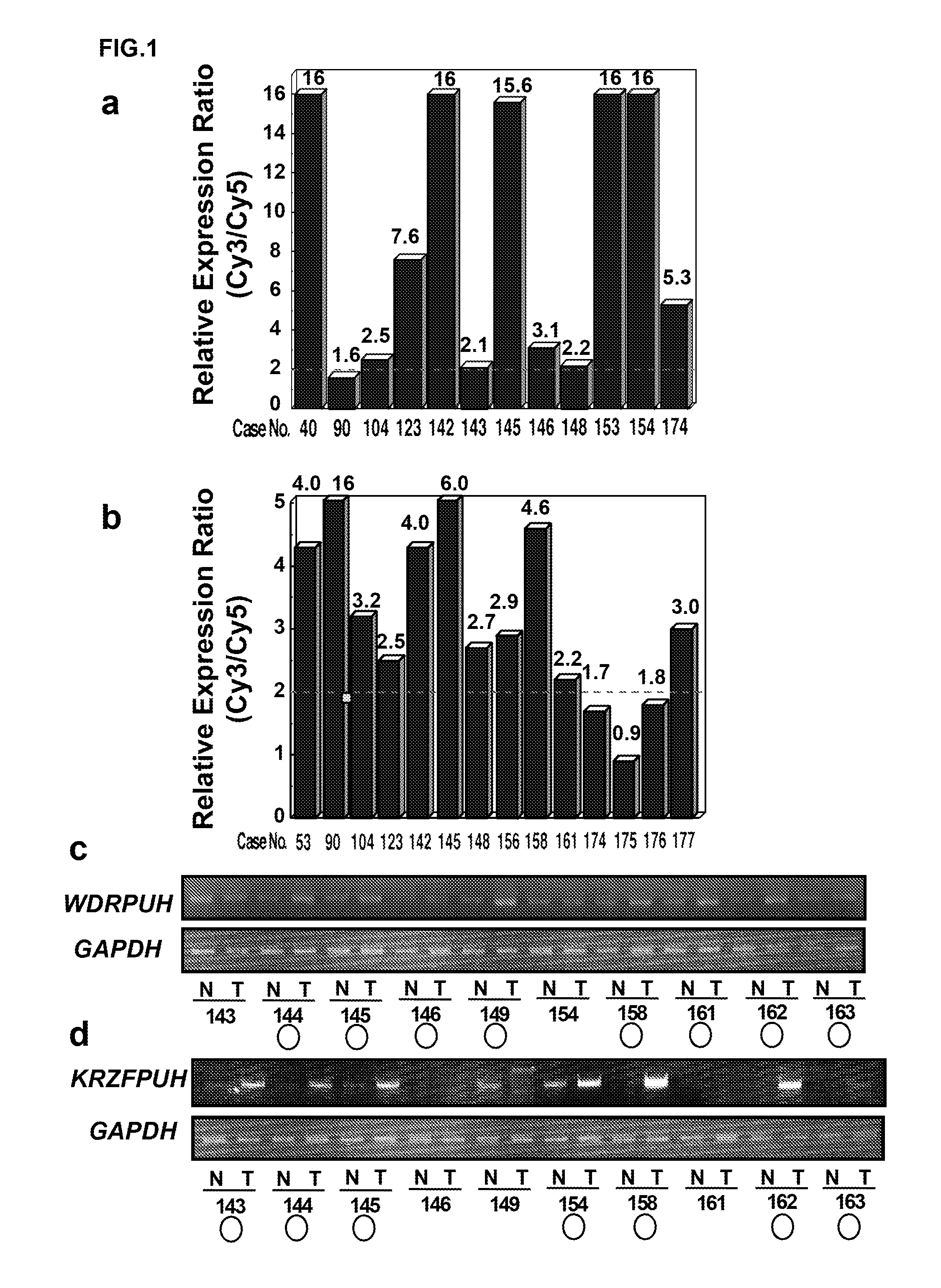
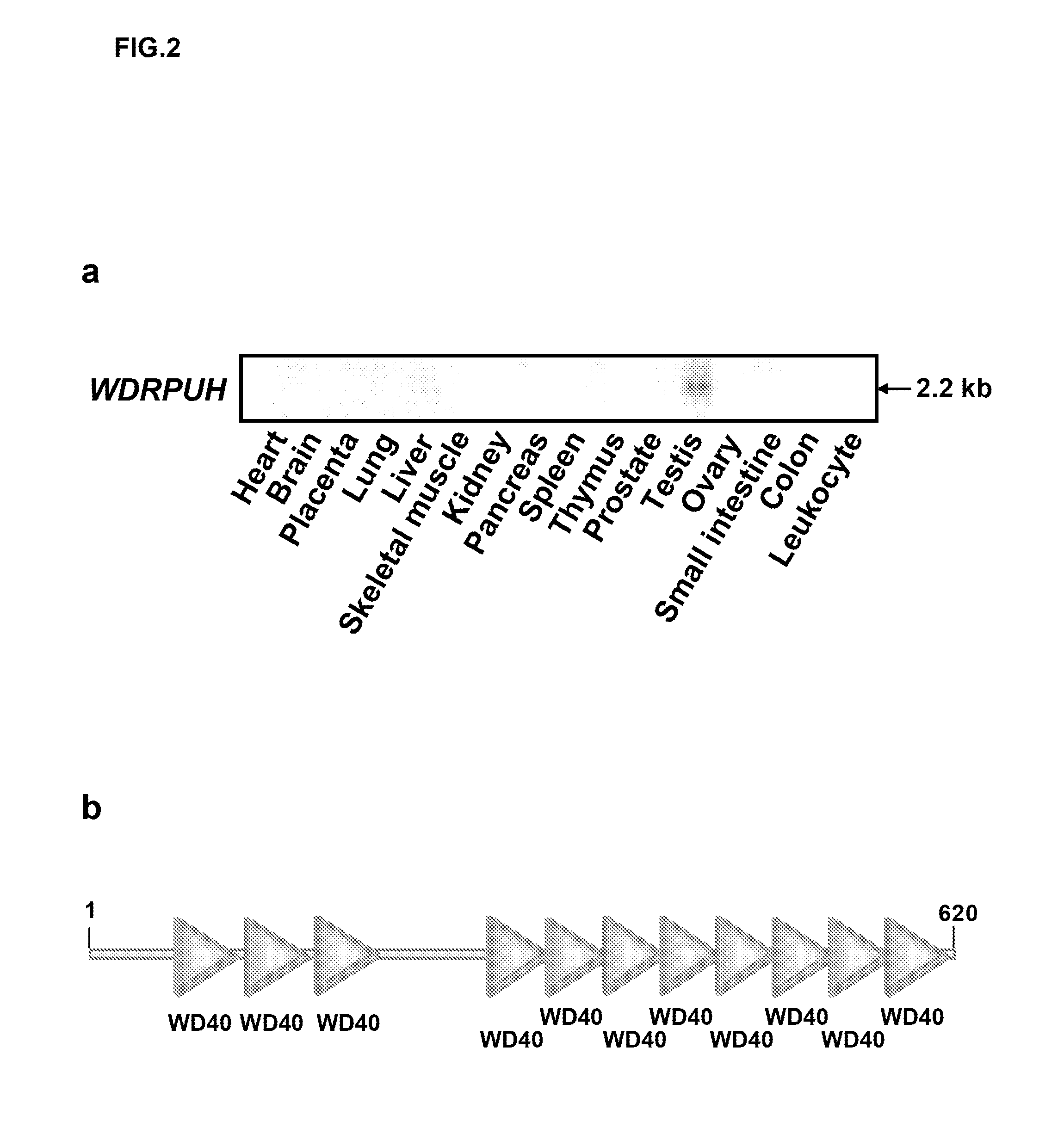
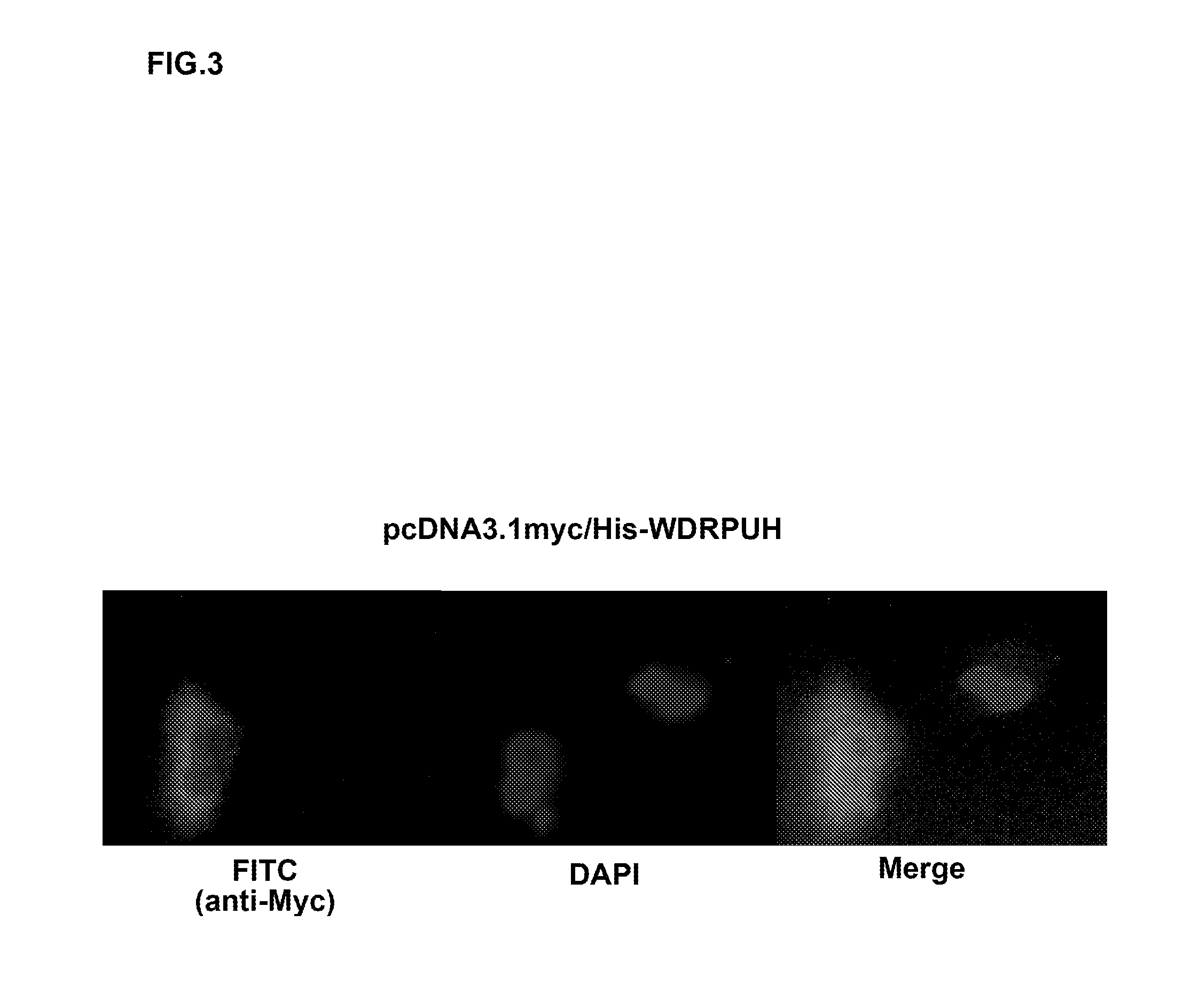
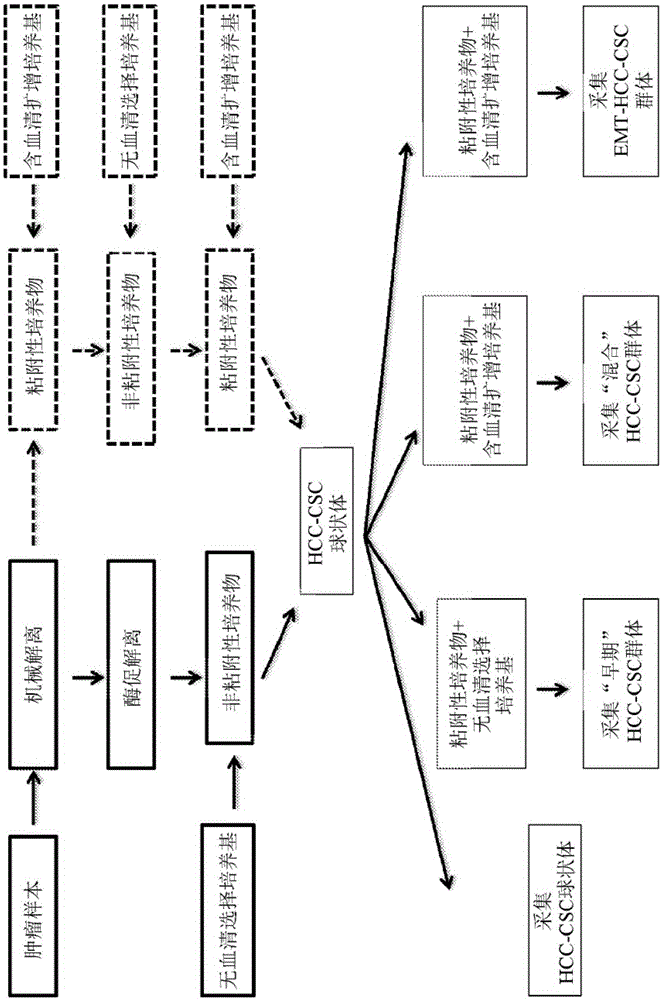
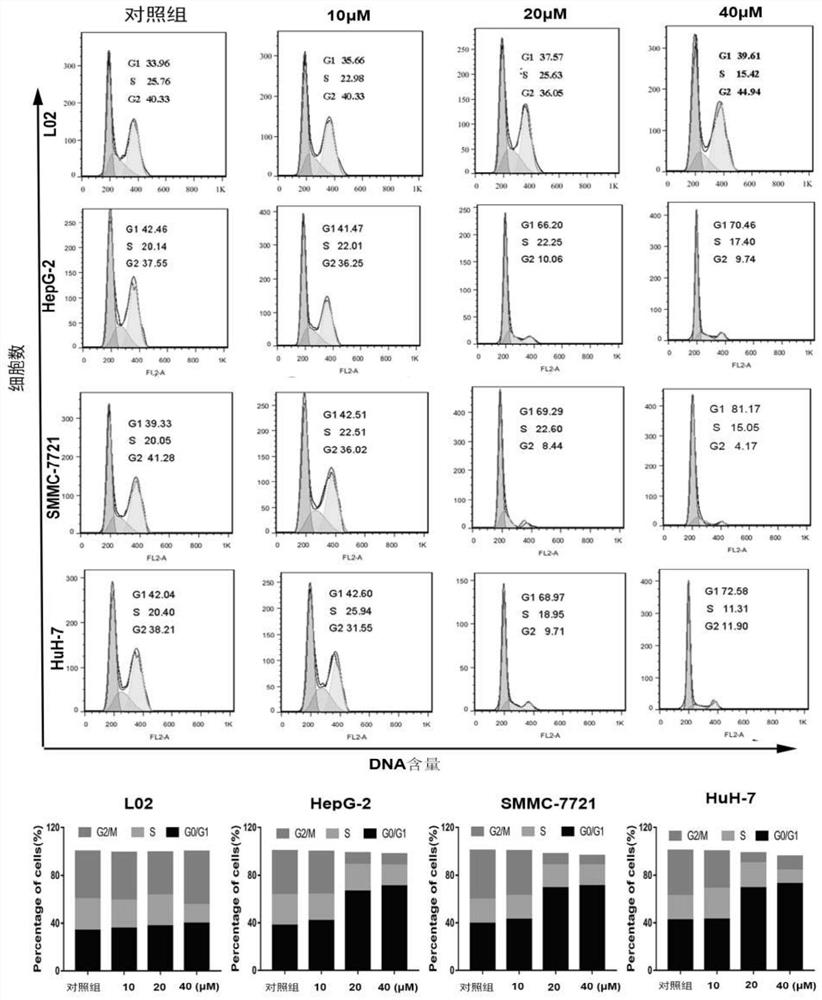
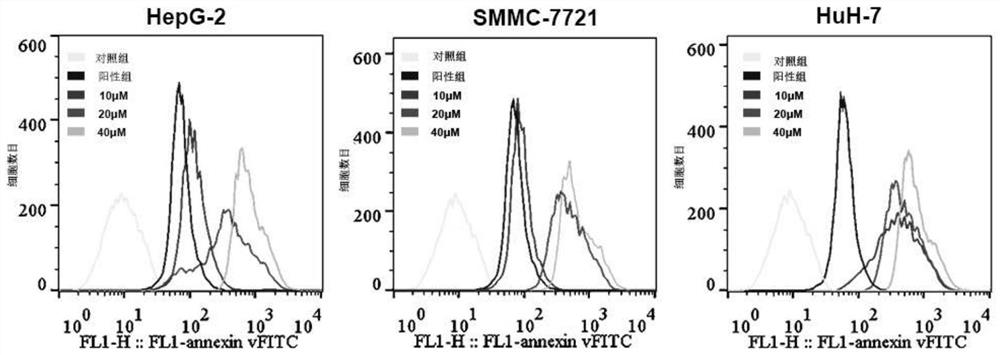
Patents
Literature
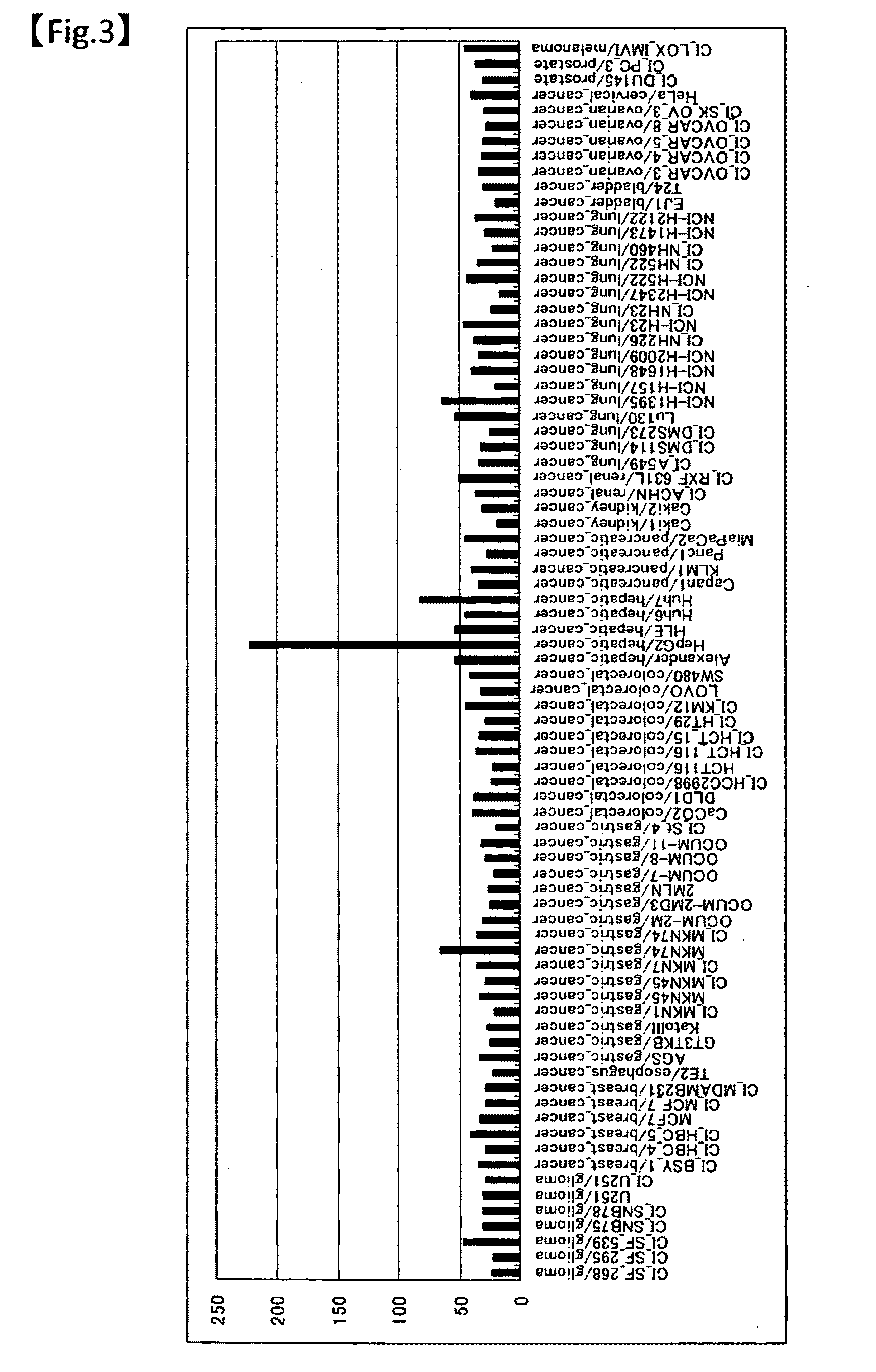
81 results about "Hepatocellular cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
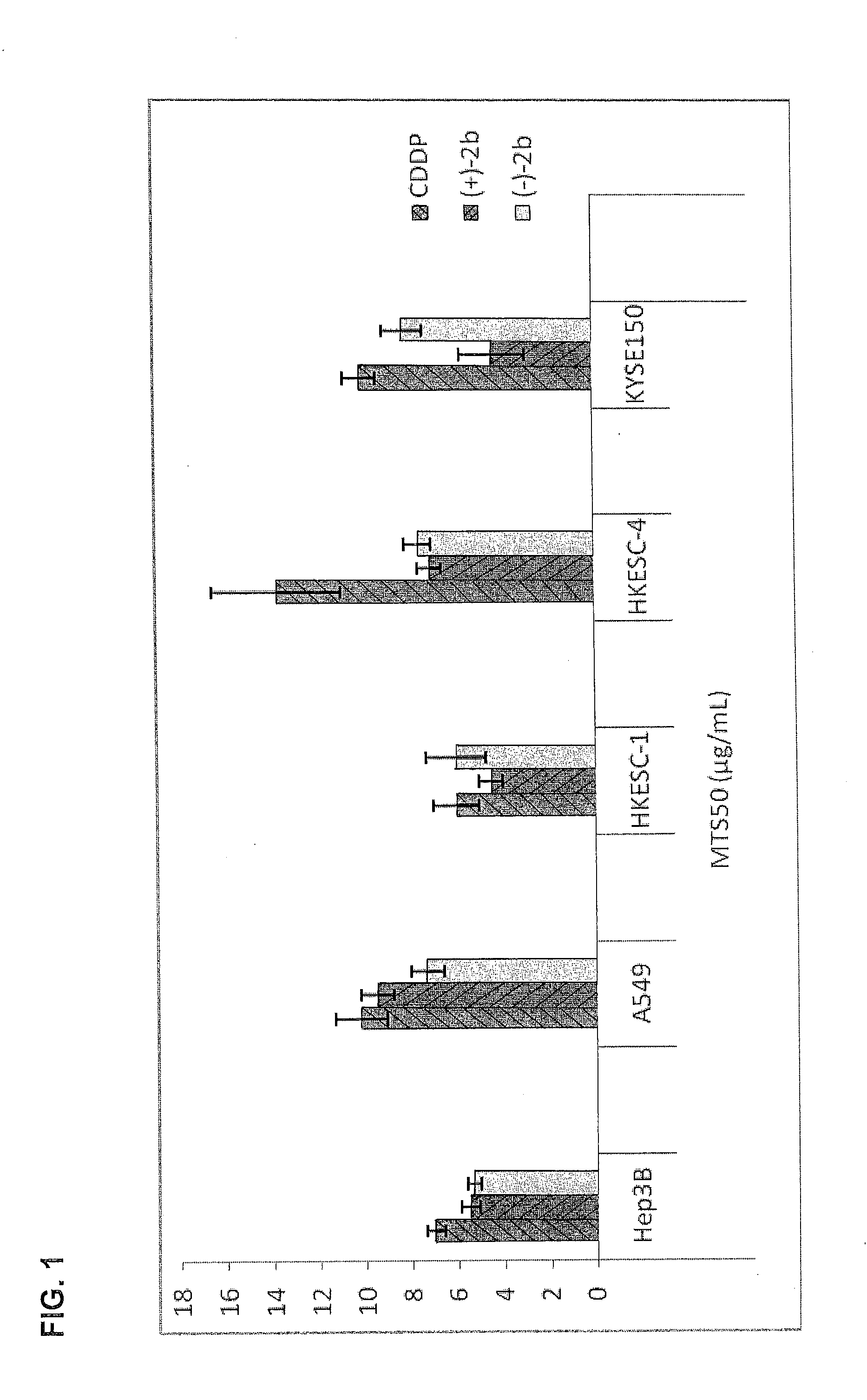
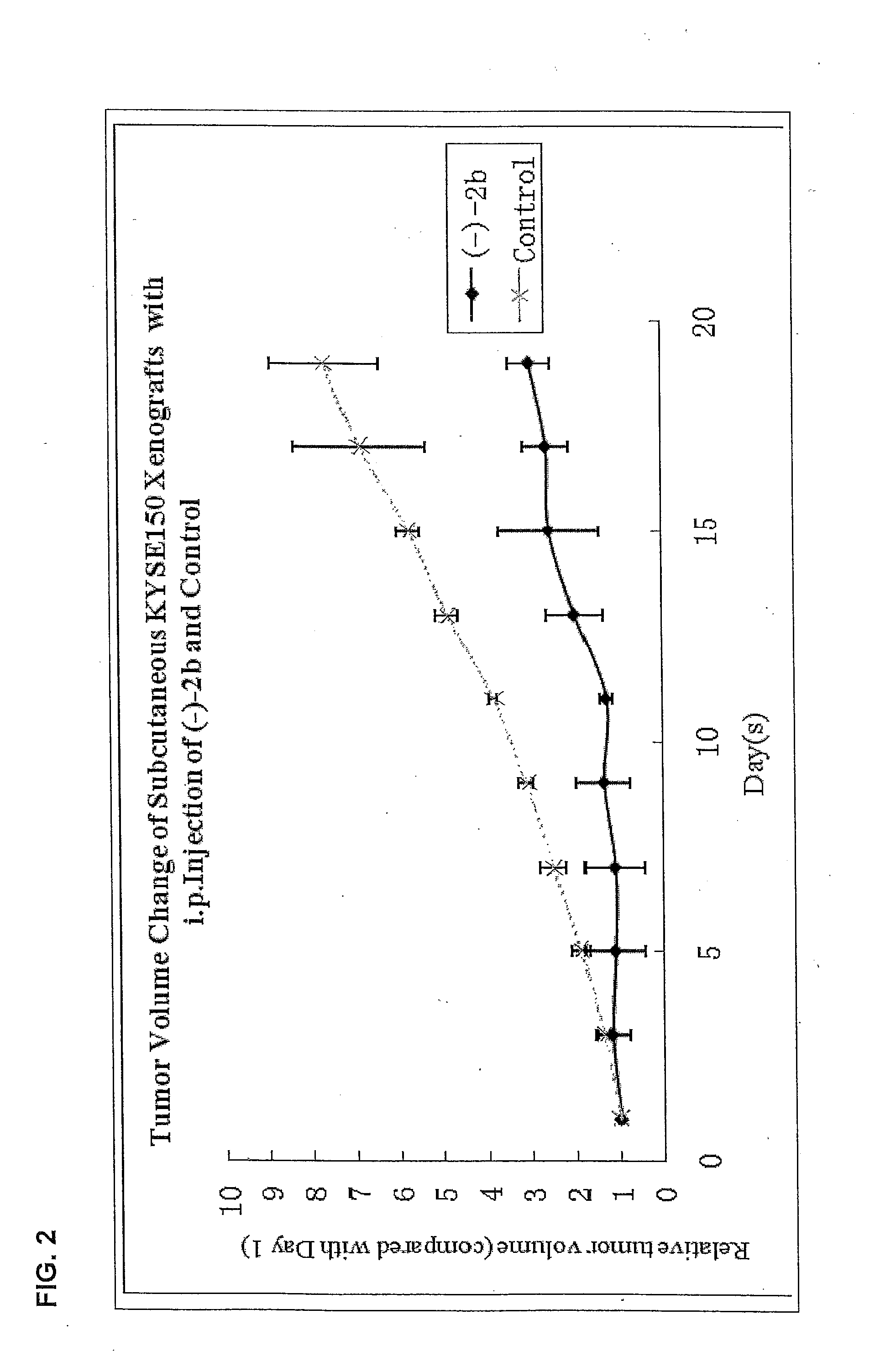
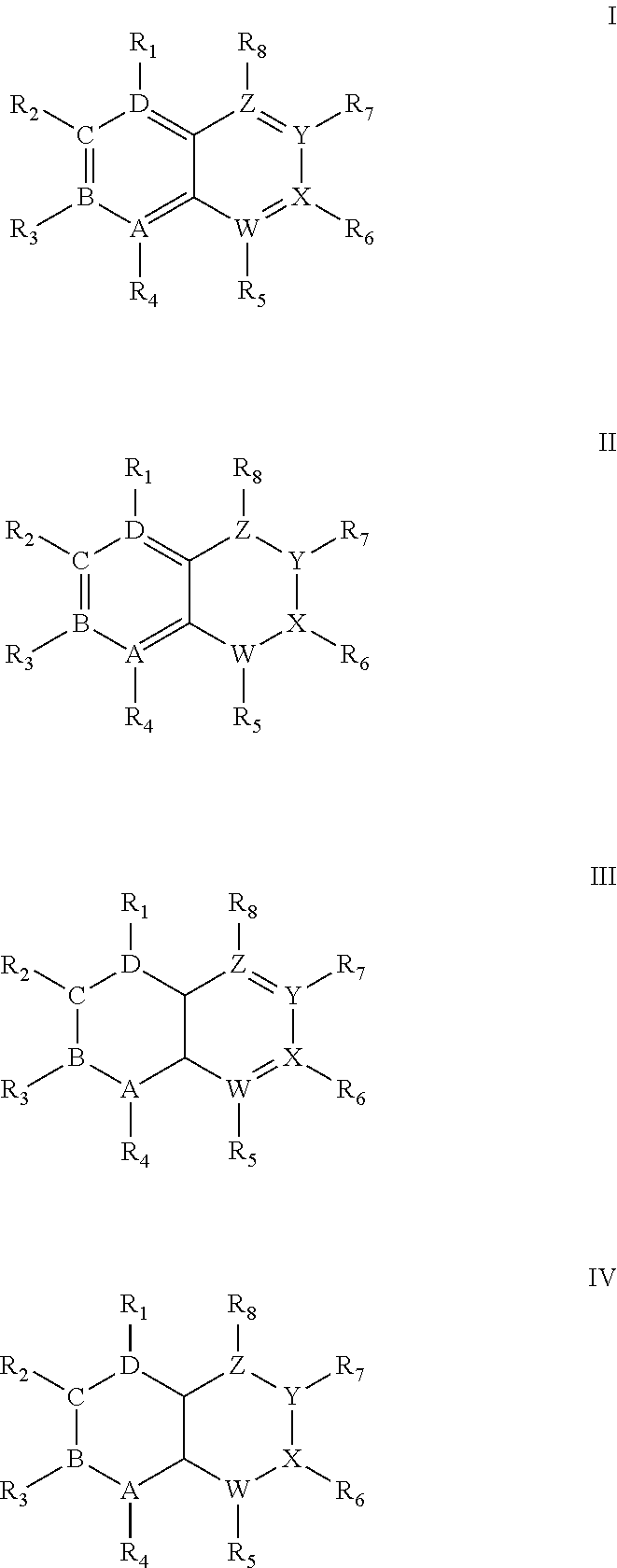
Quinoline derivatives as Anti-cancer agents
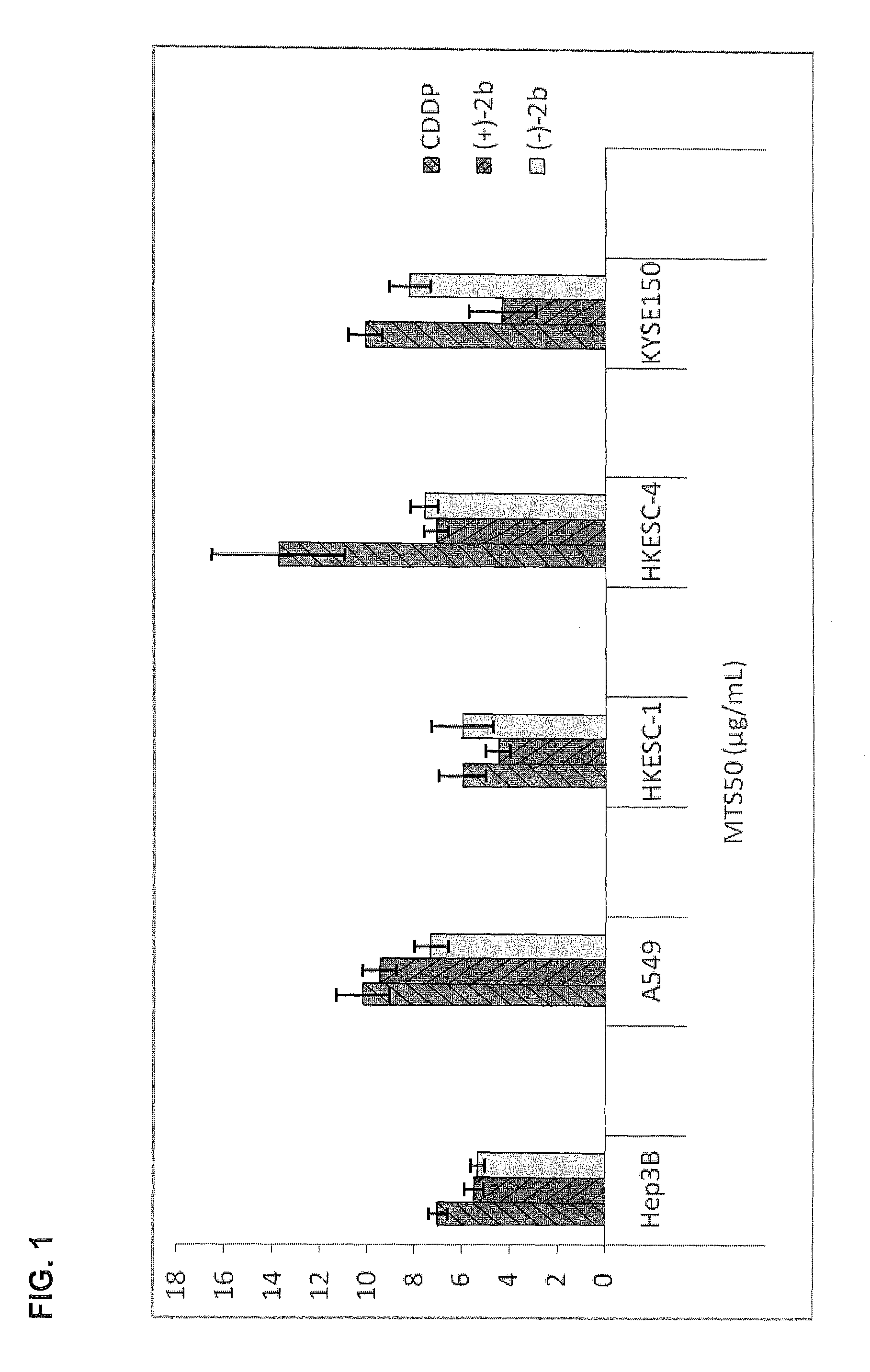
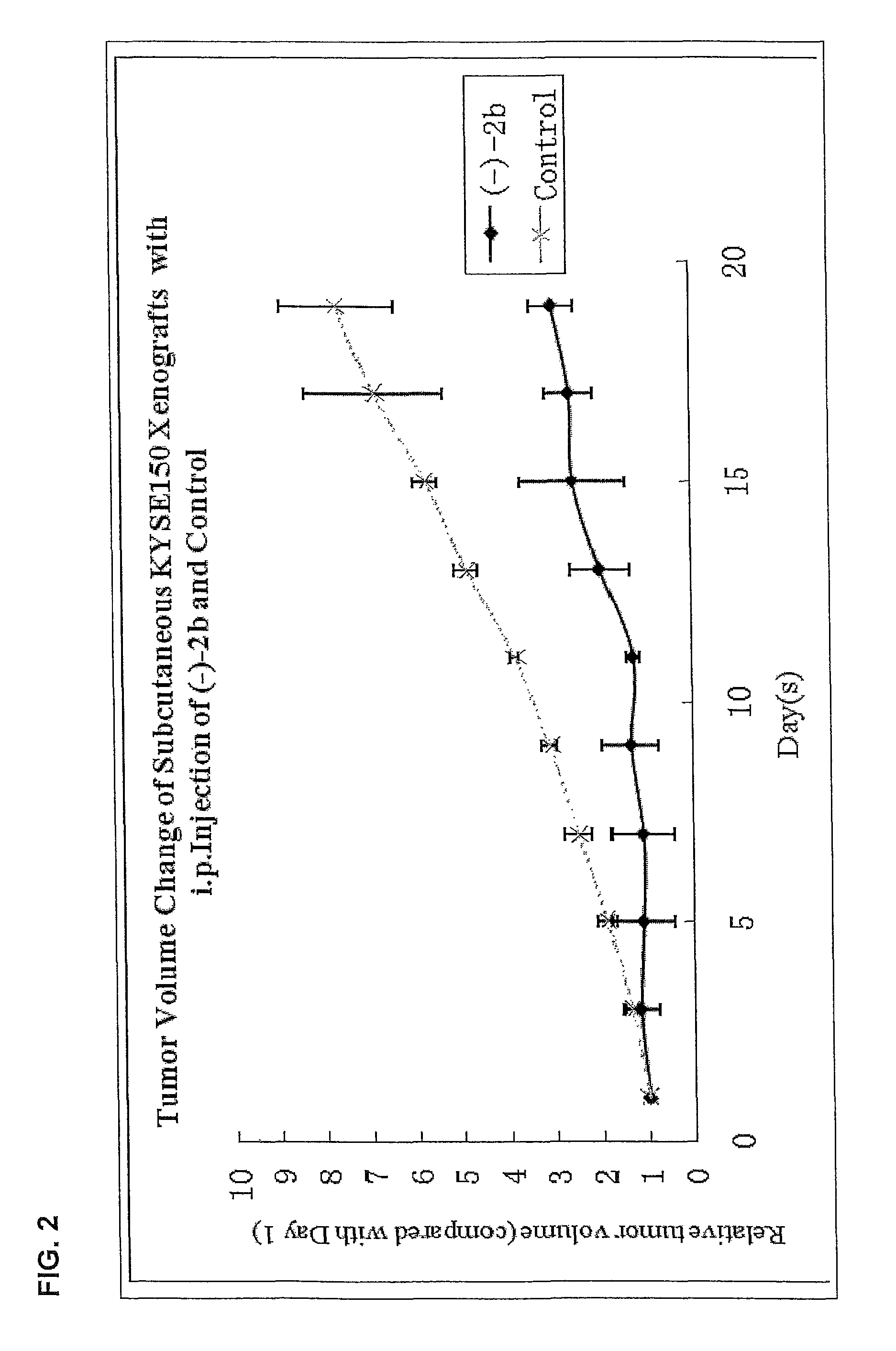
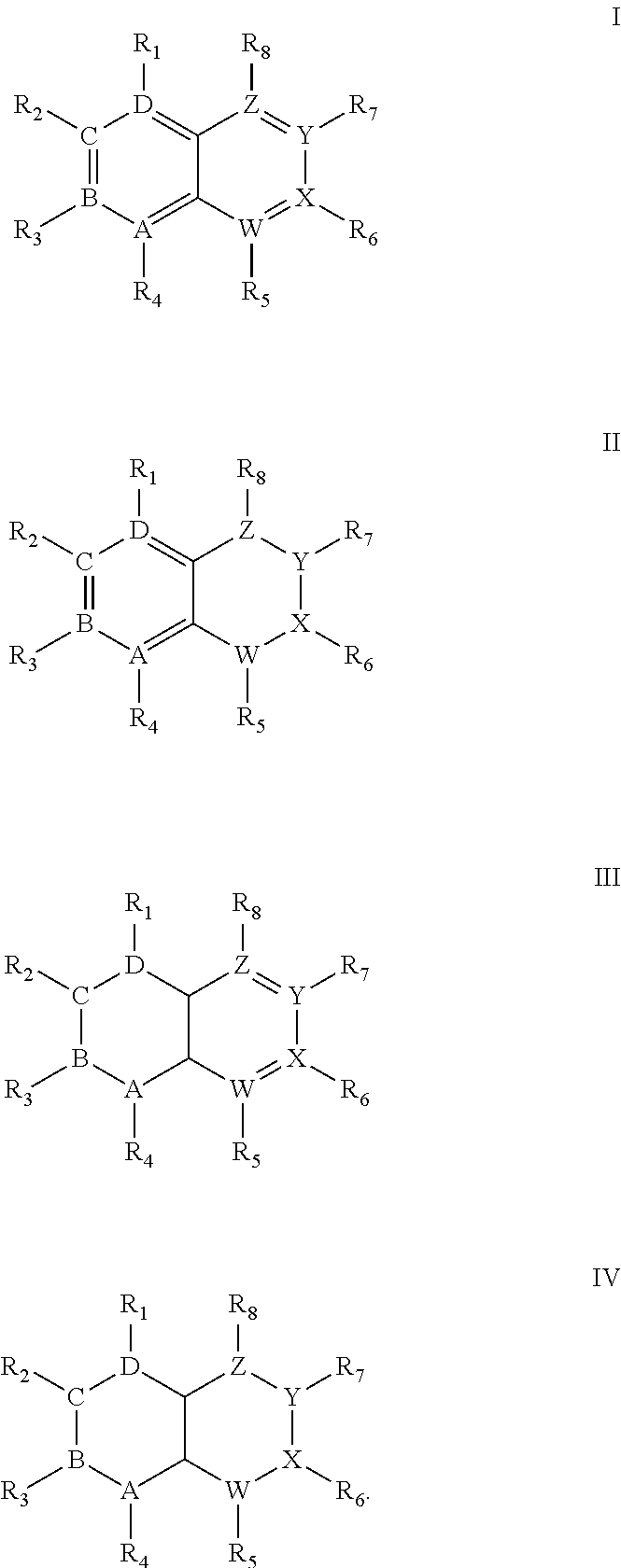
Quinoline derivatives showing anticancer activities against cancer cell lines of hepatocellular carcinoma (Hep3B), lung carcinoma (A549), esophageal squamous cell carcinoma (HKESC-1, HKESC-4 and KYSE150). The quinoline derivatives have a backbone structure of the following formulas:
Owner:THE HONG KONG POLYTECHNIC UNIV
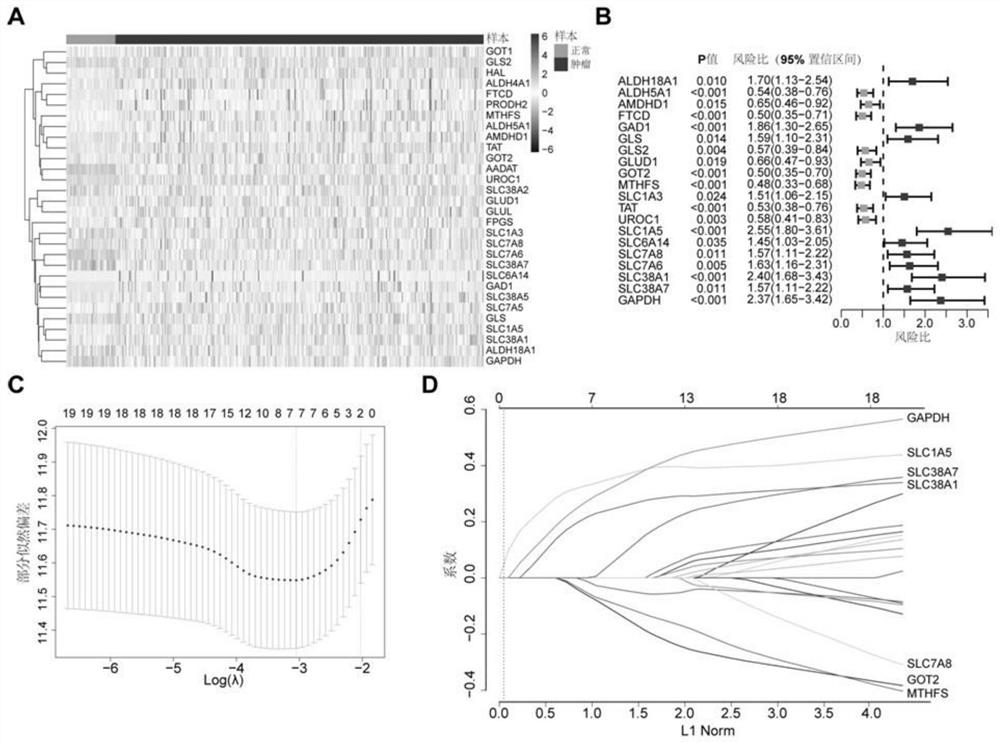
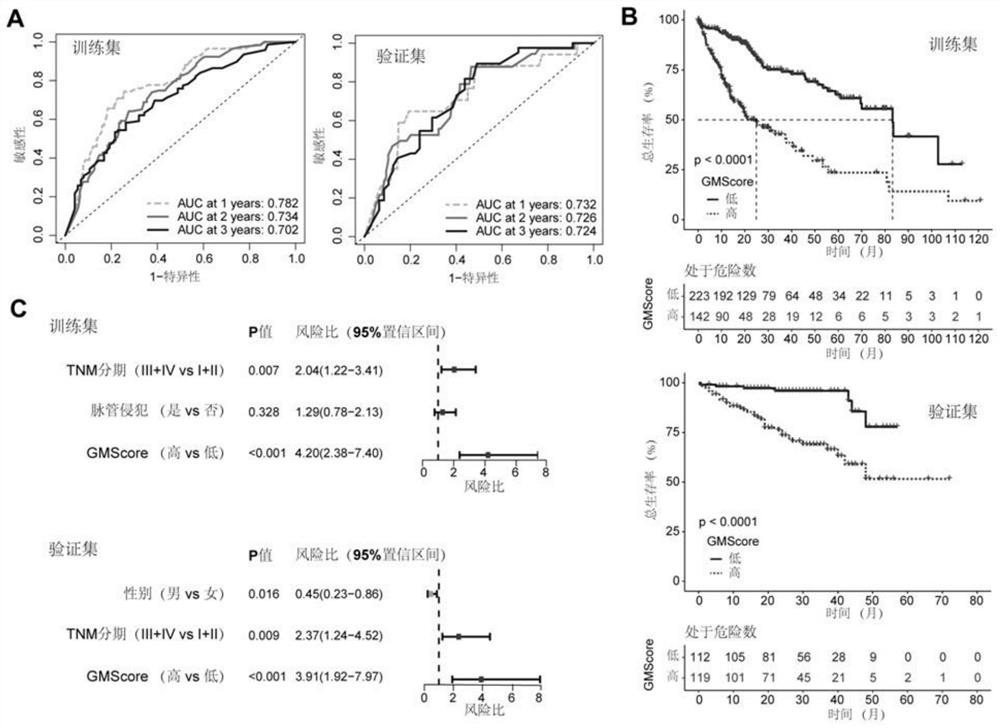
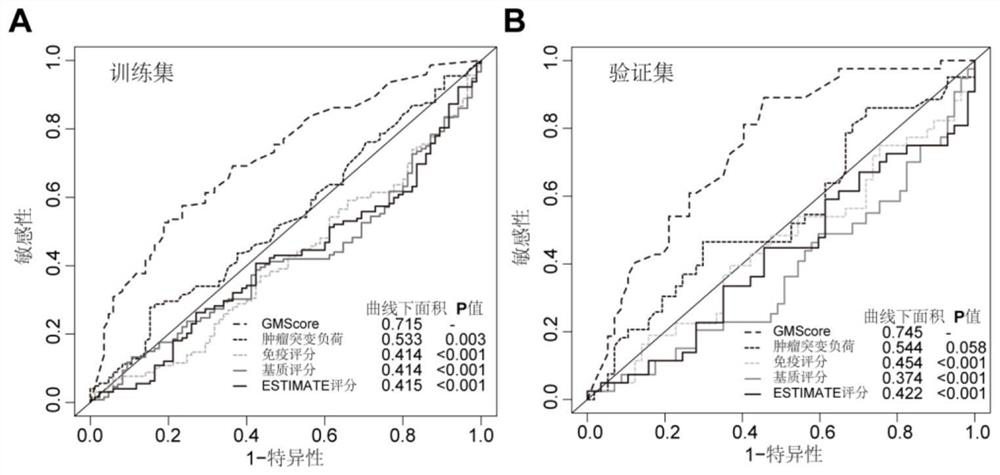
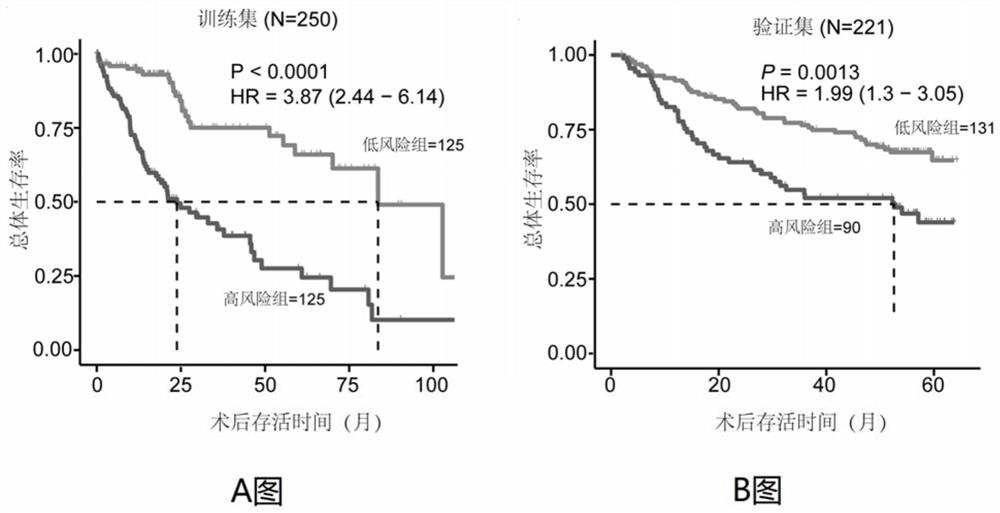
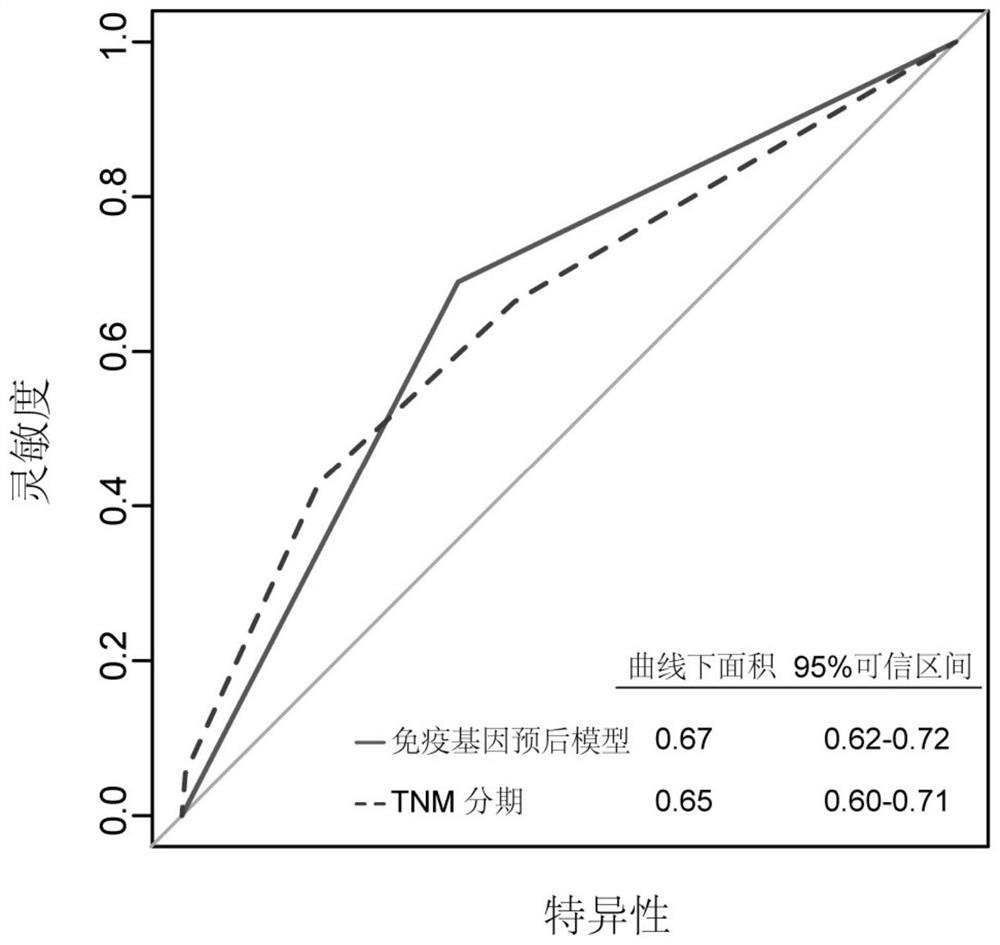
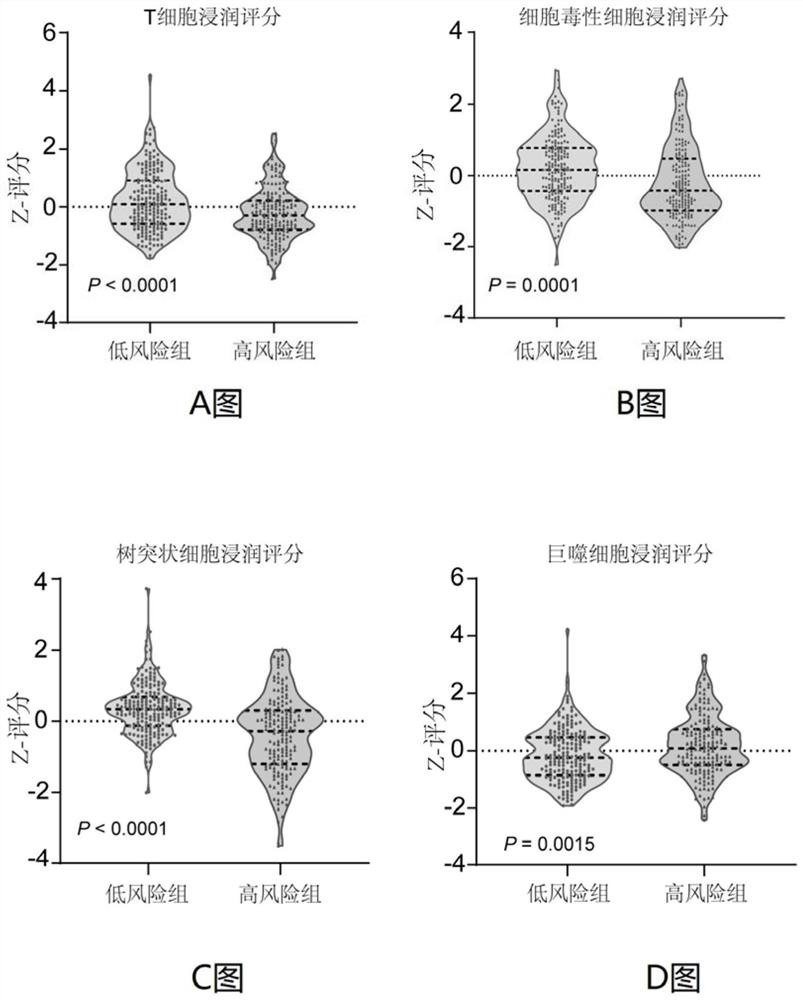
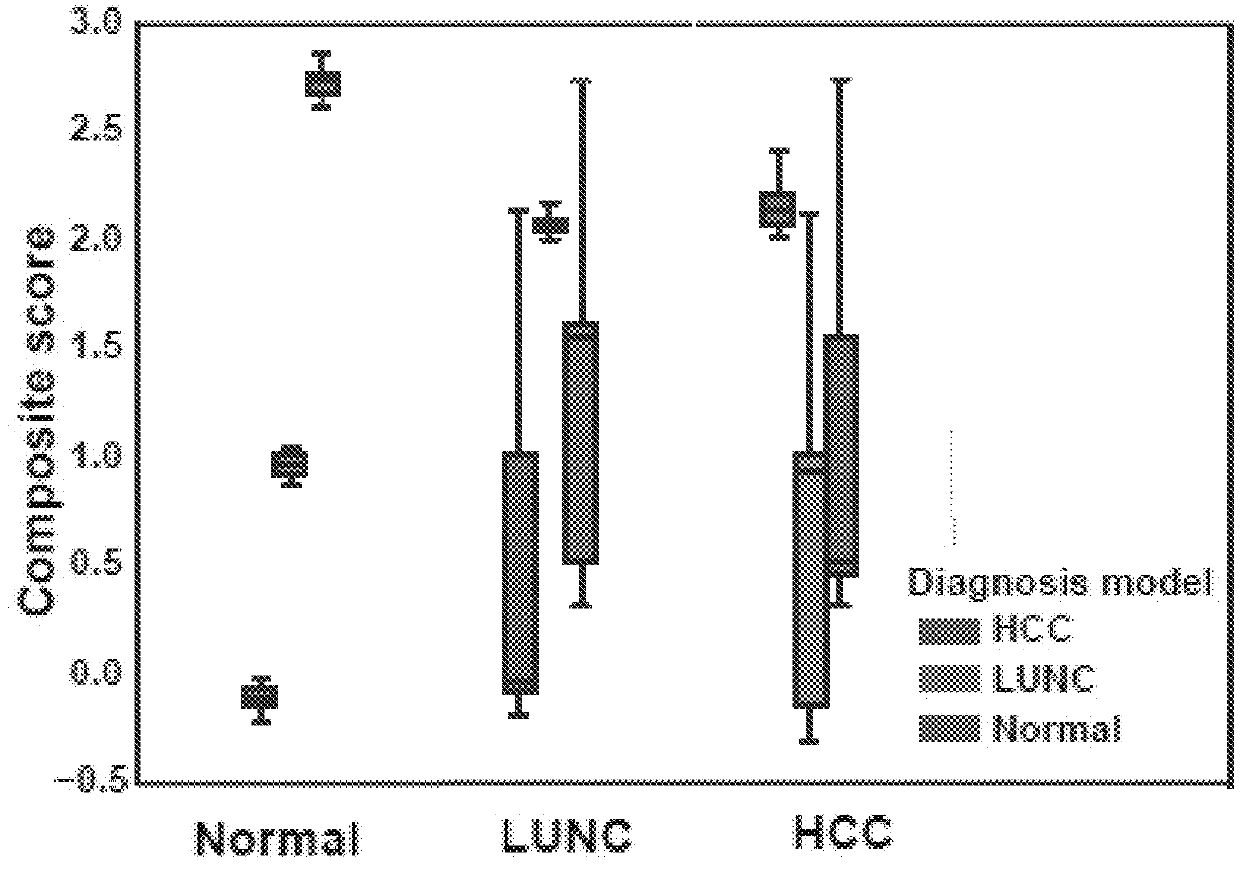
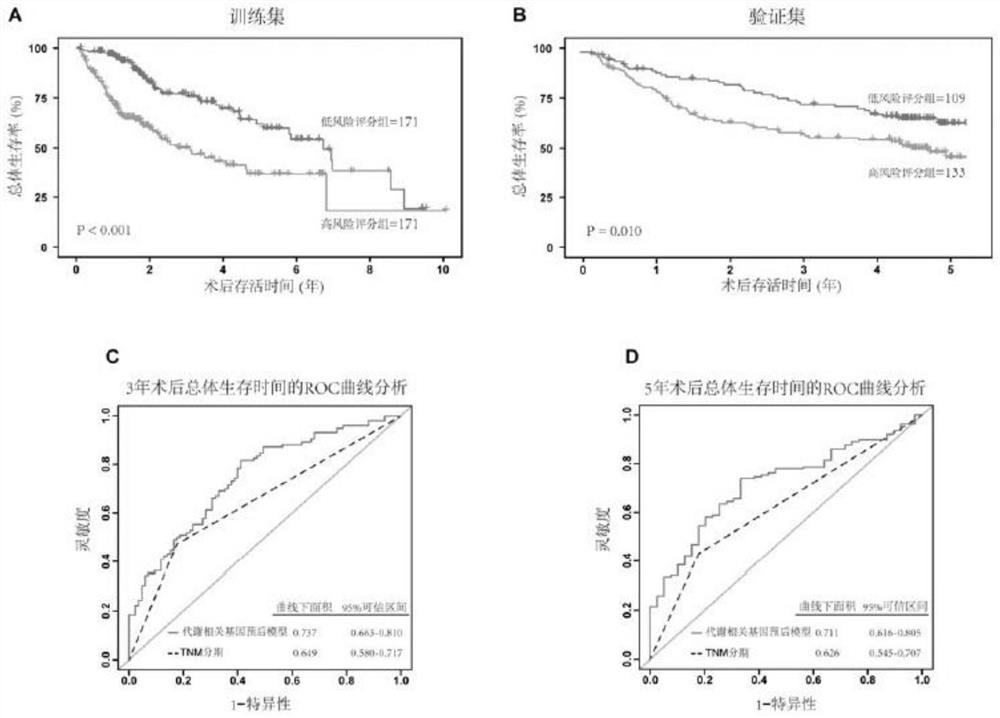
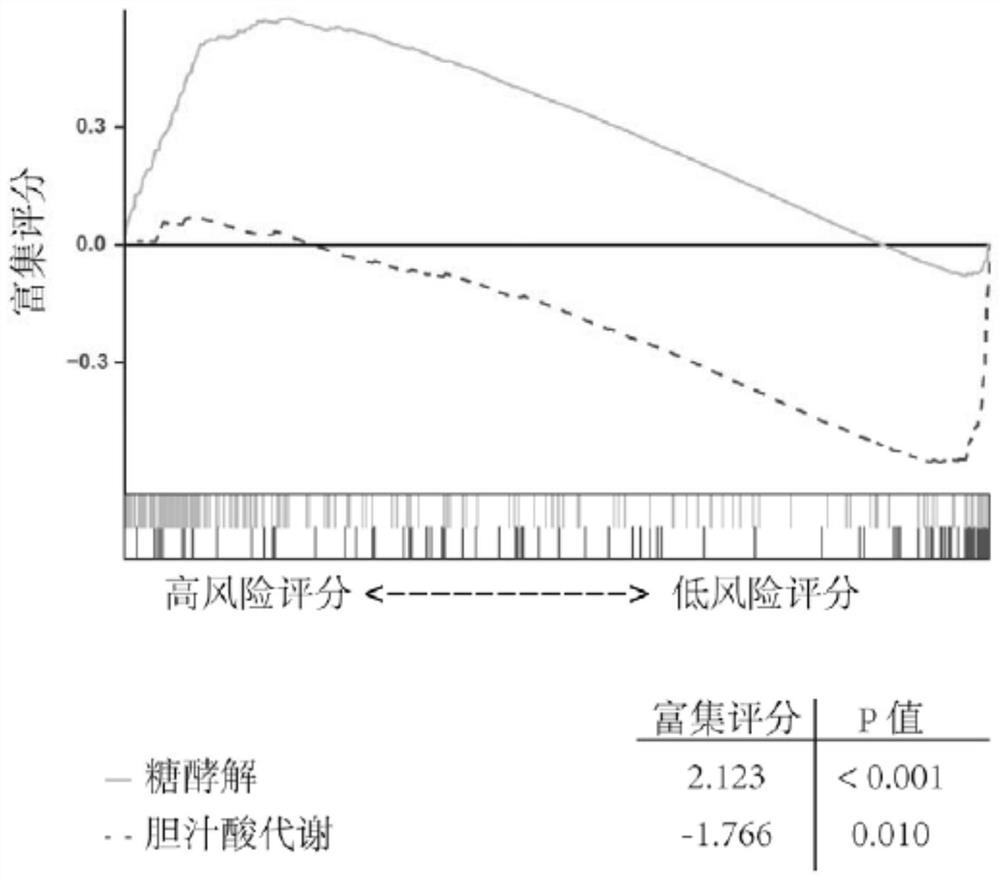
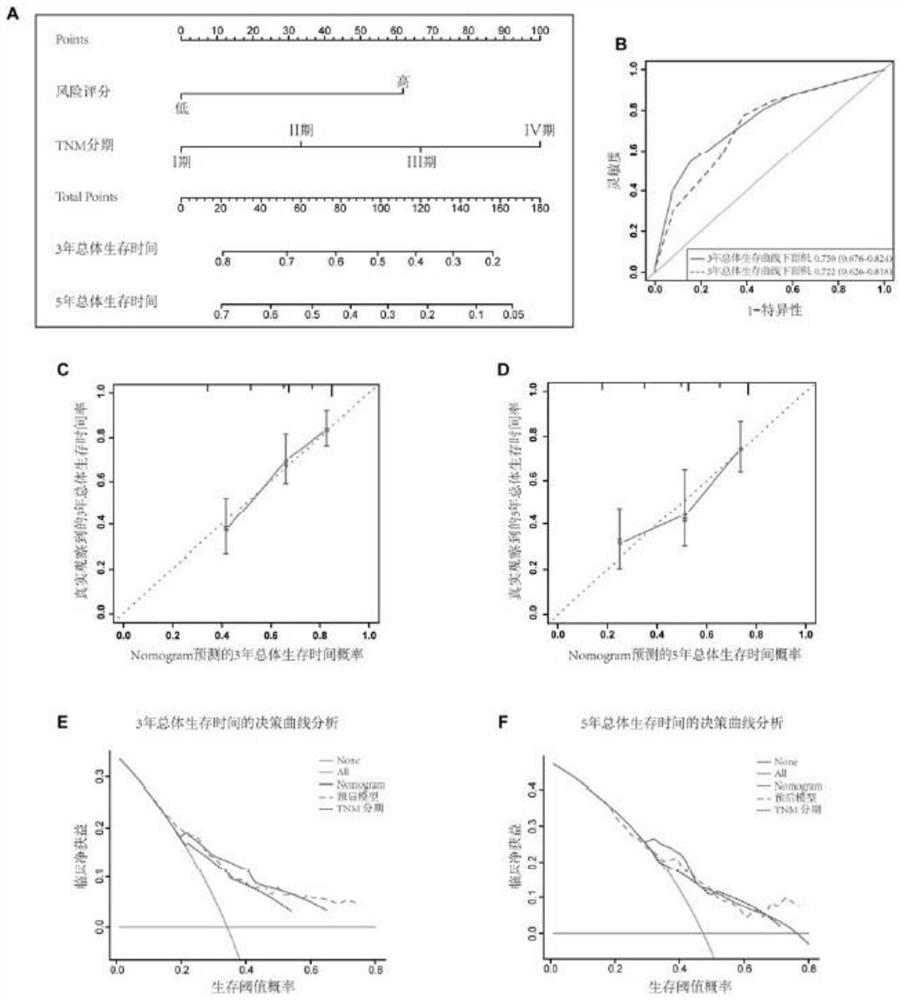
Immune gene prognosis model for predicting hepatocellular carcinoma tumor immune infiltration and postoperative survival time
ActiveCN112011616APromote the implementation of precision medicineObjective assessment of infiltrationMicrobiological testing/measurementBiostatisticsTNM staging systemMicroarray cgh
The invention relates to an immune gene prognosis model for predicting hepatocellular carcinoma tumor immune infiltration and postoperative survival time, and belongs to the technical field of biological medicines. The model can be used for evaluating the infiltration degree of immune cells in a tumor in clinical practice by detecting the expression levels of 22 specific immune related genes of ahepatocellular carcinoma patient, so that the model can be used for predicting hepatocellular carcinoma tumor immune infiltration in clinical practice and improve the prediction capability of the liver cancer immunotherapy response. The model can be used for judging the postoperative overall survival risk of a patient and guiding the formulation of a postoperative treatment strategy, and the corresponding microarray chip kit can realize the standardization and convenience of detection. Meanwhile, the immune gene prognosis model provided by the invention can increase the prediction accuracy andthe clinical net income of a hepatocellular carcinoma TNM staging system on the total survival time of three years and five years after operation. As a molecular marker for objectively and accuratelyevaluating the tumor immune state and poor prognosis risk of hepatocellular carcinoma, the model can realize accurate implementation of hepatocellular carcinoma immunotherapy and accurate prognosis prediction.
Owner:上海顿慧医疗科技发展有限公司
Diagnostics and methods for treatment of non-alcoholic hepatic steatosis and hepatic steatohepatitis, and prevention of complications thereof
ActiveUS20190255084A1Increased riskPrecise definitionPeptide/protein ingredientsDigestive systemHepatitis c viralLiver steatosis
The present invention is directed to a System characterization of NASH that combines Modeling and Biomarkers, enabling pharmaceutical compositions and methods of treatment that relate to the inhibition, resolution and / or prevention of Non Alcoholic Fatty Liver Disease (NAFLD) and Non Alcoholic Steatohepatitis (NASH). Said conditions are Liver related complications among the array of manifestations of metabolic syndromes, including Type 2 diabetes, hyperlipidemia, weight gain, abdominal obesity, insulin resistance, hypertension, atherosclerosis, fatty liver diseases and certain chronic inflammatory states that lead to these manifestations, among others. In additional aspects, the present invention relates to compositions and methods which may be used to treat, inhibit or reduce the likelihood of NASH and NAFLD complications in patients with hepatitis viral infections, including Hepatitis B and Hepatitis C viral infections, as well as the secondary disease states and / or conditions which are often associated with such viral infections, including hepatic steatosis (steatohepatitis), cirrhosis, fatty liver and hepatocellular cancer, metabolic syndrome complications including cardiovascular diseases, neurodegenerative diseases and premature ageing, among other disease states or conditions.
Owner:APHAIA PHARMA AG +1
Methylation markers for diagnosing hepatocellular carcinoma and lung cancer
ActiveUS20180274039A1Enhanced allele calling accuracyMicrobiological testing/measurementHepatocellular carcinomaOncology
Disclosed herein, in certain embodiments, are methods and kits for diagnosing a subject as having hepatocellular carcinoma (HCC) or lung cancer. In some instances, also described herein are methods of determining the prognosis of the subject having HCC or lung cancer. In additional instances, described herein are methods of determining the specific staging of HCC or lung cancer in a subject.
Owner:RGT UNIV OF CALIFORNIA +1
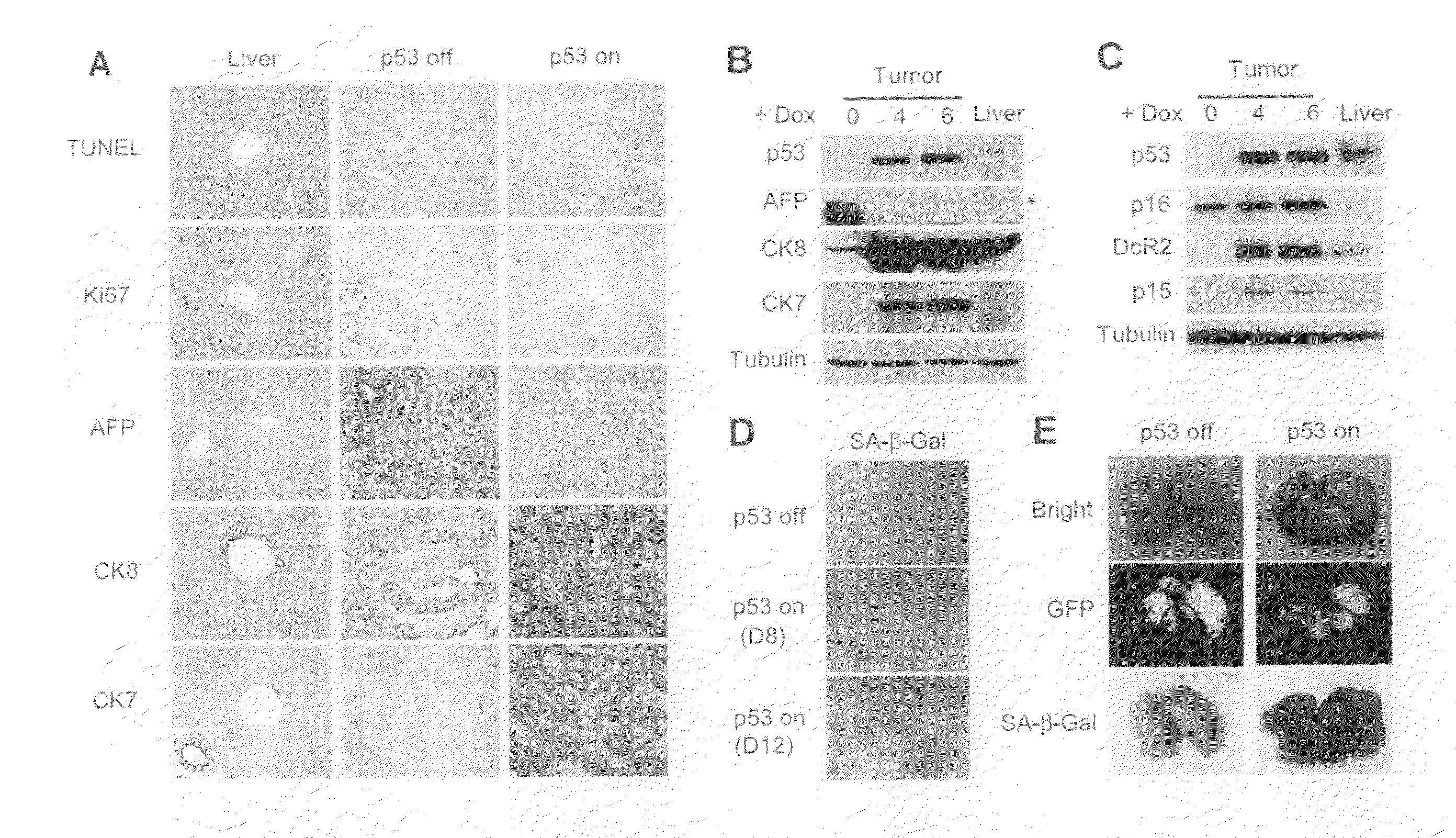
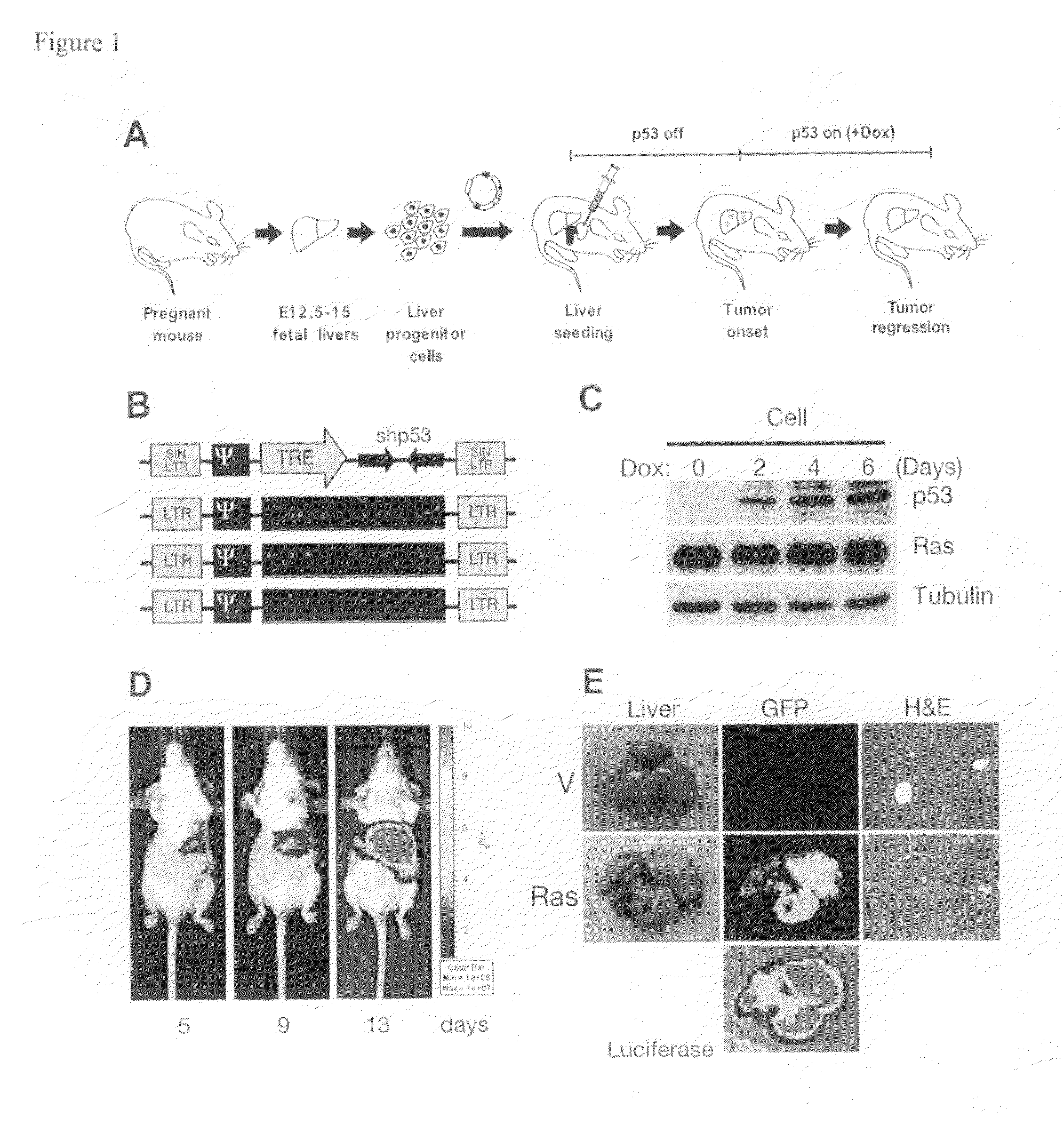
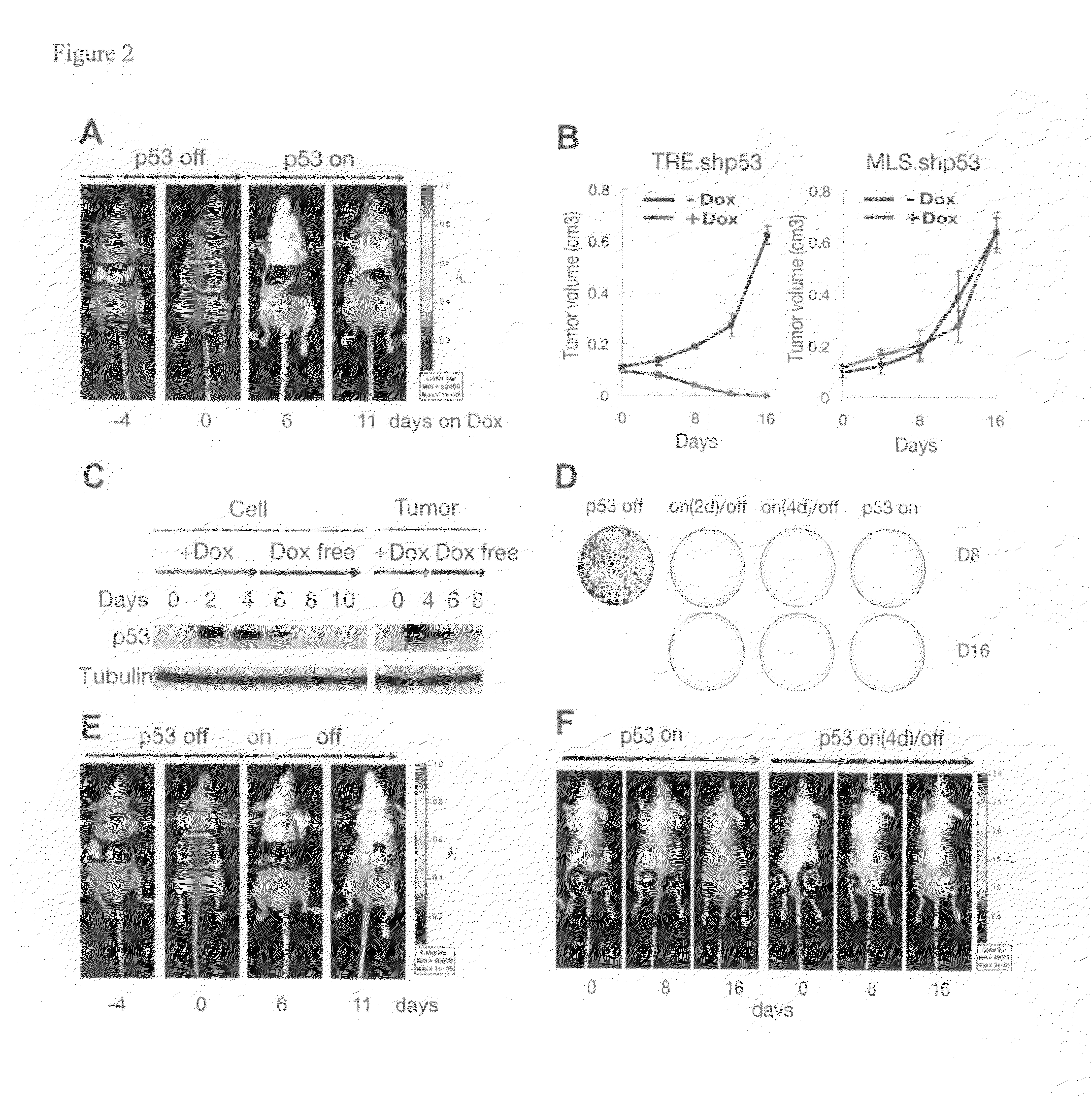
Orthotopic, controllable, and genetically tractable non-human animal model for cancer
InactiveUS20090022685A1High resolutionBiocideOrganic active ingredientsAbnormal tissue growthCooperative interaction
This invention provides a genetically tractable in situ non-human animal model for hepatocellular carcinoma. The model is useful, inter alia, in understanding the molecular mechanisms of liver cancer, in understanding the genetic alterations (e.g., in oncogenes and tumor suppressor genes) that lead to chemoresistance or poor prognosis, and in identifying and evaluating new therapies against hepatocellular carcinomas. The liver cancer model of this invention is made by altering hepatocytes to increase oncogene expression, to reduce tumor suppressor gene expression or both, preferably by inducible, reversible, and / or tissue specific expression of double-stranded RNA molecules that interfere with the expression of a target gene, and by transplanting the resulting hepatocytes into a recipient non-human animal. The invention further provides a method to treat cancer involving cooperative interactions between a tumor cell senescence program and the innate immune system.
Owner:COLD SPRING HARBOR LAB INC
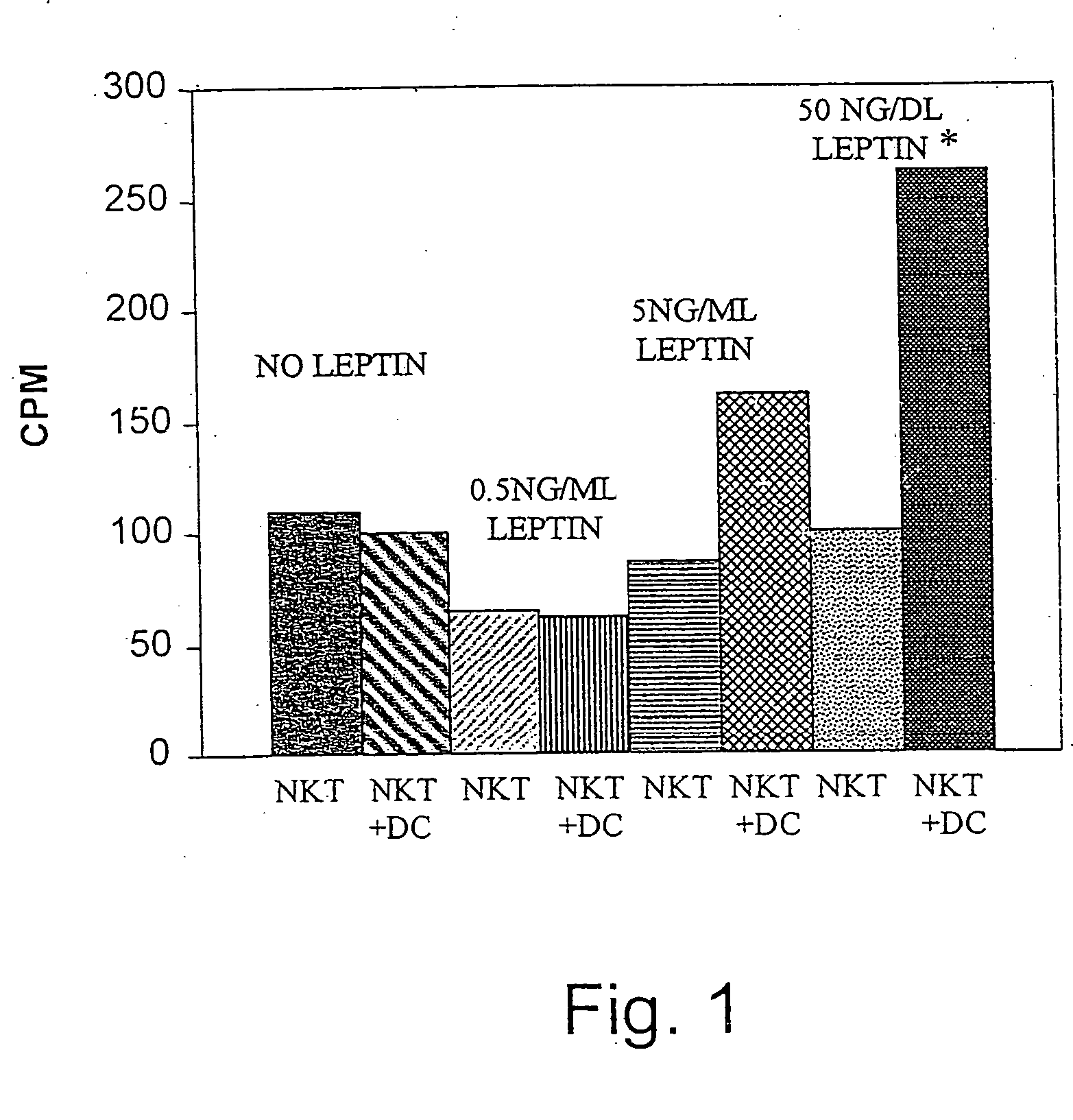
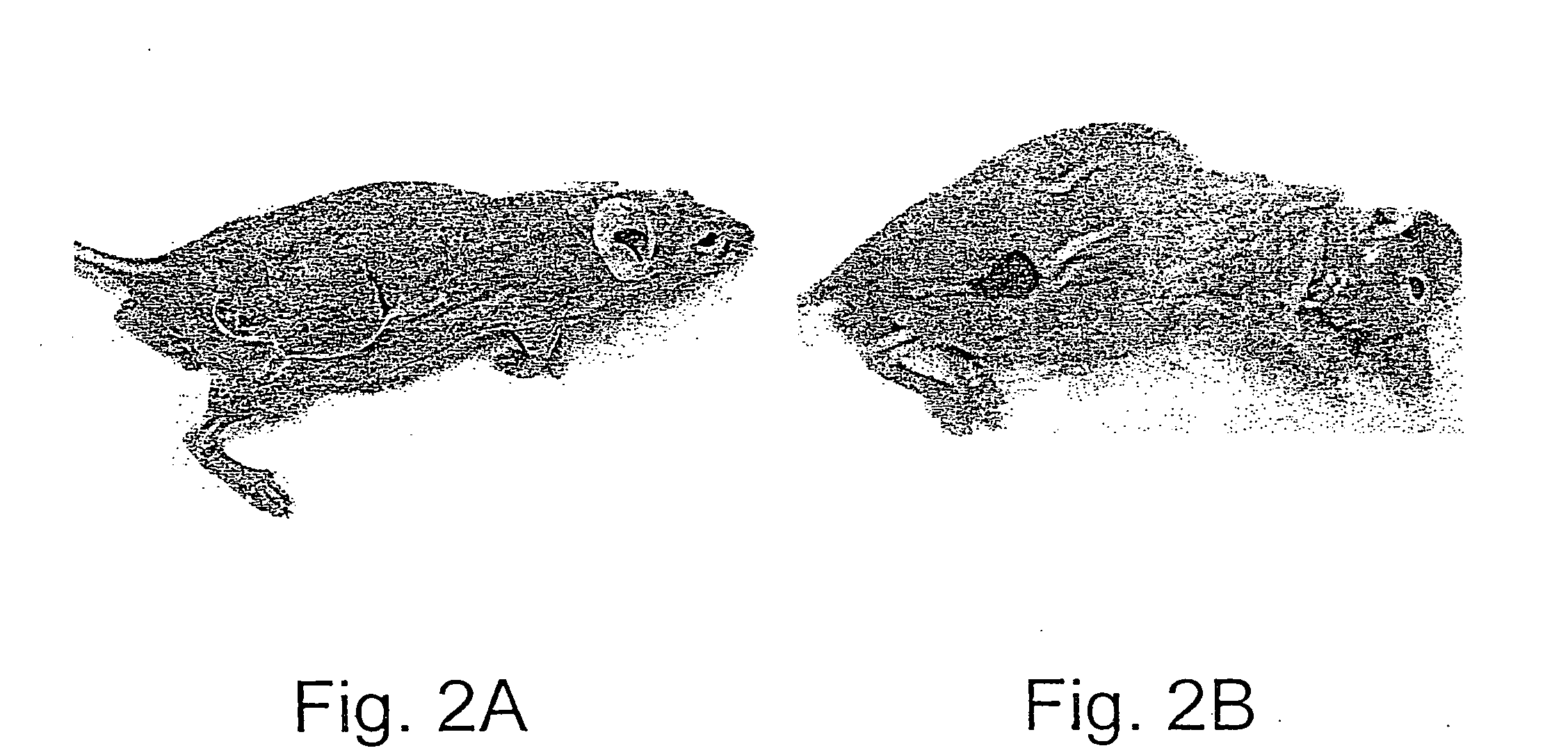
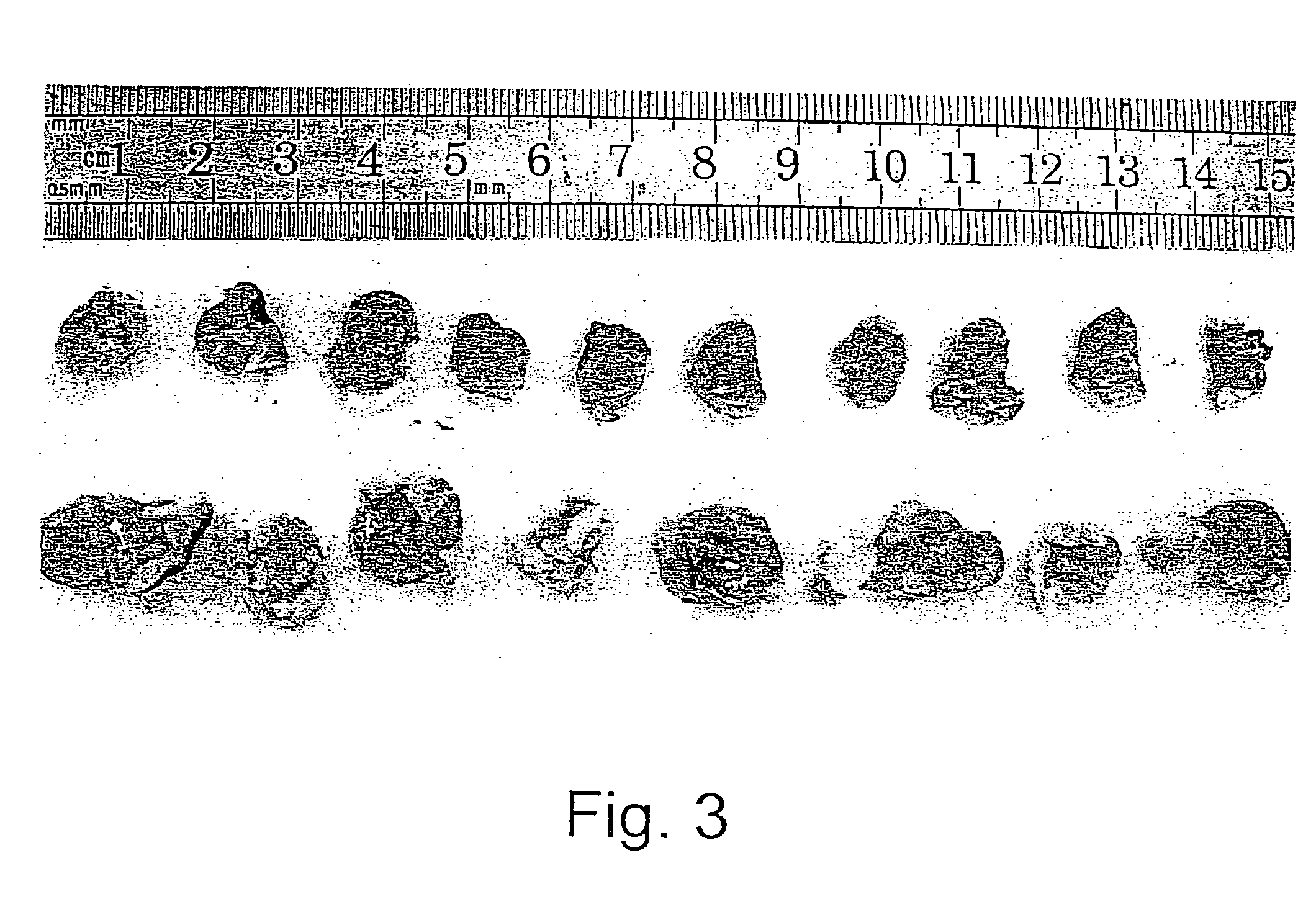
Methods and uses of leptin in immune modulation and hepatocellular carcinoma
Leptin was previously demonstrated to exert potent immune modulatory properties in several immune mediated disorders. The aim of the study was to determine leptin's anti-tumor effect in a murine model of human hepatocellular carcinoma (HCC). In vivo, Athymic T cell deficient (nude) mice transplanted with 1×106 human Hep3B cells, followed by administration of two daily intraperitoneal doses of 0.5 mg / gram leptin for 6 weeks. Leptin administration induced a significant reduction in tumor size and improved survival in nude mice. Histologically, tumors of leptin-administered mice featured increased inflammatory exudate in interphase areas. Leptin-induced tumor suppression was associated with a significant increase in peripheral natural killer (NK) cell number. Splenocytes from leptin-treated mice featured decreased expression of CIS mRNA. To determine which lymphocyte subset is a prerequisite for the anti tumor effect of leptin, T&B cell deficient (Scid) mice and T,B& NK deficient (Scid-Beige) mice were subcutaneously implanted with Hep3B tumor cells, with and without the daily intraperitoneal administration of 0.5 mg / gram leptin for 6 weeks. SCID mice featured leptin-associated tumor suppression similar to those of nude mice. In contrast, NK-deficient SCID-Beige mice developed larger tumors. To further establish natural killer cell's central role in mediation of leptin's anti-tumor effect, NK cells were incubated in vitro with increasing doses of leptin, demonstrating a dose-dependent increase in cytotoxic activity. Incubation of leptin with hepatoma cell line was found to induce a dose-dependent reduction in hepatoma cell proliferation, suggesting an additive direct anti-tumor effect. Further synergism in inhibition of hepatoma cell proliferation in vitro was achieved following addition of natural killer cells. HCC cells expressed leptin receptor mRNA, while addition of leptin induced increased mRMA expression of STAT2 and SOCS1 on tumor cell lines. Leptin administration induces a significant suppression of human HCC. This effect is mediated by induction of natural killer cell proliferation and activation, and by direct inhibition of tumor growth. Decreased natural killer cell expression of inhibitory CIS protein and over expression of the anti-proliferative STAT2 and SOCS1 proteins in HCC lines may underline both anti cancerous effects of leptin.
Owner:ENZO THERAPEUTICS
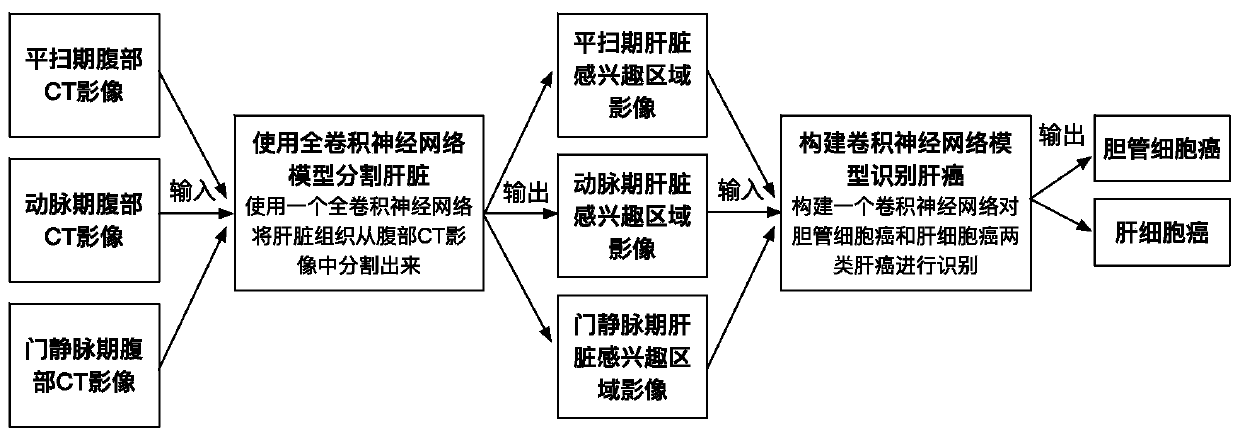
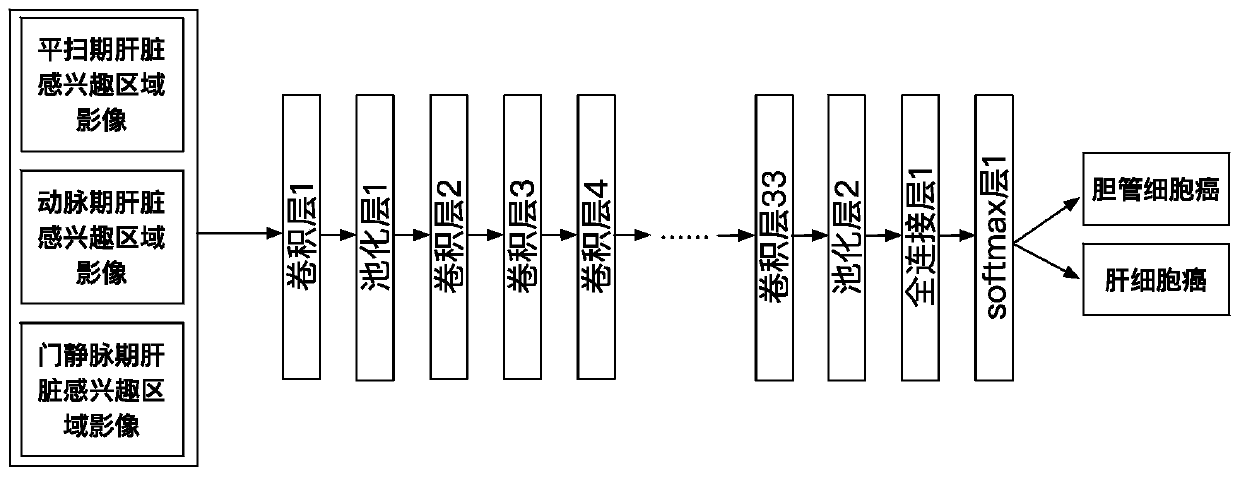
Automatic liver tumor classification method and device based on multi-stage CT image analysis
According to the liver tumor automatic classification method and device based on multi-stage CT image analysis, full-automatic bile duct cell carcinoma and hepatocellular carcinoma can be recognized,and a high-precision bile duct cell carcinoma and hepatocellular carcinoma recognition model is obtained. The method comprises the following steps: (1) acquiring a contrast-enhanced abdominal CT scanning image, storing the contrast-enhanced abdominal CT scanning image as an arterial phase, a portal vein phase and a delay phase, and carrying out definite diagnosis on liver cancer categories to which all data belong to serve as a model training gold standard; (2) constructing a three-dimensional full convolutional neural network segmentation model, and segmenting the intrinsic characteristics ofthe liver tissue in each stage from the abdominal CT image through model training learning; (3) constructing a three-dimensional convolutional neural network classification model; and inputting the image data obtained by segmentation into a classification model for training, so as to enable the model to perform joint learning and training on the cancer features in multiple periods, thereby predicting the category to which the cancer belongs, comparing the prediction result with a gold standard, and supervising the training process of the model in a loss value feedback mode.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
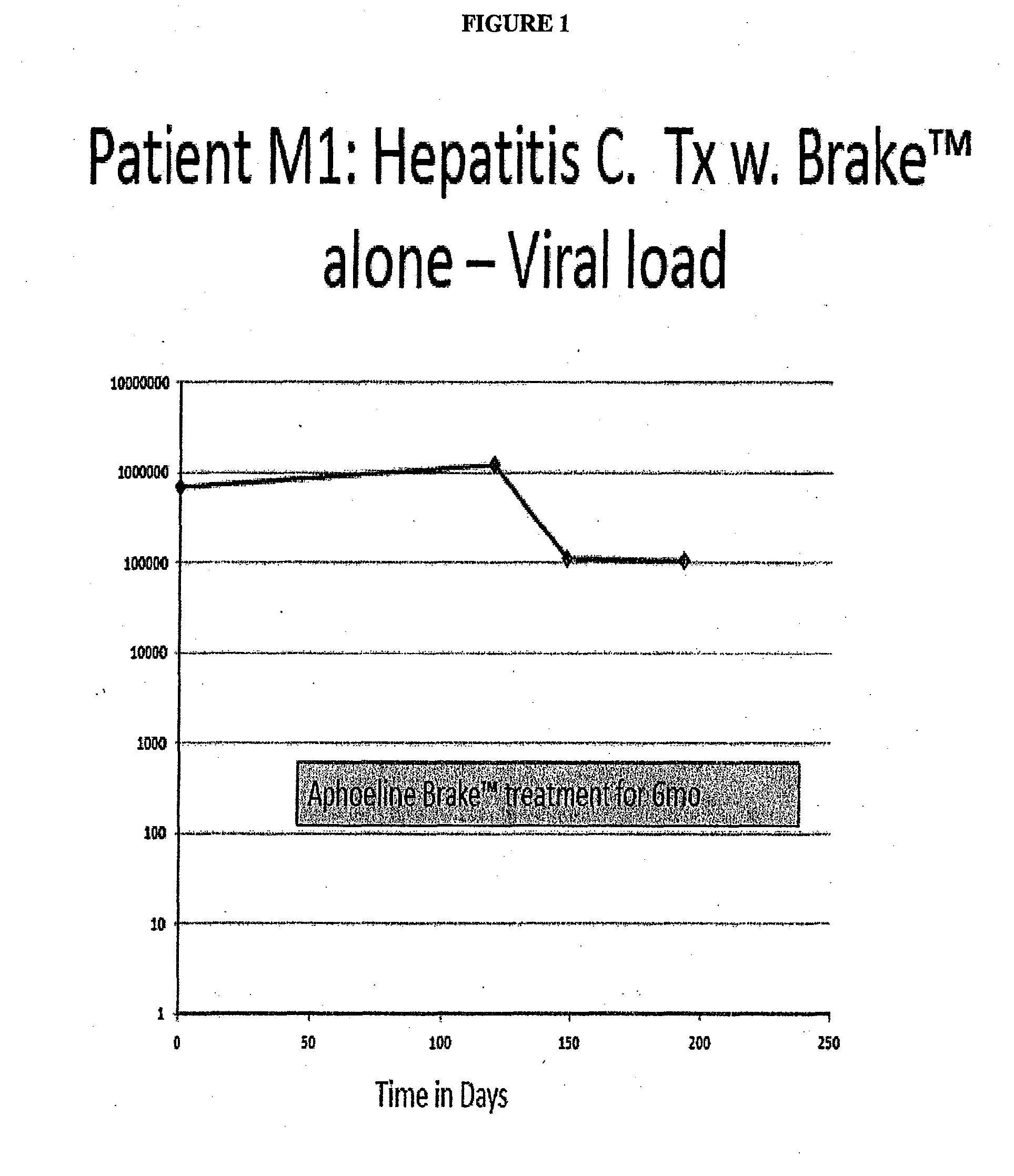
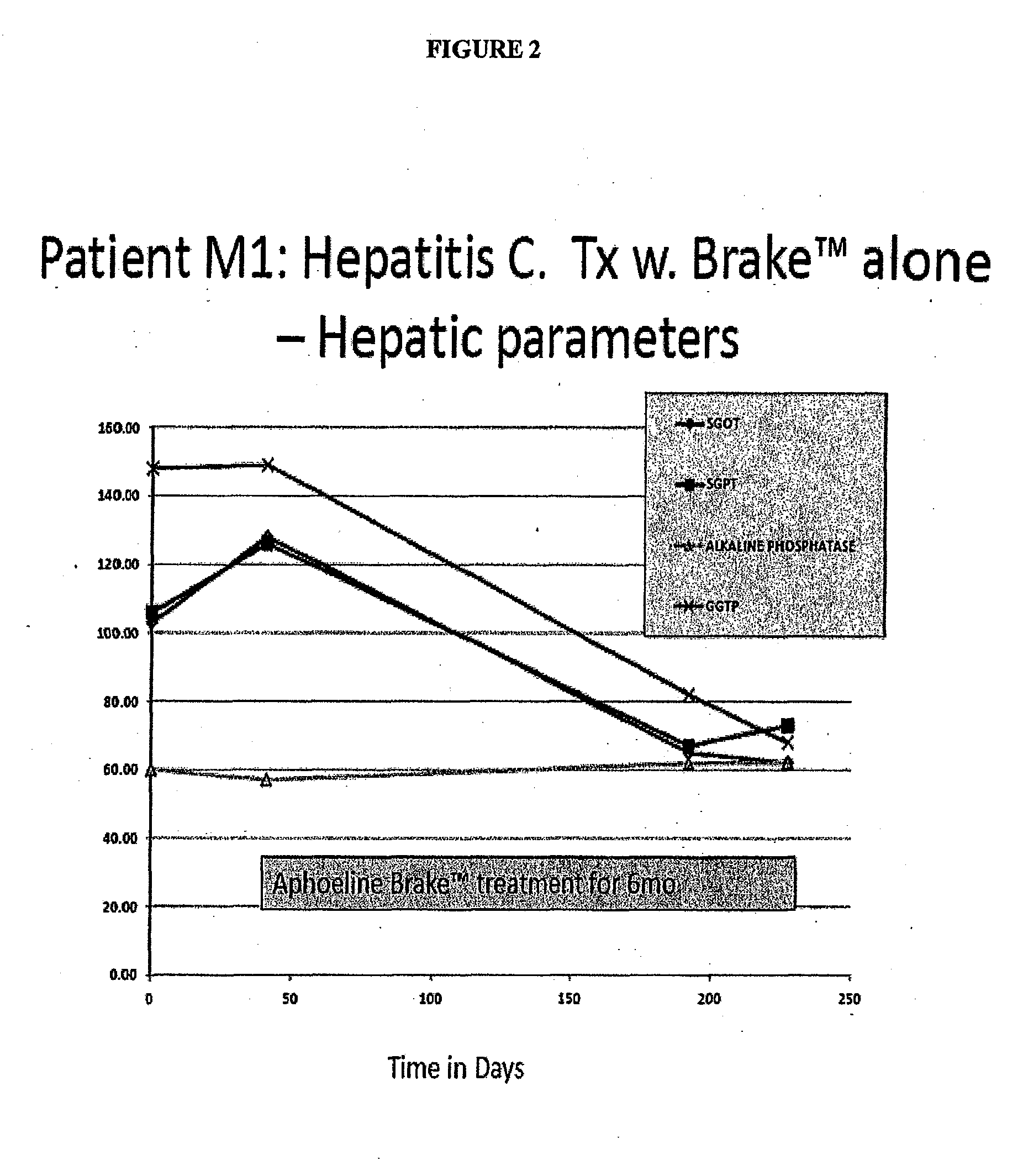
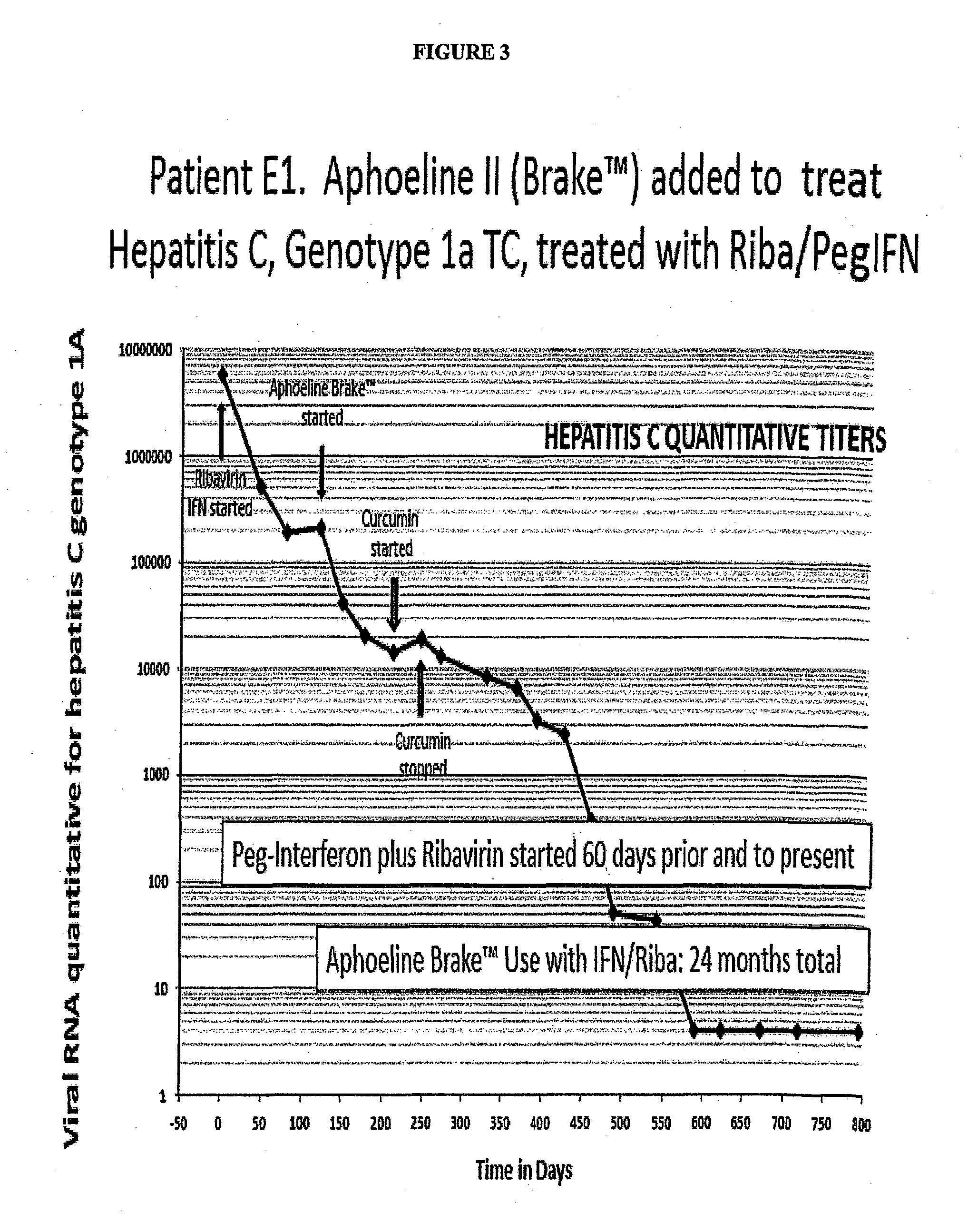
Compositions, methods of treatment and diagnostics for treatment of hepatic steatosis alone or in combination with a hepatitis c virus infection
ActiveUS20130337055A1Reduce the possibilityIncrease opportunitiesBiocidePeptide/protein ingredientsHepatocellular carcinomaInsulin resistance
The present invention is directed to pharmaceutical compositions and methods of treatment that relate to the inhibition, resolution and / or prevention of an array of the manifestations of metabolic syndromes, including Type 2 diabetes, hyperlipidemia, weight gain, obesity, insulin resistance, hypertension, atherosclerosis, fatty liver diseases and certain chronic inflammatory states that lead to these manifestations, among others. In additional aspects, the present invention relates to compositions and methods which may be used to treat, inhibit or reduce the likelihood of hepatitis viral infections, including Hepatitis B and Hepatitis C viral infections, as well as the secondary disease states and / or conditions which are often associated with such viral infections, including hepatic steatosis (steatohepatitis), cirrhosis, fatty liver and hepatocellular cancer, among other disease states or conditions.
Owner:SAPIENZA RES LLC +1
Quinoline derivatives as anti-cancer agents
Quinoline derivatives showing anticancer activities against cancer cell lines of hepatocellular carcinoma (Hep3B), lung carcinoma (A549), esophageal squamous cell carcinoma (HKESC-1, HKESC-4 and KYSE150). The quinoline derivatives have a backbone structure of the following formulas:
Owner:THE HONG KONG POLYTECHNIC UNIV
Human hepatocellular carcinoma cell line, and establishing method and application thereof
PendingCN104745530AStable traitsTumorigenicMicrobiological testing/measurementMicroorganism based processesPrimary hepatic carcinomaUnknown primary
The invention discloses a human hepatocellular carcinoma cell line, and an establishing method and application thereof. The human hepatocellular carcinoma cell line is preserved in China Center for Type Culture Collection with an accession number of CCTCC No: C201114. The establishing method comprises the following steps: 1) acquiring a fresh clinical liver cancer excision specimen, cutting the specimen into small blocks with sizes in a range of 20 to 50 mg and inoculating mammals with the small blocks; and 2) in 70 to 90 d after inoculation, killing tumor-bearing mammals, taking out tumor tissue and carrying out primary culture and subculturing of cancer cells. The human hepatocellular carcinoma cell line provided by the invention has stable properties and tumorigenicity, can realize stable several passages and successful preparation of a liver cancer animal model, can be used for analyzing in-vivo and in-vitro drug susceptibility so as to further realize establishment of mutually-related in-vivo and in-vitro drug screening platforms, and is an ideal human primary hepatic carcinoma cell line applied to fundamental research and preclinical application.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV +1
Diagnosis and treatment of cancer by using Anti-prg-3 antibody
InactiveUS20100111851A1LevelImprove the level ofIn-vivo radioactive preparationsMicrobiological testing/measurementAnticarcinogenCancer type
The present invention discloses an antibody capable of binding to the PRG-3 protein and inhibiting the growth of cells expressing the PRG-3 protein. The growth inhibitory activity is cytotoxic activity, such as antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. The present invention also discloses a pharmaceutical composition, cell growth inhibitor, and an anticancer agent comprising the antibody of the present invention as an active ingredient. Examples of the type of cancer include hepatocellular carcinoma, lung cancer, colon cancer, and glioblastoma. In addition, the present invention discloses a method for diagnosing cancer by detecting expression of the PRG-3 protein or the gene encoding the PRG-3 protein, as well as a diagnostic agent and kit for use in the method.
Owner:THE UNIV OF TOKYO +1
Method for establishing hepatocellular carcinoma postoperative risk assessment model
PendingCN112331343AGuide individualized treatmentEase of evaluationHealth-index calculationMicrobiological testing/measurementTumour metabolismParanasal Sinus Carcinoma
The invention discloses a method for establishing a hepatocellular carcinoma postoperative risk assessment model, the model comprises 10 metabolism-related gene calculation items, the sum of the expression level of each gene weighted by a corresponding coefficient is used as a risk score, the risk of poor postoperative overall survival of a patient can be judged, and tumor metabolism characteristics are reflected.
Owner:JUSBIO SCI SHANGHAI CO LTD
GPC3 receptor targeted polypeptide radioactive diagnosis or therapeutic drug
InactiveCN107019807AImprove efficiencyRadioactive preparation carriersPharmaceutical non-active ingredientsMedicineTreatment of lung cancer
The invention relates to a GPC3 (glypican-3) receptor targeted polypeptide radioactive diagnosis or therapeutic drug. The GPC3 receptor targeted polypeptide radioactive diagnosis or therapeutic drug includes: polypeptide with an amino acid sequence shown as SEQ ID NO:1; and a radionuclide or positron-emitting nuclide marker coupled to the polypeptide. The invention adopts the radionuclide labeled polypeptide as a hepatocellular carcinoma specific targeting molecular probe to show the expression situation of the GPC3 receptor in hepatocellular carcinoma tumor tissue on a living body level, and improves the effective rate of hepatocellular carcinoma oncotherapy. For lung cancer, melanoma and other GPC3 receptor-positive tumors, the GPC3 receptor targeted polypeptide can also be used for preparation of drugs for diagnosis or treatment of lung cancer and melanoma.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
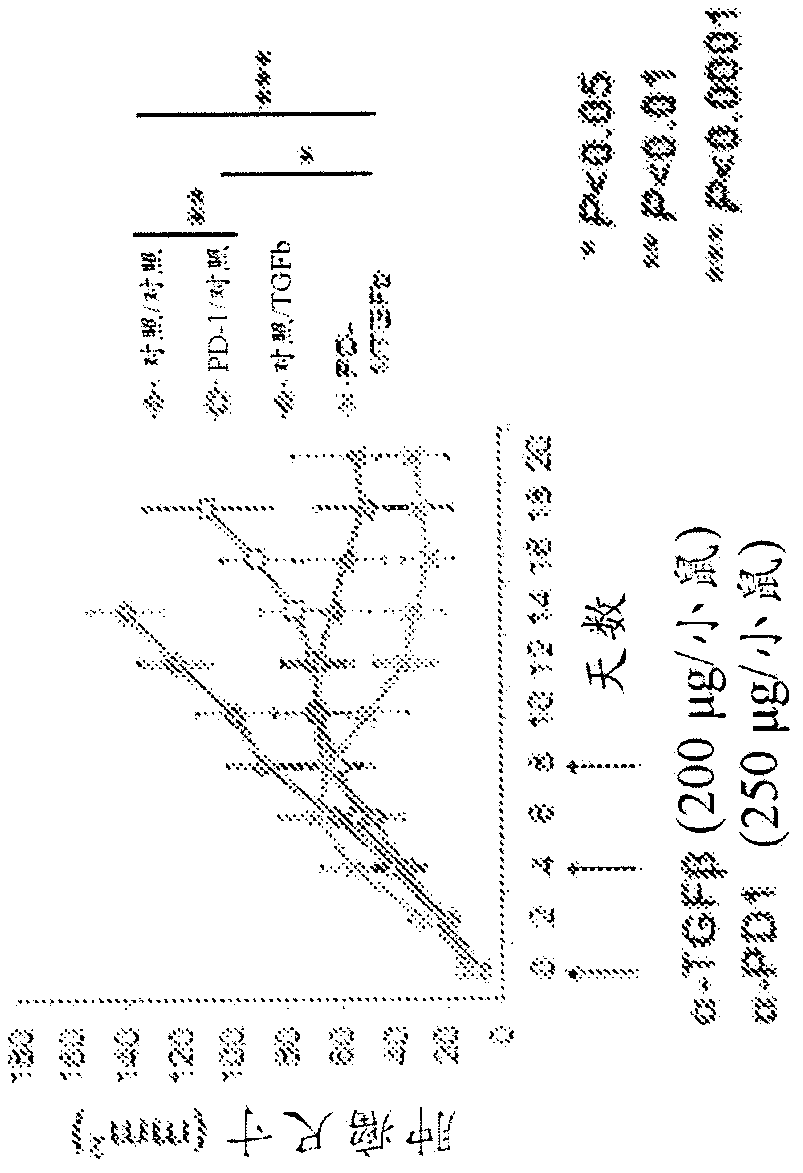
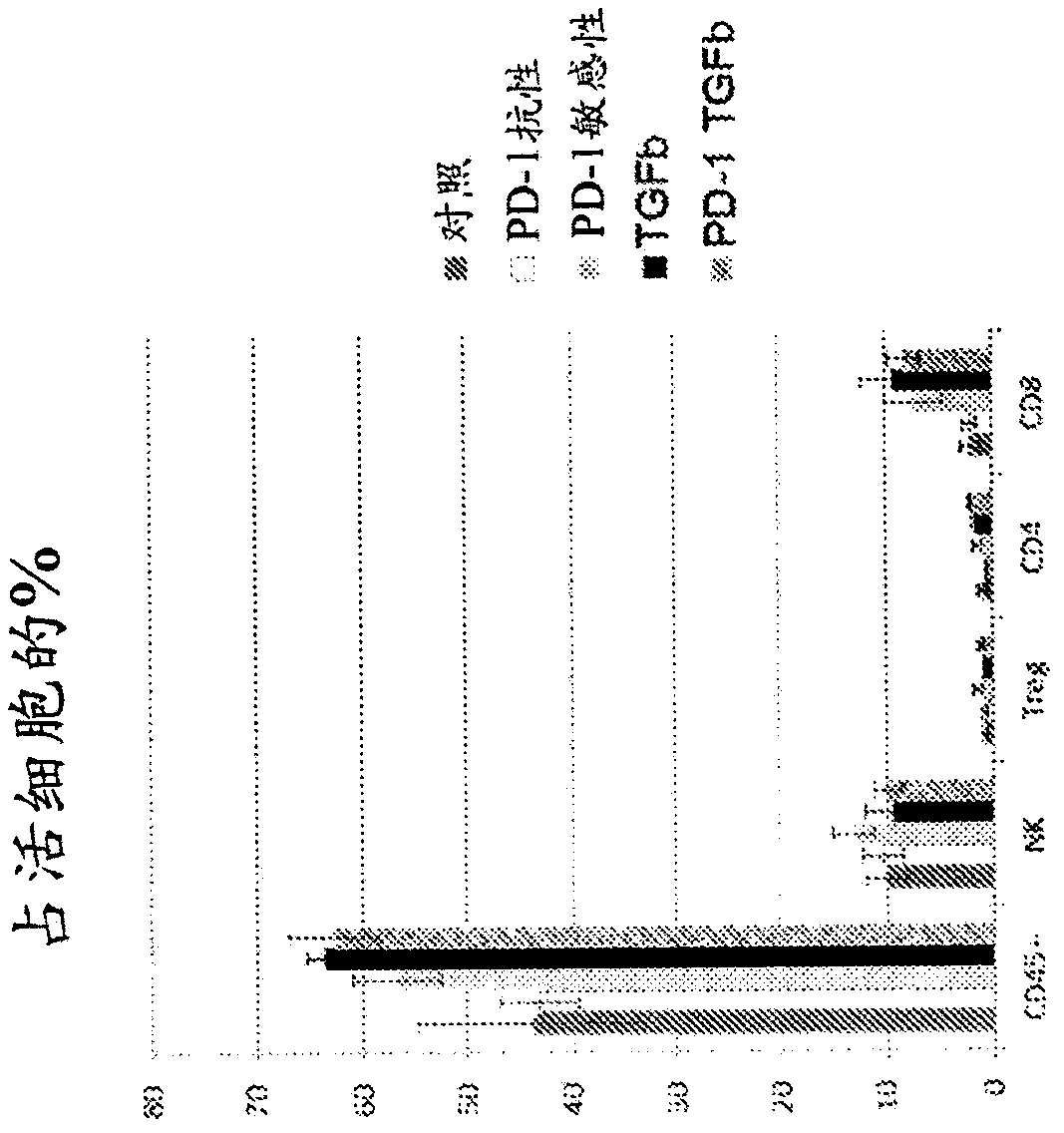
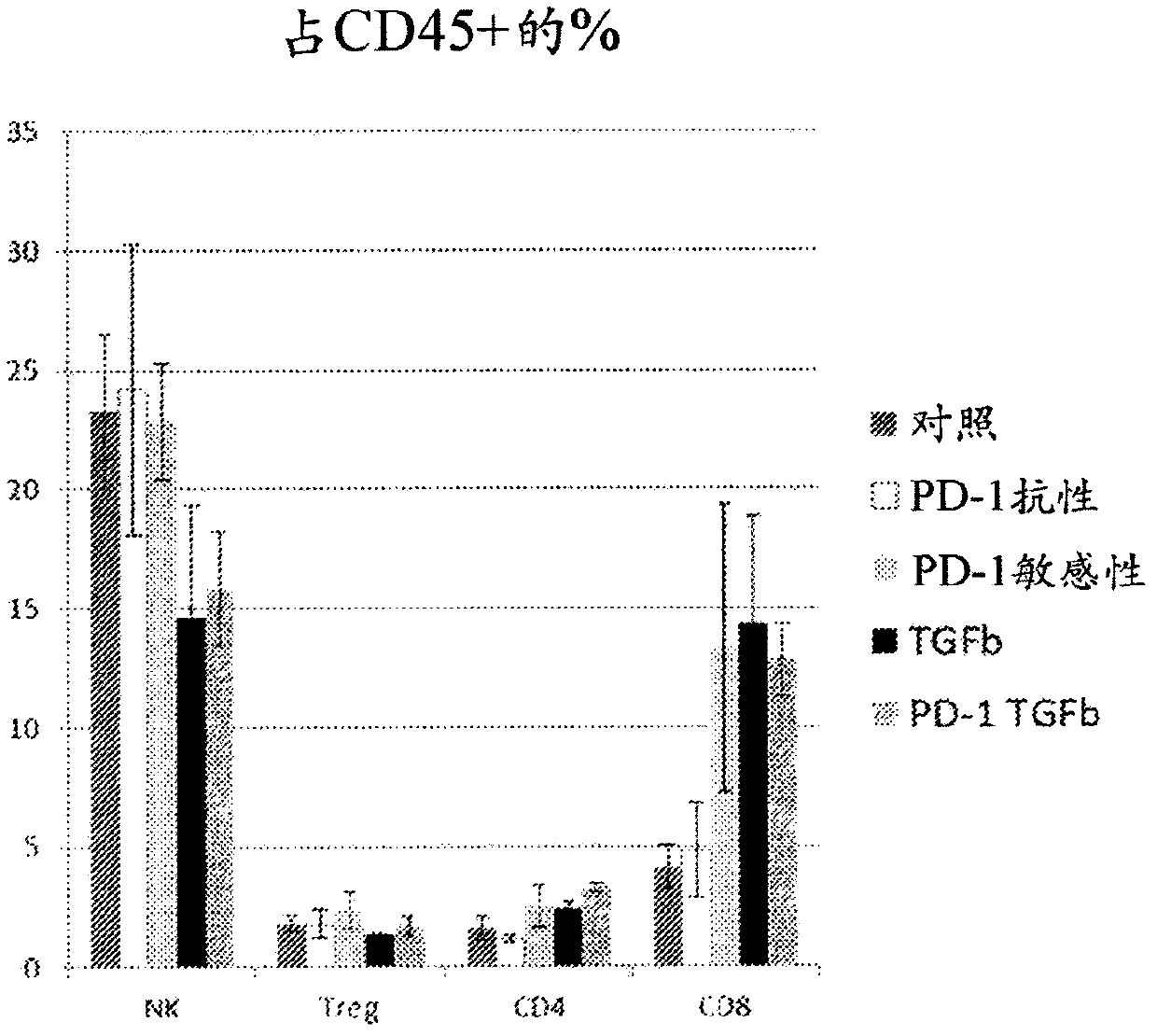
Treatment of cancer using inhibitors of tgf-beta and pd-1
ActiveCN108136001AImmunoglobulins against growth factorsAntibody ingredientsDiseaseEsophagus Cancers
The present disclosure relates, in general, to combination therapy using an inhibitor of transforming growth factor beta (TGFP) and an inhibitor of programmed cell death protein 1 (PD-1) for treatingcancer or preventing recurrence of cancer diseases such as lung cancer, prostate cancer, breast cancer, hepatocellular cancer, esophageal cancer, colorectal cancer, pancreatic cancer, bladder cancer,kidney cancer, ovarian cancer, stomach cancer, fibrotic cancer, glioma and melanoma, and metastases thereof.
Owner:XOMA TECH LTD +1
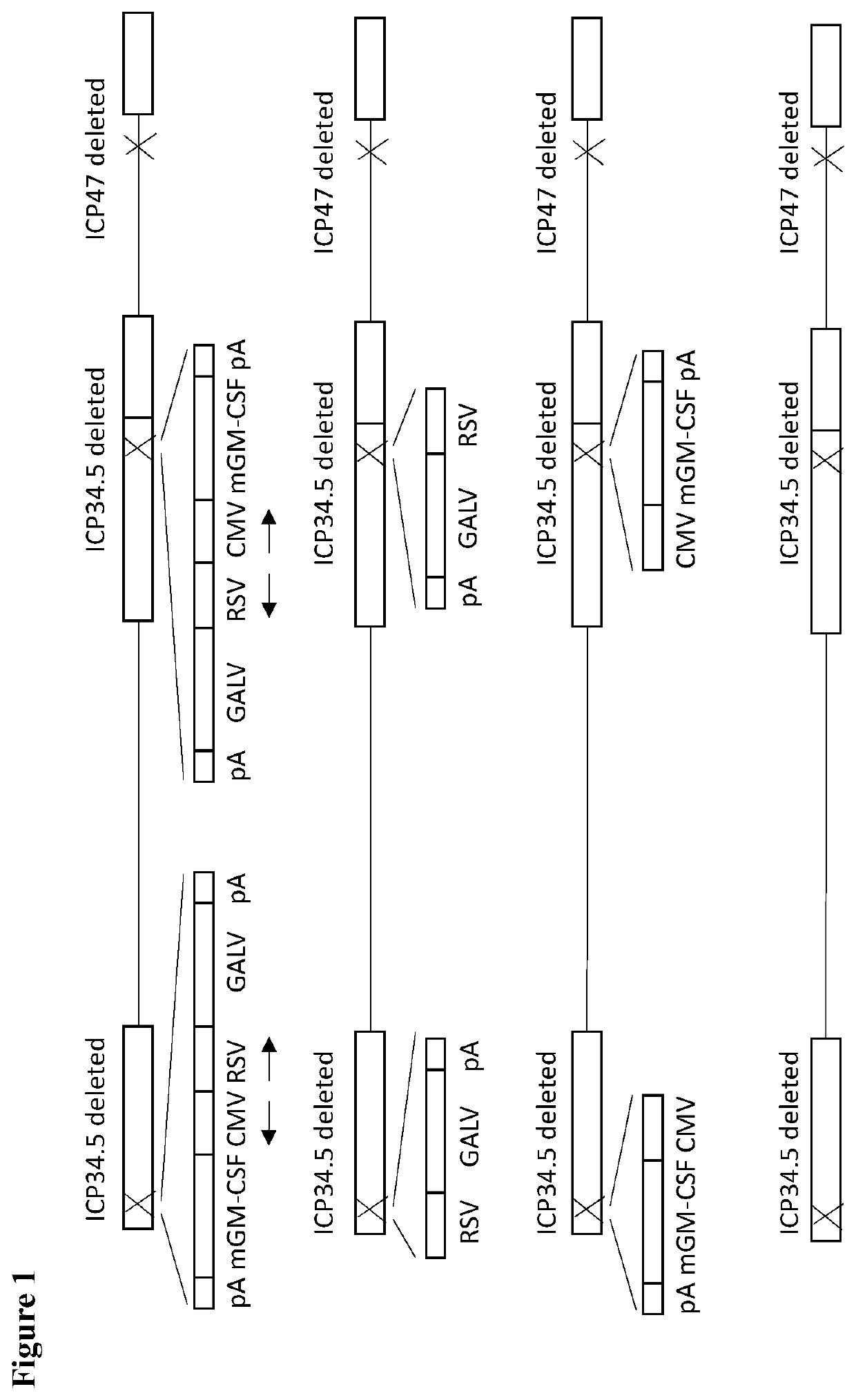
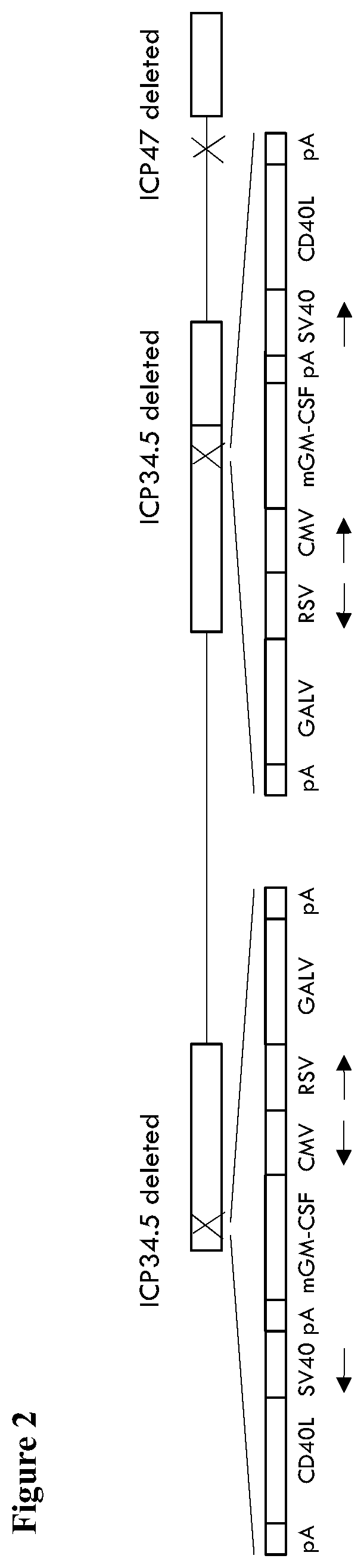
Treatment using oncolytic virus
PendingUS20210252135A1Direct effectImprove immunityPeptide/protein ingredientsViral antigen ingredientsAppendiceal carcinomaSquamous Carcinomas
An oncolytic virus for use in a method of treating or preventing cutaneous squamous cell carcinoma (CSCC), renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), small cell lung cancer (SCLC), advanced recurrent head and neck cancer, squamous cell carcinoma of the head and neck (SCCHN), nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), anal cancer, colorectal cancer (CRC), basal cell carcinoma (BCC), Merkel cell carcinoma, appendiceal carcinoma, sarcoma of the skin, recurrent melanoma after surgery, advanced or metastatic urothelialcarcinoma, liver metastases, microsatellite instability high cancer (MSI-H), mixed advanced solid tumors, virally caused cancer, locoregionally advanced cancer, pediatric cancer, cancer in patients with no or minimal pre-existing anti-cancer immunity, cancer as first line therapy, cancer in previously treated patients, cancer in patients who have not received checkpoint blockade therapy, and / or cancer in patients who have received checkpoint blockade therapy, wherein the oncolytic virus: is, or is derived from, a clinical isolate which has been selected by comparing the abilities of a panel of three or more clinical isolates of the same viral species to kill tumor cells of two or more tumor cell lines in vitro and selecting a clinical isolate which is capable of killing cells of two or more tumor cell lines more rapidly and / or at a lower dose in vitro than one or more of the other clinical isolates in the panel; comprises (i) a fusogenic protein-encoding gene; and (ii) an immune stimulatory molecule or an immune stimulatory molecule-encoding gene; comprises (i) a GM-CSF-encoding gene; and (ii) an immune co-stimulatory pathway activating molecule or an immune co-stimulatory pathway activating molecule-encoding gene; and / or comprises a gene encoding a CTLA-4 inhibitor.
Owner:REPLIMUNE
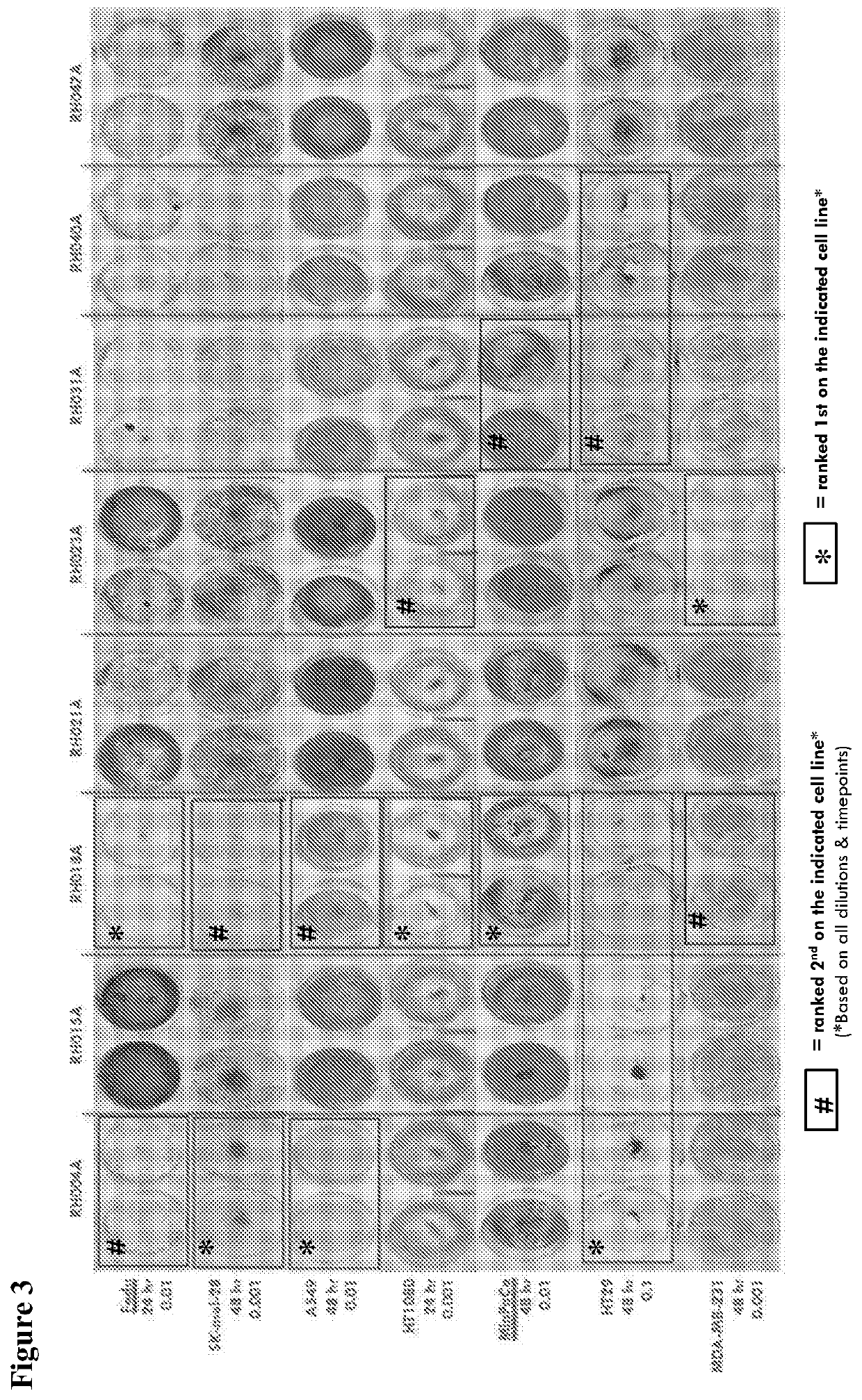
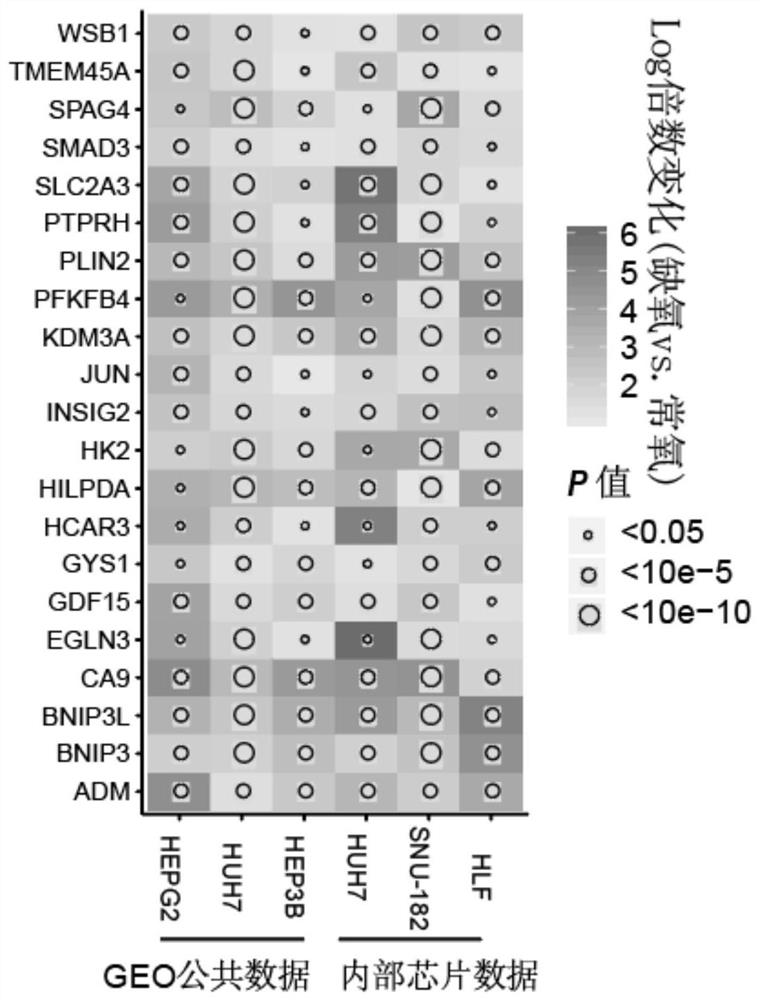
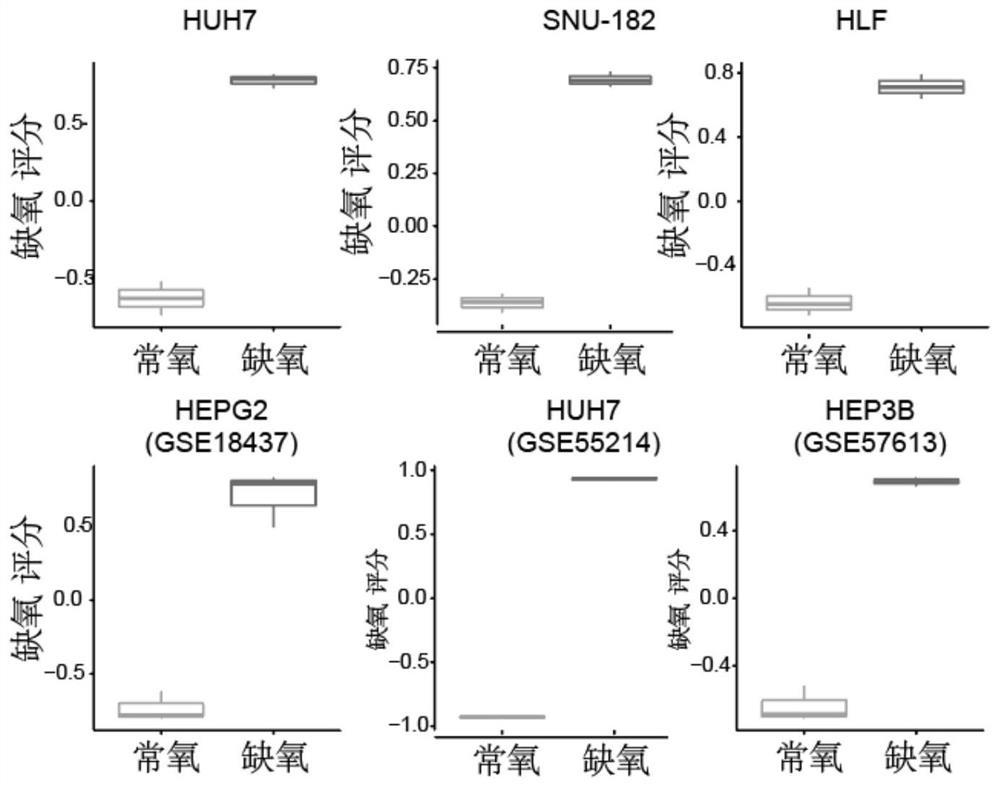
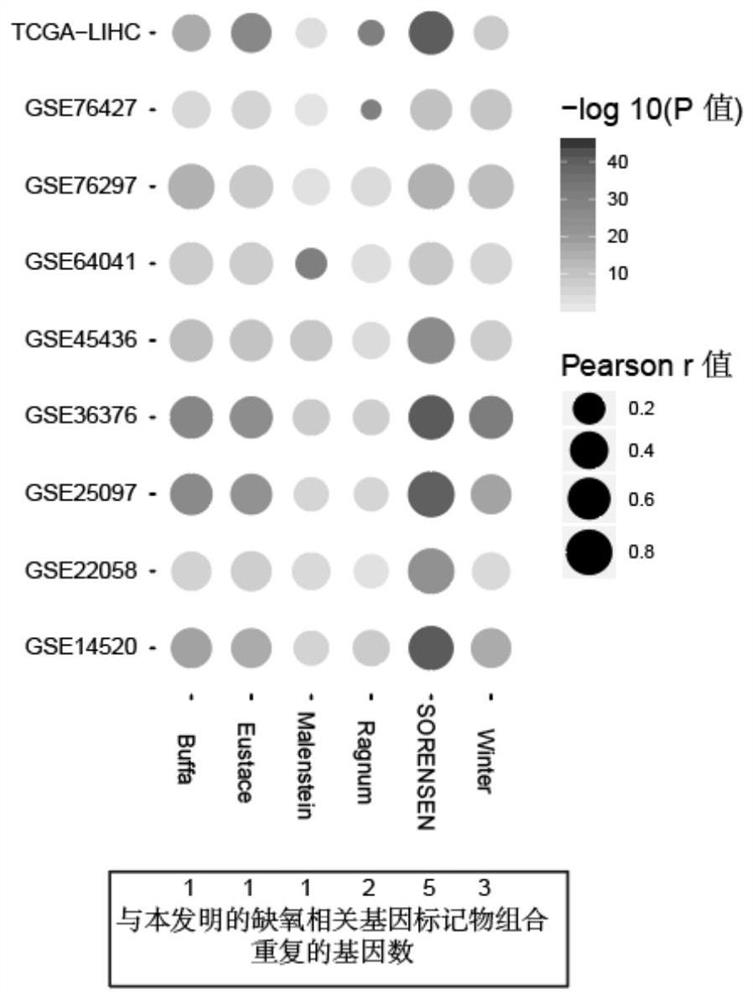
Hypoxia-related gene marker combination for hepatocellular carcinoma and application thereof
PendingCN113046436AReflects hypoxic exposureMicrobiological testing/measurementProteomicsGenetics genomicsOncology
The invention provides a hypoxia-related gene marker combination for hepatocellular carcinoma and application thereof. A hypoxia score obtained by using the hypoxia-related gene marker combination can better reflect the hypoxia exposure condition of hepatocellular carcinoma tissues, and the hepatocellular carcinoma is subjected to typing and prognosis analysis. Meanwhile, the hypoxia-related gene marker combination is used for comprehensively exploring the change of hypoxia-related molecular markers in the hepatocellular carcinoma from multiple aspects of genomics, epigenomics, transcriptomics, proteomics and the like, a more complete hepatocellular carcinoma hypoxia-related molecular landscape is described, meanwhile, the immune microenvironment change of liver cancer is analyzed, and more valuable information is provided for diagnosis and treatment of the hepatocellular carcinoma.
Owner:SHENZHEN PEOPLES HOSPITAL
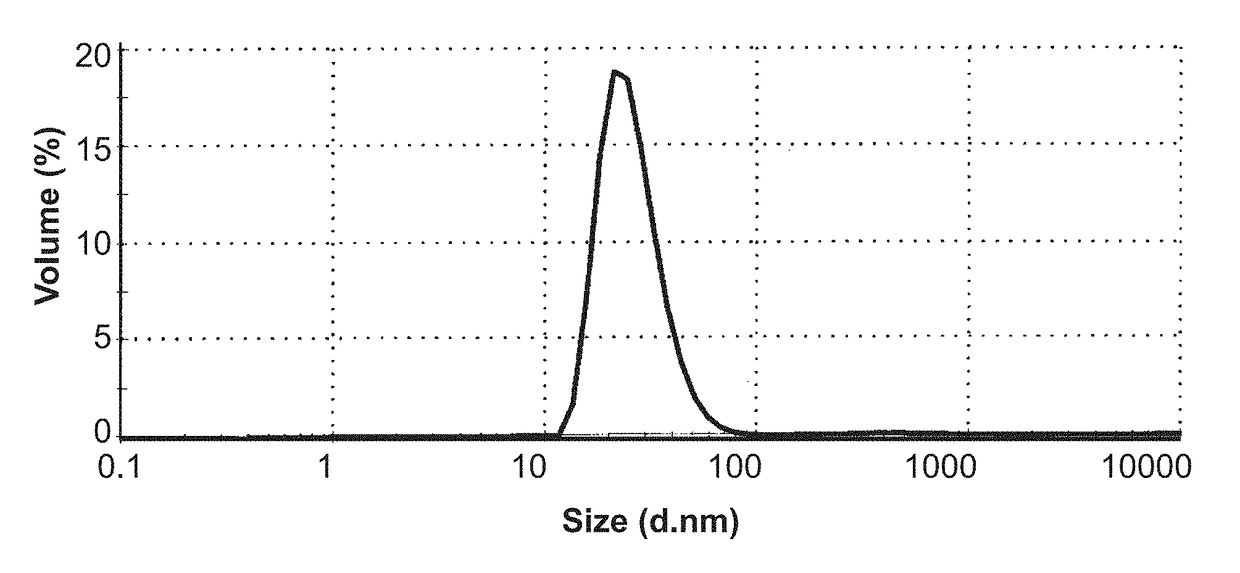
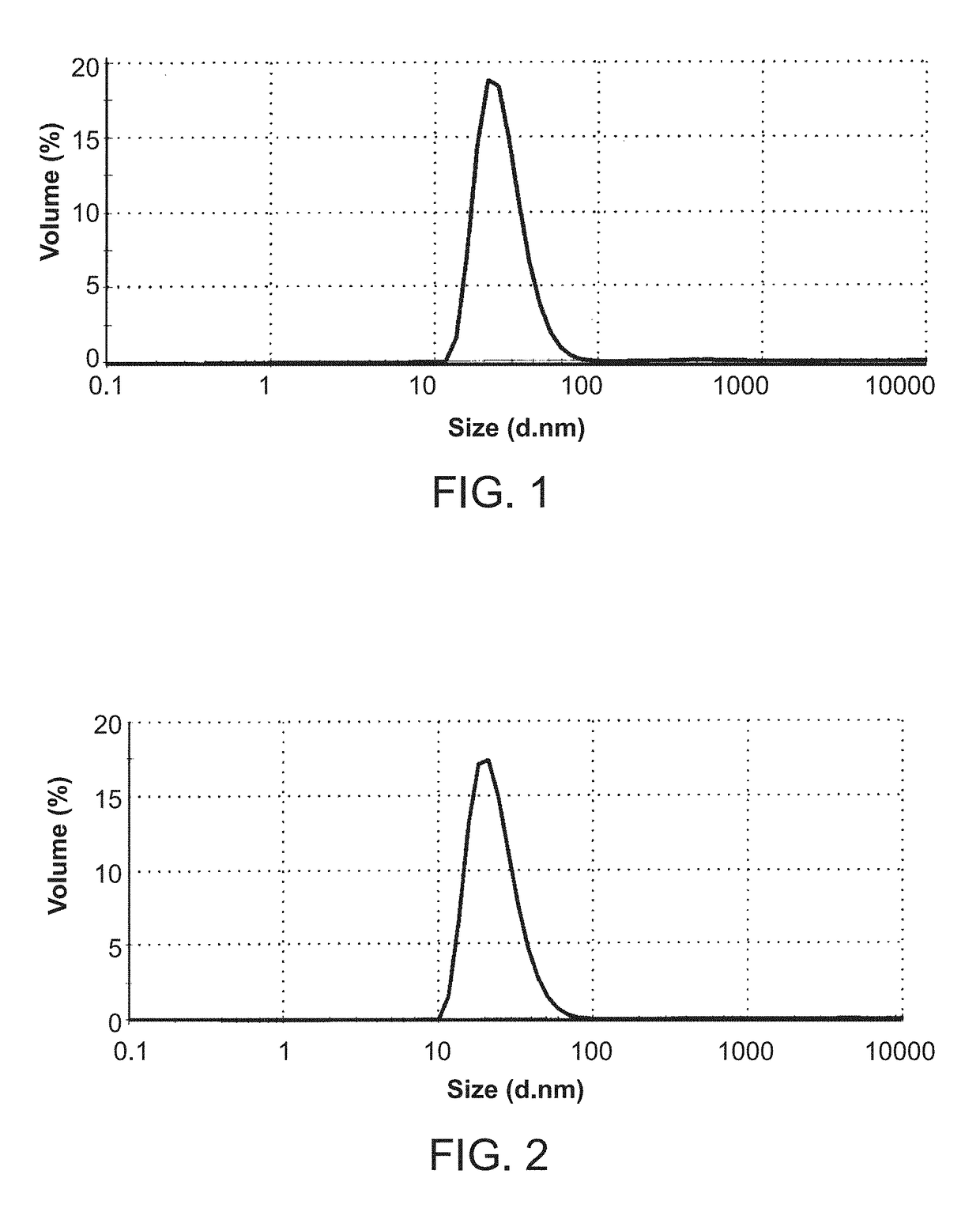
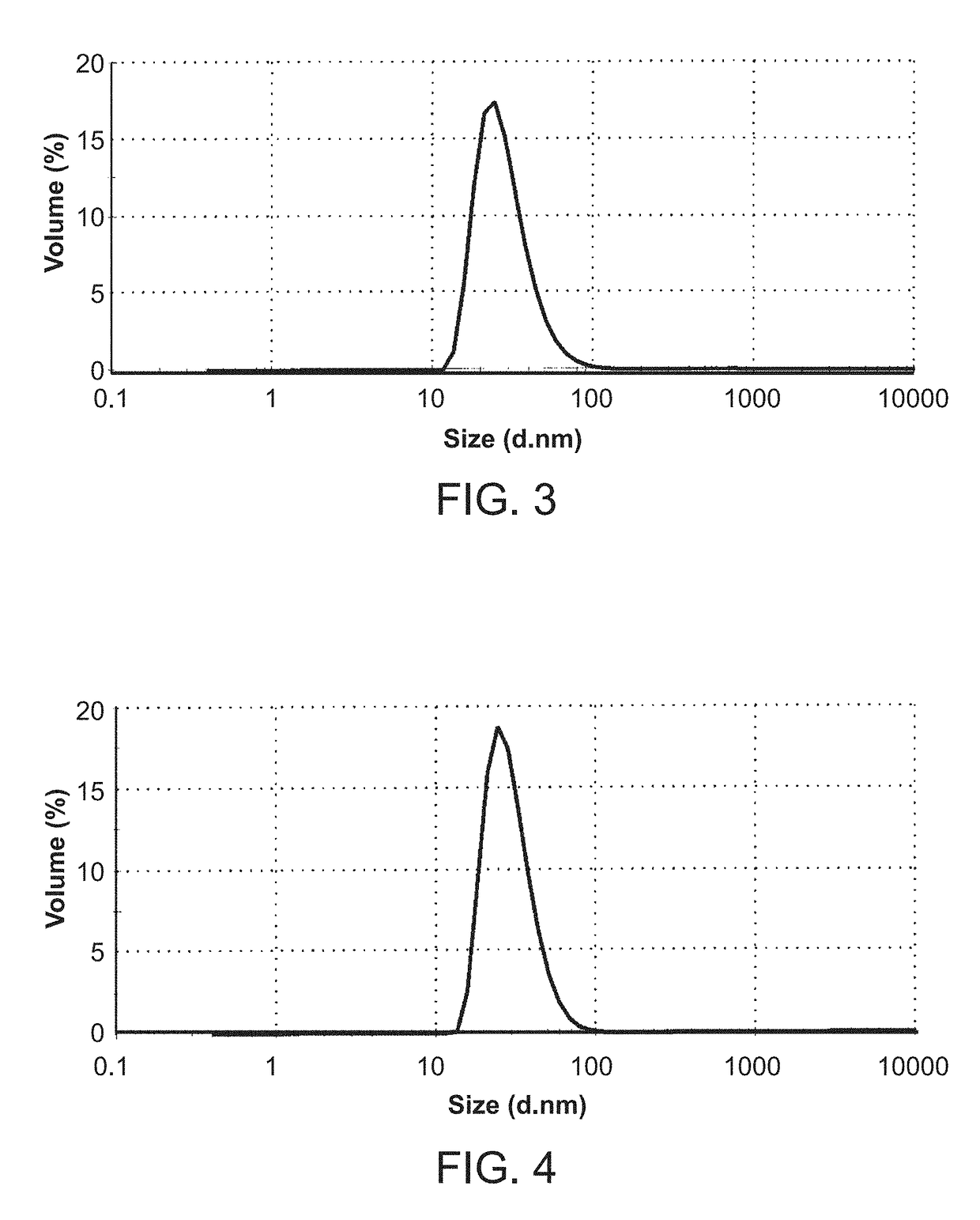
Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use
The invention relates to novel crystalline solid forms of the chemical compound N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide (Compound 1), and solvates thereof, including hydrates, that are useful for the treatment of cancer. Also disclosed are pharmaceutical compositions comprising the crystalline solid forms and processes for making the crystalline solid forms, as well as methods of using them for the treatment of cancer, particularly thyroid cancer, prostate cancer, hepatocellular cancer, renal cancer, and non-small cell lung carcinoma. The crystalline solid forms can be used to make the L-malate salt of cabozantinib.
Owner:EXELIXIS INC
Genes and polypeptides relating to hepatocellular or colorectal carcinoma
Owner:ONCOTHERAPY SCI INC
Individualized high purity hepatocellular carcinoma stem cells, methods and use of the same
The disclosure provides cancer stem cells, for use in stimulating immune response against a cancer, such as hepatocellular carcinoma (HCC). Methods for preparing and purifying the cancer stem cells are provided.
Owner:NEOSTEM ONCOLOGY
Combination liposomal pharmaceutical formulations
ActiveUS9895313B2Good curative effectLow toxicityOrganic active ingredientsPharmaceutical non-active ingredientsDocetaxel-PNPCholesterol derivative
Docetaxel and doxorubicin can be formulated in liposomal pharmaceutical compositions. In various embodiments, the pharmaceutical compositions include (i) a first liposome type comprising a first lipid layer comprising an unsaturated phospholipid, cholesterol or a cholesterol derivative, DC-cholesterol, a cationic lipid, and preferably a pegylated phospholipid, and a first active pharmaceutical ingredient (API) comprising docetaxel in the first lipid layer; and (ii) a second liposome type comprising a second lipid layer, an aqueous interior, and a second API comprising doxorubicin crystallized in the aqueous interior, (iii) where the first liposome type does not comprise doxorubicin and the second liposome type does not comprise docetaxel. The pharmaceutical composition can be used to treat a subject, for example, a human subject having cancer. The cancer can be, for example, a lung cancer, preferably non-small cell lung cancer (NSCLC), colon cancer, breast cancer, or liver cancer, preferably hepatocellular carcinoma (HCC).
Owner:CUREPORT
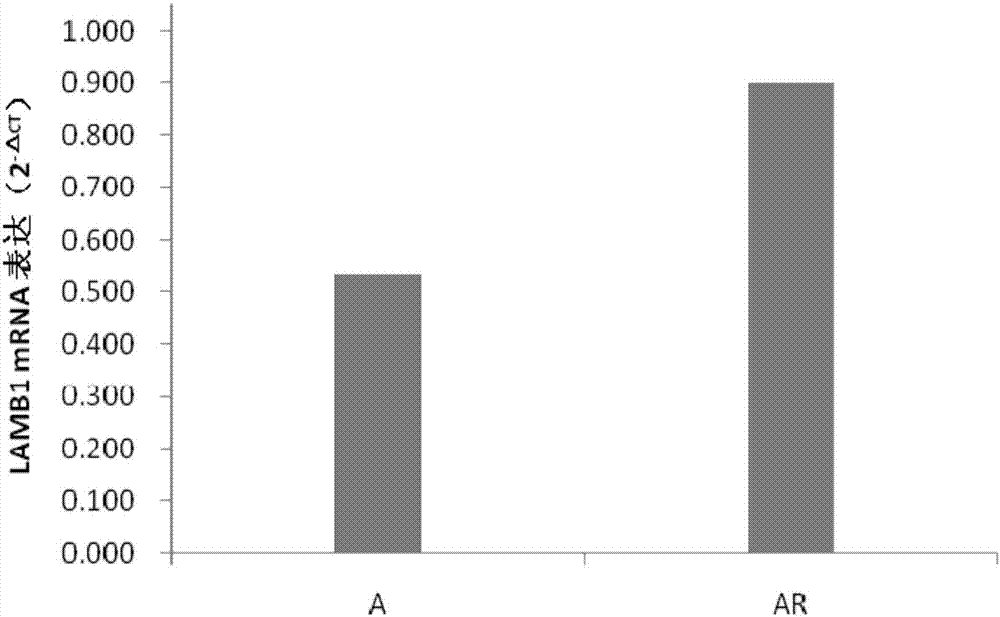
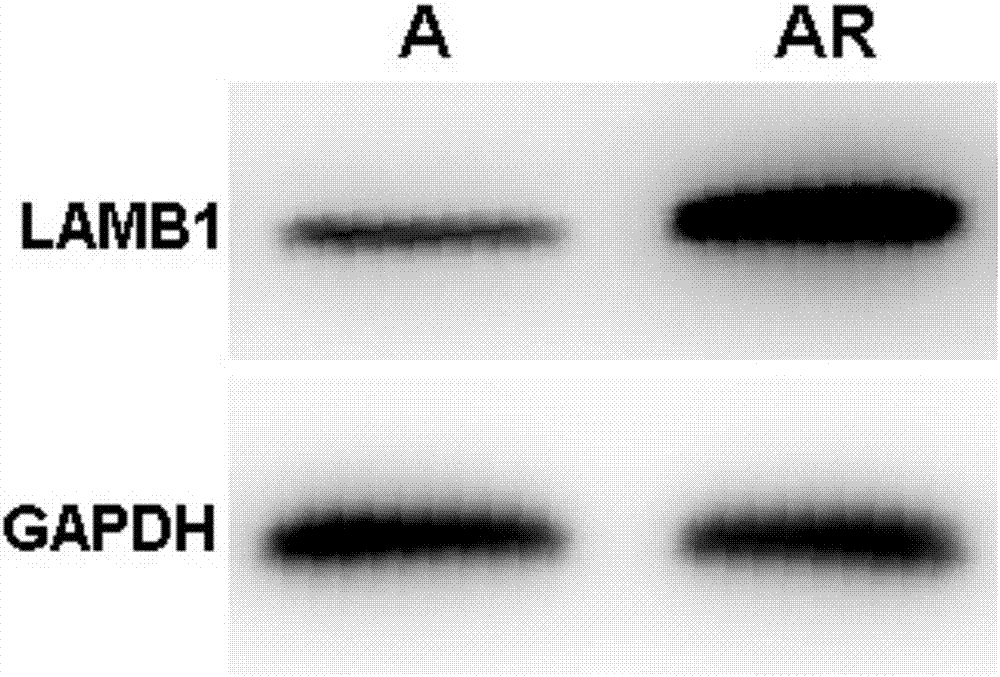
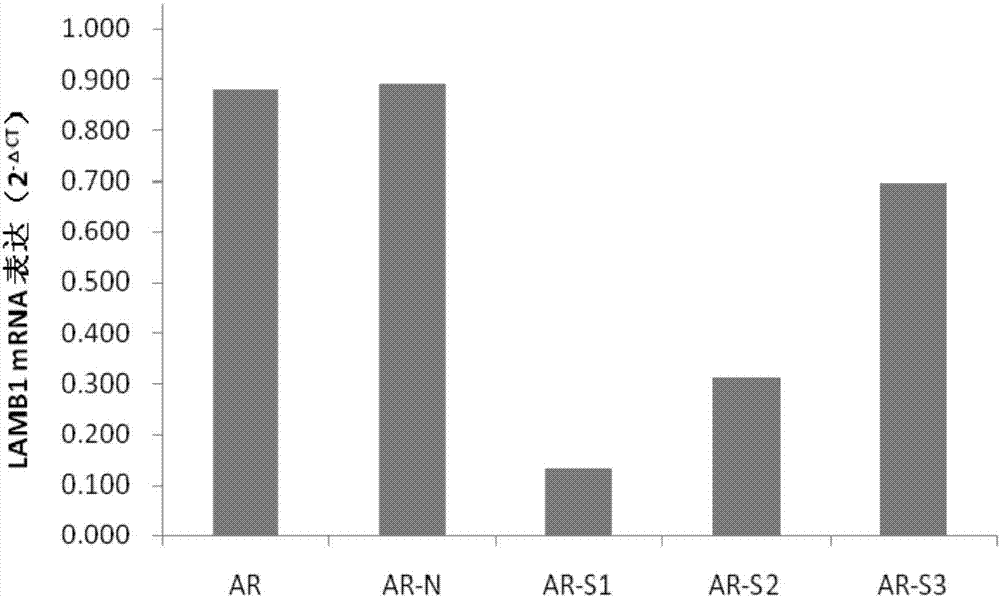
siRNA capable of specially inhibiting expression of LAMB1 gene and recombinant vector and application of siRNA
InactiveCN107354157AReduce proliferationPromote apoptosisOrganic active ingredientsGenetic material ingredientsCancer cellHepatocellular carcinoma
The invention discloses siRNA capable of specially inhibiting expression of an LAMB1 gene, a recombinant vector of the siRNA and application of the siRNA in reversing ovarian cancer taxol resistance, and belongs to the technical field of molecular biology and biological medicine. The siRNA comprises a sense strand and an antisense strand, the sense strand is 5'-UGUUUGAAAGCCGAAUCUGCG-3', and the antisense strand is 5'-CAGAUUCGGCUUUCAAACAAA-3'. According to the siRNA, mRNA and protein expression of the LAMB1 gene in a cancer cell can be inhibited specially and efficiently, proliferation of the cancer cell is reduced, cell apoptosis is increased, the invasiveness and migration capability of the cancer cell is reduced, and moreover, the resistance of an ovarian cancer cell to taxol can be reversed effectively. The invention further provides the application of the siRNA and the recombinant vector thereof in preparing medicines treating an ovarian cancer, a hepatocellular cancer, a colorectal cancer, a malignant glioma, a prostatic cancer or a gastric cancer and medicines reversing the ovarian cancer taxol resistance.
Owner:ZHEJIANG UNIV
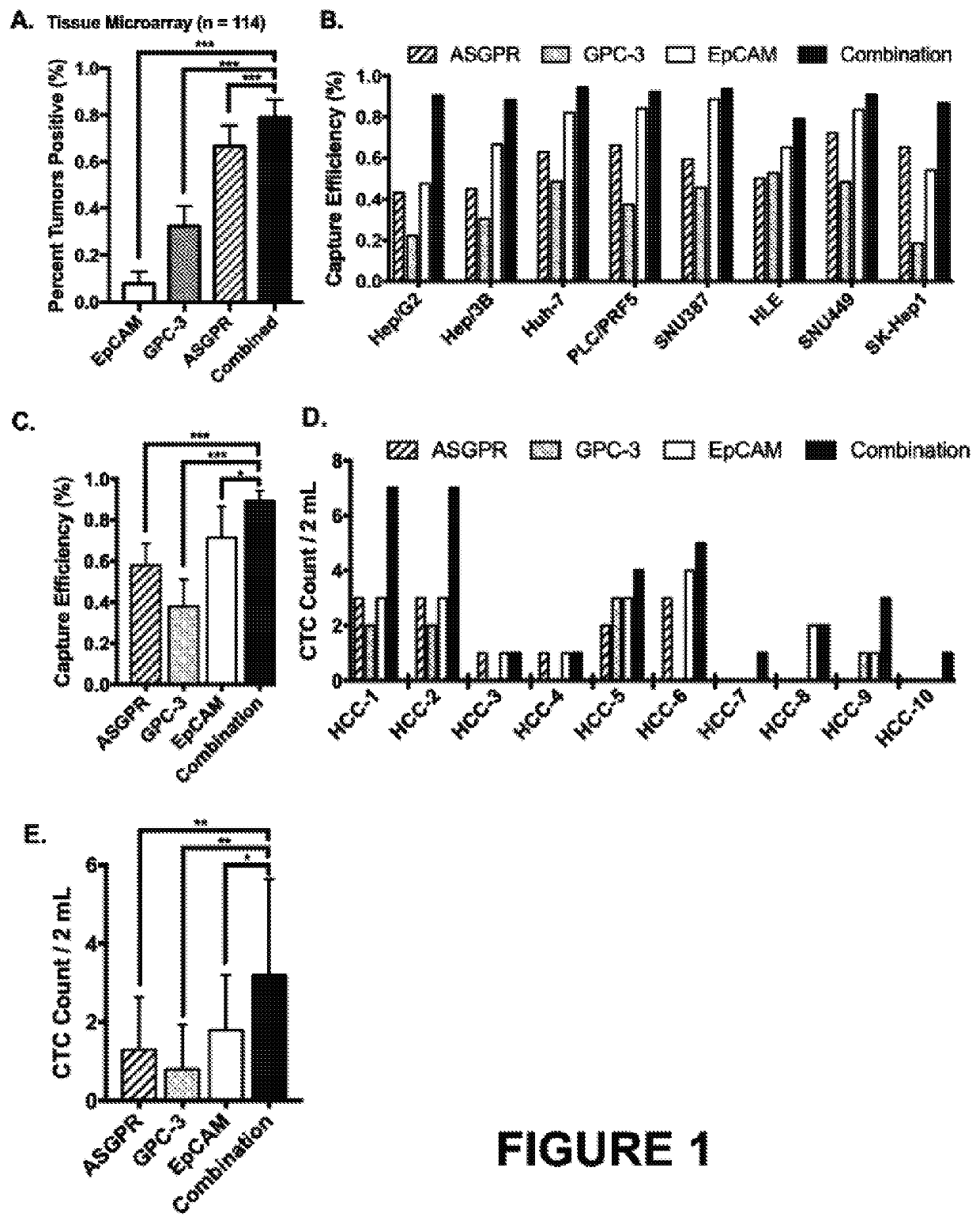
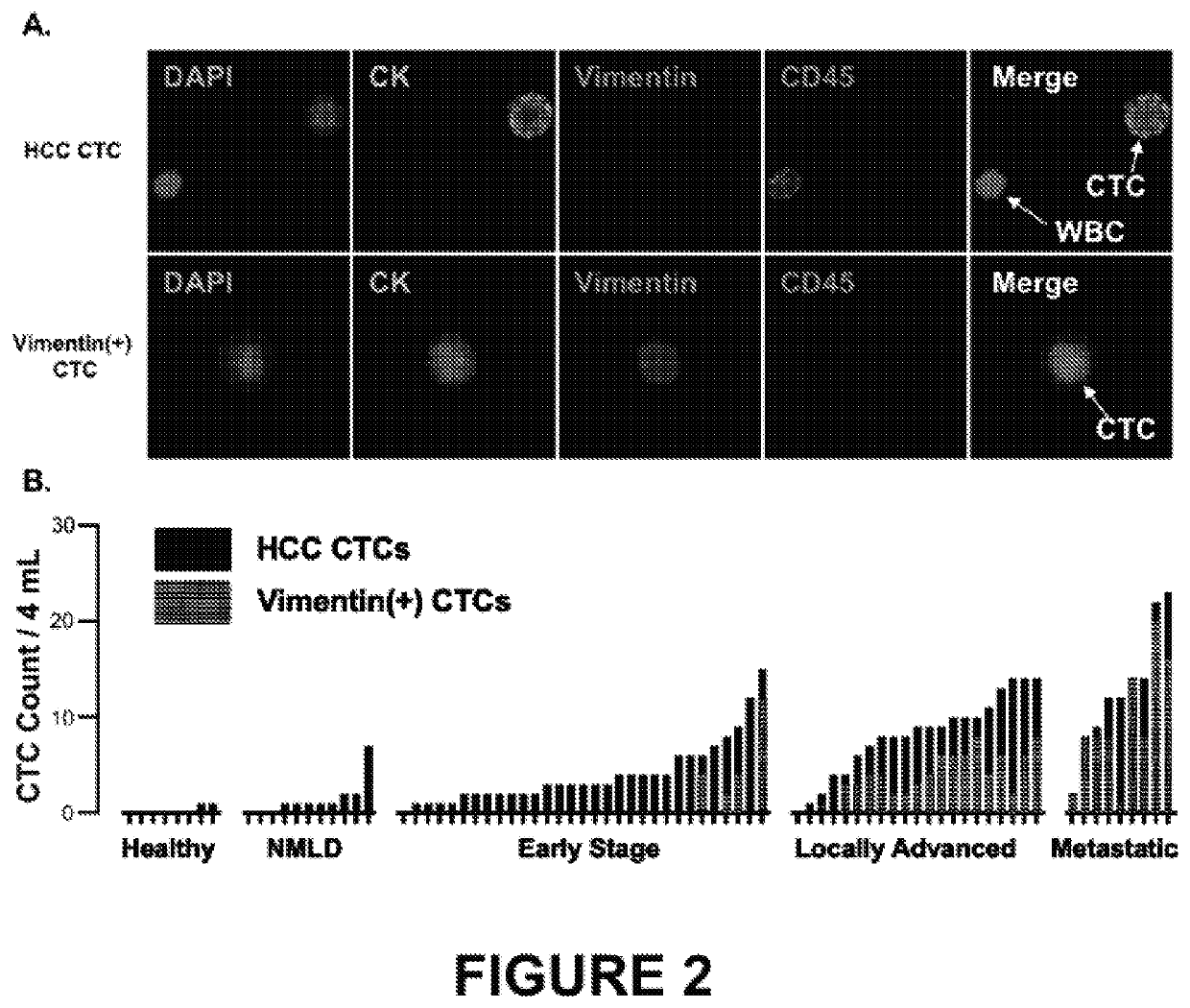
Phenotypic profiling of hepatocellular carcinoma circulating tumor cells for treatment selection
ActiveUS20200182877A1Accurate identificationEfficient captureImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisOncologyThelial cell
Methods and kits for detecting hepatocellular carcinoma recurrence or metastasis, and of measuring markers of hepatocellular carcinoma, including markers of hepatocellular carcinoma recurrence or metastasis, in a blood sample obtained from a subject by (a) isolating circulating tumor cells (CTCs) by contacting a blood sample obtained from the subject with a set of capture antibodies, wherein the capture antibodies specifically bind asialoglycoprotein receptor (ASGPR), Glypican-3, and epithelial cell adhesion molecule (EpCAM); (b) contacting the isolated CTCs with an antibody that specifically binds vimentin; and (c) measuring the number of vimentin-positive CTC.
Owner:RGT UNIV OF CALIFORNIA
Compositions, devices and kits for selective internal radiation therapy
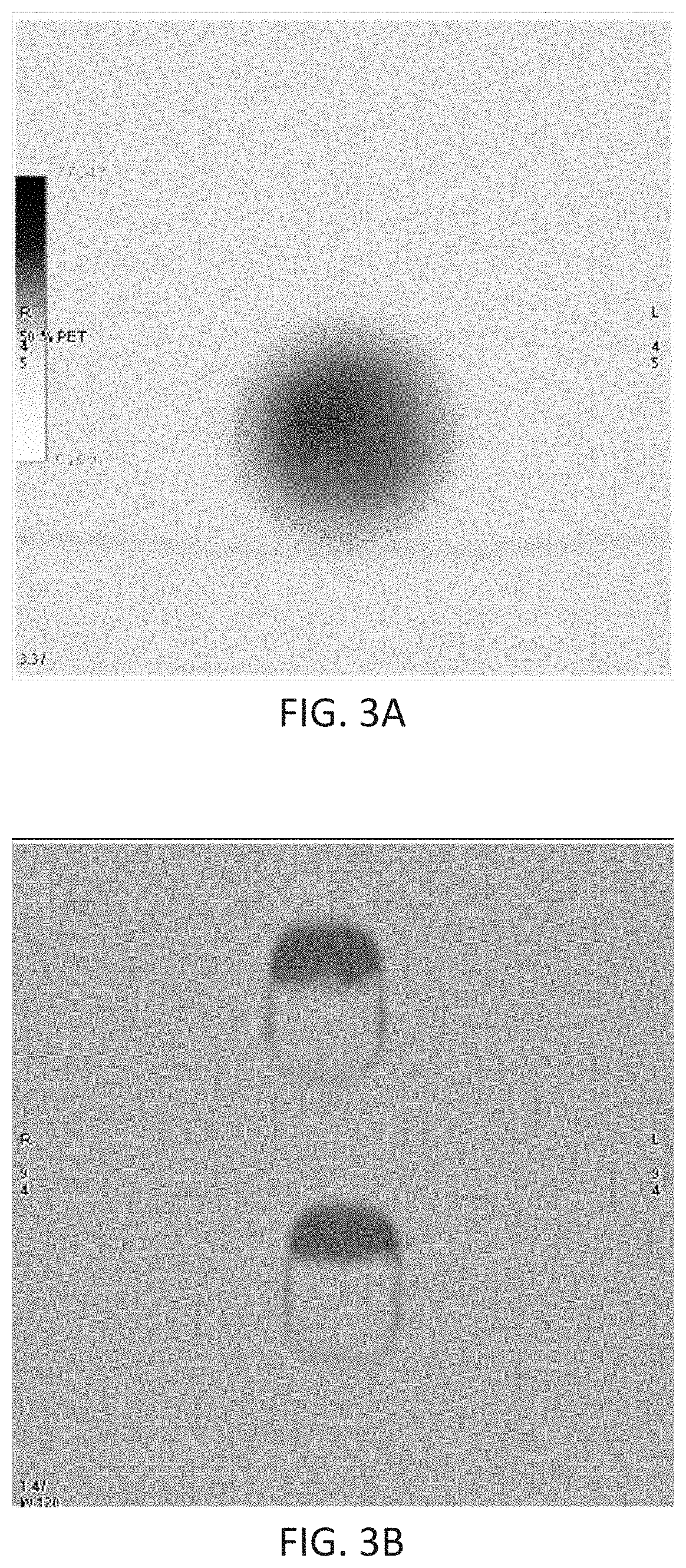
ActiveUS11141526B2Reduce chanceAssess effectivenessPowder deliveryInfusion syringesRadio isotopesDosing regimen
Systems, kits and methods for preparing an injection system and / or treating target lesions with a selective internal radiation therapy which includes a double-barrel syringe loaded with a two-component tissue glue and radioisotope loaded microspheres. The microspheres are loaded into the syringe based on the size of the target location and are administered with a needle or dual-lumen catheter. Dosing regimens for treating breast cancer lesions or surgical beds up to 130 mm in diameter and hepatocellular carcinoma lesions up to 50 mm are included.
Owner:BETAGLUE TECH SPA
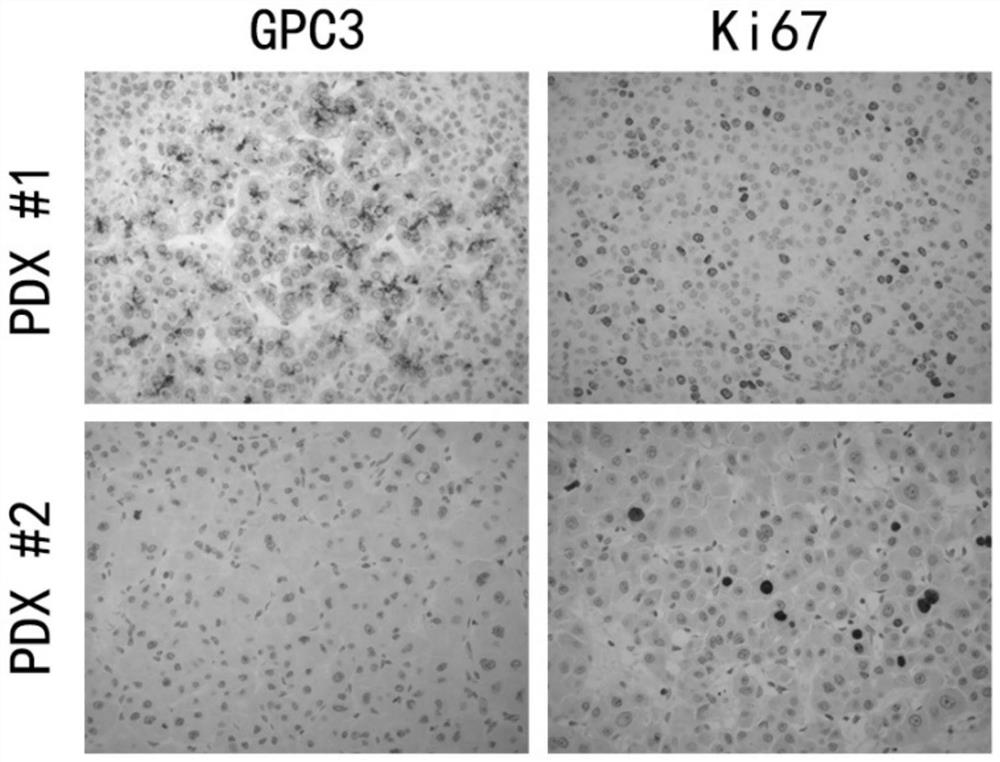
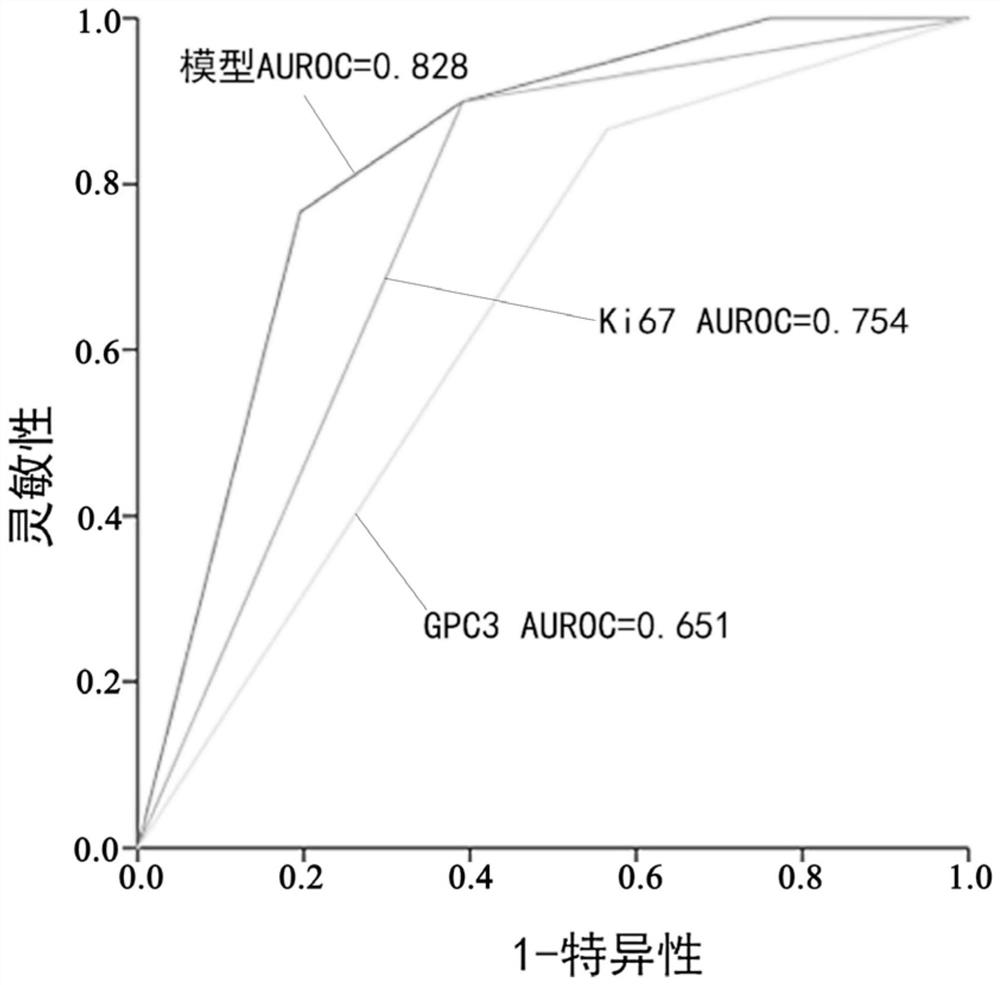
Hepatocellular carcinoma PDX model construction method
Disclosed are: a composition for determining the tumor formation rate during the construction of a hepatocellular carcinoma PDX model, which is intended for non-disease treatment; and a hepatocellularcarcinoma PDX model construction method using the composition. The composition for judging the tumor formation rate during the construction of the hepatocellular carcinoma PDX model comprises GPC3 and Ki67 in a hepatocellular carcinoma tumor tissue sample, and the tumor formation rate of PDX modeling can be judged by detecting the two proteins. According to the method for predicting tumorigenesisof the hepatocellular carcinoma PDX model, the immunohistochemical results of tumor tissues GPC3 and Ki67 of a hepatocellular carcinoma patient are interpreted, and whether tumorigenesis of the PDX model occurs or not is predicted in an early stage. The PDX model is predicted not to be nodulated in the early stage, and the PDX model can be constructed as soon as possible.
Owner:ZHEJIANG UNIV
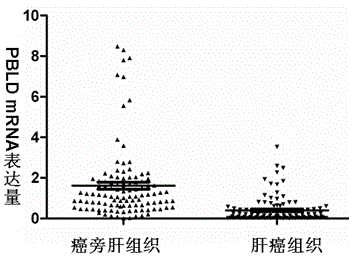
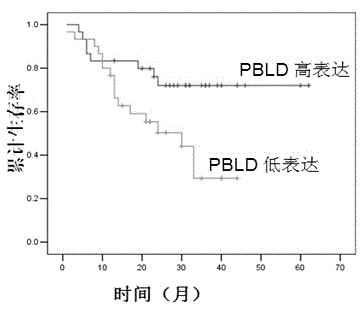
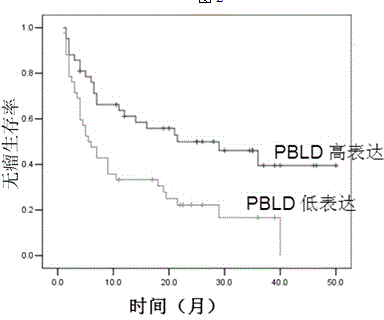
PBLD gene expression primer for primary hepatoma treatment scheme selection and/or prognosis assessment, and application thereof
InactiveCN104404138AGood treatment effectAccurate assessment of prognosisMicrobiological testing/measurementDNA/RNA fragmentationTreatment effectStatistical analysis
The invention discloses a PBLD gene expression primer for primary hepatoma treatment scheme selection and / or prognosis assessment, and an application thereof. The primer comprises an upstream primer 5'-GGGTCTGCACACGCTGTTC-3' and a downstream primer 5'-TAATGTCAACCCTTCCGTCT-3'. Prognosis can be accurately assessed through PBLD detection on a primary hepatoma patient or a biopsy tissue by using the specific primer and a kit made via using the primer according to the PBLD gene expression level in order to avoid tedious statistical analysis, and accurate curative effect assessment can be provided for a clinic doctor and the patient according to detection results in order to determine the suitable treatment scheme of the patient, guide the doctor to select an effective clinic treatment scheme, improve the treatment effect of the patient and realize individual treatment.
Owner:广州市第一人民医院
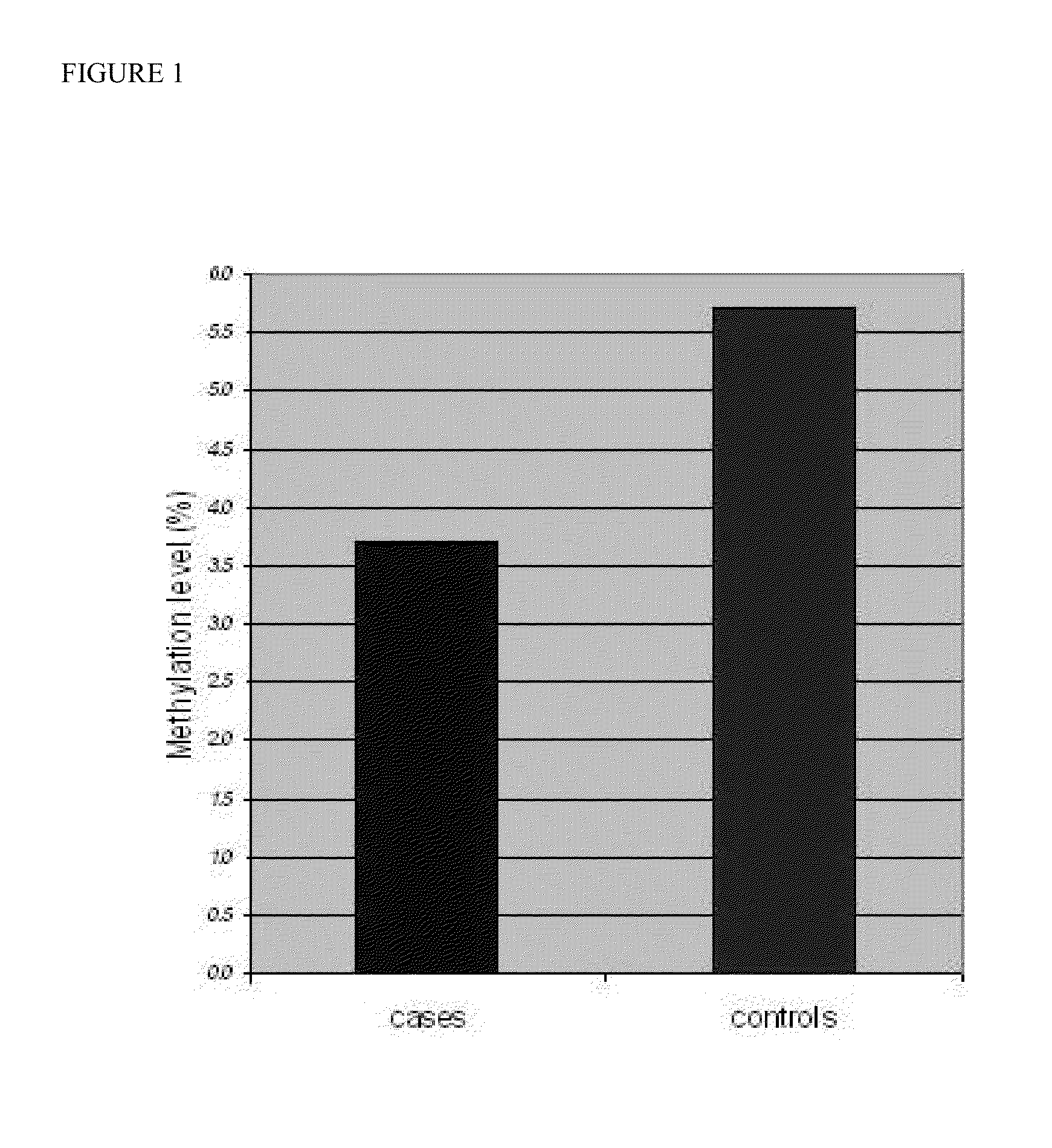
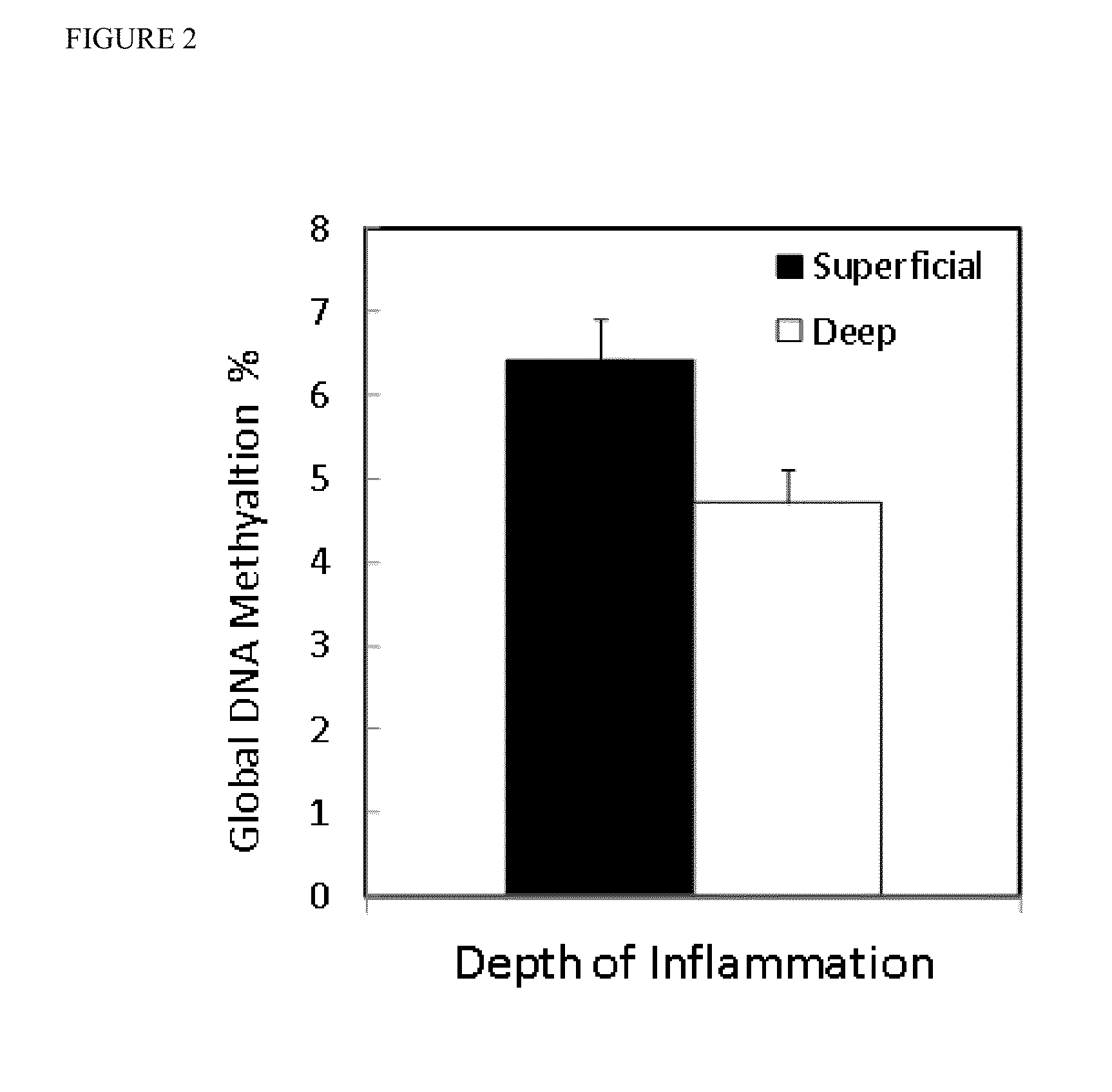
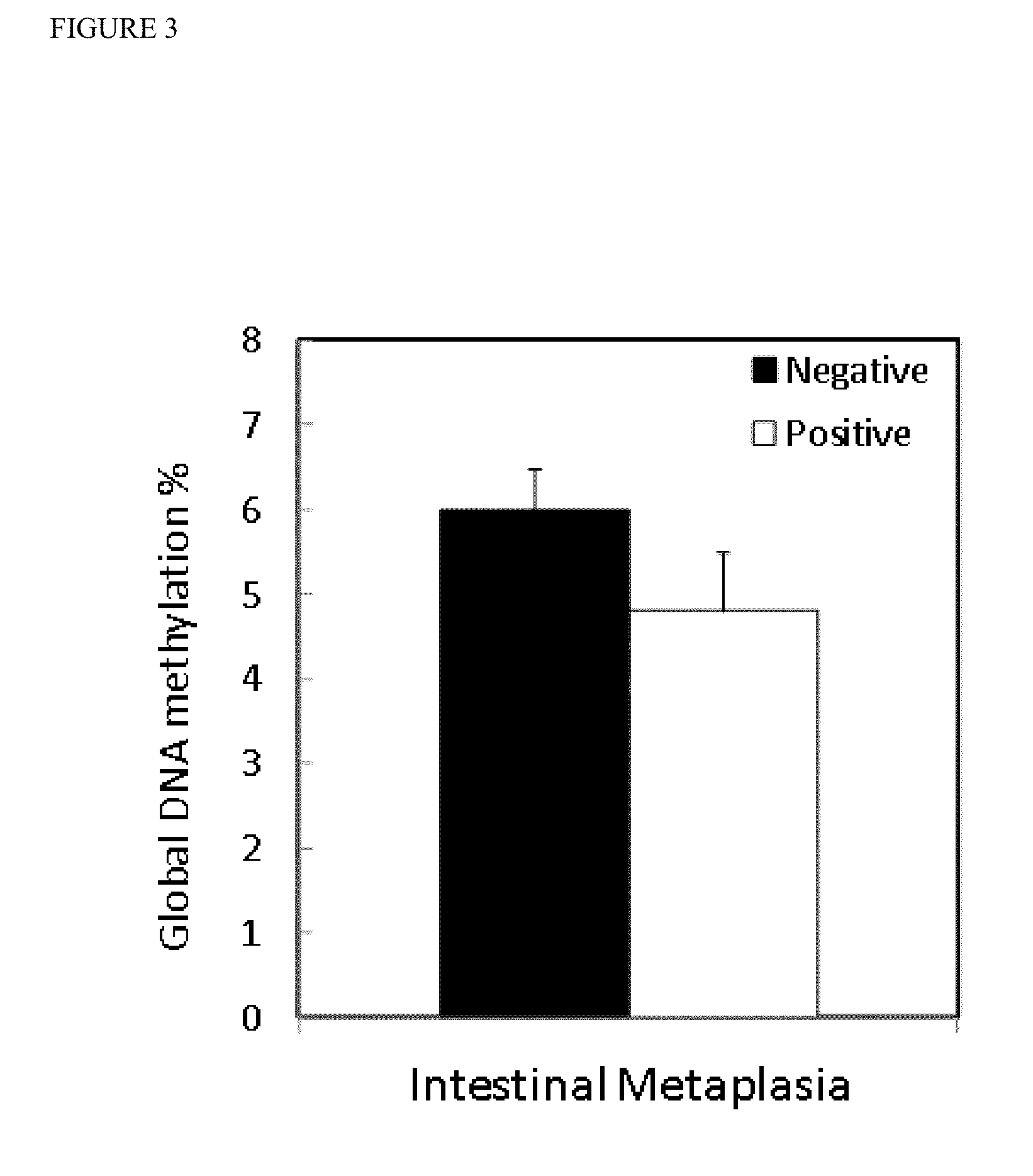
Global DNA hypomethylation and biomarkers for clinical indications in cancer
ActiveUS20130035247A1Increased riskMicrobiological testing/measurementLibrary screeningOncologyBiomarker (petroleum)
The present invention provides methods of determination of a global DNA methylation index (GDMI) in a sample from a subject, using a variety of methods which can detect global, genome-wide, and gene-specific DNA methylation to create methylation portraits that can be used for early detection, diagnosis, and clinical management in the personalized medicine space. Further, the invention provides methods of diagnosis of cancer, including gastric cancer and hepatocellular cancer in a subject, by comparing the GDMI in a sample obtained from a subject to the methylation index of standard controls. These methods allow diagnosis of gastric carcinoma and liver cancer in patients who may be asymptomatic or have inconclusive pathology, and allowing earlier treatment of the subject.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
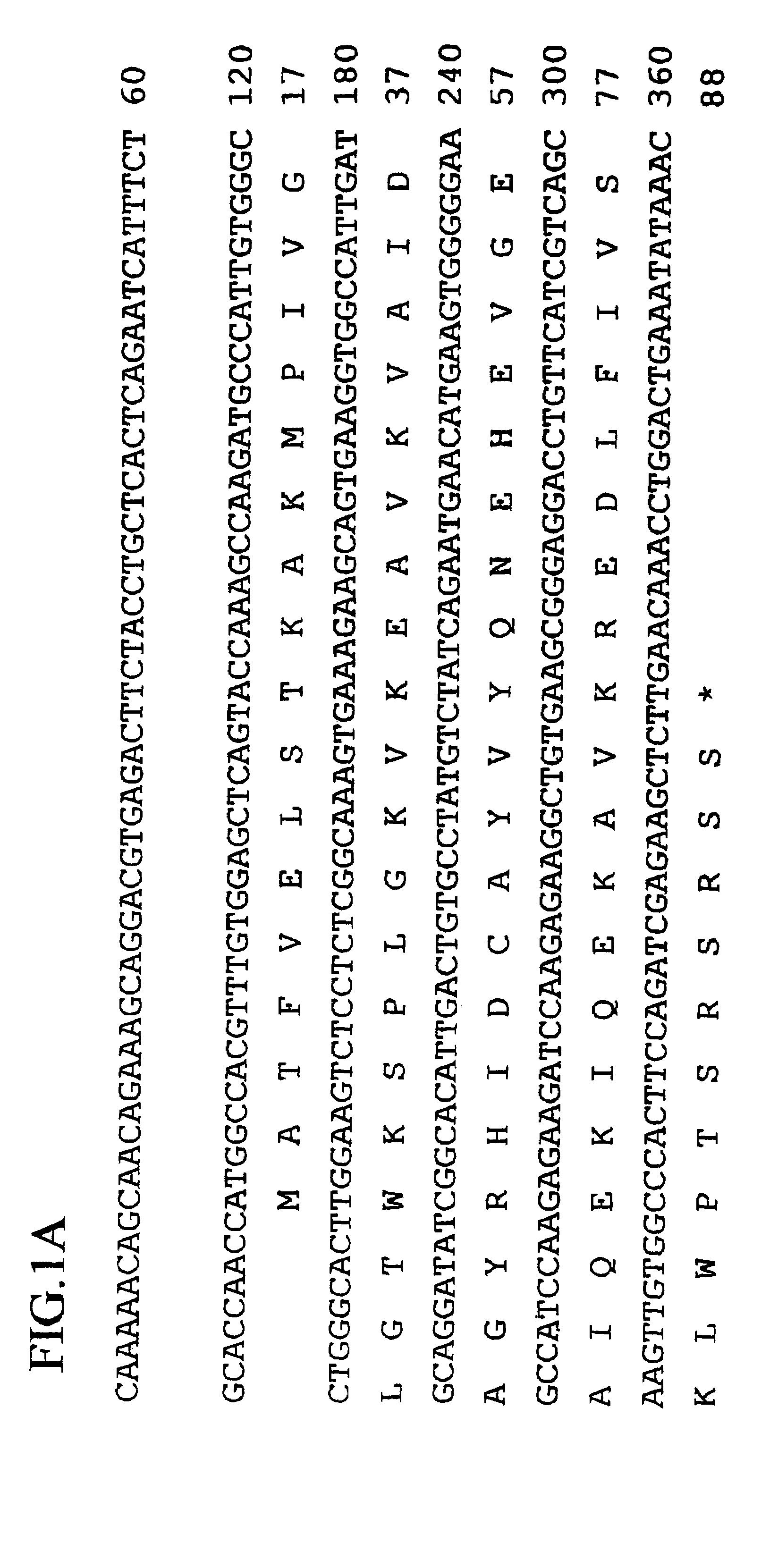
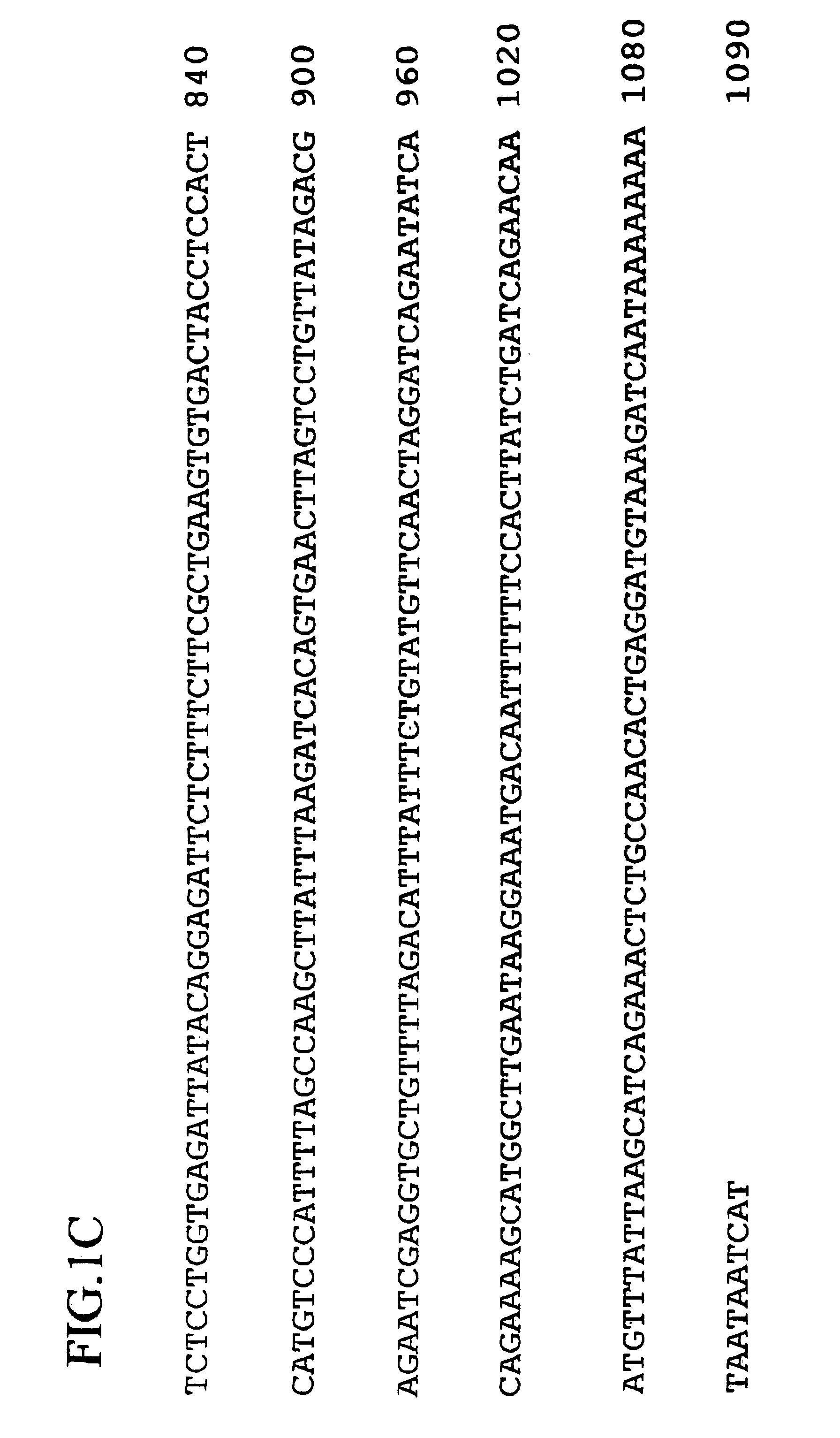
Human ARL-related gene variants associated with cancers
InactiveUS7087733B2Sugar derivativesMicrobiological testing/measurementHepatocellular carcinomaNucleic acid sequencing
The invention relates to the nucleic acid sequences of two novel human ARL-related gene variants (ARLV1 and ARLV2) and the polypeptides encoded by ARLV1 and ARLV2.The invention also relates to the process for producing the polypeptides encoded by ARLV1 and ARLV2.The invention further relates to the use of the nucleic acid of ARLV1 and ARLV2 and the polypeptide encoded by ARLV1 and ARLV2 in diagnosing diseases associated with the deficiency of human ARLV gene, in particular large cell lung cancer or hepatocellular carcinoma.
Owner:BIOPTIK TECH INC
Glutamine metabolism gene tag scoring system for predicting prognosis and treatment resistance of hepatocellular carcinoma
ActiveCN113930506AObjective assessment of overall survivalAids in treatment resistanceMicrobiological testing/measurementProteomicsOncologyChemo therapy
The invention discloses a glutamine metabolism gene tag scoring system for predicting prognosis and treatment resistance of hepatocellular carcinoma, and belongs to the technical field of biological medicine. By detecting the expression level of seven specific glutamine metabolism related genes of a hepatocellular carcinoma patient, a model can be used for judging the total lifetime of the patient, evaluating the treatment resistance of a postoperative transcatheter arterial chemoembolism patient and evaluating the immune cell infiltration degree in a tumor and the immune escape potential of a tumor cell, and the prediction capability on the liver cancer immunotherapy reaction is improved. Meanwhile, the model provided by the invention can improve the prediction accuracy of the three-year total lifetime of hepatocellular carcinoma, and compared with the prediction of the prognosis of the patient directly through the existing next-generation sequencing technology, the number of genes needing to be detected is reduced, the efficiency is improved, and the cost is reduced. As a molecular marker capable of objectively and accurately evaluating hepatocellular carcinoma treatment resistance and tumor immune state, accurate prediction and precise implementation of hepatocellular carcinoma treatment prognosis can be realized.
Owner:AFFILIATED HOSPITAL OF JIANGSU UNIV
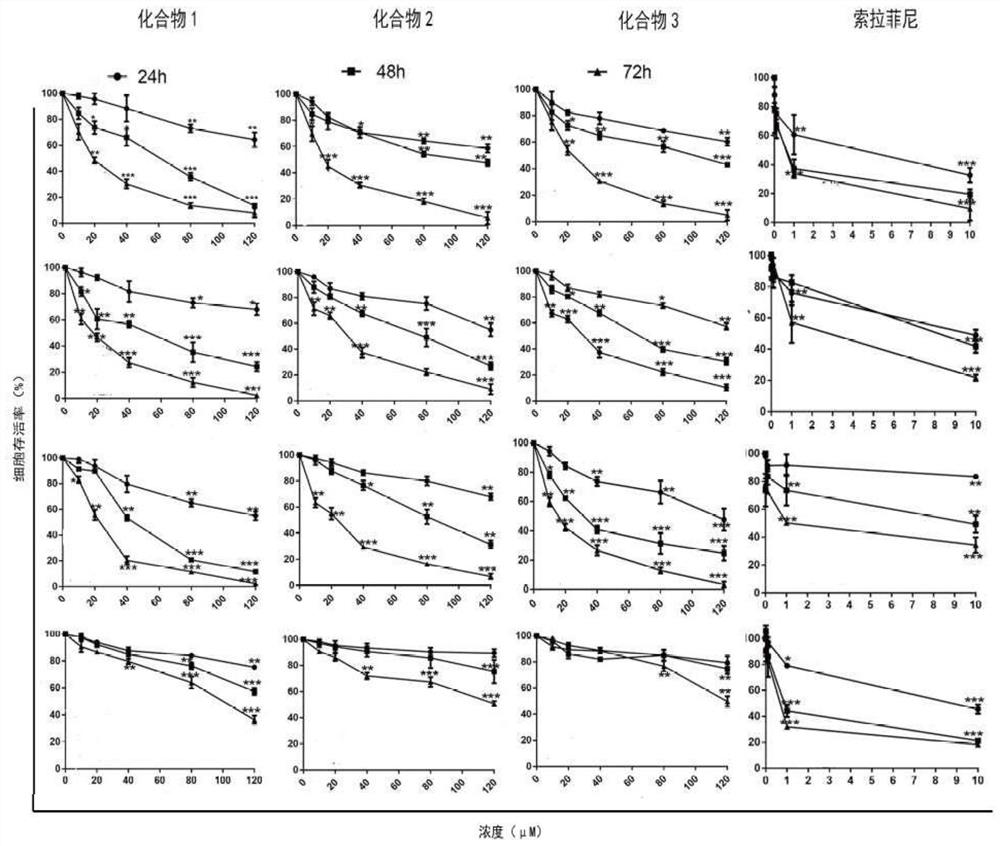
Application of 2-acyl-1-dimethylaminomethylferrocene derivative in preparation of drugs for targeted treatment of hepatocellular carcinoma
ActiveCN112870189AGrowth inhibitionGood choiceOrganic active ingredientsAntineoplastic agentsAcyl groupPharmaceutical drug
The invention discloses an application of a 2-acyl-1-dimethylaminomethyl ferrocene derivative in preparation of a drug for targeted treatment of hepatocellular carcinoma. The structural formula of the 2-acyl-1-dimethylaminomethyl ferrocene derivative is shown in the specification, wherein R is halogen. The compound can induce apoptosis of tumor cells and inhibit growth of the tumor cells through active oxygen and cell cycle arrest, and has good selectivity.
Owner:HUAQIAO UNIVERSITY
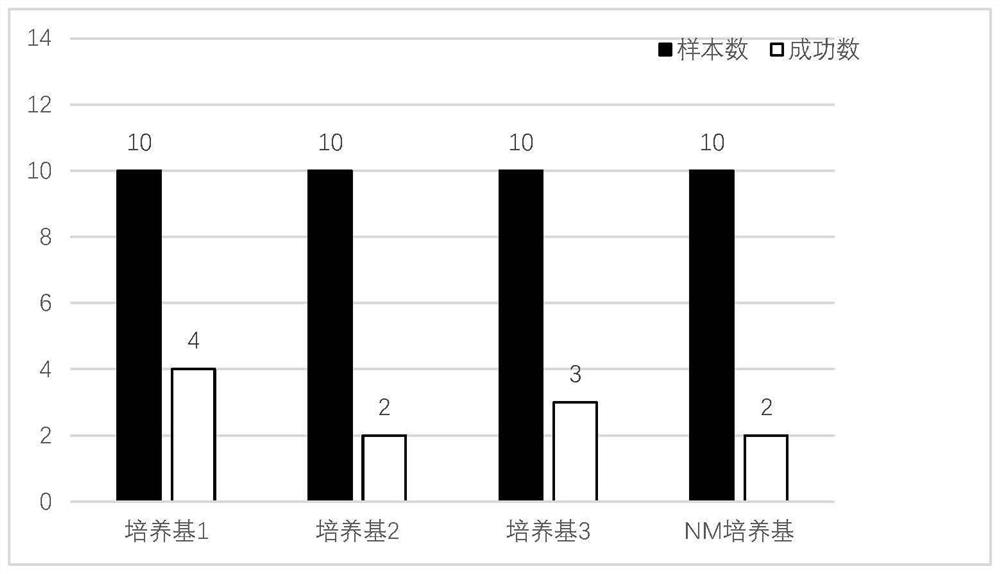
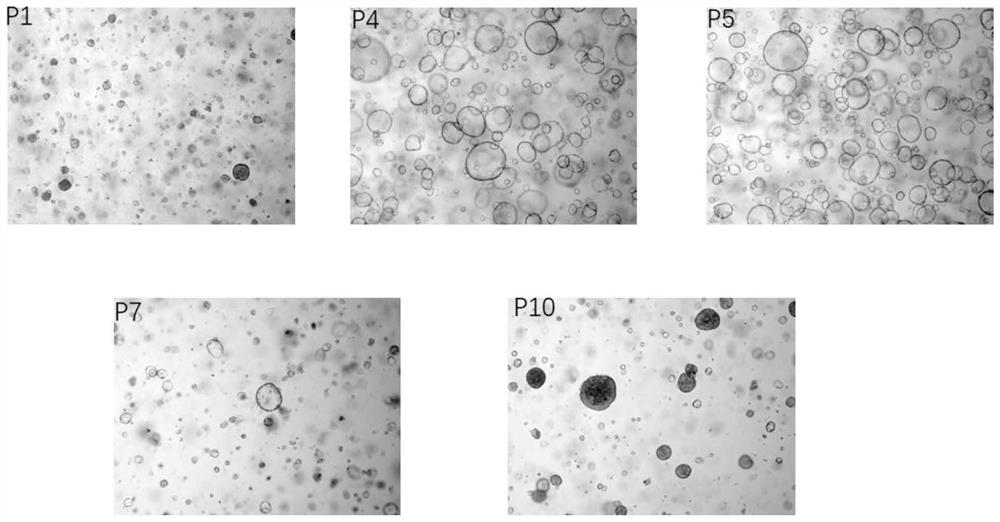
Continuous culture method and culture medium for primary hepatocellular carcinoma organoids
The invention discloses a culture medium for primary liver cancer organoids and a culture method of the primary liver cancer organoids. By adjusting the contents of various components in the culture, the organoid culture of the primary hepatocellular carcinoma cells with huge heterogeneity can be realized, and a foundation is laid for revealing the pathogenesis of hepatocellular carcinoma and screening anti-cancer drugs.
Owner:上海诺典生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use](https://images-eureka.patsnap.com/patent_img/3ea48ff4-46e9-4662-9819-cf9dff241981/US10501418-D00001.png)
![Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use](https://images-eureka.patsnap.com/patent_img/3ea48ff4-46e9-4662-9819-cf9dff241981/US10501418-D00002.png)
![Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use](https://images-eureka.patsnap.com/patent_img/3ea48ff4-46e9-4662-9819-cf9dff241981/US10501418-D00003.png)