Deuterated cabozantinib derivative, preparation method and application thereof, and intermediate of deuterated cabozantinib derivative
A technology of drugs and compounds, applied in the field of deuterated cabozantinib derivatives, applications and their intermediates, and their preparation methods, can solve the problems of hair color changes, toxic reactions, etc., to increase concentration, increase half-life, reduce Effects of drug toxicity and other side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Embodiment 1 Preparation of compounds with structures shown in formula II and malate thereof
[0136] Step 1: Preparation of a compound having a structure shown in formula XII
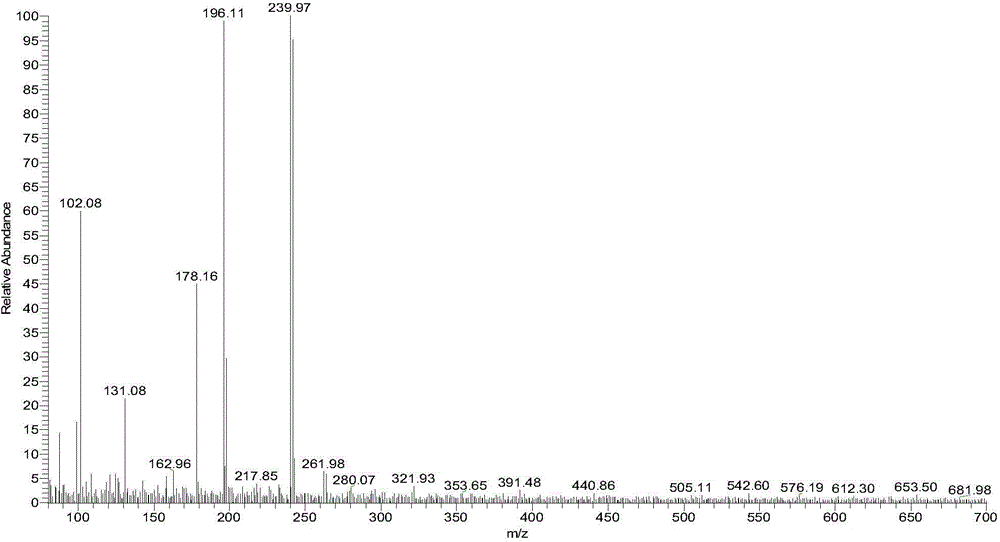
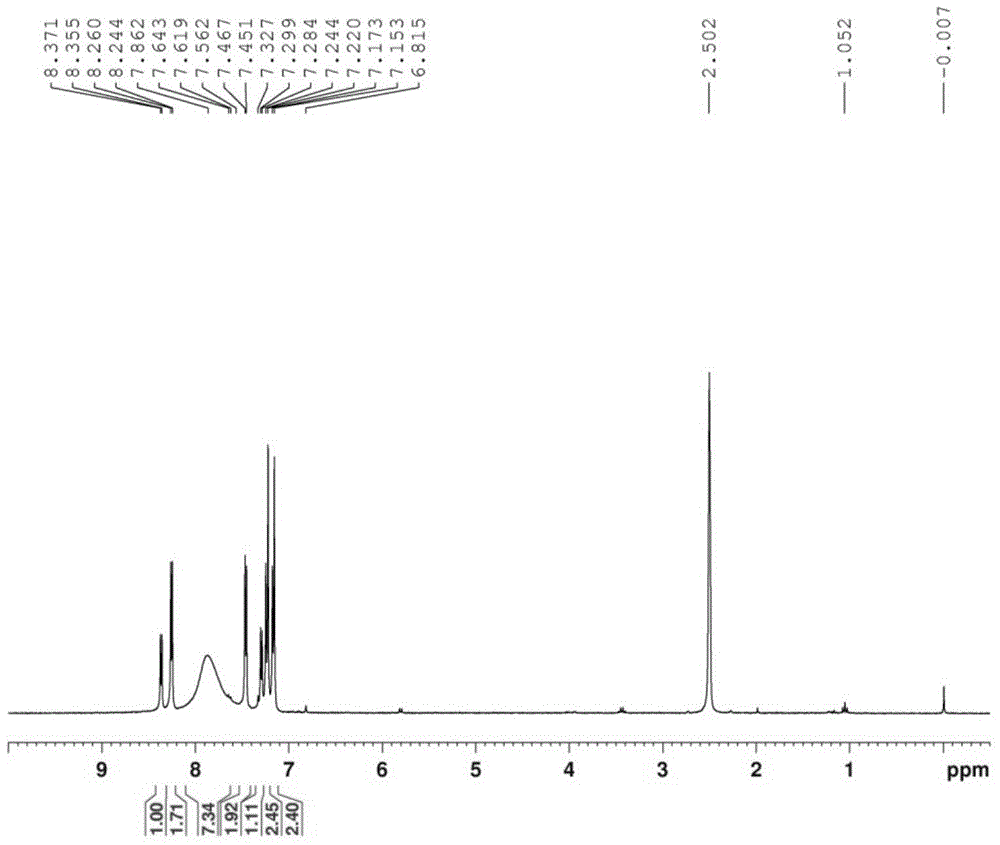
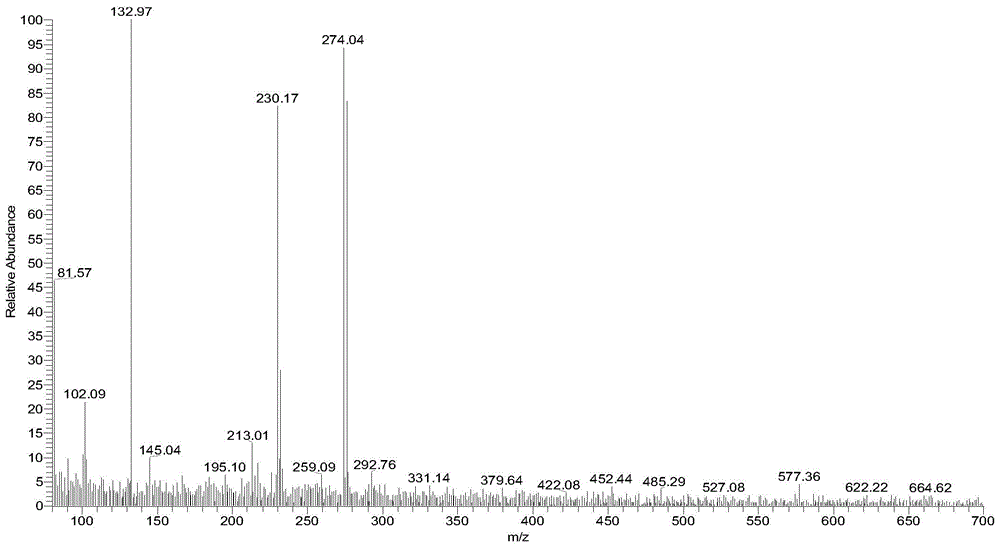
[0137] Weigh the compound (1.5 g, 6.7 mmol) having the structure shown in Formula XVIII and add it into a stuffy jar, then add 15 mL of acetic acid and 15 mL of 40% hydrobromic acid in sequence, and raise the temperature to 140° C. overnight (about 15 hours). LC-MS detected that there was no starting material, and at the same time, part of 6,7-dimethyloxy-4-bromoquinoline was generated. After directly evaporating the reaction liquid, add 25mL of water to make a slurry, then use a small amount of ammonia water to adjust the pH value to about 10, and make a slurry for 1 hour, then filter with suction to obtain a solid, use absolute ethanol (20mL) to azeotrope three times with water, and then use an oil pump Pump to constant weight. 1.3 g of white solid were obtained. Carry out LC-MS detection, ...
Embodiment 2
[0158] Example 2 Preparation of a compound having a structure shown in formula III and its malate
[0159] According to the preparation method provided in Step 1 to Step 6 in Example 1, the trideuteroiodomethane in Step 2 in Example 1 is replaced with dideuteriomethyl iodide, and the preparation is carried out to obtain a compound having the structure shown in formula III , named N-{4-[6,7-bis(dideuteriomethyl)oxy-4-quinolyl]oxyphenyl}-N-(4-fluorophenyl)-1,1-cyclo propanediformamide;
[0160]
[0161] According to the preparation method provided in Step 7 of Example 1, the malate salt of the compound having the structure shown in formula III was prepared.
Embodiment 3
[0162] Example 3 Preparation of a compound having a structure shown in formula IV and its malate
[0163] According to the preparation method provided in step 1 to step 6 in Example 1, the trideuteriodomethane in step 2 in Example 1 is replaced with deuteriodomethane for preparation, and the compound having the structure shown in formula IV is obtained, Named N-{4-[6,7-bis(deuteromethyl)oxy-4-quinolyl]oxyphenyl}-N-(4-fluorophenyl)-1,1-cyclopropane di Formamide;
[0164]
[0165] According to the preparation method provided in step 7 of Example 1, the malate salt of the compound having the structure shown in formula IV was prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Peak area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com