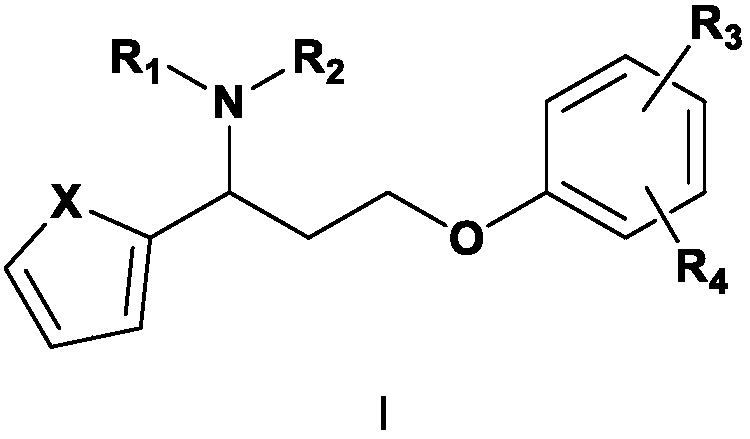
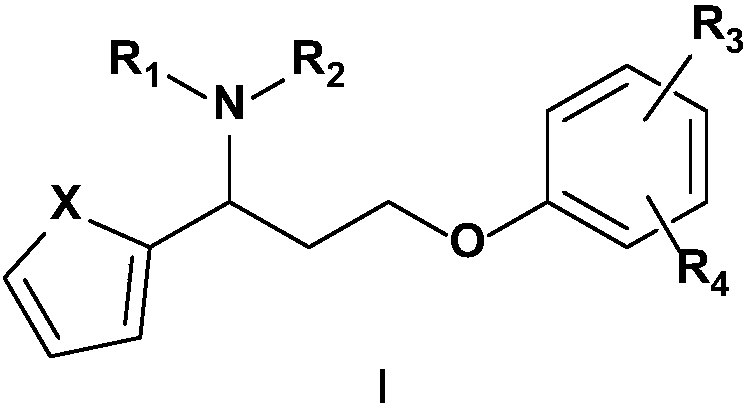
Novel 5-hydroxytryptamine and norepinephrine dual reuptake inhibitor and medical application thereof
A pharmaceutical and methyl technology, applied in the field of medicinal chemistry, can solve problems such as side effects, low response rate, and long onset time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 3-(4-methylphenoxy)-1-(thiophen-2-yl)-N,N-dimethylpropylamine hydrochloride (I 1 ) preparation
[0060]
[0061] 1.1 Preparation of 3-chloro-1-(2-thiophene)-acetone
[0062] Add trichloropropionyl chloride (63.5 g, 0.5 mol), anhydrous aluminum trichloride (100 g, 0.75 mol) and dichloromethane (400 mL) in a 1 L three-necked flask, slowly add thiophene ( 42.07g, 0.5mol) of dichloromethane solution, mechanically stirred for 3h. After the reaction was completed, the reaction solution was poured into 800 mL of ice water, stirred to separate the layers, and extracted with dichloromethane (3×200 mL). The organic phases were combined, washed with saturated sodium chloride solution (2×300 mL), dried over anhydrous magnesium sulfate, filtered with suction, and concentrated to obtain 82.5 g of crude product with a yield of 94.26%.
[0063] 1.2 Preparation of 3-chloro-1-(2-thiophene)-propanol
[0064] Add 82.5 g (0.47 mol) and 300 mL of methanol into a 1 L three-ne...
Embodiment 2
[0069] Example 2 3-(4-methylphenoxy)-1-(thiophen-2-yl)-N-methylpropylamine hydrochloride (I 2 ) preparation:
[0070]
[0071] 3.1 g (0.012 mol) of 3-(4-methylphenoxy)-1-(2-thiophene)-propanol, 1.8 g (0.018 mol) of triethylamine (TEA) and 80 mL of dry tetrahydrofuran (THF ) into a 250mL three-necked flask, and slowly dropwise added a THF solution containing 2.1g (0.018mol) of methanesulfonyl chloride under an ice-salt bath, and reacted for 5h. Then feed the dried methylamine gas into the three-necked flask to make the pH of the solution system about 13, and seal the reaction system. The reaction was stirred at room temperature for 48h. After the reaction was finished, THF was removed by distillation under reduced pressure,
[0072] Then add 200mL of water, extract with ethyl acetate (3×100mL), combine the organic phases, wash with saturated sodium chloride solution (2×100mL), dry over anhydrous magnesium sulfate, filter and distill off ethyl acetate under reduced pressur...
Embodiment 3
[0073] Example 3 3-(4-methoxyphenoxy)-1-(thiophen-2-yl)-N,N-dimethylpropylamine hydrochloride (I 3 ) preparation:
[0074]
[0075] 3.1 Preparation of 3-(4-methoxyphenoxy)-1-(2-thiophene)-propanol
[0076] According to the method of 1.3 in Example 1, 4-methoxyphenol was used instead of 4-methylphenol to obtain a white solid 7.82, with a yield of 91.2%.
[0077] 3.2 3-(4-methoxyphenoxy)-1-(thiophen-2-yl)-N,N-dimethylpropylamine hydrochloride (I 3 ) preparation
[0078] According to the method of 1.4 in Example 1, replace 3-(4-methylphenoxy)-1-(2 -thiophene)-propanol participated in the reaction to obtain 2.1 g of white solid with a yield of 65.4%. mp: 107-108°C. 1 H NMR (400MHz, DMSO-d 6 )δ ppm :7.69(d,J=5.0Hz,1H),7.34(d,J=3.3Hz,1H),7.15(m,2H),6.79(m,3H),4.81(q,J=9.3Hz,1H) ,3.90(m,2H),3.81(s,3H),2.63(s,6H),2.39-2.33(m,2H); MS(ESI,m / z):292(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com