Cabozantinib mucate and crystal form there of
A technology of mucus salt and crystal form, applied in the field of chemical medicine, can solve the problem of low solubility of free alkali, and achieve the effects of convenient long-term storage, low hygroscopicity, improved bioavailability and curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N'-(4-fluorophenyl)cyclopropane-1,1-di The preparation method of formamide mucate:
[0057] 200mg N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N'-(4-fluorophenyl)cyclopropane-1,1 - The powder of diformamide was dissolved in 8.0 mL of acetonitrile solvent system, then 84 mg of mucic acid solid was added to the solution, and magnetically stirred at room temperature to obtain it.
[0058] The mucic acid salt product prepared by the above-mentioned method has the following NMR identification data:
[0059] 1H NMR (400MHz, DMSO) δ10.18(s, 1H), 10.05(s, 1H), 8.47(d, J=5.2Hz, 1H), 7.76(d, J=9.0Hz, 2H), 7.64(dd ,J=9.1,5.1Hz,2H),7.50(s,1H),7.39(s,1H),7.23(d,J=9.0Hz,2H),7.15(t,J=8.9Hz,2H),6.43 (d,J=5.2Hz,1H),4.22(s,2H),3.94(d,J=4.8Hz,6H),3.77(s,2H),1.47(s,4H).
Embodiment 2
[0061] N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N'-(4-fluorophenyl)cyclopropane-1 of the present invention, Comparative study on the solubility of 1-dicarboxamide mucate and malate in CN102388024A:
[0062] The two salts were prepared as saturated solutions with pH 1.8 SGF (simulated gastric juice) and pH 6.5 FaSSIF (artificial intestinal fluid in fasting state) buffers. After 4 hours, measure the concentration of the sample in the saturated solution by high performance liquid chromatography, and the experimental results are as shown in table 1:
[0063] Table 1 Comparative study on solubility of cabozantinib mucate and CN102388024A malate
[0064]
[0065] Detection limit: 0.03 μg / ml
[0066] As can be seen from the above comparison results, N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxyl}benzene of the present invention after being placed in SGF and FaSSIF for 4 hours Base)-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide mucate salt has higher solubility than C...
Embodiment 3
[0068] N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N'-(4-fluorophenyl)cyclopropane-1,1-di The preparation method of formamide mucate salt crystal form A:
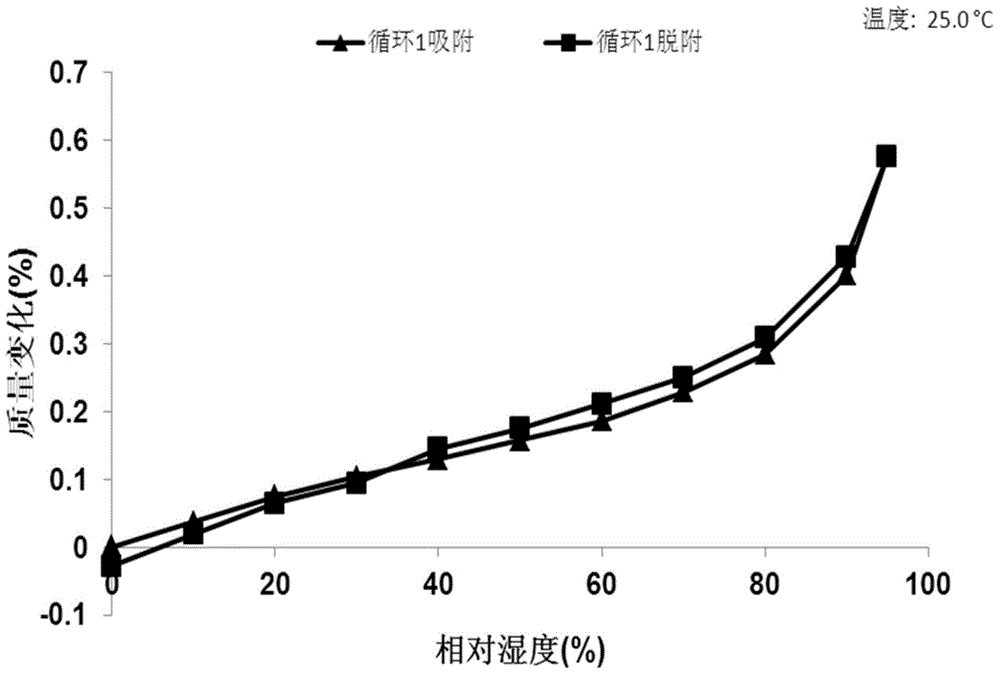
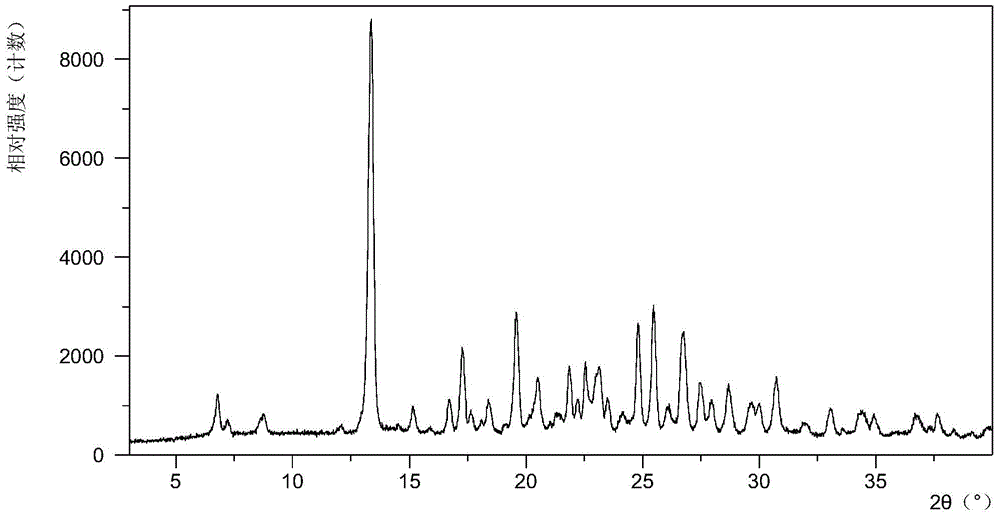
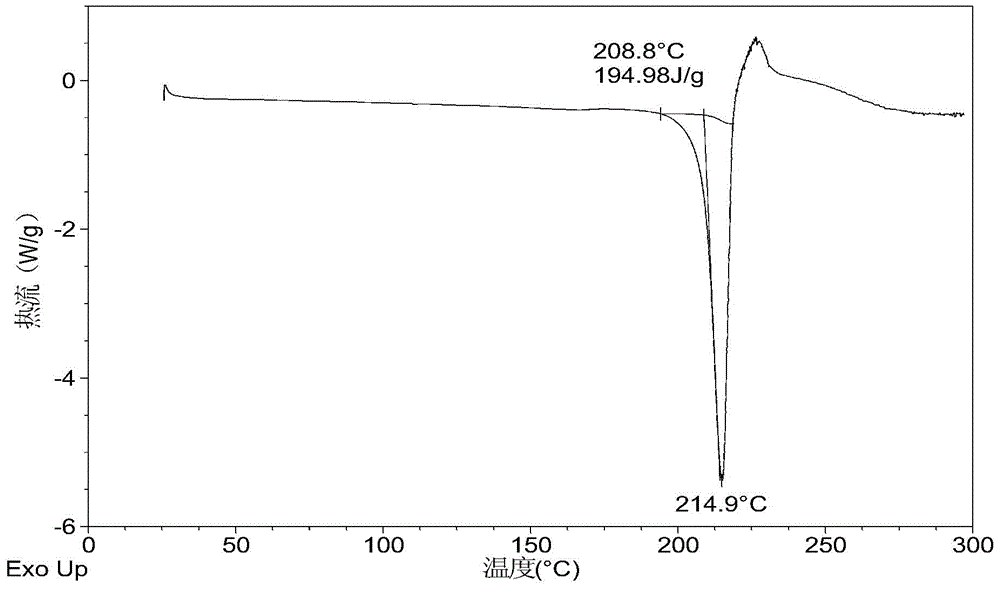
[0069] 10mg N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N'-(4-fluorophenyl)cyclopropane-1,1 - Diformamide powder was dissolved in 0.4 mL of methanol solvent system, then 4.2 mg of mucic acid solid was added, and stirred at room temperature to obtain Form A. Table 2 shows the X-ray powder diffraction data of Form A obtained in this example. Its XRPD pattern is as follows figure 1 , and its DSC graph is shown in figure 2 , and its TGA figure is shown in image 3 .
[0070] Table 2 X-ray powder diffraction data of crystal form A
[0071] 2theta
[0072] 13.28
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com