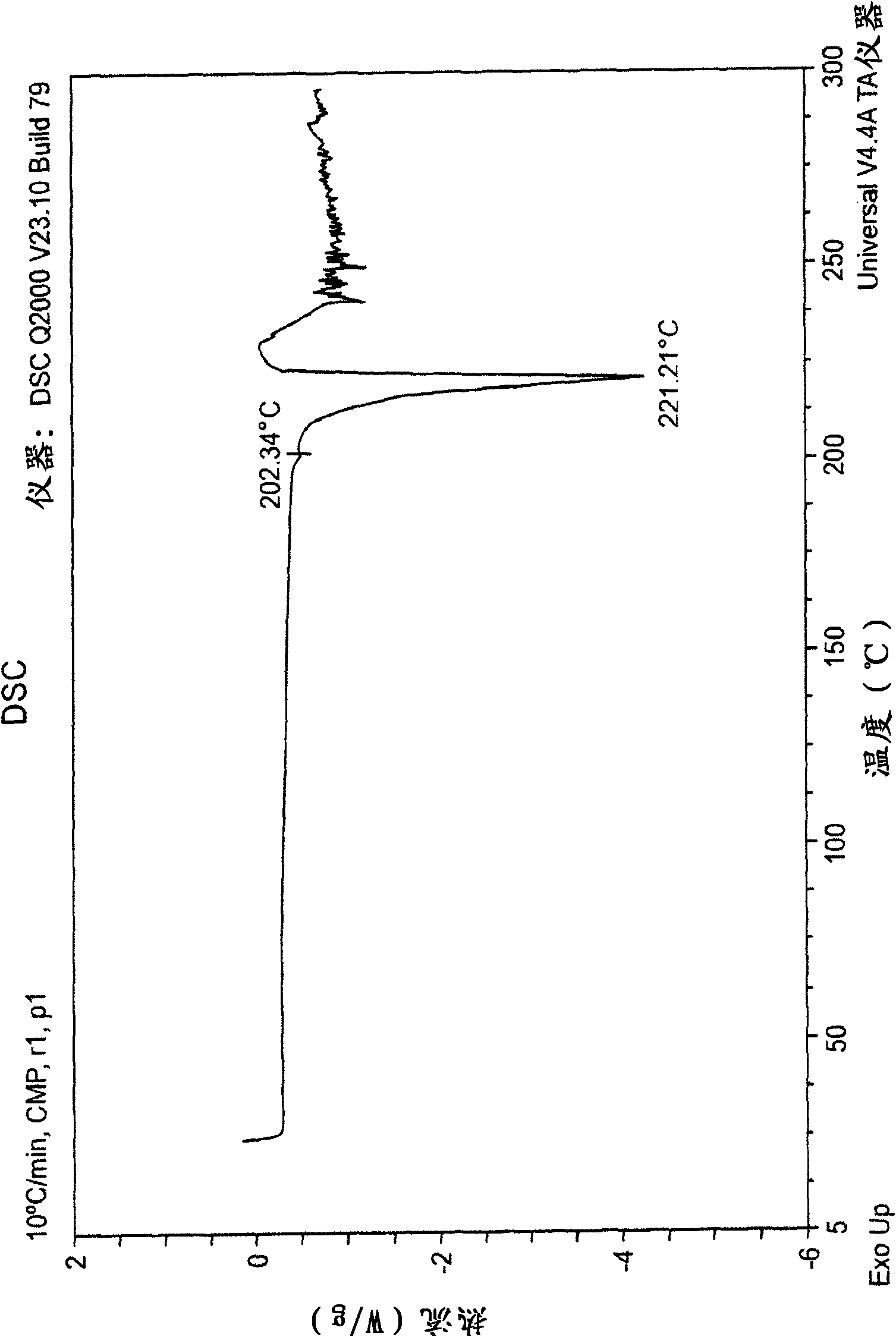
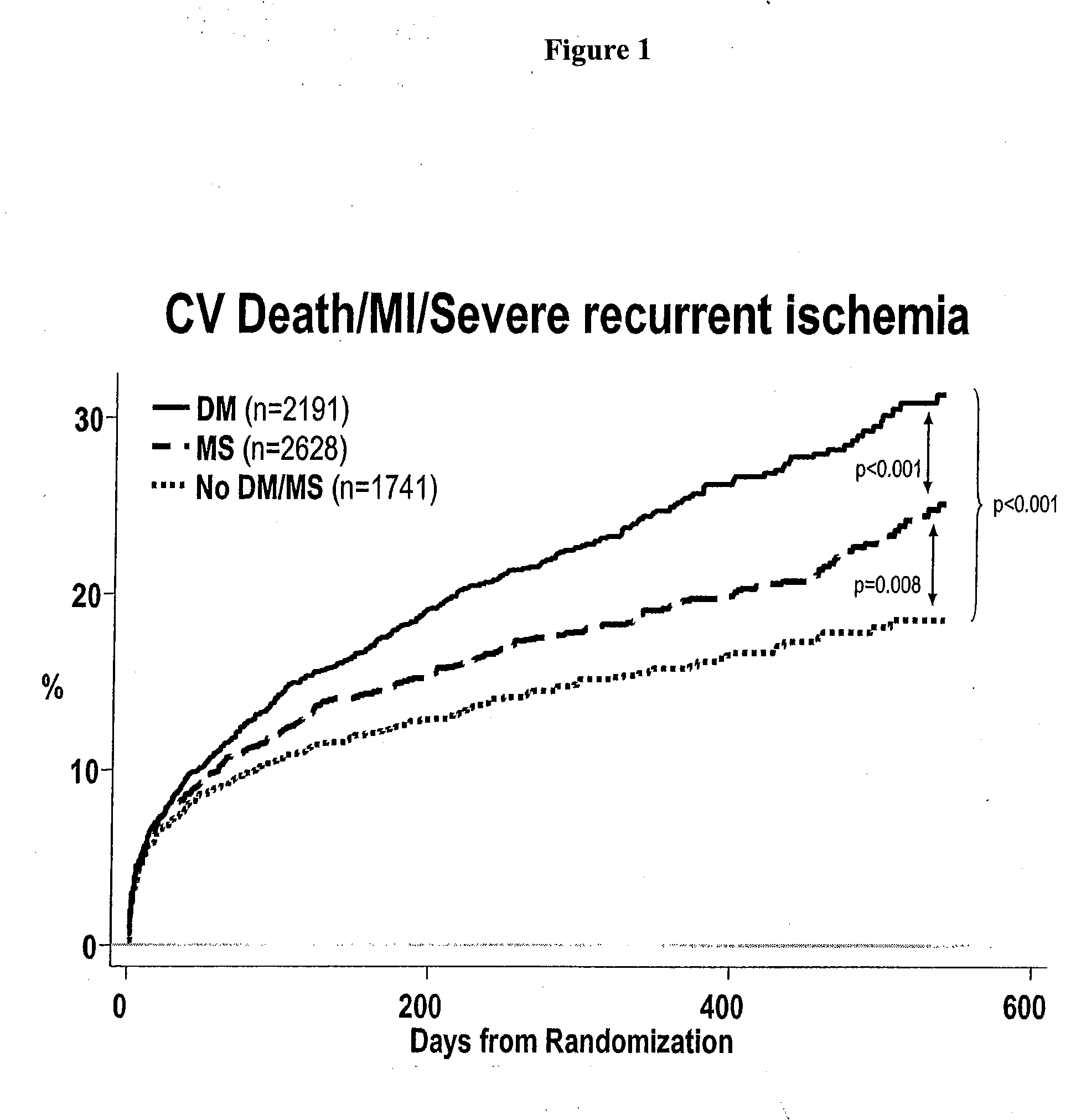
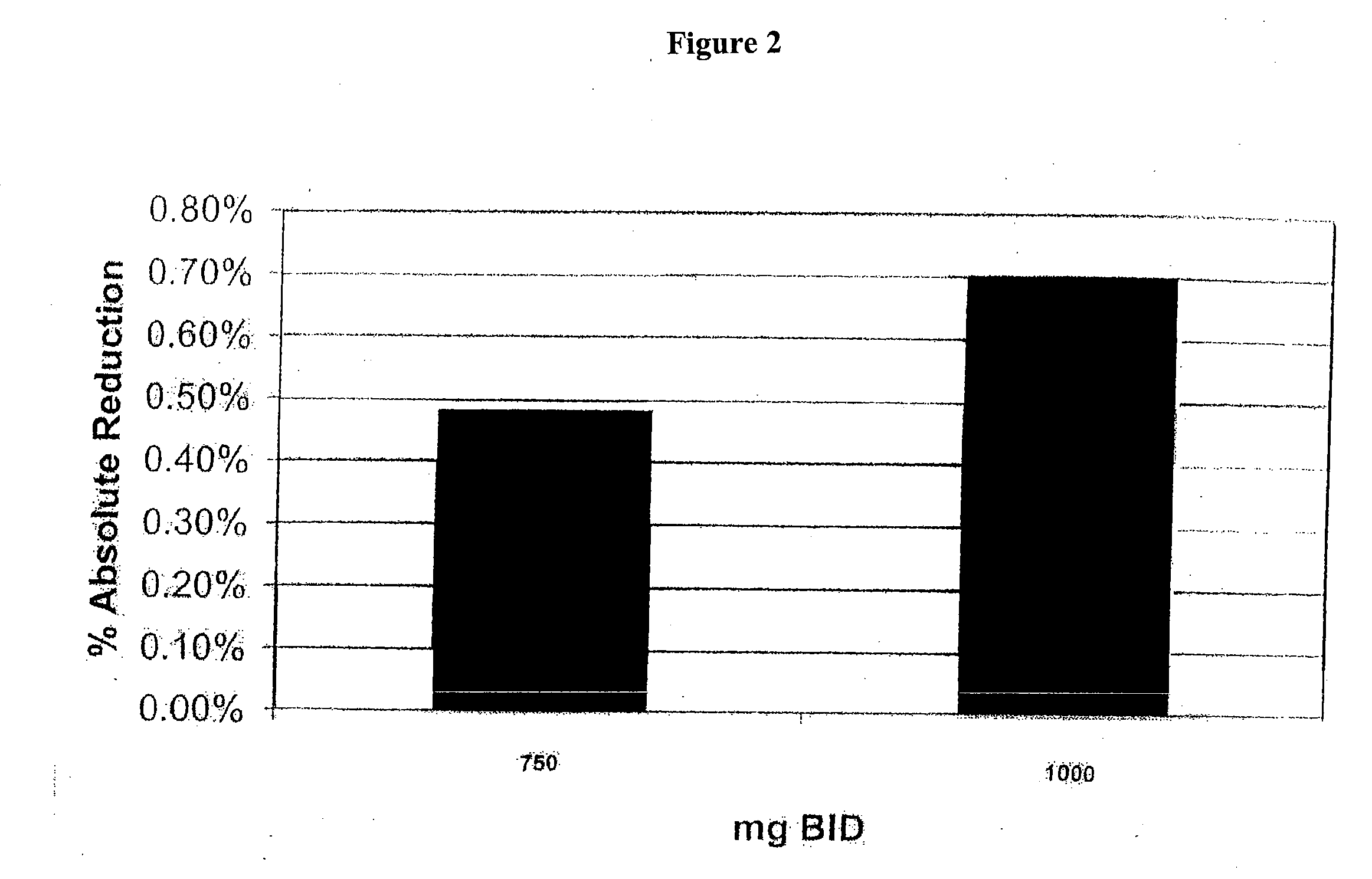
Patents
Literature
90 results about "Non diabetic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
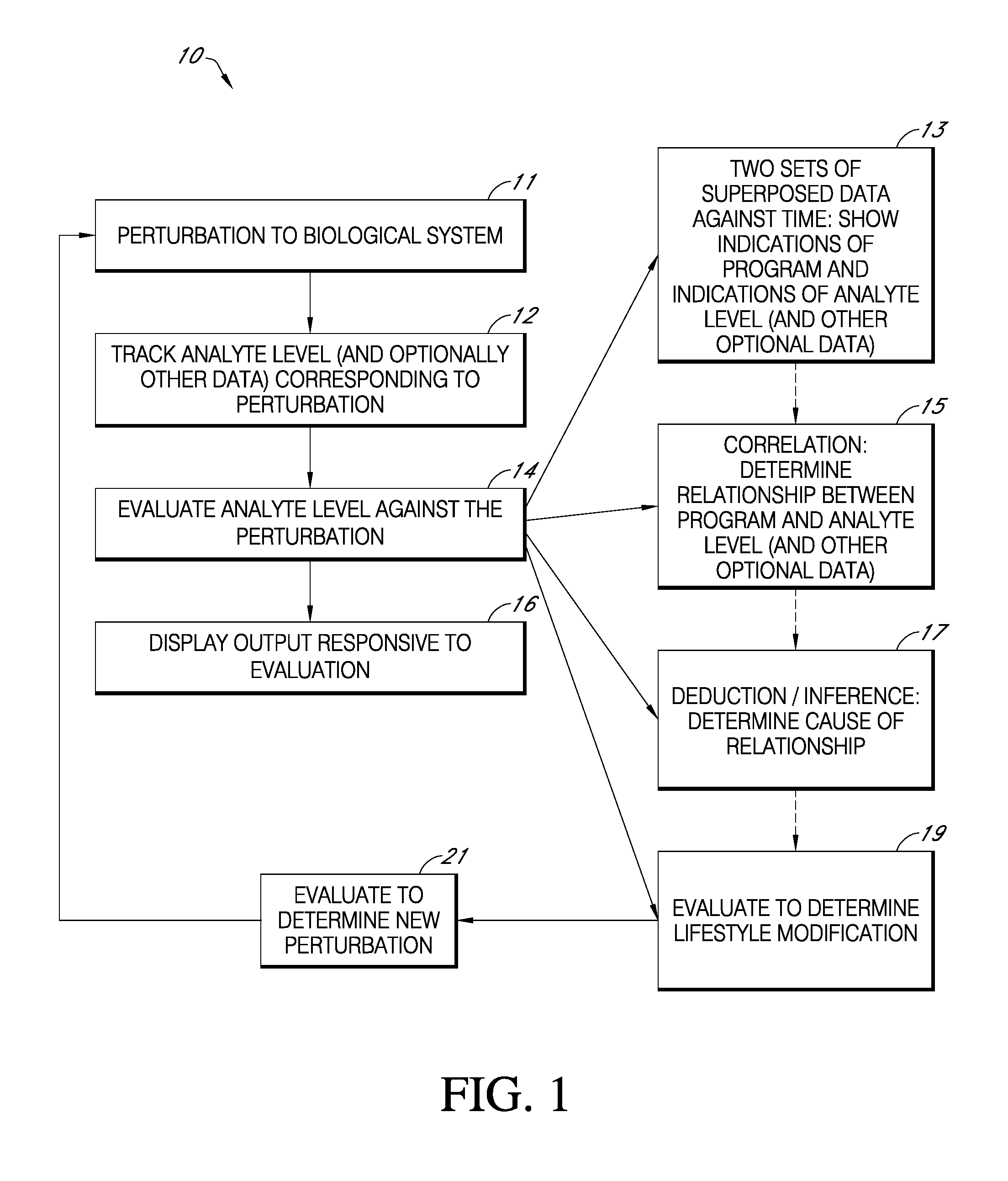
Non-diabetic hypoglycemia is a condition in which blood glucose levels are too low in non-diabetic individuals. Low blood sugar can create a variety of symptoms, ranging from light-headedness, tunnel vision, and shakiness, to more severe neurological dysfunction, because glucose is the only source...
Method for optical measurements of tissue to determine disease state or concentration of an analyte
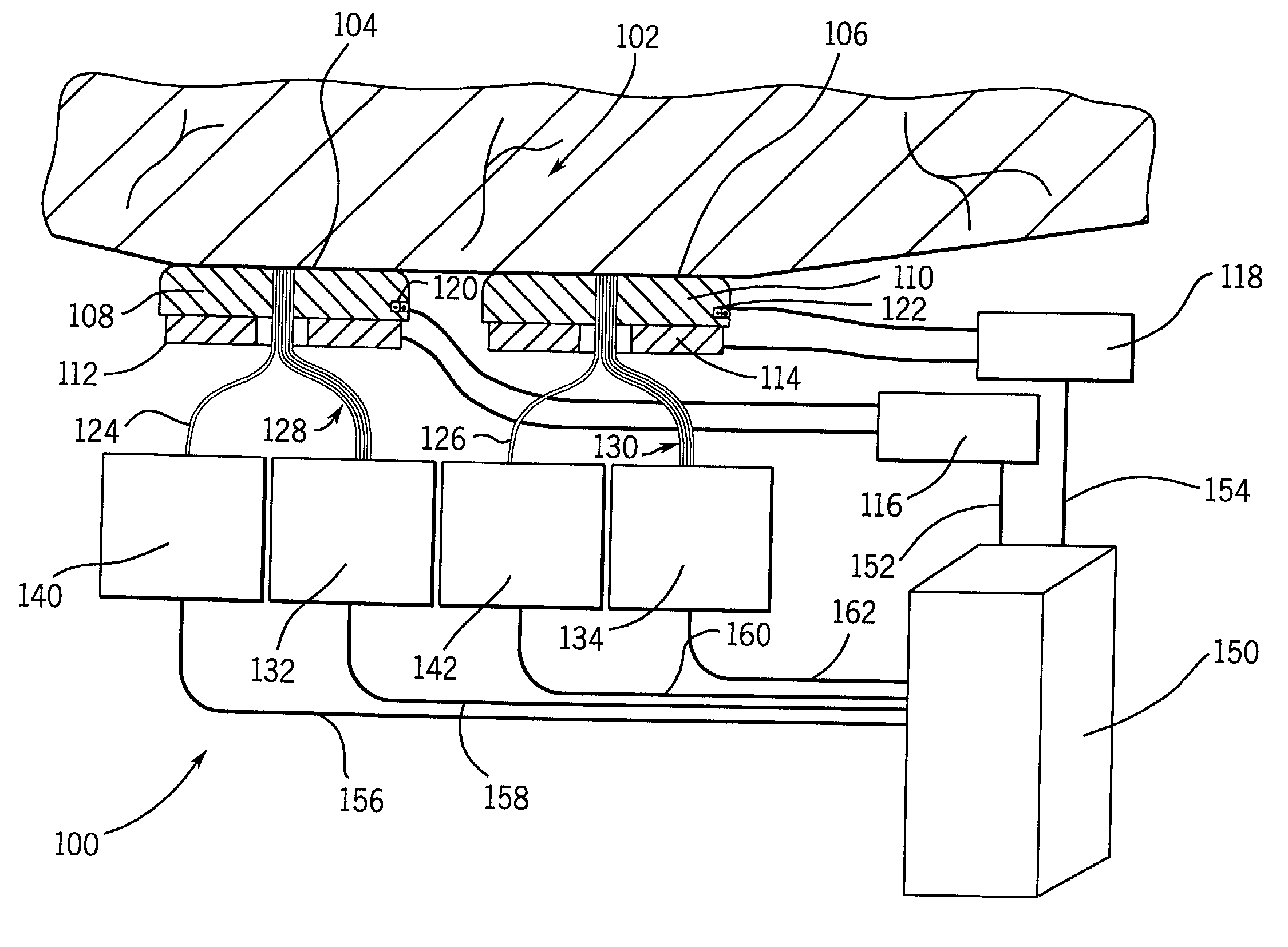
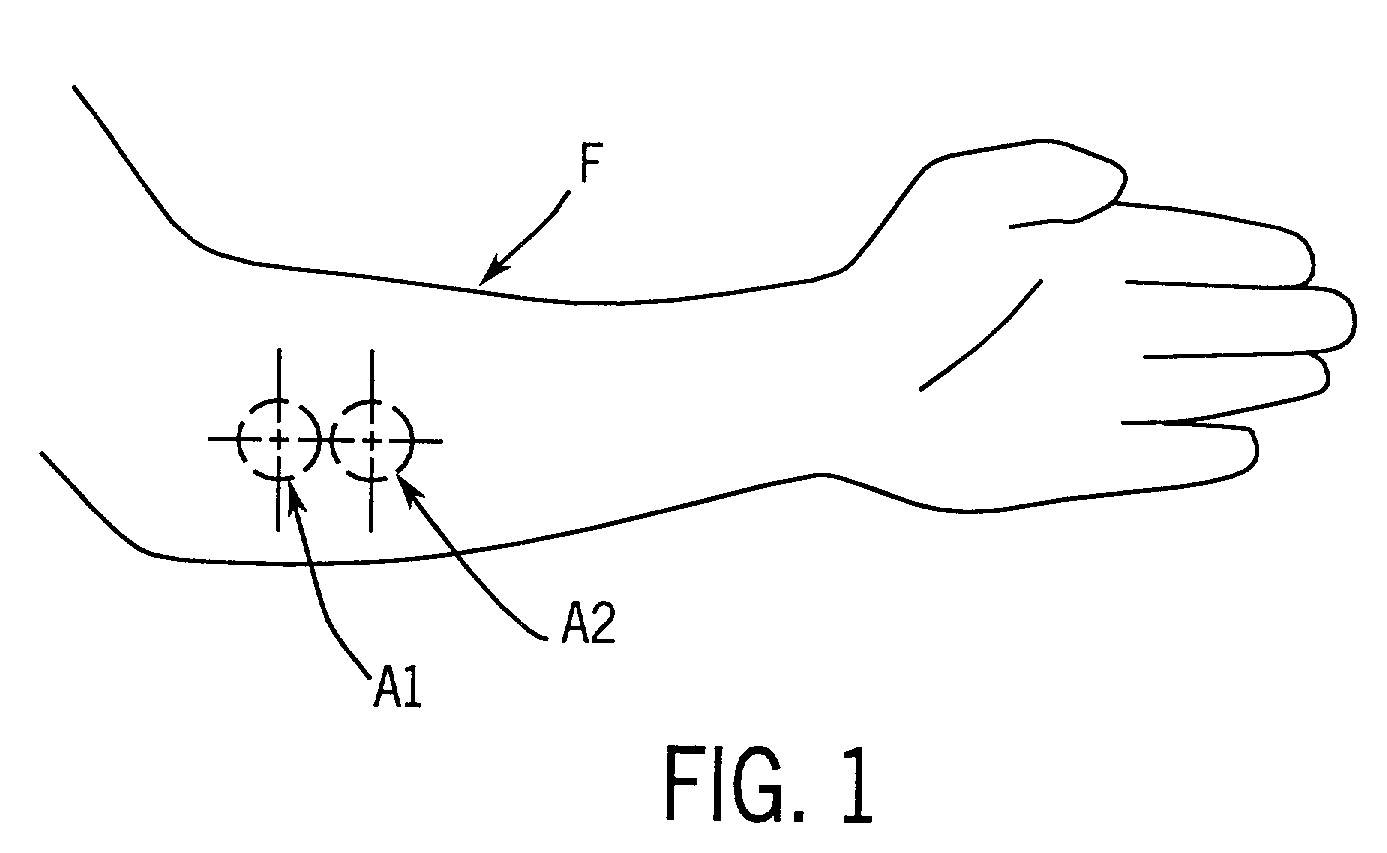
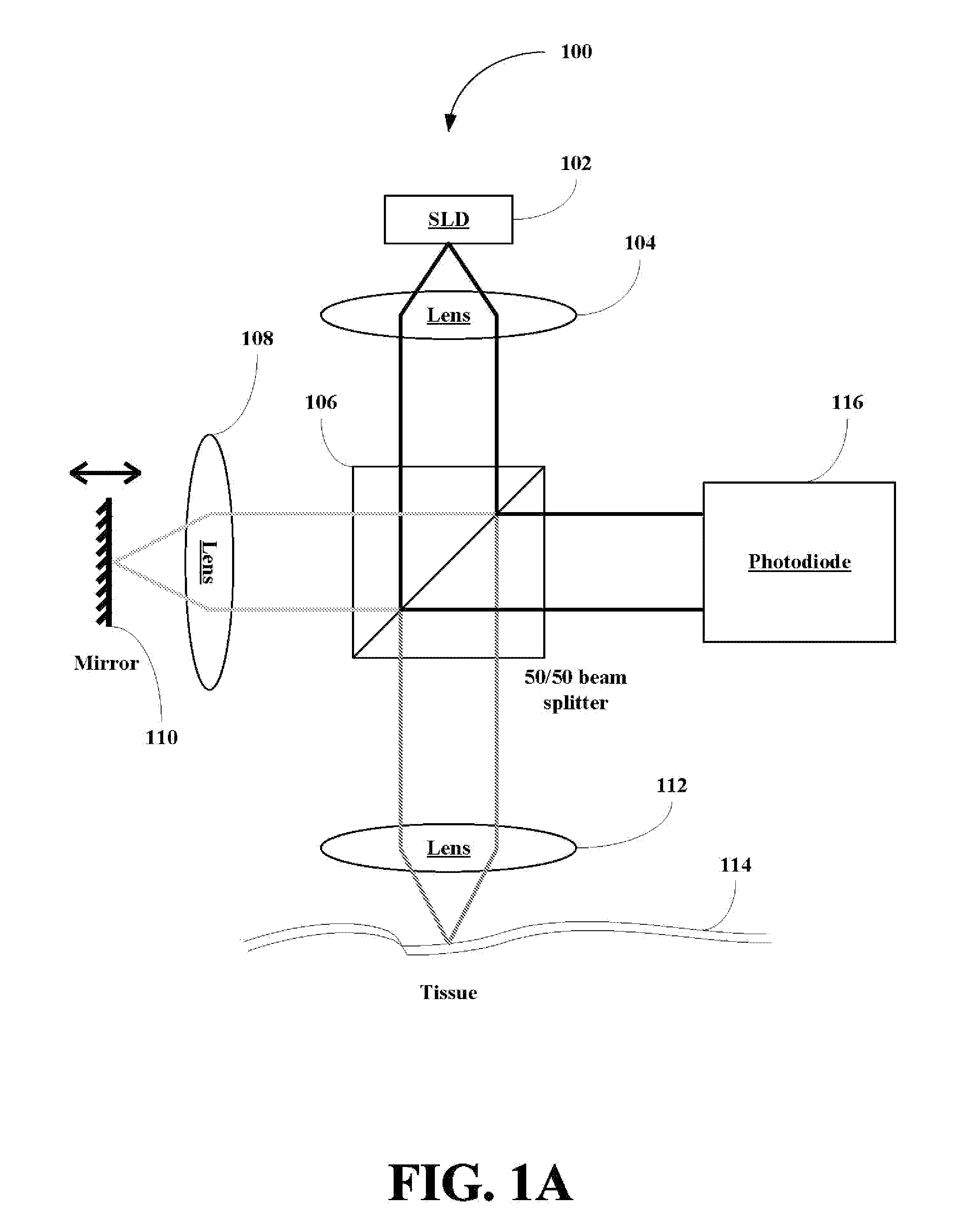
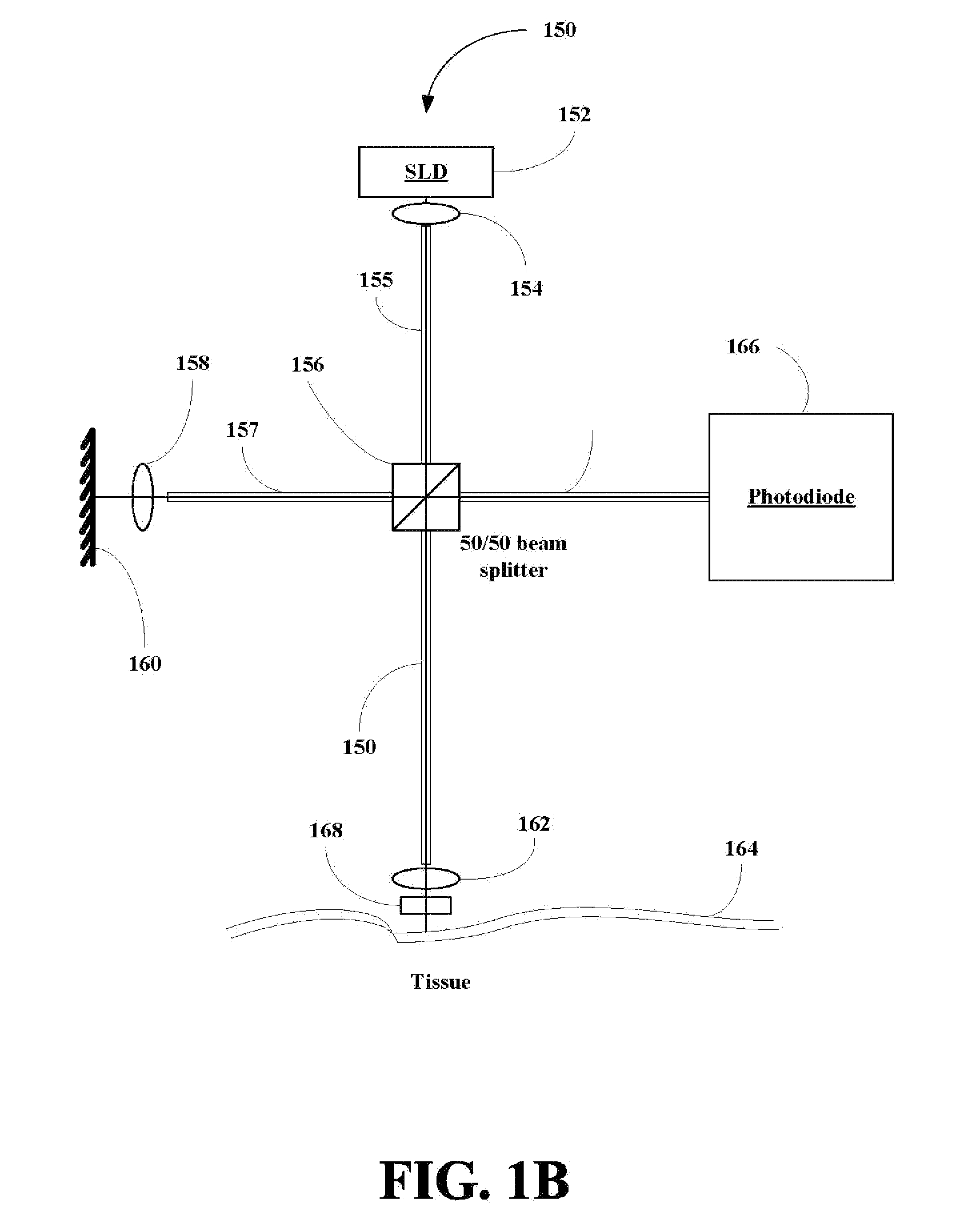
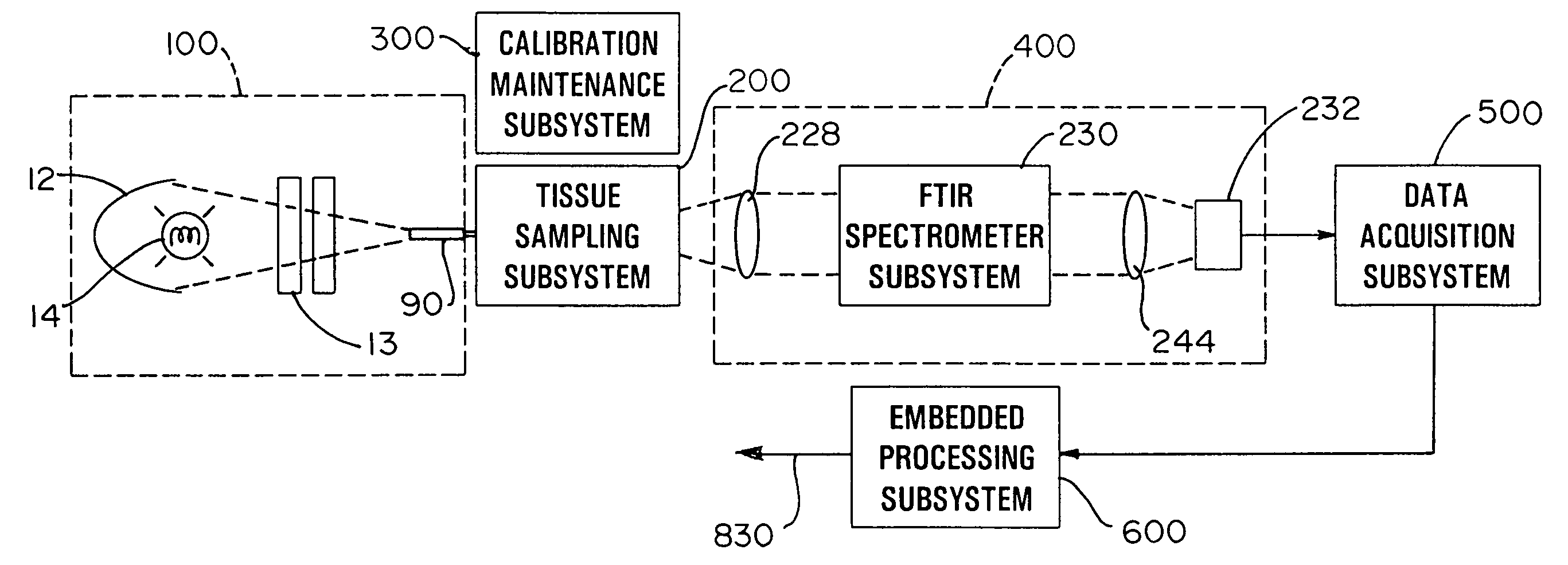
A method for collecting optical data at two morphologically similar, substantially non-overlapping, and preferably adjacent, areas on the surface of a tissue, while the temperature in each area is being maintained or modulated according to a temperature program. The optical data obtained are inserted into a mathematical relationship, e.g., an algorithm, that can be used to predict a disease state (such as the diabetes mellitus disease state) or the concentration of an analyte for indicating a physical condition (such as blood glucose level). This invention can be used to differentiate between disease status, such as, for example, diabetic and non-diabetic. The method involves the generation of a calibration (or training) set that utilizes the relationship between optical signals emanating from the skin under different thermal stimuli and disease status, e.g., diabetic status, established clinically. This calibration set can be used to predict the disease state of other subjects. Structural changes, as well as circulatory changes, due to a disease state are determined at two morphologically similar, but substantially non-overlapping areas on the surface of human tissue, e.g., the skin of a forearm, with each area being subjected to different temperature modulation programs. In addition to determination of a disease state, this invention can also be used to determine the concentration of an analyte in the tissues. This invention also provides an apparatus for the determination of a disease state, such as diabetes, or concentration of an analyte, such as blood glucose level, by the method of this invention.
Owner:ABBOTT DIABETES CARE INC
Continuous noninvasive glucose monitoring in diabetic, non-diabetic, and critically ill patients with oct
InactiveUS20070232872A1Efficient removalIncrease contrastDiagnostic recording/measuringSensorsCritically illAnalyte
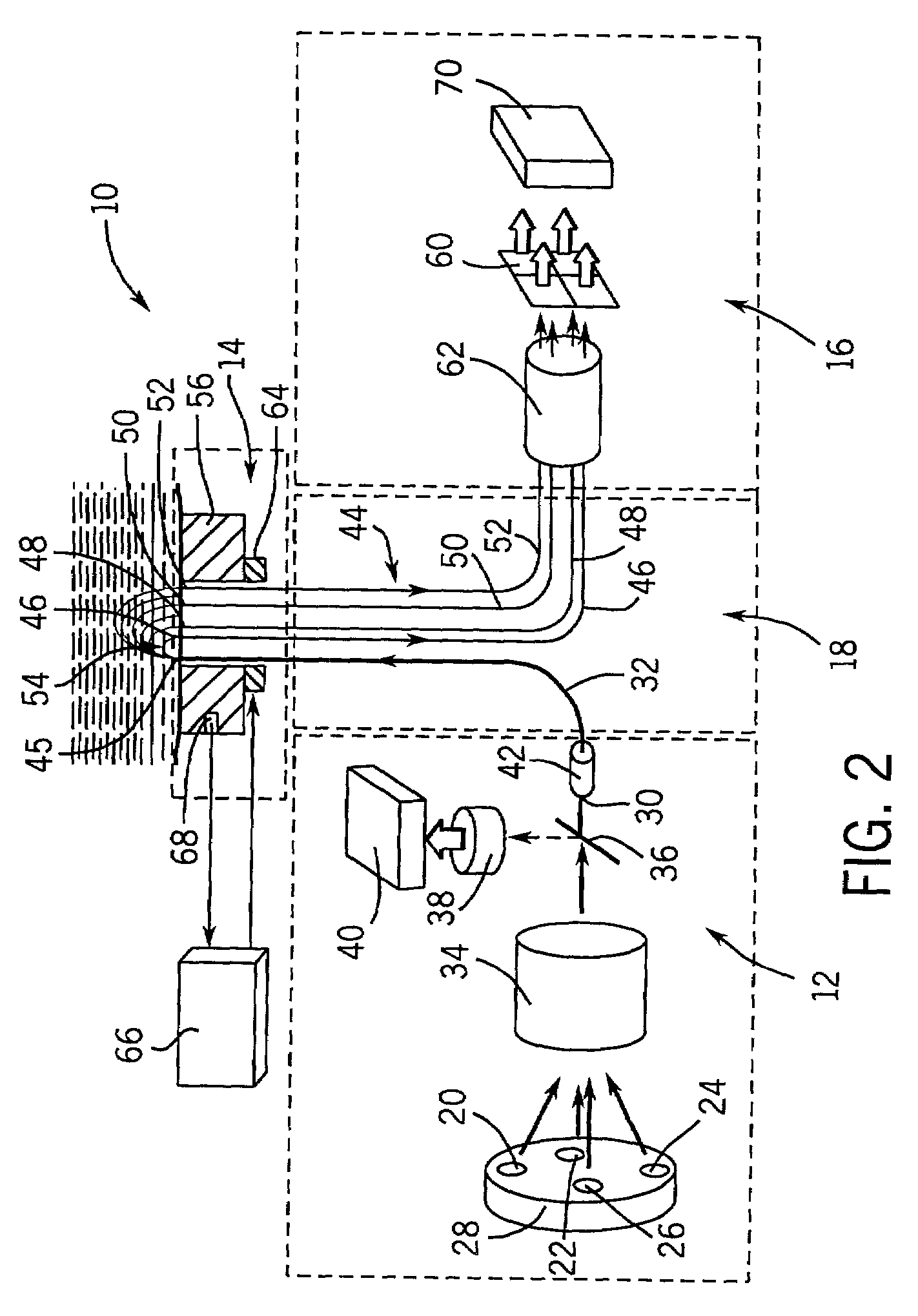
New optical coherence tomography (OCT) techniques are disclosed which are designed to improve OCT glucose concentration measure accuracy and are capable of being performed on a continuous basis. New multi-wavelength optical coherence tomography (OCT) techniques are also disclosed and designed to reduce artifacts do to water. New optical coherence tomography (OCT) techniques are also disclosed for determining local profusion rates, local analyte transport rates and tissue analyte transport rates as a measure of tissue health, disease progression and state and tissue transplantation effectiveness.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compositions and methods for treating non-alcoholic steatohepatitis
ActiveUS20150051143A1Improve steatosisImproving lobular inflammation conditionBiocidePeptide/protein ingredientsBiliary tractFatty acid
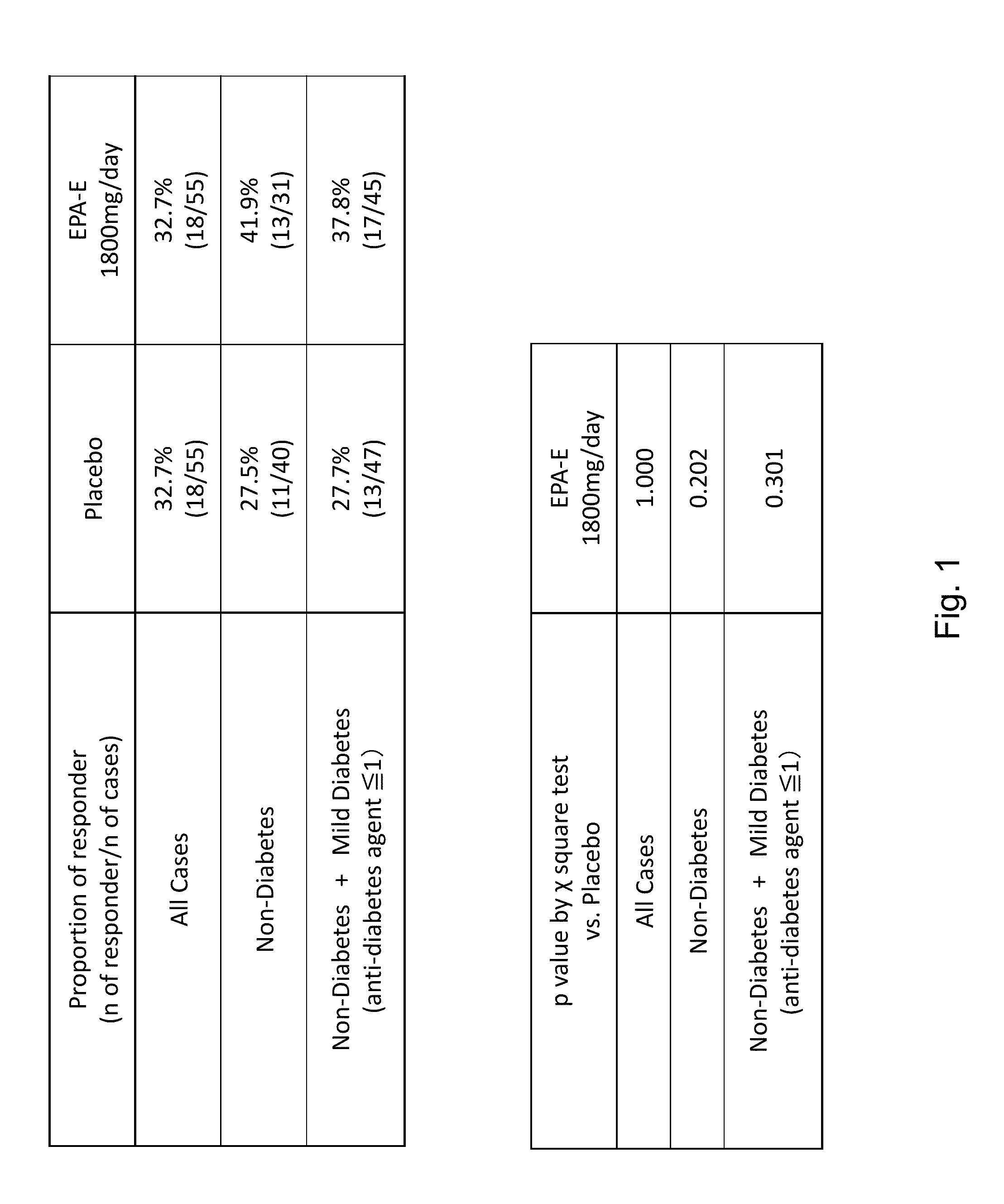
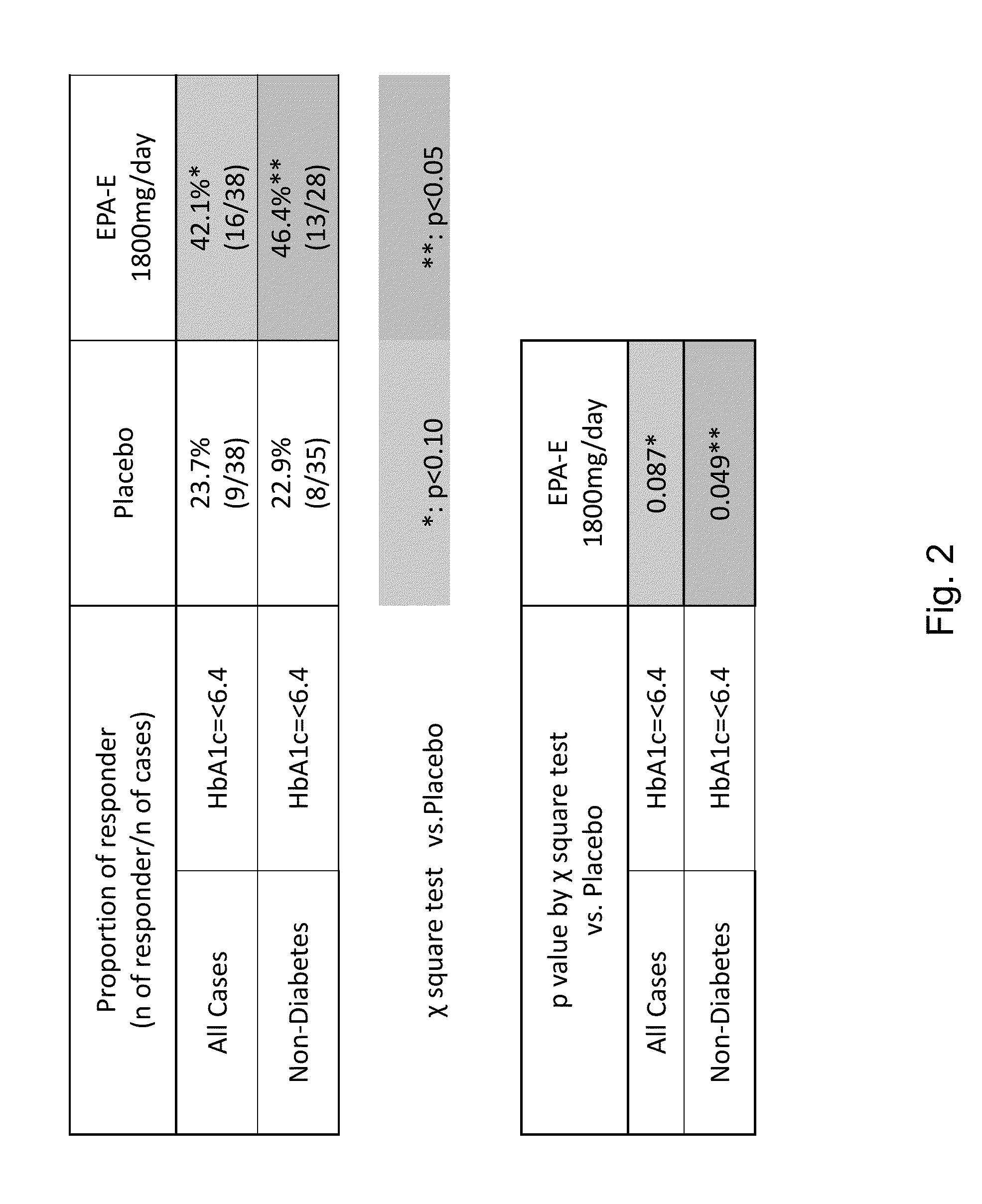
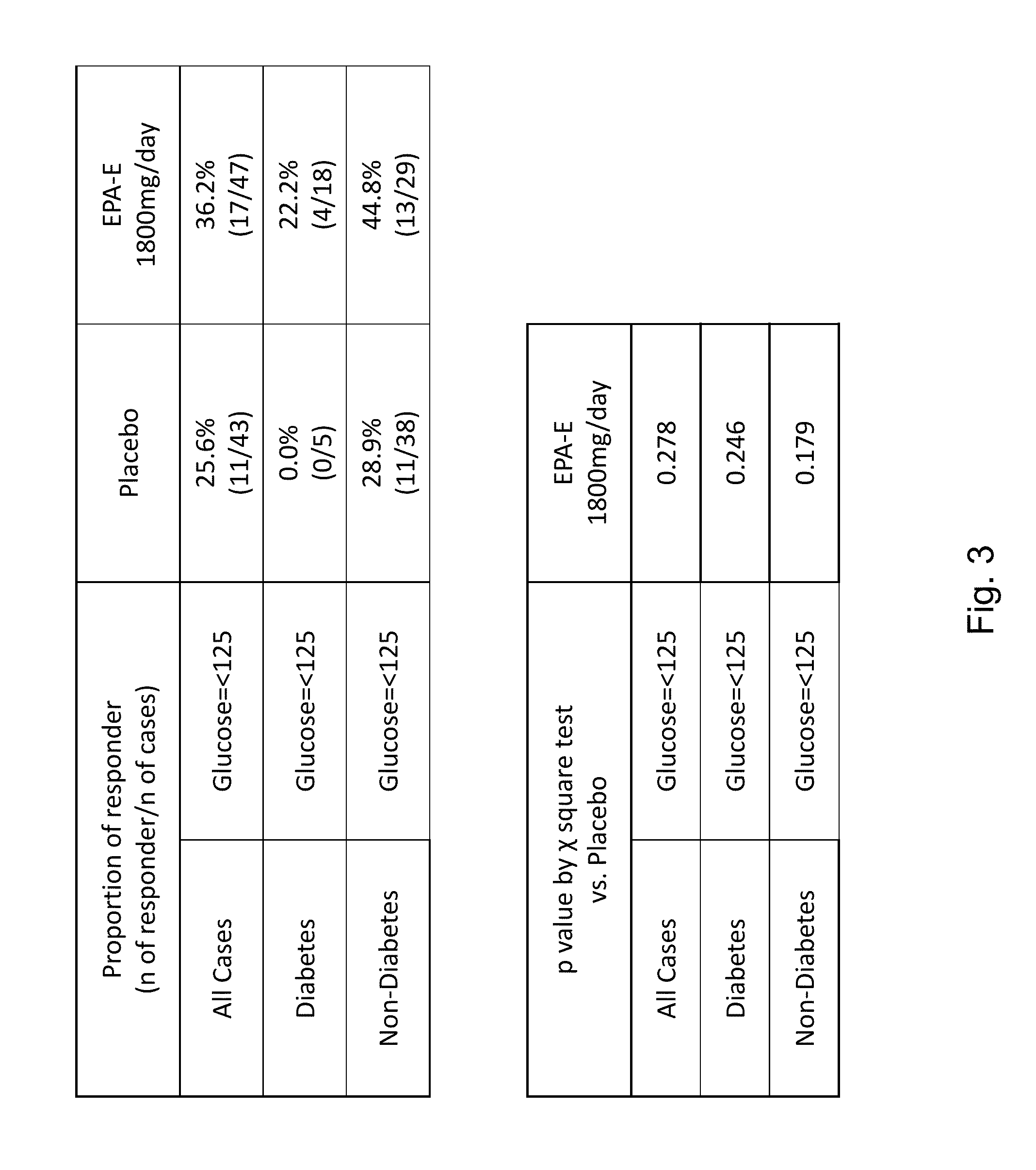
The disclosure provides for a method for treating a fatty liver disorder in a subject in need thereof, comprising selecting a subject having or suspected of having a fatty liver disease or disorder, wherein the subject is non diabetic, pre-diabetic, mildly diabetic, or has normal or substantially normal biliary tract function; and administering a therapeutically effective amount of a pharmaceutical composition comprising ethyl eicosapentanoate (EPA-E). In some cases EPA-E present may be at least 40% by weight in total of the fatty acids and their derivatives.
Owner:AMARIN PHARMA IRELAND
Apparatus and method for spectroscopic analysis of tissue to detect diabetes in an individual
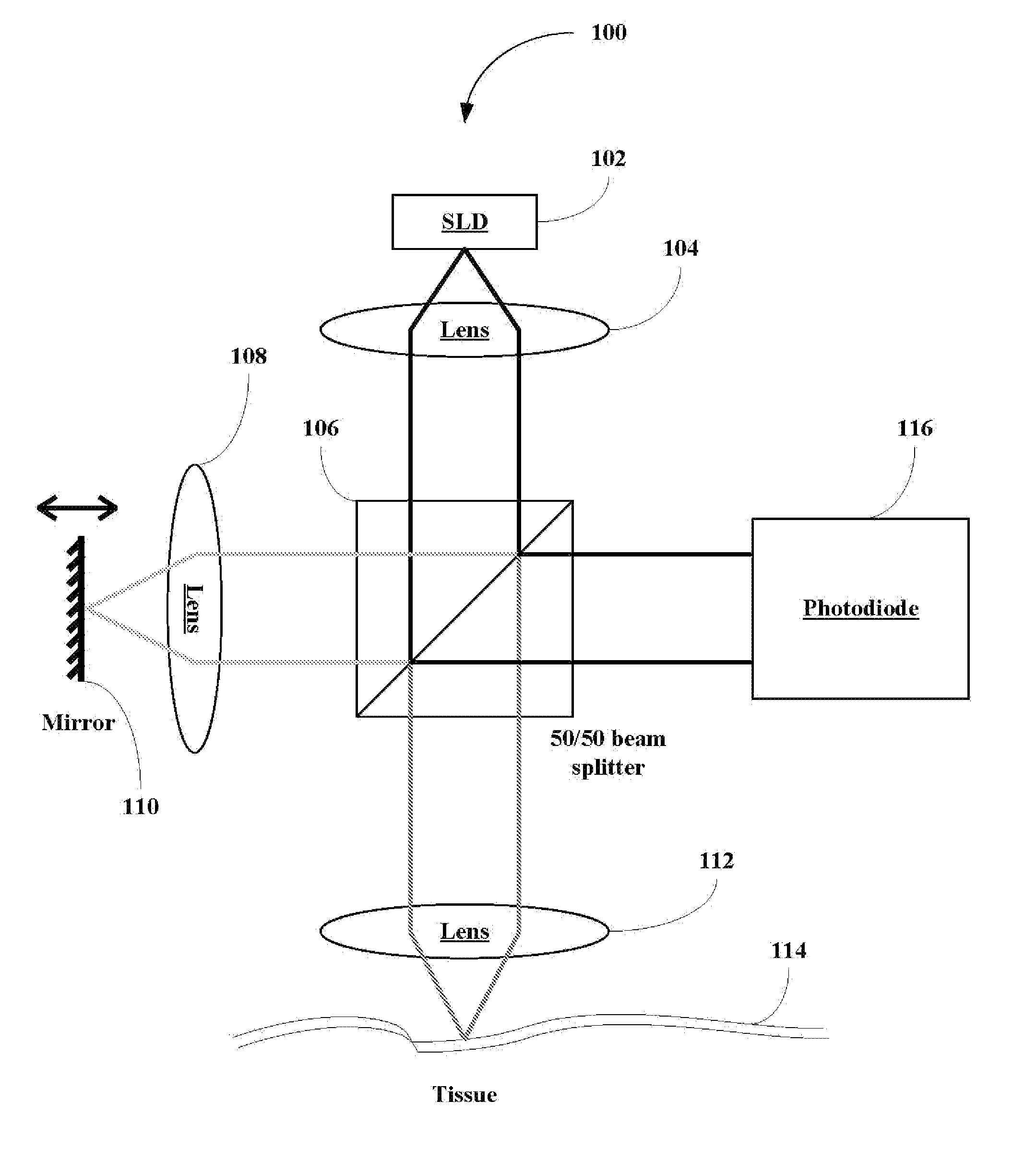
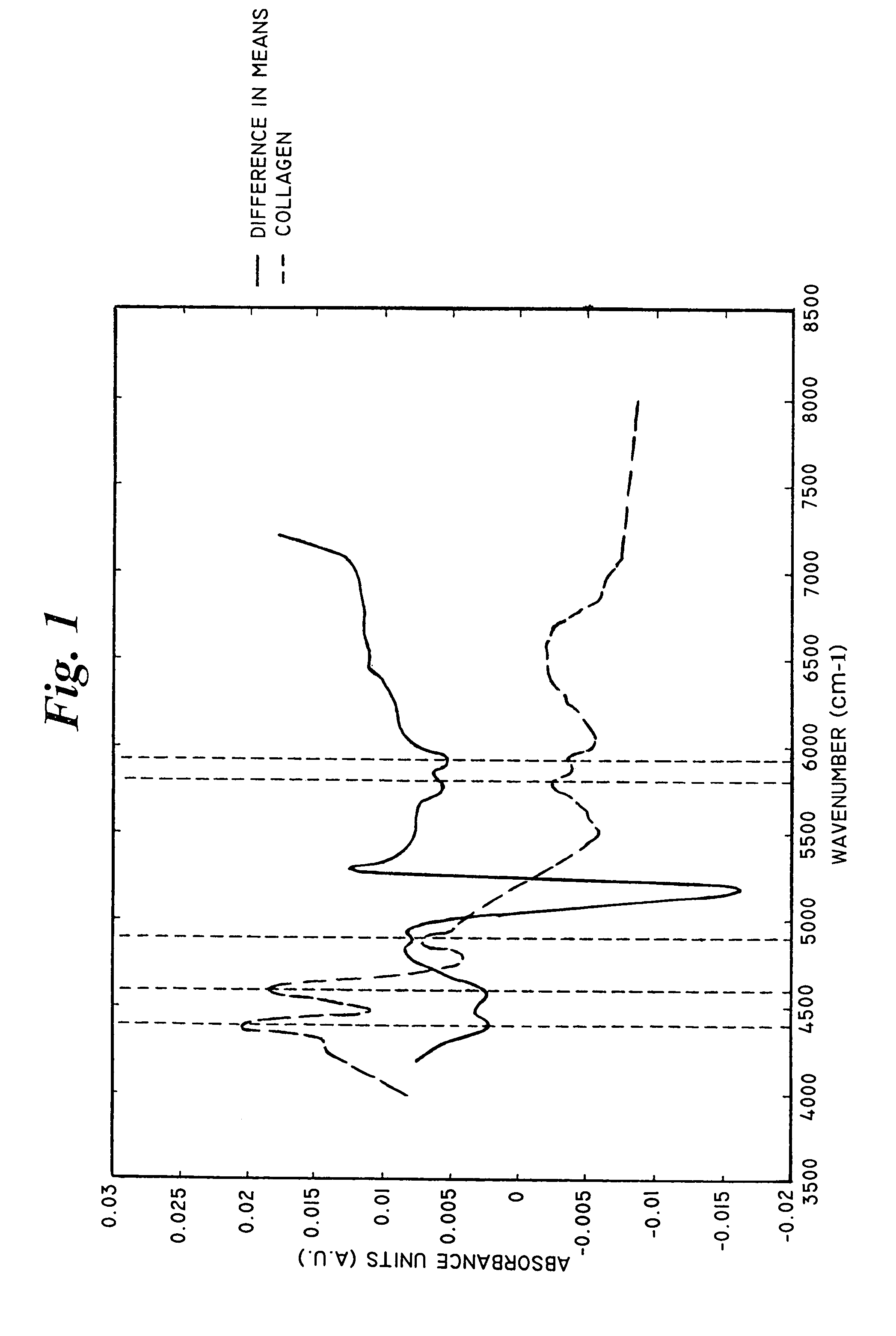
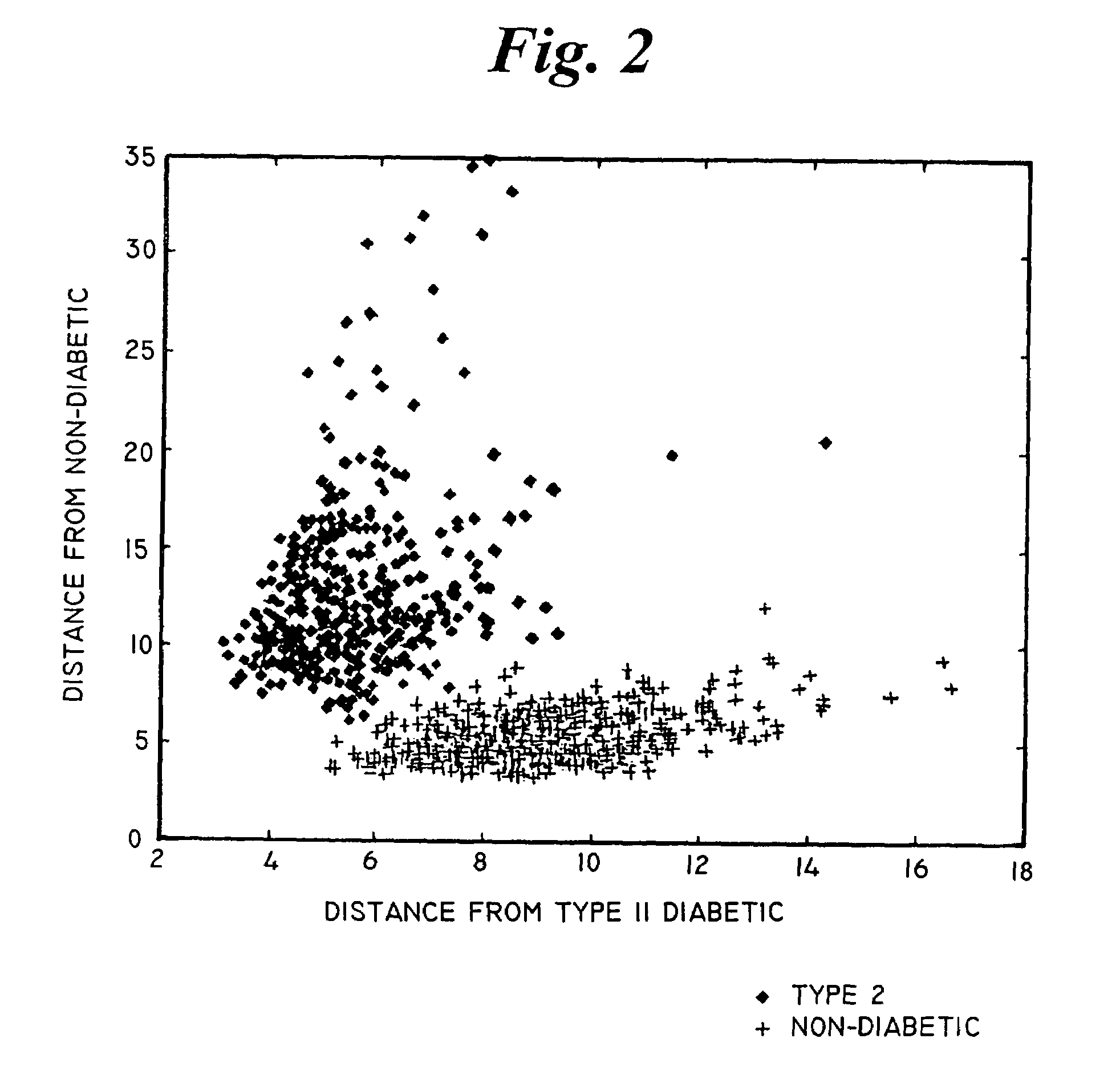
Apparatus and methods for spectroscopic analysis of human tissue to classify an individual as diabetic or non-diabetic, or to determine the probability, progression or level of diabetes in an individual. Tissue optical information of an individual, including at least a measurement of at least one wavelength or group of wavelengths indicative of glycosylated collagen content in tissue, is analyzed using multivariate techniques. The multivariate techniques include an algorithm developed from optical information from individuals having a known disease state. At least one factor in the algorithm is dependent on or a function of the measurement of the at least one wavelength or group of wavelengths indicative of glycosylated collagen content in tissue from the optical information of individuals forming the database.
Owner:VERALIGHT INC
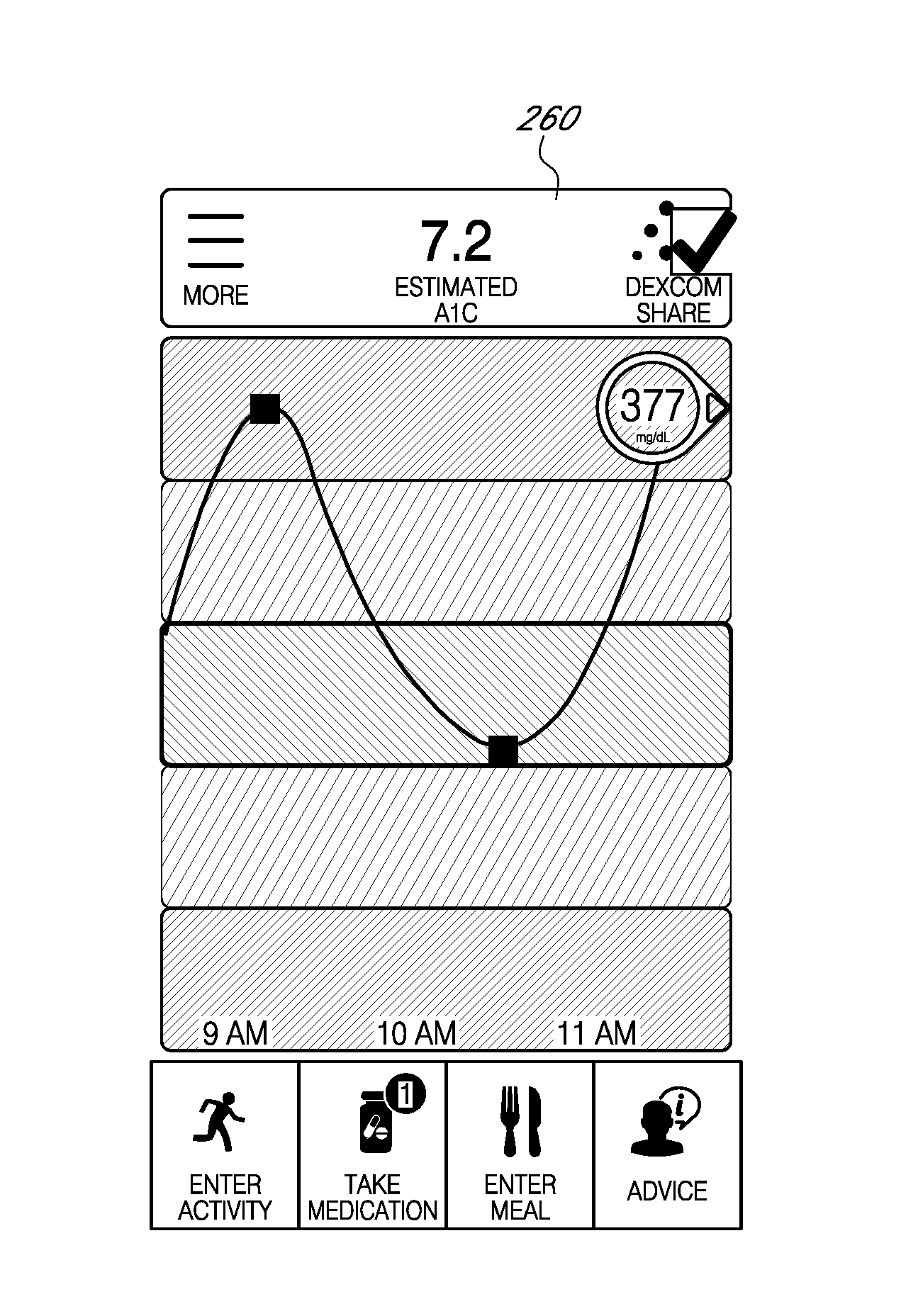
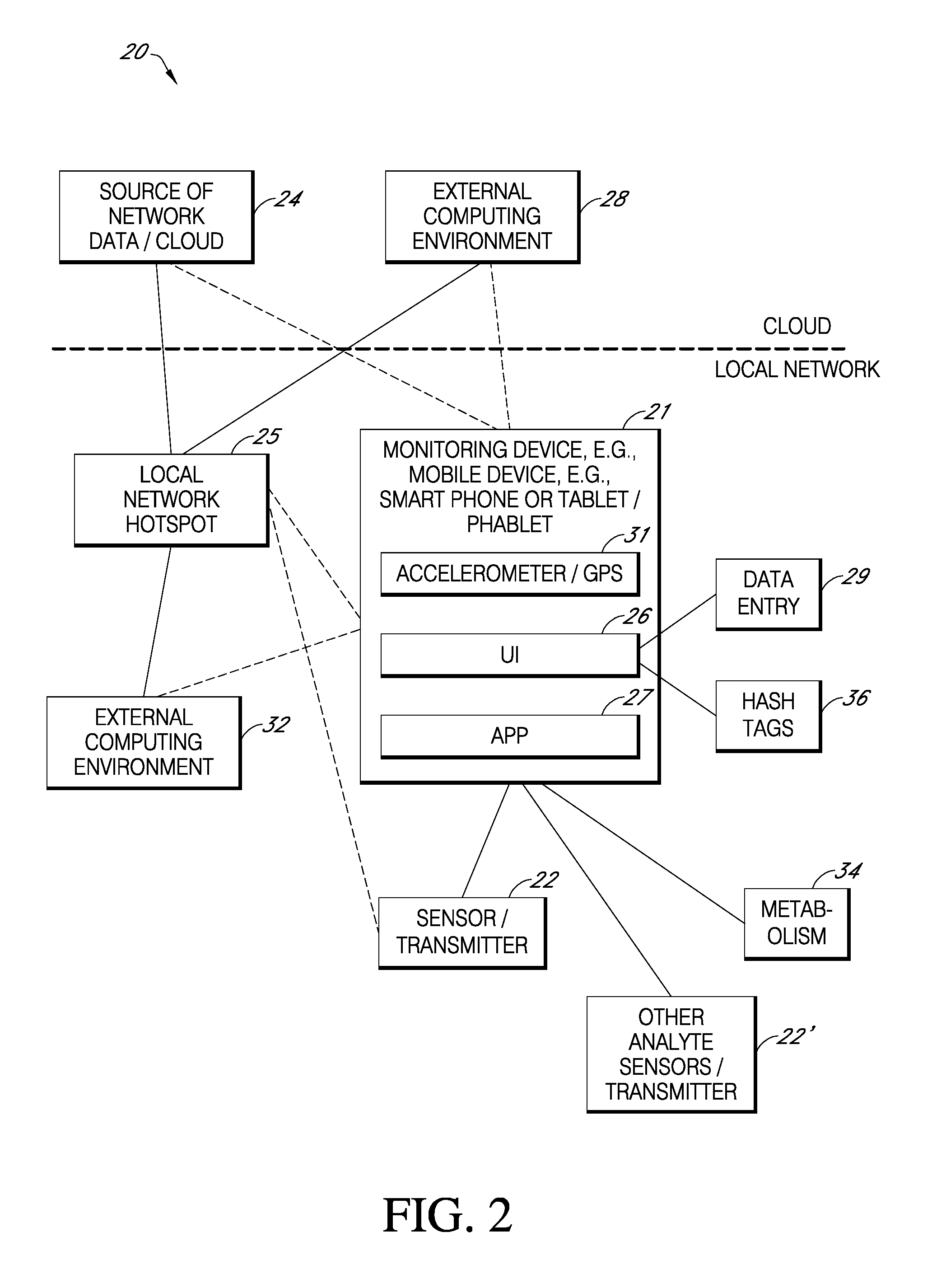
System and method for educating users, including responding to patterns
ActiveUS20160324463A1Easy to detectBenefit their healthPhysical therapies and activitiesMechanical/radiation/invasive therapiesHabitNon diabetic
Provided are systems and methods using which users may learn and become familiar with the effects of various aspects of their lifestyle on their health, e.g., users may learn about how food and / or exercise affects their glucose level and other physiological parameters, as well as overall health. In some cases the user selects a program to try; in other cases, a computing environment embodying the system suggests programs to try, including on the basis of pattern recognition, i.e., by the computing environment determining how a user could improve a detected pattern in some way. In this way, users such as type II diabetics or even users who are only prediabetic or non-diabetic may learn healthy habits to benefit their health.
Owner:DEXCOM
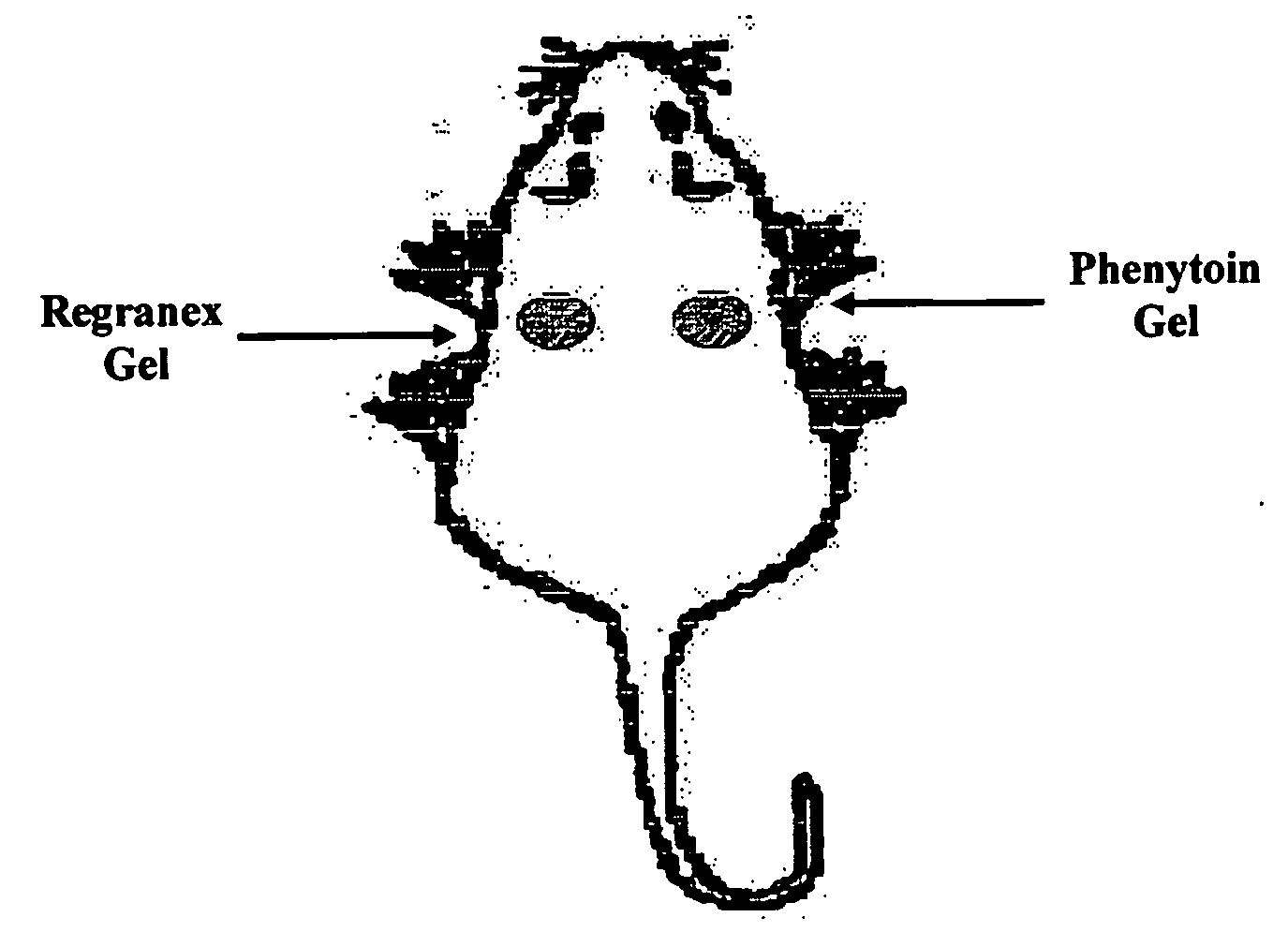
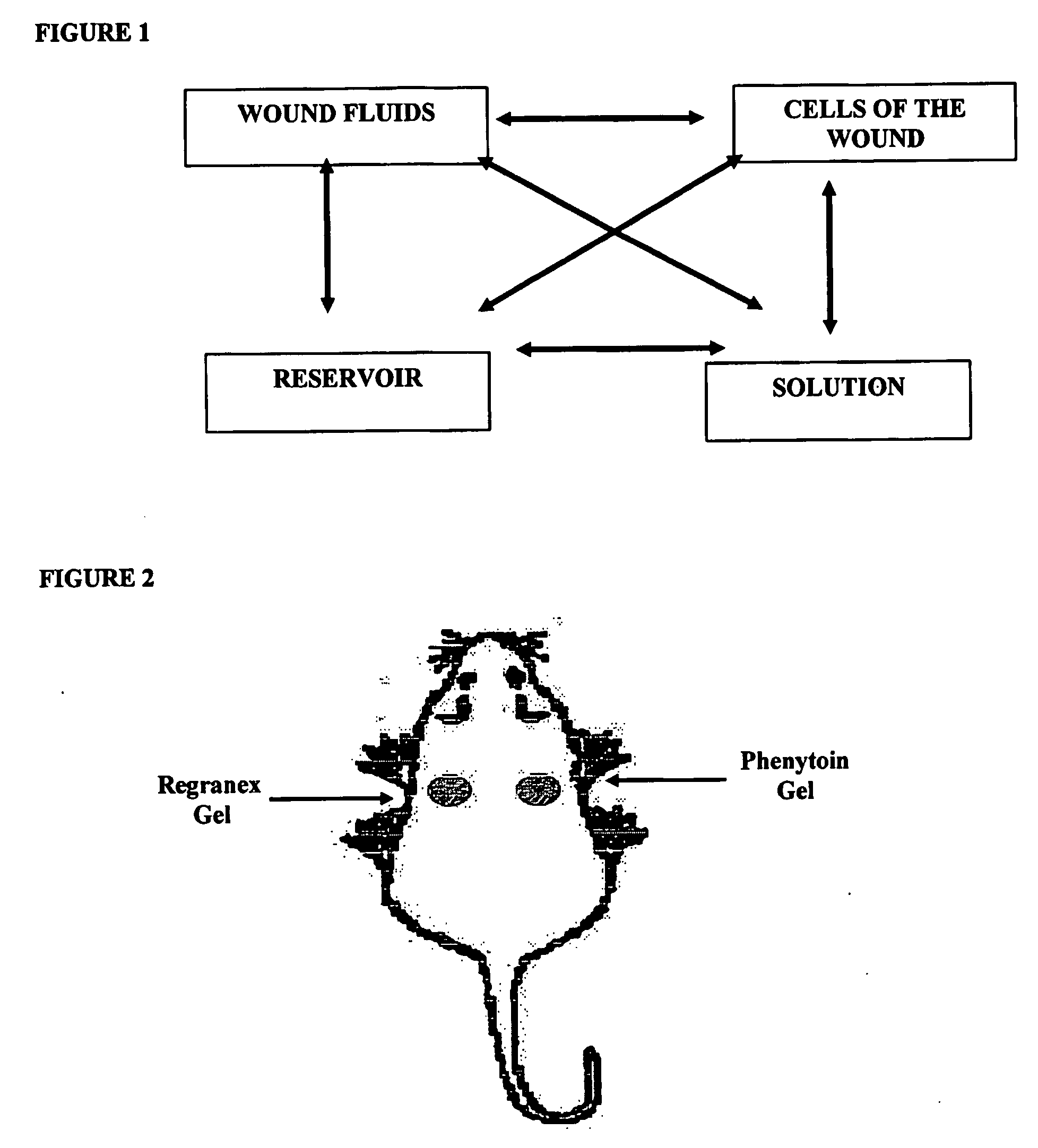
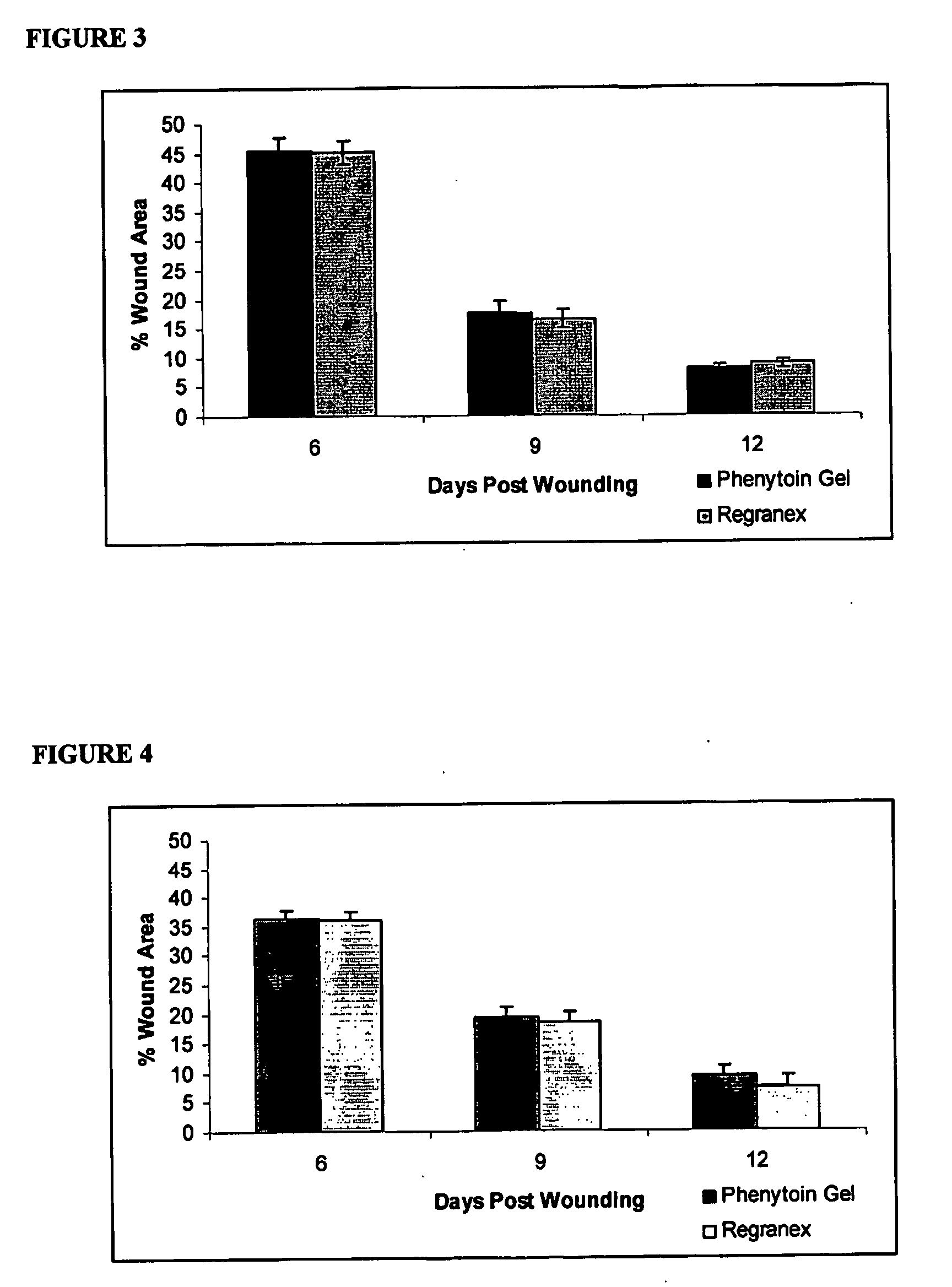
Phenytoin Formulations, and Uses Thereof in Wound Healing
InactiveUS20090022779A1Promote wound healingCut woundOrganic active ingredientsPowder deliveryWound healingPhenylbutyrate
A formulation of phenyloin suitable for topical application to a wound comprises a reservoir of phenyloin entrapped within a stabilising matrix, and an amount of dissolved phenyloin, wherein the dissolved phenyloin is in chemical equilibrium with the phenyloin entrapped within the stabilised matrix. The stabilised matrix may comprise a gel matrix, especially a gel matrix in which the polymer of the gel forms ion-pairs with the phenyloin. Also described are methods of treating a wound in diabetic and non-diabetic patients using a formulation according to the invention.
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
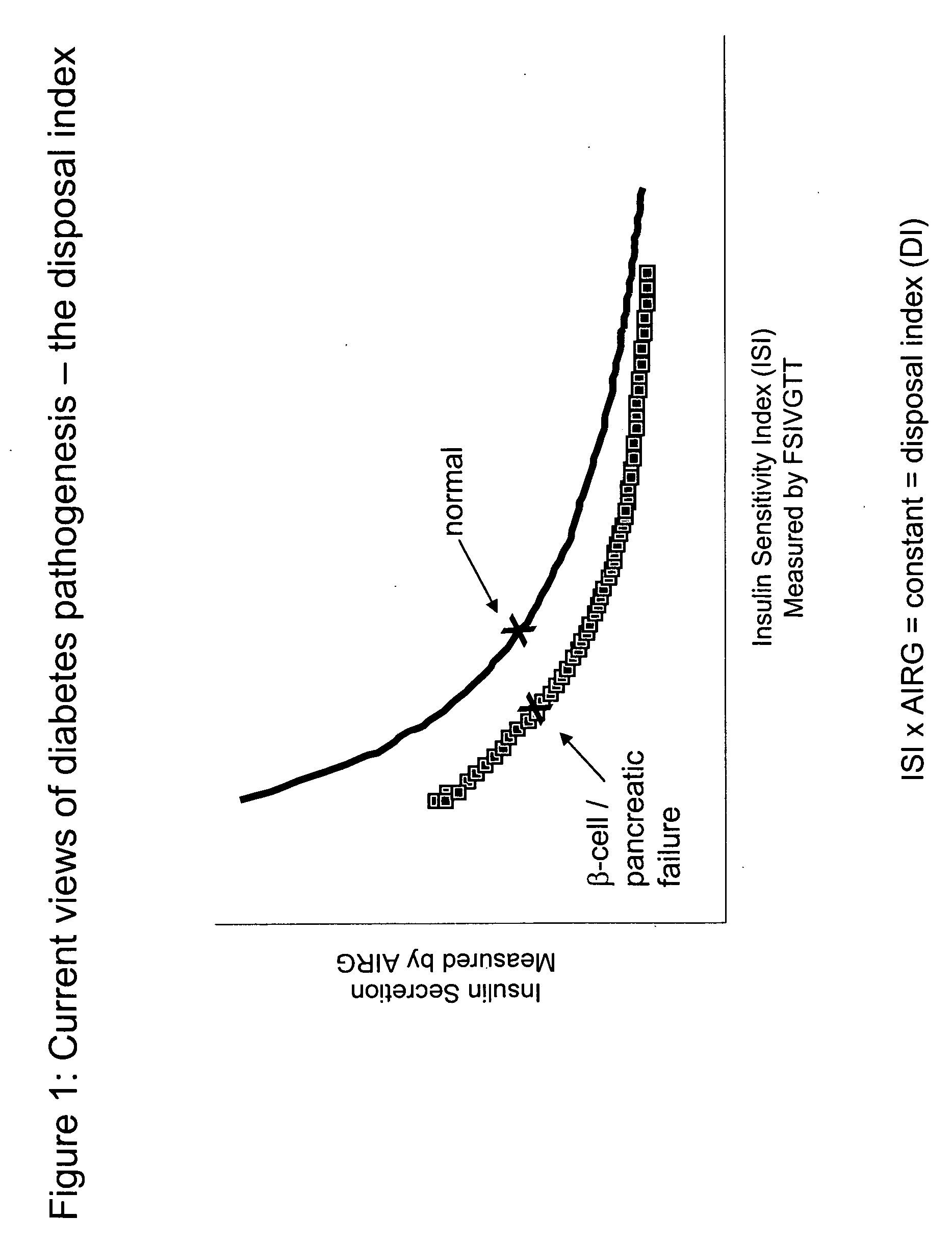
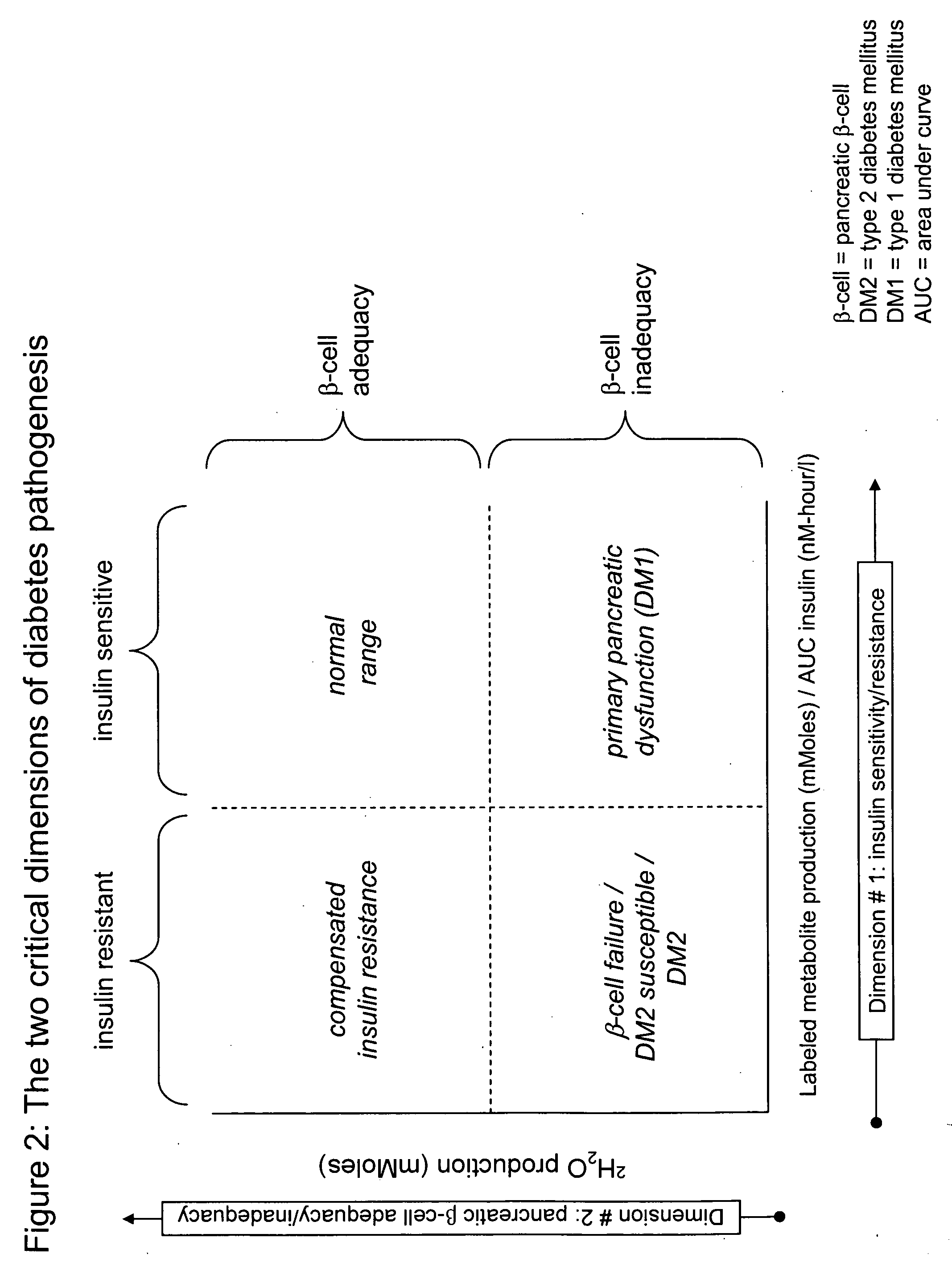
Monitoring two dimensions of diabetes pathogenesis seperately or concurrently (insulin sensitivity and beta-cell sufficiency): uses in diagnosis, prognosis, assessment of disease risk, and drug development
InactiveUS20060280682A1Improve responseReduce sensitivityCompounds screening/testingDisease diagnosisDisease riskDisease progression
Provided are methods for determining concurrently with a simple, minimally invasive test, the adequacy of pancreatic beta-cell compensation and / or the presence of tissue insulin resistance in a subject human or an experimental animal. The methods allow for the determination of a subject's or experimental animal's susceptibility to developing type 2 diabetes mellitus (DM2) or to progression to more advanced forms of DM2. Among other uses, the methods allow for diagnostic classification of subjects for decisions regarding therapeutic interventions, clinical differentiation between type 1 DM and DM2, clinical monitoring of treatments intended to reduce risk of developing DM2 in non-diabetic subjects, clinical monitoring of agents intended to improve existing DM2 and to prevent progression of DM2, clinical development and testing of new compounds, candidate agents, or candidate therapies for preventing progression to DM2 or disease progression in existing DM2, and preclinical screening of candidate agents or candidate therapies in experimental animals to identify and characterize agents having insulin-sensitizing properties, pancreatic stimulatory or regenerative properties or other desirable actions.
Owner:RGT UNIV OF CALIFORNIA
Methods for treating disorders using plant extracts
InactiveUS20050069598A1High affinityInhibit gastric emptyingAntibacterial agentsBiocideMammalFractionation
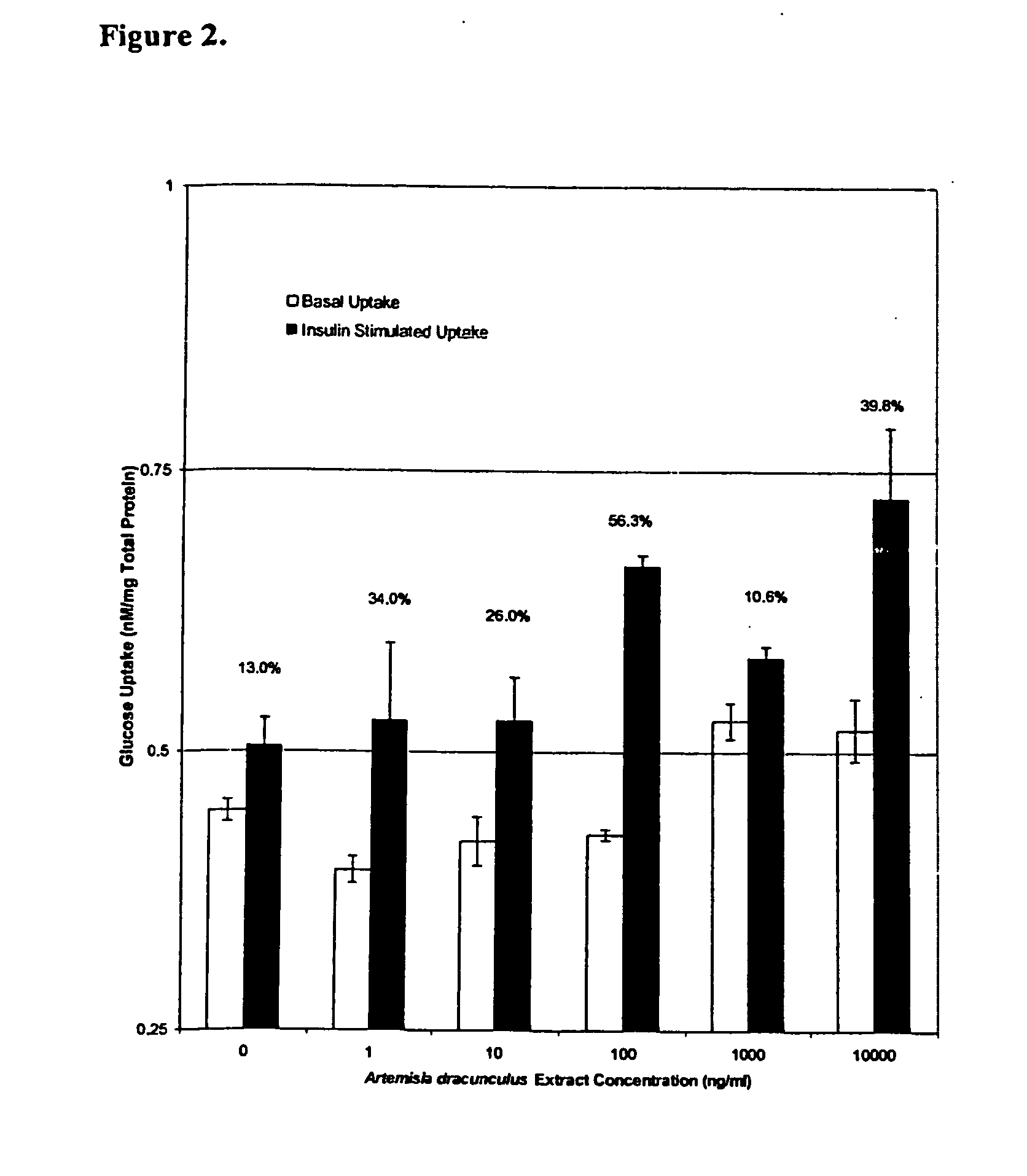
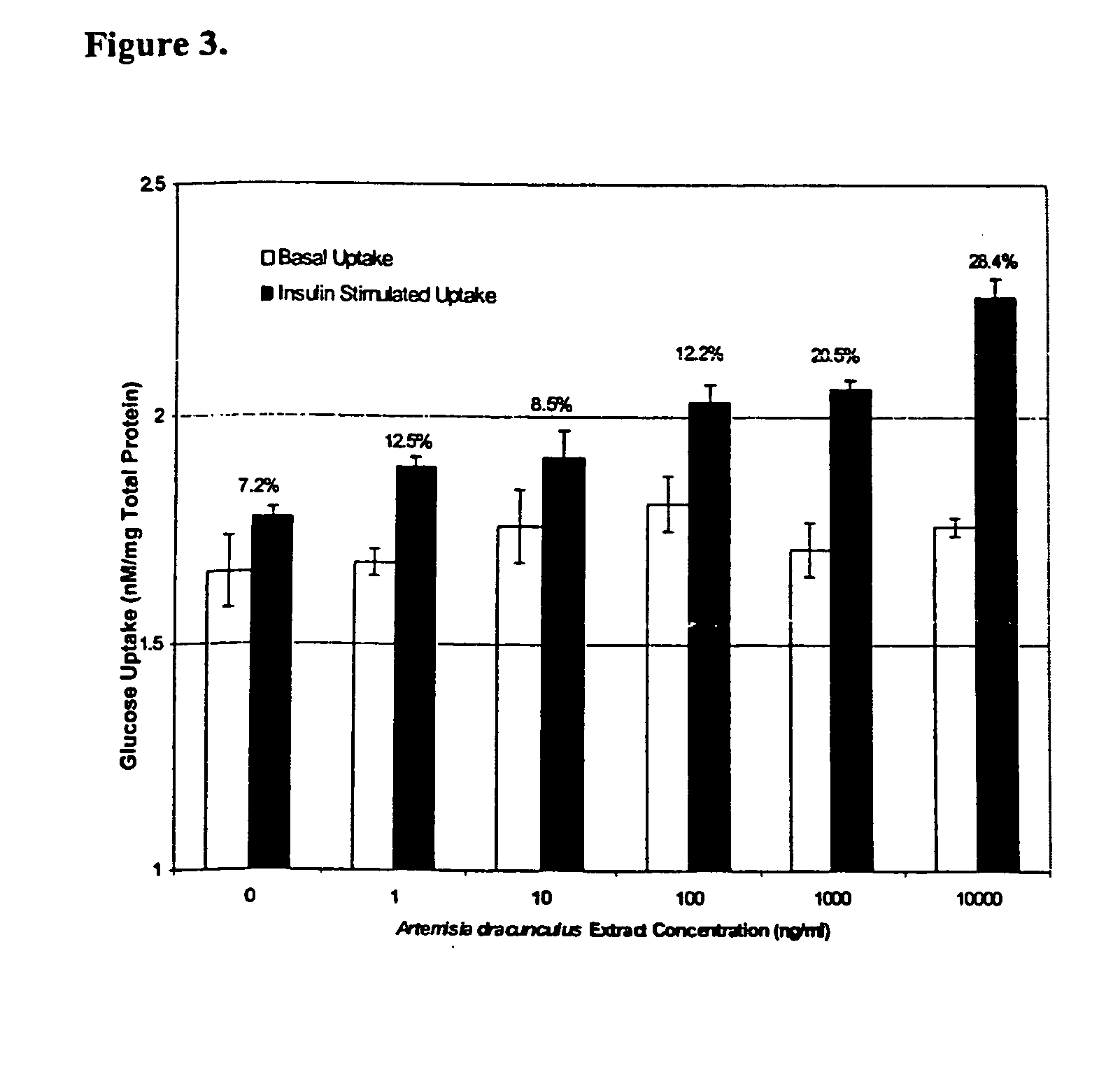
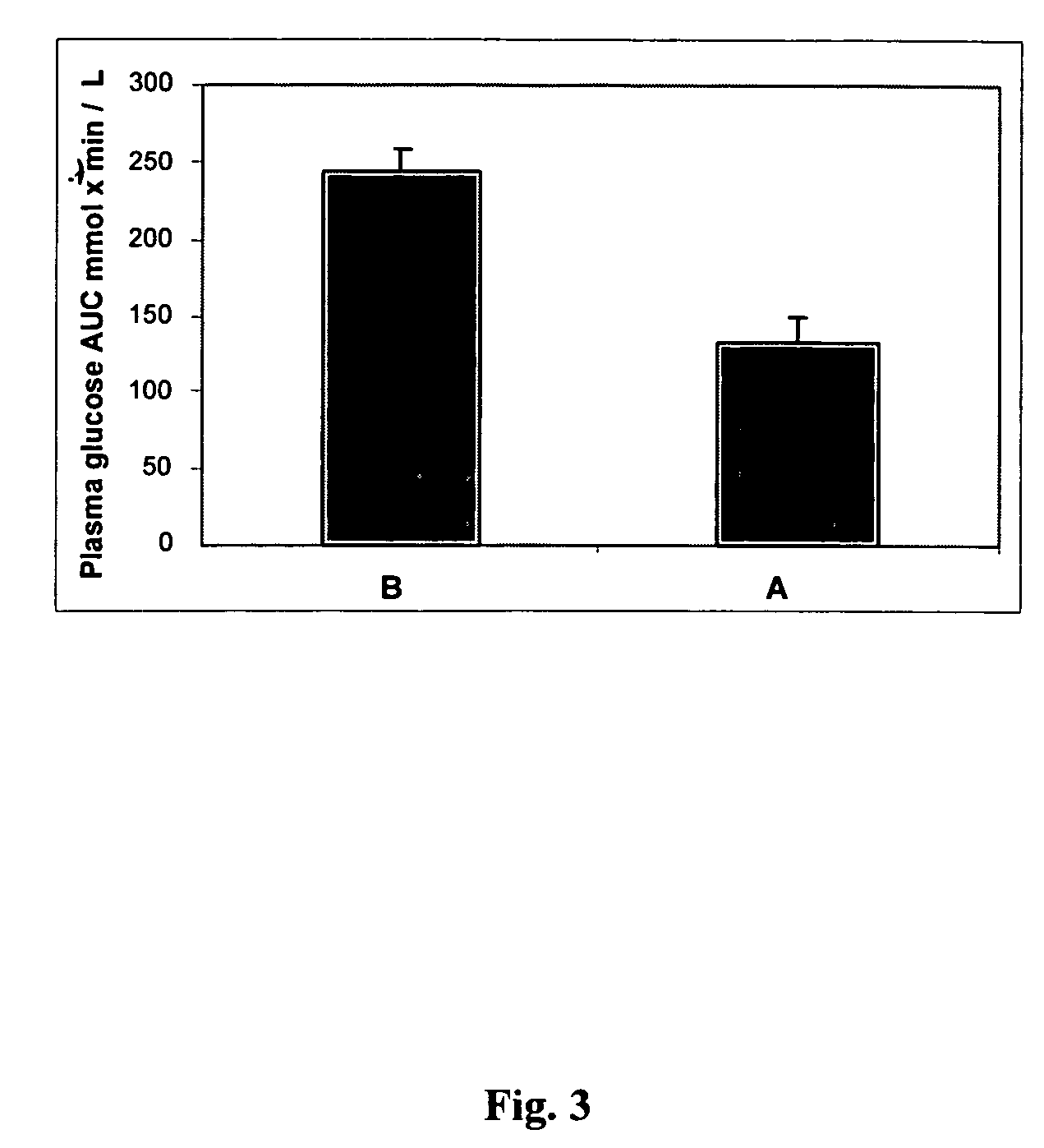
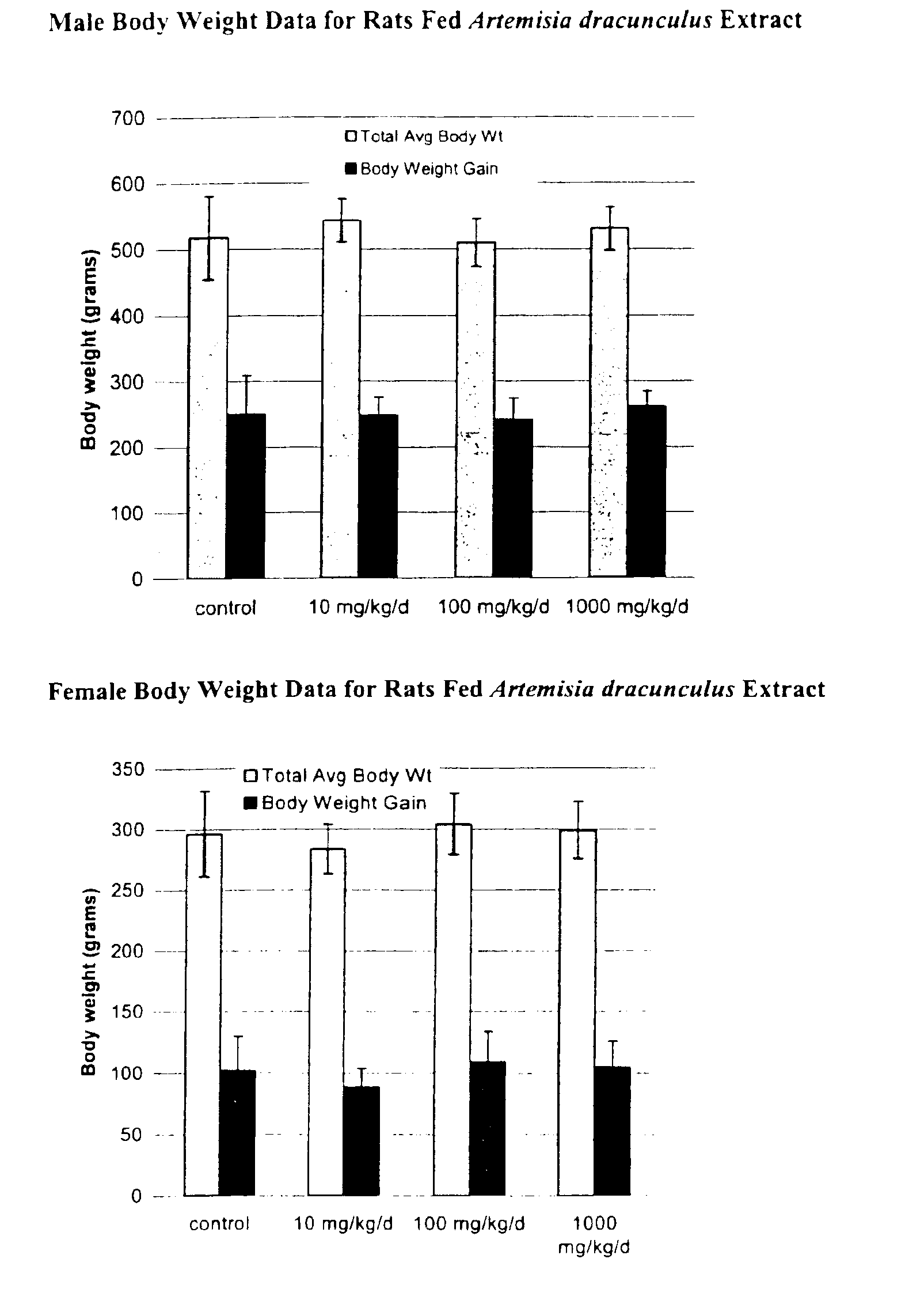
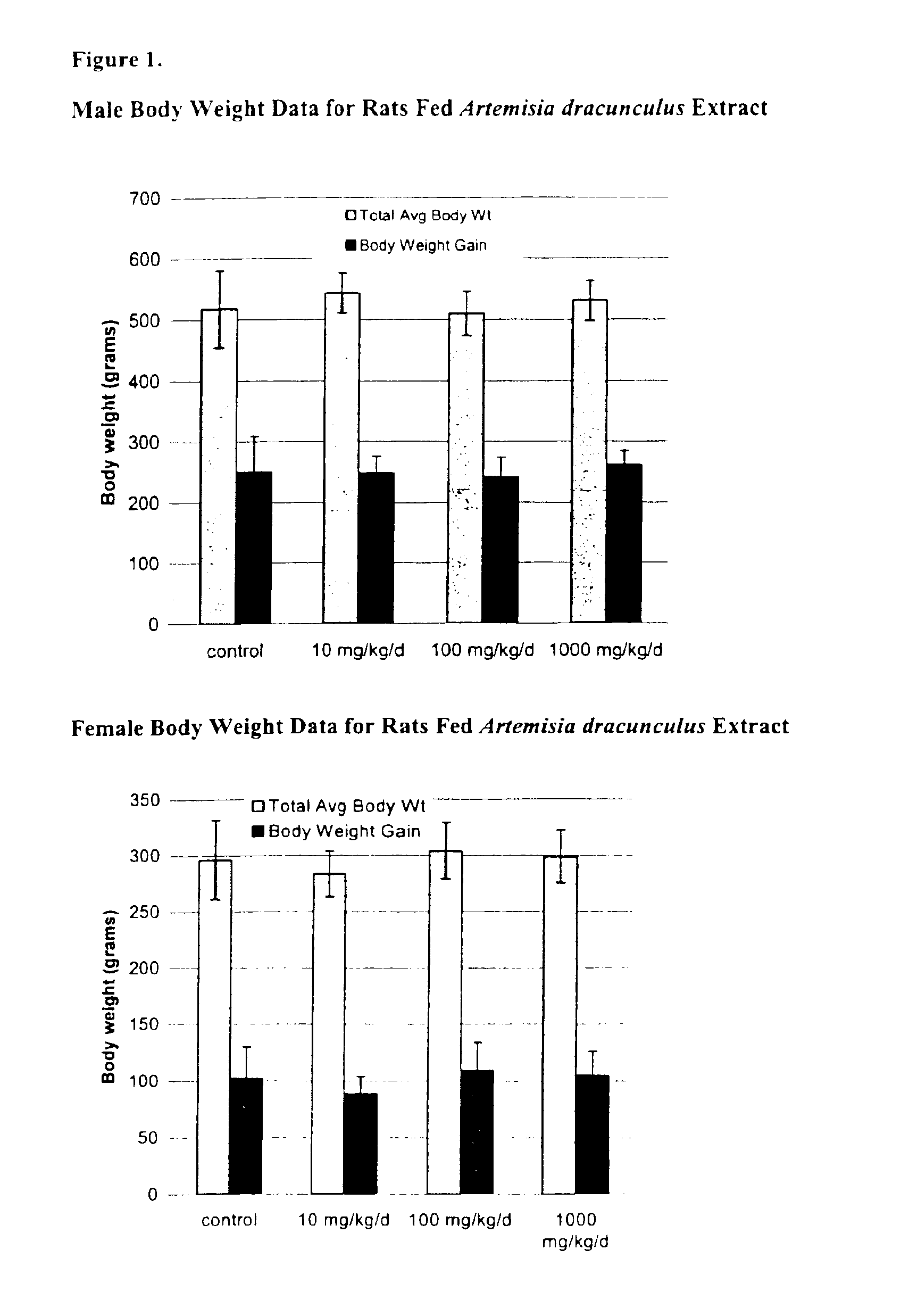
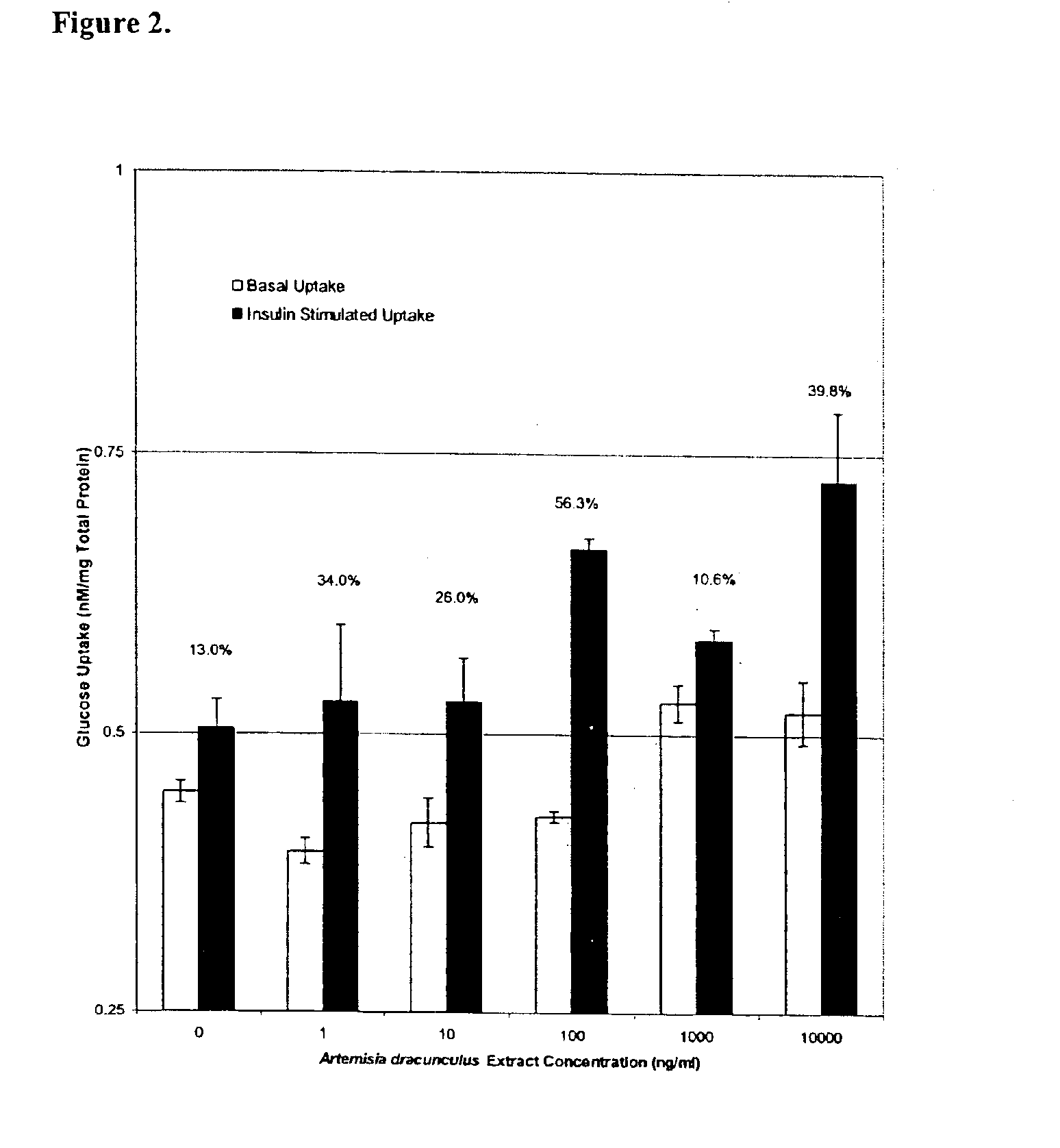
The present invention provides materials and methods relating to mildly polar fluid extracts of plant materials, such as Artemisia plant species, useful in methods for treating diabetes and methods for modulating the activity of glucagon-like peptide-1 (GLP-1), and in methods for modulating phosphoenol pyruvate carboxykinase (PEPCK) activity in a diabetes-specific manner. The extracts are generally non-toxic and non-mutagenic and may be administered to diabetics with beneficial effect on blood glucose levels. The extracts may also be administered to non-diabetics without deleterious effect. The plants are easily grown with a minimum of time, labor, and cost. Extracts are inexpensively and quickly prepared without the need for fractionation to remove potentially deleterious compounds, and the extracts may be administered to mammals such as humans through various routes, in a variety of forms, and at convenient concentrations.
Owner:RIBNICKY DAVID M +1
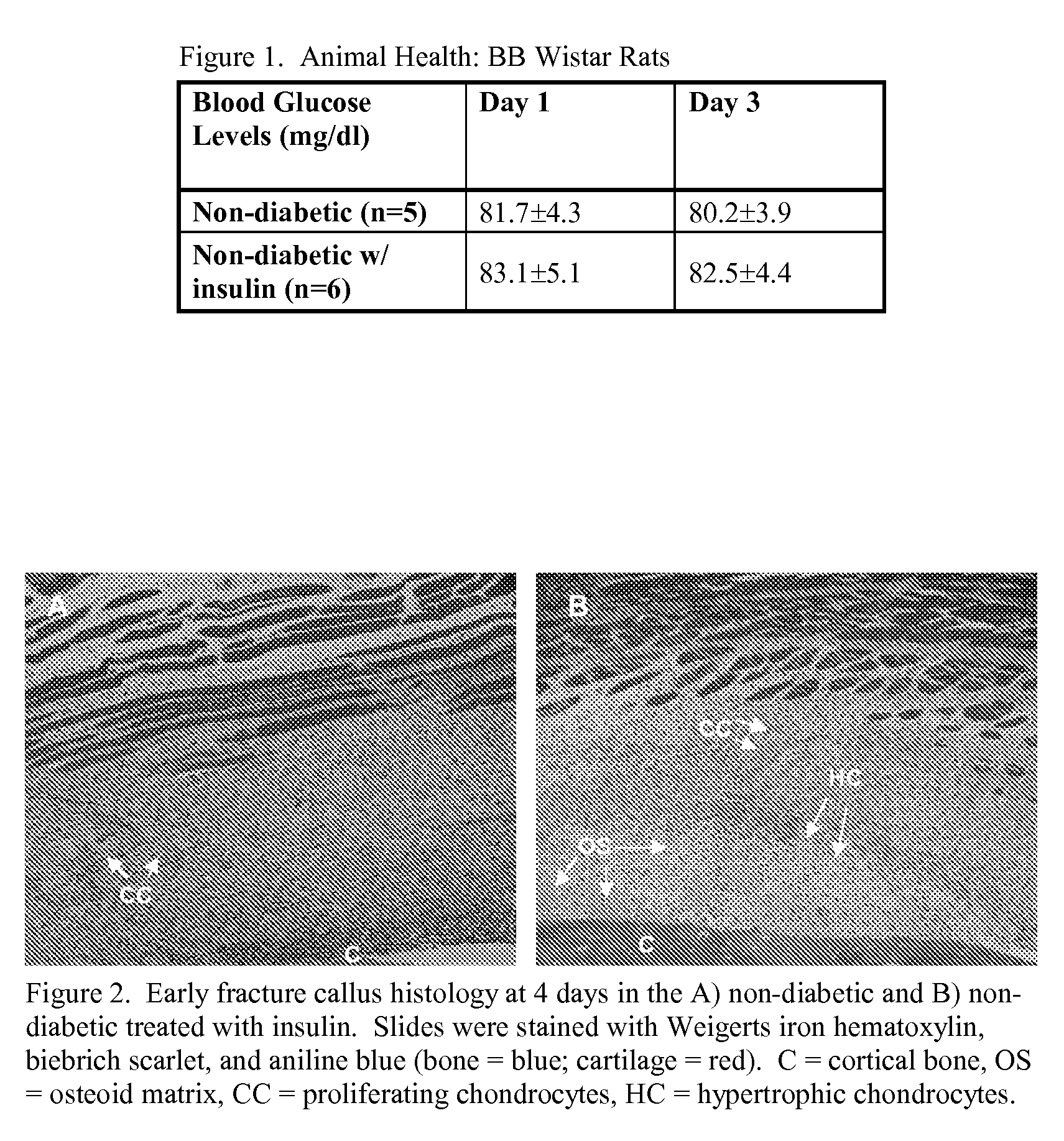
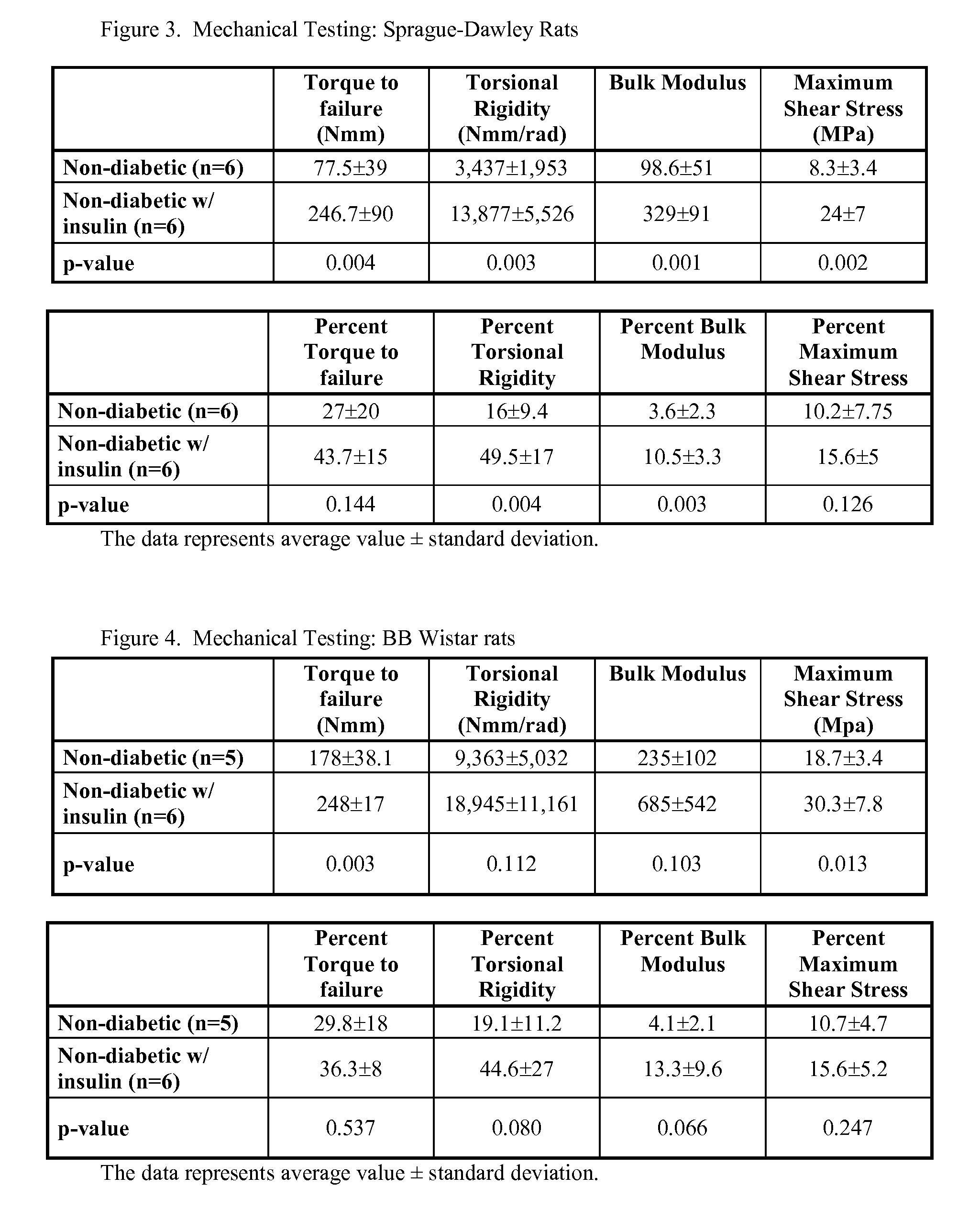
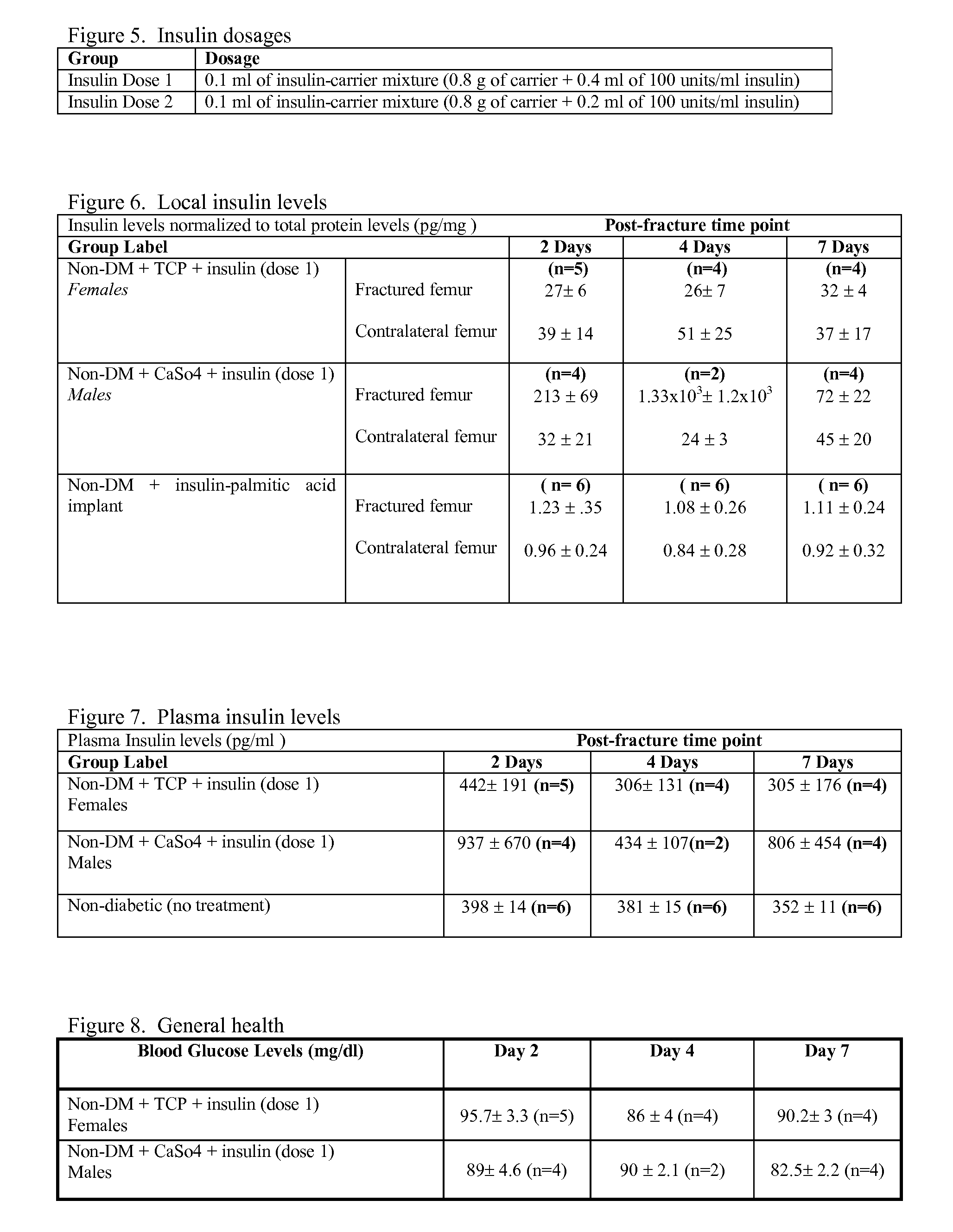
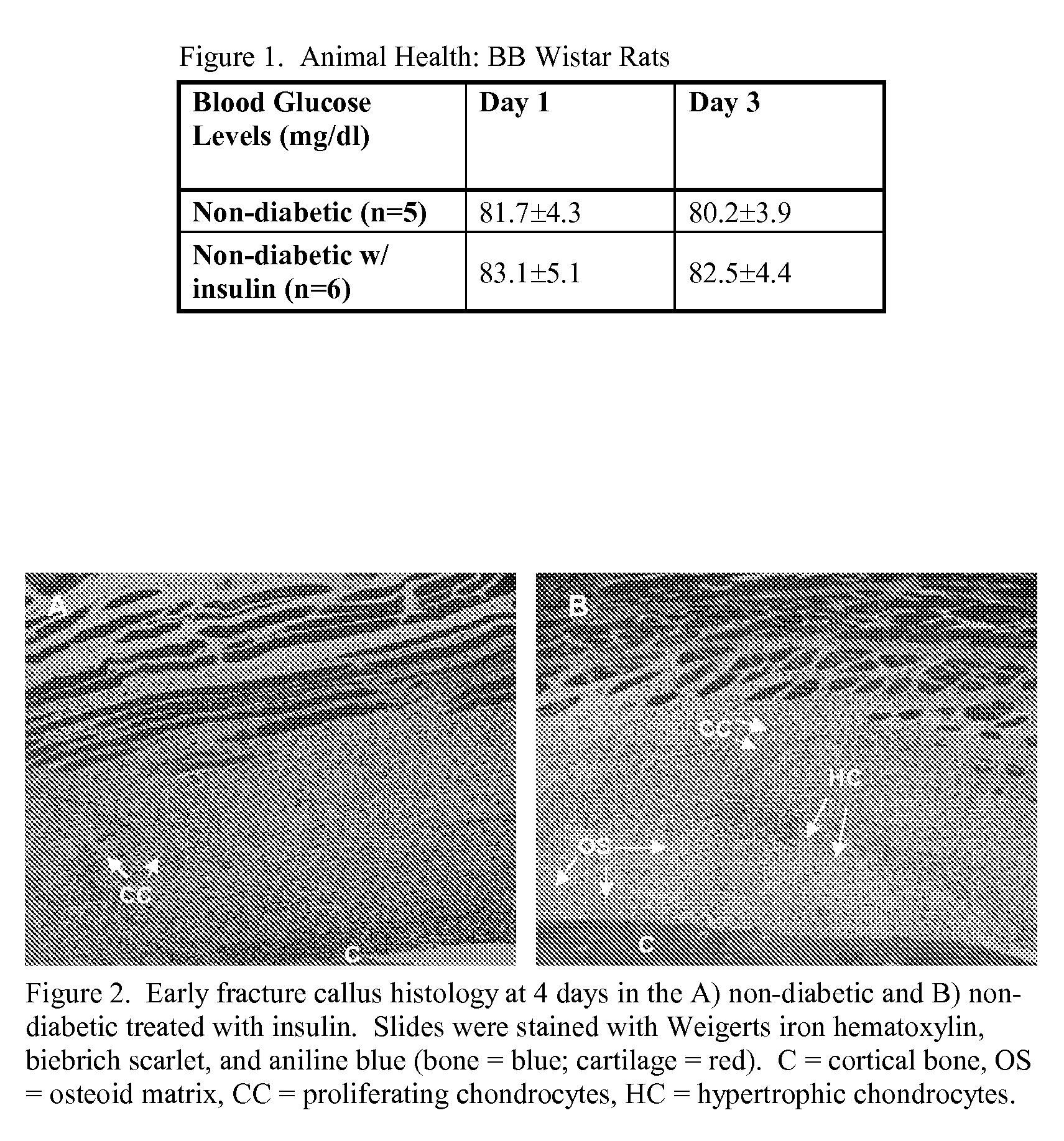
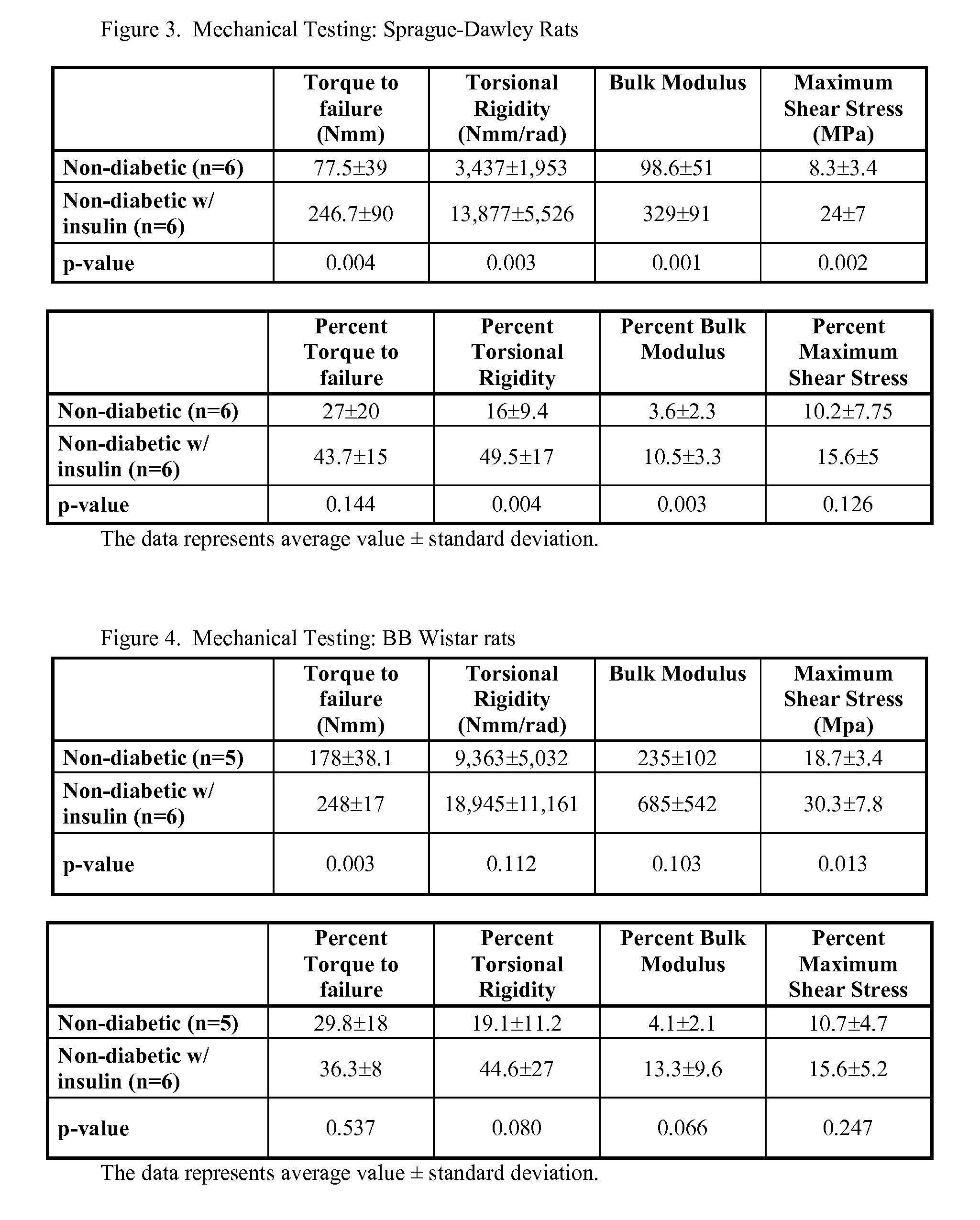
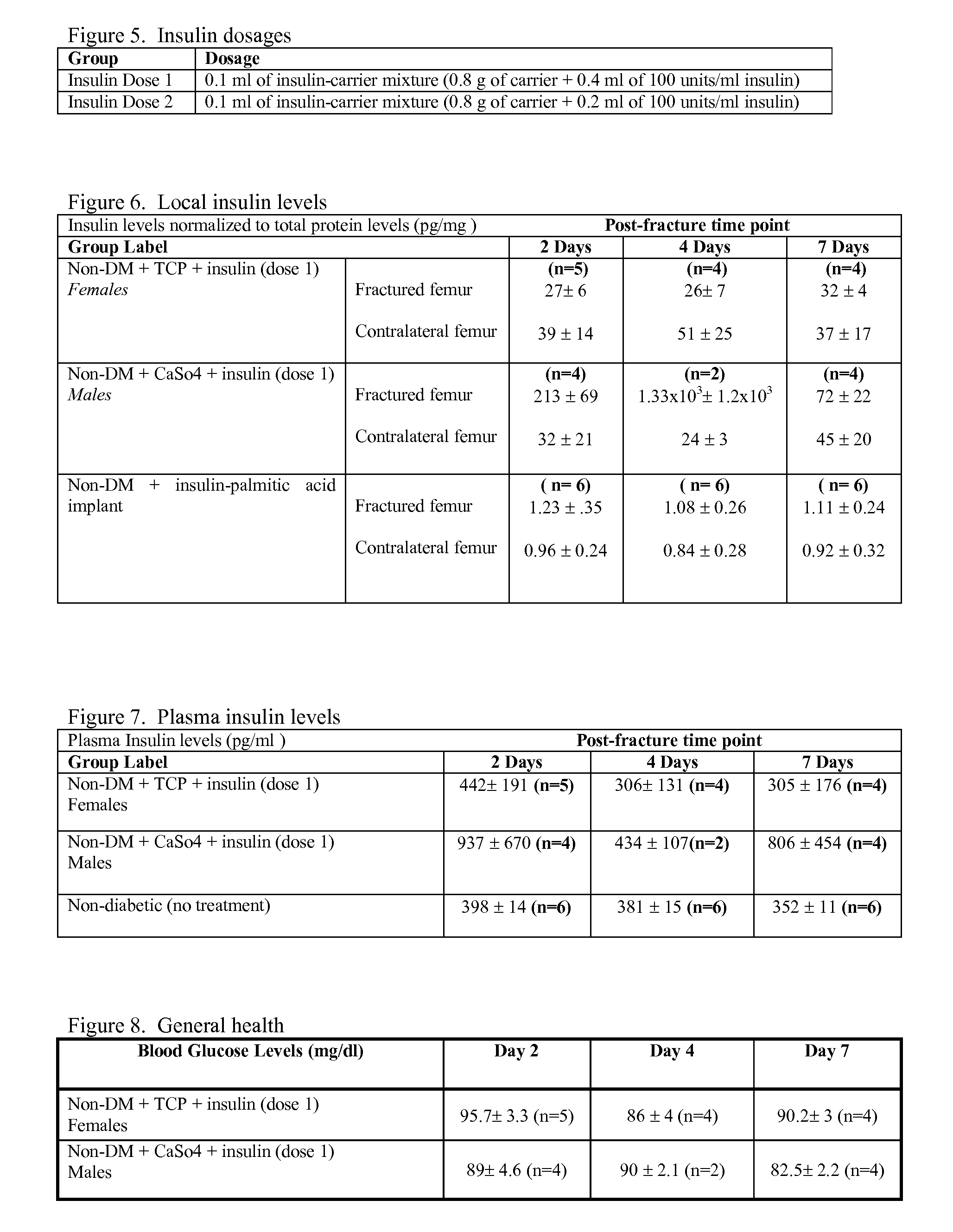
Localized Insulin Delivery for Bone Healing
ActiveUS20090214468A1Accelerate bone healingPeptide/protein ingredientsPharmaceutical delivery mechanismBone healingNon diabetic
A method of promoting bone healing in a non-diabetic patient in need thereof by locally administering a therapeutically effective amount of insulin to the patient. A drug delivery device, which includes insulin and a pharmaceutically acceptable carrier, wherein the device is adapted for localized administration of insulin to a patient in need thereof is also presented.
Owner:RUTGERS THE STATE UNIV
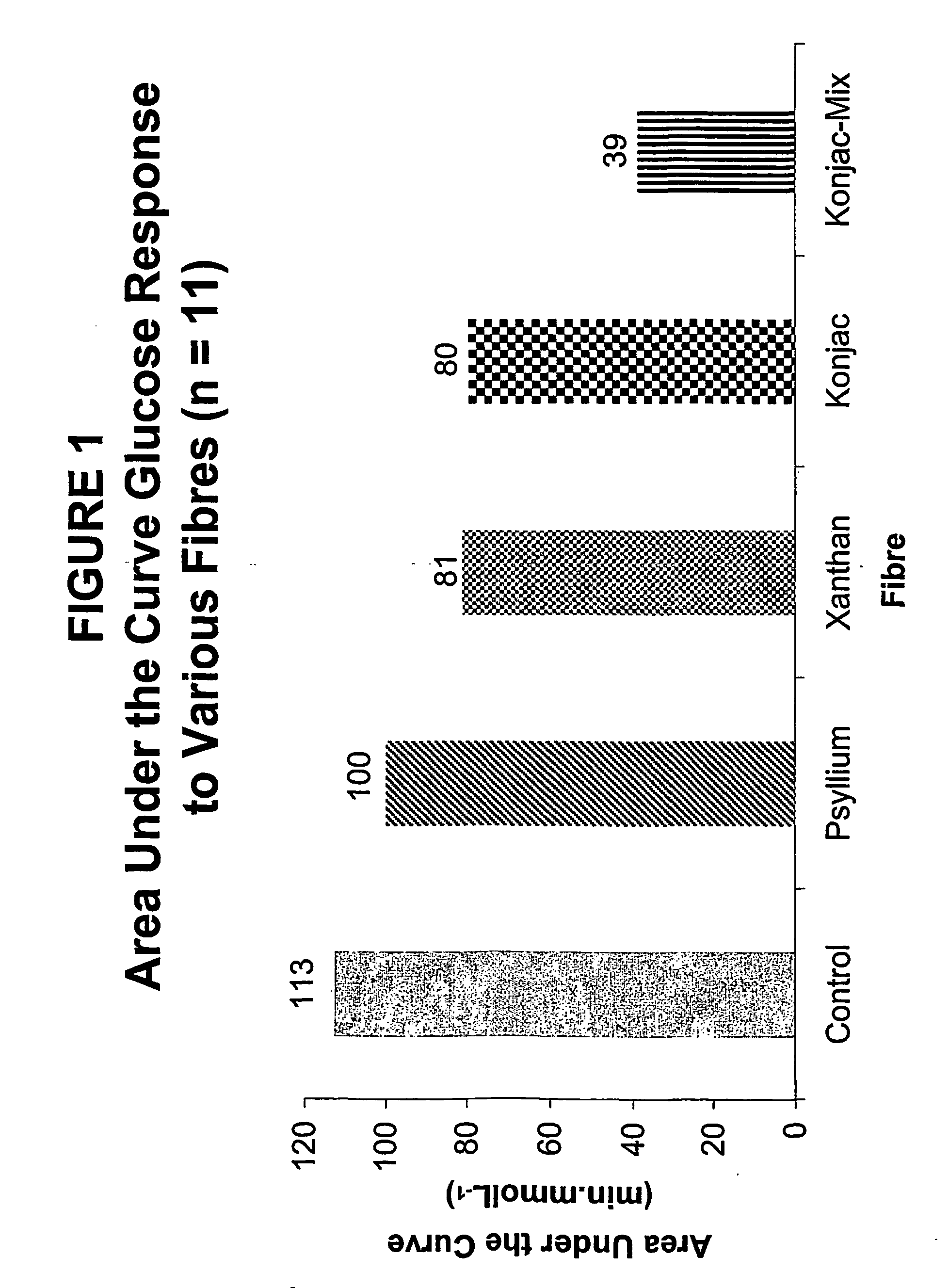
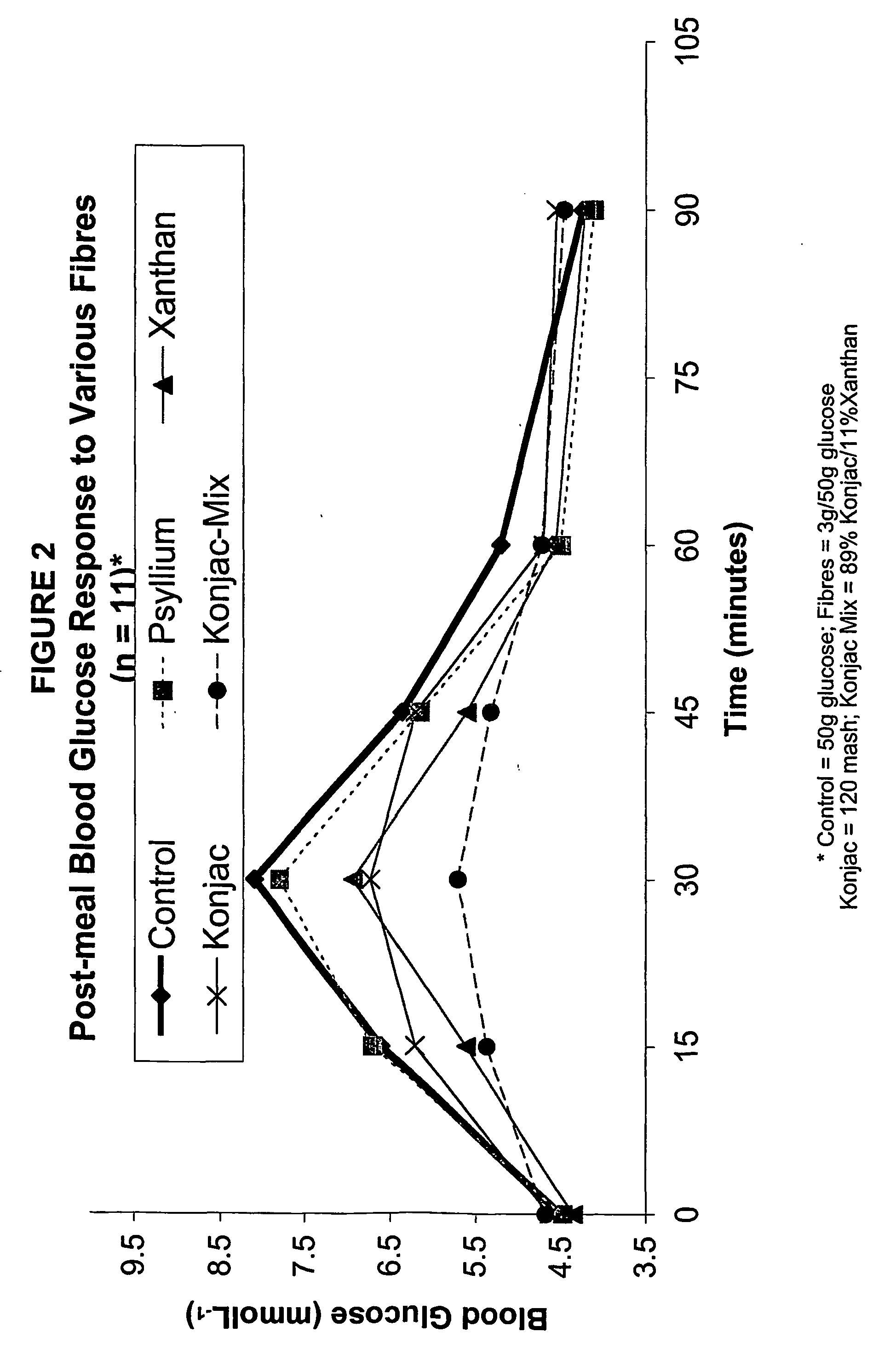
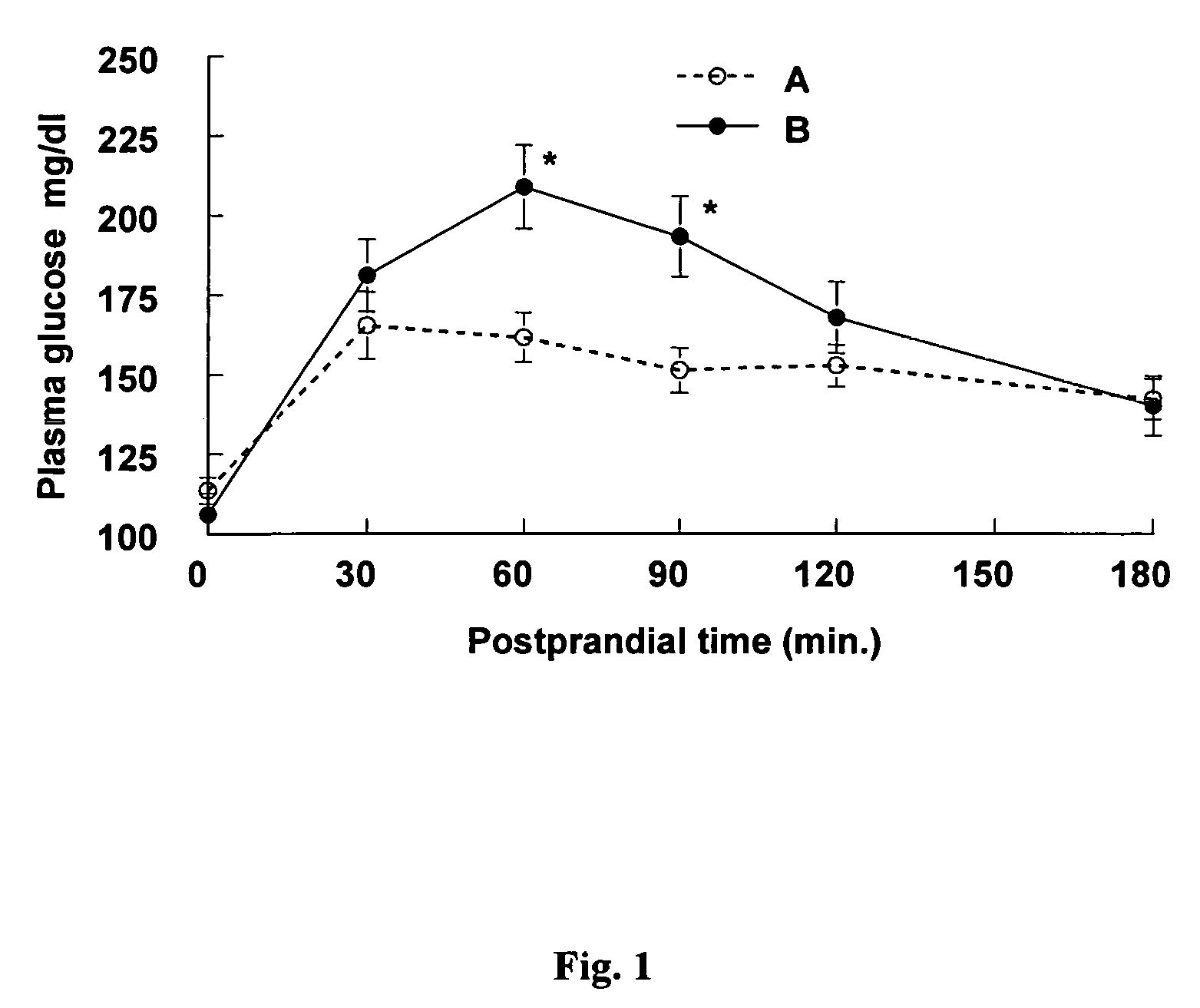
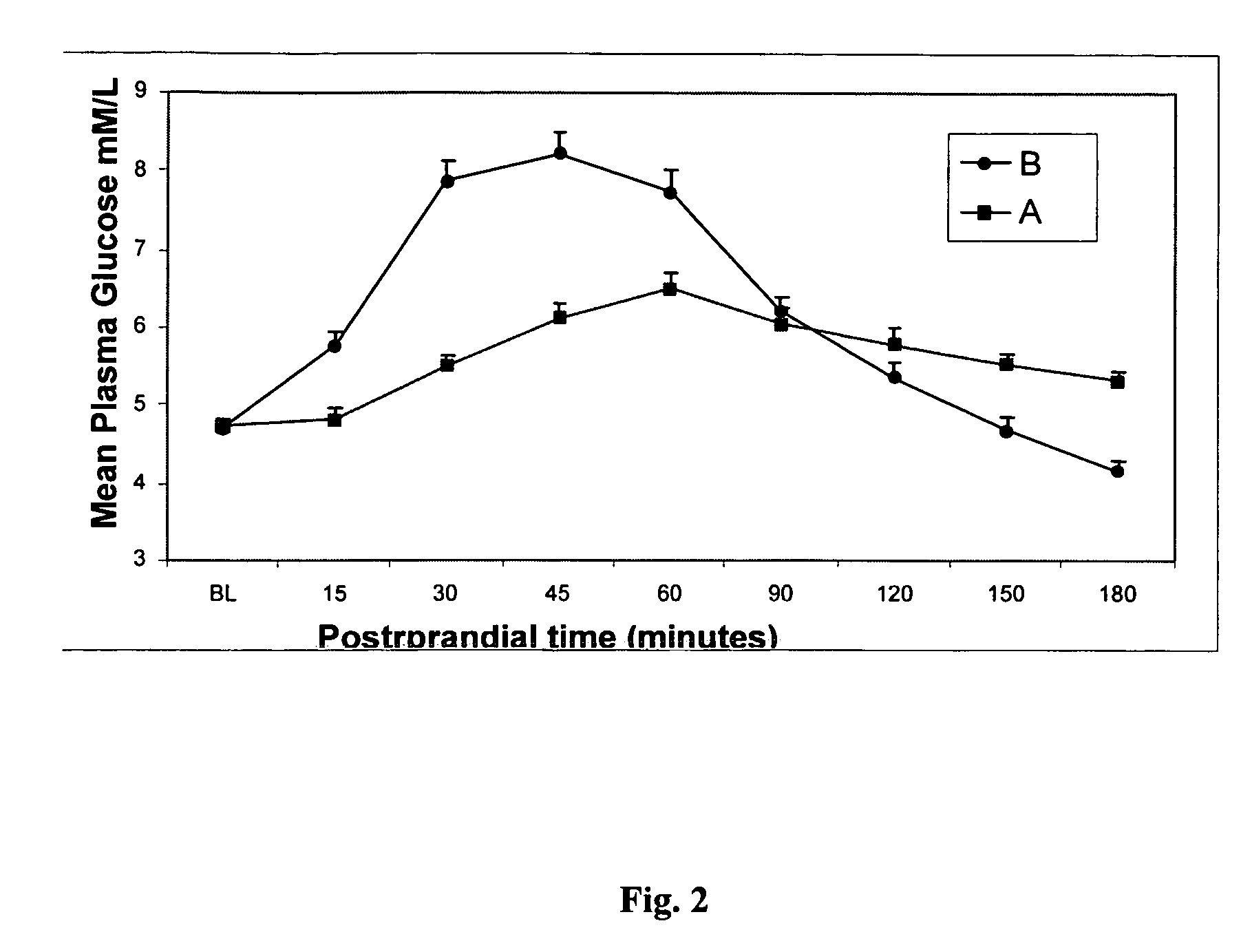
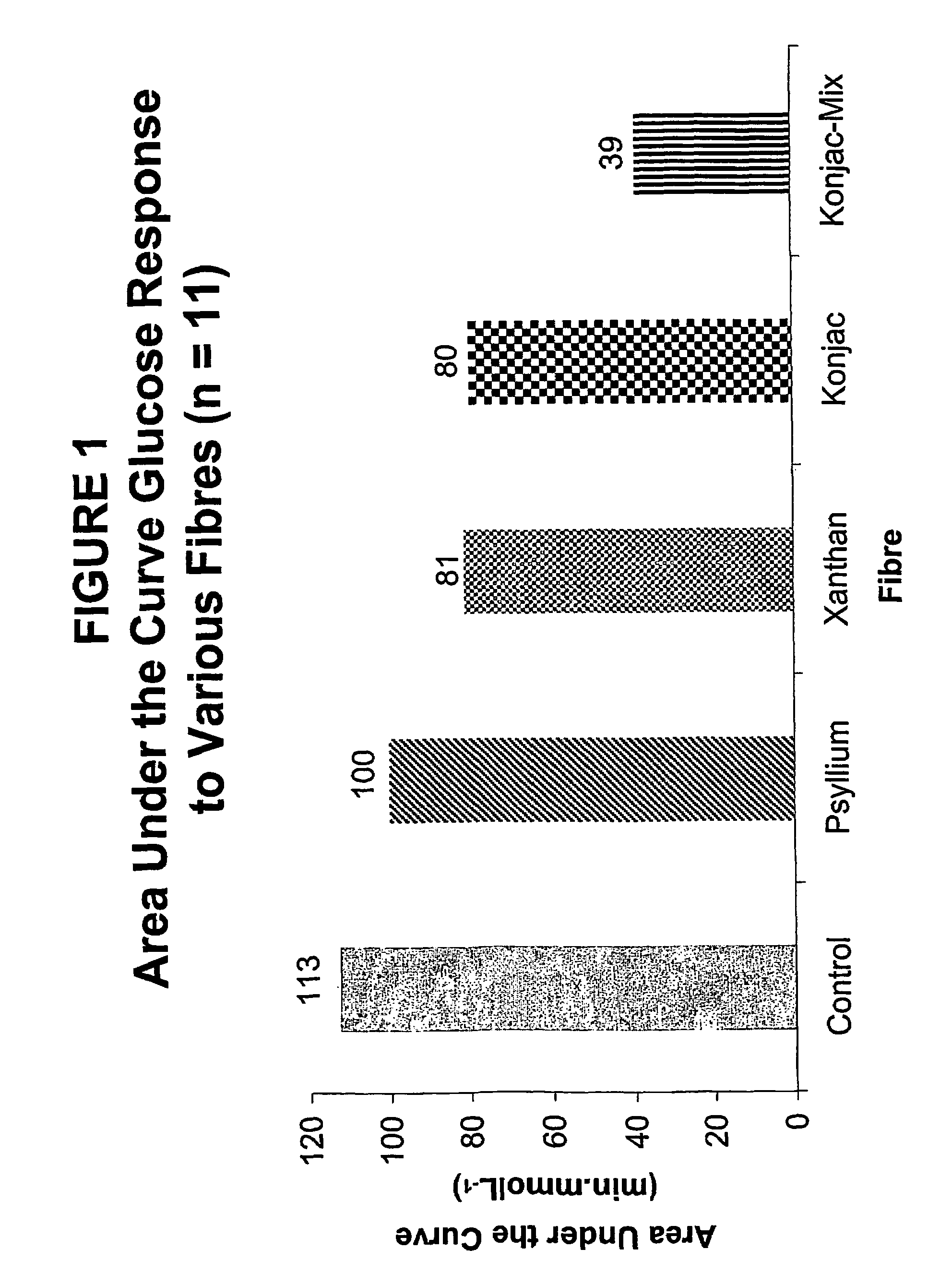
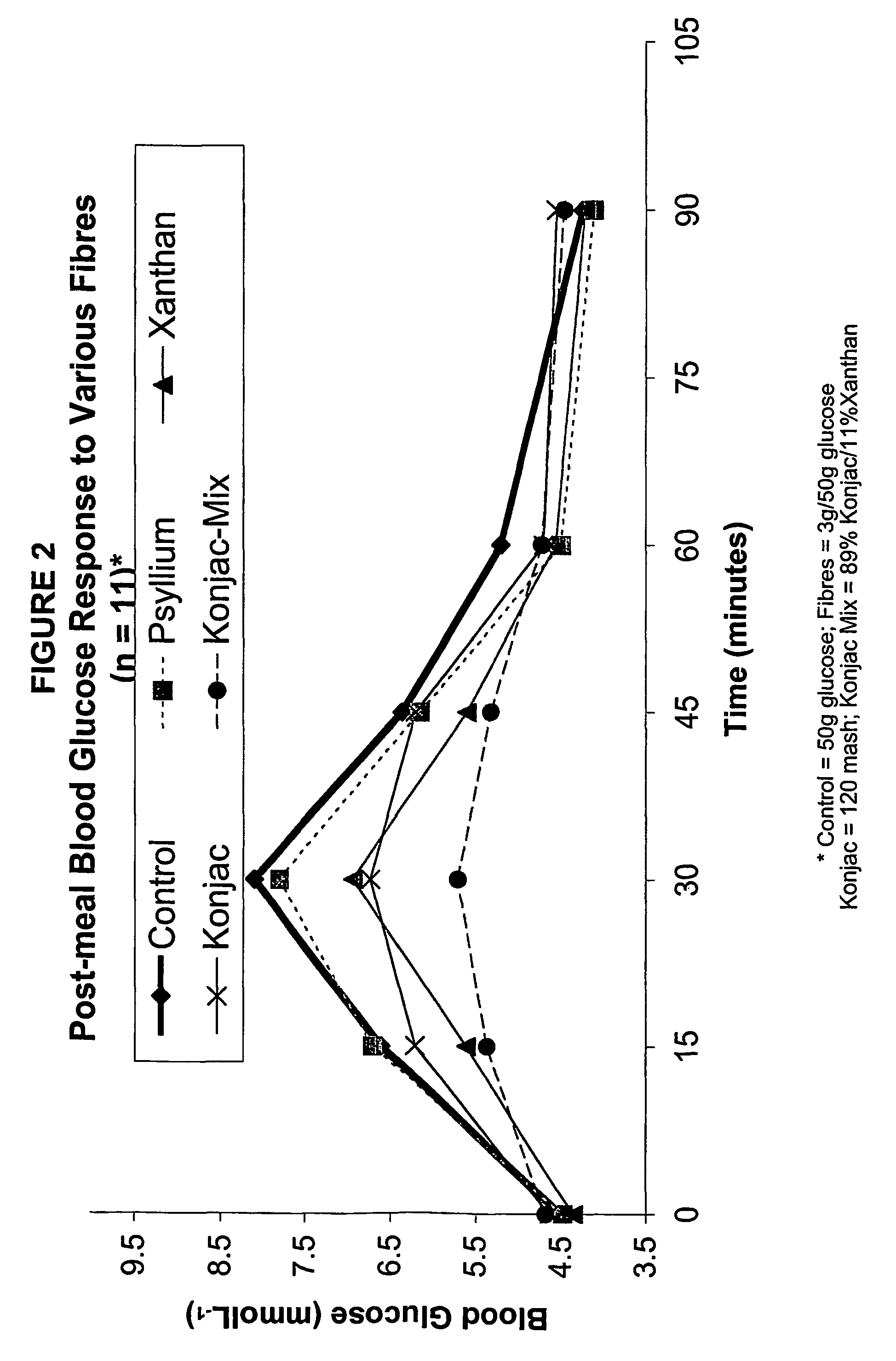
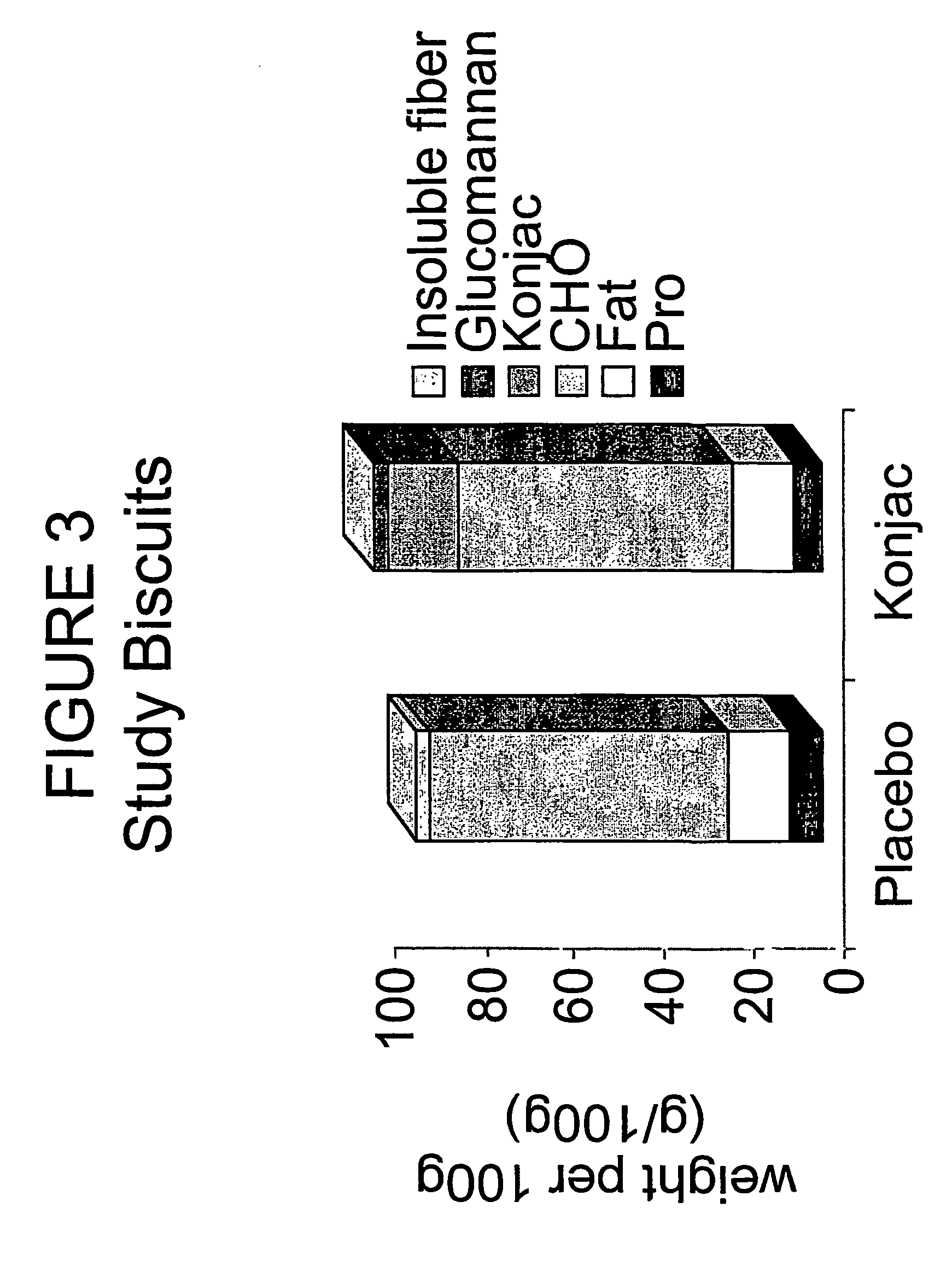
Konjac mannan and ginseng compositions and methods and uses thereof
InactiveUS20050020535A1Improve treatment outcomesLowering blood-glucosePowder deliveryBiocideNitric oxideAmorphophallus
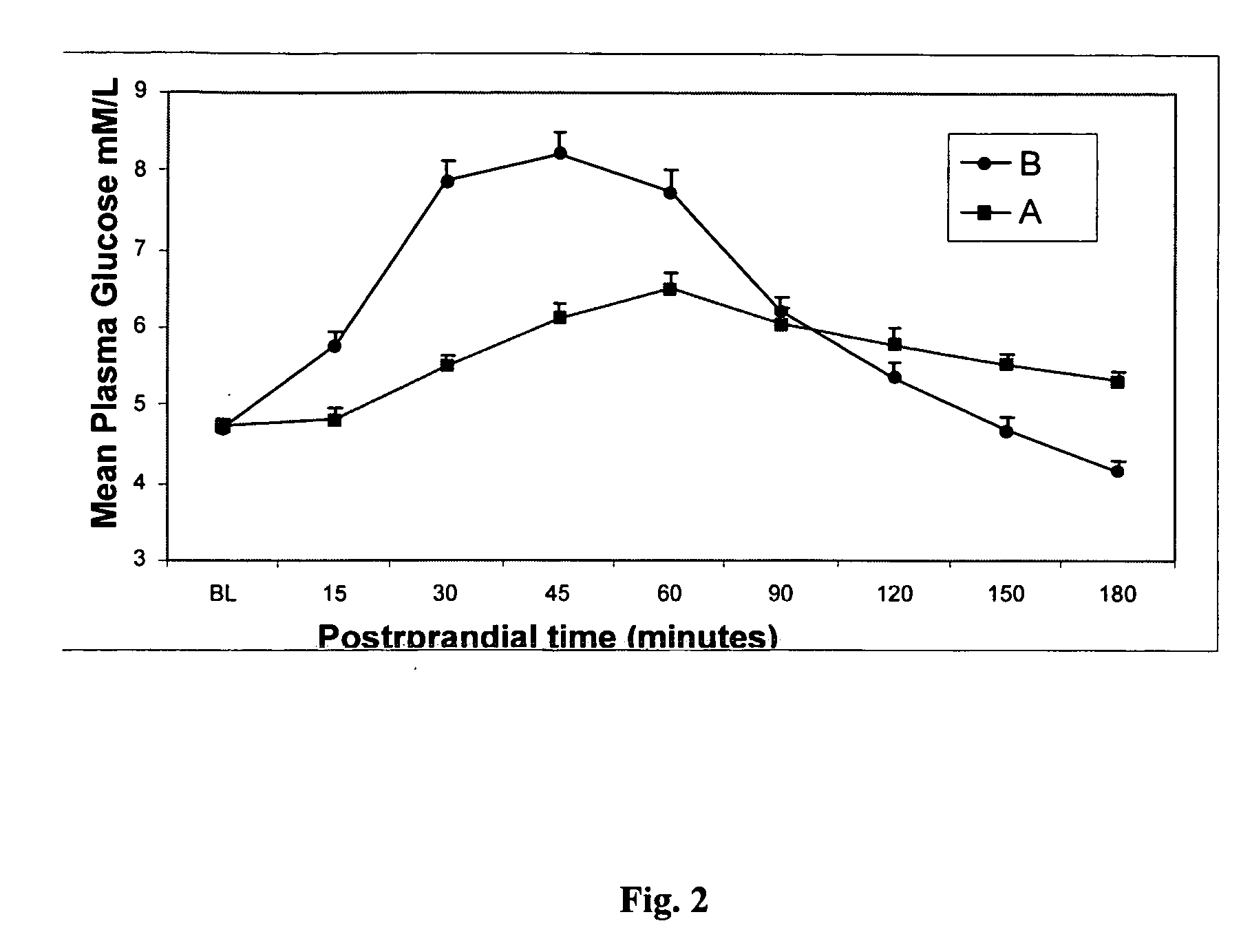
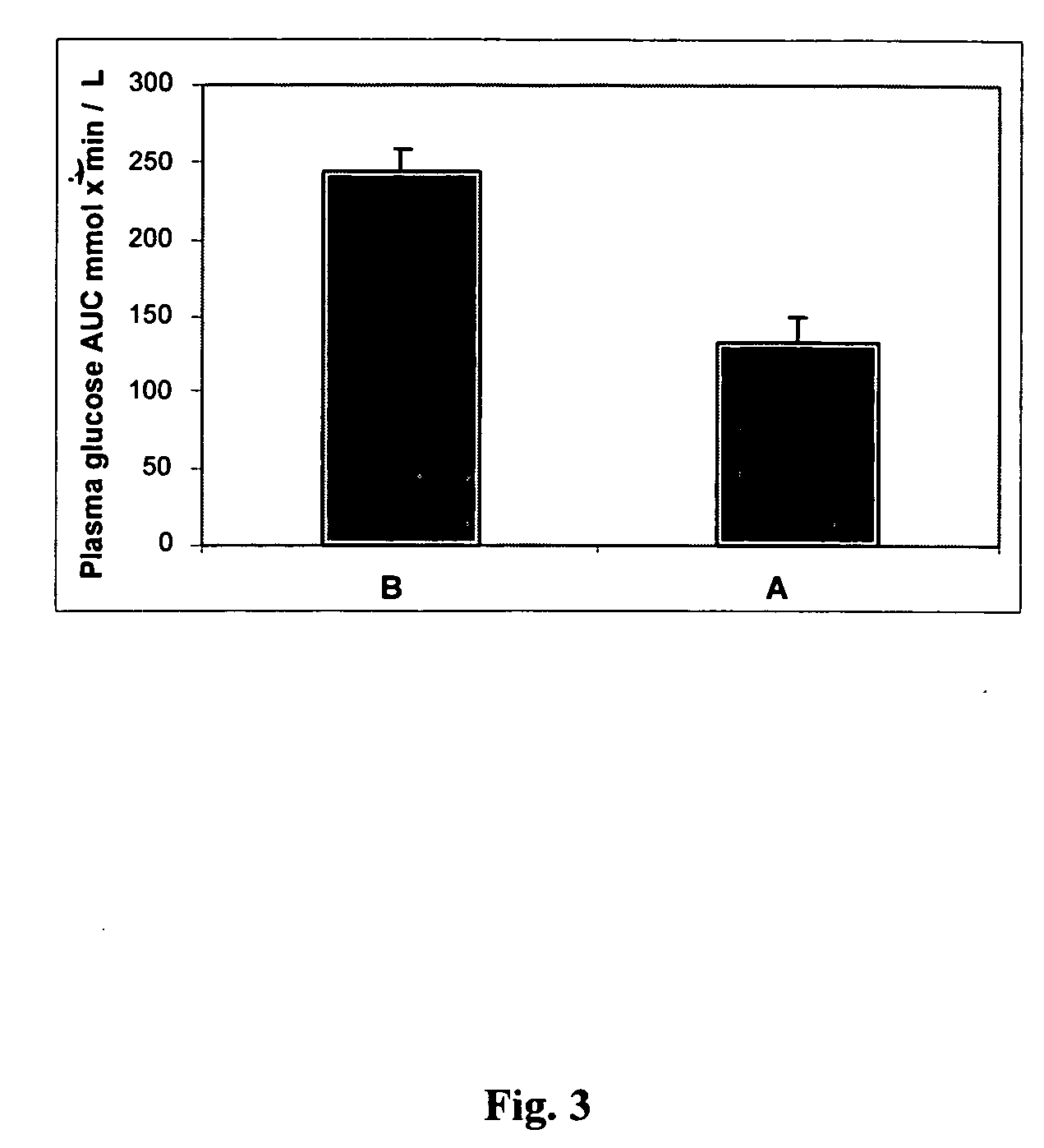
Described are a number of compositions, namely a konjac mannan mixture, a ginseng composition, and a composition comprising konjac mannan and American ginseng. Methods of use of these compositions are described including their use for reducing blood glucose in non-diabetic and diabetic individuals, as well as reducing postprandial blood glucose in such individuals. Applications in the treatment of hyperlipidemia, high blood pressure, an increase in nitric oxide, Syndrome X and cardiovascular disease are also described. Various other applications of the compositions and methods are also described.
Owner:VUKSAN HLDG
Methods of using gamma cyclodextrin to control blood glucose and insulin secretion
Owner:ABBOTT LAB INC
Method for treating type 2 diabetes with an extract of Artemisia
InactiveUS6893627B2Increasing glucose uptakeMethod securityAntibacterial agentsBiocideMammalFractionation
The present invention provides materials and methods relating to mildly polar fluid extracts of plant materials, such as Artemisia plant species, useful in methods for treating diabetes and methods for modulating the activity of glucagon-like peptide-1 (GLP-1), and in methods for modulating phosphoenol pyruvate carboxykinase (PEPCK) activity in a diabetes-specific manner. The extracts are generally non-toxic and non-mutagenic and may be administered to diabetics with beneficial effect on blood glucose levels. The extracts may also be administered to non-diabetics without deleterious effect. The plants are easily grown with a minimum of time, labor, and cost. Extracts are inexpensively and quickly prepared without the need for fractionation to remove potentially deleterious compounds, and the extracts may be administered to mammals such as humans through various routes, in a variety of forms, and at convenient concentrations.
Owner:RUTGERS THE STATE OF UNIVESITY OF NEW JERSEY
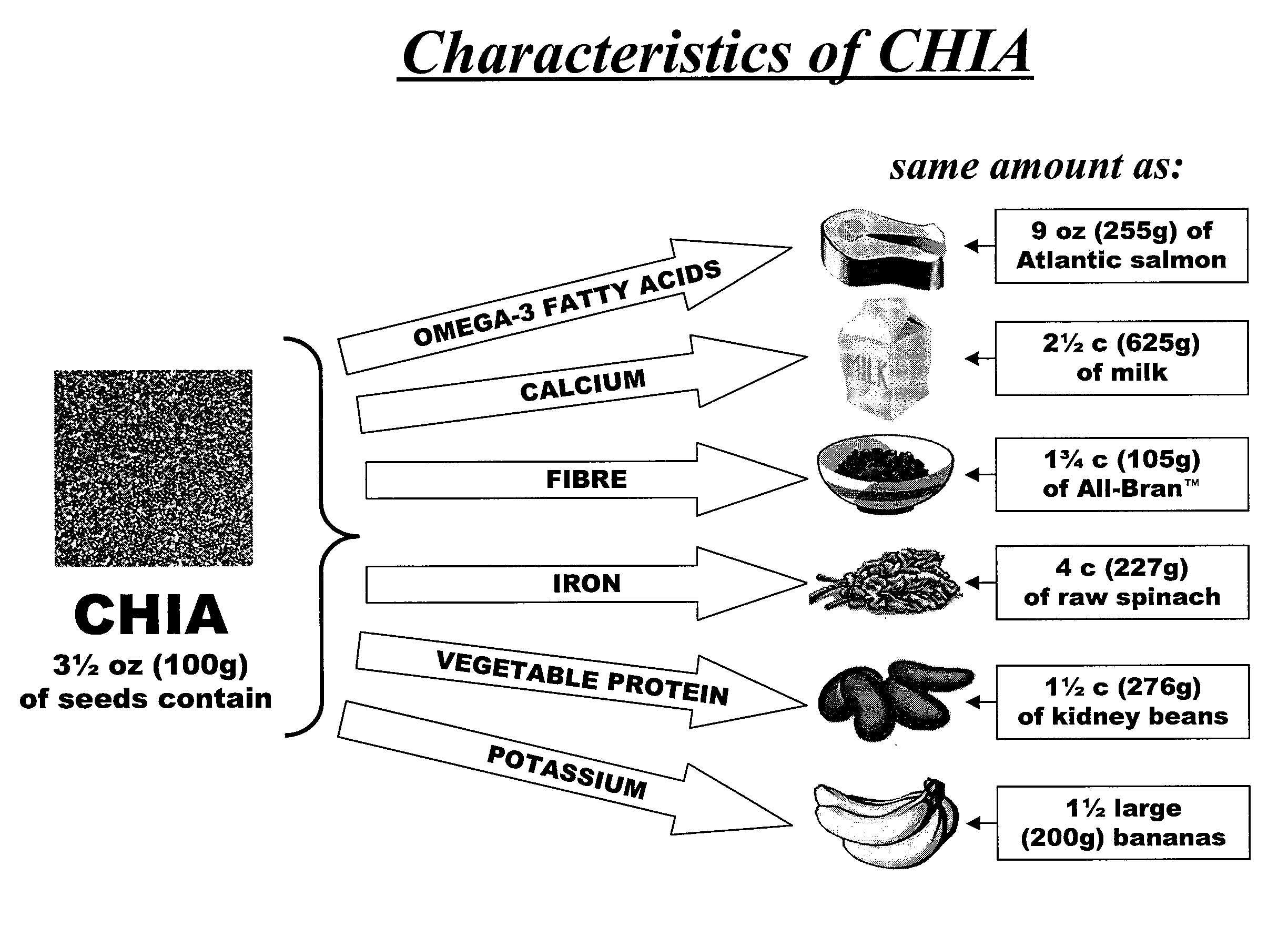
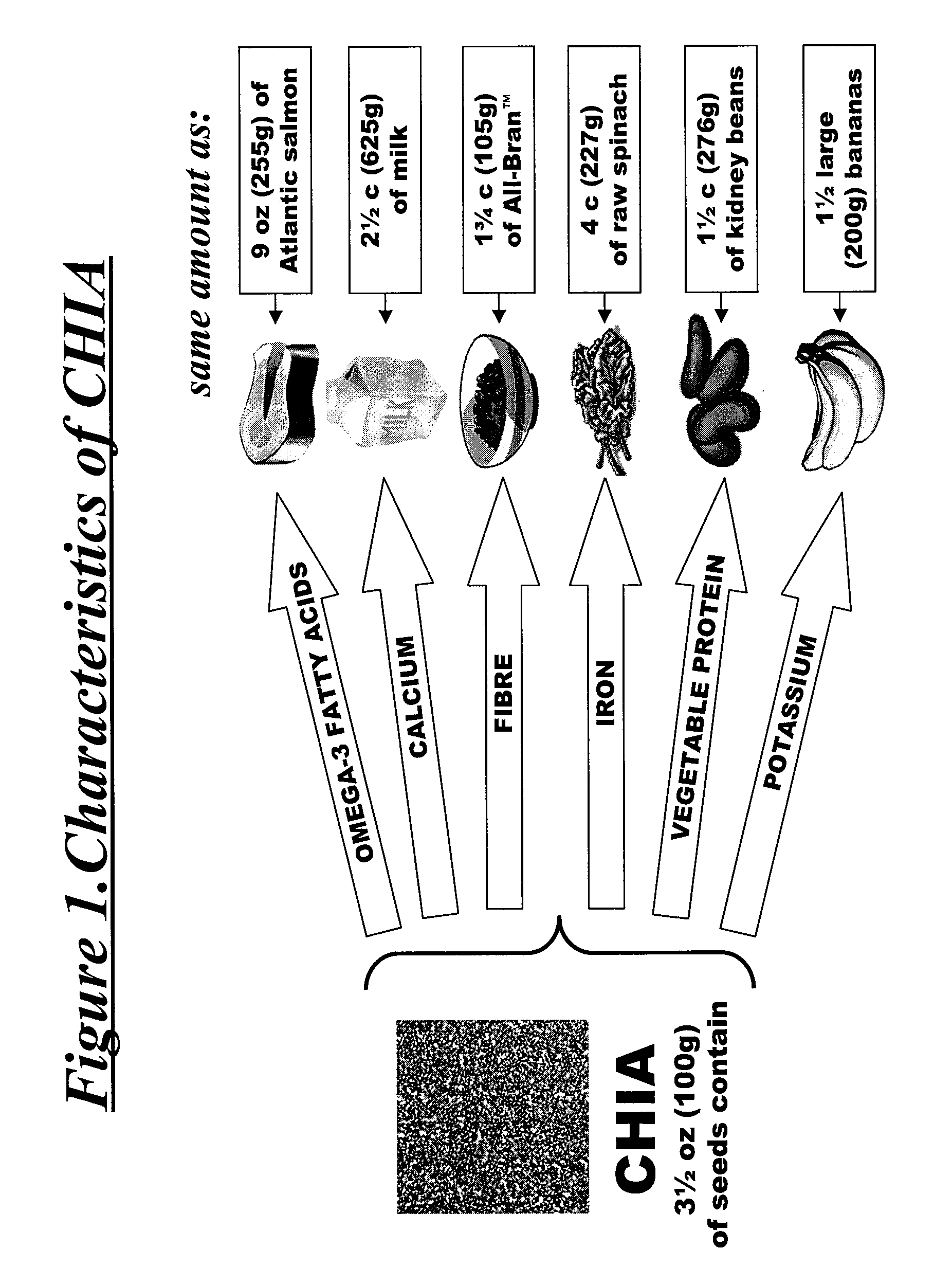
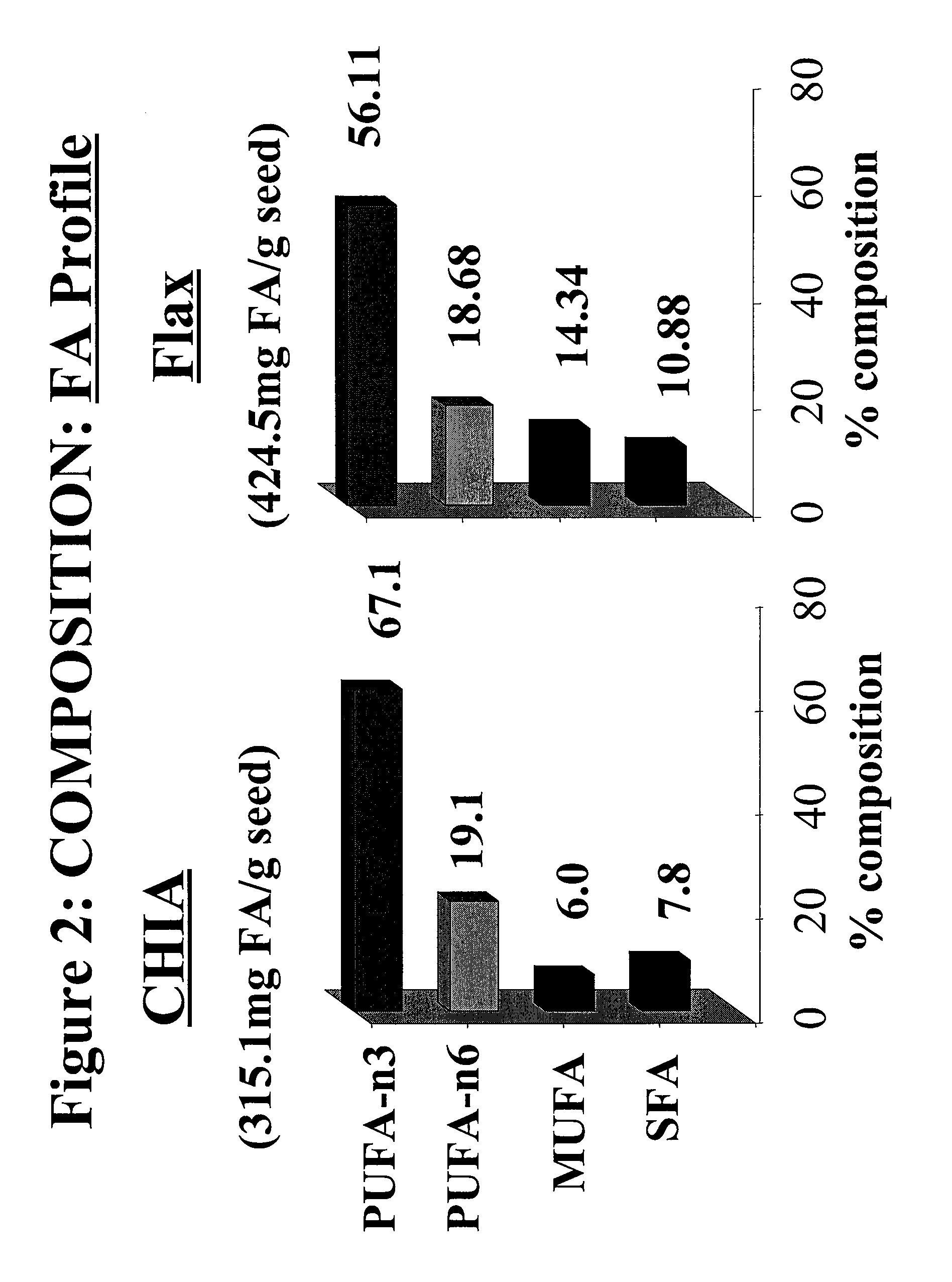
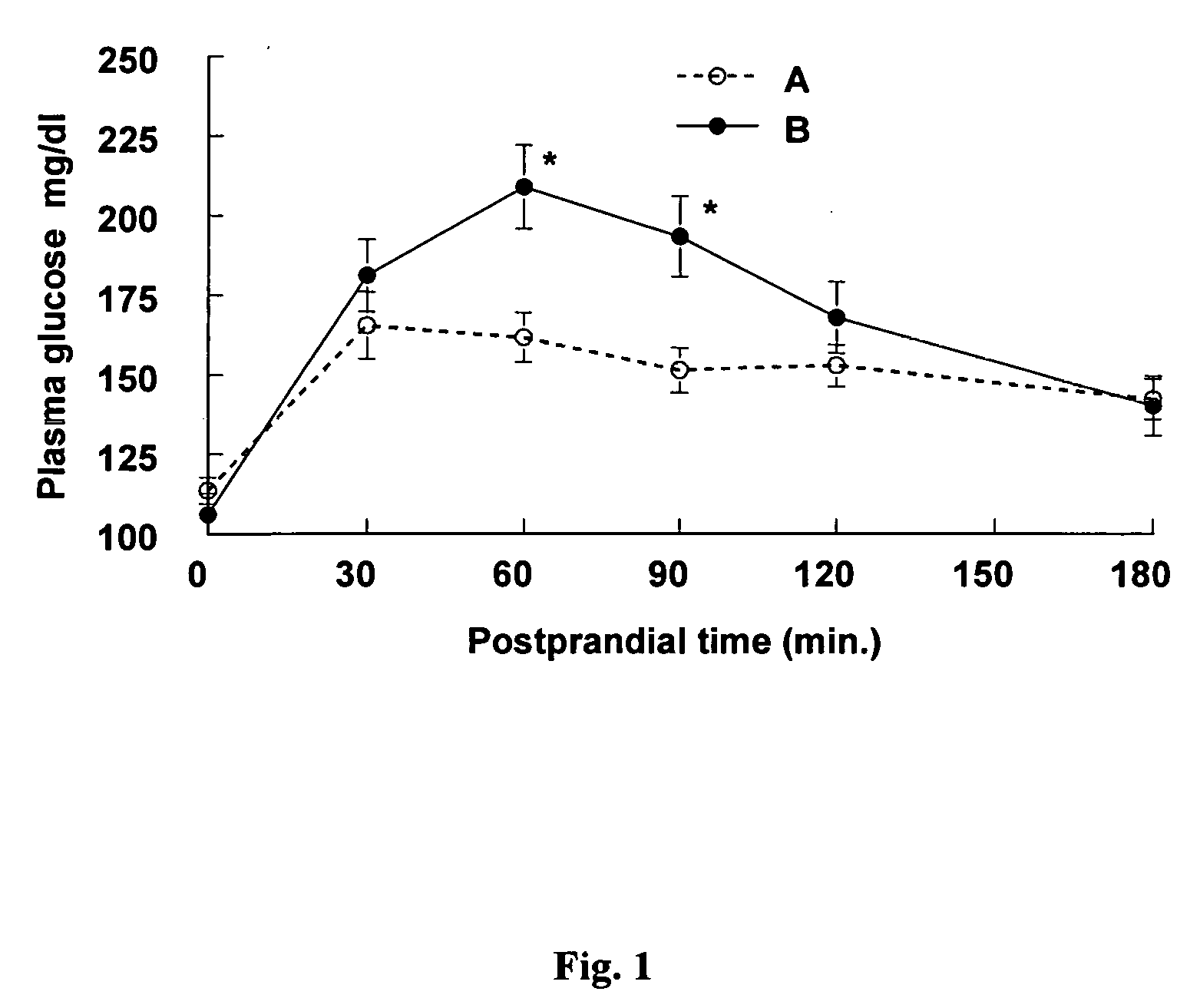
Salvia hispanica I (Chia) in the management and treatment of cardiovascular disease, diabetes and associated risk factors
InactiveUS20080305190A1Improve treatment outcomesReduces fasting and postprandial blood glucoseBiocideMetabolism disorderInflammatory factorsFactor ii
Described is use of Salvia hispanica L. (Chia) for controlling, in one embodiment reducing, blood glucose levels, preferably post-prandial blood glucose levels. This is useful in both non-diabetic and diabetic individuals, but especially in diabetic individuals. Also described is the use of chia in reducing postprandial blood glucose, insulin sensitivity, blood pressure, and oxidative stress in such individuals. The present invention further found that Chia can be used to improve endothelial function, coagulation, fibrinolysis and iron status. The present invention further encompasses the use of Chia in the treatment and / or management of diabetes and / or the treatment and management of diabetes associated conditions or risk factors, such as one or more of the following: blood pressure and blood glucose levels, post-prandial glycemia, inflammatory factors (C-reactive protein), coagulation (fibrinogen, factor VIII, von Willenbrand factor), and fibronolytic factors (such as t-PA), iron status and endothelial function, (such as increase in nitric oxide generation). In one embodiment the invention relates to dietary approaches to such treatment and management.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Methods of using gamma cyclodextrin to control blood glucose and insulin secretion
InactiveUS20050215523A1Blood glucose levelProlonged glycemic responseOrganic active ingredientsBiocideCyclodextrinDuodenum length
Disclosed are methods of producing a blunted postprandial glycemic response in an individual, and / or reducing postprandial insulin secretion, said methods comprising administering to the individual a nutritional or other product comprising gamma-cyclodextrin. Also disclosed are similar other methods directed toward the use of such products to provide weight and appetite control, to normalize blood glucose levels in individuals with impaired glucose tolerance, to minimize nighttime hypoglycemia in diabetic and non-diabetic patients, to prevent reactive hypoglycemia in susceptible non-diabetics, to normalize blood glucose levels in individuals with gestational diabetes or impaired glucose tolerance during gestation, and / or to provide a prolonged glycemic response during exercise. The methods are based upon the discovery that gamma-cyclodextrins are rapidly metabolized and absorbed in the small intestine, but subsequently result in a surprisingly blunted postprandial glycemic response and reduced insulin secretion.
Owner:ABBOTT LAB INC
Fenugreek seed extract to lower blood cholesterol
A method of lowering blood cholesterol in a non-diabetic patient by at least 30% is described. The method involves orally administering for 30 consecutive days a fenugreek seed extract composition. Various methods of preparation and various formulations are described. Physiologically effective pharmaceutical compositions and beverages containing fenugreek seed extracts and other active components are also disclosed.
Owner:ABURDEINEH S GEORGE
Coxsackie B virus and type 1 diabetes
InactiveUS20100047273A1Slow and delay and progressionSlow and delay symptomSsRNA viruses positive-senseSugar derivativesCOXSACKIE A VIRUSAutoimmune responses
Type 1 diabetes mellitus is characterized by loss of pancreatic insulin-producing beta cells, resulting in insulin deficiency. The usual cause of this beta cell loss is autoimmune destruction. Coxsackie virus has been detected in human pancreatic beta cells and causes insulitis. This non-destructive islet inflammation does not itself cause diabetes, but this disease will occur if viral infection is followed by a separate autoimmune response. The insulitis is mediated mainly by natural killer cells. Islets from coxsackie virus positive samples displayed reduced insulin secretion in response to glucose and other secretagogues. Virus extracted from positive islets was able to infect beta cells from human islets of non-diabetic donors, causing viral inclusions and signs of pyknosis.
Owner:RAPPUOLI RINO +2
Methods of treatment and pharmaceutical composition
The invention relates a pharmaceutical composition comprising a combination of:(i) the AT 1-antagonist valsartan or a pharmaceutically acceptable salt thereof; and(ii) a NEP inhibitor or a pharmaceutically acceptable salt thereof and optionally a pharmaceutically acceptable carrier and to a method for the treatment or prevention of a condition or diseaseselected from the group consisting of hypertension, heart failure, such as (acute and chronic) congestive heart failure, left ventricular dysfunction and hypertrophic cardiomyopathy, diabetic cardiac myopathy, supraventricular and ventricular arrhythmias, atrial fibrillation, atrial flutter, detrimental vascular remodeling, myocardial infarction and its sequelae, atherosclerosis, angina (whether unstable or stable), renal insufficiency (diabetic and non-diabetic), heart failure, angina pectoris, diabetes, secondary aldosteronism, primary and secondary pulmonary hypertension, renal failure conditions, such as diabetic nephropathy, glomerulonephritis, scleroderma, glomerular sclerosis, proteinuria of primary renal disease, and also renal vascular hypertension, diabetic retinopathy, the management of other vascular disorders, such as migraine, peripheral vascular disease, Raynaud's disease, luminal hyperplasia, cognitive dysfunction, such as Alzheimer's, glaucoma and stroke, comprising administering a therapeutically effective amount of the pharmaceutical composition to a mammal in need thereof.
Owner:NOVARTIS PHARM CORP
Localized insulin delivery for bone healing
ActiveUS7763582B2Peptide/protein ingredientsPharmaceutical delivery mechanismWound healingNon diabetic
A method of promoting bone healing in a non-diabetic patient in need thereof by locally administering a therapeutically effective amount of insulin to the patient. A drug delivery device, which includes insulin and a pharmaceutically acceptable carrier, wherein the device is adapted for localized administration of insulin to a patient in need thereof is also presented.
Owner:RUTGERS THE STATE UNIV
Novel choline cocrystal of epalrestat
The invention relates to a novel choline cocrystal of 5-[(lZ.2E)-2-methyl-3-phenylpropenylidene]-4-oxo-2-thioxo-3-thiazolidineacetic acid. The preparation and characterization of the novel choline cocrystal according to various embodiments of the invention is described. The invention also relates to pharmaceutical compositions containing the novel choline cocrystal and the therapeutic use of the novel choline cocrystal to treat and / or prevent various conditions, including treating and / or preventing diabetic complications, treating and / or preventing homocystinuria reducing levels of homocysteine in blood serum, inhibiting aldose reductase, and affording cardioprotection in non-diabetic patients.
Owner:BIONEVIA PHARMACEUTICALS INC
Fenugreek seed extract to lower blood cholesterol
A method of lowering blood cholesterol in a non-diabetic patient by at least 30% is described. The method involves orally administering for 30 consecutive days a fenugreek seed extract composition. Various methods of preparation and various formulations are described. Physiologically effective pharmaceutical compositions and beverages containing fenugreek seed extracts and other active components are also disclosed.
Owner:ABURDEINEH S GEORGE
Method of treating diabetes
InactiveUS20080009503A1Reduce developmentLower Level RequirementsOrganic active ingredientsPowder deliveryAcute hyperglycaemiaBlood plasma
Methods are provided for lowering plasma level of HbA1c in a diabetic, pre-diabetic, or non-diabetic patient suffering from at least one cardiovascular disease and slowing or delaying the development of or worsening of hyperglycemia in a diabetic, pre-diabetic, or non-diabetic patient.
Owner:GILEAD SCI INC
Treatment Of Diabetic Patients With A Drug Eluting Stent And Adjunctive Therapy
ActiveUS20130224255A1Reduce inflammationImprove responsivenessStentsBiocideVascular diseaseCo administration
Embodiments of the present invention include methods for the treatment, prevention, or amelioration of vascular disease in diabetic patients. The methods include both implantation of a stent including a first drug. Some embodiments include additional therapy, such as the co-administration of another drug. Some embodiments involve different stent selection for a diabetic patient compared to a non-diabetic patient.
Owner:ABBOTT CARDIOVASCULAR
Biomaker composition for detecting diabetic retinopathy and diagnostic kit therefor
InactiveUS20100179307A1Provide informationDepsipeptidesPeptide preparation methodsThyroxine-binding globulinDiabetic retinopathy
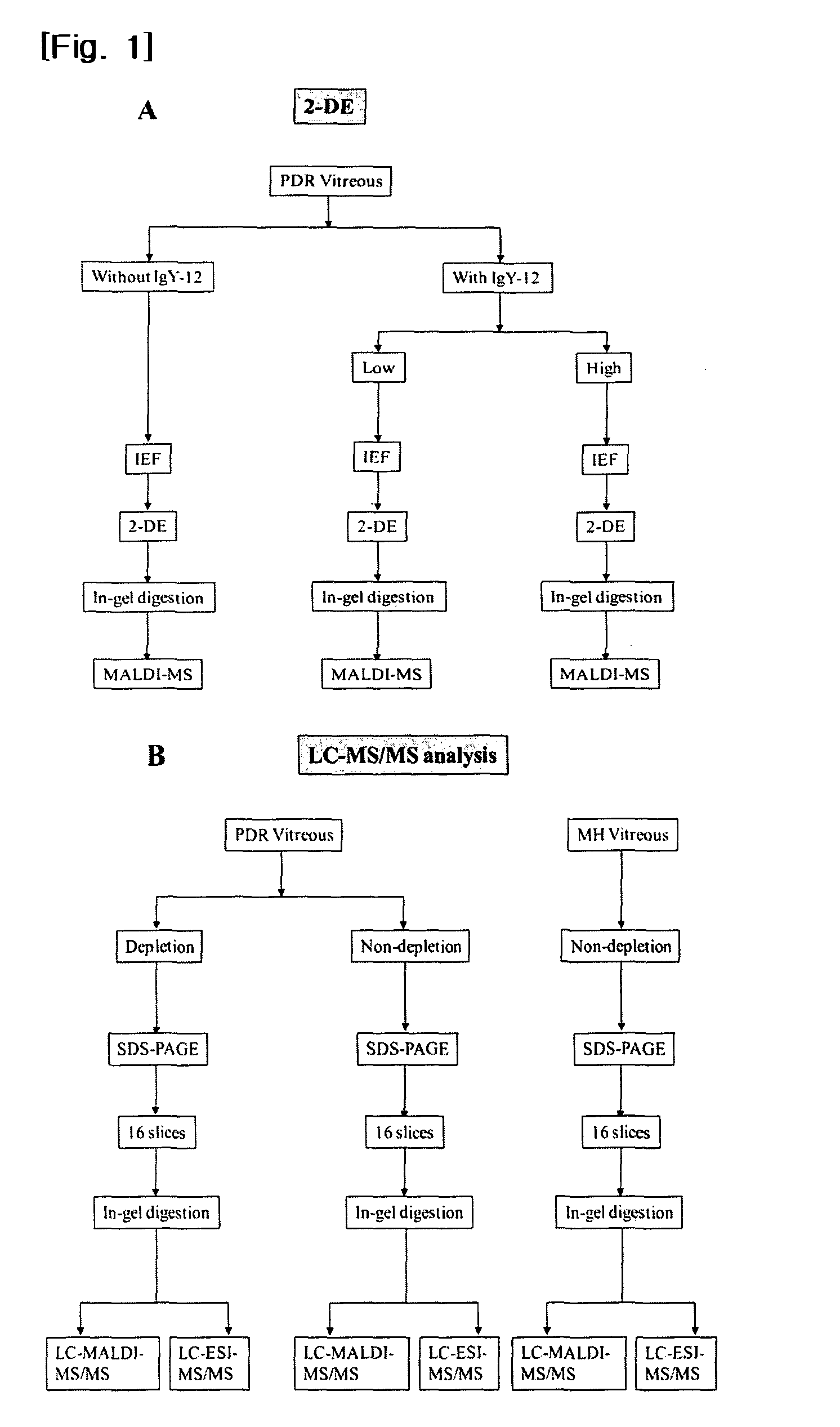
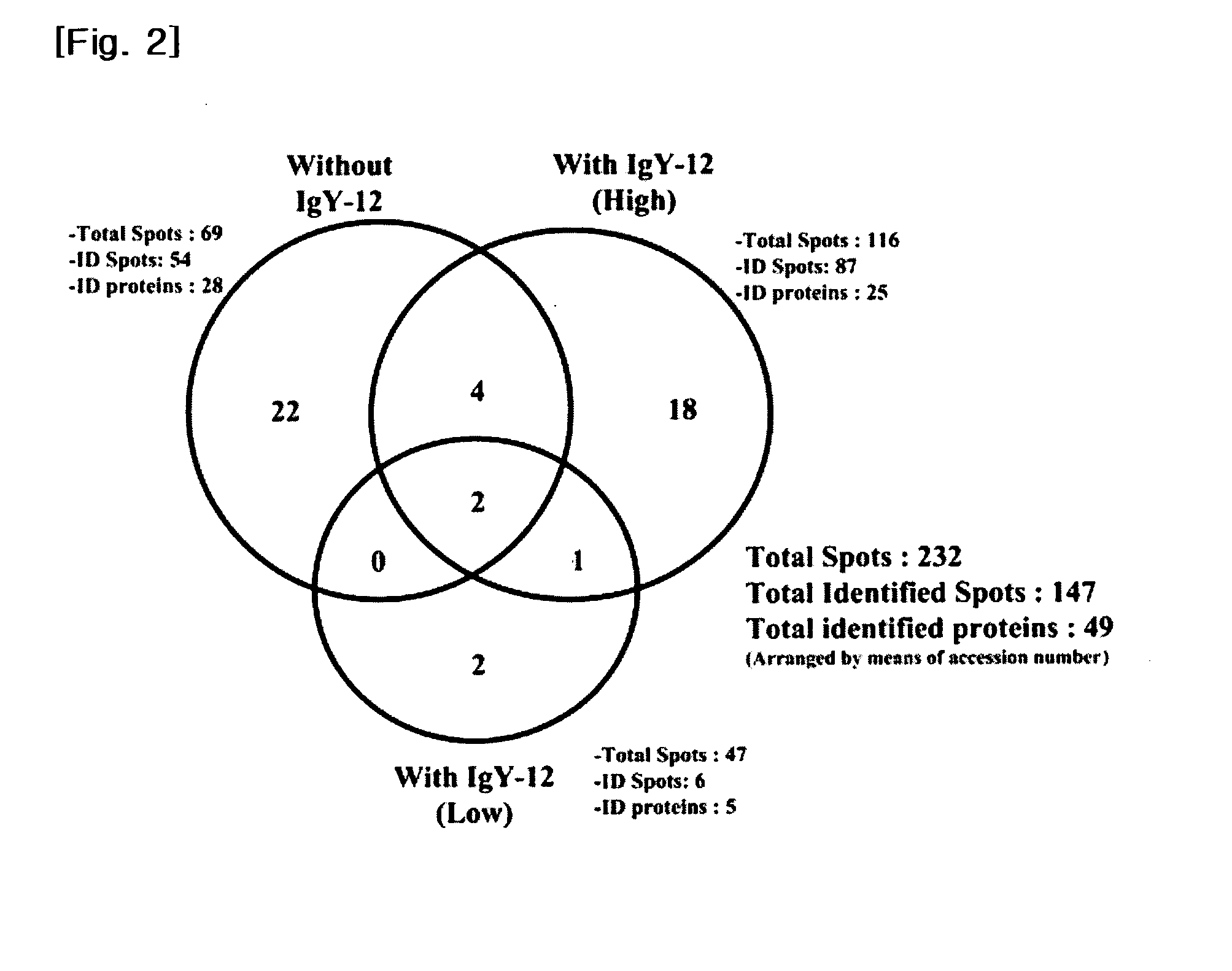
The present invention provides a biomarker composition for detecting diabetic retinopathy comprising at least one protein selected from the group consisting of proteins as set forth in SEQ ID NOS: 1 to 169. And also, the present invention provides a kit for diagnosing diabetic retinopathy, comprising a molecule specifically binding to at least one protein selected from the group consisting of proteins as set forth in SEQ ID NOS: 1 to 169. It has been newly found that 105 proteins as set forth in SEQ ID NOS: 1 to 105 are significantly over-expressed in the vitreous humors obtained from PDR patients, while 64 proteins as set forth in SEQ ID NOS: 106 to 169 are significantly over-expressed in those obtained from normal people. Therefore, the proteins can be used for biomarker capable of detecting diabetic retinopathy. The biomarker can provide fundamental information in researching vitreoretinal disorders, such as diabetic retinopathy. Especially, the newly found proteins may be applied to a kit for diagnosing diabetic retinopathy with a molecule specifically binding thereto, e.g., a monoclonal antibody. And also, it has been newly found that the levels of thyroxine-binding globulin precursor (TBG) in both vitreous and plasma of PDR and NPDR states and in plasma of diabetes mellitus state, are outstandingly higher than in non-diabetic control (MH or normal control). Therefore, TBG may be applied to a diabetes mellitus biomarker, and a kit for diagnosing diabetes mellitus with a molecule specifically binding thereto.
Owner:SEOUL NAT UNIV R&DB FOUND
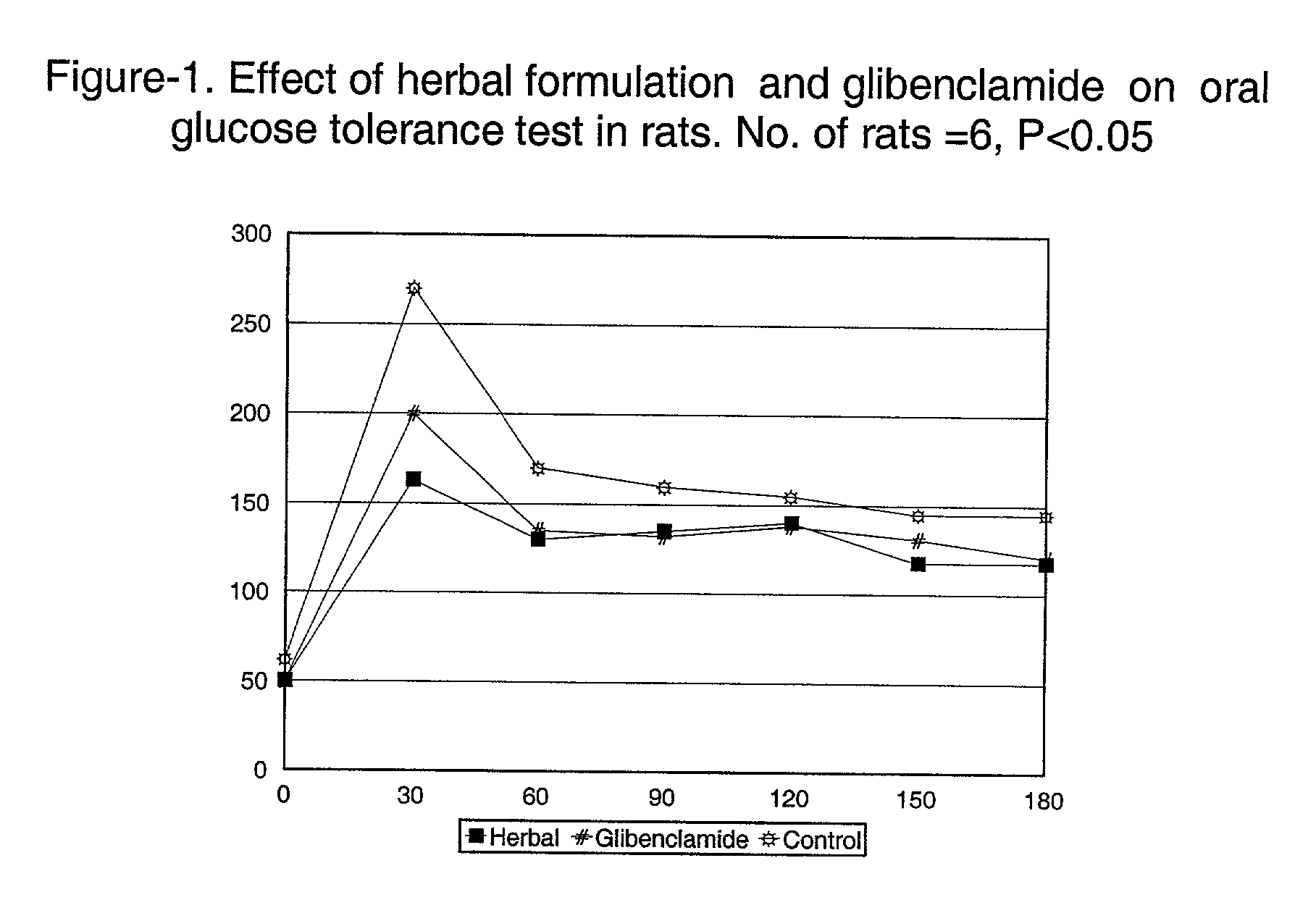
Therapeutic/edible compositions comprising herbal ingredients and methods for treating hyperglycemia
InactiveUS6989160B2Inexpensive and easily digestibleBiocideAnimal repellantsAcute hyperglycaemiaInsulin dependent
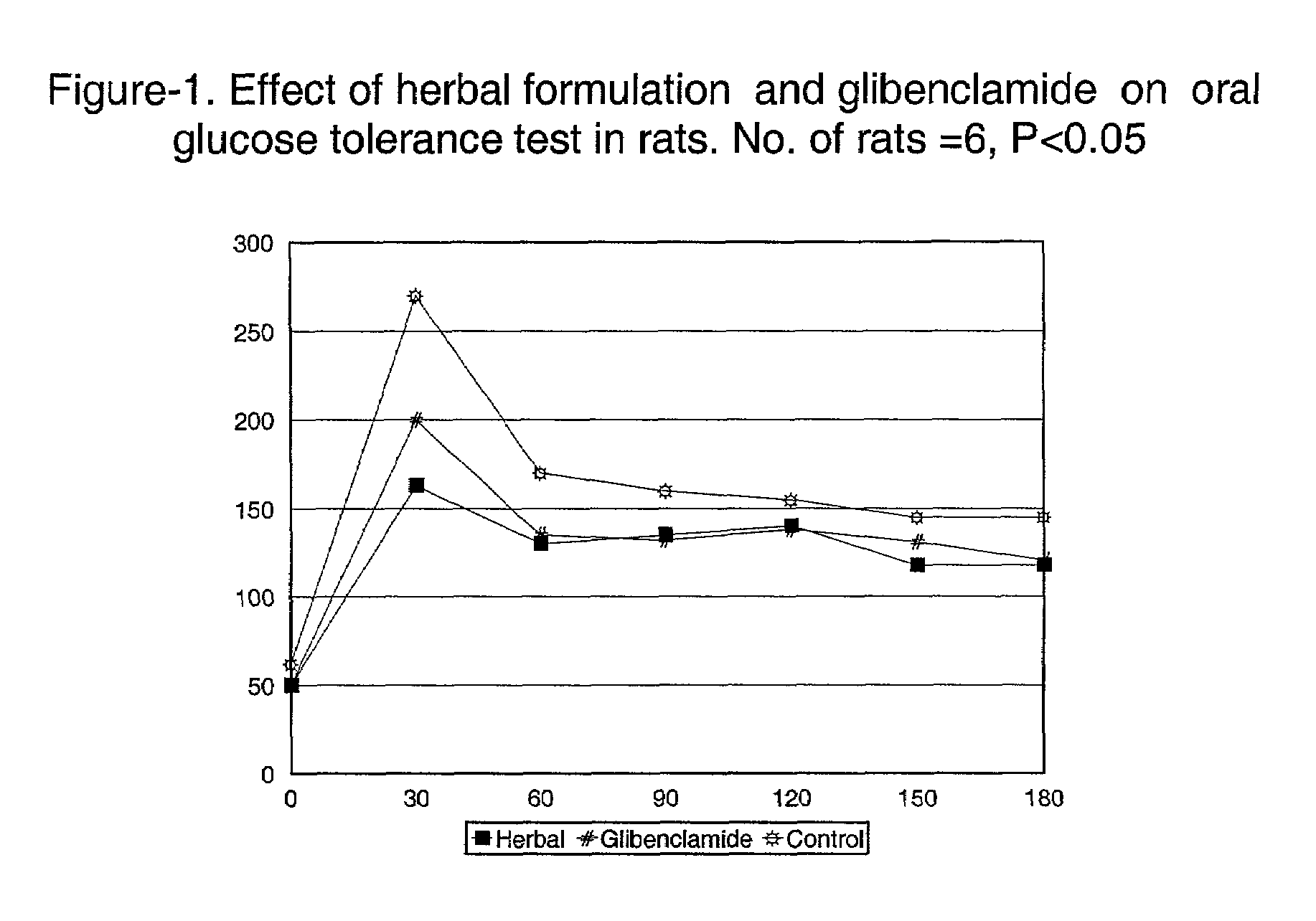
A therapeutic or an edible composition comprising at least three of the five herbs selected from Prunus amygdales, Ocimum sanctum, Azadirachta indica, Aegle marelose and Vitus vinefera, is provided for the treatment of hyperglycemia, especially for non-insulin dependent diabetic subjects, and the herbal composition has significantly reduced the blood glucose levels in both diabetic and non-diabetic experimental subjects having elevated blood glucose levels.
Owner:COUNCIL OF SCI & IND RES
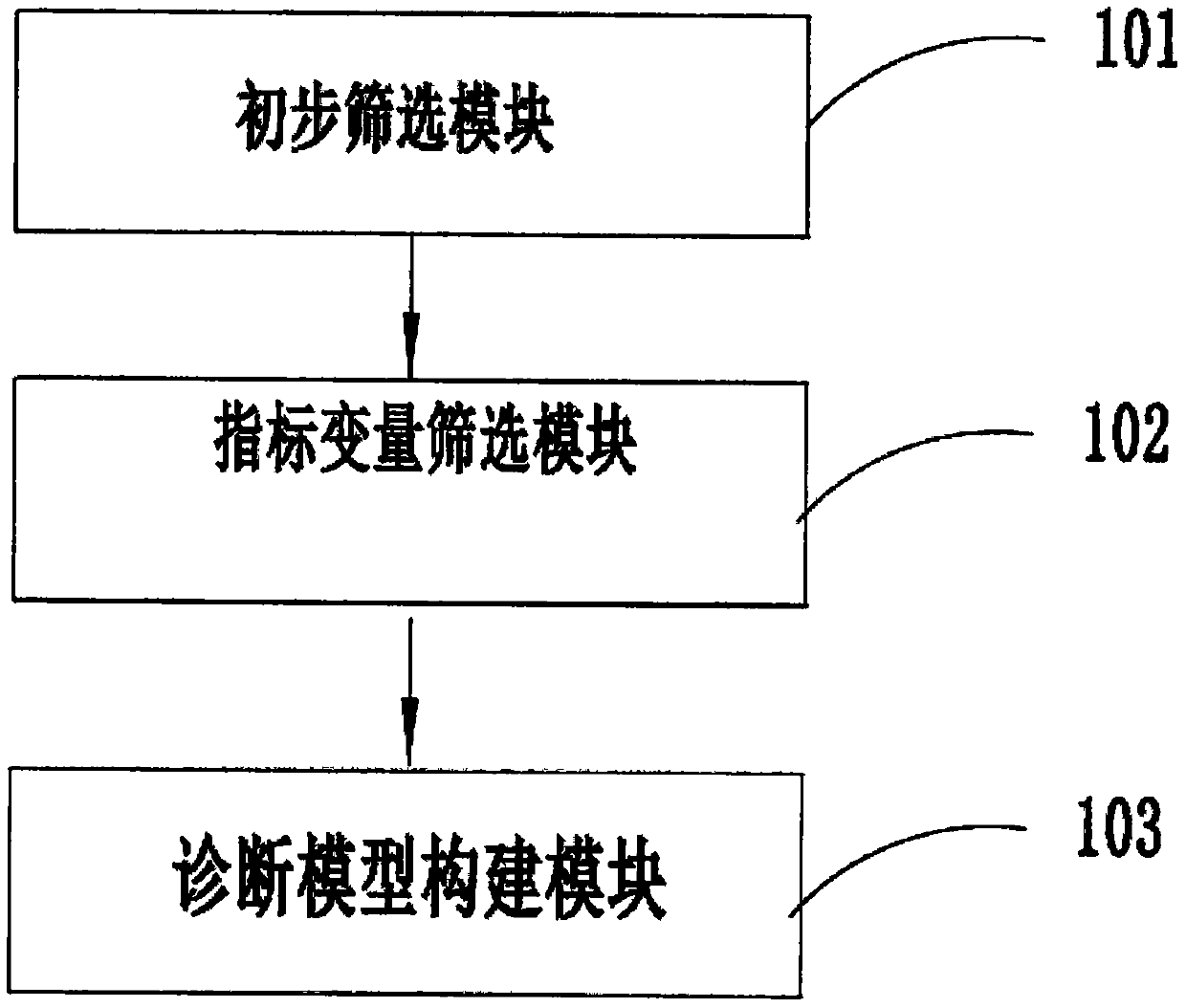
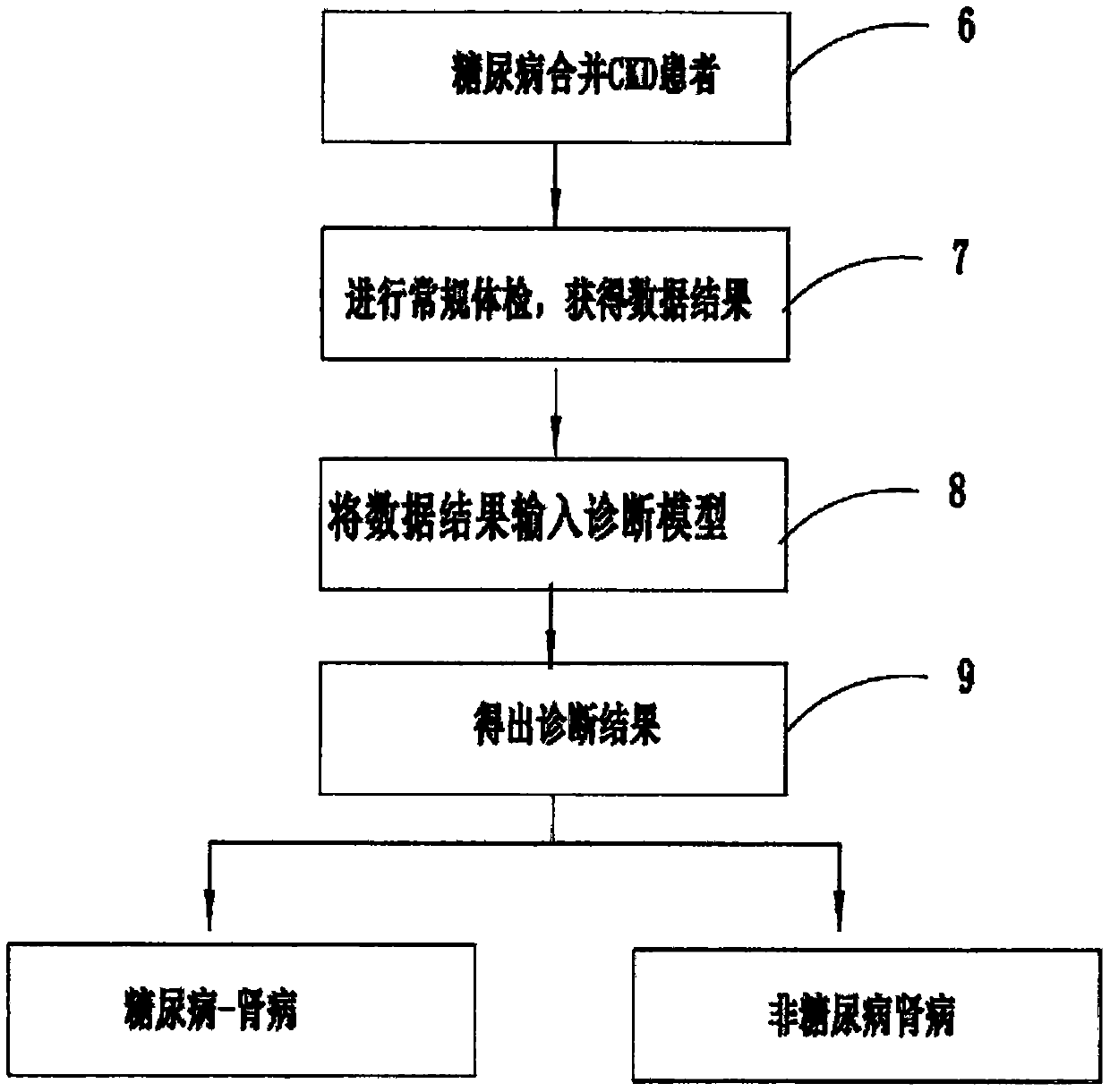
Diabetic nephropathy and non-diabetic nephropathy differential diagnosis device
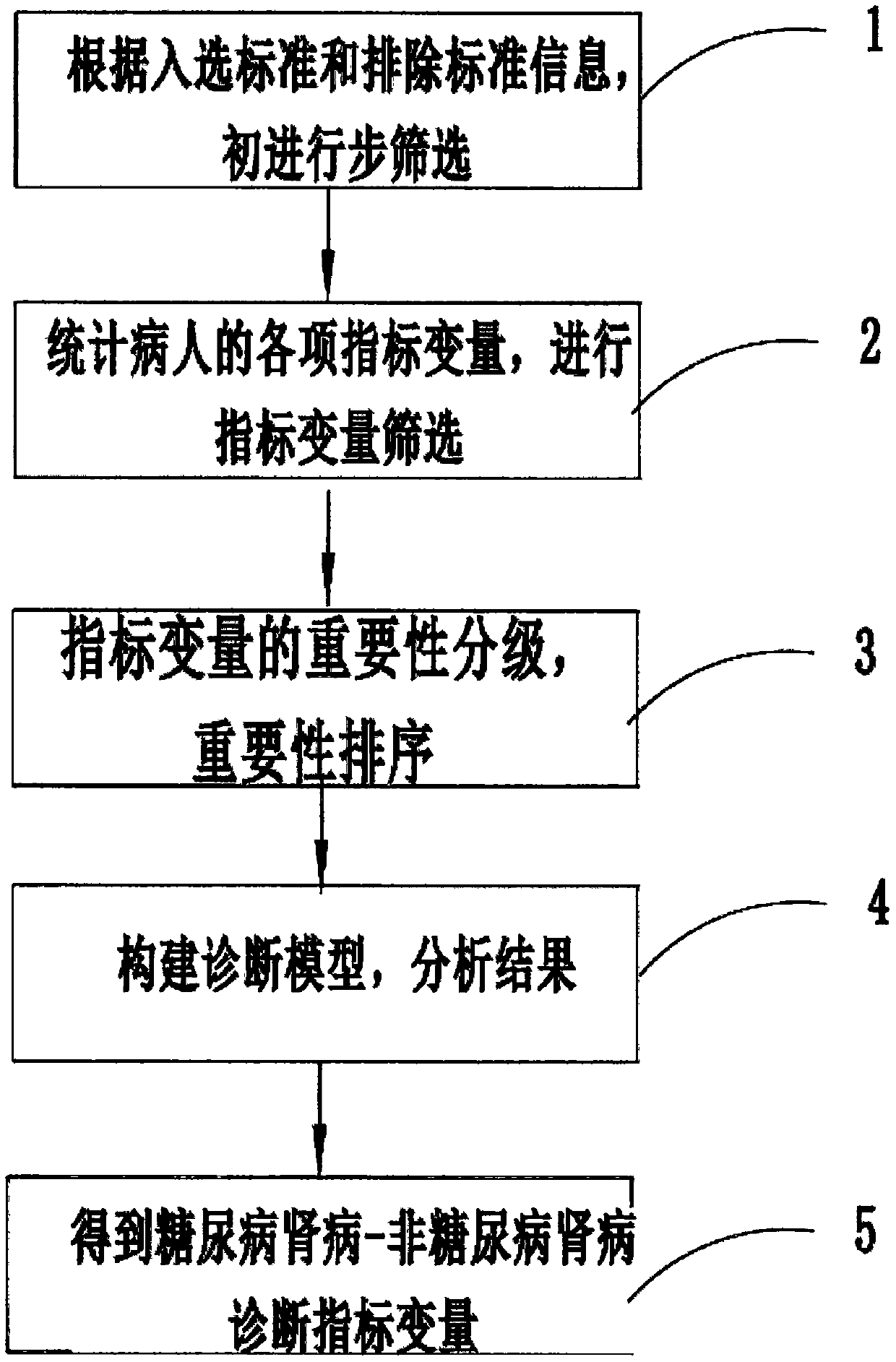
PendingCN110634563ARelieve painAccurate diagnosisMedical automated diagnosisDiagnosis standardsPuncture Biopsy
The invention provides a diabetic nephropathy-non-diabetic nephropathy differential diagnosis device and relates to the biological detection technology field. Defects that a standard in a traditionalchronic kidney disease clinical practice guide (KDOQI) is not objective and unified and is not suitable for serving as a diagnosis standard, and a traditional kidney puncture biopsy technology is large in trauma, can cause complications after operation and is high in technical difficulty. In a diabetic nephropathy-non-diabetic nephropathy differential diagnosis process provided in the invention, anew index is selected by adopting a machine learning method to construct a diabetic nephropathy and non-diabetic nephropathy differential diagnosis model, pain of a patient can be reduced, a diagnosis result is accurate, an application range is wide, and a good auxiliary diagnosis effect is provided for clinical practice.
Owner:GENERAL HOSPITAL OF PLA
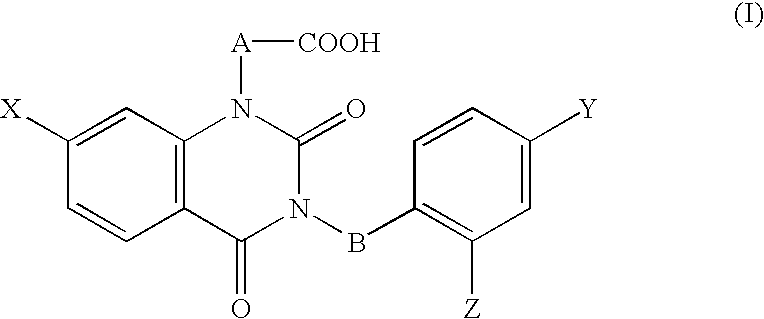
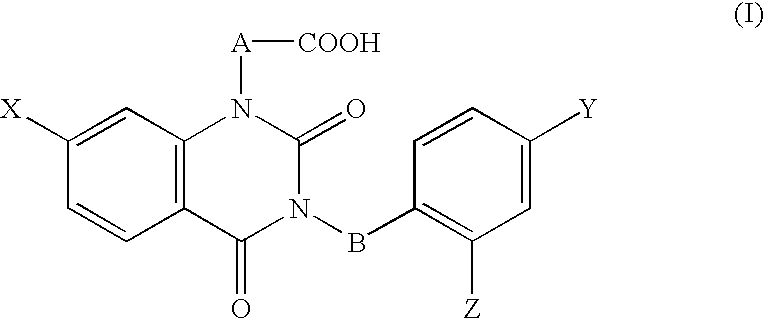
Ophthalmic composition
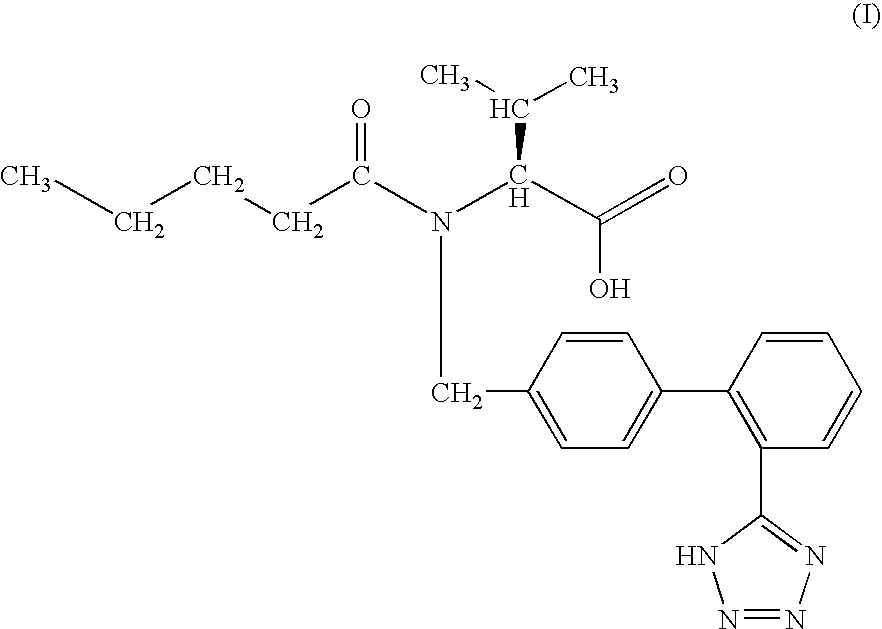
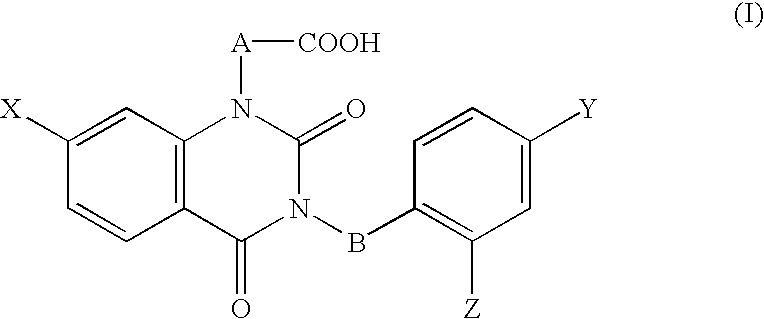
The ophthalmic composition of this invention are used for treatment of diabetic corneal lesion and / or for treatment of deteriorated corneal esthesia, which comprises, as an active ingredient, a compound represented by the general formula (I):whereinA and B are independently lower alkylene,X, Y, and Z are independently halogen,or a pharmacologically acceptable salt thereof.In addition, the ophthalmic composition of this invention are used for treatment of non-diabetic corneal lesion, for treatment of dry eye syndrome, and / or for treatment of hypolacrimation which comprises, as an active ingredient, an aldose reductase inhibitor.The ophthalmic compositions of this invention are effective for treatment of at least one disease selected among corneal lesion, deteriorated corneal esthesia, dry eye syndrome, and hypolacrimation.
Owner:R TECH UENO
Materials and methods for treating vascular leakage in the eye
The invention is directed to a method of prophylactically or therapeutically treating an animal for vascular leakage in an eye. The method comprises administering to an animal in need thereof an expression vector comprising a nucleic acid sequence encoding pigment epithelium-derived factor (PEDF) such that vascular leakage in the eye of the animal is treated prophylactically or therapeutically. The invention also provides a method of prophylactically or therapeutically treating an animal for vascular leakage in an eye. The method comprises periocularly administering to an animal in need thereof PEDF such that vascular leakage of the animal is treated prophylactically or therapeutically. The invention also provides a method of prophylactically or therapeutically treating an animal for non-diabetic vascular leakage in an eye. The method comprises administering to an animal in need thereof PEDF such that non-diabetic vascular leakage in the eye is treated prophylactically or therapeutically.
Owner:GEN VEC INC
Konjac-mannan and ginseng compositions and methods and uses thereof
InactiveUS7326404B2Improve treatment outcomesLowering blood-glucosePowder deliveryBiocideNitric oxideAmorphophallus rivieri
Owner:VUKSAN HLDG
Therapeutic/edible compositions comprising herbal ingredients and methods for treating hyperglycemia
InactiveUS20030180399A1Promote digestionLower blood sugar levelsBiocideUnknown materialsAcute hyperglycaemiaAdditive ingredient
A therapeutic or an edible composition comprising at least three of the five herbs selected from Prunus amygdales, Ocimum sanctum, Azadirachta indica, Aegle marelose and Vitus vinefera, is provided for the treatment of hyperglycemia, especially for non-insulin dependent diabetic subjects, and the herbal composition has significantly reduced the blood glucose levels in both diabetic and non-diabetic experimental subjects having elevated blood glucose levels.
Owner:COUNCIL OF SCI & IND RES
Nutraceutical treatments for diabetic and non-diabetic wound healing
InactiveUS20080003303A1Promote growthPromote healingHeavy metal active ingredientsBiocideWound healingMedicine
In an embodiment of the present invention, a nutraceutical formula comprising a combination of chromium, zinc, berry extract, Polygonum cuspidatum extract, aloe vera, chlorophyll and L-arginine combined synergistically to reduce inflammation in mammals suffering from diabetes. In another embodiment of the present invention, a nutraceutical formula comprising a of zinc, chromium, berry extract, Polygonum cuspidatum extract, aloe vera, chlorophyll and L-arginine combined synergistically to enhance wound healing in mammals suffering from diabetes. In an alternative embodiment of the present invention, a nutraceutical formula comprising a of zinc, chromium, berry extract, Polygonum cuspidatum extractive, aloe vera, chlorophyll and L-arginine combined synergistically to reduce inflammation in mammals not suffering from diabetes. In another embodiment of the present invention, a nutraceutical formula comprising a combination of zinc, chromium, berry extract, Polygonum cuspidatum extractive, aloe vera, chlorophyll, and L-arginine combined synergistically to enhance wound healing in mammals not suffering from diabetes.
Owner:INTERHEALTH NUTRACEUTICALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com