Treatment of cancer using inhibitors of tgf-beta and pd-1
A β inhibitor, PD-1 technology, applied in medical preparations containing active ingredients, antibody medical ingredients, chemical instruments and methods, etc., can solve problems that hinder the effectiveness of immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
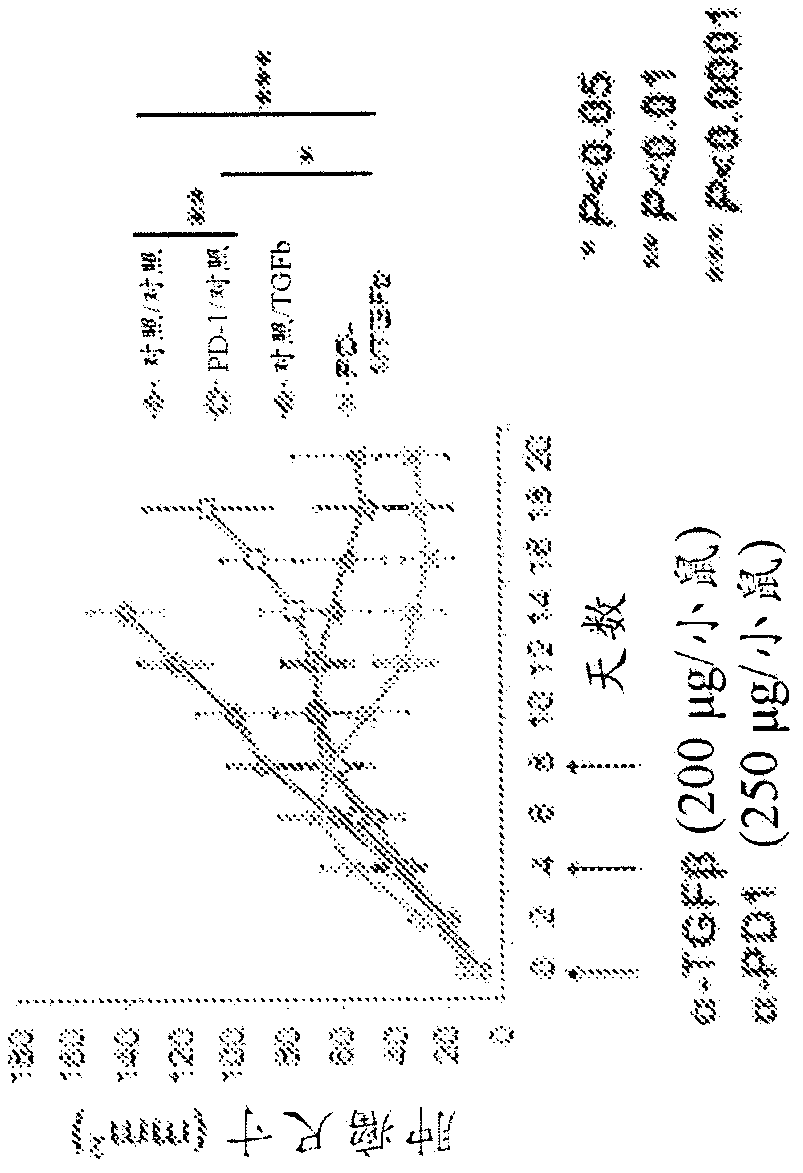
[0325] Example 1: Combination therapy of TGFβ inhibitors and PD-1 inhibitors in chemically induced cutaneous squamous cell carcinoma (cSCC).
[0326]To confirm the effect of combined TGFβ inhibitor and PD-1 inhibitor therapy, FVB mice were injected subcutaneously with the chemically induced KrasG13C-driven cSCC tumor cell line 168 (cultured ≤2 passages in vitro). When tumors reached approximately 3mm-5mm in diameter (approximately 2-3 weeks after implantation), anti-PD-1 (α-PD-1) antibody (250 μg, clone: RMP1- 14, catalog number: BE0146, BioXCell Company), pan-specific α-TGFβ1,2,3 (anti-TGFβ) antibody alone (200 μg) or combined α-PD-1 antibody and pan-specific α-TGFβ1,2, 3. Antibody treatment of mice, three treatments at 4-day intervals (mice were injected on day 0, day 4 and day 8, n=7 for each group). Tumor dimensions were subsequently measured using calipers. α-PD-1 monotherapy inhibited tumor growth compared with control mice, but tumor regression did not persist after...
Embodiment 2
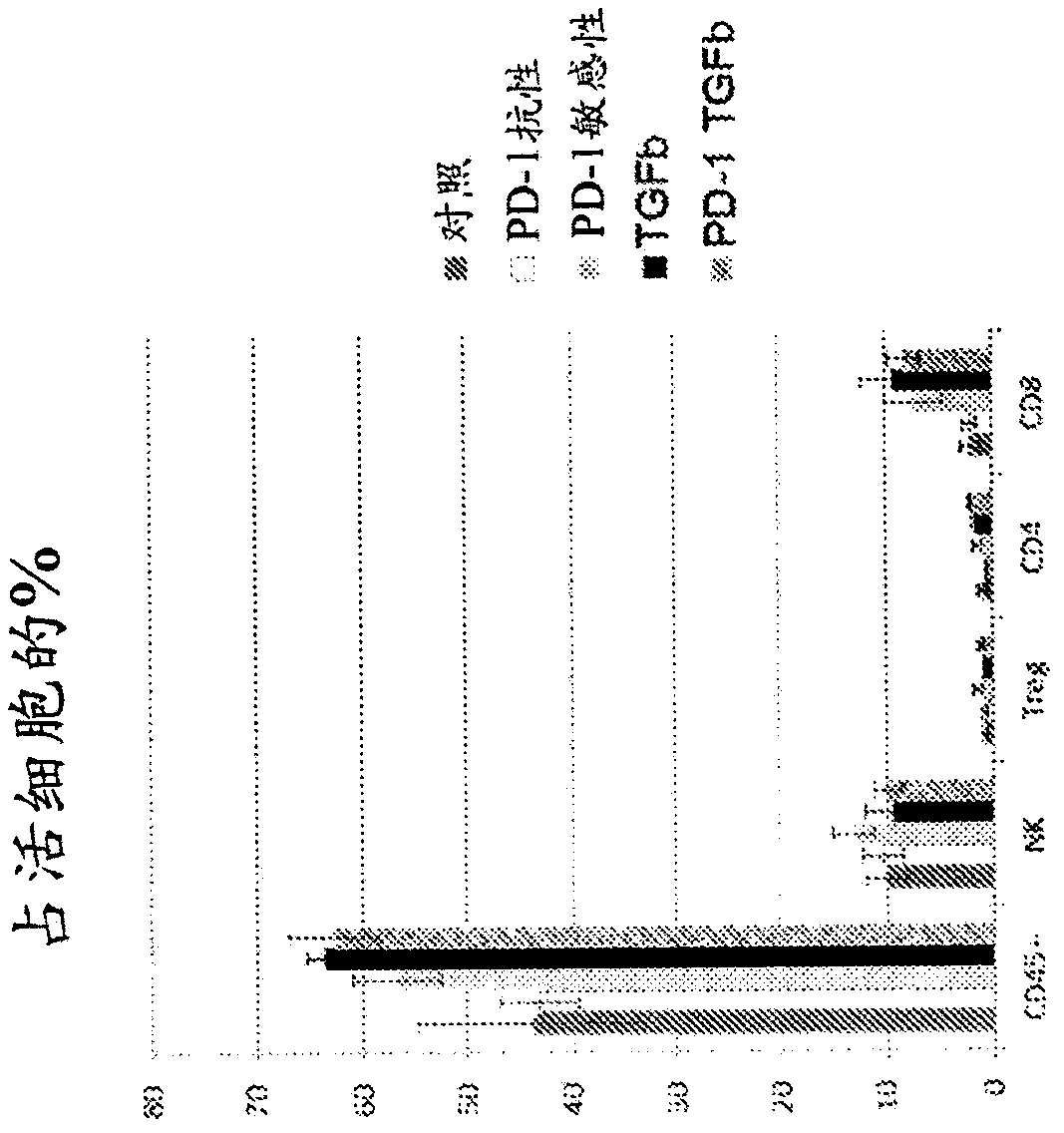
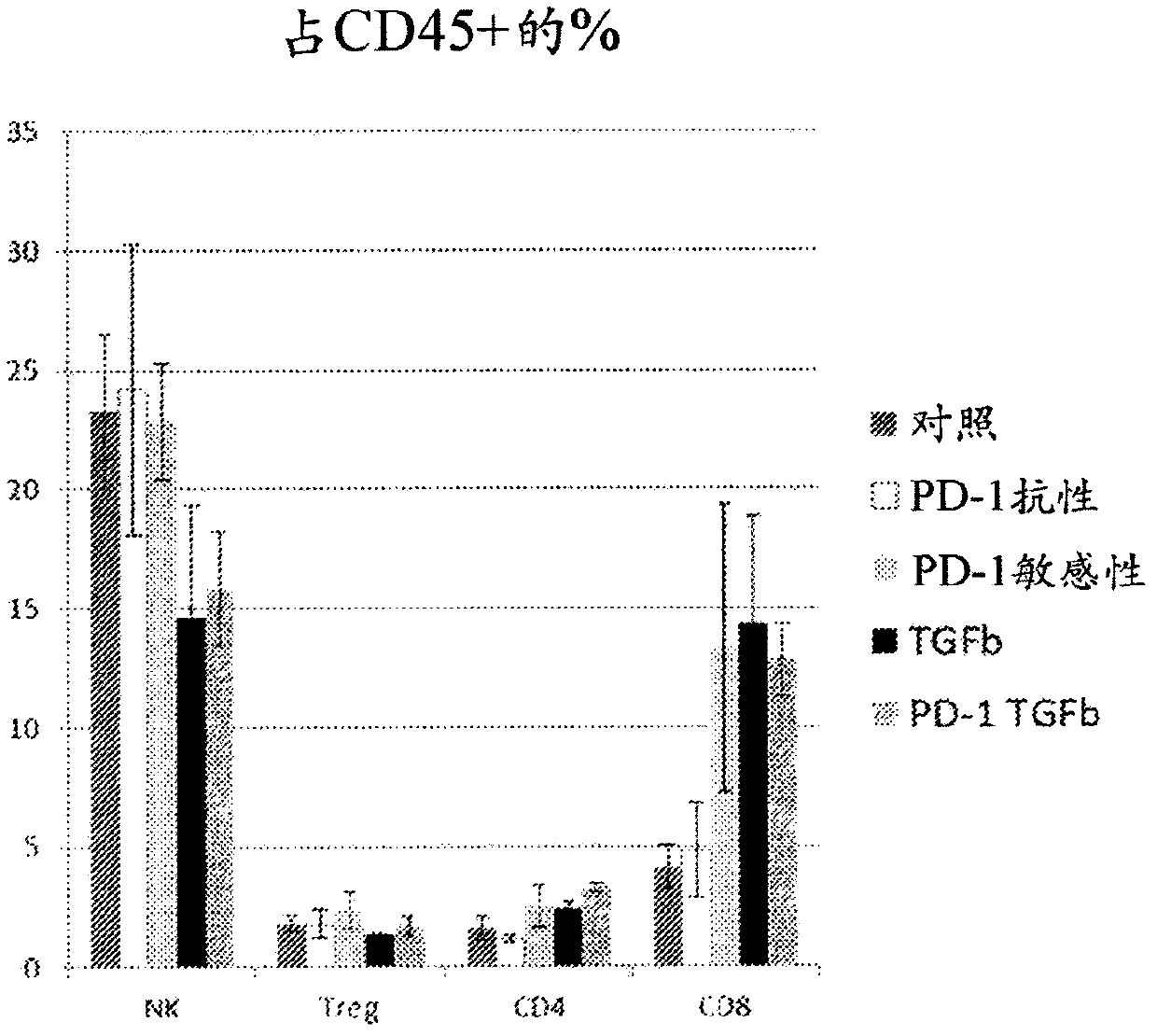
[0327] Example 2: Differential response of individual tumors to immunotherapy.
[0328] Data from Figure 1 were divided into "responders" and "progressors" to show the range of responses to α-PD-1 immunotherapy and / or α-TGFβ immunotherapy (Figure 2). Regression of implanted tumors was observed in 3 out of 7 treated mice in the case of α-TGFβ monotherapy and α-PD1 and α-TGFβ combination therapy. Furthermore, in the case of combined α-PD1 and α-TGFβ treatment, complete tumor regression was observed in approximately 50% of cases, with no further tumor growth four weeks after administration without additional drug doses (i.e. tumor size continues to decrease).
[0329] These results show that the combination of α-TGFβ antibody and α-PD-1 antibody is more effective than single antibody therapy in promoting tumor regression and, surprisingly, in preventing cancer recurrence.
Embodiment 3
[0330] Example 3: Combination treatment with α-TGFβ / α-PD-1 reduces tumor size in chemically induced (DMBA-TPA) cutaneous squamous cell carcinoma (cSCC) mice.
[0331] In addition to using an allograft model, a mouse skin model of direct chemically induced carcinogenesis was used to evaluate the antitumor effects of pan-specific α-TGFβ1,2,3α-TGFβ / α-PD-1 combination therapy. The DMBA-TPA (12-O-tetradecanoyl-phorbol-13-acetate)-induced cSCC model has been well characterized (Balmain A et al., Princess Takamatsu Symp., 22:97-108, 1991; Burns PA et al., Oncogene, 6(12):2363-9, 1991; Yuspa SH et al., Dermatol Symp Proc., 1(2):147-50, 1996; Frame S et al., Philos Trans R Soc Lond BBiol Sci.353 (1370):839-45, 1998) and is widely used to identify genetic and molecular mechanisms of cancer initiation and progression that would not be possible by studying human cancer or simpler models such as tumor allografts . This model is initiated by the DMBA mutation of Hras codon 61 in >90% of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com