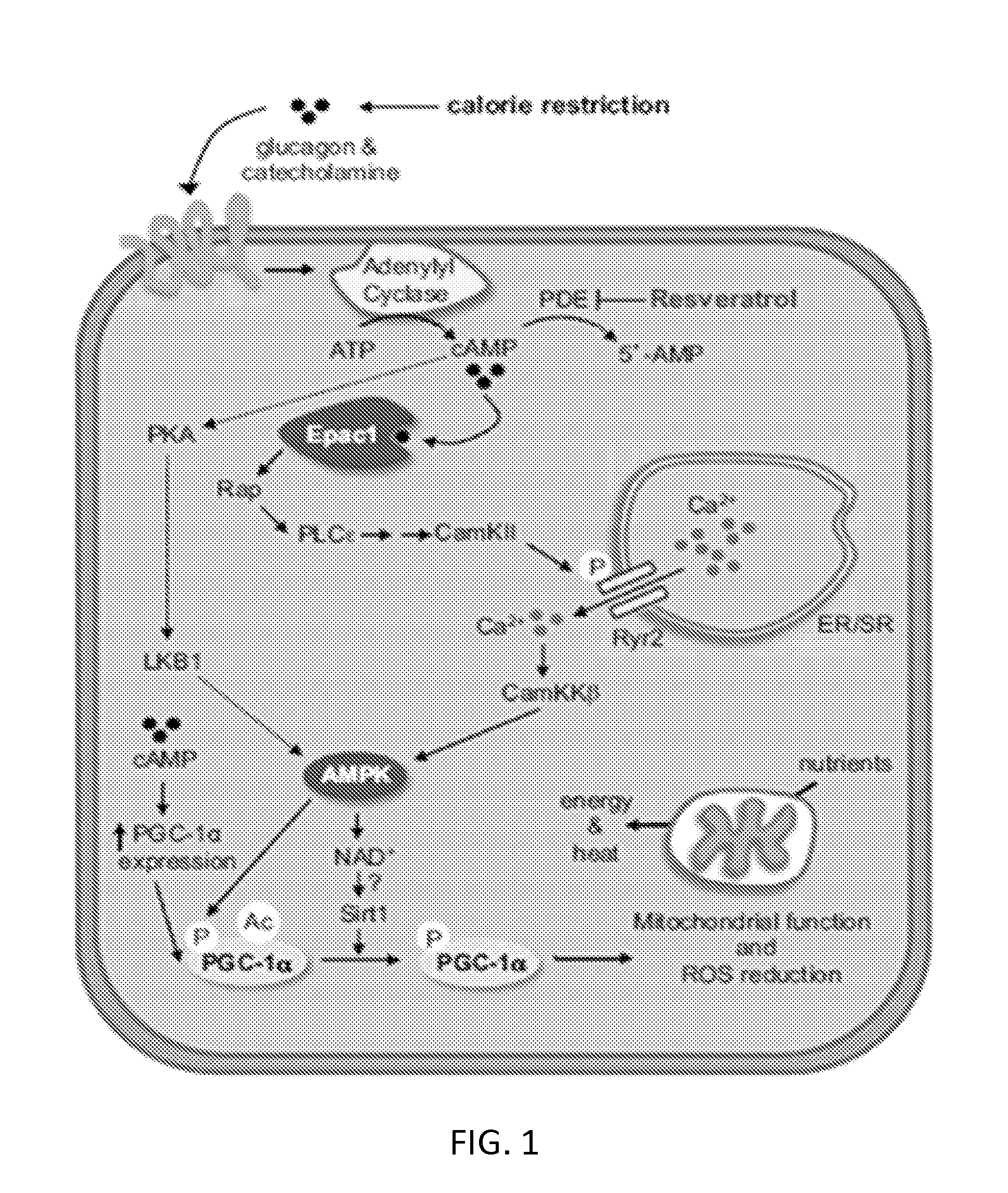
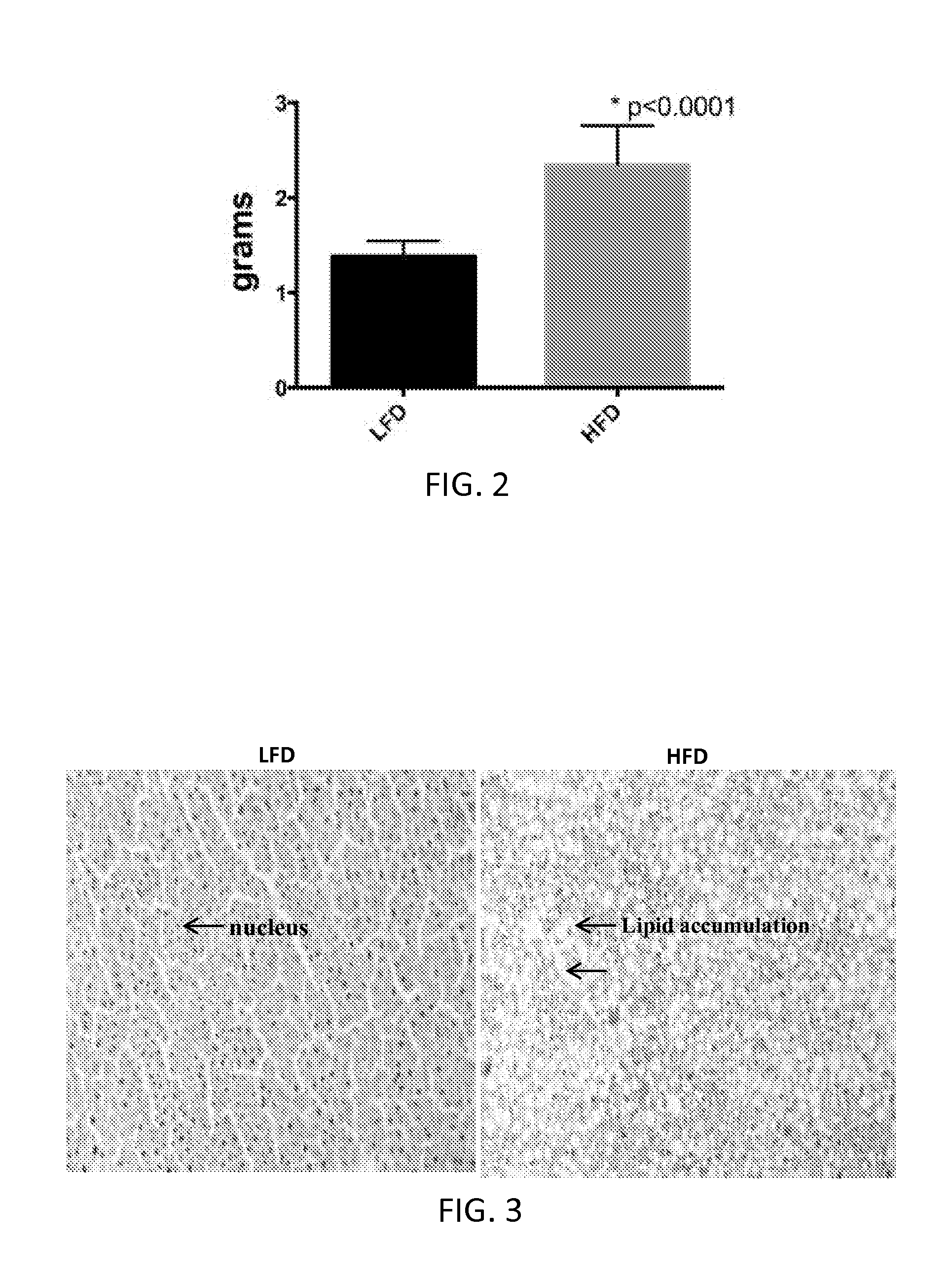
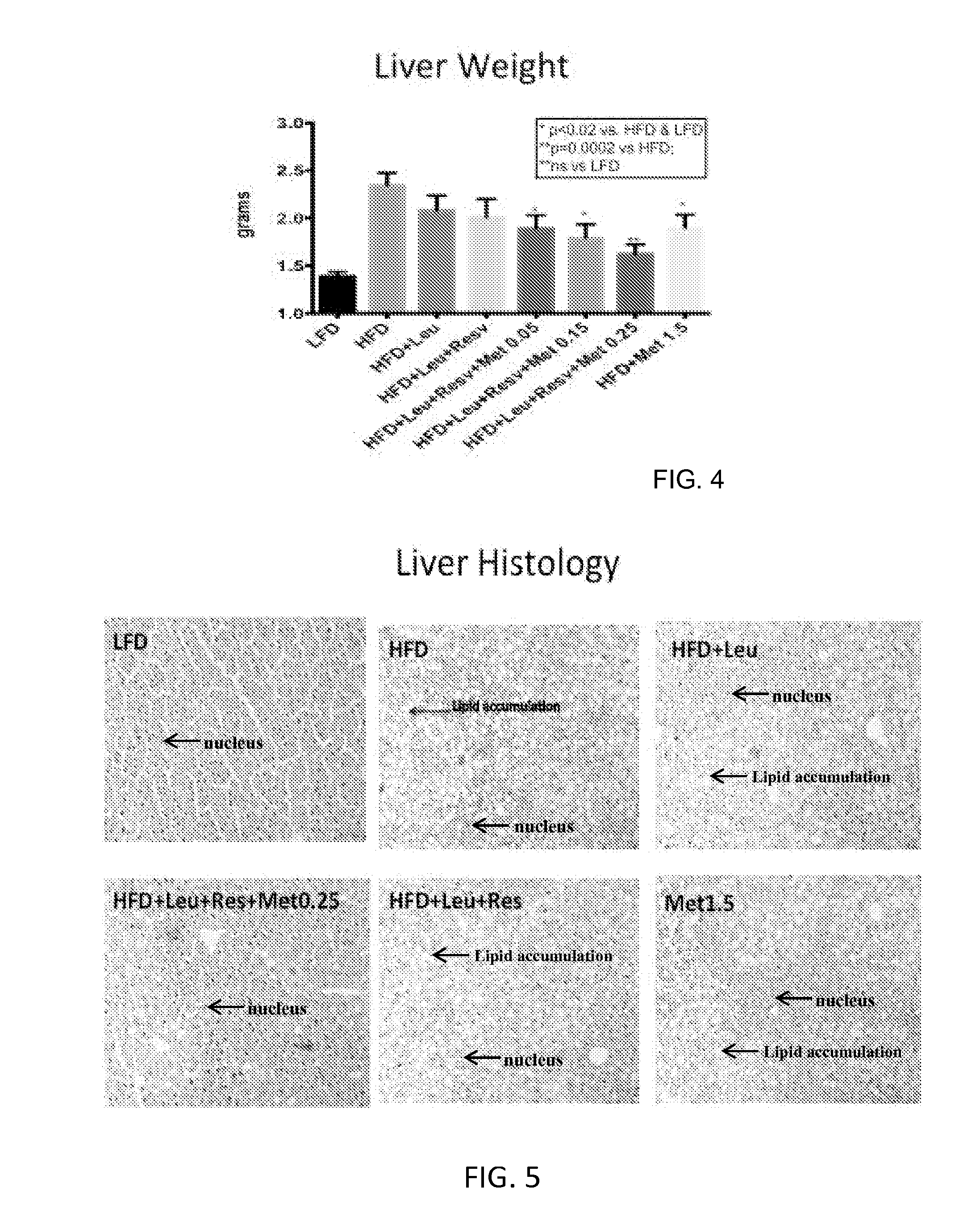
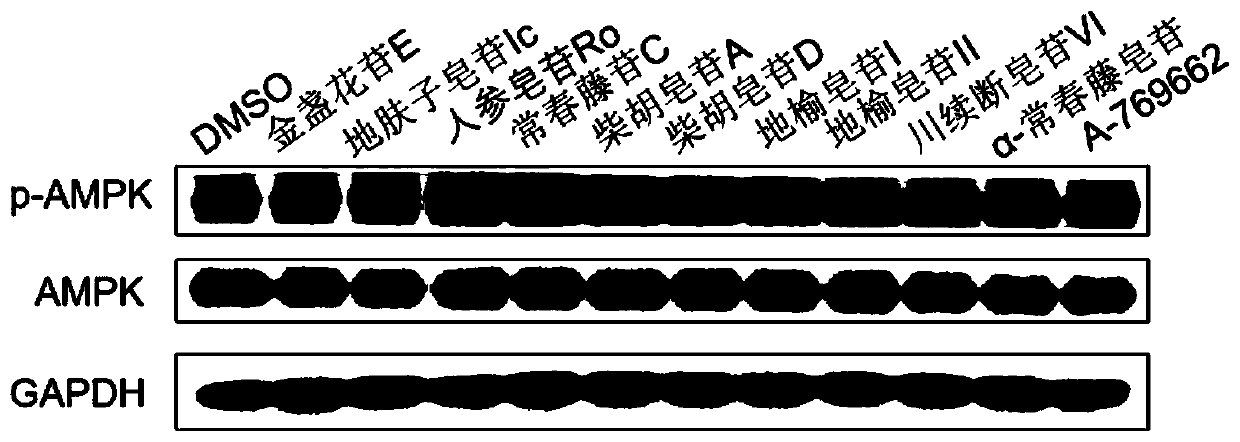
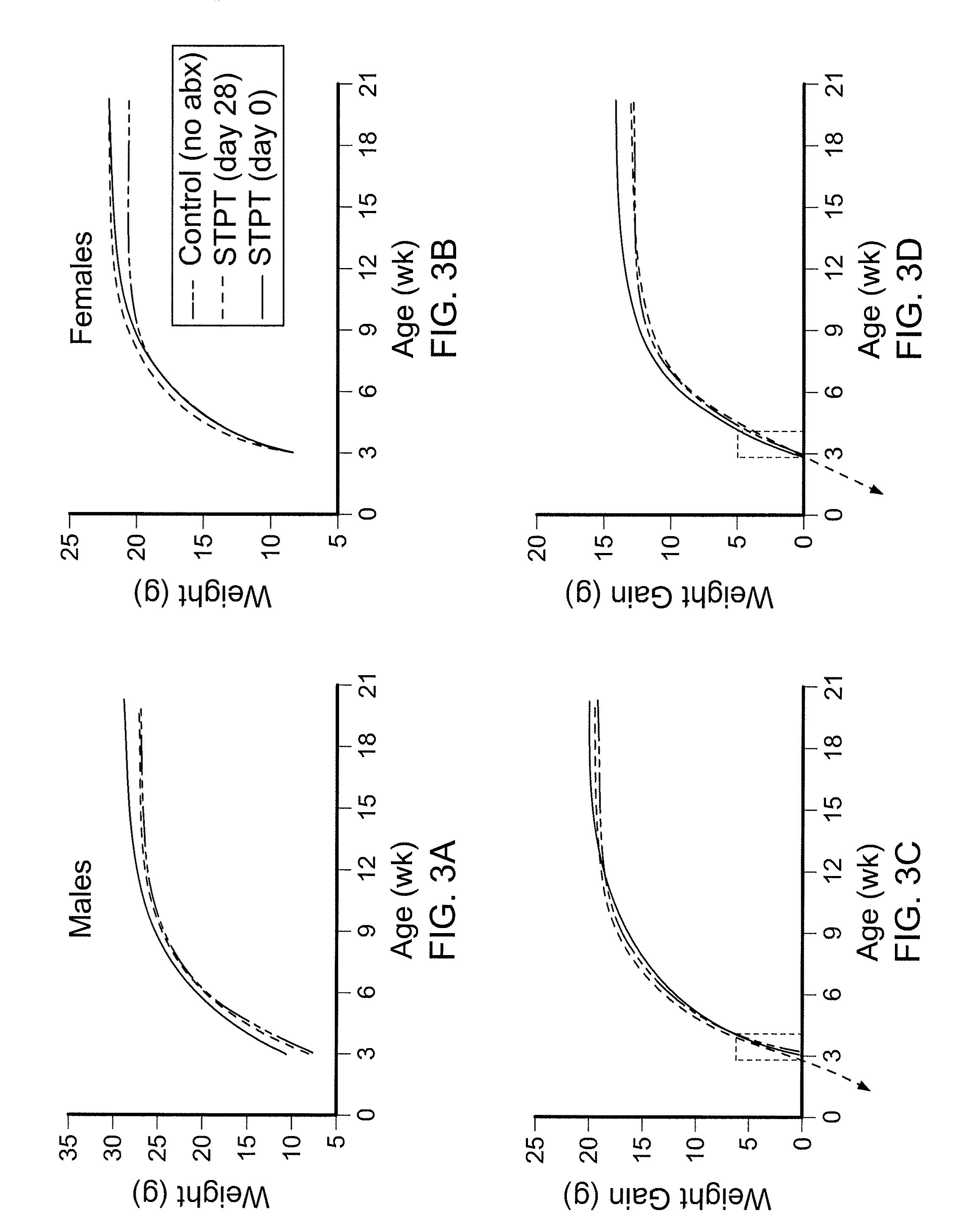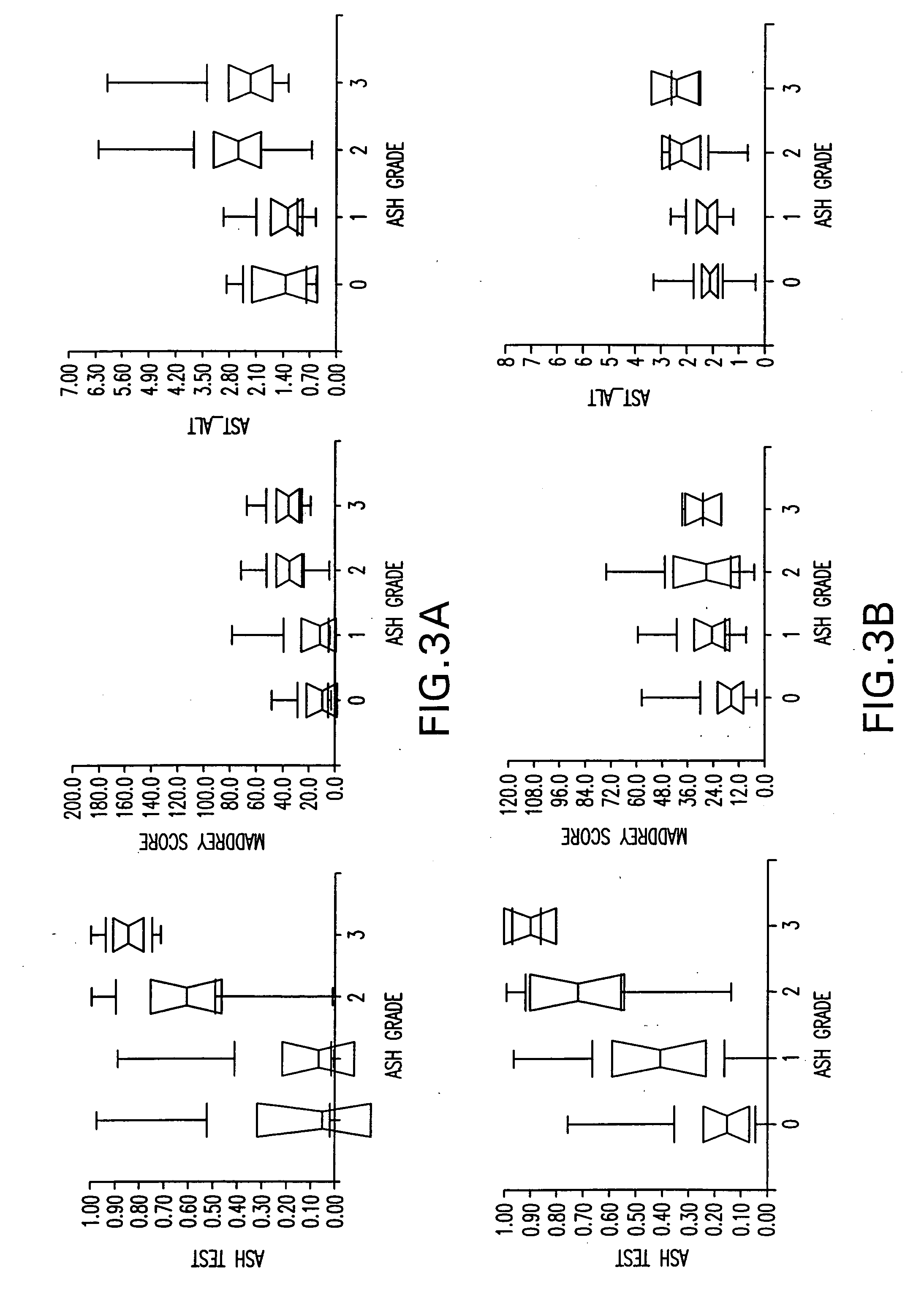Patents
Literature
538 results about "Non alcoholic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-alcoholic drink. An alcohol-free or non-alcoholic drink is a version of an alcoholic drink made without alcohol, or with the alcohol removed or reduced to almost zero.
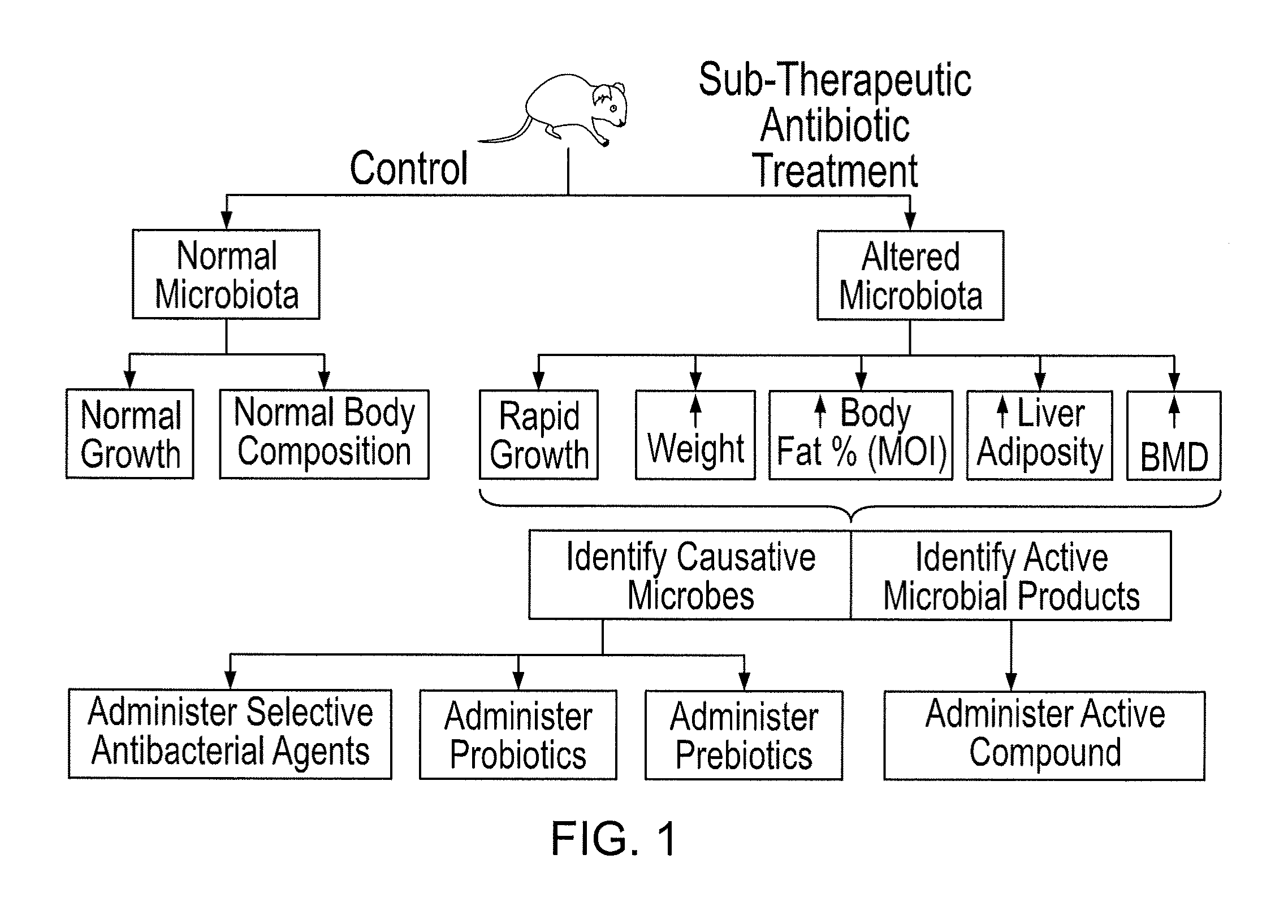
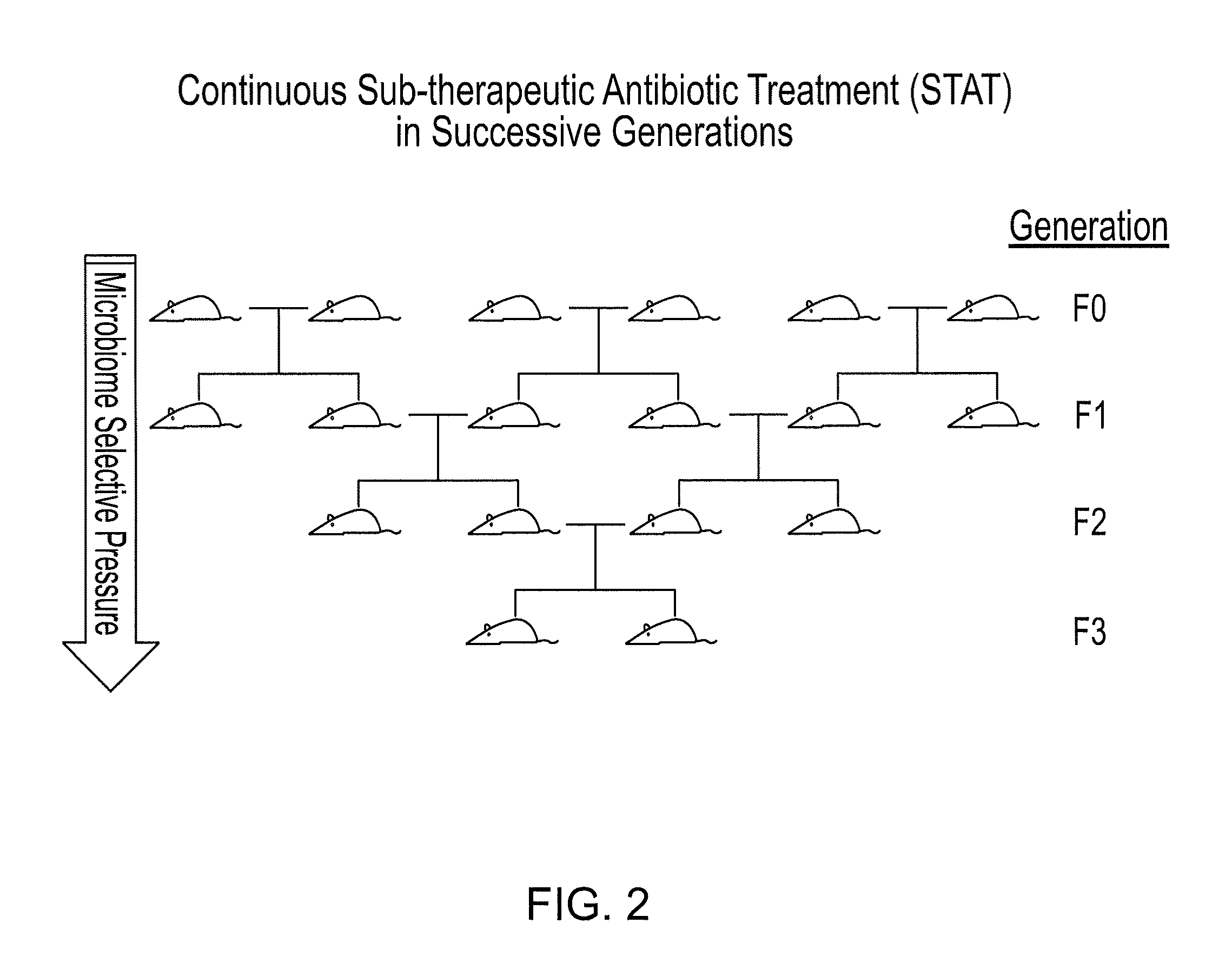
Compositions and methods for treating obesity and related disorders by characterizing and restoring mammalian bacterial microbiota
ActiveUS20110280840A1Increased use of antibioticIncreasing adult height and muscle massBiocideMetabolism disorderIntestinal microorganismsBone formation
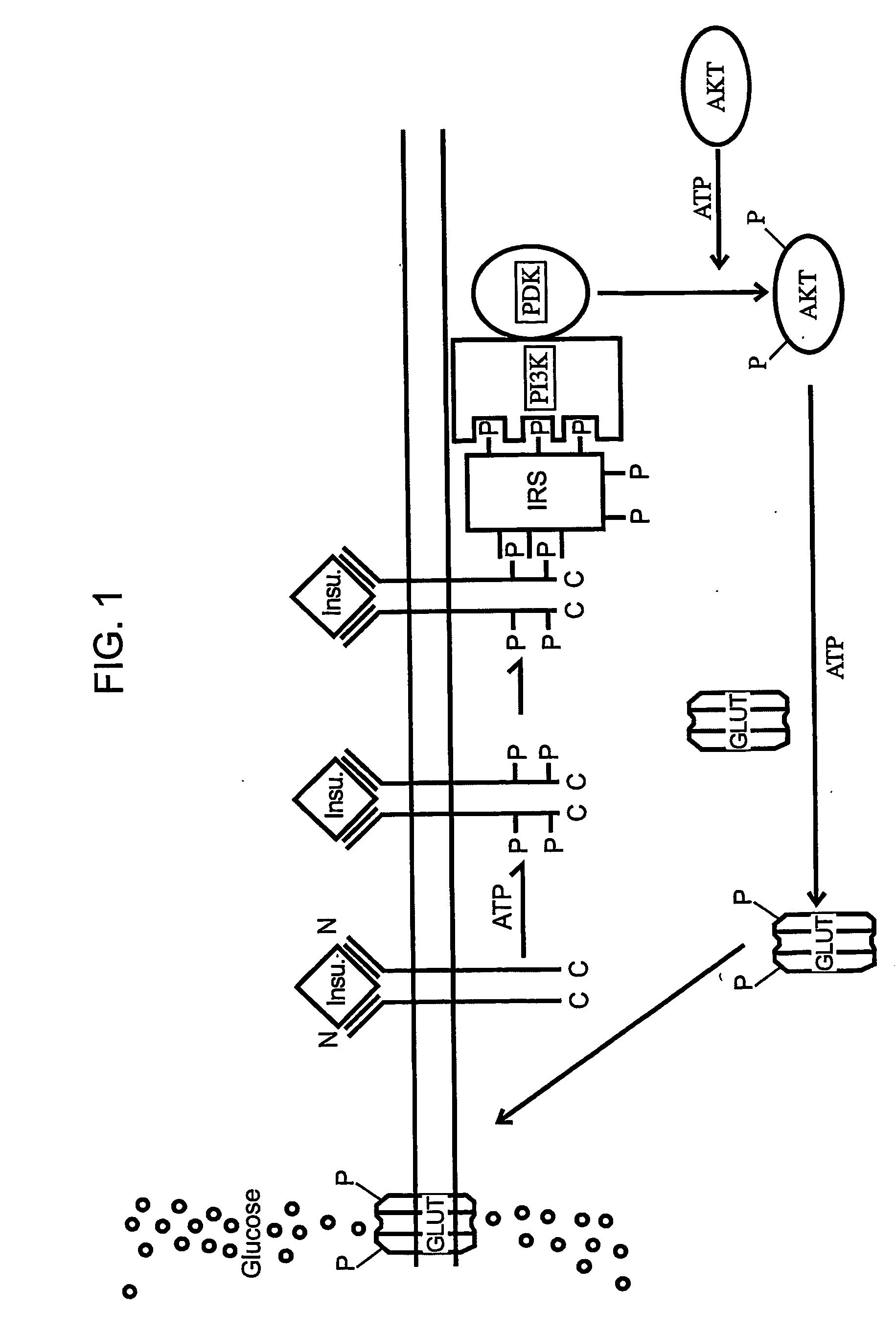
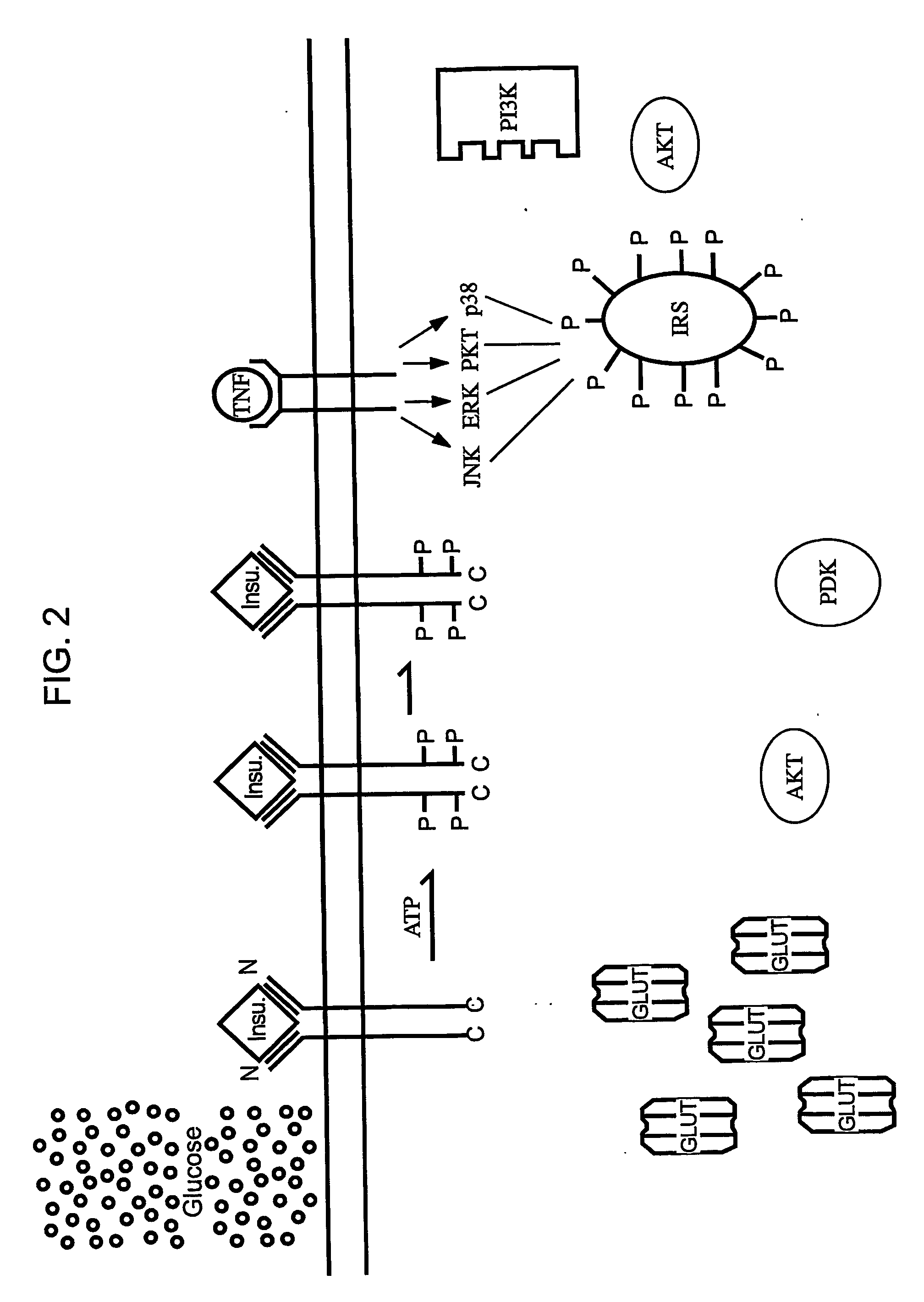
The present invention relates to characterizing changes in mammalian gastrointestinal microbiota associated with antibiotic treatment and various disease conditions (such as obesity, metabolic syndrome, insulin-deficiency or insulin-resistance related disorders, glucose intolerance, diabetes, non-alcoholic fatty liver, abnormal lipid metabolism, short stature, osteoporosis, and other disorders of bone formation and mineralization, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of probiotics, prebiotics, or narrow spectrum antibiotics / anti-bacterial agents that are capable of restoring healthy mammalian bacterial gastrointestinal microbiota.
Owner:NEW YORK UNIV
Prophylactic/ameliorating or therapeutic agent for non-alcoholic steatohepatitis
InactiveUS20110082119A1Slow downGood treatment effectBiocideAntipyreticNonsteroidal Antiinflammatory Drugs/NSAIDsBULK ACTIVE INGREDIENT
A highly safe and effective prophylactic / ameliorating or therapeutic agent for NACH and the method for using the same are provided.A prophylactic / ameliorating or therapeutic agent for NASH containing a combination of at least one first ingredient selected from the group consisting of an ω3PUFA and pharmaceutically acceptable salts and esters thereof and at least one second ingredient selected from the group consisting of (a) a biguanide hypoglycemic agent, (b) a nonsteroidal anti-inflammatory drug, (c) a 3-hydroxy-3-methyl glutaryl coenzyme A reductase inhibitor, and (d) an angiotensin II receptor blocker as the active ingredients; and its method of use.
Owner:MOCHIDA PHARM CO LTD
Prophylactic/ameliorating or therapeutic agent for non-alcoholic steatohepatitis
InactiveUS20110105510A1Safe effectiveStrong specificityBiocideSenses disorderPDE4 InhibitorsBULK ACTIVE INGREDIENT
Owner:MOCHIDA PHARM CO LTD
Compositions and methods for treating non-alcoholic steatohepatitis
ActiveUS20150051143A1Improve steatosisImproving lobular inflammation conditionBiocidePeptide/protein ingredientsBiliary tractFatty acid
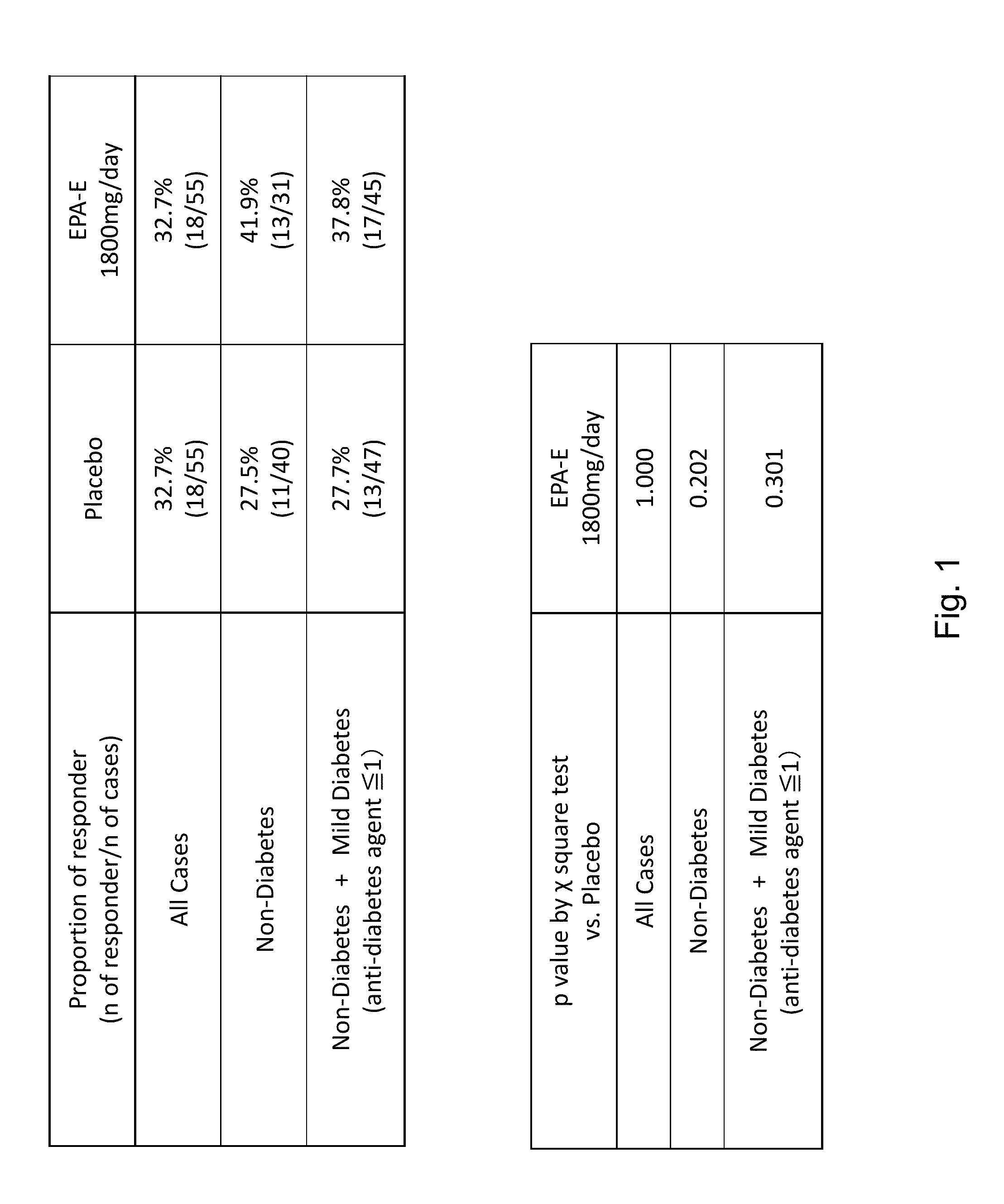
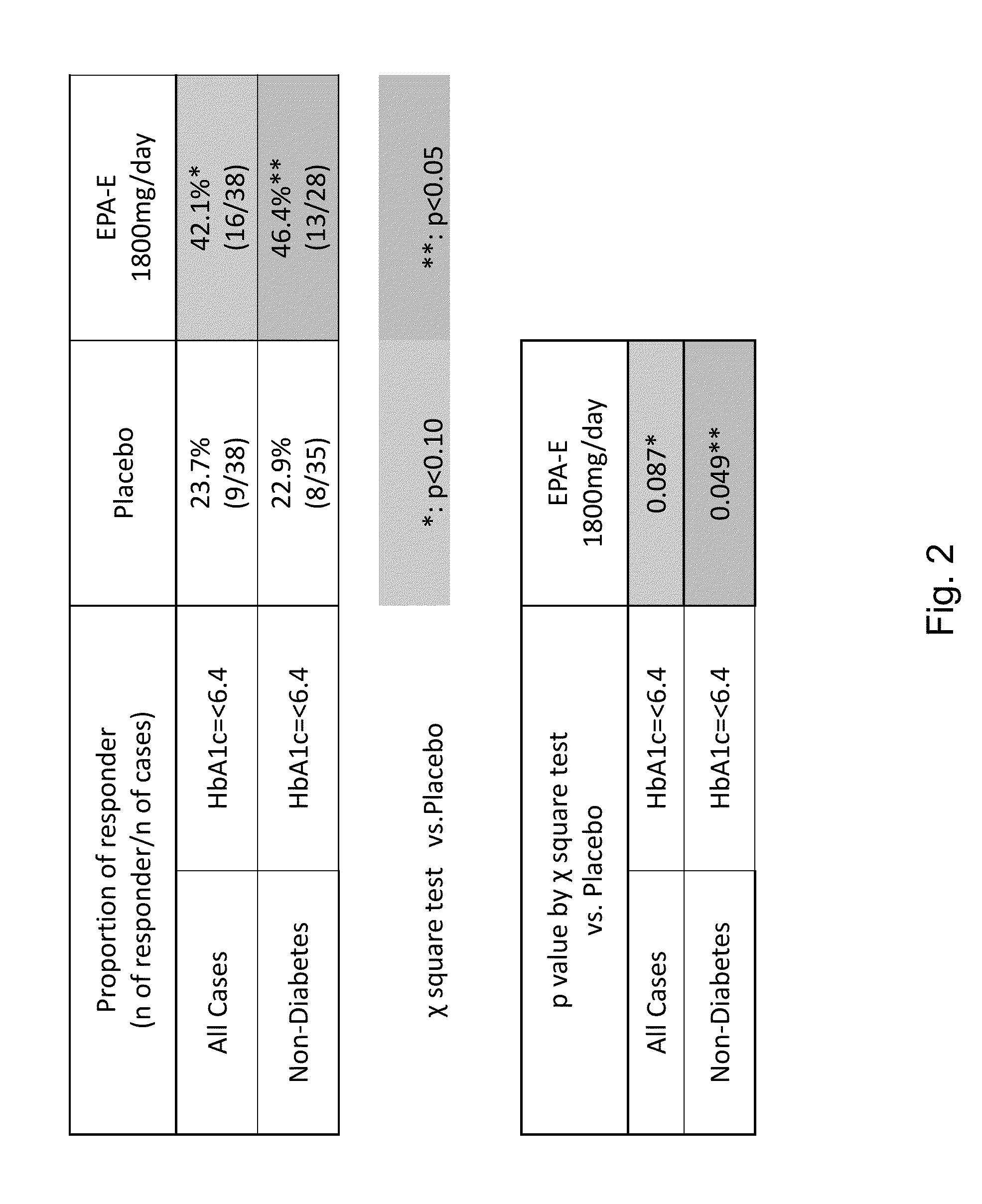
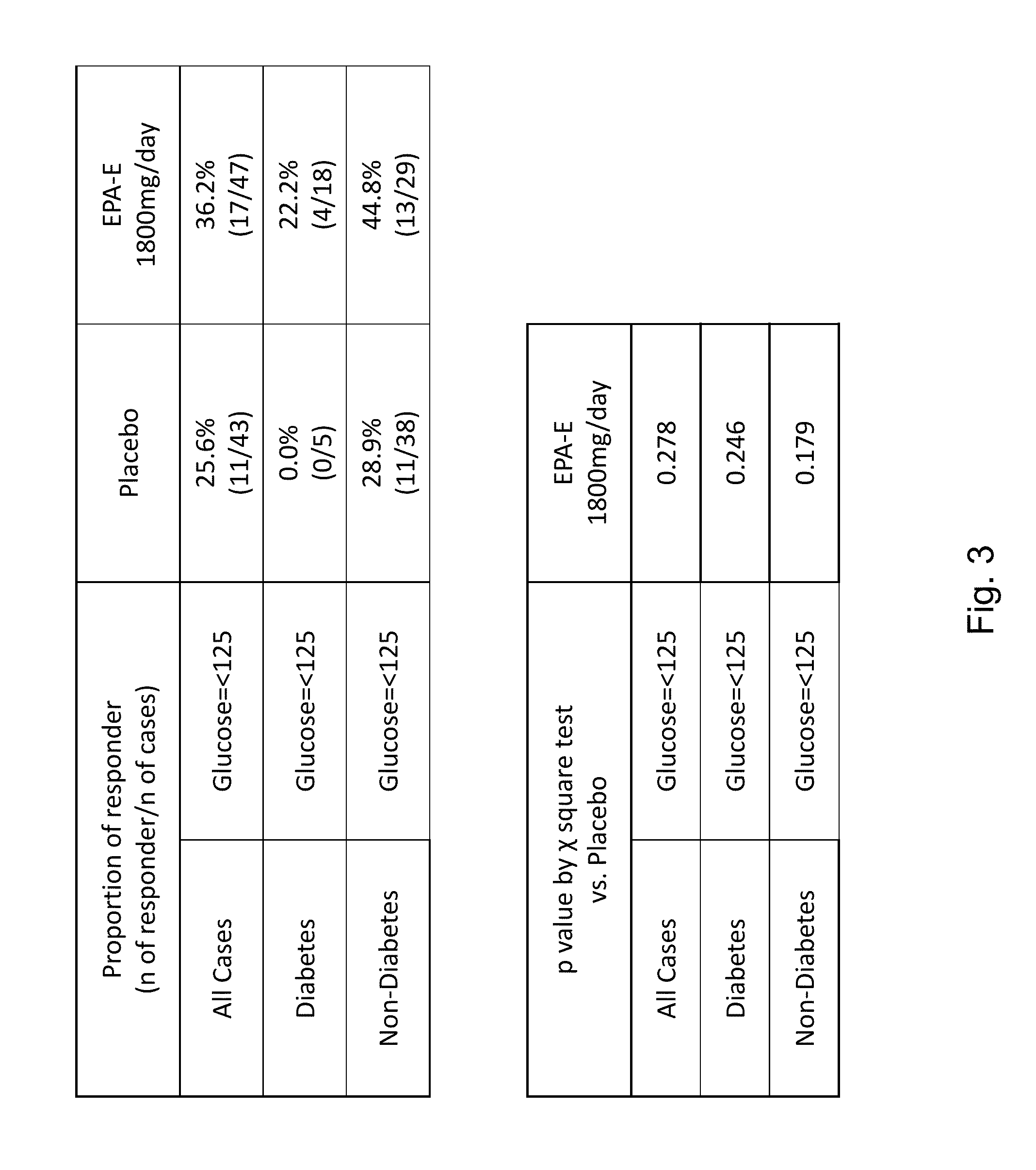
The disclosure provides for a method for treating a fatty liver disorder in a subject in need thereof, comprising selecting a subject having or suspected of having a fatty liver disease or disorder, wherein the subject is non diabetic, pre-diabetic, mildly diabetic, or has normal or substantially normal biliary tract function; and administering a therapeutically effective amount of a pharmaceutical composition comprising ethyl eicosapentanoate (EPA-E). In some cases EPA-E present may be at least 40% by weight in total of the fatty acids and their derivatives.
Owner:AMARIN PHARMA IRELAND
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS20050123628A1Reduce oxidative stressReduce lipid peroxidationBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
Marker associated with non-alcoholic steatohepatitis
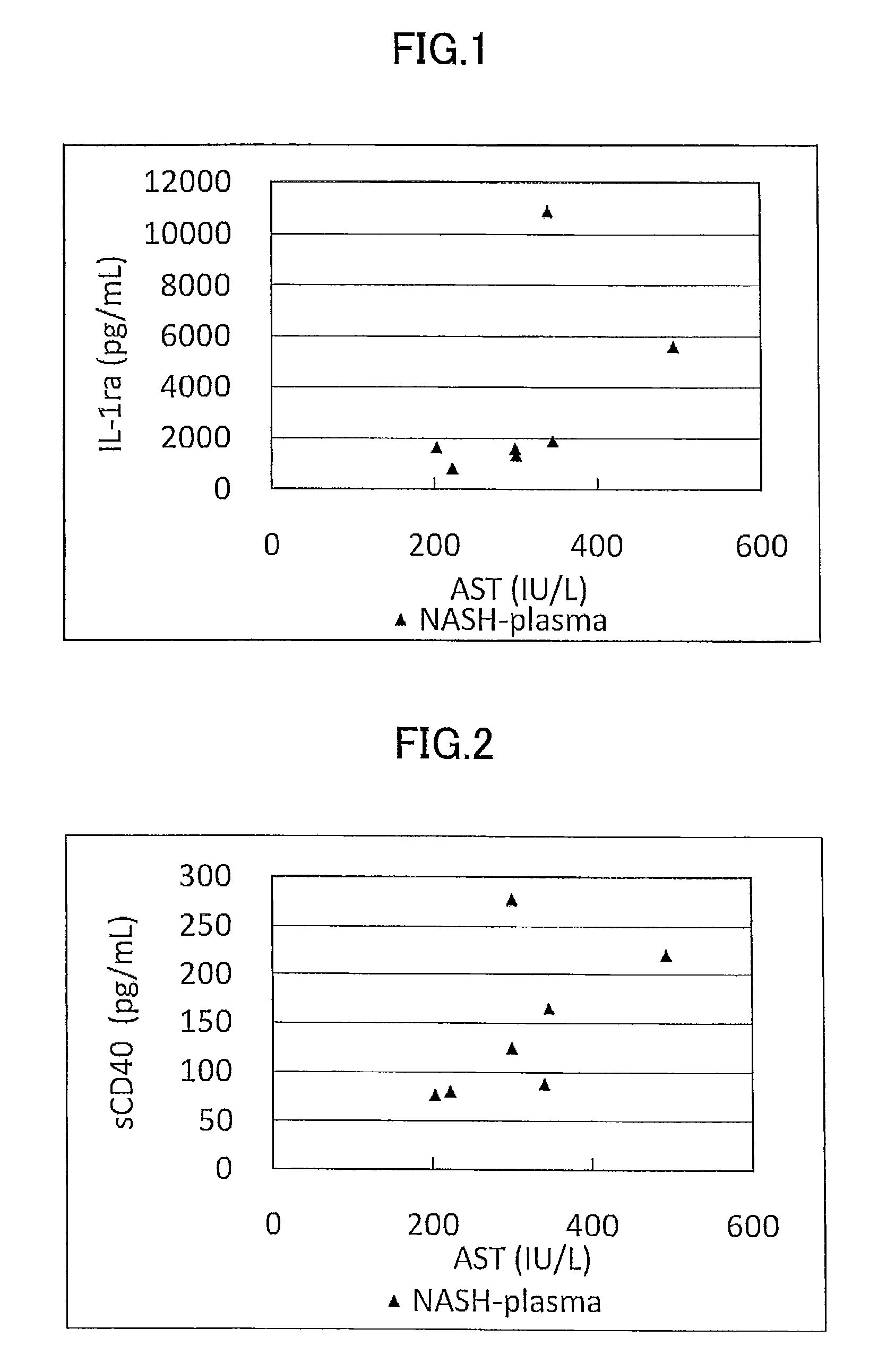
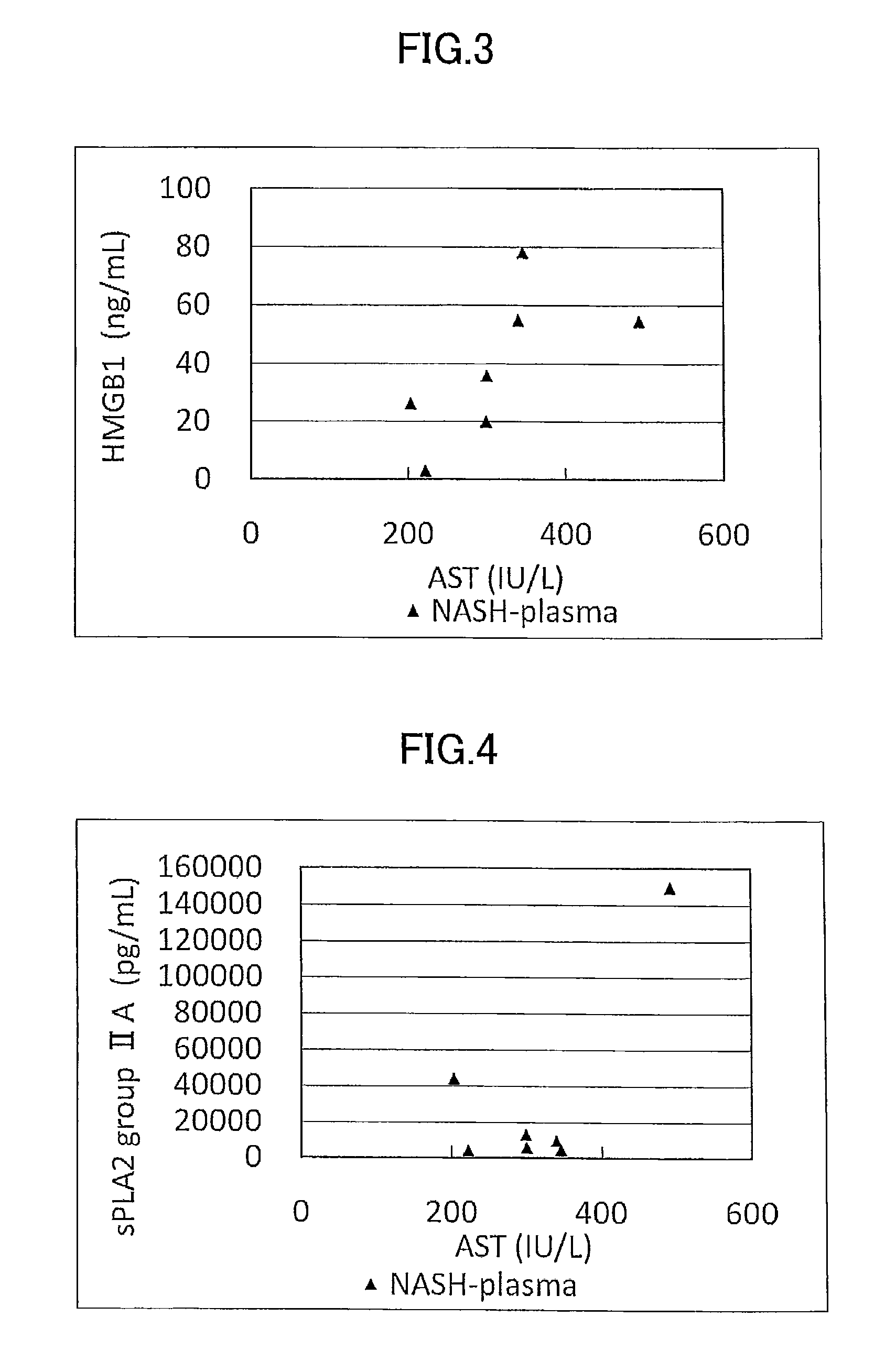
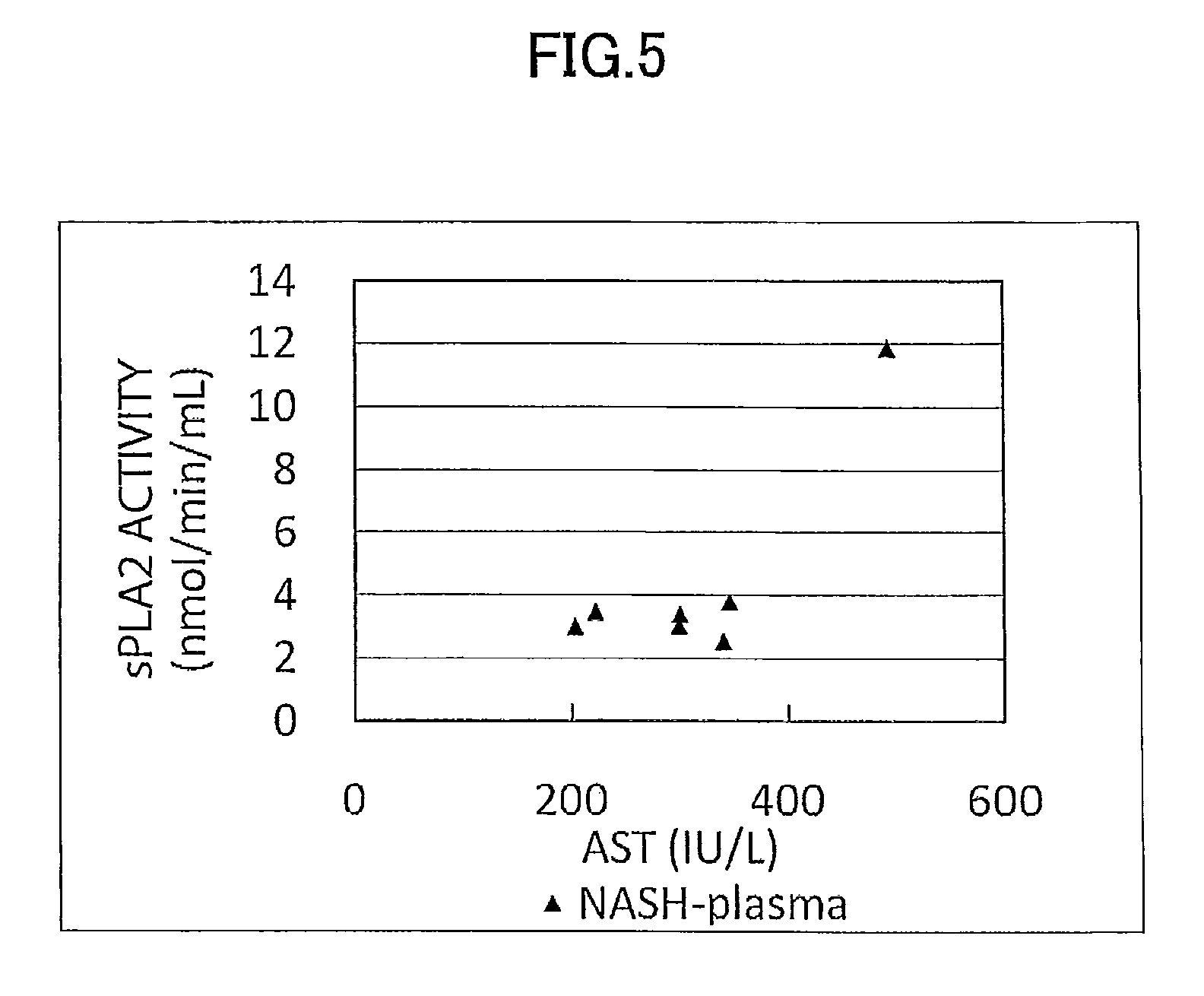
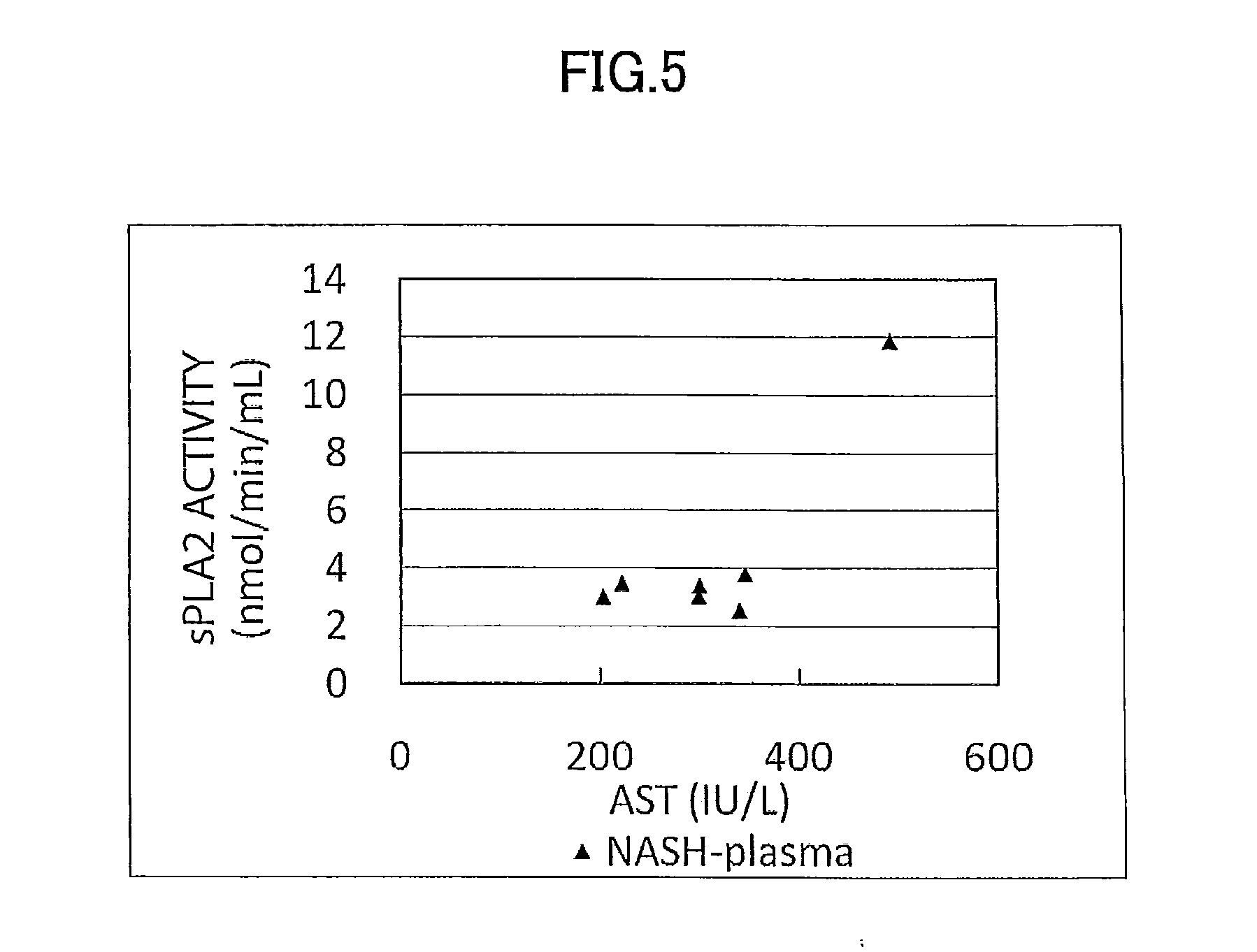
ActiveUS9060981B2Easy to detectEase of evaluationOrganic active ingredientsMicrobiological testing/measurementPediatricsNon alcoholic
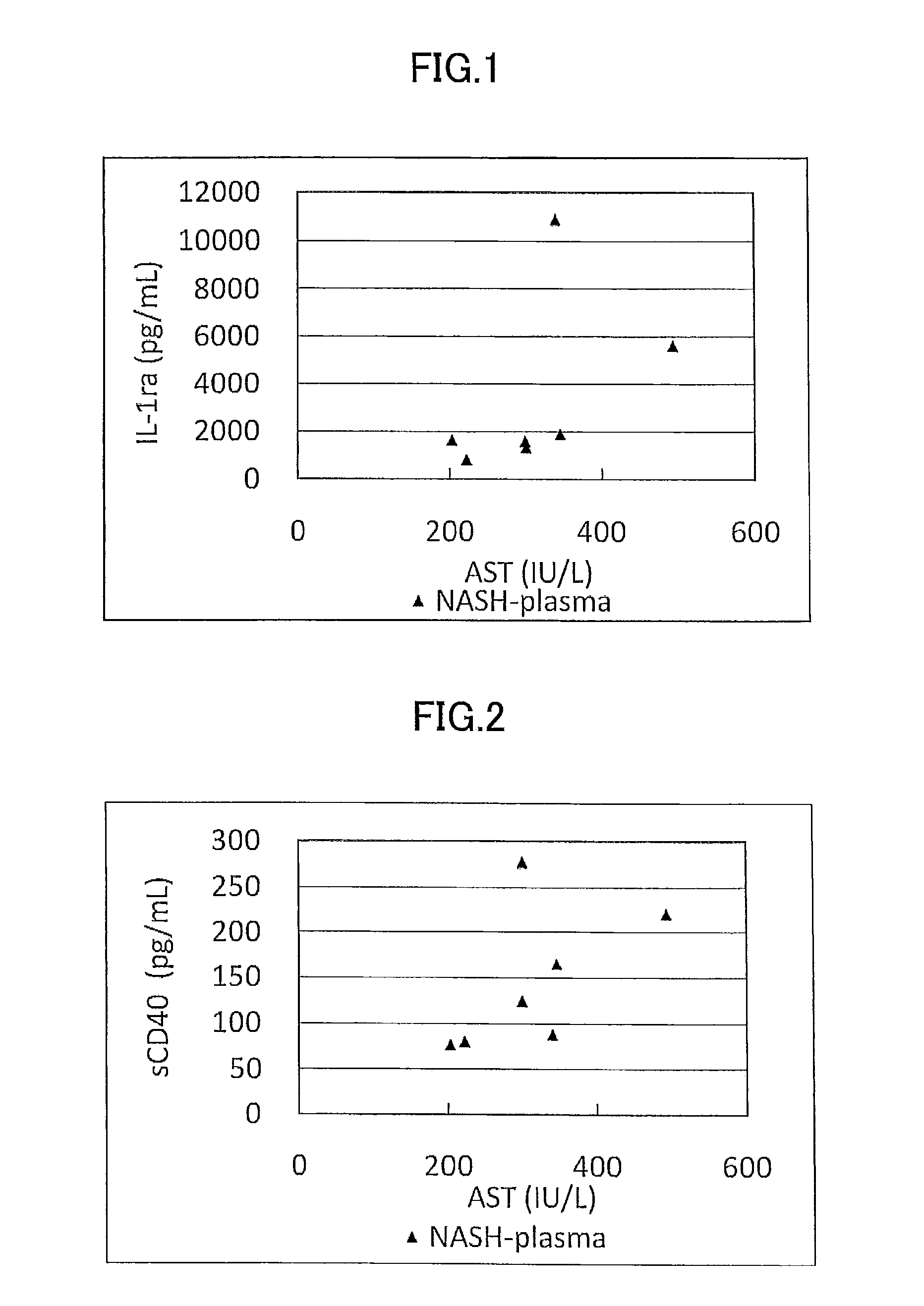
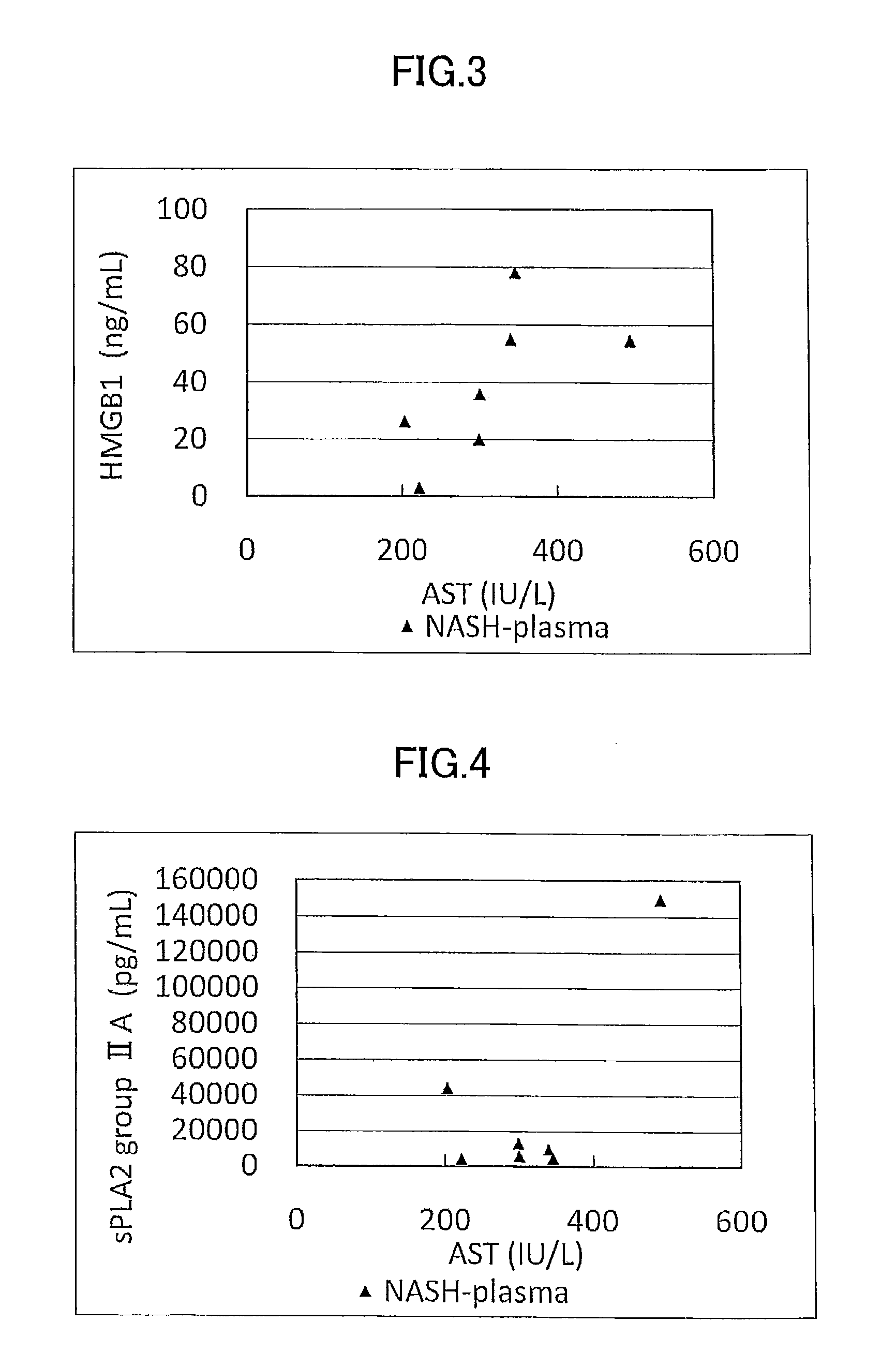
Disclosed is a novel NASH marker for use in a method for detecting NASH or evaluating the severity of NASH, which utilizes at least one factor selected from the group consisting of an IL-1 receptor antagonist, sCD40, HMGB1, sPLA2 group IIA and an sPLA2 activity as the marker. Also disclosed is a method for detecting NASH or evaluating the severity of NASH in a subject, which utilizes the marker.
Owner:AMARIN PHARMA IRELAND
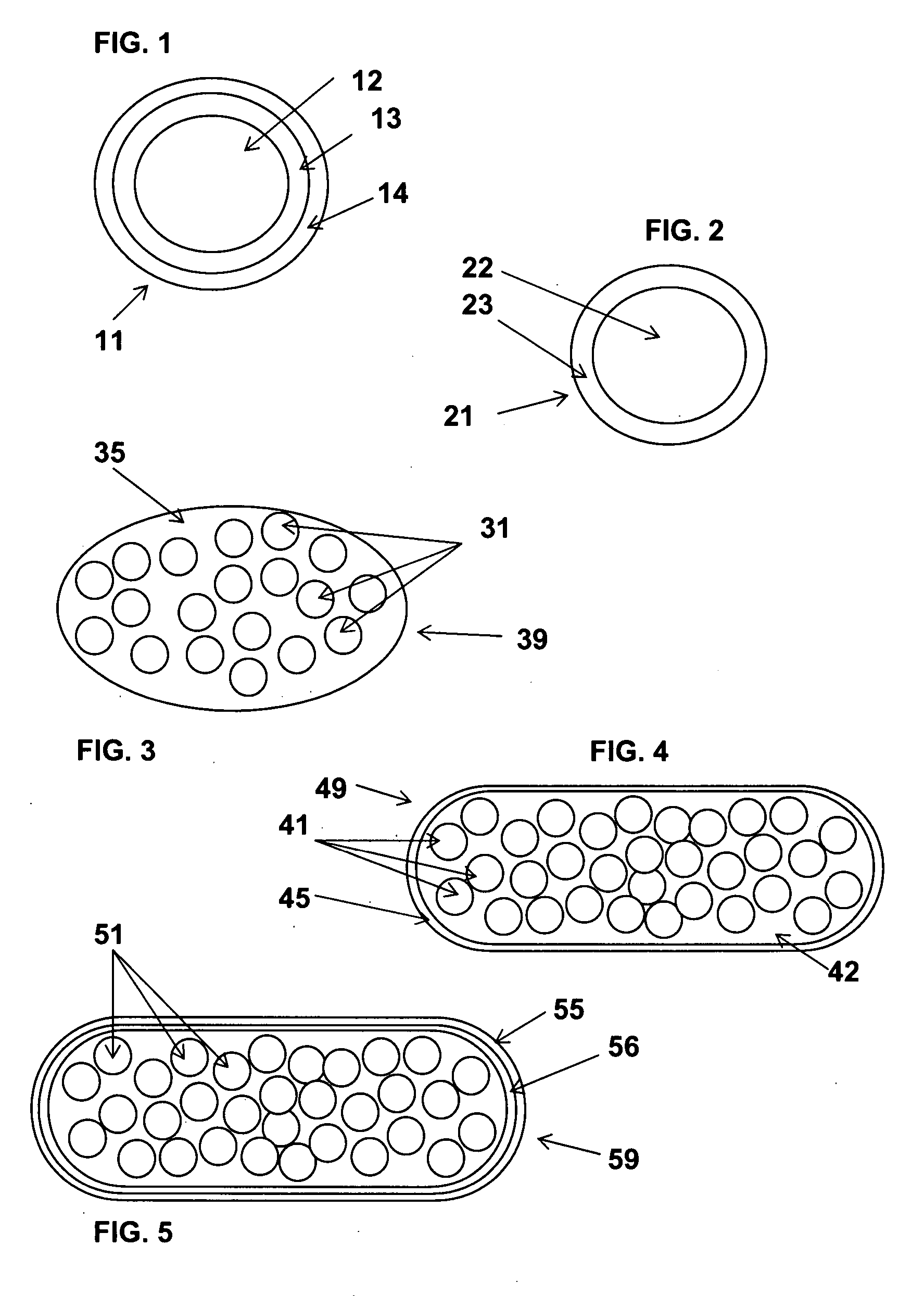
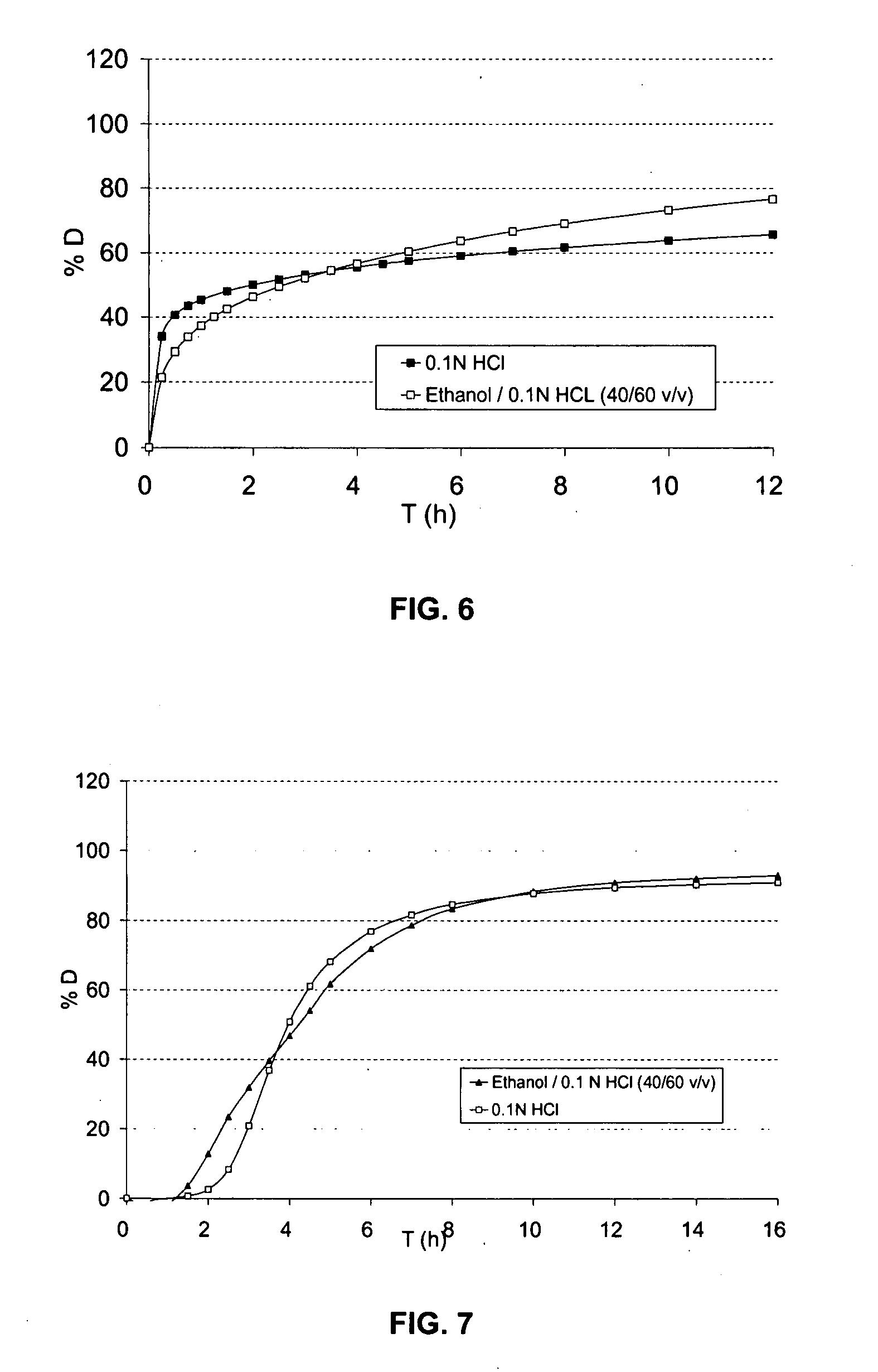
Multimicroparticulate pharmaceutical forms for oral administration
InactiveUS20070264346A1Great therapeutic safetyGood effectOrganic active ingredientsPowder deliveryAlcohol freeMicroparticle
The object of the present invention is to minimize the risks of dose dumping associated with the concomitant consumption of alcohol and certain modified-release pharmaceutical or dietetic forms. The invention relates to an oral form comprising microparticles of the reservoir type for the modified release of at least one active principle (AP), characterized in that it is resistant to immediate dumping of the dose of AP in the presence of alcohol. In particular, the oral form according to the invention is characterized in that the time taken to release 50% of the AP in an alcoholic solution is not reduced more than 3-fold relative to the time taken to release 50% of the AP in an alcohol-free aqueous medium. The form comprises an agent D, which is a pharmaceutically acceptable compound whose hydration or solvation rate or capacity is greater in an alcohol-free aqueous medium than in alcoholic solution
Owner:FLAMEL IRELAND
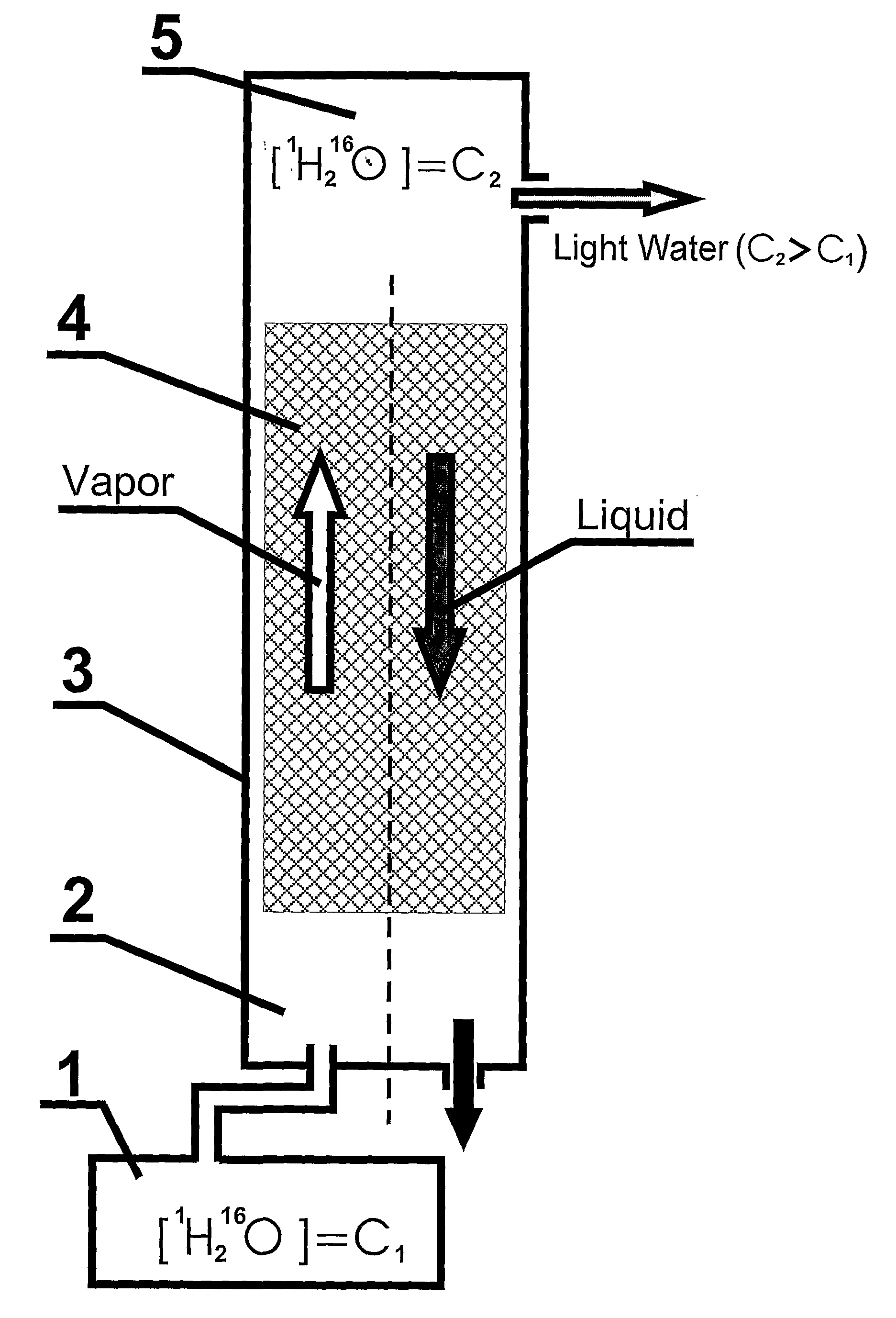
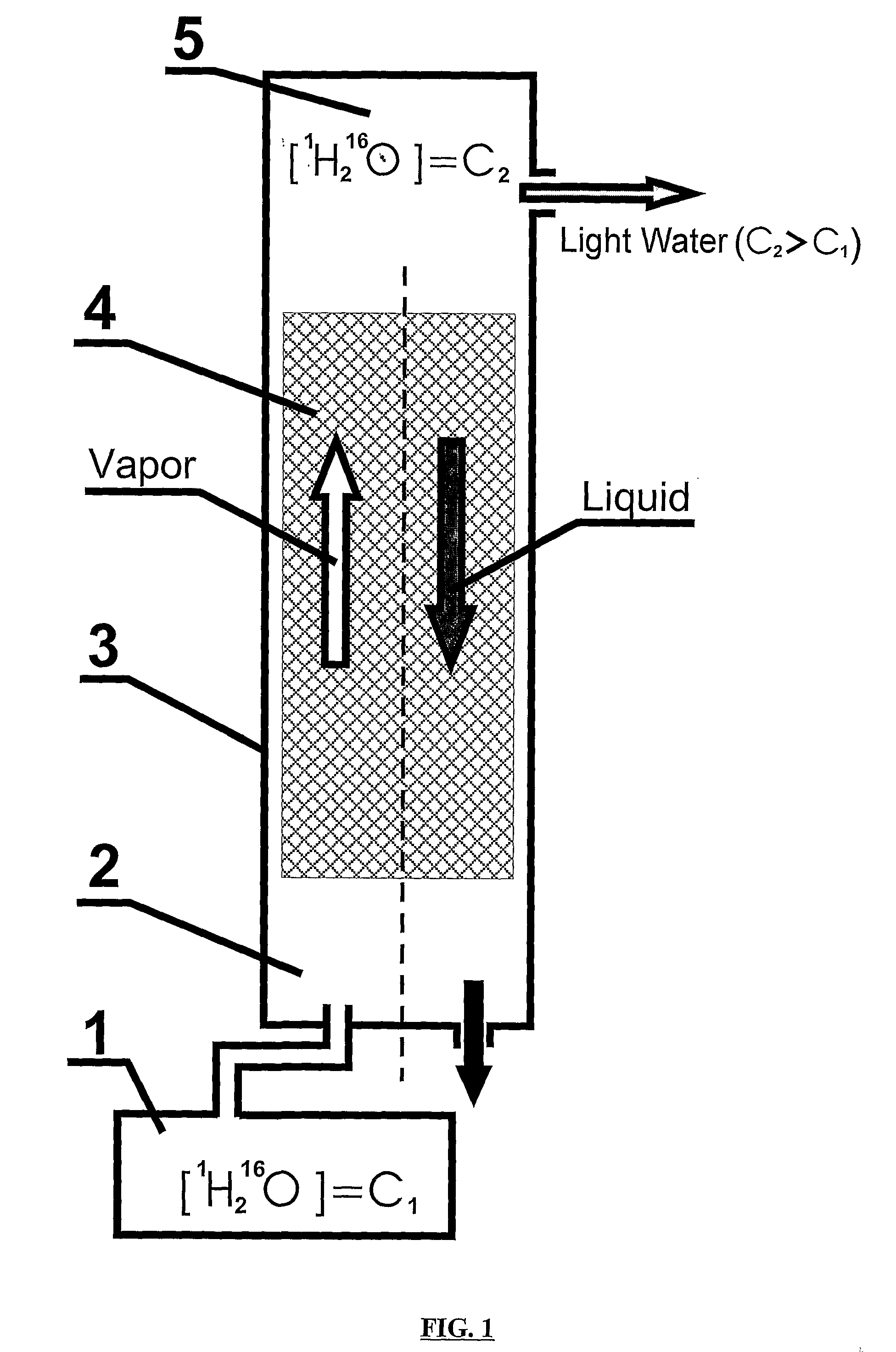
Non-Alcoholic Beverage Enriched With 1H216O
InactiveUS20080138470A1Improve palatabilityIncrease contentInorganic active ingredientsFatty substance preservation using additivesAlcohol drinkFruit juice
The present invention relates to production of non-alcoholic beverage enriched with 1H216O in comparison with typical non-alcoholic beverage composition. This is provided by addition to alcoholic beverage highly pure light water comprising 1H216O from about 99.76% to about 99.99% by weight of water, while the content of 1H216O in typical water is no more than 99.575 by weight of water. According to the present invention non-alcoholic beverage enriched with 1H216O in an amount no less than 99.76% by weight of water, includes drinking water, table drinking water, mineralized water, mineral water, mineral table water, treatment-prophylactic mineral water, mineral-medicinal water; blended beverage which is table beverage, beverage for special purposes, refreshing beverage, cool beverage, tonic, lemonade, non-alcoholic cocktail; and beverage which is juice, nectar, kissel, mors, tea, kvass, non-alcoholic beer. The taking non-alcoholic beverages enriched with 1H216O, wherein the content of 1H216O is no less than 99.76% by weight of water of said non-alcoholic beverage prepared in accordance with present invention improves human wellness and life quality.
Owner:SOLOVIEV SERGEY PAVLOVICH
PPAR activity regulators
The object of the present invention is to provide PPAR (peroxisome proliferator-activated receptor) activity regulators, which can be widely used for improving insulin resistance and preventing / treating various diseases such as diabetes, metabolic syndromes, hyperlipemia, high-blood pressure, vascular disorders, inflammation, hepatitis, fatty liver, liver fibrosis, NASH (non-alcoholic steatohepatitis) and obesity.The present invention provides PPAR activity regulators which comprise an acylamide compound having the specific structure, prodrugs thereof, or pharmaceutically acceptable salts thereof.
Owner:AJINOMOTO CO INC
Skin formulation
The skin formulation is a non-alcoholic composition for treating and alleviating skin disorders, including dermatitis, rough skin, cracking, itching and psoriasis. The formulation includes only natural ingredients. All oils in the formulation are unadulterated or minimally processed and do not result in irritation to the skin or other harmful side effects. The compositions can be formulated as a lotion, lotion bar, or a soap.
Owner:SPRINGSTEAD PATRICIA R
Compositions for treating infected skin and mucous membrane comprising an anti-microbial agent and an essential oil
The invention provides a composition of matter for treating infected skin and mucousal membranes, said composition comprising at least one anti-microbial drug; and at least one essential oil, in combination with a substantially, alcohol-free carrier system, said carrier being selected from a liquid carrier or a semi-solid carrier, said carrier system being selected from isotonic system and a moderately hypertonic system.
Owner:J P M E D
Method of reducing drug-induced adverse side effects in a patient
InactiveUS20060252670A1Good treatment effectReduce adverse side effectsBiocideSenses disorderSide effectPeroxisome Proliferation
The invention relates to the discovery that farnesoid X receptor (FXR) agonists can be used in combination with peroxisome proliferation activated receptor gamma (PPARγ) agonists to reduce drug-induced adverse side effects in patients suffering from conditions such as insulin resistance, Type II diabetes, metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and heart disease. Particularly, the present invention encompasses methods for treating patients suffering from drug-induced adverse side effects with selective PPARγ, dual PPARα / γ and pan PPARα / γ / δ agonists in combination with FXR agonists.
Owner:INTERCEPT PHARMA INC
Methods of Treating Tnf-Mediated Disorders
The present invention provides methods of treating TNF-α-mediated disorders, the methods generally involving administering to an individual in need thereof effective amounts of pirfenidone or a pirfenidone analog and a second therapeutic agent that reduces TNF-α synthesis or that reduces TNF-α binding to a TNF receptor. The present invention further provides methods for treating non-alcoholic steatohepatitis, the method generally involving administering to an individual in need thereof an effective amount of pirfenidone. The present invention further provides methods of treating end-stage or advanced Type II diabetes, the methods generally involving administering to an individual in need thereof effective amounts of pirfenidone and insulin.
Owner:INTERMUNE INC
Marker associated with non-alcoholic steatohepatitis
ActiveUS20120231471A1Easy to detectEase of evaluationOrganic active ingredientsMicrobiological testing/measurementPediatricsNon alcoholic
Disclosed is a novel NASH marker for use in a method for detecting NASH or evaluating the severity of NASH, which utilizes at least one factor selected from the group consisting of an IL-1 receptor antagonist, sCD40, HMGB1, sPLA2 group IIA and an sPLA2 activity as the marker. Also disclosed is a method for detecting NASH or evaluating the severity of NASH in a subject, which utilizes the marker.
Owner:AMARIN PHARMA IRELAND
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS7078064B2Reduce peroxidationReduce stressBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
Nuclear sulfated oxysterol, potent regulator of lipid homeostasis, for therapy of hypercholesterolemia, hypertriglycerides, fatty liver diseases, and atherosclerosis
ActiveUS8399441B2Lower Level RequirementsImprove suppression propertiesOrganic active ingredientsMetabolism disorderLipid formationMetabolite
The sulfated oxysterol 5-cholesten-3β, 25-diol 3-sulphate, a nuclear cholesterol metabolite that decreases lipid biosynthesis and increases cholesterol secretion and degradation, is provided as an agent to lower intracellular and serum cholesterol and / or triglycerides, and to prevent or treat lipid accumulation-associated inflammation and conditions associated with such inflammation. Methods which involve the use of this sulfated oxysterol to treat conditions associated with high cholesterol and / or high triglycerides and / or inflammation (e.g. hypercholesterolemia, hypertriglyceridemia, non-alcoholic fatty liver diseases, atherosclerosis, etc.) are also provided.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Application of GLP-1R/GCGR double-target agonist polypeptide to treatment of fatty liver diseases, hyperlipidemia and arteriosclerosis
ActiveCN106046145AImprove biological activityExtended half-lifePeptide/protein ingredientsMetabolism disorderFibrosisDrug biological activity
The invention relates to application of a polypeptide compound having double agonist effects on a glucagon-like peptide-1 receptor and a glucagon receptor. The polypeptide compound has the characteristics of high enzymolysis stability, high bioactivity, no adverse reaction, etc., can alleviate abnormal rise in the levels of total cholesterol and triglyceride in blood induced by diabetes and high-meal diet, lowers the level of liver enzyme, improves liver damage and fibrosis stage and is applicable to prevention or treatment of diseases like non-alcoholic fatty liver diseases, hyperlipidemia and arteriosclerosis.
Owner:SHENZHEN TURIER BIOTECH CO LTD
Preparation method of high purity bulleyaconitine A
ActiveCN101555227AHigh purityReduce manufacturing costNervous disorderOrganic chemistrySolvent vaporEthyl acetate
The invention relates to a preparation method of high purity bulleyaconitine A. The preparation method comprises the following steps: root of radix aconiti feri of Yunnan is crushed, and acid alcoholic solvent or alcohol water solution is used for diacolation, and supersound or thermal refluxing extraction. The extracting solution is decompressed and concentrated to be non-alcoholic at the temperature of 80 to 90 DEG C, cooled down to room temperature, to ensure the relative density to be 1.00 to 1.30; standing is performed for 10 to 20 hours; the extracting solution is filtered; the pH is adjusted to 6.0 to 8.0 with basifier; and then the extracting solution is extracted with ethyl acetate or chloroform. The ethyl acetate or chloroform liquid is contracted, column chromatography is performed to the obtained paste, silica gel or neutral alumina are used as fillers, any one of solvent vapor-acetone-diethylamine, ligarine-acetone-diethylamine or cyclohexane-acetone-diethylamine is used for eluation, the bulleyaconitine A in the collected liquid is constantly checked during the thin layer chromatography, the collected liquid containing the bulleyaconitine A is merged, the solvent is concentrated, carbinol or ethanol is dissolved, filtering is performed, crystallization is performed at room temperature, filtering is performed, the crystal is repeatedly rinsed with carbinol or ethanol, and the final product is obtained. The purity of the obtained product is high, the production cost is low, and the toxicity of the used organic solvent is low.
Owner:KPC PHARM INC
Composite nourishing dense liquid of common oats and preparation method thereof
ActiveCN101317657ALower blood cholesterolBlood sugar controlFood preparationPolygonum fagopyrumWheat germ
The invention provides oat composite nutrition slurry and a preparation method thereof, which relates to a non-alcoholic drink. The raw material comprises oat, sesame, soya, brown rice, buckwheat, wheat germ, white granulated sugar, liquid native starch anti-aging agent and composite phosphate. The oat, the sesame, the soya, germinate brown rice, the buckwheat and the wheat germ are roasted; the roasted oat, soya, germinate brown rice, buckwheat and wheat germ are crushed and compounded into paste by adding water to prepare slurry A; the roasted sesame and wheat germ are grinded so as to obtain sesame wheat germ flour; the liquid native starch anti-aging agent is uniformly mixed with the white granulated sugar where hot water is added so as to obtain the liquid native starch anti-aging agent solution; the composite phosphate is dissolved by water so as to gain the composite phosphate solution; the slurry A is mixed with the sesame wheat germ flour where the liquid native starch anti-aging agent solution and the composite phosphate solution are added so as to obtain slurry B; the white granulated sugar is dissolved into syrup C by water; the slurry B and the syrup C are added with water for constant volume so as to obtain compounding solution; and the steps as follows are carried out to prepare the oat composite nutrition slurry: cooling, pre-heating, degassing, homogenizing, sterilizing, filling and packaging.
Owner:XIAMEN HUIERKANG FOOD
Methods of use and nutritional compositions of touchi extract
InactiveUS20090148545A1Reduce complicationsReduce post-prandial glucose excursionBiocideAntiviralsPhysiologyConstipation
Disclosed is a method and composition for nutritional compositions containing -glucosidase inhibitors, and more specifically Touchi Extract and its uses in the treatment of many disorders. These disorders include diabetes, hyperlipidemia, obesity, Metabolic syndrome / Syndrome X, COPD, malabsorption, Crohn's disease, diarrhea, constipation, irritable bowel syndrome, human immunodeficiency virus, cystic fibrosis, non-alcoholic steatohepatitis, polycystic ovarian syndrome including associate infertility, and erectile dysfunction. Further, -glucosidase inhibitors, and more specifically Touchi Extract can be used to aid healing in critical care patients and for general wound healing. Additionally, -glucosidase inhibitors, including Touchi Extract can be used to enhance athletic performance.
Owner:NESTEC SA
Treatment of diseases
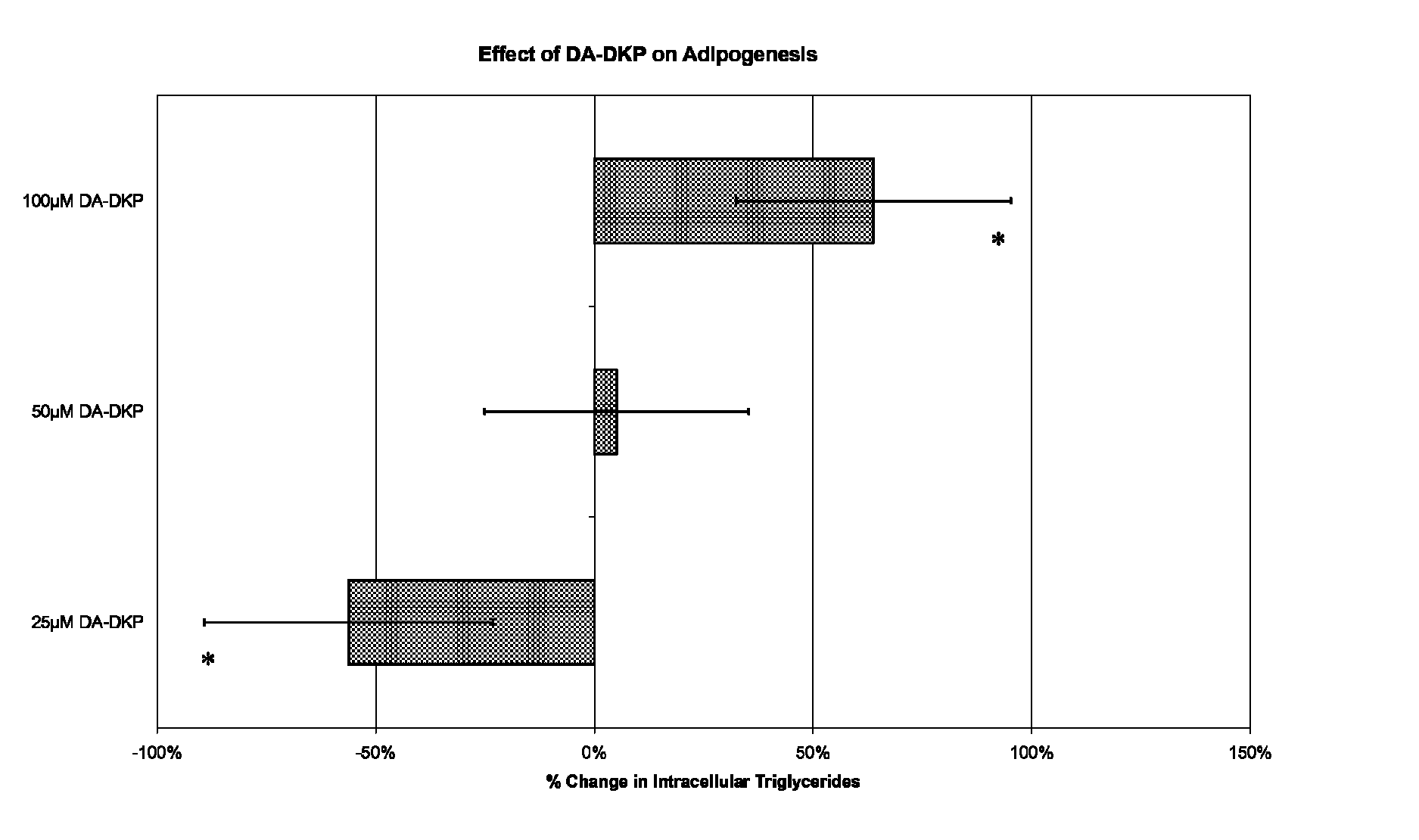
The invention provides (i) a method of treating metabolic syndrome in an animal, (ii) a method of suppressing the appetite of an animal, (iii) a method of treating obesity in an animal, (iv) a method of reducing the weight of an animal in need thereof, (v) a method of reducing a blood lipid level in an animal in need thereof, (vi) a method of treating non-alcoholic steatohepatitis in an animal, and (vii) a method of inhibiting adipogenesis. The methods comprise administering an effective amount of an active ingredient, wherein the active ingredient comprises a diketopiperazine, a prodrug of a diketopiperazine or a pharmaceutically-acceptable salt of either of them to the animal. The invention also provides a kit comprising a container holding a diketopiperazine, a prodrug of a diketopiperazine or a pharmaceutically-acceptable salt of either of them; and instructions for administration. The diketopiperazines have the formula given in the application.
Owner:AMPIO PHARMA
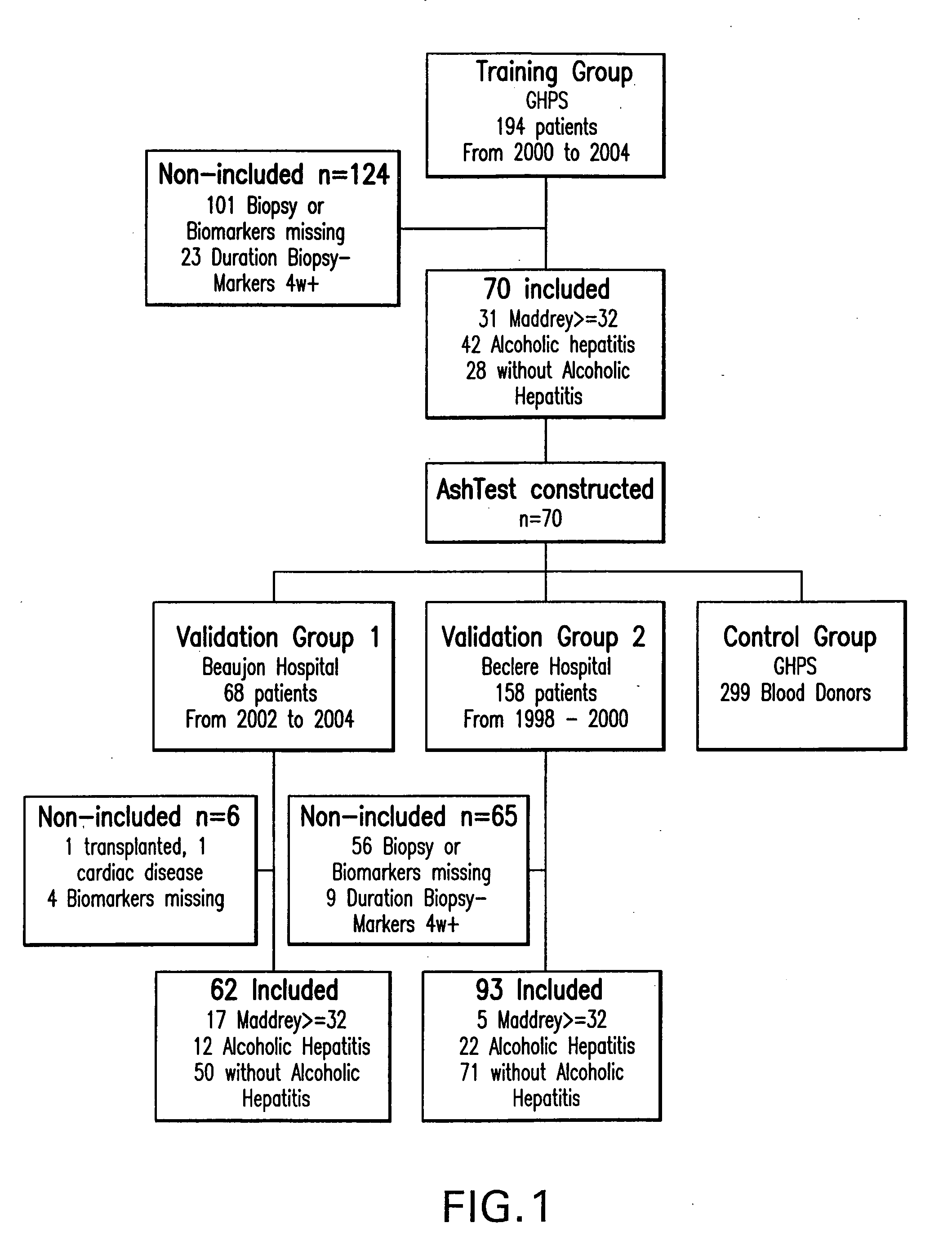
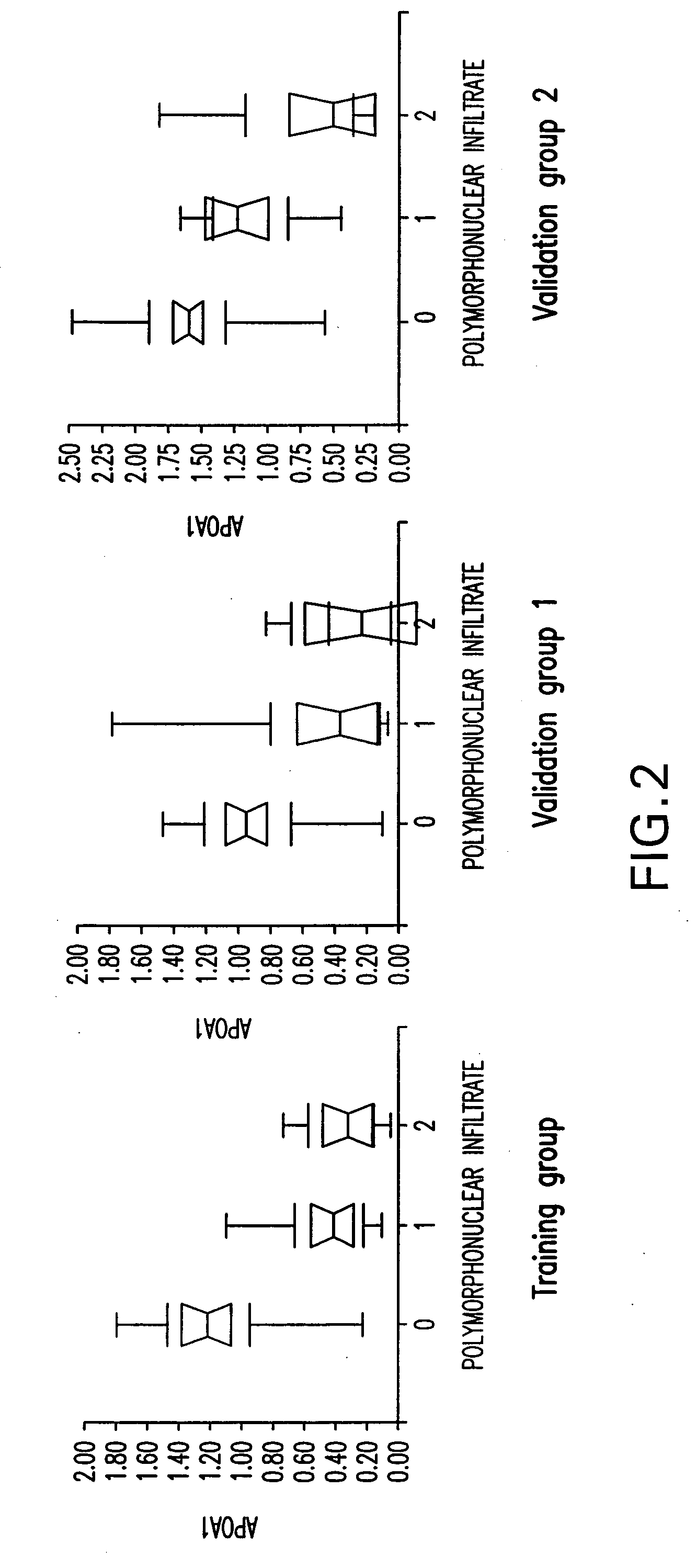
Diagnosis method of alcholic or non-alcoholic steato-hepatitis using biochemical markers
ActiveUS20060172286A1Reduce in quantityLow costMicrobiological testing/measurementDiagnostic recording/measuringLiver fibrosisBiochemical markers
The present invention is drawn to a new diagnosis method for detecting the extent of alcoholic or non-alcoholic steato-hepatitis in a patient, in particular in a patient suffering from a disease involving alcoholic or non-alcoholic steato-hepatitis or who already had a positive diagnosis test of liver fibrosis and / or presence of liver necroinflammatory lesions, by using the serum concentration of easily detectable biological markers. The invention is also drawn to diagnosis kits for the implementation of the method.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
Skin formulation
The skin formulation is a non-alcoholic composition for treating and alleviating skin disorders, including dermatitis, rough skin, cracking, itching and psoriasis. The formulation includes only natural ingredients. All oils in the formulation are unadulterated or minimally processed and do not result in irritation to the skin or other harmful side effects. The compositions can be formulated as a lotion, lotion bar, or a soap.
Owner:SPRINGSTEAD PATRICIA R
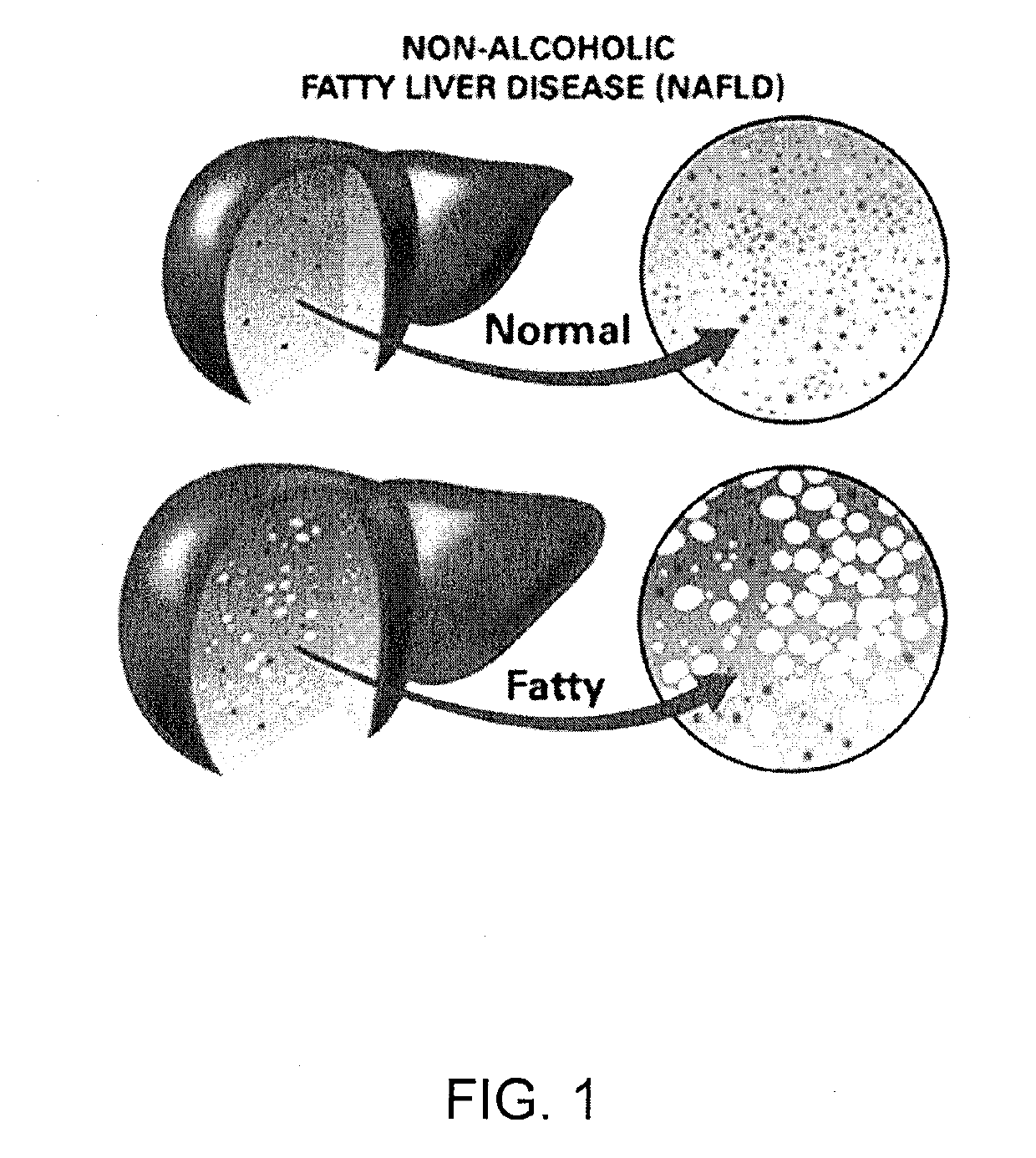
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20190117709A1Inhibit progressEarly detectionOrganic active ingredientsDigestive systemFiberMonoacylglycerol acyltransferase
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
Non-alcoholic monocrystalline silicon flock making additive
InactiveCN102586888AImprove permeabilityGood emulsificationAfter-treatment detailsFinal product manufactureAlcoholMetallurgy
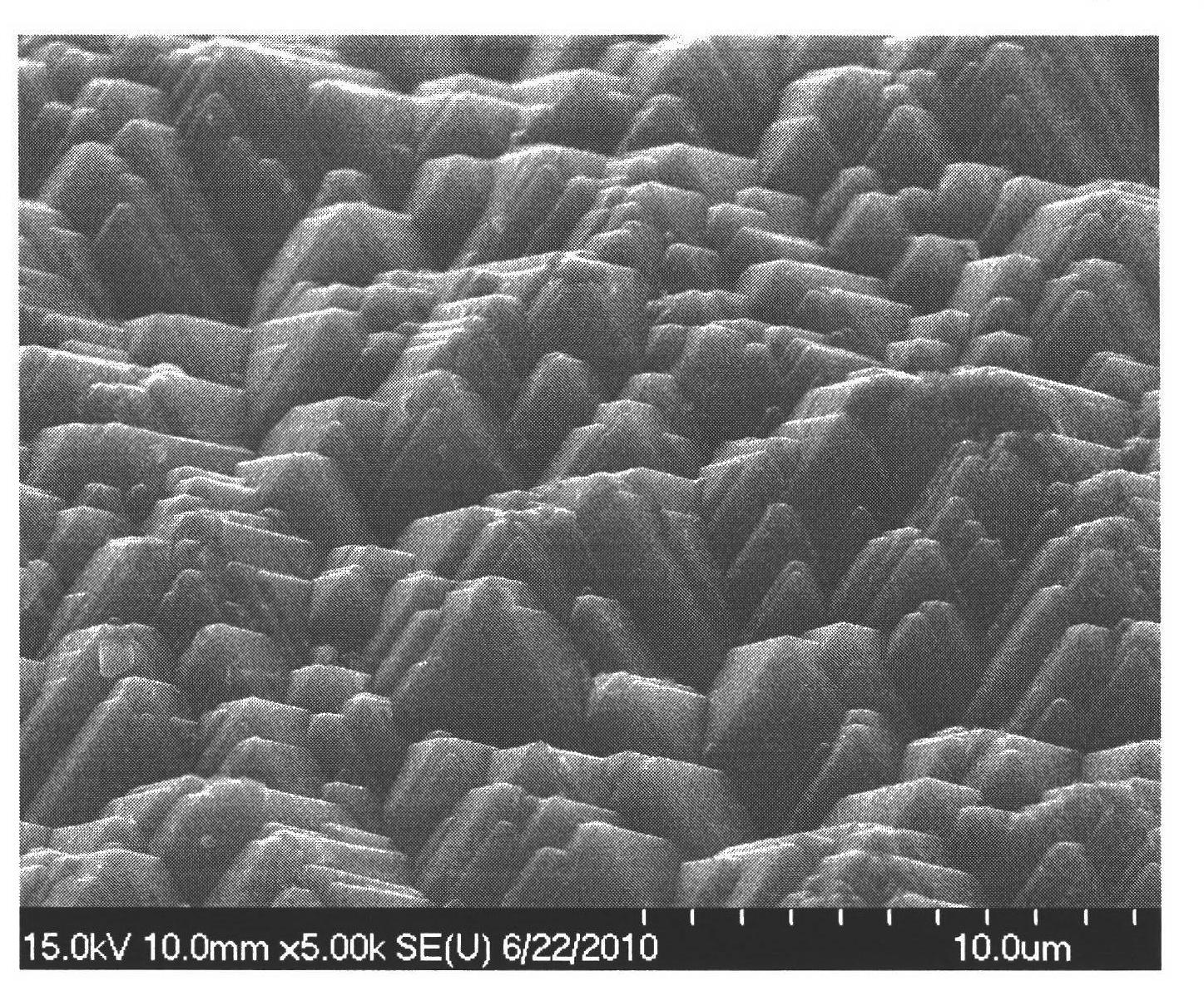
The invention provides relates to the technical field of production of crystalline silicon solar batteries, in particular to a non-alcoholic monocrystalline silicon flock making additive. The non-alcoholic monocrystalline silicon flock making additive consists of citric acid (sodium citrate), sodium dodecyl benzene sulfonate and deionized water. When the non-alcoholic monocrystalline silicon flock making additive is applied to manufacturing of suede of a monocrystalline wafer, uniform, thin and dense suede pyramid can be obtained without using alcohol such as isopropanol or ethanol, so the flock manufacturing cost can be reduced, environmental pollution caused by the alcohol is avoided, the process of the crystalline silicon solar battery is stable, and good practical value is achieved.
Owner:苏州先拓光伏科技有限公司
Method for wetting hydrophobic porous polymeric membranes to improve water flux without alcohol treatment
A method is provided for substantially instantaneously wetting hydrophobic, porous polymeric membranes and for rendering hydrophobic membranes hydrophilic. The method involves treating the membrane with a non-alcoholic aqueous solution of a low molecular weight surfactant, and then drying the treated membrane. The low molecular weight surfactant exhibits high polymer affinity for the hydrophobic membrane substrate as well as high water solubility; a preferred surfactant is sodium dodecylbenzenesulfonate (SDBS). The method is particularly useful for treating hydrophobic membranes such as those made of polyolefins, fluorinated or chlorinated polymers, polysulfone, or polyethersulfone, preferably having a pore size of about 0.01 microns to about 1 micron. A wettable membrane is thus provided as the aqueous surfactant solution is absorbed into the hydrophobic membrane.
Owner:HYDRANAUTICS
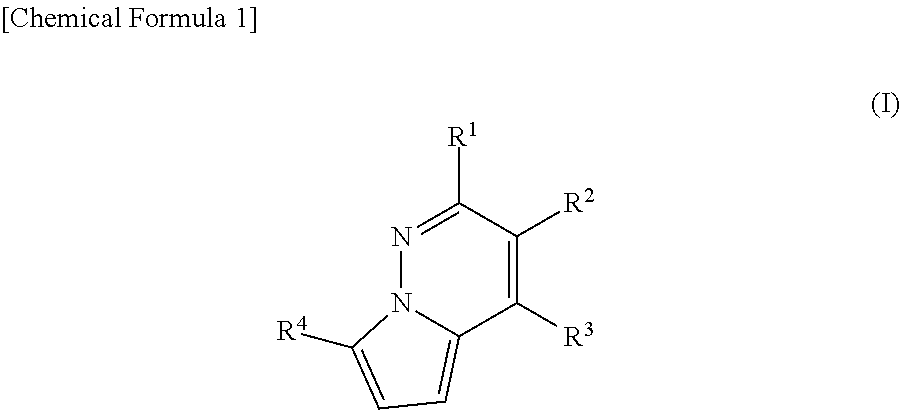
Treatment for non-alcoholic-steatohepatitis
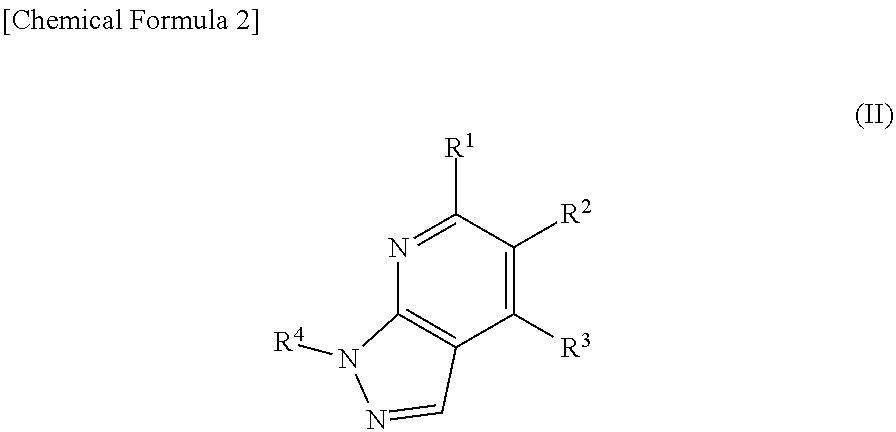
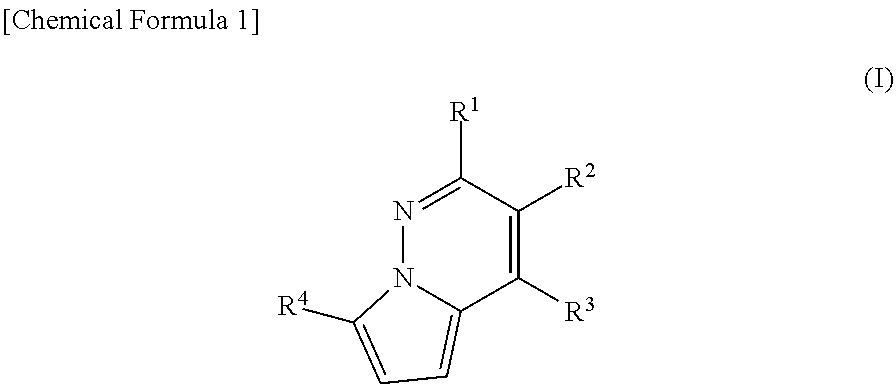
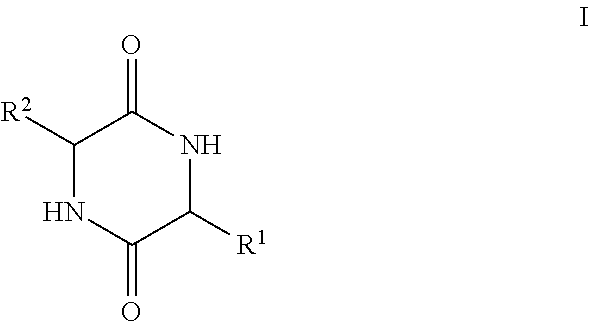
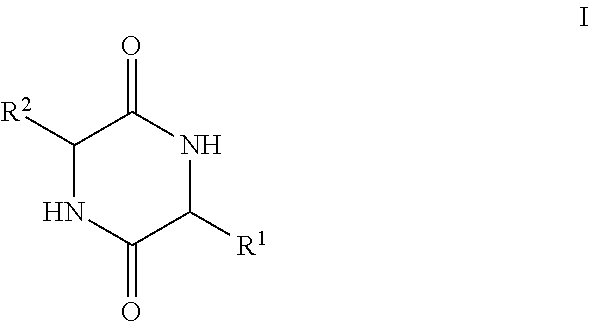
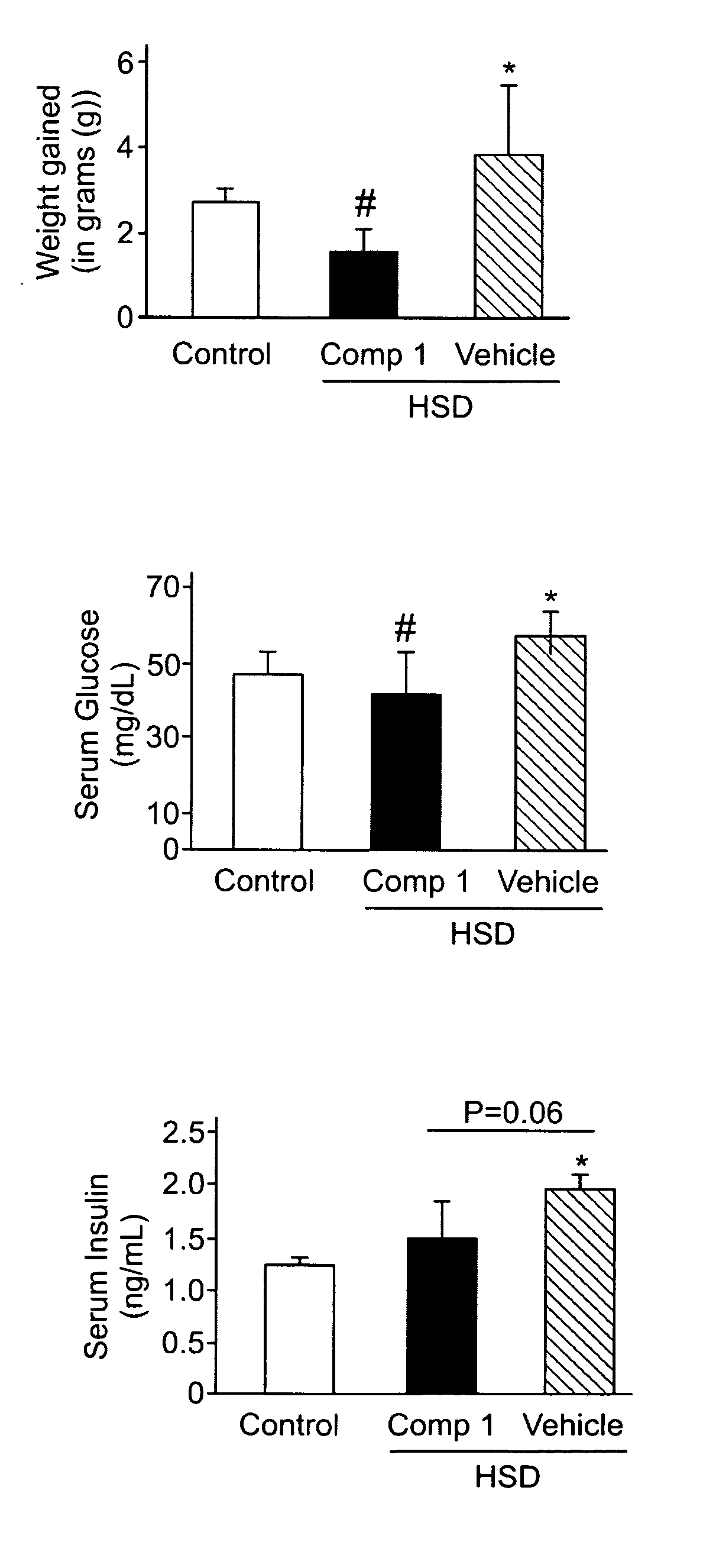
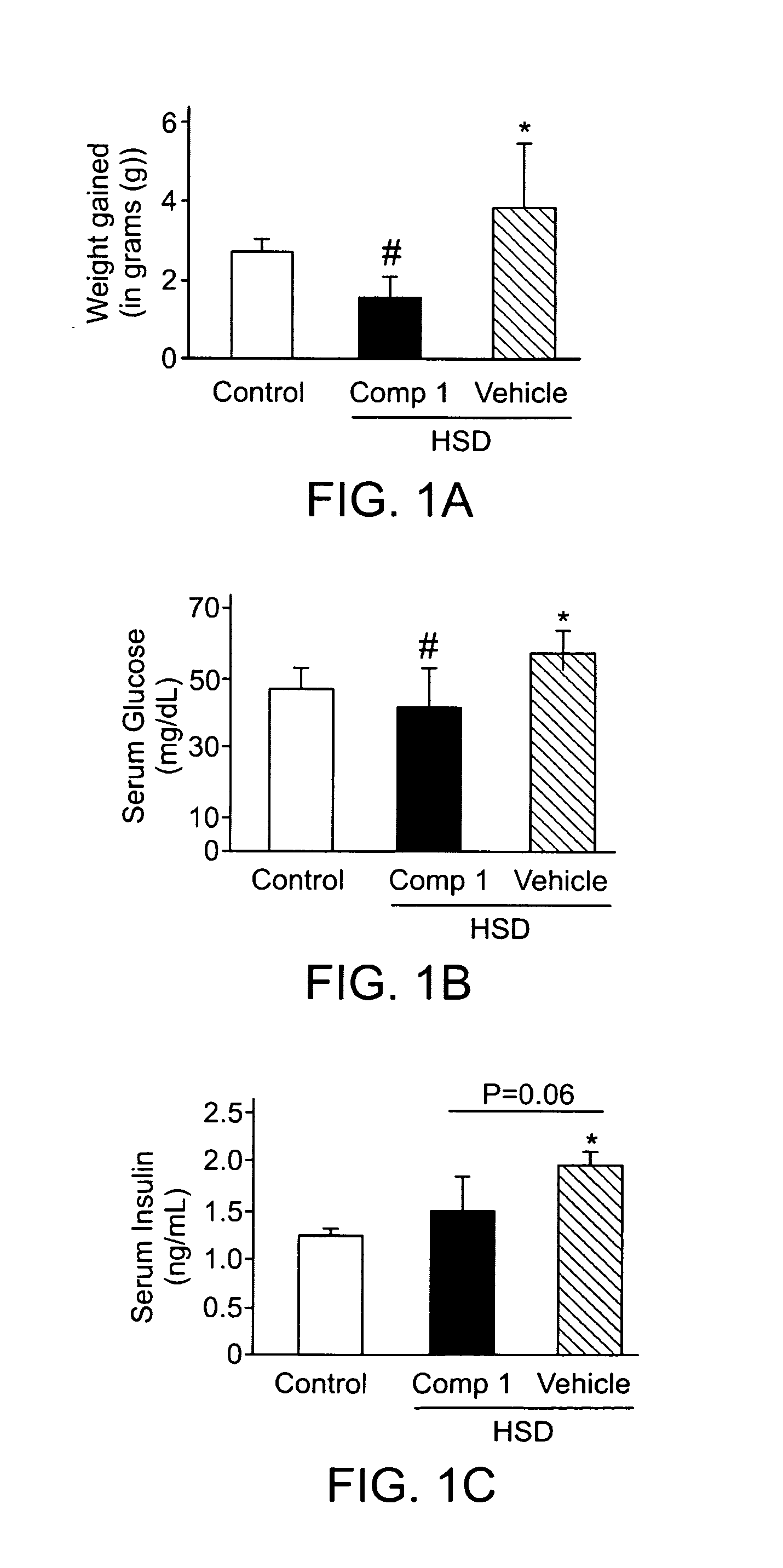
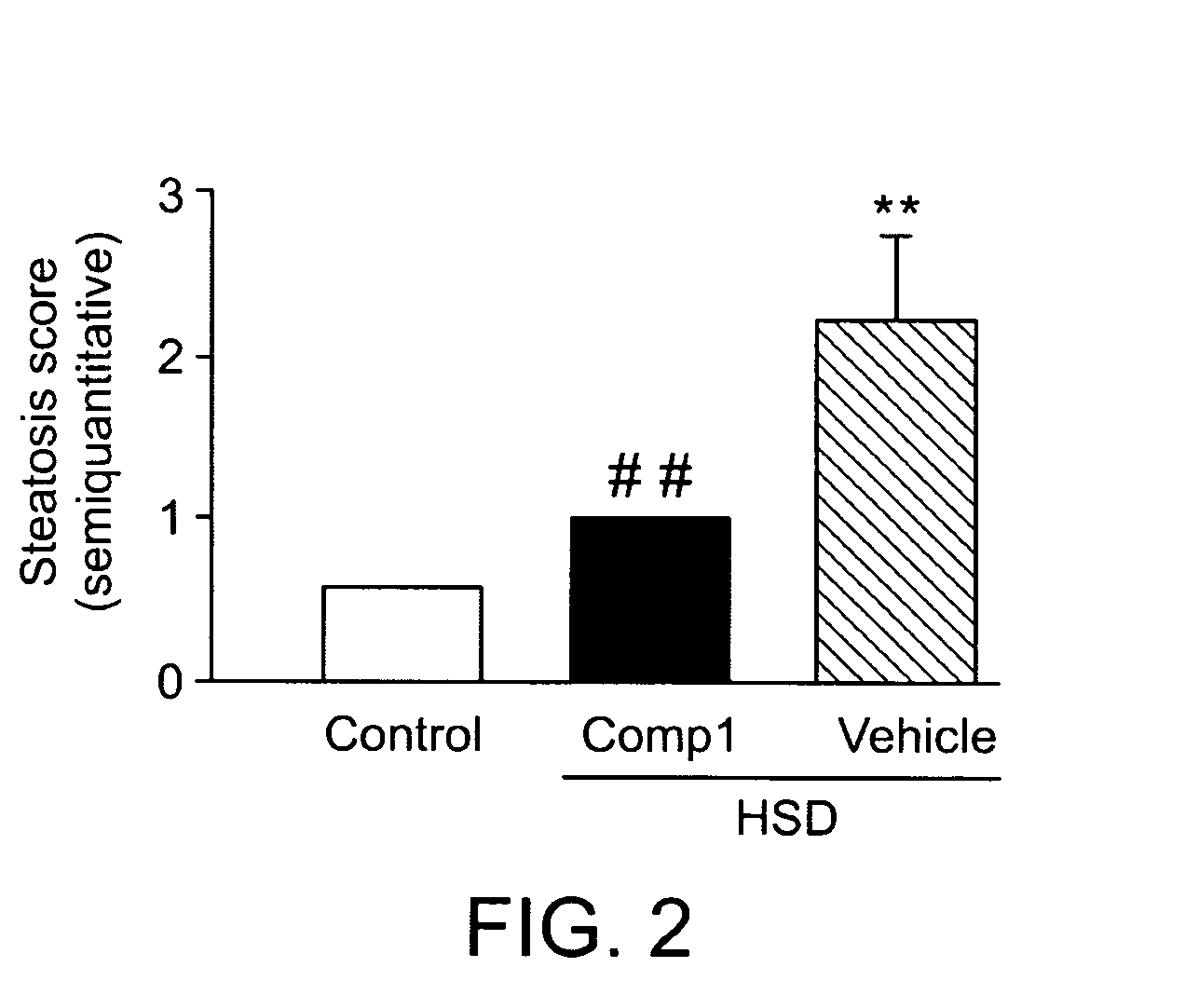
The present invention provides methods of treating a subject with non-alcoholic fatty liver disease (NAFLD), insulin resistance, obesity or hyperlipidemia, comprising administering to the subject an effective amount of a compound according to Formula I:or a physiologically acceptable salt thereof.
Owner:ARES TRADING SA
Alcohol-free slightly-alcoholic oral care composition and a process for preparing same
InactiveUS20130224125A1Extends antimicrobial efficacy of coatingImprove efficacyCosmetic preparationsToilet preparationsAlcohol freeWater insoluble
An aqueous, heat and cold stable, non-alcoholic or slightly-alcoholic microemulsion based antimicrobial mouthwash composition with improved antimicrobial efficacy. The composition comprises a unique water-soluble matrix composite, at least one water-immiscible or water-insoluble antimicrobial agent, and optionally, a preservative or preservative system, a weak carboxylic acid, a coloring agent and other additives. Examples of antimicrobial agents include menthol, thymol, eucalyptol and / or methyl salicylate. A process for preparing the oral care composition is also disclosed.
Owner:ISP INVESTMENTS INC
Compositions and methods for the reduction or prevention of hepatic steatosis
ActiveUS20160067201A1Lower Level RequirementsReduction of non-alcoholic steatohepatitis (NASH)BiocidePeptide/protein ingredientsSteatosisMetabolite
Methods useful for reducing or preventing non-alcoholic steatohepatitis or hepatic steatosis are provided herein. Such methods may comprise administering to a subject in need thereof a sirtuin pathway activator and / or PDE5 inhibitor alone or in combination with an amount of a branched amino acid in free amino acid form, or a metabolite thereof. Also provided herein are compositions and kits for practicing any of the methods described herein.
Owner:NUSIRT SCI
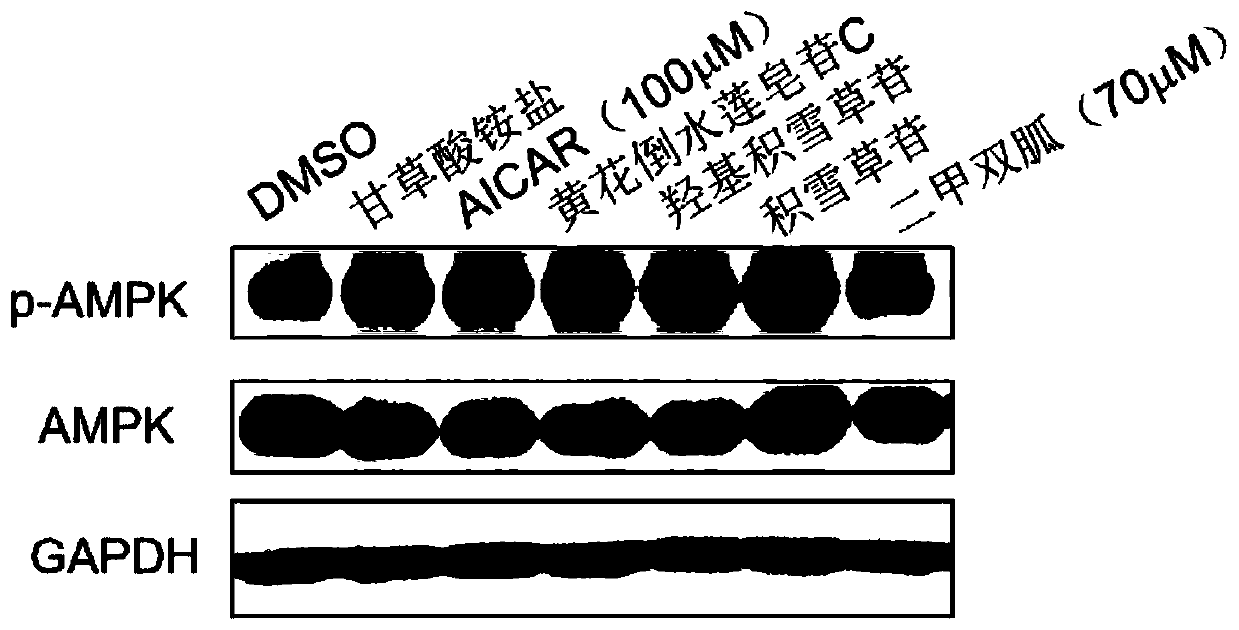
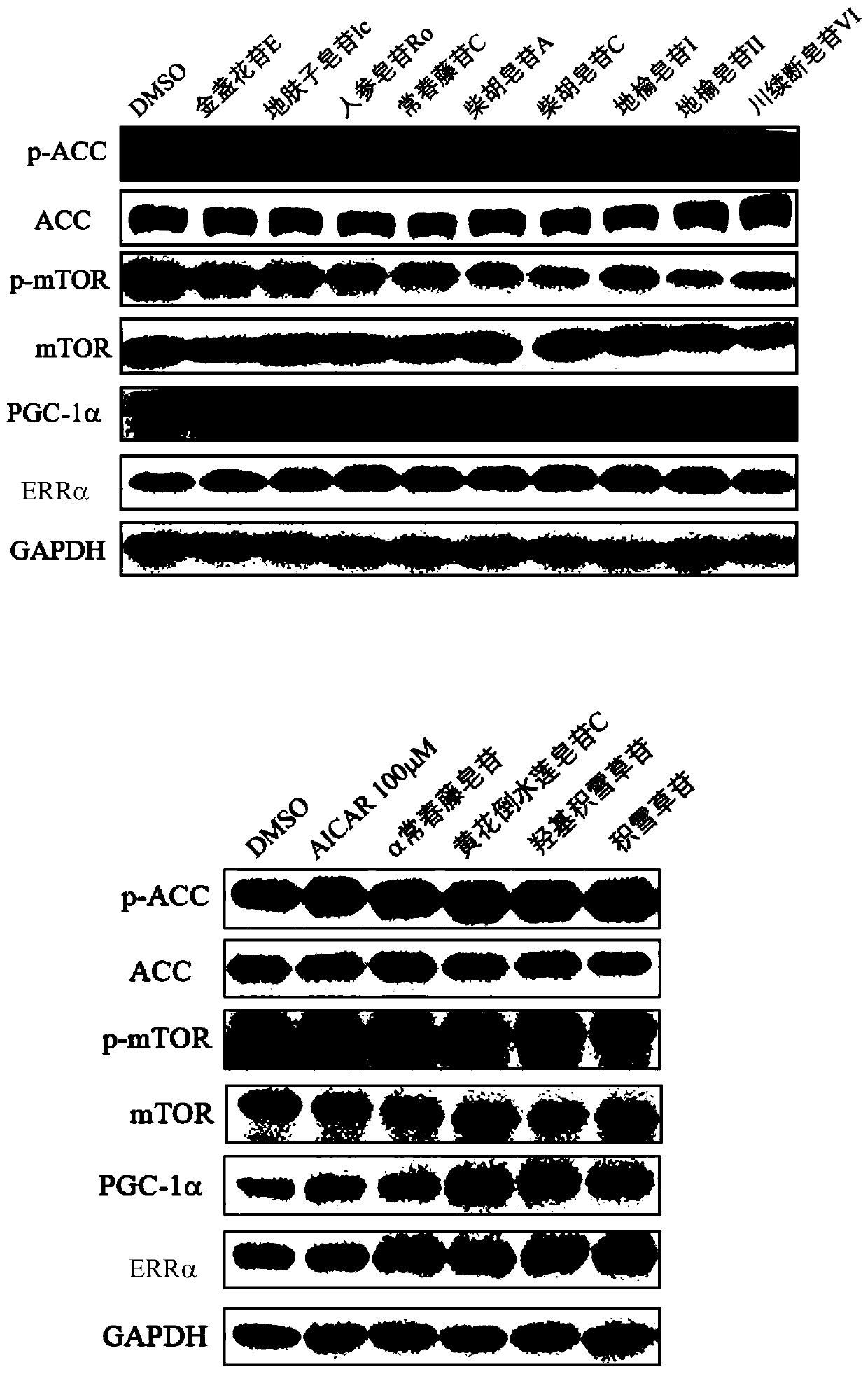
Medical use of pentacyclic triterpenoid saponin and pharmaceutical composition thereof
InactiveCN110200981AImprove securityGood curative effectOrganic active ingredientsSenses disorderBovine respiratory diseaseDiabetic complication
The present invention discloses an application of pentacyclic triterpenoid saponin represented by formulas (I) to (XIII) for the preparation of a medicine for preventing or treating AMPK-mediated diseases including fatty liver disease, inflammatory bowel disease, respiratory disease, diabetic complications and polycystic kidney disease. The compounds of formula (I) to (XIII) are especially usefulfor nonalcoholic simple fatty liver, nonalcoholic steatohepatitis, non-alcoholic steatohepatitis-induced cirrhosis, ulcerative colitis, Crohn's disease, chronic obstructive Pulmonary diseases, asthma,idiopathic pulmonary fibrosis, cystic fibrosis, allergic rhinitis, diabetic nephropathy, diabetic cardiomyopathy, diabetic ulcers, and autosomal dominant polycystic kidney disease. A pharmaceutical composition for preventing and treating the AMPK-mediated diseases of the present invention comprises a therapeutically effective amount of the compounds of the formula (I) to (XIII) or a pharmaceutically acceptable salt or a solvate thereof as an active ingredient and a pharmaceutically acceptable excipient.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com