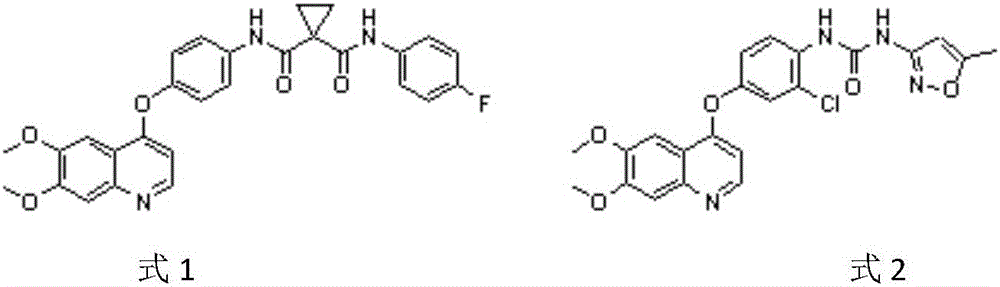
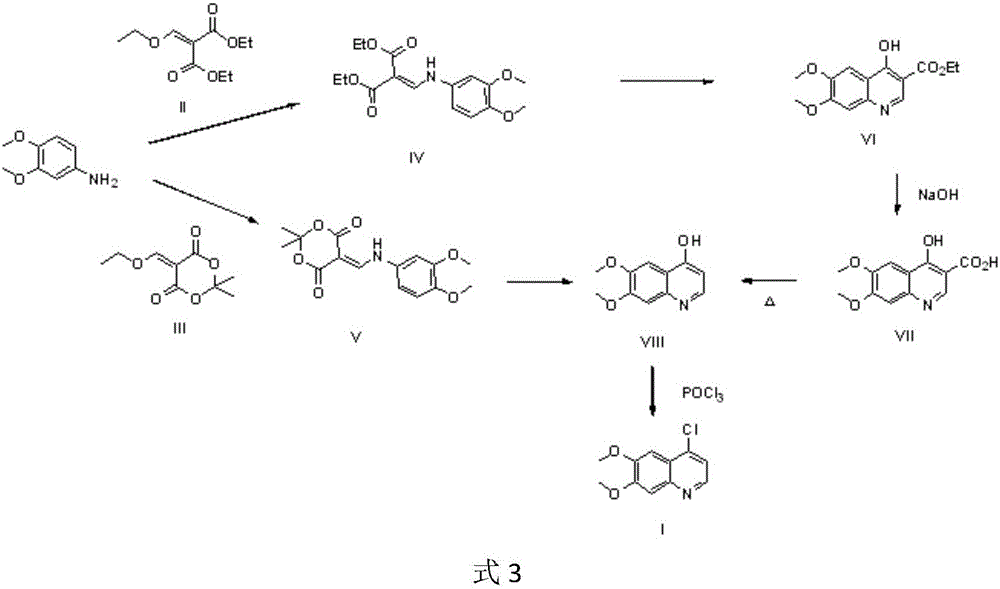
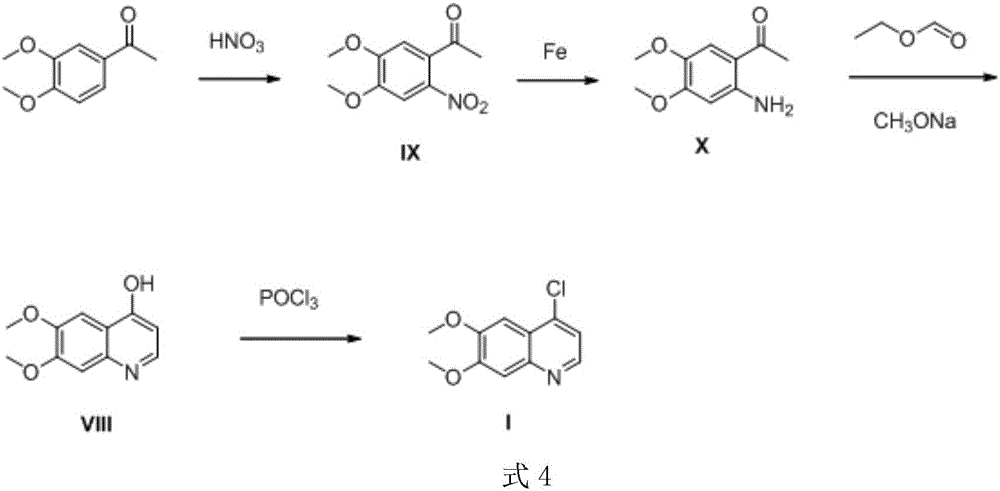
Preparation method of 4-chloro-6,7-dimethoxyquinoline
A technology of dimethoxyquinoline and dimethoxy, applied in the preparation of 4-chloro-6,7-dimethoxyquinoline, the key to the preparation of antitumor drugs cabozantinib and tivozanib In the field of intermediates, it can solve the problems of high cost of industrial preparation, a large amount of industrial waste residue, and high cost of industrialization, and achieve the effect of simple and convenient post-processing method, simple process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of 2-nitro-4,5-dimethoxyacetophenone (1)
[0040] Add 3,4-dimethoxyacetophenone (18.0g, 0.1mol) into a 500mL three-neck flask, add acetic acid (180mL) at 20°C, stir, heat to 60°C, and wait until the solids are completely dissolved , add 65wt% nitric acid (14.6mL, 0.21mol) to mix at this temperature, track the reaction with thin layer chromatography (TCL), the developer of TLC is ethyl acetate:petroleum ether=1:8, TLC detection wavelength is 254nm, After 3.5 h, TCL showed that the reaction was complete. The reaction solution was slowly poured into a stirred 400mL ice-water mixture in a beaker, and a yellow solid was generated. After stirring for half an hour, suction filtered to obtain a yellow solid, which was dried at 50°C to obtain 2-nitro-4,5-dimethoxyacetophenone (19.1 g) with a yield of 84.7% as a light yellow solid.
[0041] 1 H NMR (400MHz, DMSO-d 6 ): δ=2.52(s,3H), 3.90(s,3H), 3.93(s,3H), 7.23(s,1H), 7.64(s,1H); ESI-MS(m / z) 226.1[M +H]...
Embodiment 2
[0043] Preparation of 1-(4,5-dimethoxy-2-nitrophenyl)-3-(dimethylamino)propen-1-one (1)
[0044] Add 2-nitro-4,5-dimethoxyacetophenone (18.0g, 0.08mol) into a 250mL three-necked flask, add toluene (100mL) at 20°C, stir to dissolve, add N,N- Dimethylformamide dimethyl acetal (25.6g, 0.21mol), heated to 120°C for reaction, using TCL (the developer of TLC is dichloromethane:methanol:triethylamine=10:1:0.1, the detection wavelength is 254nm) to track the reaction, TCL detection after 4h showed that the reaction was over, the reaction solution was placed at 20°C and stirred, and after about 1h of precipitation, a yellow solid was produced, which was filtered by suction and dried to obtain a yellow solid 1-(4,5-dimethoxy -2-nitrophenyl)-3-(dimethylamino)propen-1-one (17.4 g, 77.7%).
[0045] 1 H NMR (400MHz, CDCl 3 ):δ=2.87(s,3H),3.10(s,3H),3.98(s,3H),3.99(s,3H),5.25(d,J=12.2Hz,1H),6.86(s,1H) ,7.30(brs,1H),7.60(s,1H).ESI-MS(m / z)281.2[M+H] + ,561.3[2M+H] + ,583.2[2M+Na] + .
Embodiment 3
[0047] Preparation of 4-hydroxy-6,7-dimethoxyquinoline (1)
[0048] 1-(4,5-dimethoxy-2-nitrophenyl)-3-(dimethylamino)propene-1-one (18.0g, 0.064mol) was added to a 1000mL eggplant-shaped bottle, at 20 At ℃, add 8g of Raney nickel and 600mL of tetrahydrofuran. After evacuating for 3 times, inject hydrogen gas, heat to 38℃, and track with TCL (TLC developer is dichloromethane:methanol=15:1, detection wavelength is 254nm). After 10 hours, TCL detection showed that the reaction was complete. The reaction solution was filtered with diatomaceous earth, and the diatomaceous earth was washed with tetrahydrofuran (25mL×2), and the tetrahydrofuran was evaporated to dryness to obtain a yellow solid 4-hydroxy-6,7-dimethoxy Quinoline crude product, the crude product was added to 140mL of ethyl acetate and ethanol mixed solution with a volume ratio of 1:1, and recrystallized and purified in the mixed solution to obtain off-white solid 4-hydroxyl-6,7- Dimethoxyquinoline 11.6g, yield 88.3%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com