Application of 2-acyl-1-dimethylaminomethylferrocene derivative in preparation of drugs for targeted treatment of hepatocellular carcinoma
A technology of dimethylaminomethyl ferrocene and hepatocellular carcinoma, which is applied in the field of medicinal chemistry, can solve the problems of toxic side effects, poor targeting and selectivity of cancer cells, poor therapeutic effect of hepatocellular carcinoma, etc., and achieve growth inhibition, The effect of good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This example refers to Synthesis of Ferrocene Derivatives with Planar Chirality via Palladium-Catalyzed Enantioselective C-H Bond Activation, Org. Lett.16 (2014) 5164-5167 to synthesize 2-acyl-1-dimethylaminomethyl ferrocene derivatives with the following structural formula :
[0024]
[0025] The preparation of 2-acyl-1-dimethylaminomethylferrocene derivatives and Sorafenib in this example: first dissolve the compound with a small amount of DMSO, and then use DMEM culture solution to prepare the original solution with a concentration of 20mM. solution. Then prepare different final concentrations according to the needs of the experiment, and store them in a -20°C refrigerator in the dark for future use.
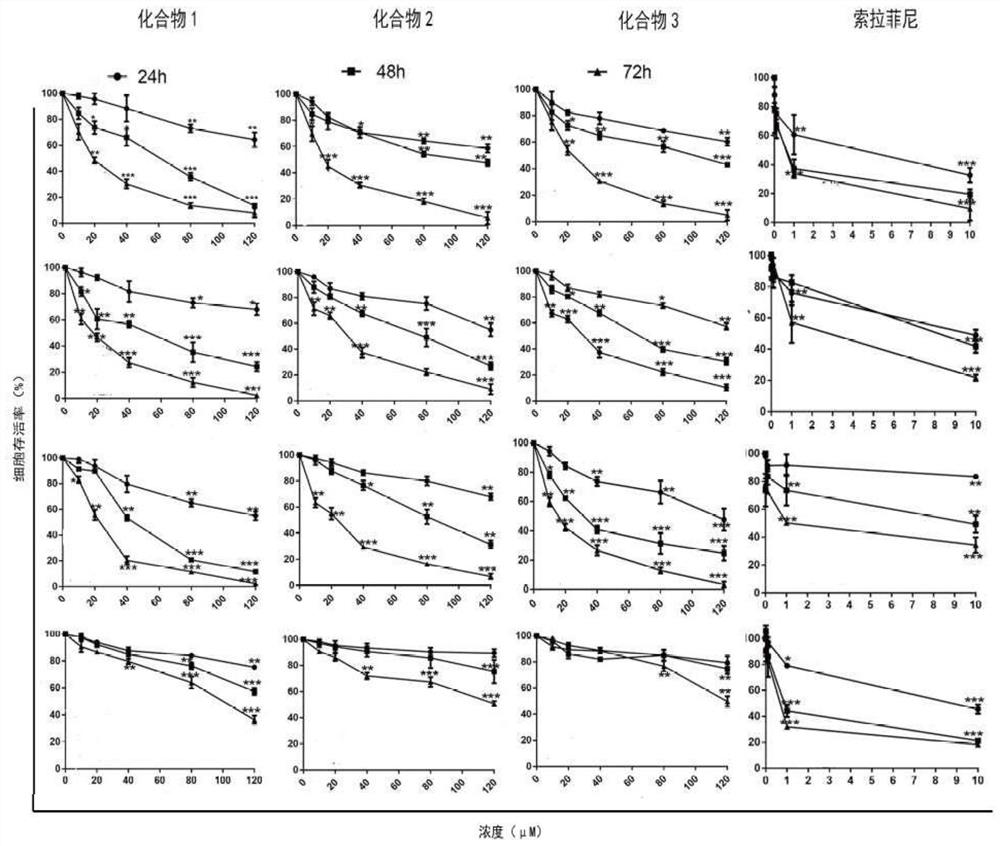
[0026] In vitro anti-hepatoma cell activity detection of the above-mentioned 2-acyl-1-dimethylaminomethylferrocene derivatives: Human hepatoblastoma cells (HepG2, SMMC-7721 and HuH-7) were cultured in DMEM medium , adding 10% FBS. Human normal hepatocyte L02 was ...
Embodiment 2
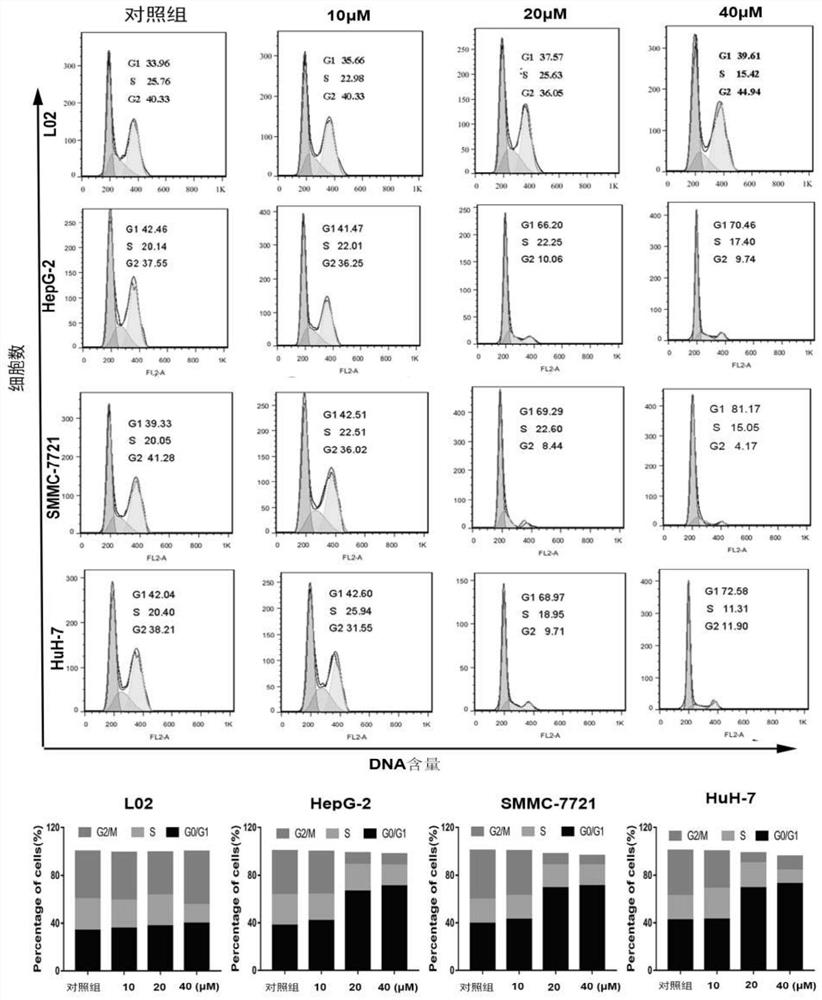
[0032] Select the compound 1 with the best activity and the best selectivity from Example 1, and use flow cytometry to detect its cell cycle arrest: take HepG-2, SMMC-7721 and HuH-7 and L02 in the logarithmic growth phase Cells were routinely digested to make a single-cell suspension, counted, and the cell concentration was adjusted to 10,000 cells / mL, inoculated in a 6-well cell culture plate at 2 mL per well, and placed at 37°C, 5% CO 2 The cells were cultured in a concentration-saturated humidity incubator. After 12 hours, the original culture solution was discarded, and the cells were divided into a control group and a compound group (compound 1). For the control group, add 2 ml of DMEM medium containing 10% FBS in each well of the cell culture plate; for the compound group, add 2 ml of the DMEM medium of 10% FBS in each well of the cell culture plate, and then add the mother solution (compound 1) at a concentration of : 1 μL, 2 μL and 4 μL, and mixed thoroughly so that th...
Embodiment 3
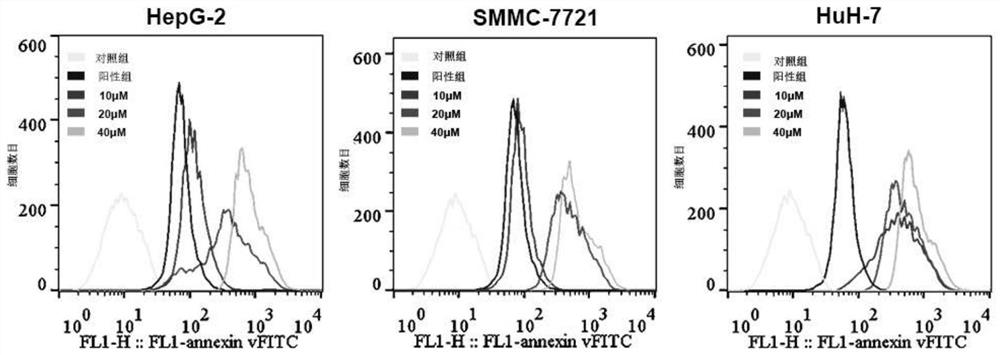
[0034] Select compound 1 with the best activity and the best selectivity from Example 1 to treat the cells, and use flow cytometry to detect intracellular reactive oxygen species: take the logarithmic growth phase of liver cancer cells (HepG-2, SMMC-7721 and HuH- 7), routinely digested to make a single cell suspension, counted, adjusted the cell concentration to 10,000 cells / mL, inoculated 2 mL per well in a 6-well cell culture plate, and placed it at 37 ° C, 5% CO 2 The cells were cultured in a concentration-saturated humidity incubator. After 24 hours, the original culture solution was discarded, and the cells were divided into a control group and a compound group (compound 1). For the control group, 2ml of 10% serum medium was added to each well of the cell culture plate; for the compound group, 2ml of 10% serum medium was added to each well of the cell culture plate, and then the mother solution (compound 1) was added at a concentration of 1 μL, 2 μL and 4 μL, and mixed th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com