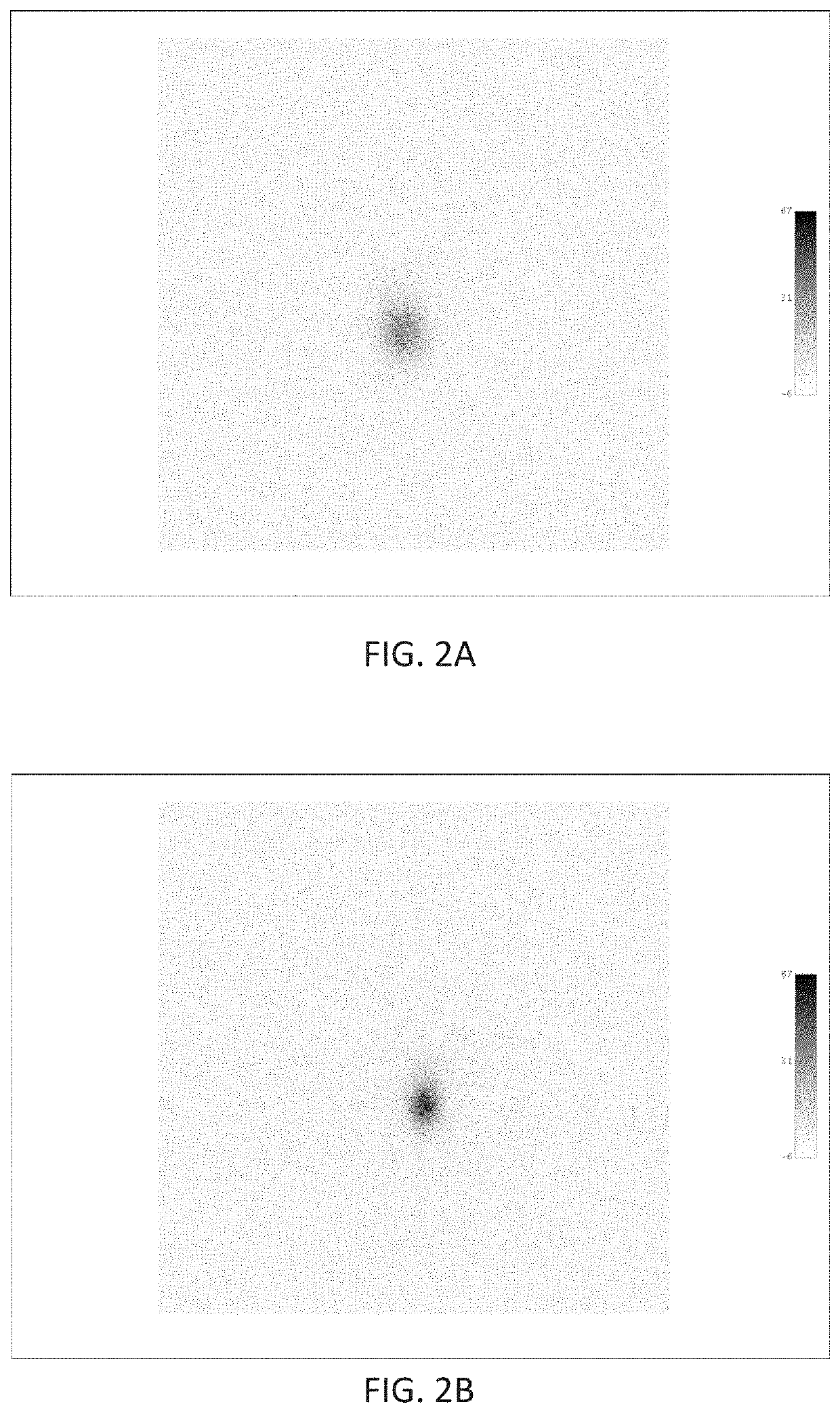
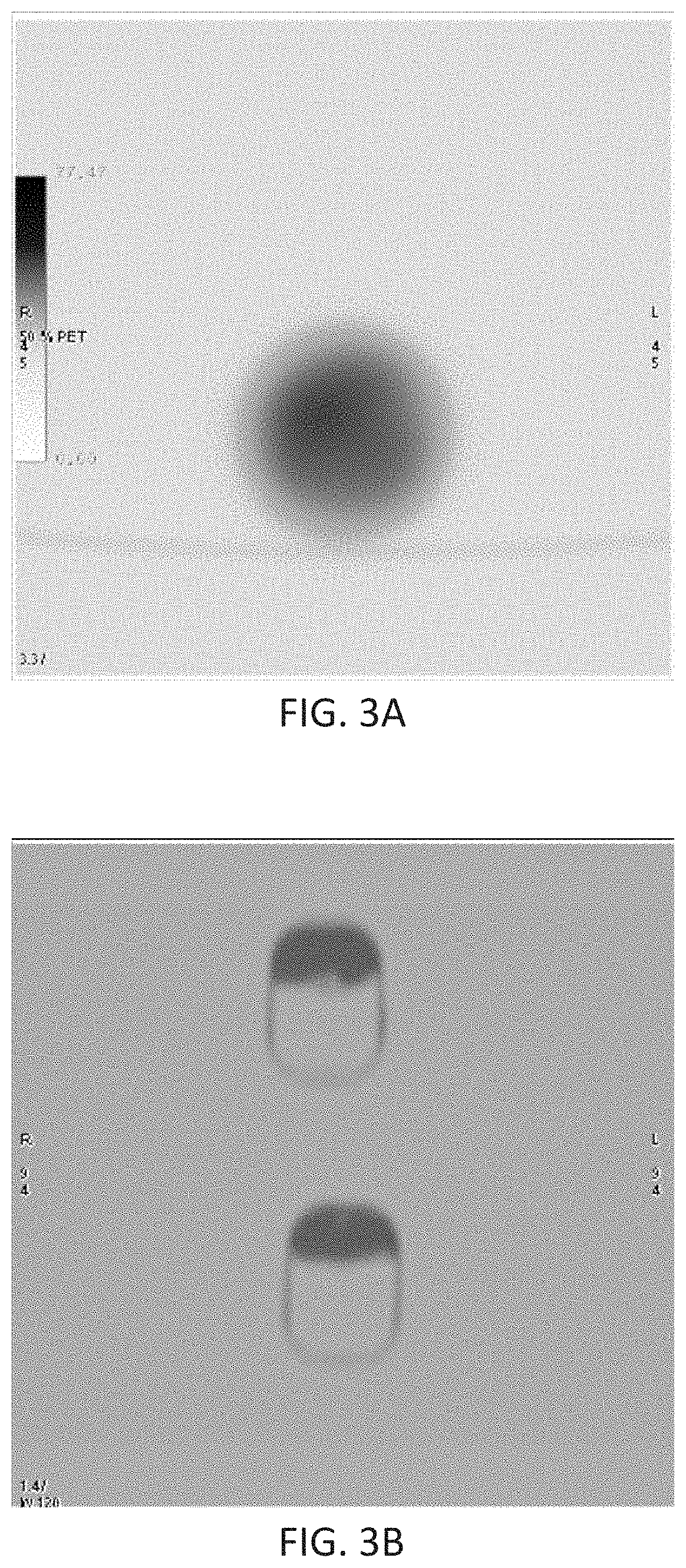
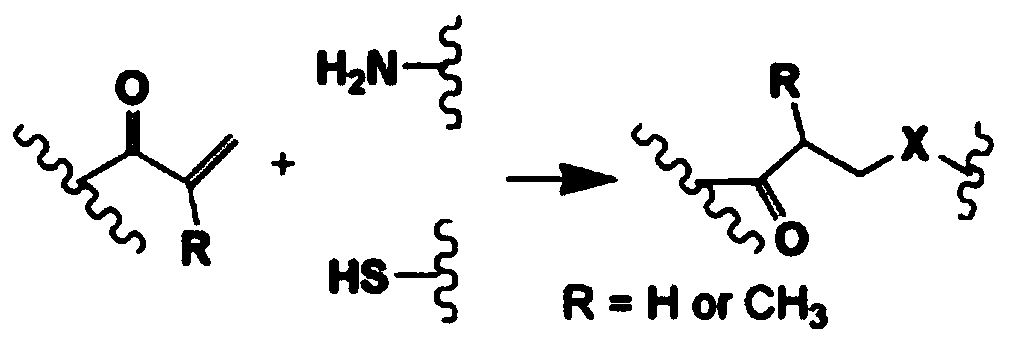
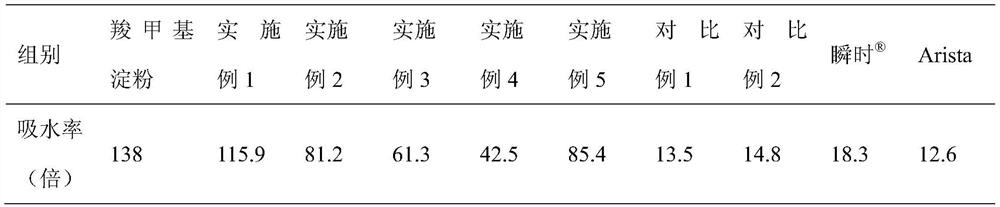
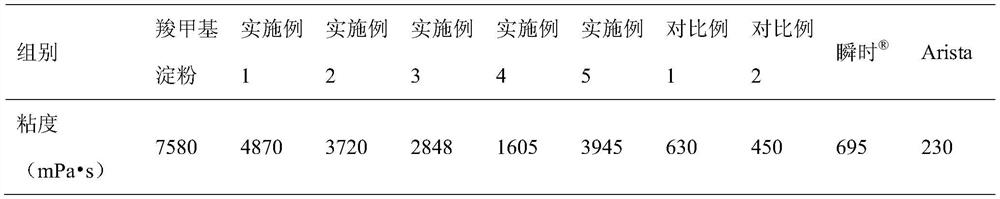
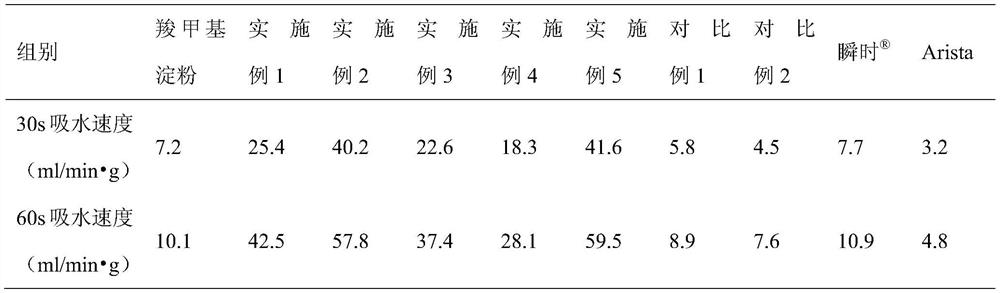
Patents
Literature
45 results about "Tissue glue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tissue Adhesive Glue. Tissue adhesive glue has been a revolutionary step in the management of lacerations. Tissue glue is indicated for low tension wounds, or occasionally higher tension wounds that have been properly undermined and layered.
Methods for tissue welding using laser-activated protein solders
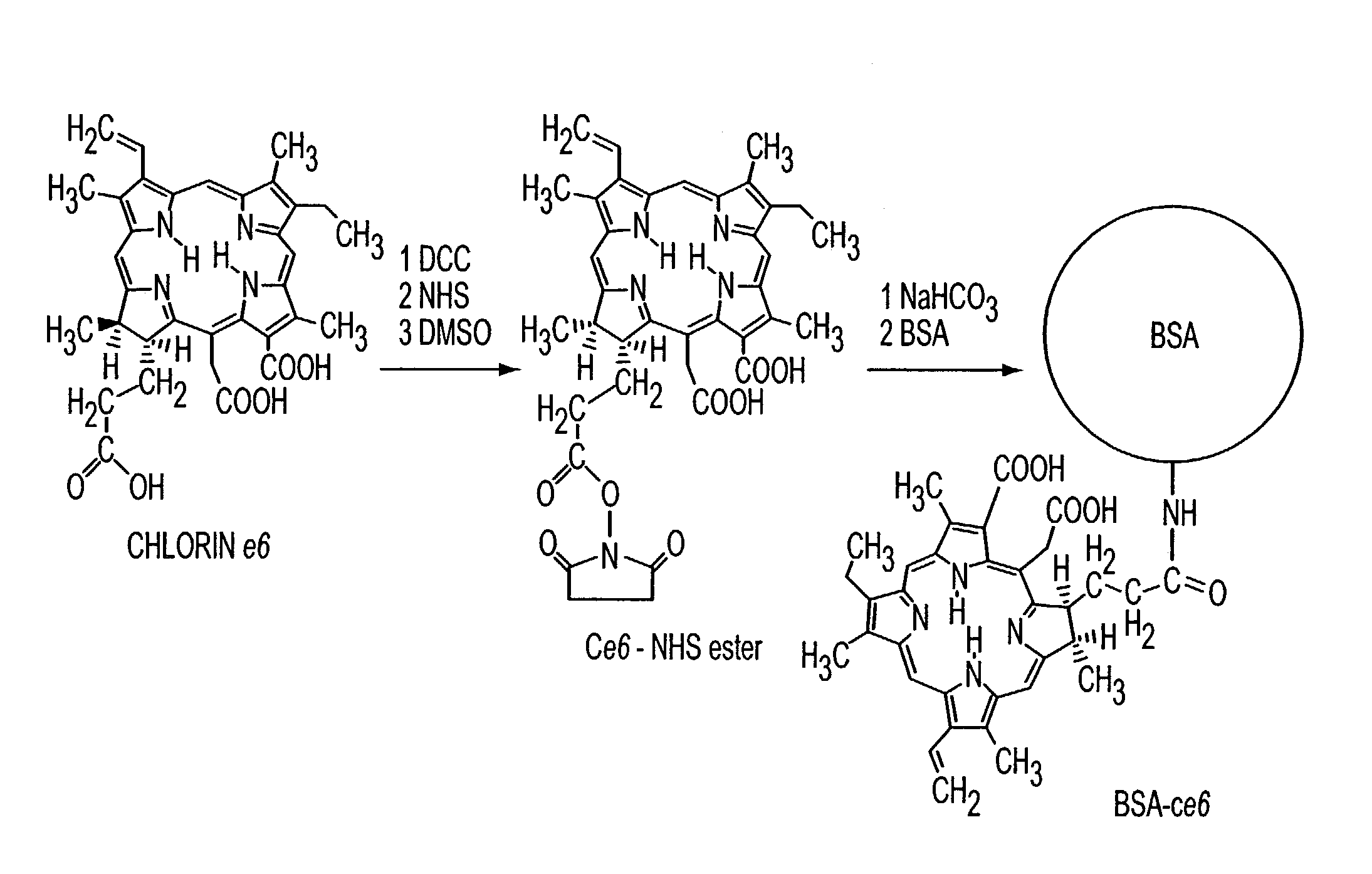
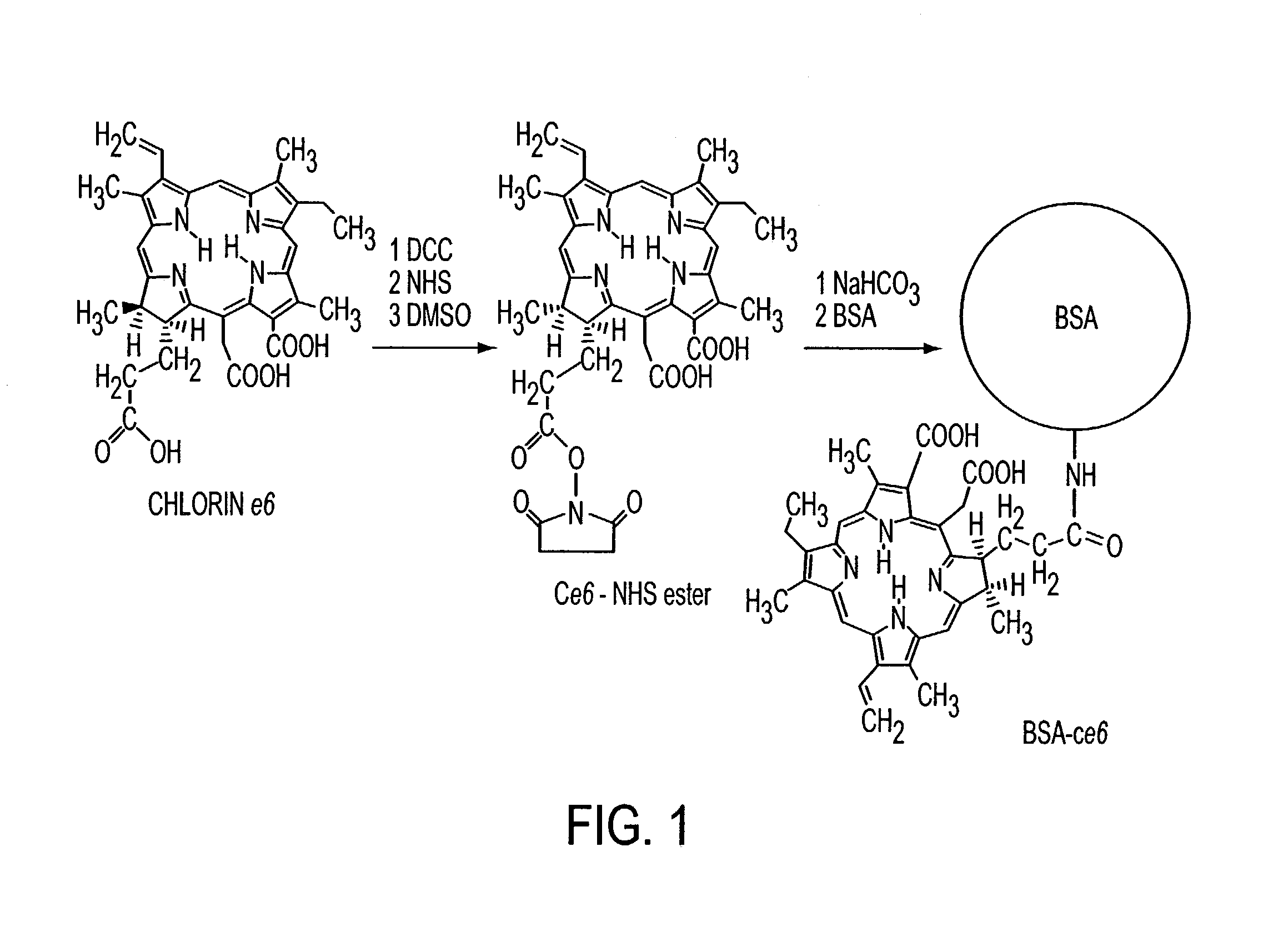
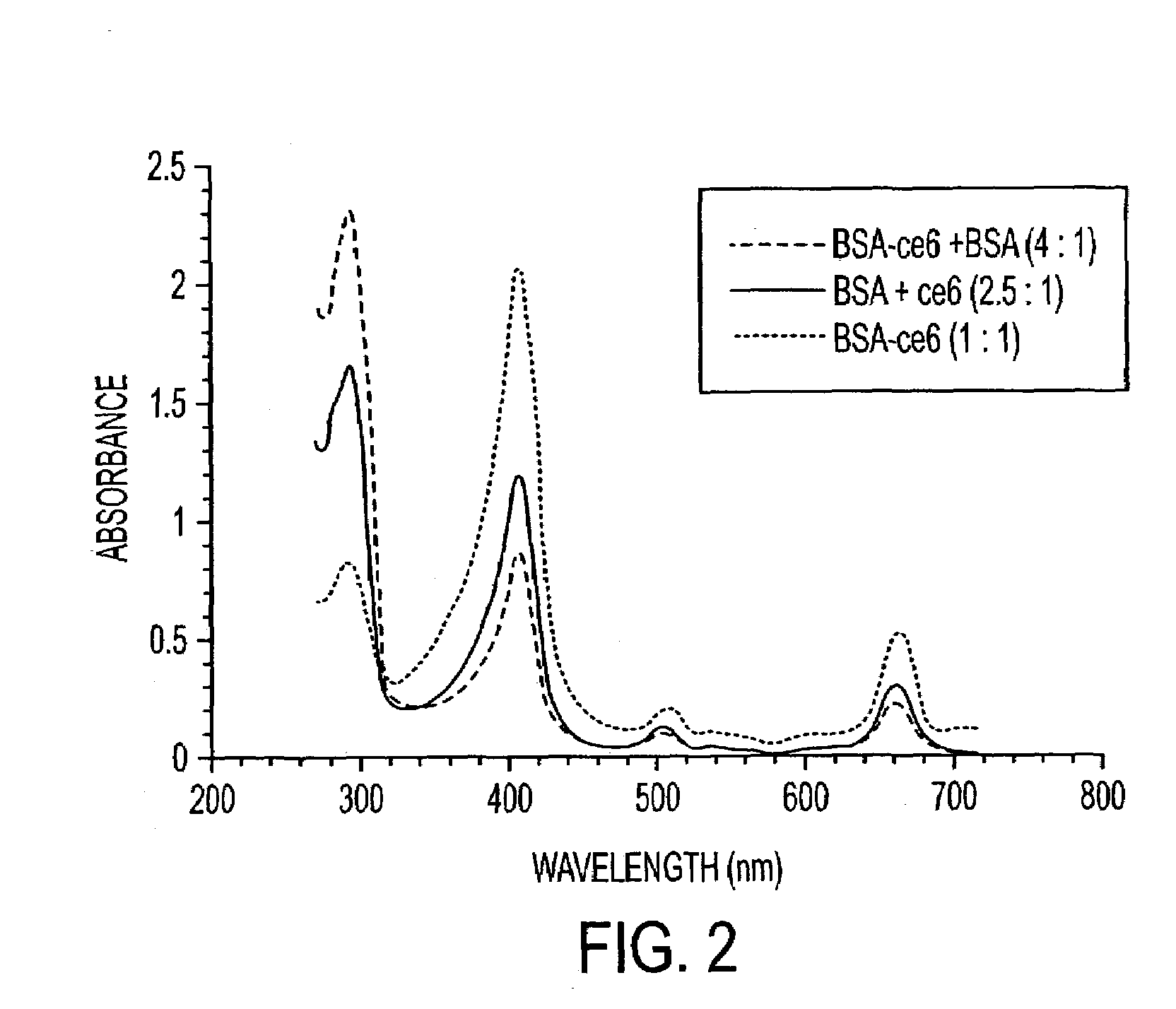
Various tissue glues have drawback such as toxicity, causing inflammatory reactions or insufficient bonding strength. The present invention is directed to methods of form tissue adhesion by administering to tissues compositions comprising proteins conjugated to one or more novel photosensitizers and irradiating the composition. The composition may further comprise one or more proteins not conjugated to the photosensitizer. Additionally, the present invention relates to compositions and methods wherein increased ratios of protein to photosensitizer enhance weld strength.
Owner:THE GENERAL HOSPITAL CORP
Biocompatibility pre-gelatinized modified starch and preparation thereof
InactiveCN101497670AAbsorbentSpeed up absorptionSurgical adhesivesFibre treatmentBiocompatibility TestingHigh pressure
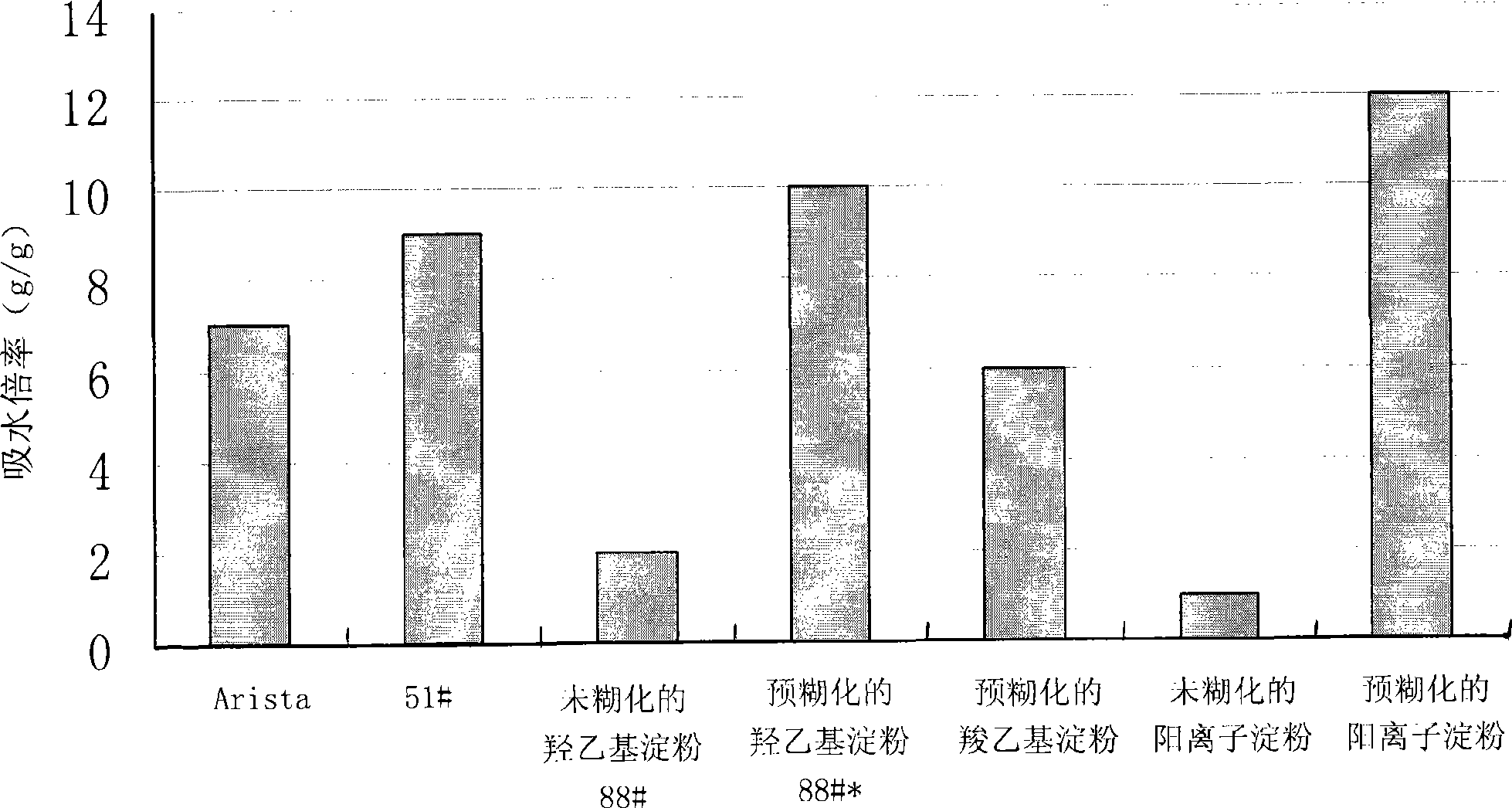
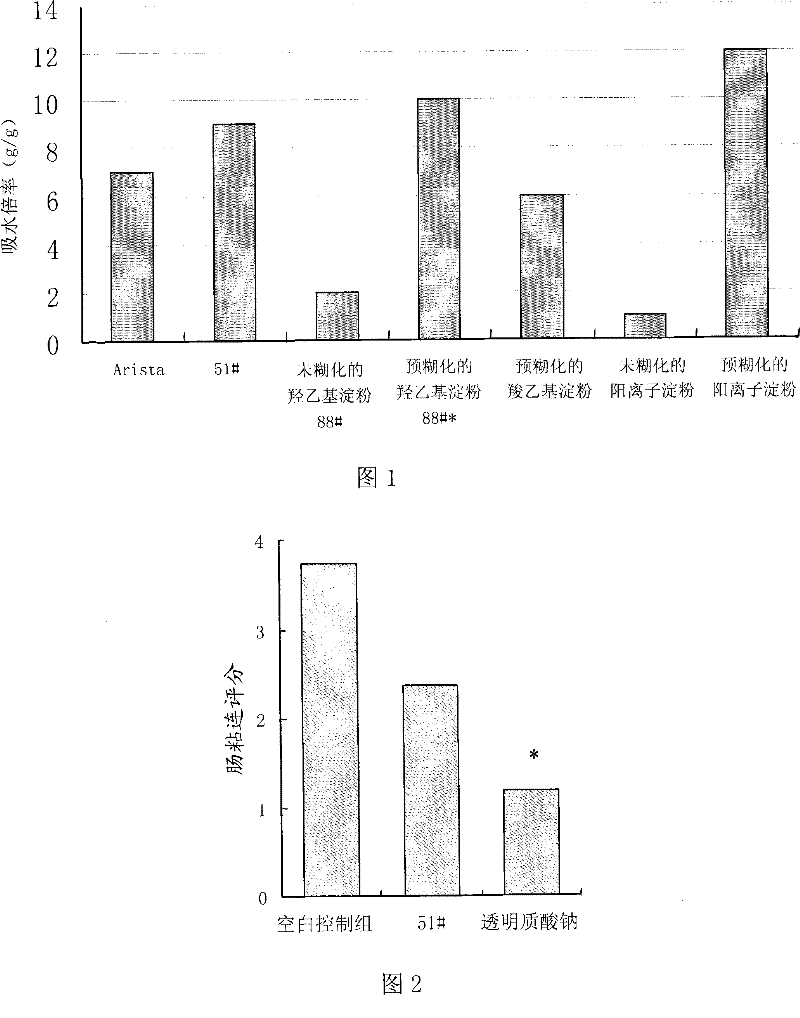
The invention relates to a biocompatible pre-gelatinized modified starch. The water absorbency is not less than one time, and the biocompatible pre-gelatinized modified starch is taken as biocompatible hemostatic material, biocompatible anti-blocking material, biocompatible tissue-healing promoting material, biocompatible surgical sealant or biocompatible wound closure tissue glue. The invention has the advantages that the biocompatible pre-gelatinized modified starch is directly acted on the wounded area with blood for immediately stopping bleeding, has obviously increased water absorbency and speed of water absorption and greater viscosity and stickiness and further plays the role in preventing the tissue and the blood vessel from being damaged during the process of stopping bleeding; the modified starch is easy to swell or dissolve in water, and is washed by normal saline after the bleeding stopping so as to reduce the residual in the body, to be favorable for wound healing and to avoid the pain due to tearing the gauze and the bandage out; the pre-gelatinized modified starch has the actions of bacterial resistance and anti-inflammatory; and the pre-gelatinized modified starch is stable, not easy to decompose, long in guarantee period, convenient for storage, resistant at high pressure and low pressure, resistant at high temperature and low temperature and not easy to change the physicochemical characteristics.
Owner:纪欣
Compositions comprising a tissue glue and therapeutic agents
InactiveUS6919067B2Minimize exposureSurgical adhesivesPharmaceutical delivery mechanismLocal radiotherapyMedicine
The present invention concerns compositions comprising a radiotherapeutic agent, or an agent which can be converted to a radiotherapeutic, and a tissue glue. The compositions of the present invention are particularly useful for providing local radiotherapy. The present invention also concerns methods of using the compositions of the invention, particularly for radiotherapy.
Owner:MOLECULAR SYNTHETICS
Polyamino acid based mussel bionic tissue adhesive and preparation method thereof
ActiveCN109355057AEasy to prepareProcess parameters are easy to controlNon-macromolecular adhesive additivesCross-linkDopamine
The invention provides a polyamino acid based mussel bionic tissue adhesive and a preparation method thereof. The polyamino acid based mussel bionic tissue adhesive is prepared by the following stepsthat a polyamino acid based grafted dopamine prepolymer is synthesized; a compound cross-linking agent is prepared; the prepolymer water solution with the concentration of 30-50% by weight and the compound cross-linking agent water solution are mixed evenly according to the ratio of 1-2 to 1, and the polyamino acid based mussel bionic tissue adhesive is prepared through cross linked gelatinization. For solving the existing problems of tissue adhesives, the preparation method of the polyamino acid based mussel bionic tissue adhesive is prepared. The tissue adhesive has the high bonding strengthunder physiological conditions, creatively utilizes MgO nano particles to form a crosslinking system and is endowed with intrinsic antibacterial property and biological activity.
Owner:HARBIN ENG UNIV
Wound suturing-free tissue adhesive
The present invention relates to a wound non-suturing tissue glue, in the concrete, it relates to an alkyl alpha-cyanoacrylate tissue glue containing anti-bacterial agent and deodorant. Its composition contains 40.0-85.0 portions of alkyl alpha-cyanoacrylate, 0.7-6.5 portions of strength improving agent, 0.5-7.5 portions of plasticity improving agent, 0.1-22.5 portions of anti-bacterial agent and 0.02-9.5 portions of deodorant. The anti-bacterial agent can be one or more than two kinds of cefmetazole, erythromycin ethylsuccinate, acetylspiramycin and chlorhexidine iodine and the deodorant can be one or more than two kinds of Chinese angelica root essential oil, cinnamon oil and myrrh essential oil.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU UNIV OF CHINESE MEDICINE
Tissue construct, methods of producing and using the same
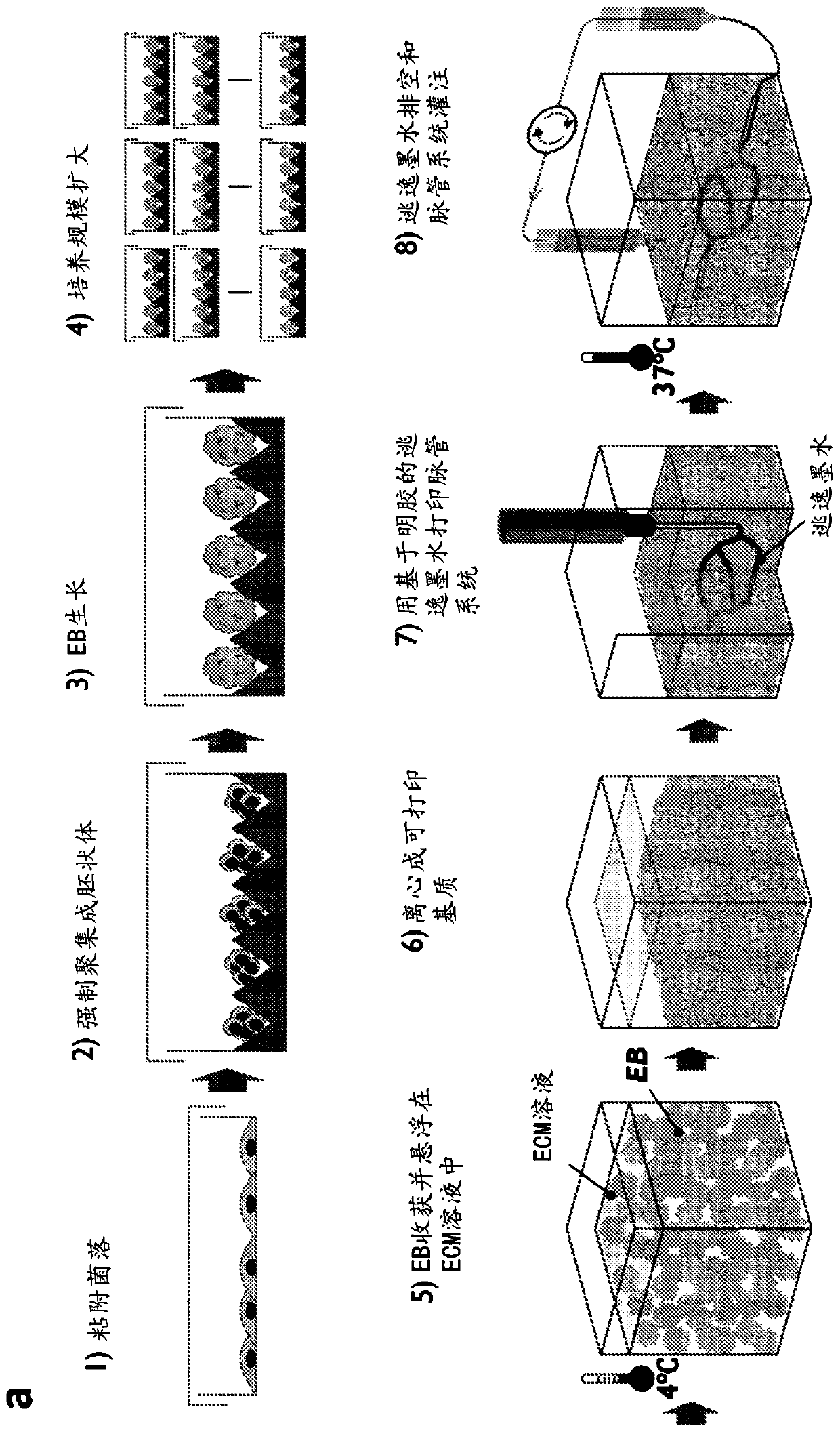
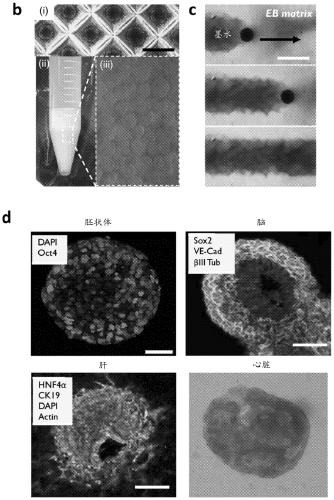
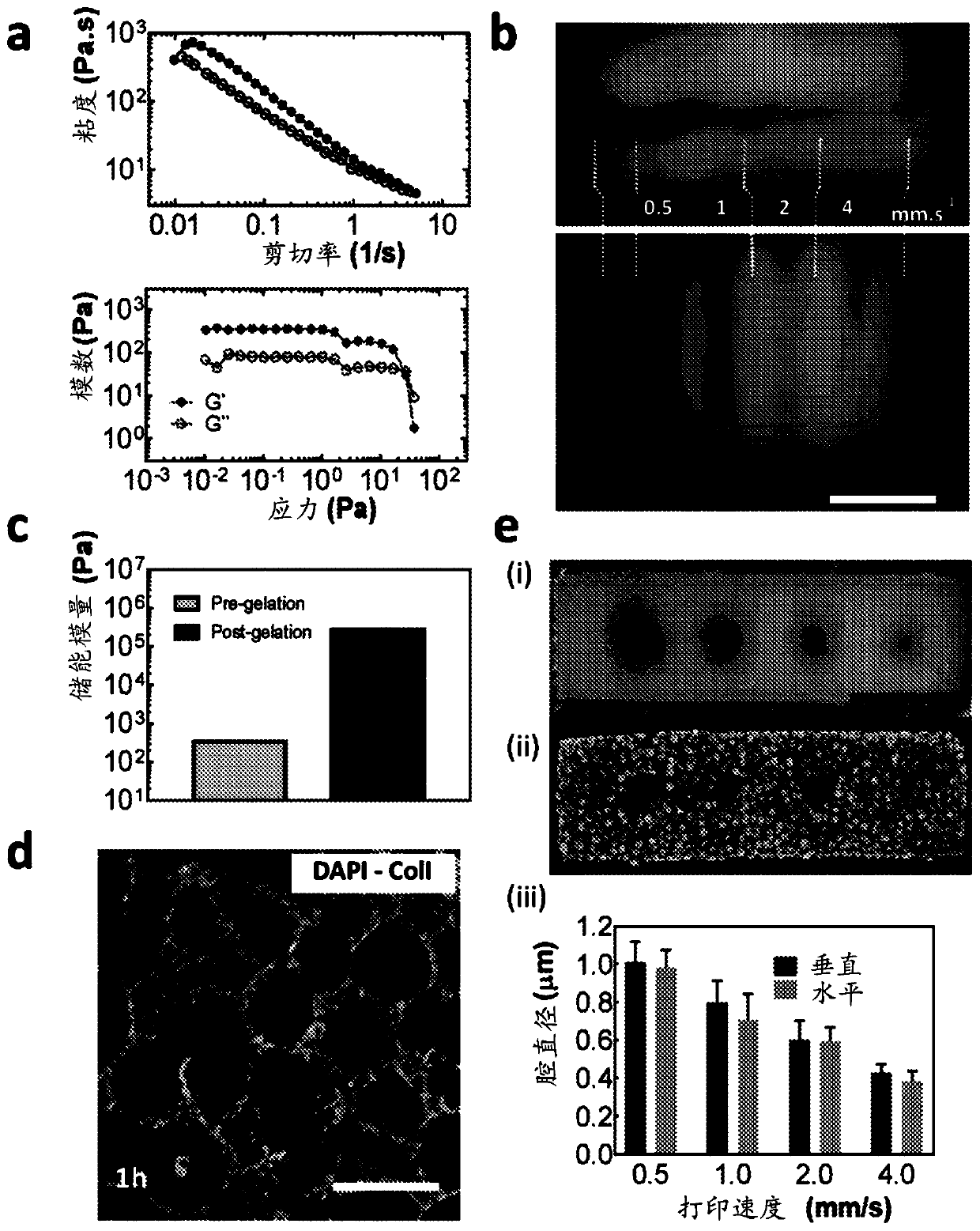
Described are methods for producing tissue constructs, tissue constructs produced by the methods, and their use. The described method of producing a tissue construct comprises providing a granular tissue, depositing one or more filaments on or in the granular tissue, each filament comprising an ink, and gelling or fusing the granular tissue, thereby producing the tissue construct.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
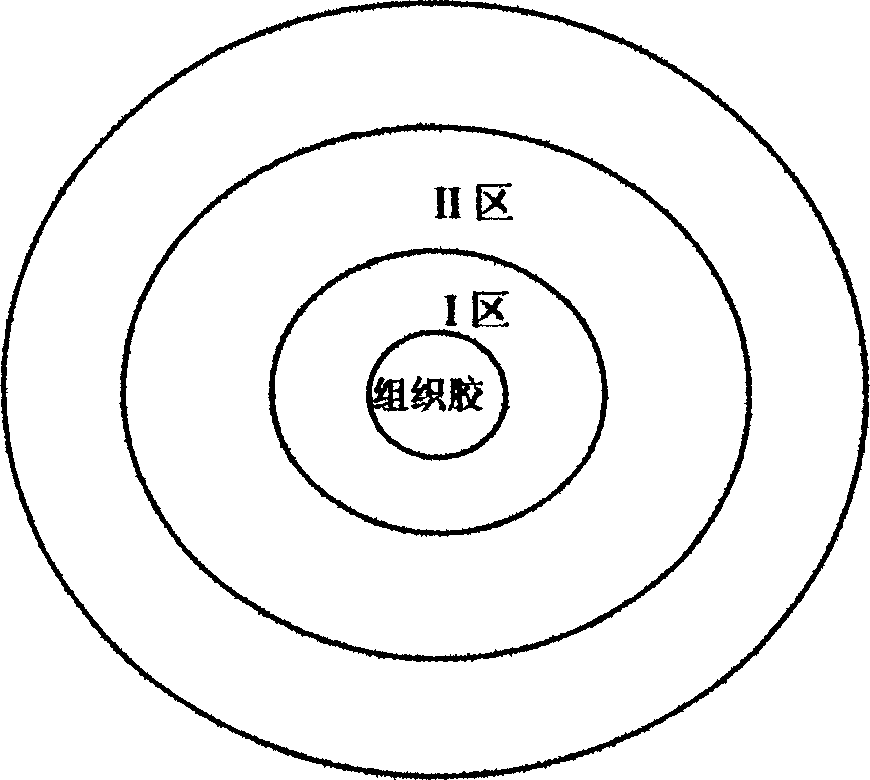
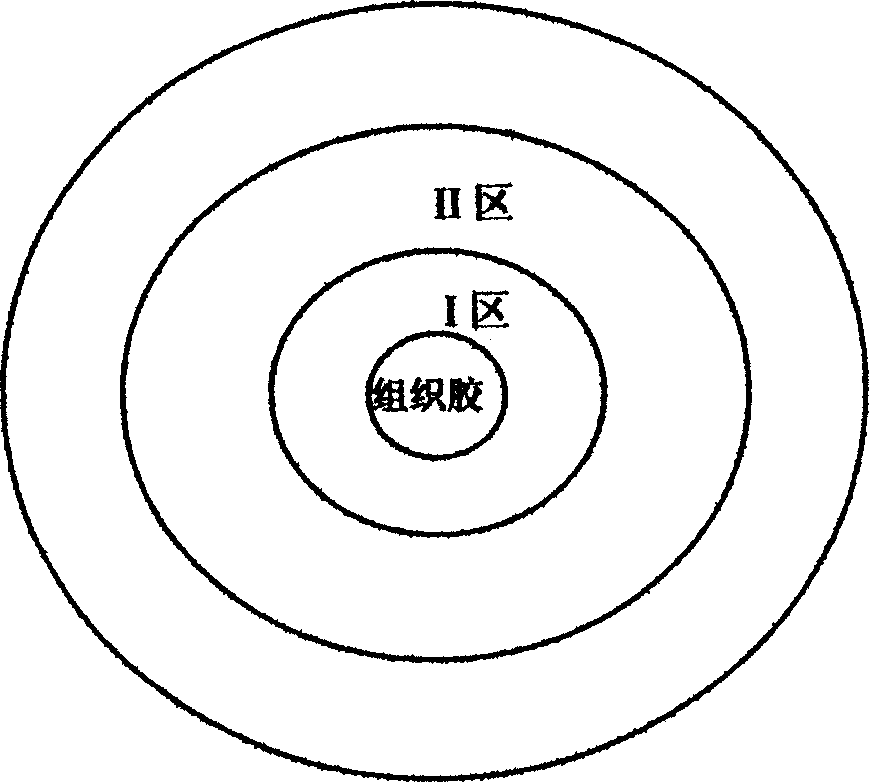
Phantom model containing tree-shaped pipeline structure
The invention belongs to the field of medical care and health, and relates to a phantom model, particularly to a phantom model containing a tree-shaped pipeline structure. The phantom model provided by the invention includes a peripheral tissue colloid model and a hollow pipeline model, the hollow pipeline model is a pipeline directly formed inside the peripheral tissue colloid model, and no partition membrane or partition wall exists between the hollow pipeline model and peripheral tissue glue. The model has the advantage of good sound transmission performance, effectively guarantees the quality of a sonogram echogram, and can effectively simulate important pipeline structures in a human body (such as liver), thereby obtaining better simulation, and enabling the range of application of the model to be wider, and the phantom model can be used as a sign structure in melting technology planning, and can also be used for training of a cavity radiographic technology.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
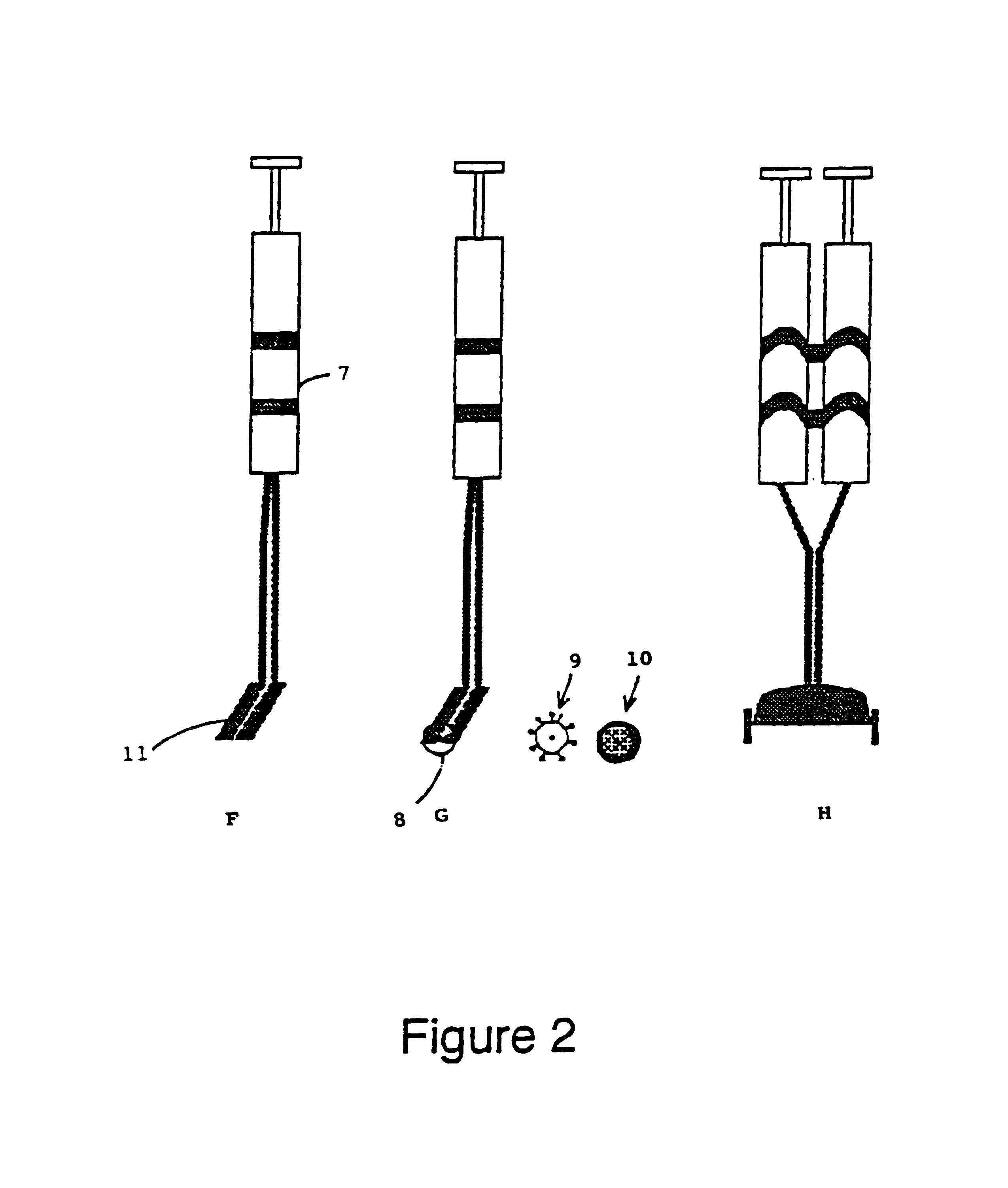
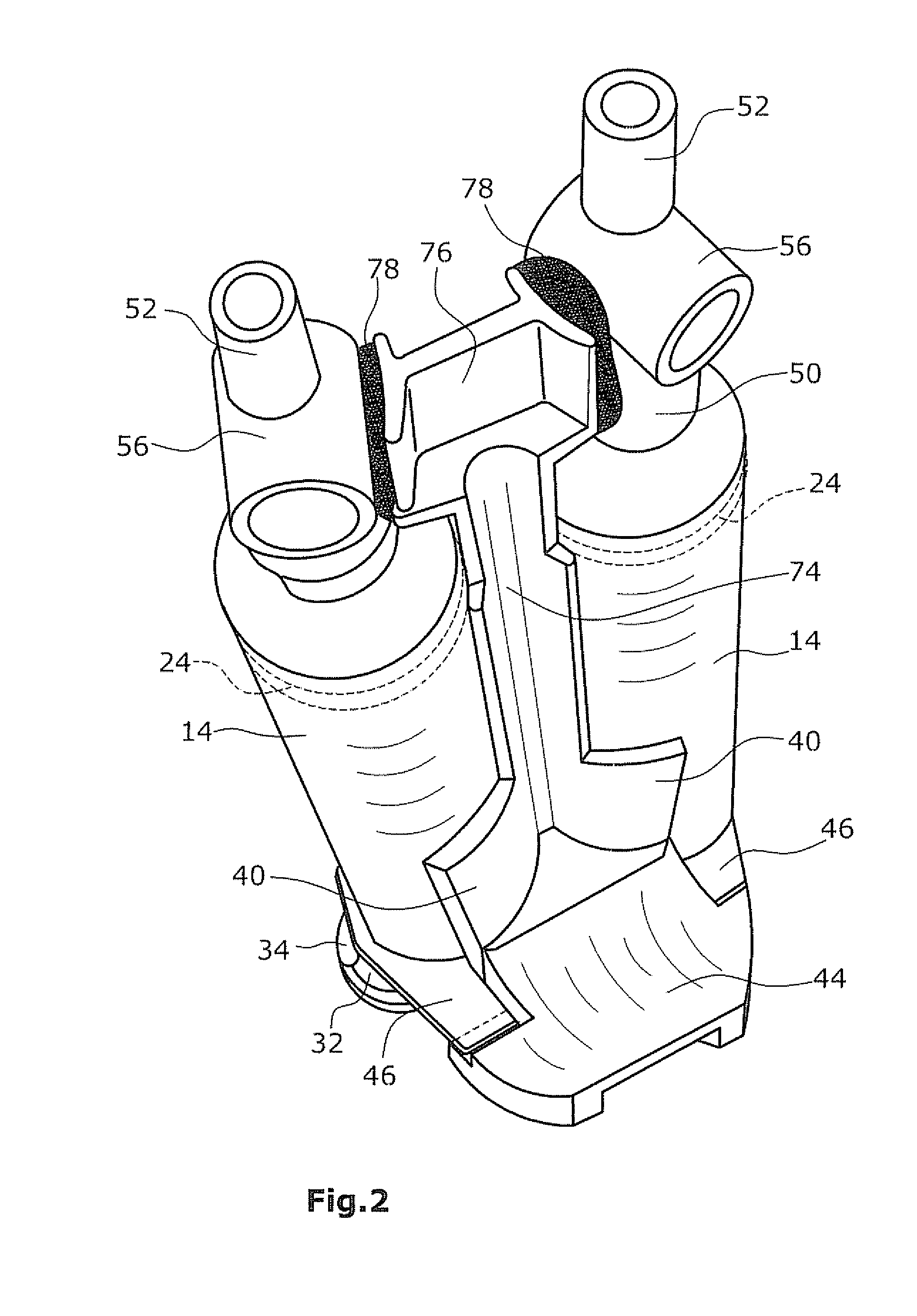
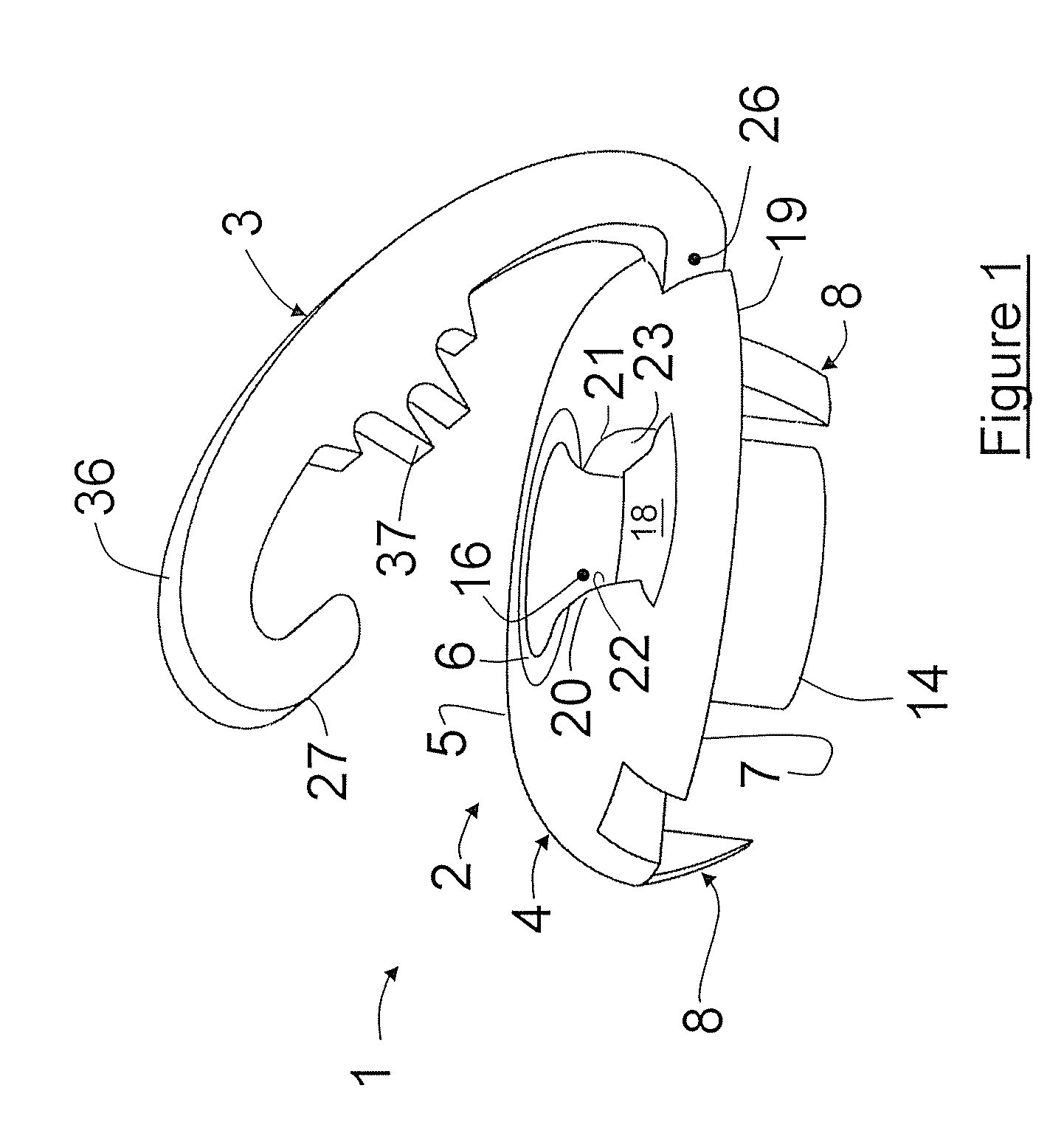
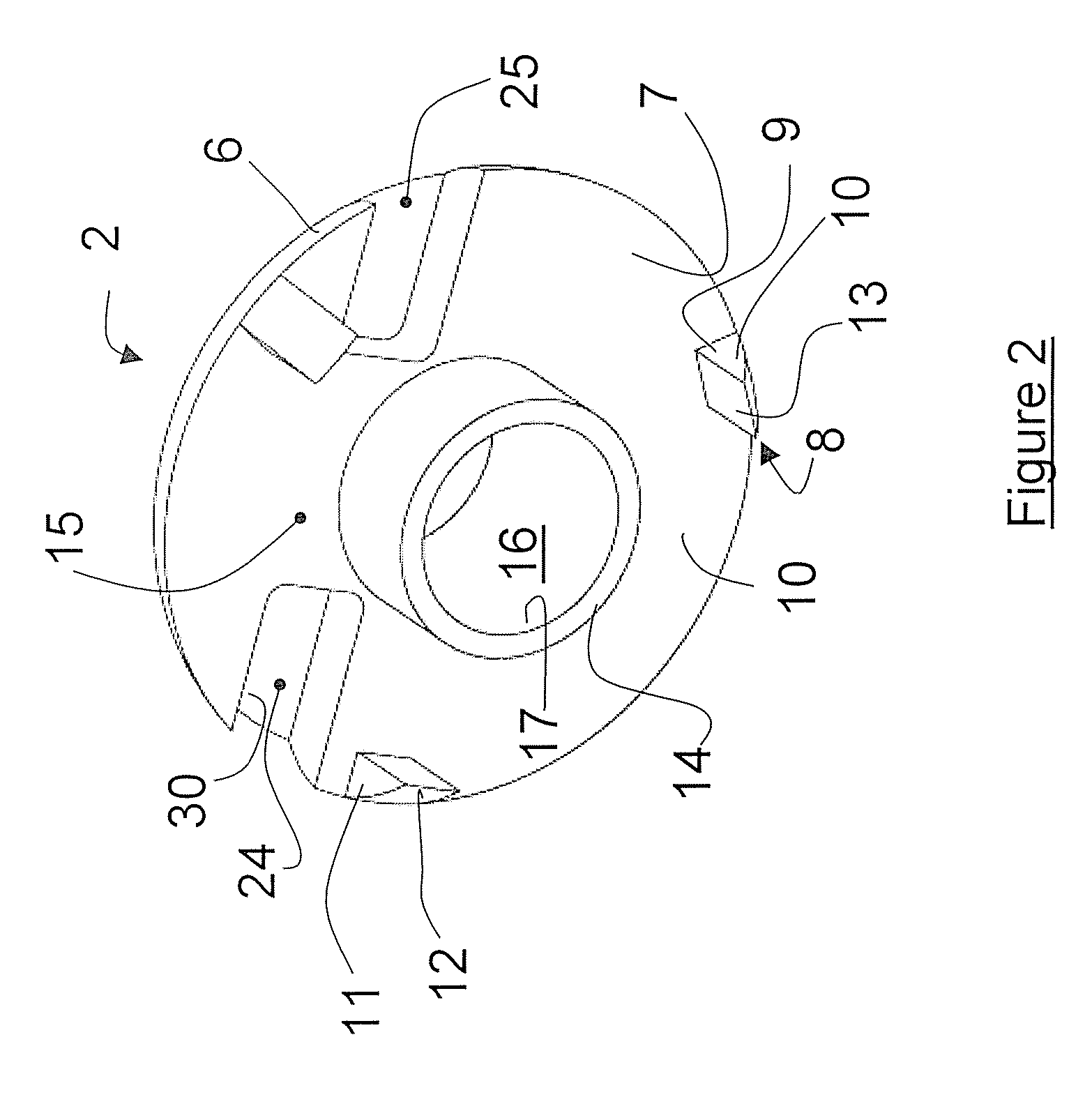
Applicator device for applying a multi-component fluid
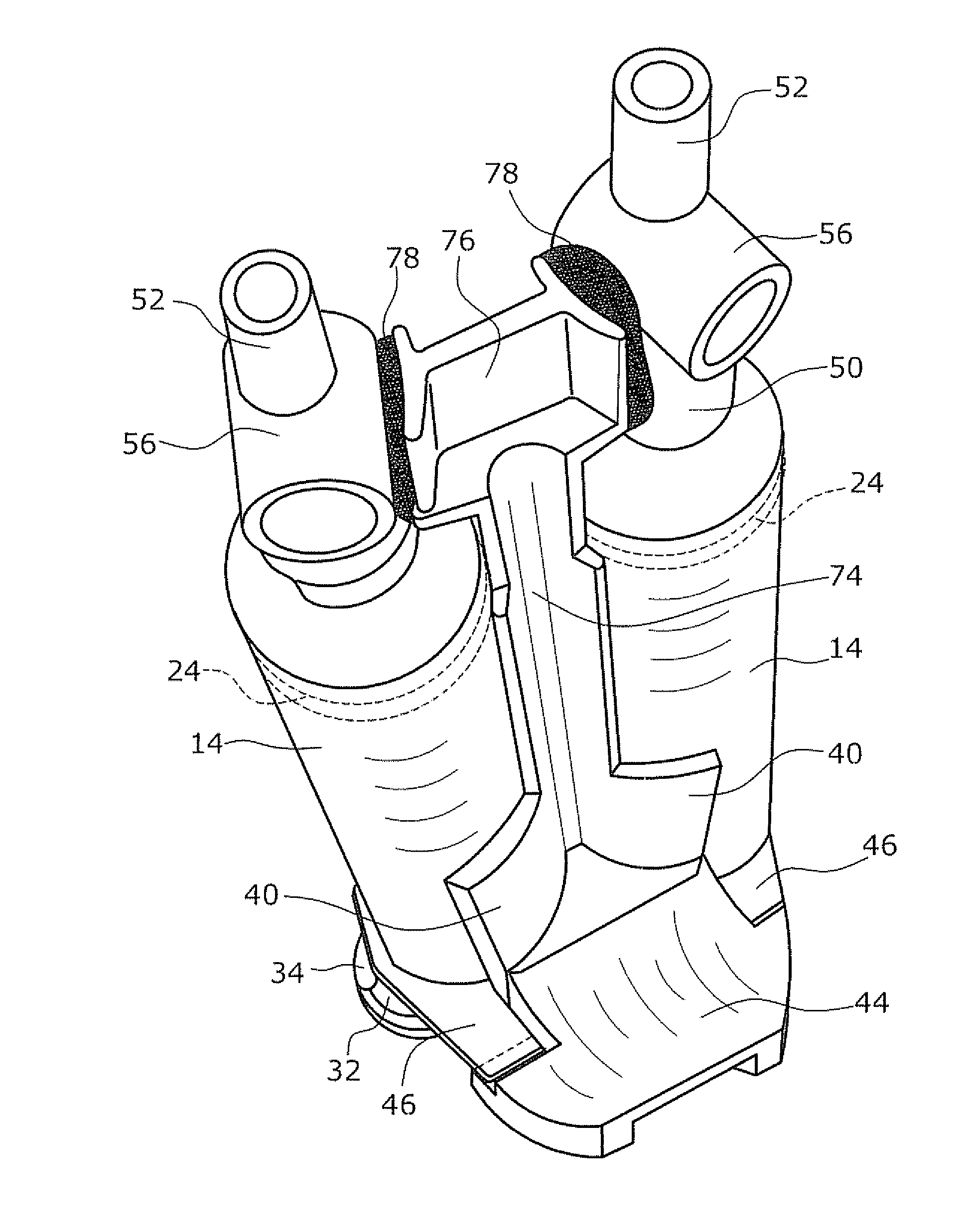
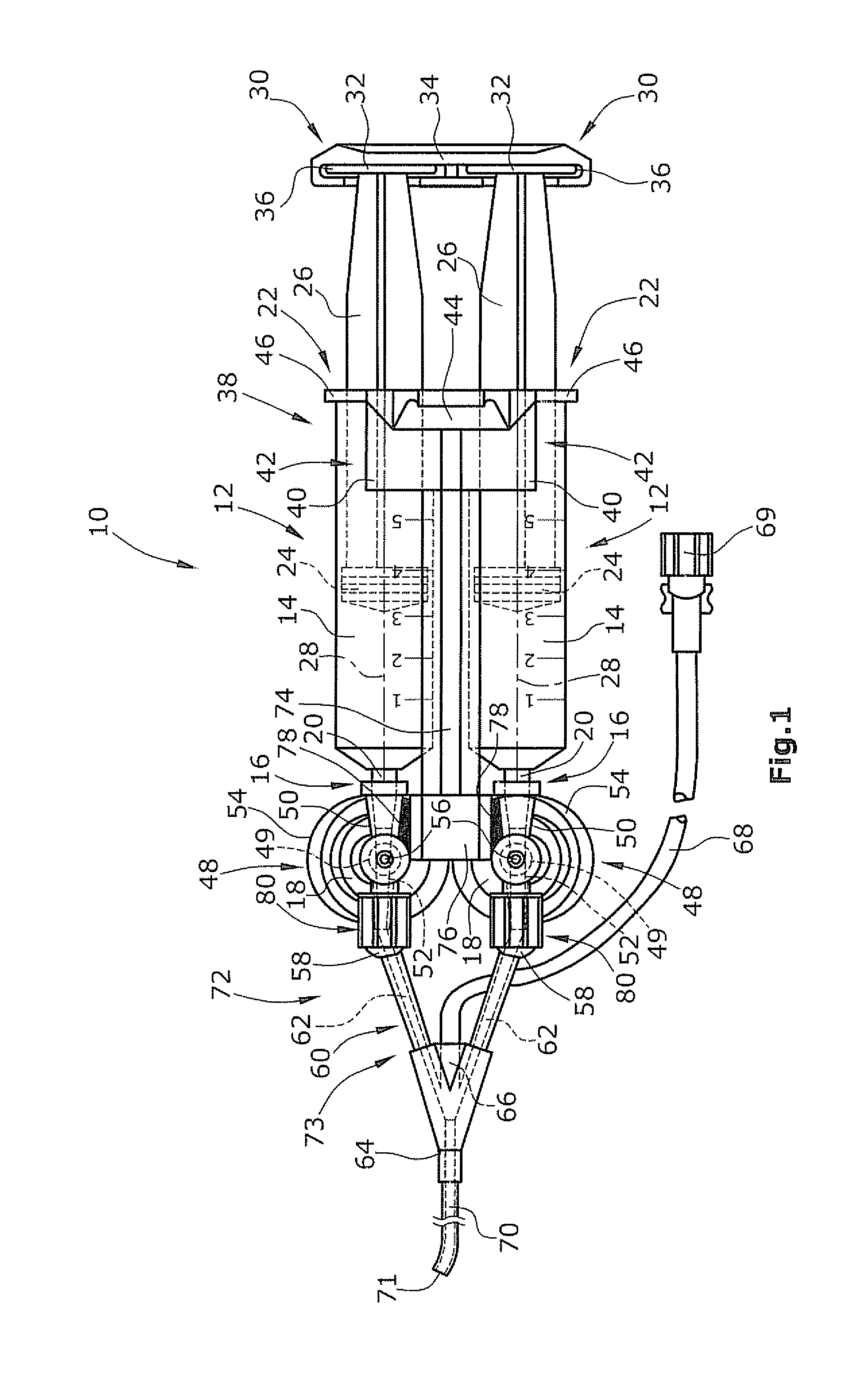
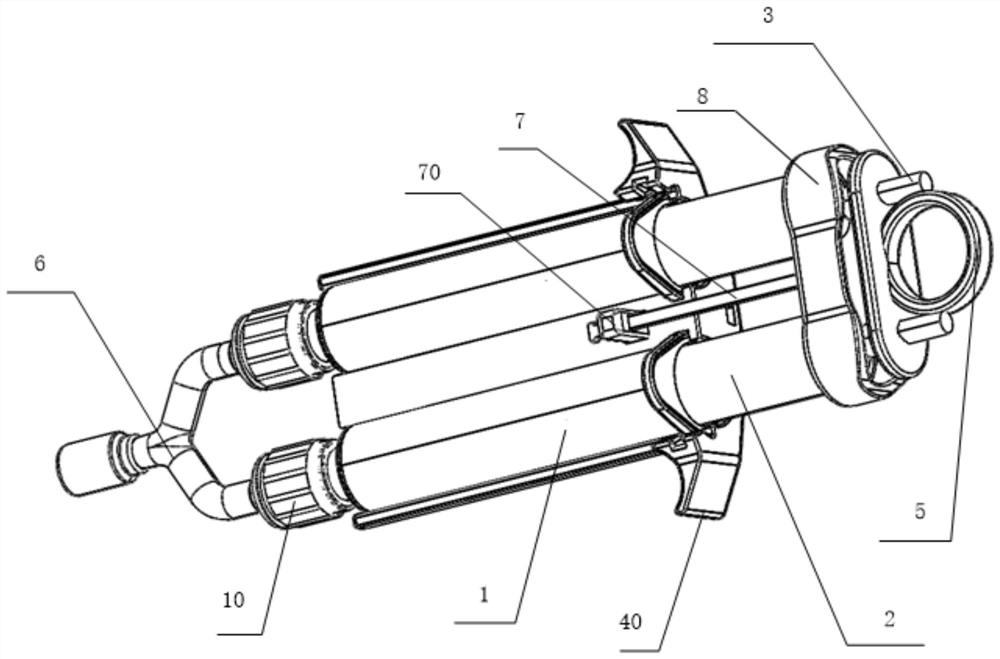
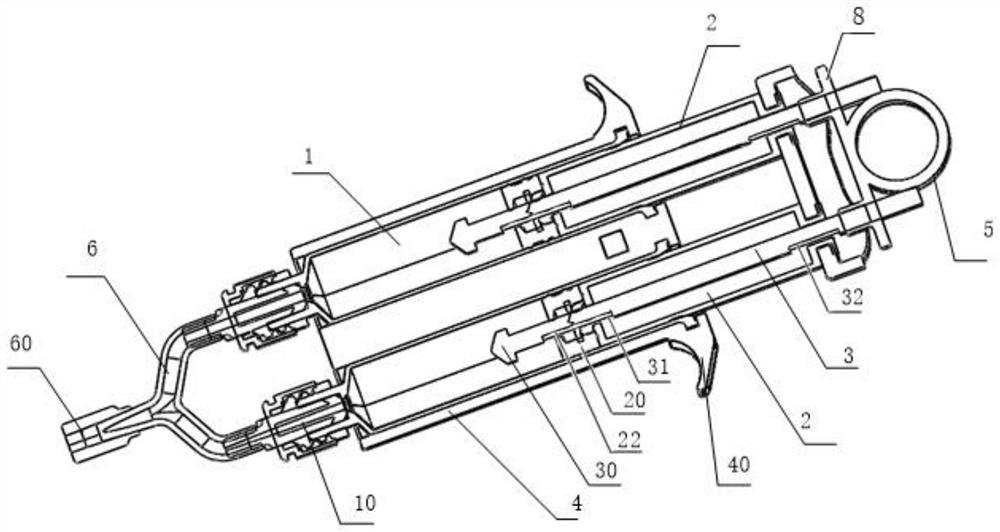
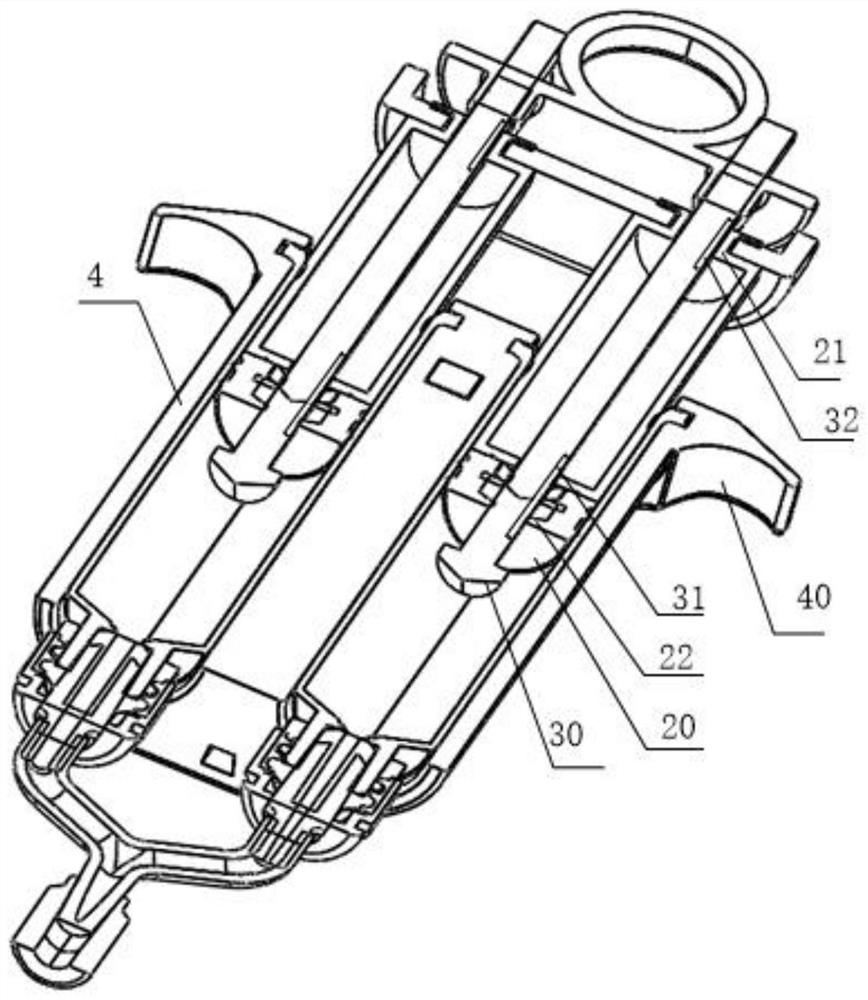
Disclosed is an applicator device (10) for applying a multi-component fluid, especially a multi-component tissue glue, comprising a plurality of substantially cylindrical supply containers (12) for respectively one component of the fluid to be applied, each supply container (12) having a front end (16) with an outlet opening (18), a rear end (22) opposite to the front end (16), and a slidably displaceable piston (24) arranged within the supply container (12) and having a piston rod (26) extending out of the rear end (22) for operating the piston (24). Moreover, the applicator device includes a manifold (72) having terminal ends (50,58) with a first port for fluid connection with the front ends (16) of the supply containers (12), the manifold (72) further having internal channels (62) extending from the first ports of the terminal ends (50,58) to an outlet site. Finally, also a holding element (38) for holding the supply containers (12), and a coupling element (74) extending from the holding element (38) and having a connection end (76) connected to the manifold (72) are provided, wherein the connection end (76) of the coupling element (74) is bonded to the manifold (72).
Owner:OMRIX BIOPHARM
Antibactrial wound suturing-free tissue adhesive
The present invention relates to an anti-bacterial wound non-suturing tissue glue. In the concrete, it relates to an alkyl alpha-cyanoacrylate tissue glue containing anti-bacterial agent. Its composition contains 30.0-80.0 portions of alkyl alpha-cyanoacrylate, 0.5-7.5 portions of strength improving agent, 1.0-8.5 portions of plasticity improving agent and 0.5-25.0 portions of anti-bacterial agent. The anti-bacterial agent is one or more than two kinds of cefmetazole, erythromycin ethylsuccinate, acetylspiramycin and chlorhexidine iodine.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Flexible recording electrode and preparation method and implantation method thereof
PendingCN110786846AReduce volumeImprove flexibilityDiagnostic recording/measuringSensorsBiomedical engineeringRecording electrode
The invention provides a flexible recording electrode. The flexible recording electrode comprises an adapter, a plurality of pin headers, and a plurality of electrode wires, the plurality of pin headers are arranged on the adapter at intervals, one ends of the plurality of electrode wires are in corresponding connection with the plurality of pin headers, one ends, away from the plurality of pin headers, of the plurality of electrode wires are bonded by a tissue glue, and a portion, not covered with the tissue glue, of the electrode wires is covered with a protective glue. The flexible recording electrode has a small volume, so that the electrical activity of a target brain area with a small area can be recorded, and the load on the animal's head can be reduced. The flexible recording electrode has good flexibility, the wound electrode wires are fixed by the tissue glue, the tissue glue can be degraded in the brain tissue, the electrode wires recover the flexibility, and can follow themovement of the brain tissue, and thereby the damage to the brain tissue is reduced. The structure is simple, and the cost is low. The invention also provides a preparation method and an implantationmethod of the flexible recording electrode.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Polyphenol-silk fibroin-polyethylene glycol medical tissue adhesive as well as preparation method and application thereof
PendingCN114344556AHigh bonding strengthImprove cohesionSurgical adhesivesAbsorbent padsPolymer scienceCarboxyl radical
According to the polyphenol-silk fibroin-polyethylene glycol medical tissue adhesive, the preparation method and the application, under the combined action of polyethylene glycol and a polyphenol compound, silk fibroin is rapidly separated out from a solution and forms a first-layer network structure with the polyphenol compound under the hydrophobic-hydrogen-bond action; meanwhile, a second-layer network structure is formed by the polyethylene glycol and the polyphenol compound through strong hydrogen-bond interaction, meanwhile, silk fibroin is subjected to self-crosslinking under the action of the carboxyl activating agent, cohesion of the adhesive is increased, and therefore the polyphenol-silk fibroin-polyethylene glycol medical tissue adhesive with the adhesion performance is obtained. The raw materials and the preparation process of the medical tissue adhesive are non-toxic and harmless, the product is non-irritant to a human body and has excellent biocompatibility, the preparation method is simple, and technological parameters are easy to control.
Owner:HARBIN ENG UNIV
Variable cross-section porous strip-shaped suture line for promoting tendon healing and preparation method thereof
ActiveCN111529752AReduce cuttingPromote ingrowthSuture equipmentsSurgical needlesSuturing needleTendon healing
The invention relates to a variable cross-section porous strip-shaped suture line for promoting tendon healing and a preparation method thereof, and belongs to the technical field of biomedical repairmaterials. The suture line comprises a suture line body and a hydrogel coating, and the suture line body is arranged to be of a three-section variable cross-section structure. Two ends of the sutureline body are arranged to be in a fine line shape which is easily connected with a suture needle so as to easily penetrate through tissues, and the middle portion is arranged to be in a porous band shape facilitating tissue ingrowth and reducing tendon cutting. The hydrogel coating which serves as tissue glue and has antibacterial, anti-inflammatory and healing-promoting effects is arranged on theouter surface of the suture line. The prepared suture line is good in operability and small in tissue cutting, is beneficial for promoting tendon healing, and has good application prospects.
Owner:DONGHUA UNIV
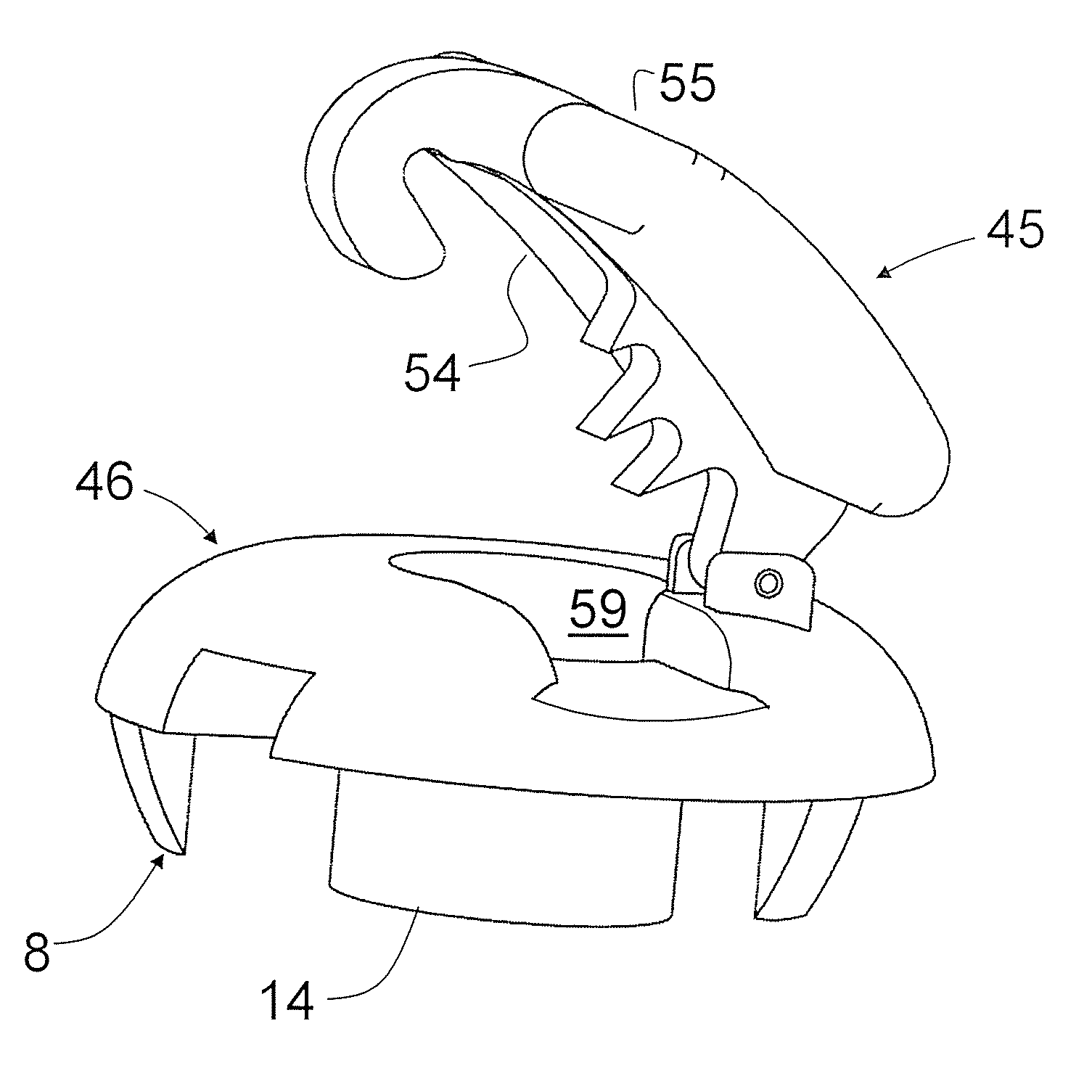
Soft tissue fixation device
ActiveUS8603115B2Quickly and easily attachedSimple structureLigamentsMusclesTissue glueSoft tissue fixation
A soft tissue fixation device for use in ACL or CrCL, reconstruction has a base member provided with a passageway extending perpendicularly from its top surface through its bottom surface. The passageway is sized to allow soft tissue to be inserted through the passageway. The fixation device also includes an affixing member attachable to the base member. The base member has a notched section in the top surface extending from the passageway to a first perimeter section of the base member sized to accommodate at least a portion of the graft. Either surgical grade tissue glue or at least one perpendicularly extending spike is used to secure the base member to the bone. The base member is also provided with a sleeve whose interior wall surfaces form a part of the passageway and is sized to be inserted into the bone opening. The affixing member is provided with a series of teeth members extending downward from its lower surface. The teeth members are positioned so that when the affixing member is attached to the base member the teeth members will extend across and into the notched section of the top surface of the base member. The opposite ends of the affixing member is shaped to fit into aligned notches positioned along perimeter sections of the base member bottom surface for attaching the clip member to the base member.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
A soft tissue fixation device
ActiveUS20080027441A1Quickly and easily attachedSimple structureLigamentsMusclesTissue glueSoft tissue fixation
A soft tissue fixation device for use in ACL or CrCL, reconstruction has a base member provided with a passageway extending perpendicularly from its top surface through its bottom surface. The passageway is sized to allow soft tissue to be inserted through the passageway. The fixation device also includes an affixing member attachable to the base member. The base member has a notched section in the top surface extending from the passageway to a first perimeter section of the base member sized to accommodate at least a portion of the graft. Either surgical grade tissue glue or at least one perpendicularly extending spike is used to secure the base member to the bone. The base member is also provided with a sleeve whose interior wall surfaces form a part of the passageway and is sized to be inserted into the bone opening. The affixing member is provided with a series of teeth members extending downward from its lower surface. The teeth members are positioned so that when the affixing member is attached to the base member the teeth members will extend across and into the notched section of the top surface of the base member. The opposite ends of the affixing member is shaped to fit into aligned notches positioned along perimeter sections of the base member bottom surface for attaching the clip member to the base member.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Compositions comprising a tissue glue and therapeutic agents
InactiveUS20050002860A1Minimize exposureSurgical adhesivesPharmaceutical delivery mechanismLocal radiotherapyMedicine
The present invention concerns compositions comprising a radiotherapeutic agent, or an agent which can be converted to a radiotherapeutic, and a tissue glue. The compositions of the present invention are particularly useful for providing local radiotherapy. The present invention also concerns methods of using the compositions of the invention, particularly for radiotherapy.
Owner:FILLER AARON GERSHON +1
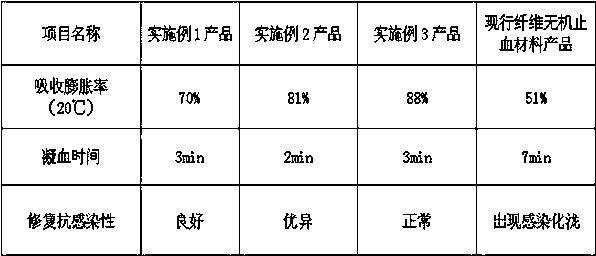
Bionic cloth for stopping bleeding
InactiveCN108379646AImprove magnetismImprove durabilityPharmaceutical delivery mechanismAbsorbent padsVitamin CPolyvinyl alcohol
The invention belongs to the technical field of medical products, relates to a material for stopping bleeding and in particular relates to bionic cloth for stopping bleeding. The bionic cloth for stopping bleeding has a multilayered structure and comprises a repairing dissolving layer, an isolation and ventilation layer, a bleeding stopping medicine layer and a covering outer layer in sequence, wherein the repairing dissolving layer is cyanoacrylate tissue glue; the isolation and ventilation layer is formed by weaving cotton and hemp fibers; the bleeding stopping medicine layer is a medical treatment medicament; the covering outer layer is cotton and hemp fiber cloth; the repairing dissolving layer, the isolation and ventilation layer, the bleeding stopping medicine layer and the coveringouter layer are stuck and cured together through crosslinking; the bleeding stopping medicine layer is prepared from the following components in percentage by mass: 22 to 24 percent of mannitol, 0.5 to 12.6 percent of chitosan, 9 to 22 percent of vitamin C, 5.2 to 6.5 percent of dexamethasone, 2.0 to 2.2 percent of thrombin, 2.0 to 3.2 percent of polyvinyl alcohol, 0.2 to 0.5 percent of sodium dodecyl sulfonate, 0.5 to 7.2 percent of modified zeolite and the balance of starch micro-pore polysaccharide granules. After the product absorbs a lot of blood, the volume is expanded so that blood vessel ruptures or wounds are sealed and a barrier is provided for a bleeding stopping opening; the bionic cloth has a more rapid healing speed and a better effect.
Owner:朱梅佳
Culture of tissue engineering skin and detection method of proliferation activity
InactiveCN101392237AObjective detection of proliferative activityDoes not affect growthArtificial cell constructsVertebrate cellsStainingCuticle
The invention discloses a detection method of tissue engineering skin culture and proliferation activity, which comprises the following process: first three-dimensional host materials containing basilar membrane are prepared as the substitute of dermis, namely dermis tissue of human with epidermis being removed, and keratinocyte is cultivated; the keratinocyte is inoculated to the surface of the dermis tissue of human with epidermis being removed and coated with mixed tissue glue, sampling is carried out for the detection of the skin proliferation activity after the culture of air-liquid surface culture mode, the culture is stopped after the culture is continued for a week, and then the skin is picked out for skin proliferation activity detection. A fresh culture specimen picked is put into BrdU culture liquid and a culture box of 37 DEG C for breeding, immnohistochemical staining is carried out after 24 hours, proliferation cells are observed under a microscope, the quantity of proliferation cells is calculated by using a computer imaging system, and tissue engineering skin structure is observed by the method of histology simultaneously. The detection method of tissue engineering skin culture and proliferation activity can detect the impact of different culture conditions and relevant factors on the tissue engineering skin cell proliferation by quantization.
Owner:陆洪光
Visible sacculus sleeve for esophageal and gastric varices
InactiveCN103494629AGood curative effectEasy injectionBalloon catheterSurgeryVeinBleeding gastric varices
A visible sacculus sleeve for esophageal and gastric varices comprises a sleeve body, an inflation pipe I, an inflation connector I, an inflation connector II and an inflation pipe II, wherein one end of the sleeve body is connected with a handheld end, a treatment window is arranged at the other end of the sleeve body, a far-end sacculus is arranged on one side of the treatment window, a near-end sacculus is arranged on the other side of the treatment window, the inflation pipe I and the inflation pipe II are arranged on the two sides of the inner wall of the sleeve body respectively, one end of the inflation pipe I is connected with the far-end sacculus, the other end of the inflation pipe I is connected with the inflation connector I, one end of the inflation pipe II is connected with the near-end sacculus, and the other end of the inflation pipe II is connected with the inflation connector II. According to the visible sacculus sleeve, a bleeding point can be accurately pressed with the help of the sleeve body and one of the sacculi till bleeding is stopped under an endoscope, the other sacculus presses the wall of the oesophagus so that an oesophagus vein can be blocked, and the probability of ectopic embolism is lowered. According to the visible sacculus sleeve, doctors in most primary hospitals can conveniently master the tissue glue injection technology and the sclerosis injection technology, and safety is improved in an operation.
Owner:刘雄昌
Compositions, devices and kits for selective internal radiation therapy
ActiveUS11141526B2Reduce chanceAssess effectivenessPowder deliveryInfusion syringesRadio isotopesDosing regimen
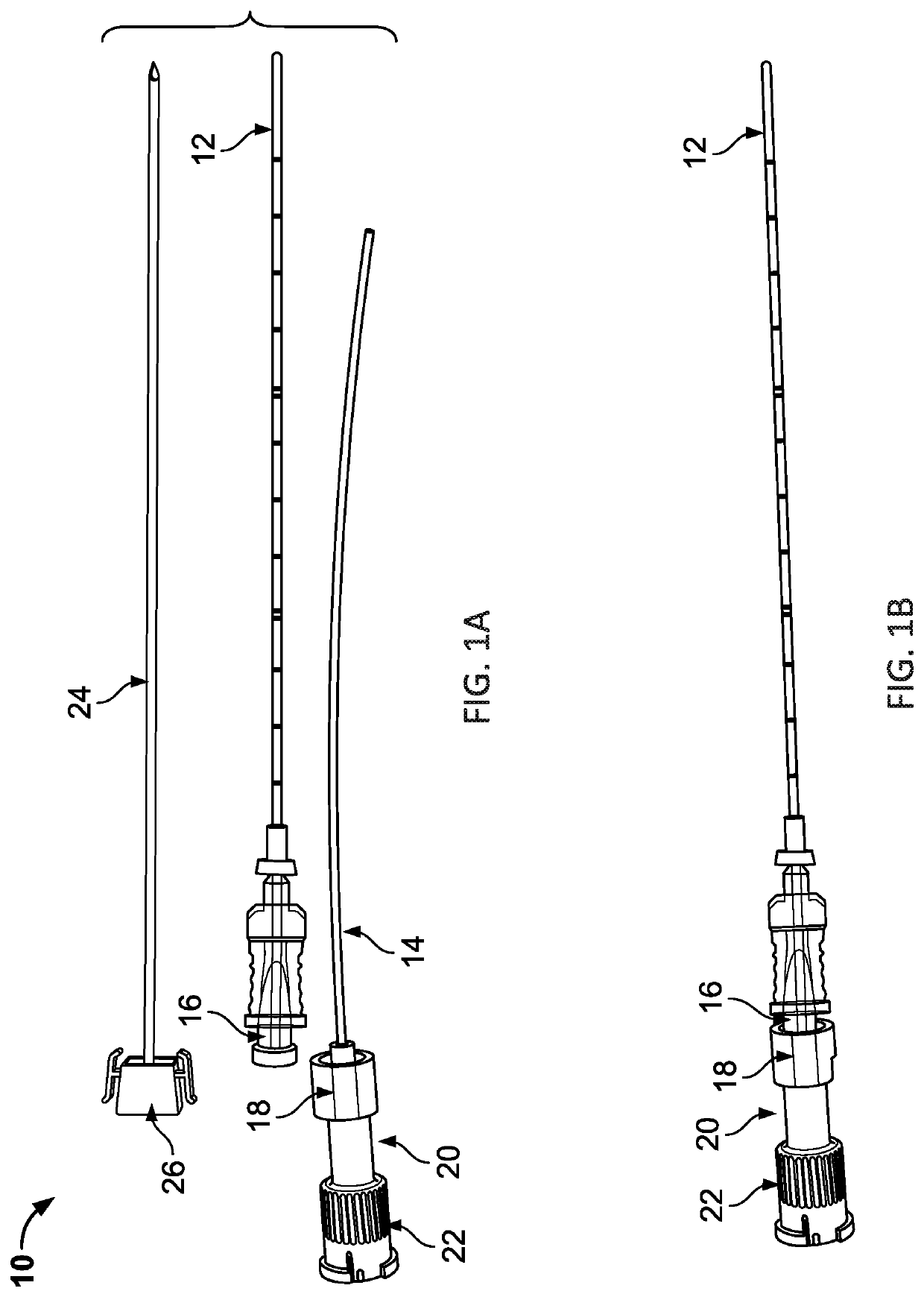
Systems, kits and methods for preparing an injection system and / or treating target lesions with a selective internal radiation therapy which includes a double-barrel syringe loaded with a two-component tissue glue and radioisotope loaded microspheres. The microspheres are loaded into the syringe based on the size of the target location and are administered with a needle or dual-lumen catheter. Dosing regimens for treating breast cancer lesions or surgical beds up to 130 mm in diameter and hepatocellular carcinoma lesions up to 50 mm are included.
Owner:BETAGLUE TECH SPA
Injector special for endoscopic tissue glue injection
PendingCN108785802ASave operating timeAvoid contactInfusion syringesTreatment demandEndoscopic treatment
Endoscopic tissue glue injection is an effective method for gastric fundal varices treatment and requires a 'sandwich' method, and hyperosmotic drugs need to be injected rapidly and powerfully after tissue glue injection. At present, injectors of 2 ml have very high requirements for endoscopic treatment operators, cooperative persons and medicine suction nurses in use, the needle plugging risk also exists during injector change, and the endoscopic treatment demand cannot be very well met. At present, no reasonable special injector exists in the market. The invention provides an injector special for endoscopic tissue glue injection. The injector special for endoscopic tissue glue injection comprises a large-diameter injection needle cylinder (1), a small-diameter injection needle cylinder (2) and a fixing insertion piece (3). The injection is simple and convenient to use, prevents tissue glue from being in contact with air, reduces the risk that an injection needle is plugged by solidifying tissue glue and can meet the demand for endoscopic tissue glue injection.
Owner:张丹
Adhesive and preparation method thereof
InactiveCN111803700AEnhanced interactionHigh mechanical strengthSurgical adhesivesPolymer scienceProtein molecules
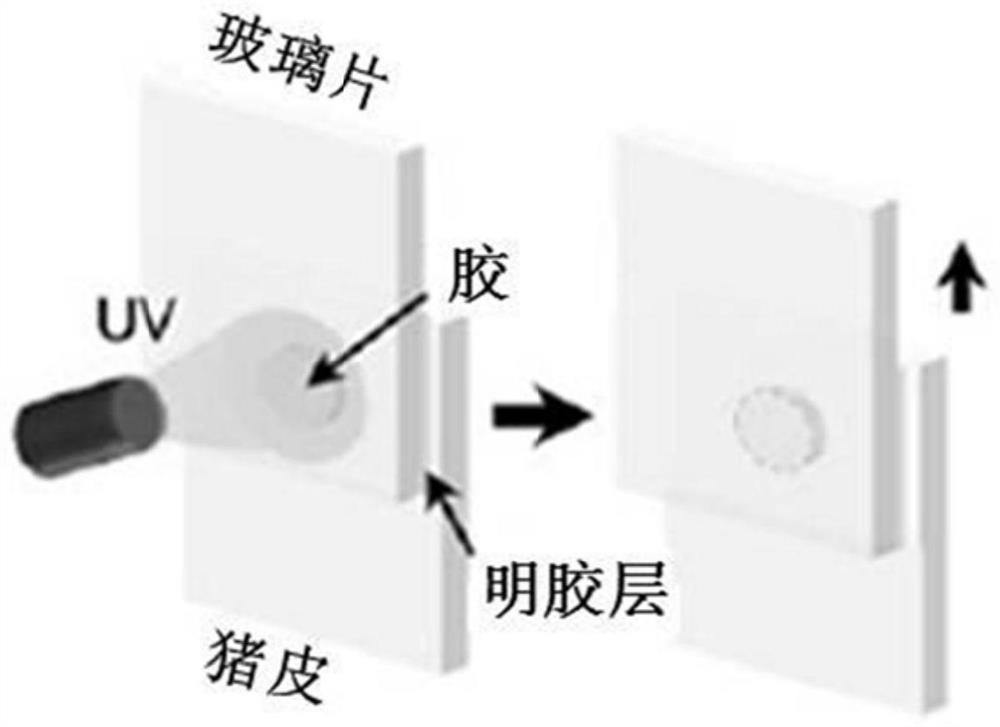
The invention provides an adhesive and a preparation method thereof. The adhesive comprises bovine serum albumin, a cross-linking agent and diazirine. In the adhesive disclosed by the invention, the diazirine can quickly generate carbene under the irradiation of ultraviolet light, the carbene and groups such as-COOH and-NH2 which are close to the carbine are subjected to an insertion reaction to generate covalent bonds, and the interaction force between bovine serum protein molecules and between protein and tissues is enhanced, so that the mechanical strength and the bonding strength of the tissue adhesive are enhanced.
Owner:GUANGDONG PROV MEDICAL INSTR INST
Preparation method of degradable tissue glue based on polylactic acid and polycaprolactone copolymer
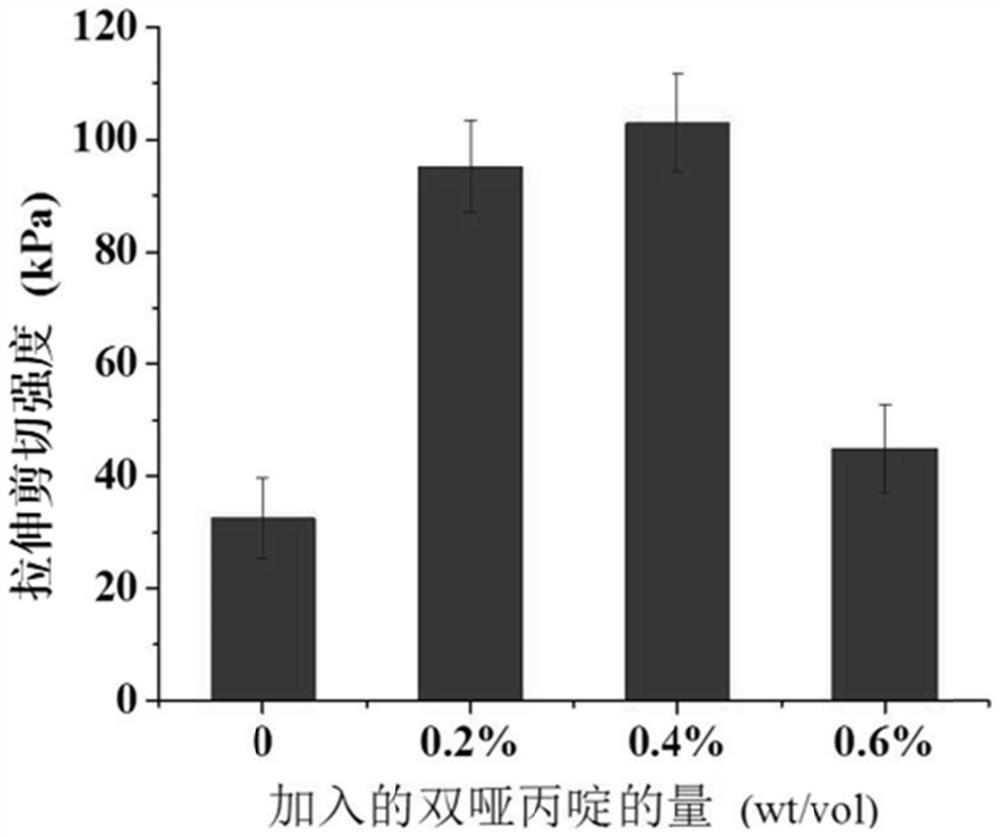
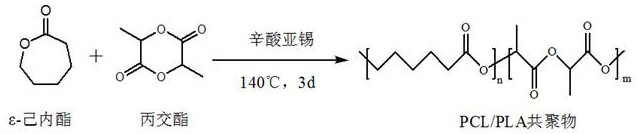
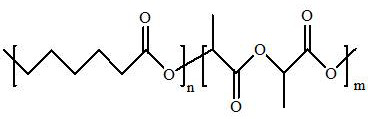
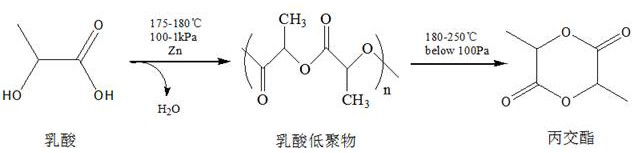
InactiveCN111671967AWith double-sided adhesive effectAdhesion plays a roleSurgical adhesivesPolymer scienceLactide
The invention relates to the technical field of tissue glue manufacturing, in particular to a preparation method of degradable tissue glue based on polylactic acid and polycaprolactone copolymer. Thepreparation method comprises the following steps: S1, synthesizing PCL / PLA copolymer through bulk ring-opening polymerization reaction of epsilon-caprolactone and DL-lactide under high-temperature and vacuum conditions; and S2, purifying the PCL / PLA copolymer. The copolymer has a double-sided bonding effect in actual use, has good biocompatibility, can play a bonding role within a certain time,has good viscoelasticity and holding power, and has high peel strength. The copolymer has a double-sided bonding effect in actual use, has good biocompatibility, can play a bonding role within a certain time, and has good viscoelasticity and holding power, and high peel strength.
Owner:SHANDONG ZHUSHI PHARMA GRP CO LTD
Biocompatibility pre-gelatinized modified starch and preparation thereof
InactiveCN101497670BImprove water absorptionHigh viscositySurgical adhesivesFibre treatmentBiocompatibilityBlood vessel
The invention relates to a biocompatible pre-gelatinized modified starch. The water absorbency is not less than one time, and the biocompatible pre-gelatinized modified starch is taken as biocompatible hemostatic material, biocompatible anti-blocking material, biocompatible tissue-healing promoting material, biocompatible surgical sealant or biocompatible wound closure tissue glue. The invention has the advantages that the biocompatible pre-gelatinized modified starch is directly acted on the wounded area with blood for immediately stopping bleeding, has obviously increased water absorbency and speed of water absorption and greater viscosity and stickiness and further plays the role in preventing the tissue and the blood vessel from being damaged during the process of stopping bleeding; the modified starch is easy to swell or dissolve in water, and is washed by normal saline after the bleeding stopping so as to reduce the residual in the body, to be favorable for wound healing and to avoid the pain due to tearing the gauze and the bandage out; the pre-gelatinized modified starch has the actions of bacterial resistance and anti-inflammatory; and the pre-gelatinized modified starch is stable, not easy to decompose, long in guarantee period, convenient for storage, resistant at high pressure and low pressure, resistant at high temperature and low temperature and not easy to change the physicochemical characteristics.
Owner:纪欣
Micro-infusion method and device thereof based on epididymis duct intracavity environment experiment
InactiveCN104323868ASolve difficult to buySolve the domestic plasticineDiagnostic recording/measuringSensorsBlood vesselTissue glue
The invention relates to a micro-infusion method and a device thereof based on epididymis duct intracavity environment experiment. In international reports, the live micro-infusion method on the function research of epididymis ducts adopts the steps of by utilizing a dissecting microscope, separating the epididymis duct from the epididymis duct tail part, forming an incision, inserting a catheter, fixing by plasticine, separating an accompanying blood vessel near an spermaduct, and inserting a catheter, so the difficulty in intubation operation is larger. The method comprises the following steps of separating the epididymis duct from the epididymis duct tail part; using a glass micro-infusion tube, drawing and thinning a head part, and connecting the tail part with a silicone tube, a syringe and a micro injection pump; directly inserting a micro-infusion pipe head part into the epididymis duct, dripping a tissue glue for fixing, and filling interference materials; directly inserting the silicone tube without separation of the accompanying blood vessel near the spermaduct, and fixing by the tissue glue; finally, backflushing the spermaduct, and collecting epididymis duct liquid at the opening of the injection part of the epididymis duct. The method and the device have the advantages that the micro-infusion operation under the live environment is optimized, the operation is simple, the implementation is easy, the required material is purchased domestically, the experiment cycle is greatly shortened, and the experiment efficiency is improved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Tissue glue applying device
PendingCN114177510AFacilitate instant generationInstant generationTransportation and packagingSurgeryInjection portEngineering
The invention provides a tissue glue applying device. The tissue glue applying device comprises two injection assemblies and a mixer, the two injection assemblies are connected with the mixer through the injection ports; each injection assembly comprises an injection shell, a sliding column and a push rod; the sliding column is inserted into the injection shell from the opening and can slide along the injection shell; the two ends of the sliding column are an insertion end and a push-pull end respectively; the insertion end is located in the injection shell, and the push rod is installed at the push-pull end. A liquid inlet hole is formed in the insertion end; a plug for sealing the liquid inlet hole is arranged on the push rod; the push rod slides relative to the containing cavity so that the plug can open the liquid inlet hole, and the injection shell and the containing cavity can be communicated through the liquid inlet hole. The two injection assemblies are used for storing multiple tissue glue raw materials respectively, the liquid inlet hole can be opened through the sliding effect of the push rod, powder and liquid are mixed to generate tissue glue, and therefore the tissue glue can be conveniently and instantly generated and used, and the advantages that the liquid levels are mutually mixed and fully react, and application is rapid are achieved.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Compositions, devices and kits for selective internal radiation therapy
ActiveUS20210128819A1Reduce chanceAssess effectivenessPowder deliveryInfusion syringesRadio isotopesDosing regimen
Systems, kits and methods for preparing an injection system and / or treating target lesions with a selective internal radiation therapy which includes a double-barrel syringe loaded with a two-component tissue glue and radioisotope loaded microspheres. The microspheres are loaded into the syringe based on the size of the target location and are administered with a needle or dual-lumen catheter. Dosing regimens for treating breast cancer lesions or surgical beds up to 130 mm in diameter and hepatocellular carcinoma lesions up to 50 mm are included.
Owner:BETAGLUE TECH SPA
Water-based tissue adhesives
An adhesive composition described herein comprises an aqueous solvent; and a population of first nano particles dispersed in the aqueous solvent, the first nano particles comprising a negative or positive charge, and an average size in three dimensions of 1 nm to 1000 nm. In some embodiments, a population of second nano particles is dispersed in the aqueous solvent, the second nano particles comprises a negative or positive charge opposite the charge of the population of first nano particles. In some embodiments, the population of first nano particles comprises an average first size in three dimensions, and the population of second nano particles comprise an average second size in three dimensions that is different from the average first size of the first nano particles.
Owner:阿莱奥生物医学工程有限公司
A medical absorbable polysaccharide composite material and its application
ActiveCN109498833BHigh saturated water absorption multipleFast water absorptionSurgical adhesivesPharmaceutical delivery mechanismFreeze-dryingBiocompatibility
Owner:济南格莱威医疗科技有限公司
Polyphenol-based medical tissue adhesive as well as preparation method and application thereof
PendingCN114369441AHigh bonding strengthFast bondingSurgical adhesivesNon-macromolecular adhesive additivesPolyethylene glycolBiocompatibility
The invention discloses a polyphenol-based medical tissue adhesive as well as a preparation method and application thereof, and belongs to the field of biomedical material preparation and biomedical application. The adhesive is prepared from a polyphenol compound solution, inorganic nanoparticles and a polyethylene glycol solution under specific conditions. The prepared polyphenol-based medical adhesive has the characteristics of high bonding strength, excellent hemostasis and antibacterial property, good oxidation resistance, safety, no toxicity, good biocompatibility, low price and the like, is simple and easy to use and operate, can be used for most damaged tissues such as skin, blood vessels, visceral organs, muscles, bones and the like, and has the advantages of wide application prospect and the like. The adhesive has the effects of adhering tissues, covering wounds, stopping bleeding, resisting bacteria, resisting oxidation and the like, can be used as a hemostatic, antibacterial and antioxidant adhesive material, and has a wide clinical application prospect.
Owner:HARBIN ENG UNIV
Formulations for histatin protectives and therapeutics
InactiveUS20170239331A1Reduce developmentAvoid injuryLaser surgeryTetracycline active ingredientsDiseaseAerosol spray
Owner:VISUS THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com