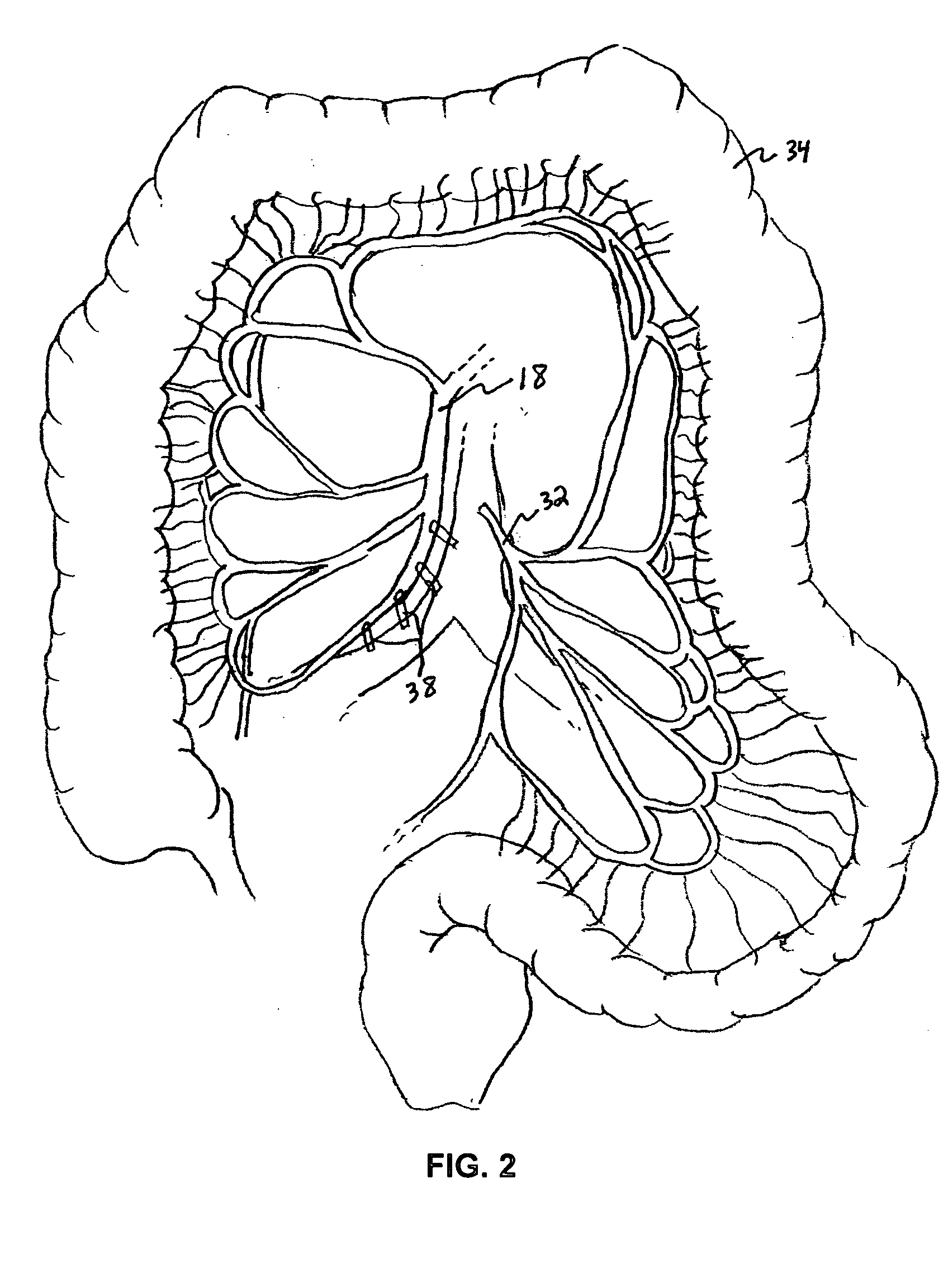
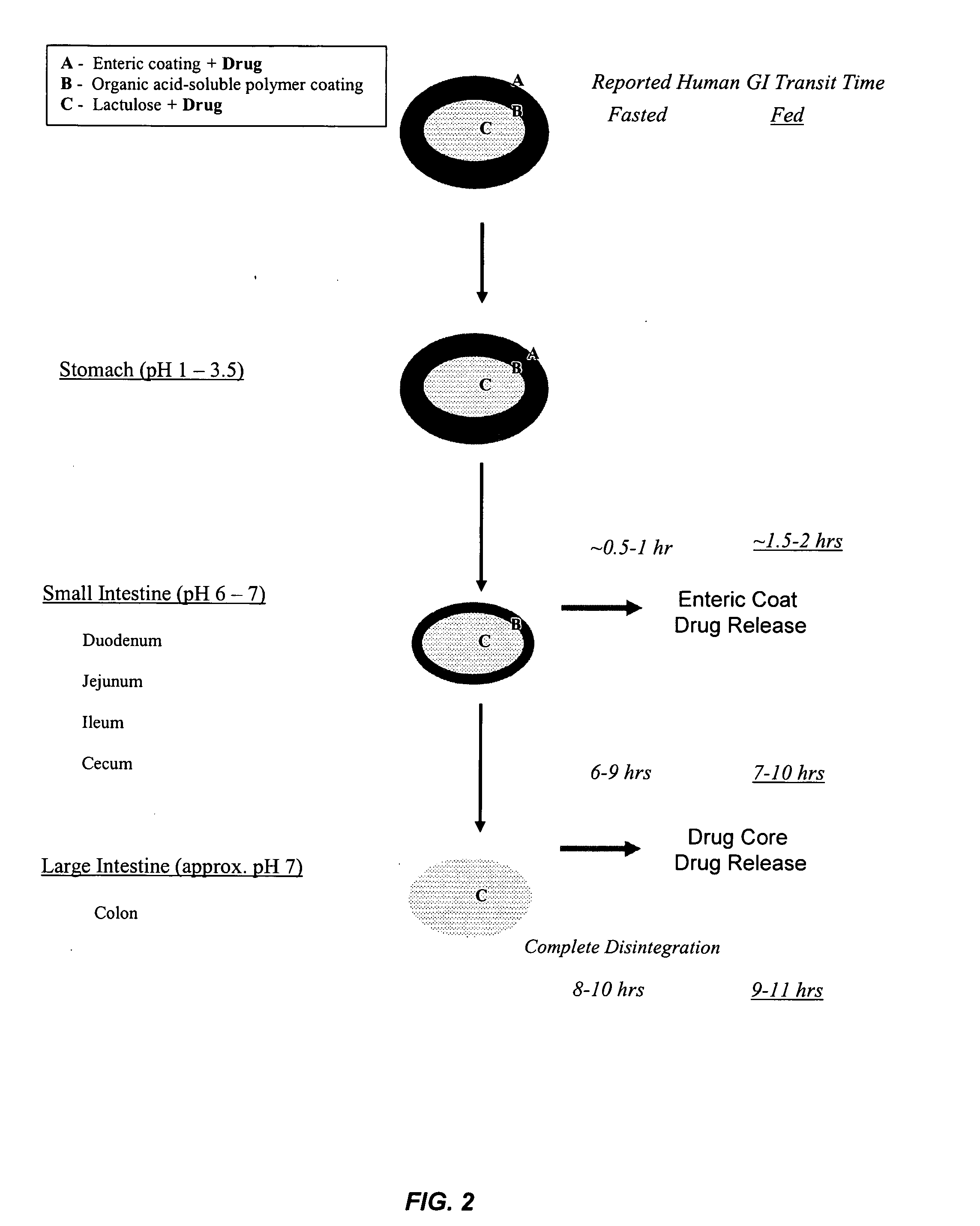
Patents
Literature
227 results about "Duodenum length" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
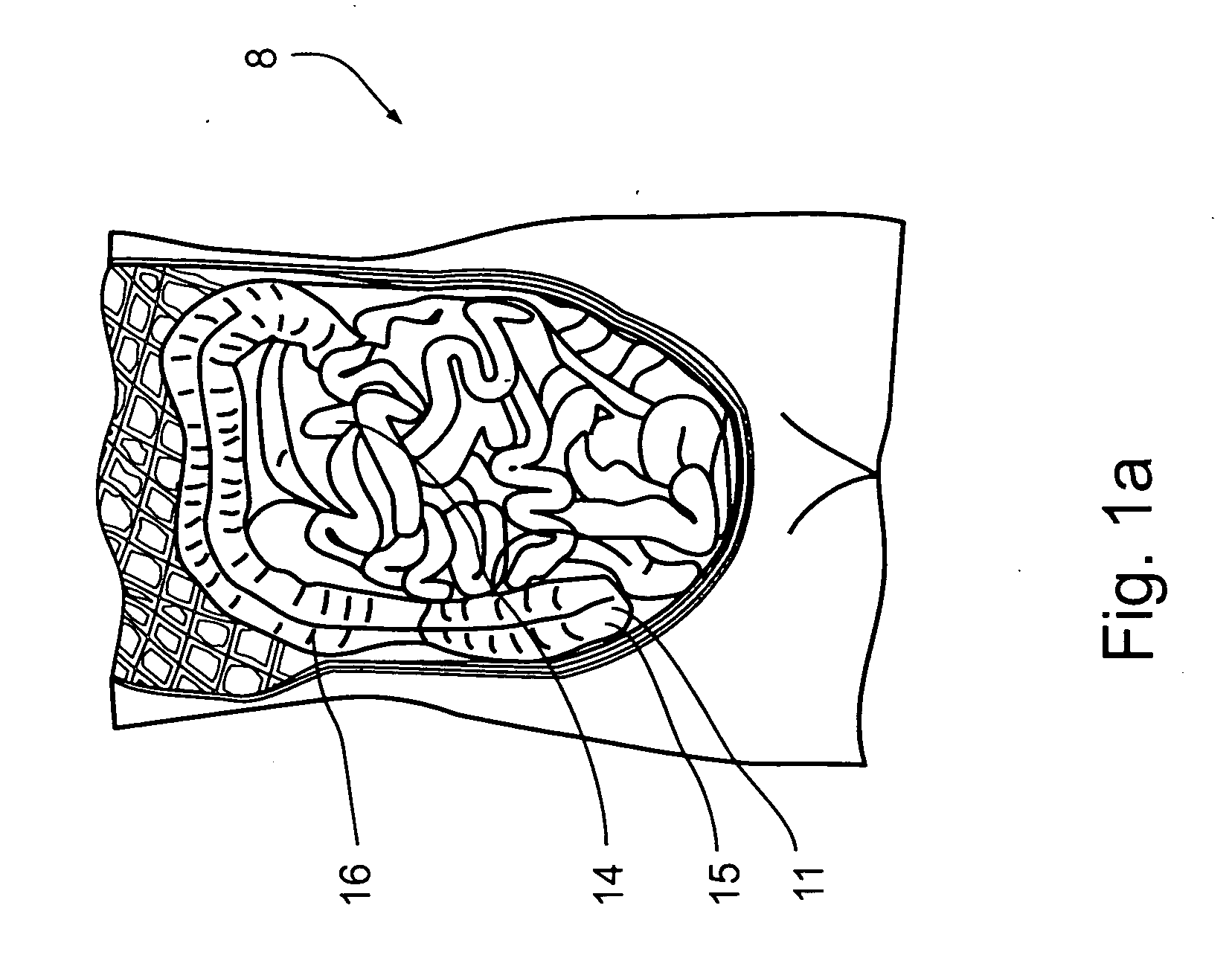
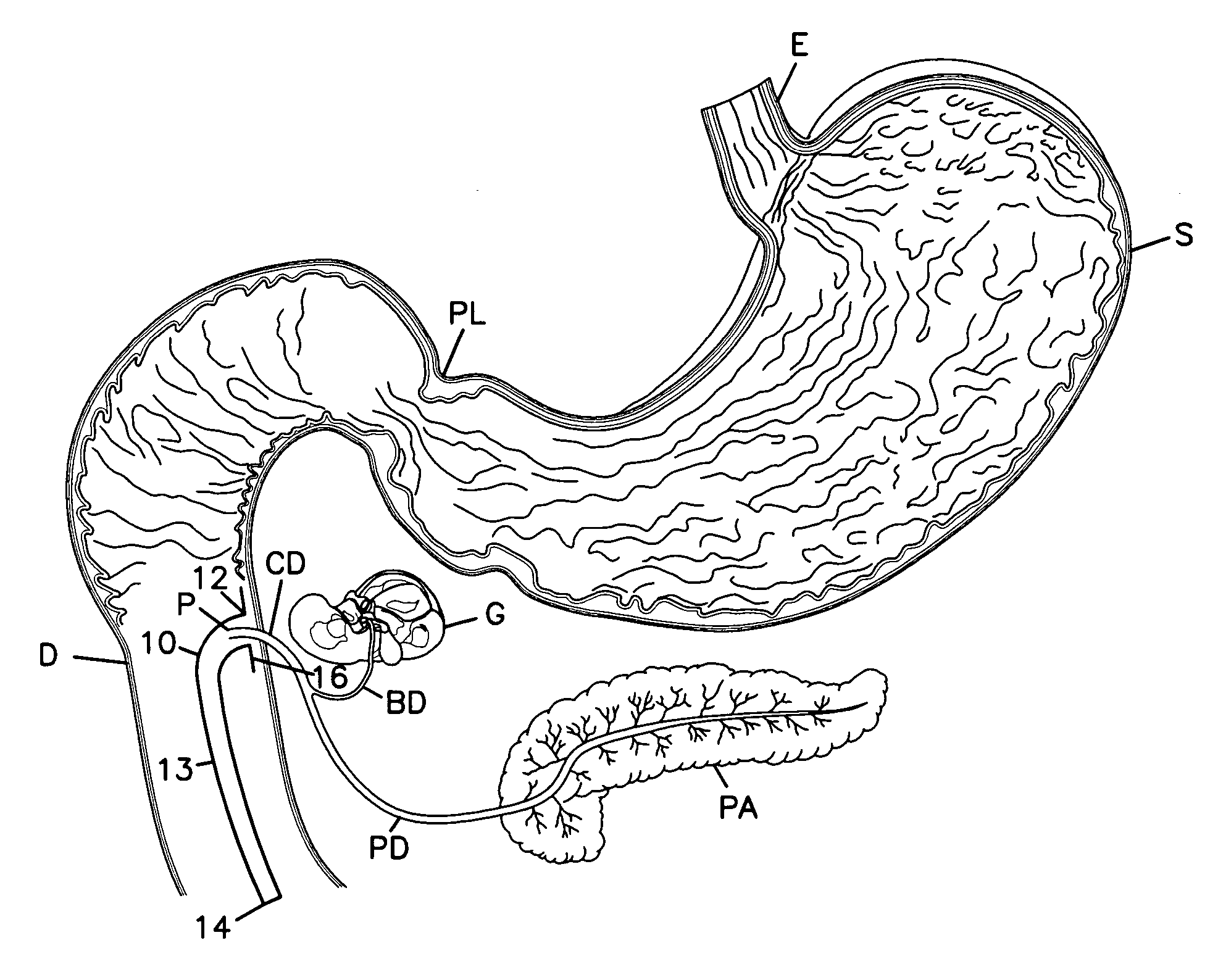
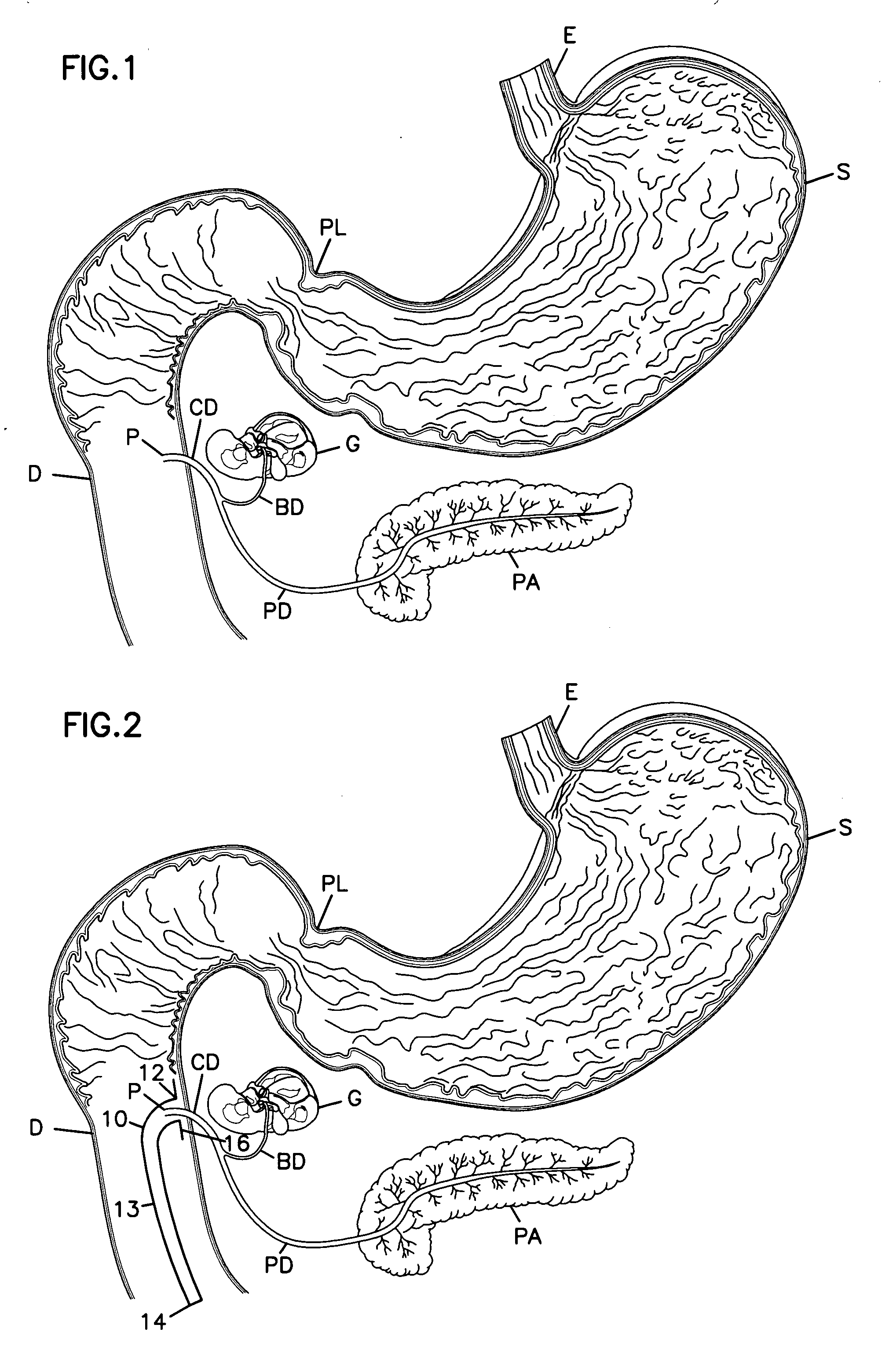
The duodenum is a short structure ranging from 20 cm (7.9 inches) to 25 cm (9.8 inches) in length, and shaped like a "C". It surrounds the head of the pancreas. It receives gastric chyme from the stomach, together with digestive juices from the pancreas (digestive enzymes) and the liver (bile).
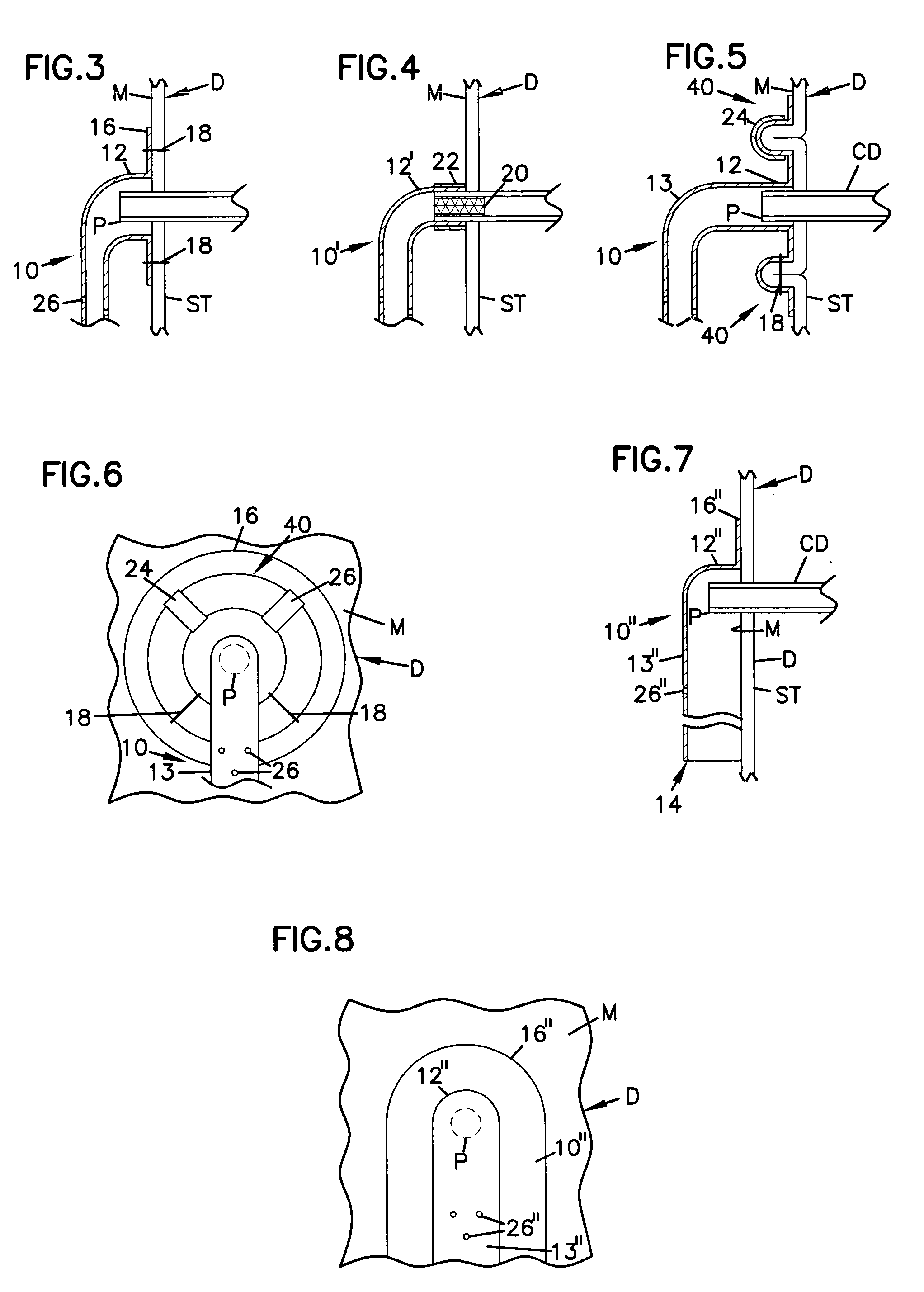
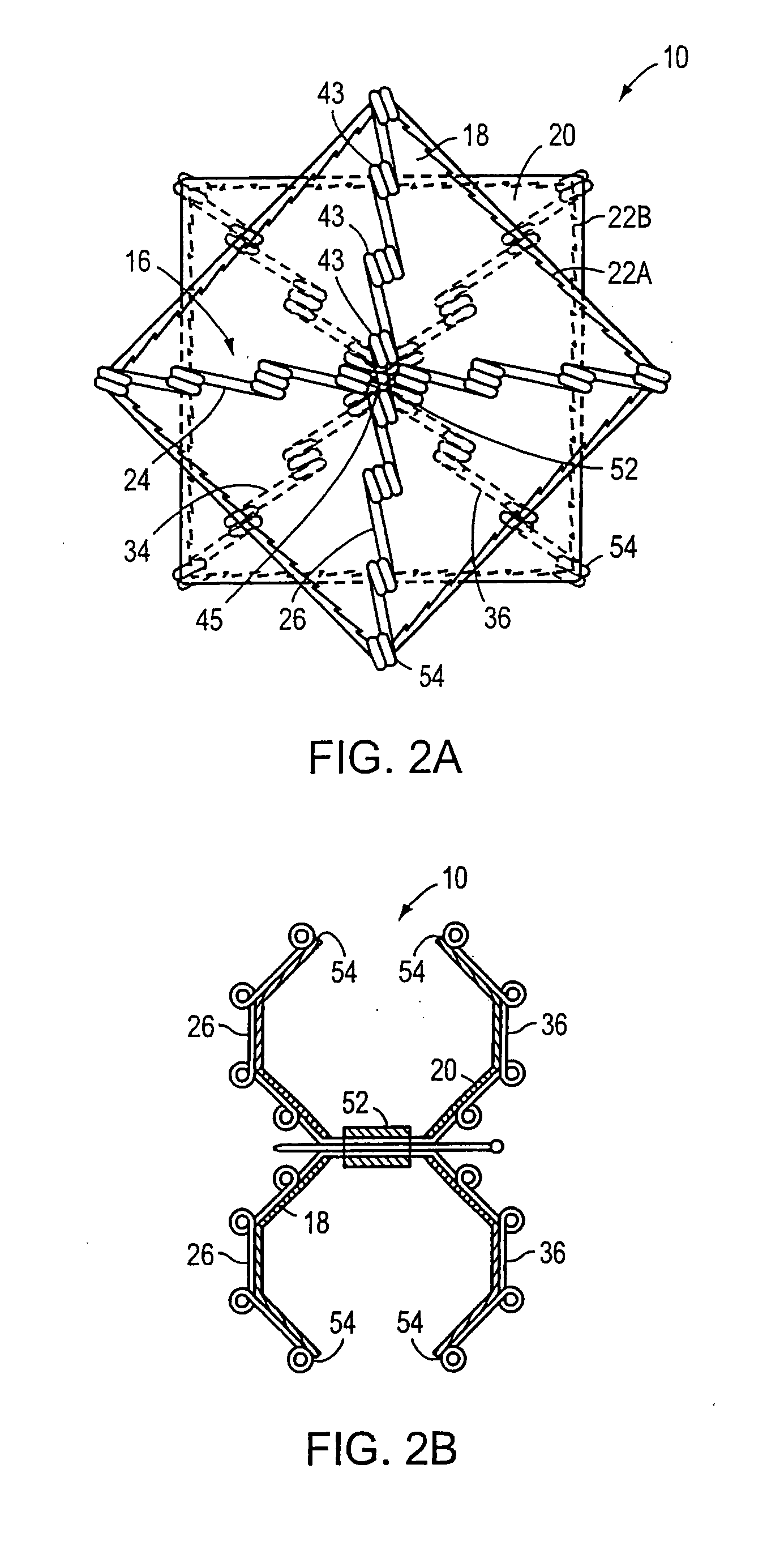
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050049718A1Effectively reducing stomach volumeStimulating intestinal responseMedical devicesTubular organ implantsIntestinal structureMorbid obesity
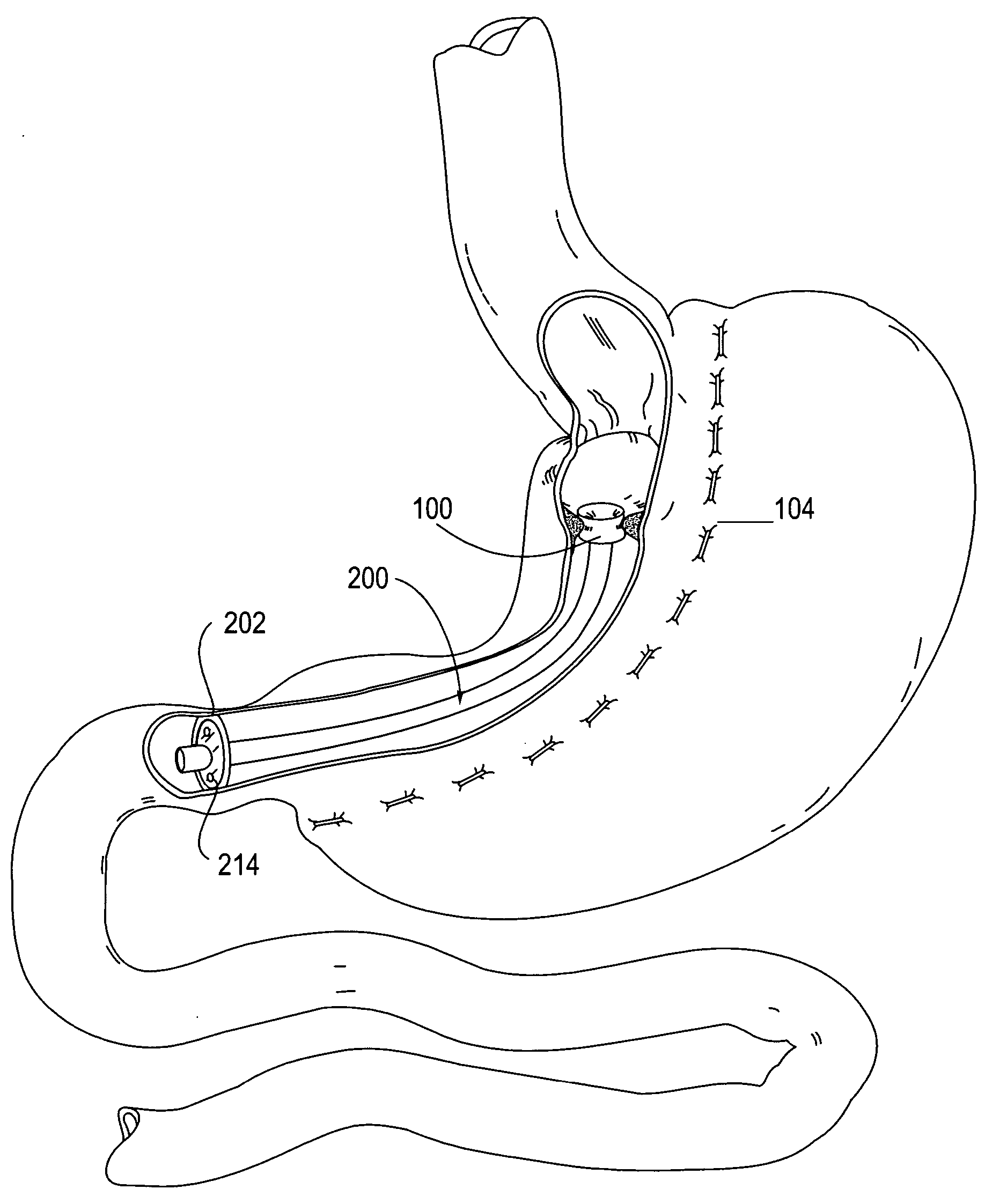
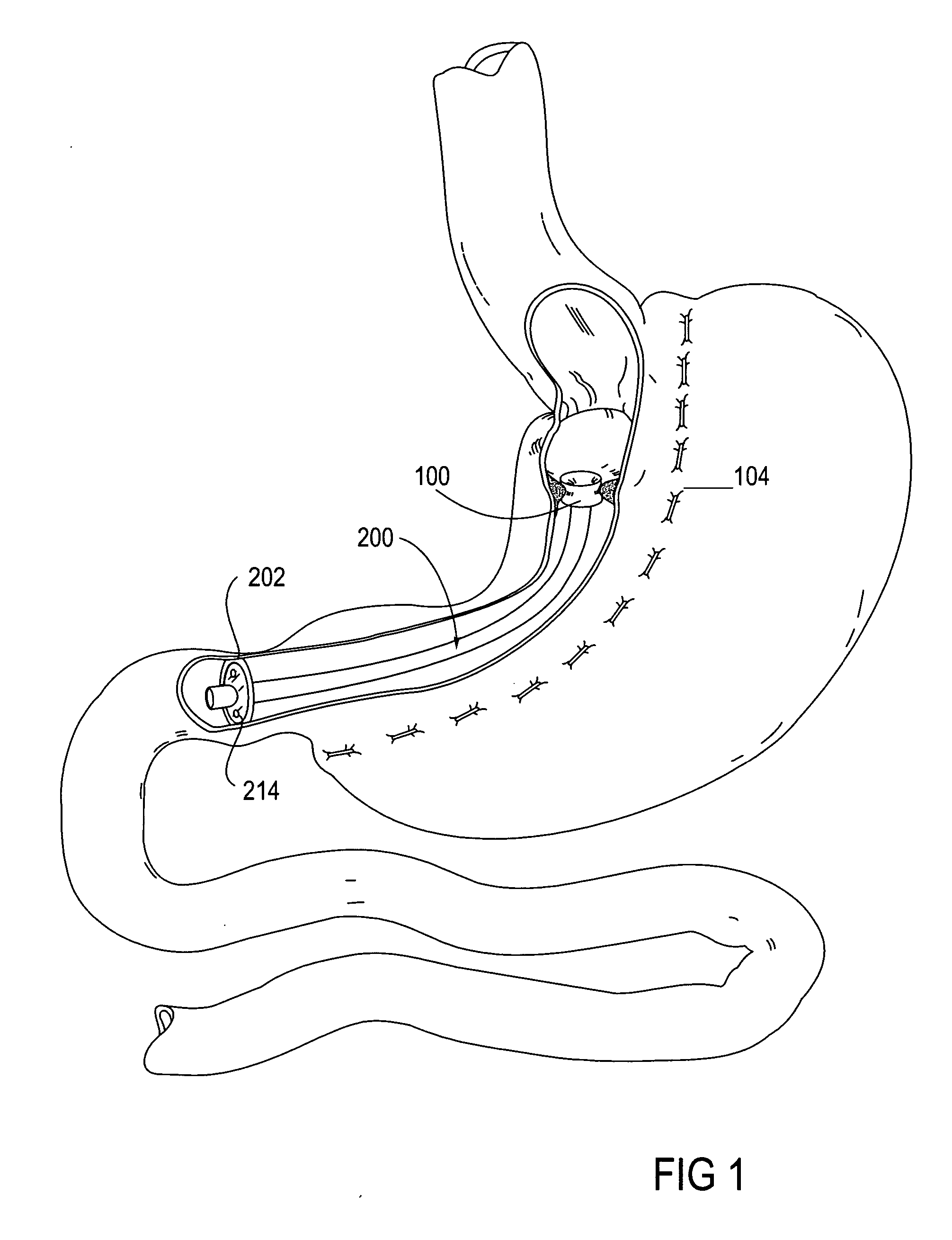
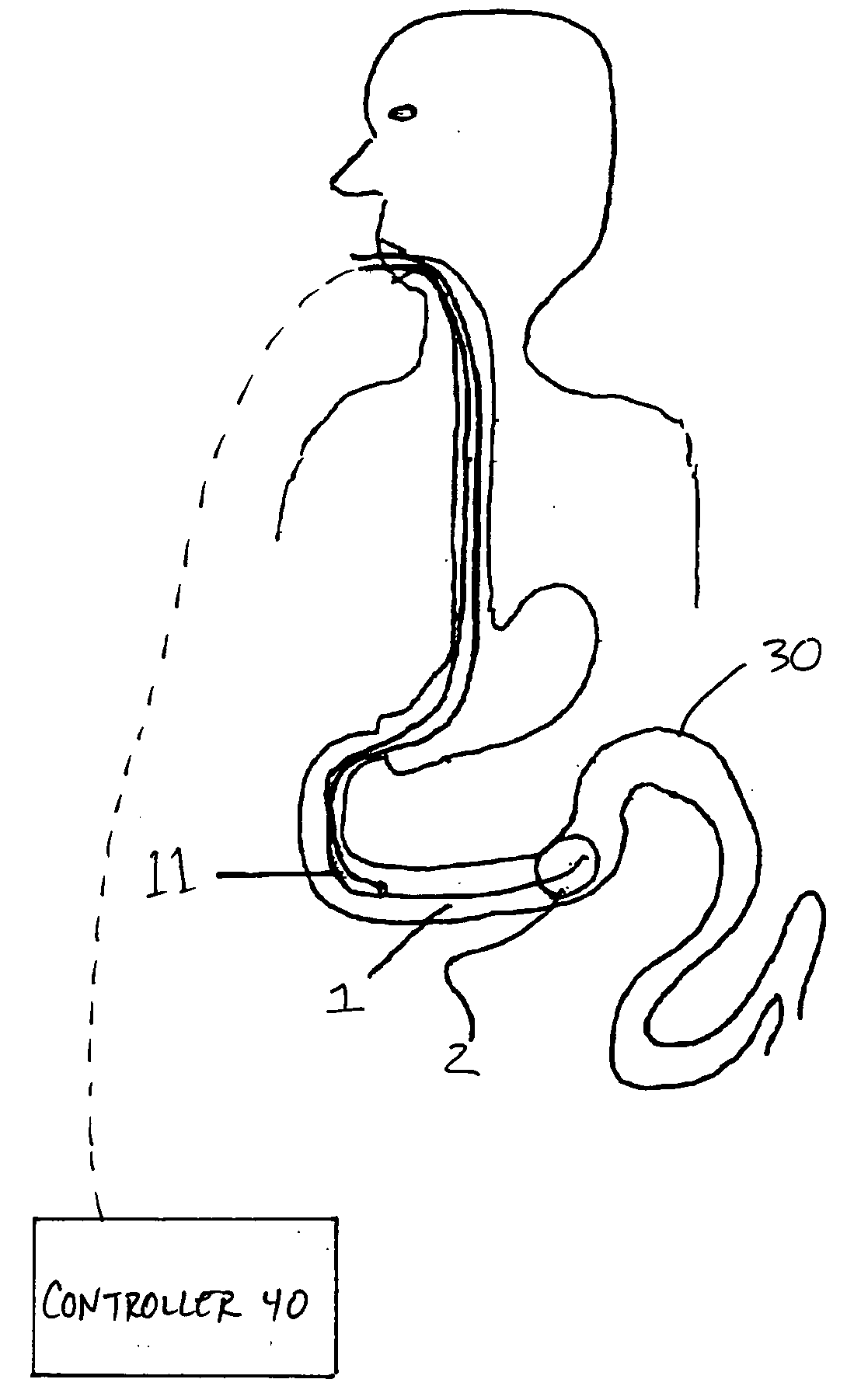
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include a gastric sleeve device, an intestinal sleeve device, and a combined gastrointestinal sleeve device.
Owner:VALENTX
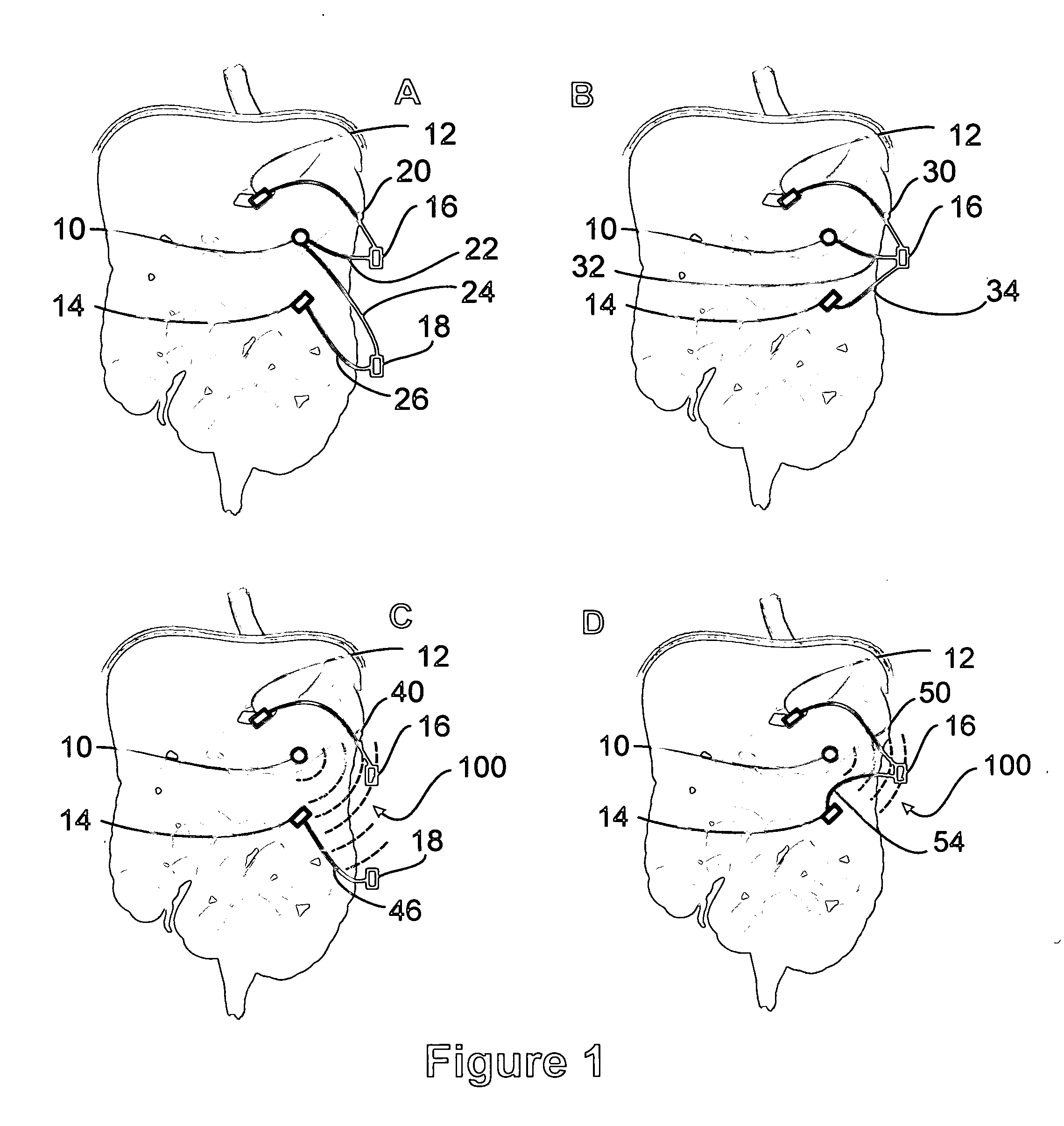
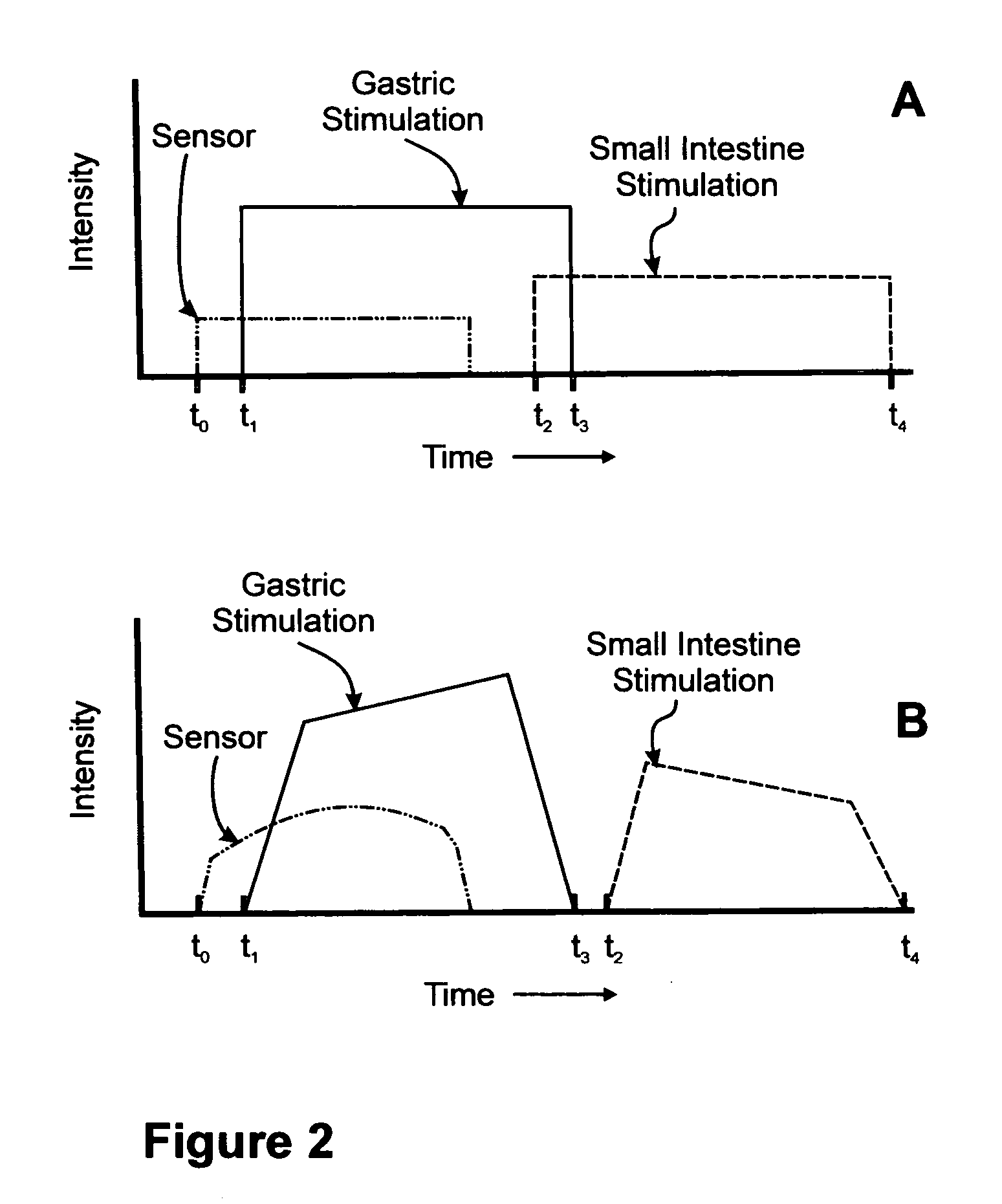
Sensor based gastrointestinal electrical stimulation for the treatment of obesity or motility disorders
InactiveUS20050222638A1Reduce riskAvoid stimulationElectrotherapyArtificial respirationGastroparesisMotility
A method for treatment of obesity, especially morbid obesity, gastroparesis and other syndromes related to motor disorders of the stomach. The method of this invention utilizes a sensor to detect food entering the patient's stomach, thereby the sensor communicates with and activates at least one electrical stimulation device attached to either the stomach or the small intestine.
Owner:MEDTRONIC TRANSNEURONIX
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050240279A1Effectively reducing stomach volumeStimulating intestinal responseTubular organ implantsNon-surgical orthopedic devicesIntestinal structureStoma
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device and a combined gastrointestinal sleeve device.
Owner:VALENTX
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS8734429B2Rapid drug releasePoor absorptionMedical devicesPressure infusionIntestinal wallsDrugs preparations
Owner:RANI THERAPEUTICS
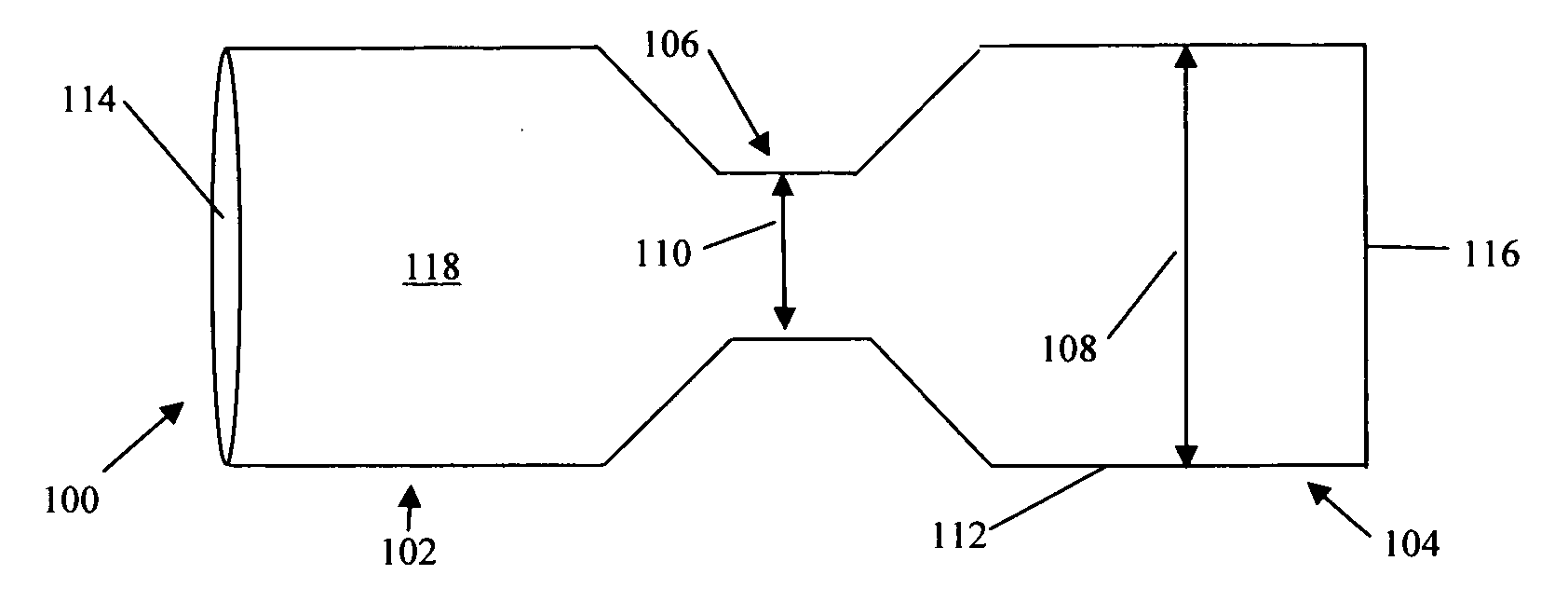
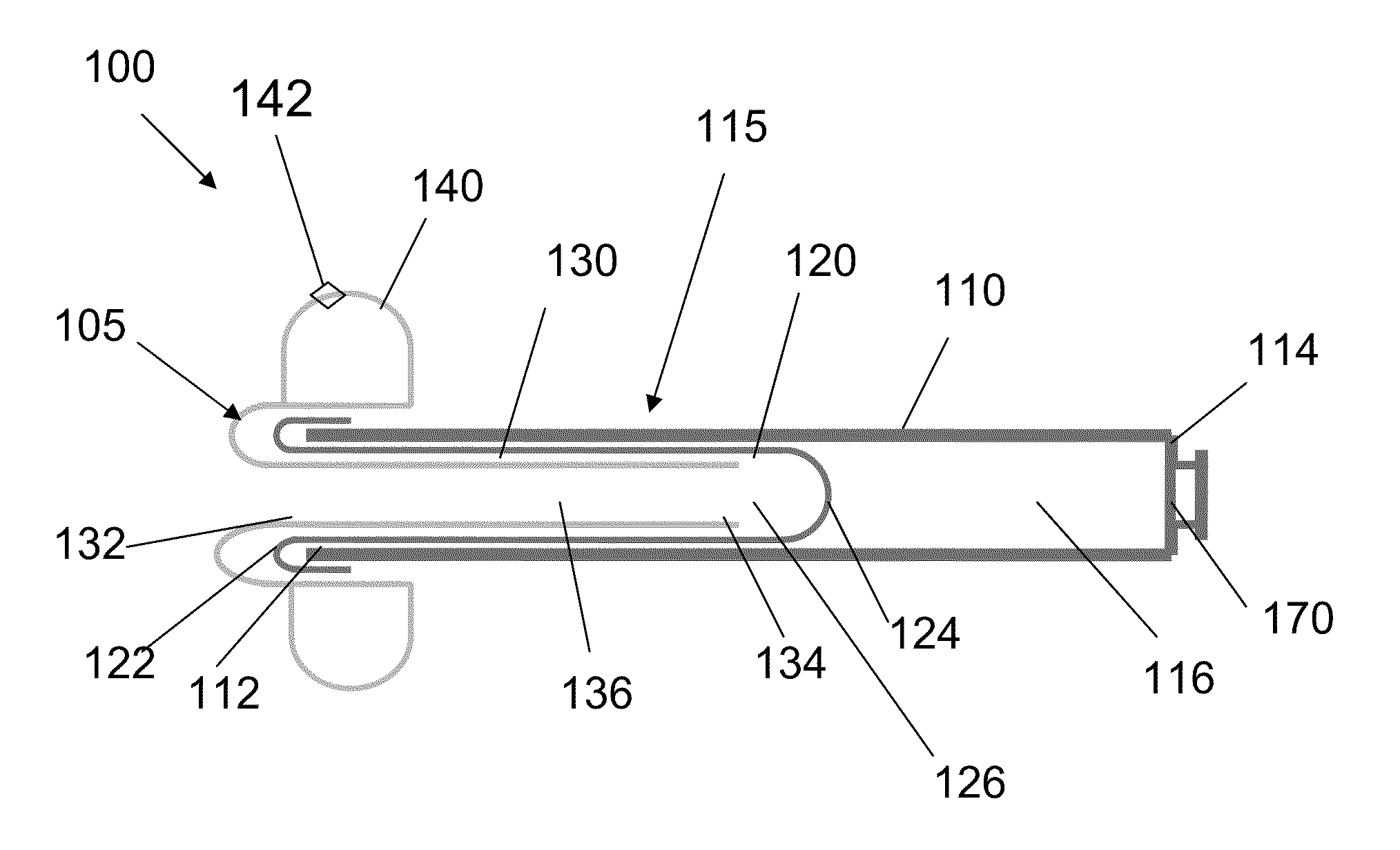
System, system devices, and methods for regulating nutrient absorption and caloric intake
InactiveUS20050197714A1Easy to superviseRegulate nutrient absorption and caloric intakeTubular organ implantsObesity treatmentSmall intestineNon invasive
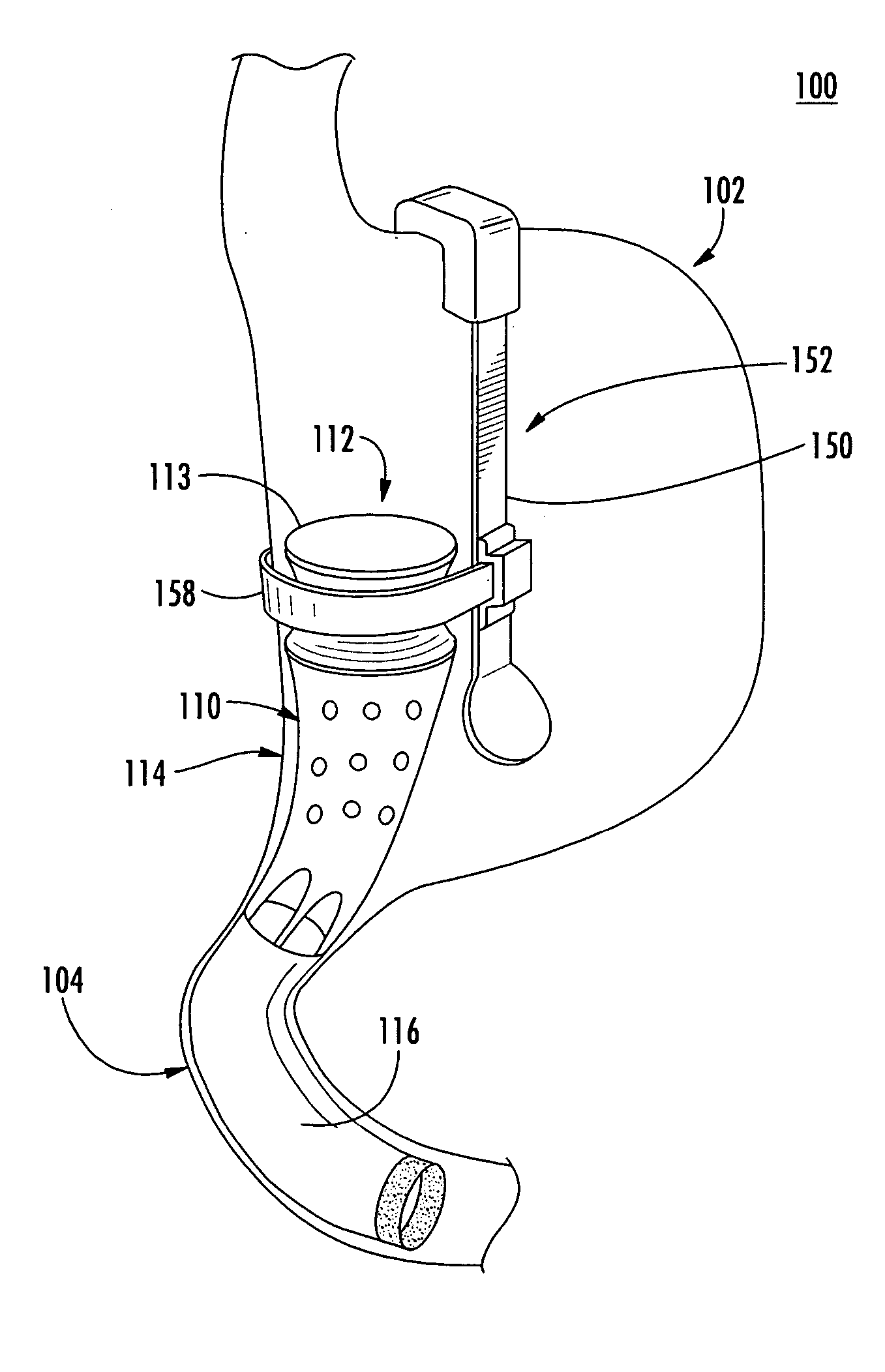
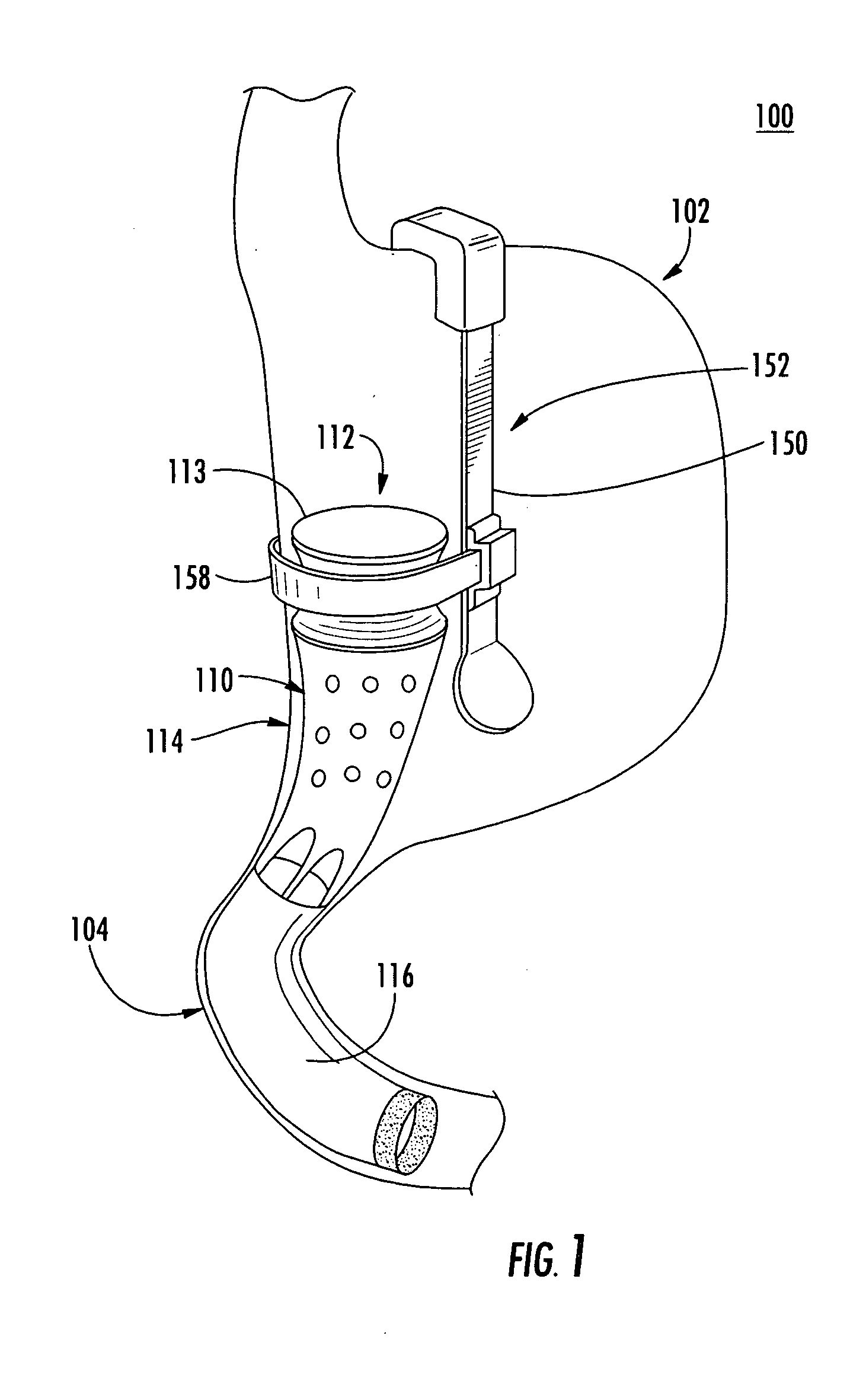
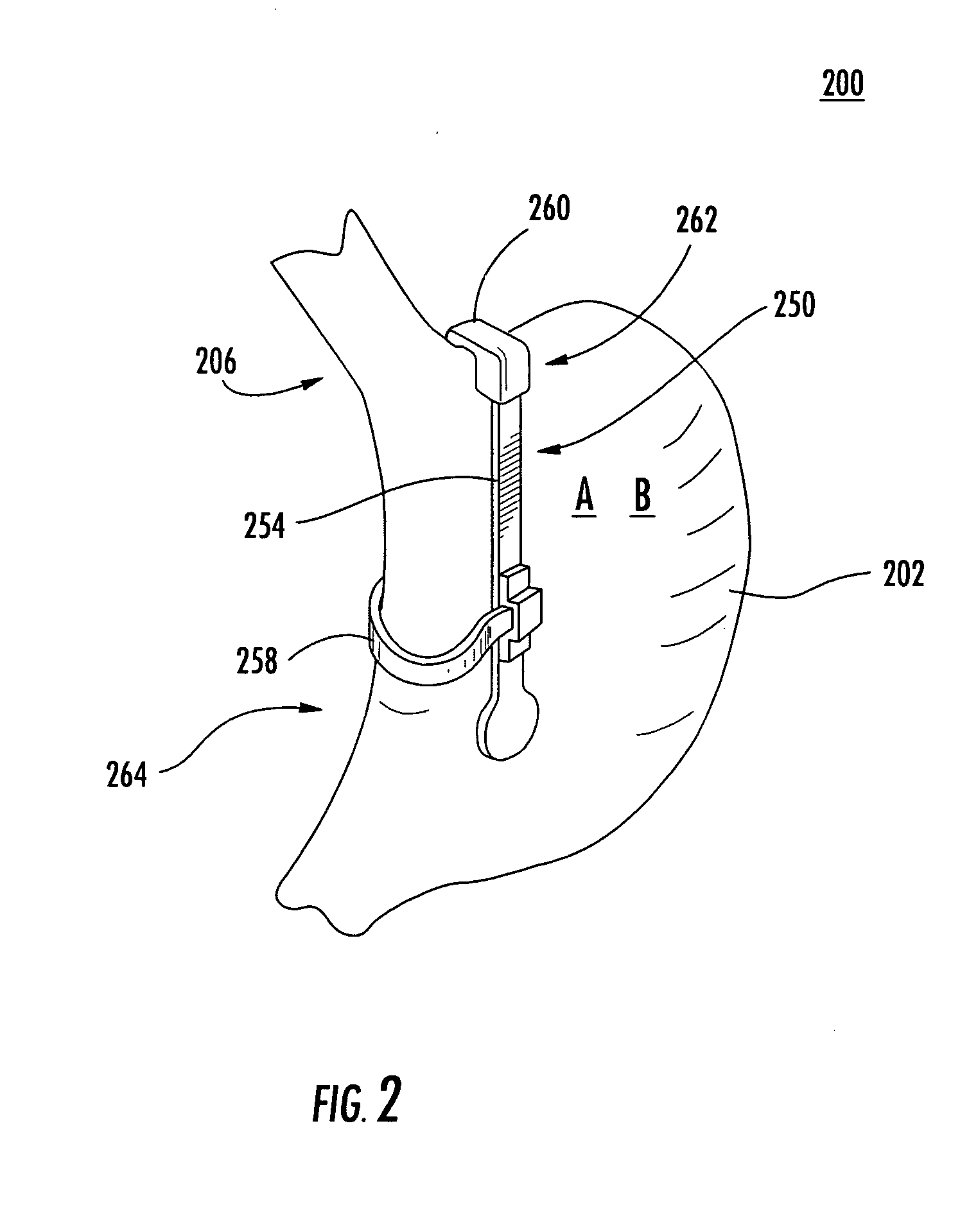
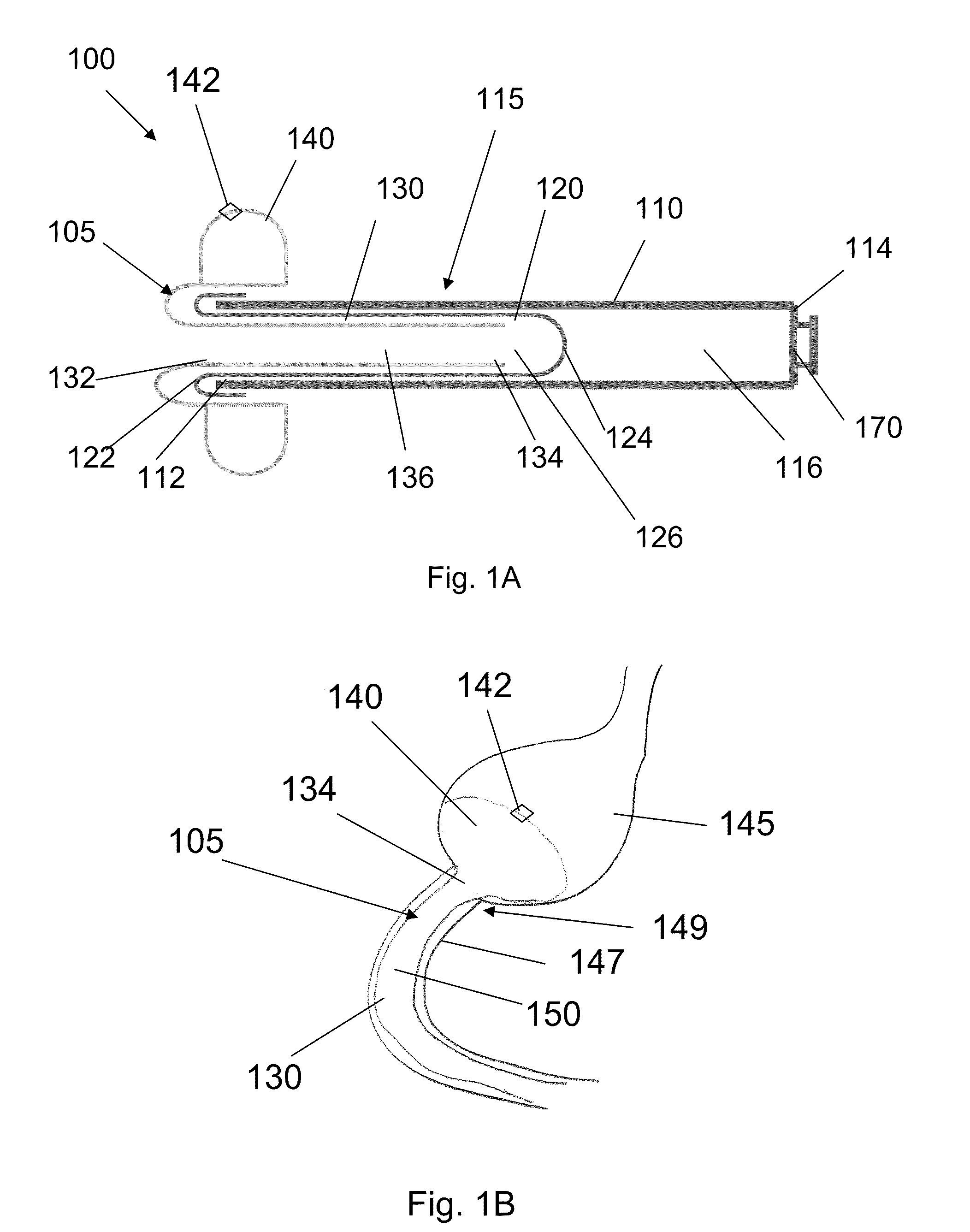
A system (100) for regulating absorption and caloric intake can include an elongated tube (110) having a stomach portion (114) and a lower intestine portion (116) where the elongated tube defines a passage (114) to guide ingested material through the stomach (102) and through a portion of the small intestine (104). The system also includes a non-invasive stomach stricture device (150) having a clamping structure (152) for regulating the rate of flow of ingested material through the elongated tube where the clamping structure reduces the capacity of the passage by clamping a portion of the elongated tube through the exterior of the stomach.
Owner:PRECISION MEDICAL DEVICES INC
Ingestible device platform for the colon
ActiveUS20050266074A1Enhance the imageUltrasonic/sonic/infrasonic diagnosticsSurgeryAbnormal tissue growthOptical fluorescence
An ingestible pill platform for colon imaging is provided, designed to recognize its entry to the colon and expand in the colon, for improved imaging of the colon walls. On approaching the external anal sphincter muscle, the ingestible pill may contract or deform, for elimination. Colon recognition may be based on a structural image, based on the differences in diameters between the small intestine and the colon, and particularly, based on the semilunar fold structure, which is unique to the colon. Additionally or alternatively, colon recognition may be based on a functional image, based on the generally inflammatory state of the vermiform appendix. Additionally or alternatively, pH, flora, enzymes and (or) chemical analyses may be used to recognize the colon. The imaging of the colon walls may be functional, by nuclear-radiation imaging of radionuclide-labeled antibodies, or by optical-fluorescence-spectroscopy imaging of fluorescence-labeled antibodies. Additionally or alternatively, it may be structural, for example, by visual, ultrasound or MRI means. Due to the proximity to the colon walls, the imaging in accordance with the present invention is advantageous to colonoscopy or virtual colonoscopy, as it is designed to distinguish malignant from benign tumors and detect tumors even at their incipient stage, and overcome blood-pool background radioactivity.
Owner:SPECTRUM DYNAMICS MEDICAL LTD
Slowly digesting starch and fermentable fiber
ActiveUS20070196437A1Prevention and treatmentBenefit their healthOrganic active ingredientsBiocideDigestible starchAdditive ingredient
Compositions which provide slowly digestible starch and a source of fermentable dietary fiber. Microparticles in which starch is entrapped in a crosslinked matrix to provide dietary benefit. Such microparticles are used to deliver glucose to targeted regions in the small intestine for beneficial physiological effects, and fermentable dietary fiber to the colon to improve colon health and to treat diseases of the colon. Microparticles can be employed to selectively deliver fermentable dietary fiber to targeted portions of the colon. A method for making the microparticles is provided as well as methods for using the microparticles for controlled digestion of starch on ingestion in the small intestine and methods for using the microparticles to deliver dietary fiber. The microparticles with entrapped starch provide a low glycemic index and extended glucose release in food products and food ingredients. The microparticles with entrapped starch can, in particular, be used as an ingredient in foods that are to be cooked.
Owner:PURDUE RES FOUND INC +1
Stomach peristalsis device and method
InactiveUS20060129237A1Facilitate expedite mixing breaking downPassage is slowedLigamentsMusclesPylorusPeristalsis
The invention relates to an implantable stomach prosthesis for surgically replacing or augmenting all or part of the antrum and / or pylorus of a stomach. The prosthesis controls the passage of food from the stomach to the small intestine. The prosthesis may be configured to chum ingested material and release it from the stomach through a prosthetic pyloric valve. At least one expandable member is arranged to be expanded to control the passage of food and / or to mimic the churning action of a patient's stomach. The prosthesis includes an outer support structure, a flexible inner member forming a conduit for the movement of material, and at least one expandable member located between the outer support structure and inner member. An implantable pump system is provided for inflating and deflating the expandable member(s).
Owner:PYTHON MEDICAL
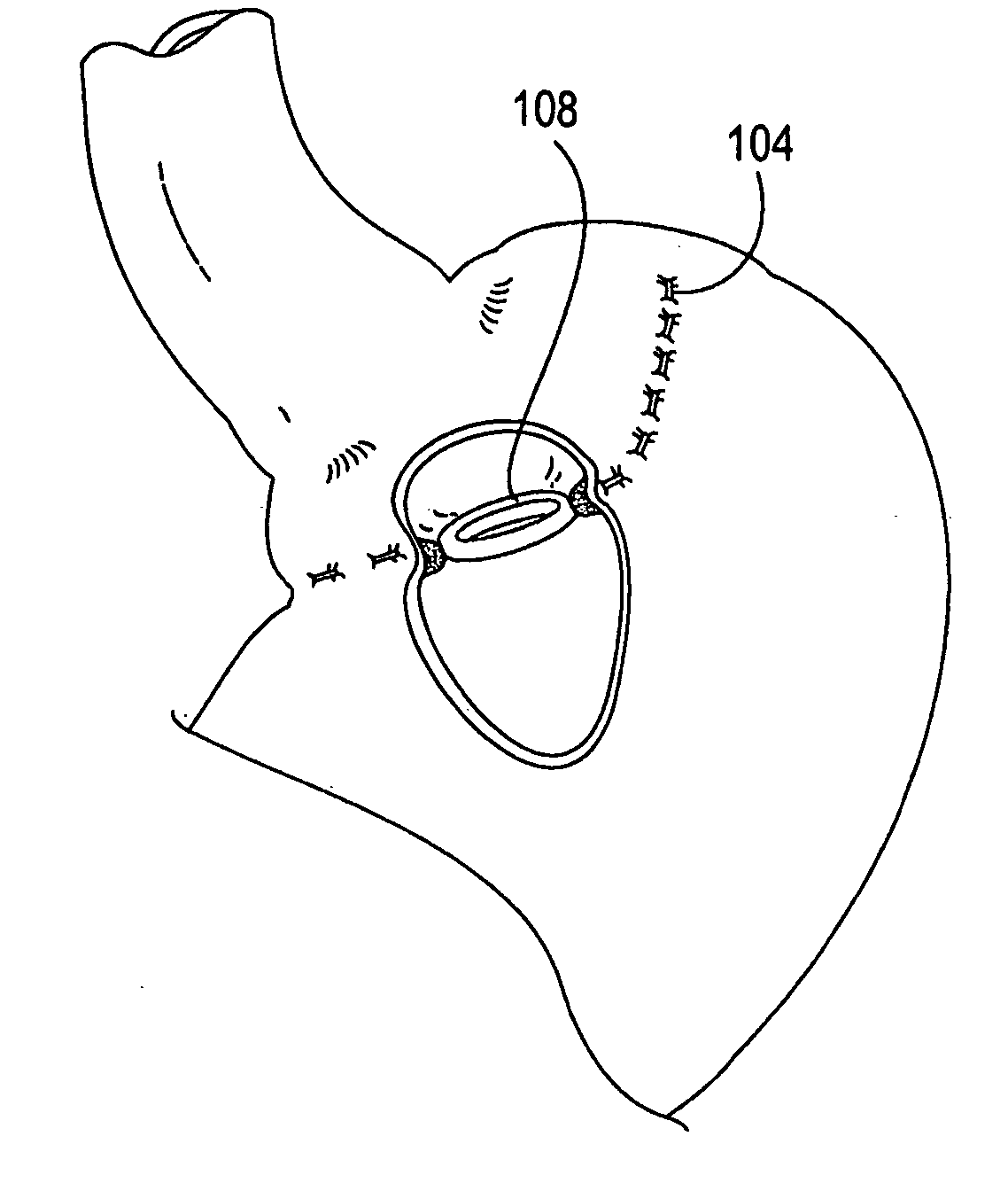
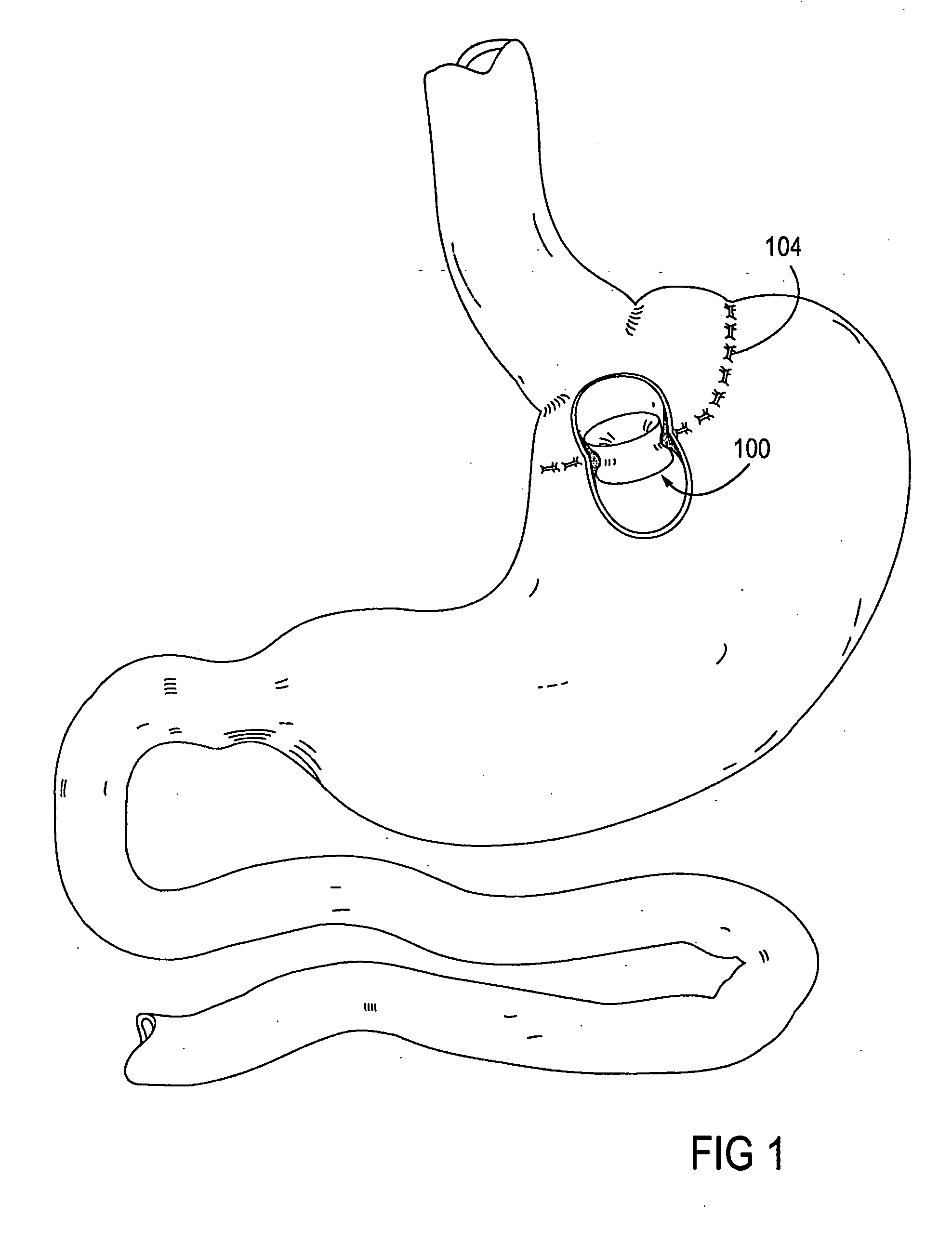
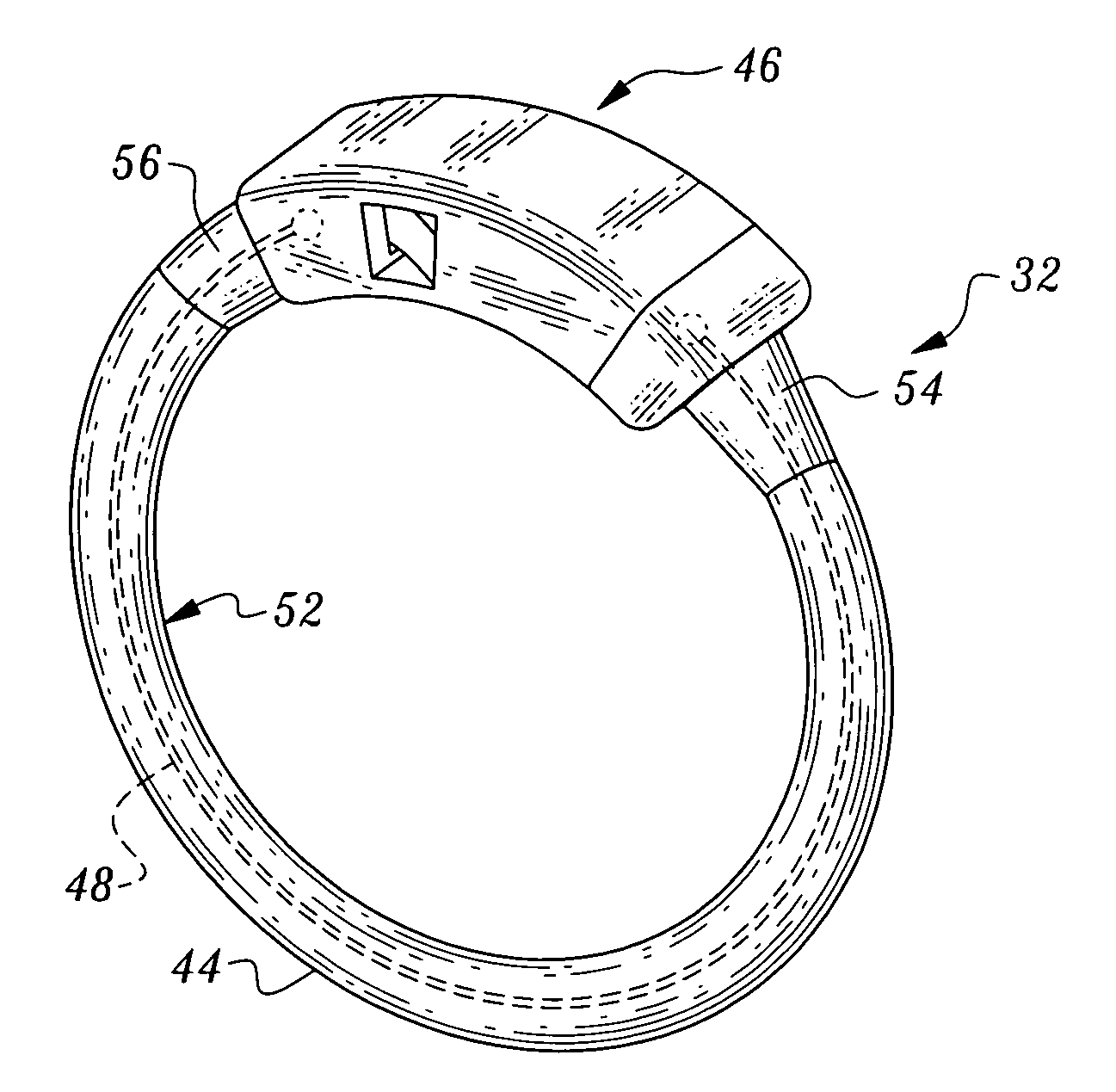
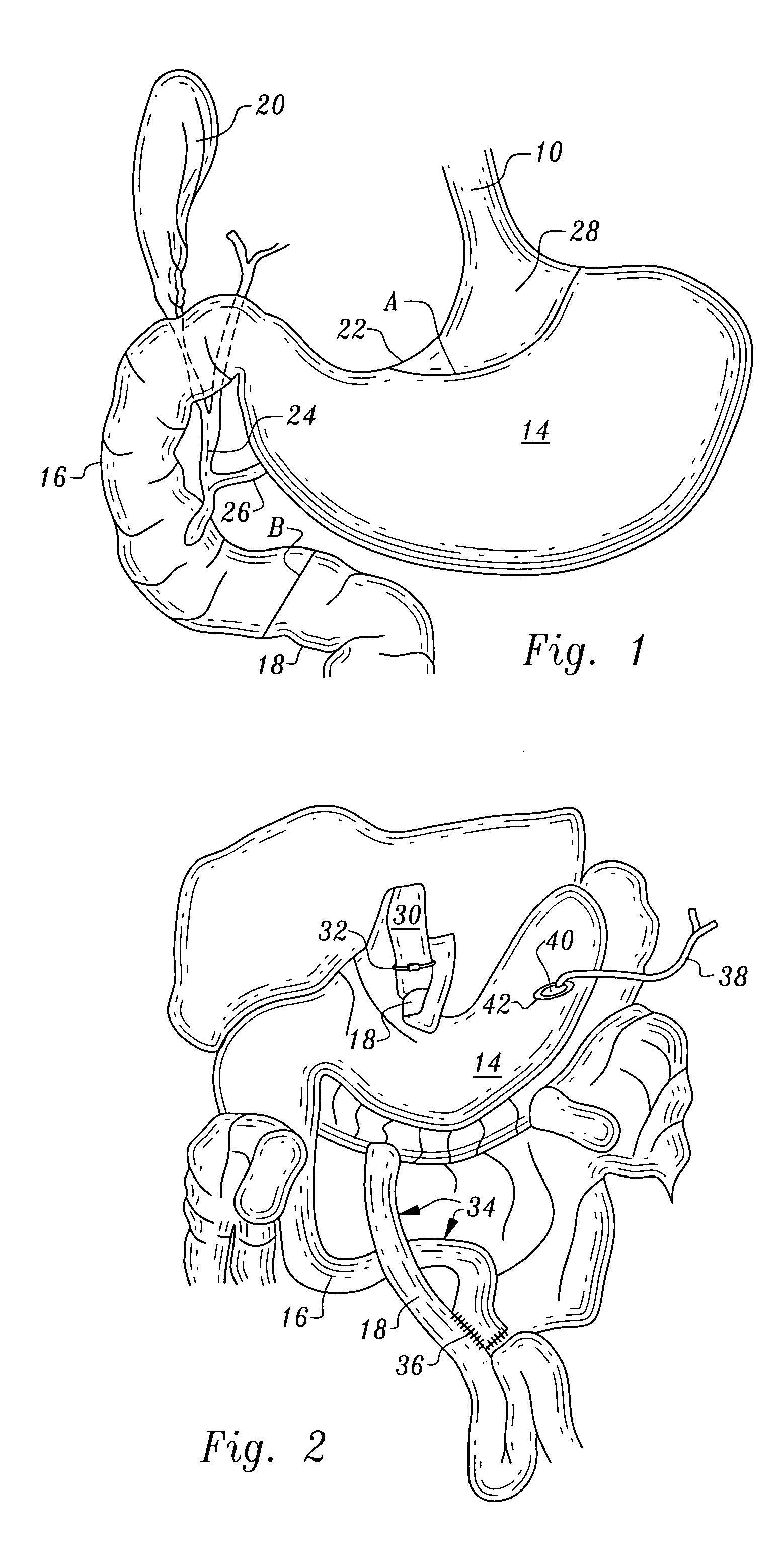
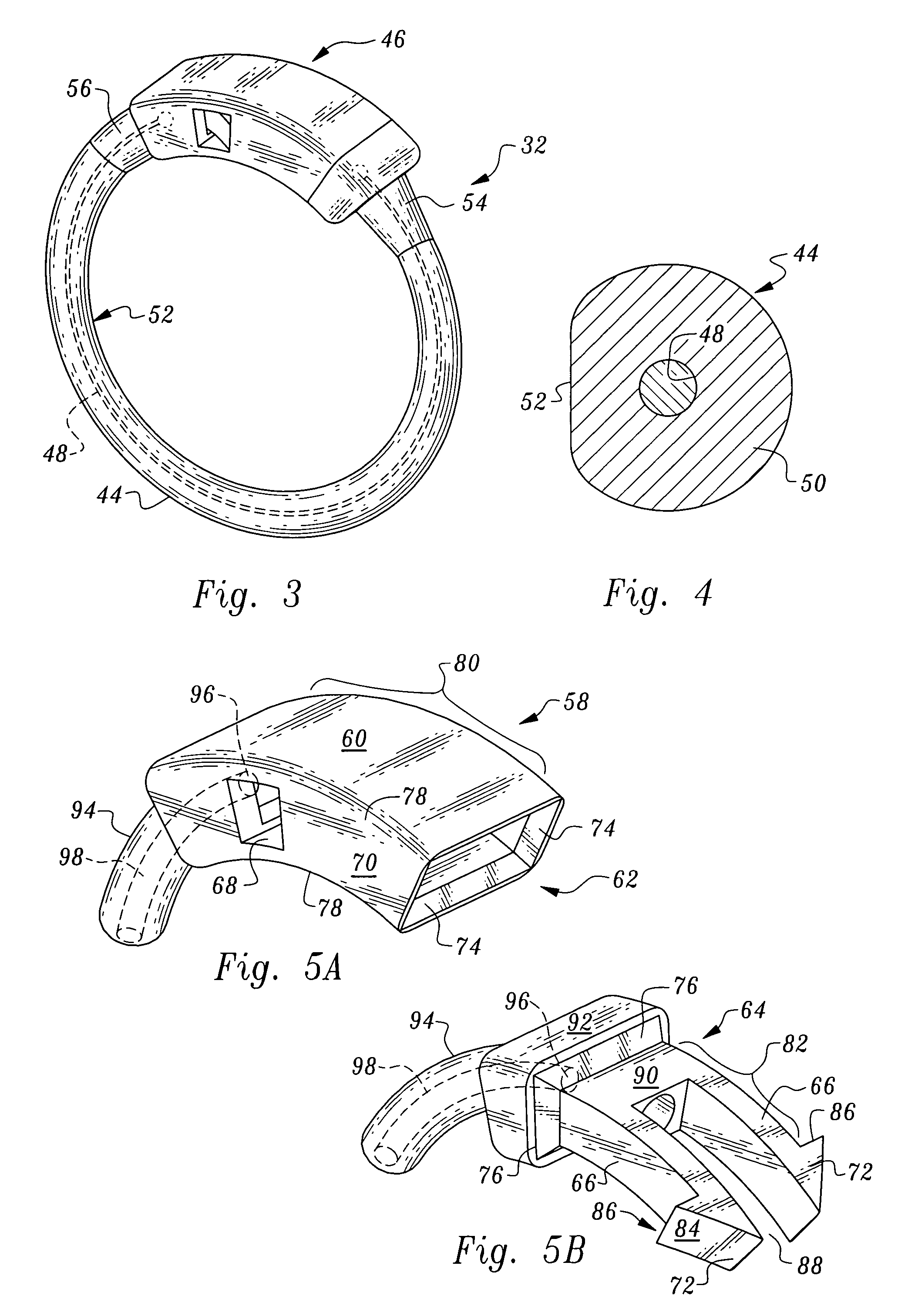
Gastric bypass band and surgical method
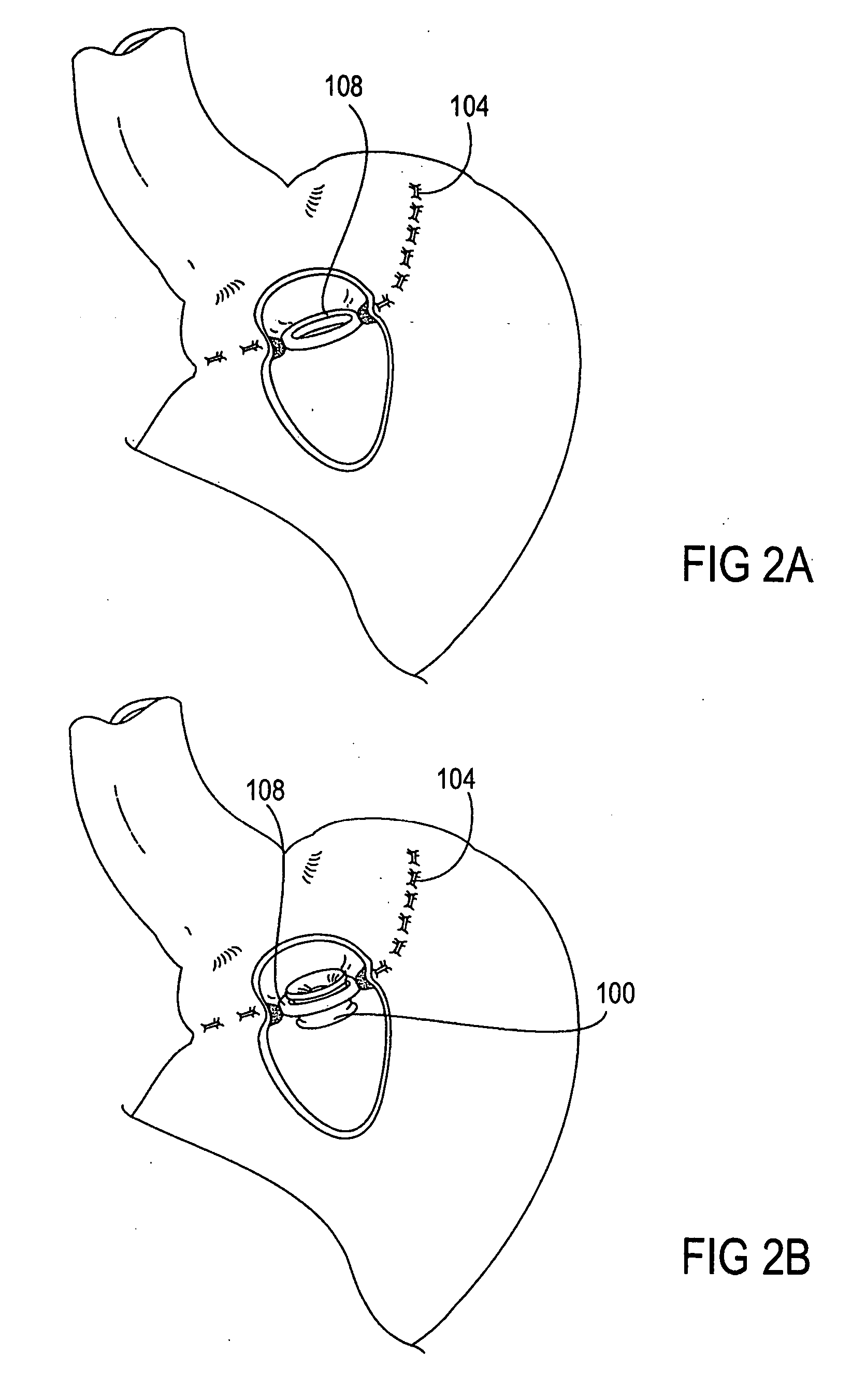
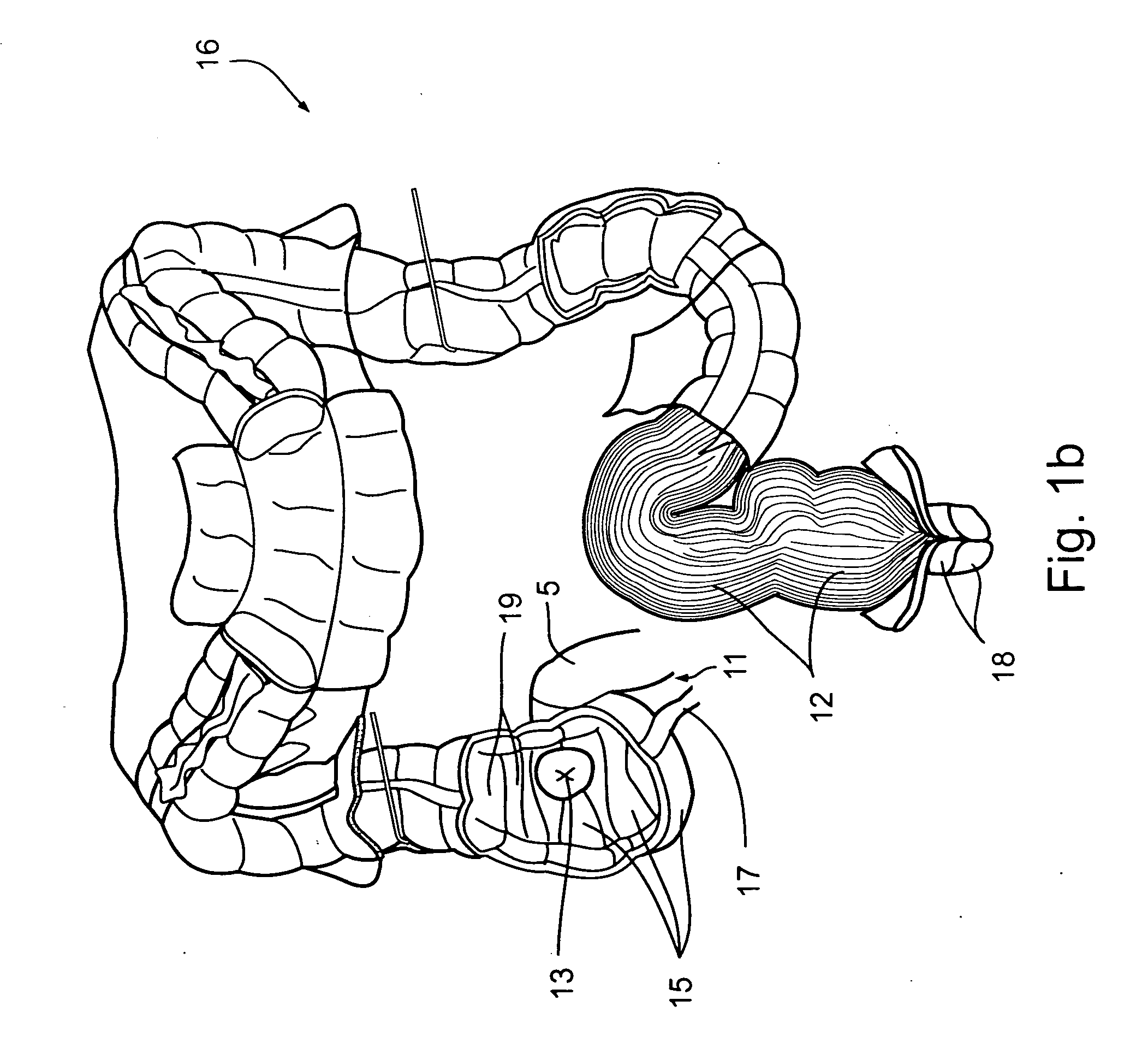
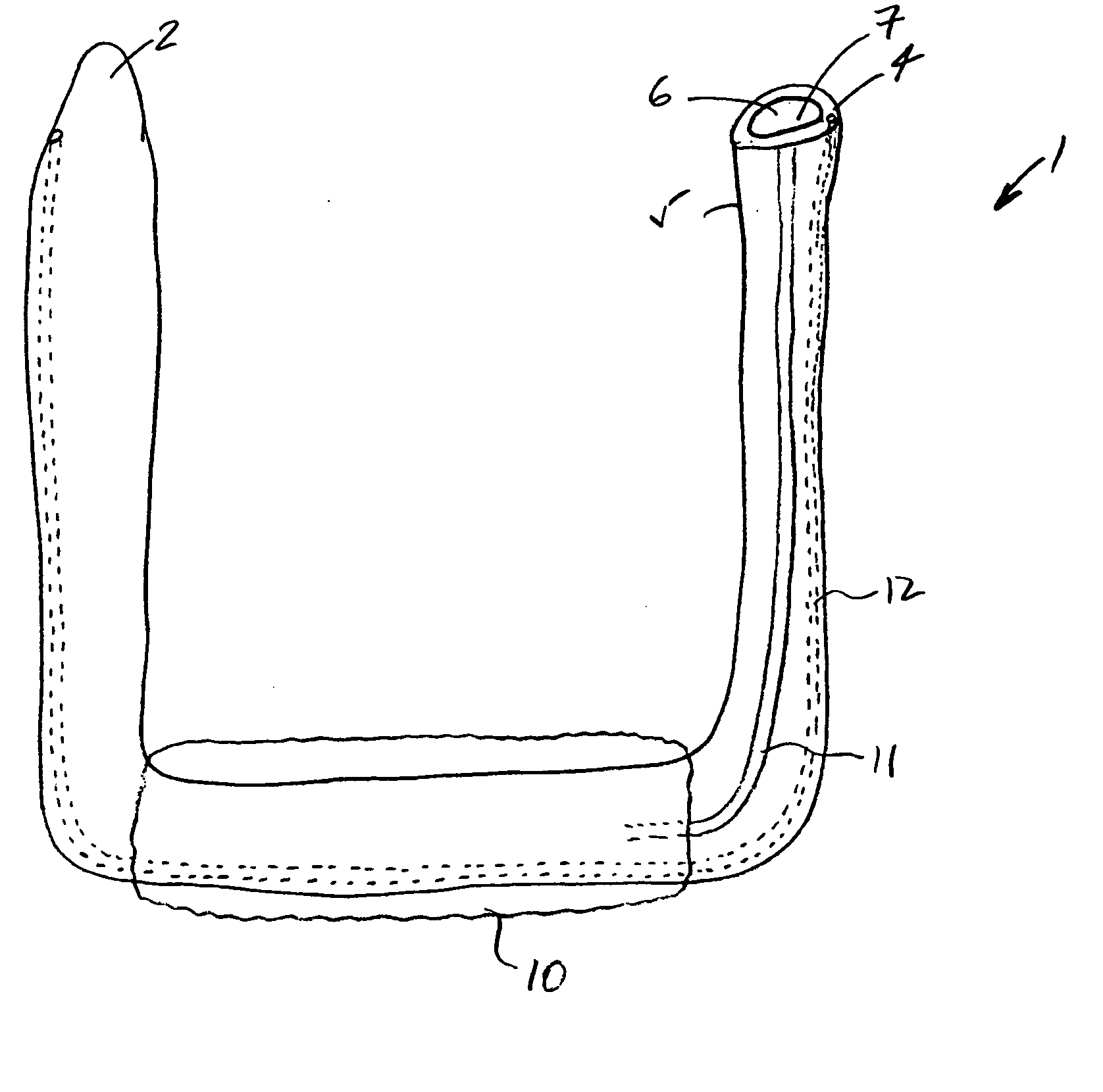
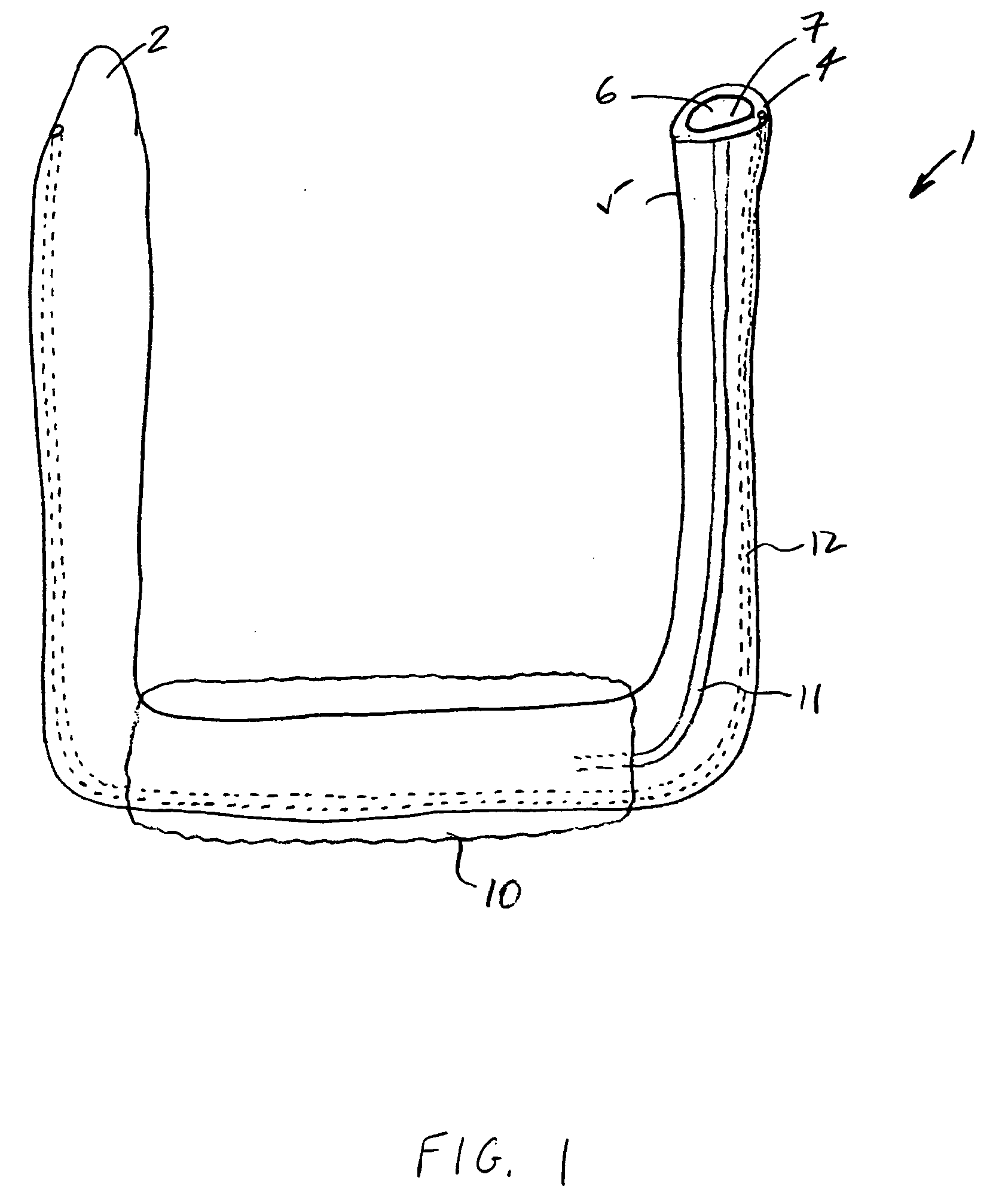
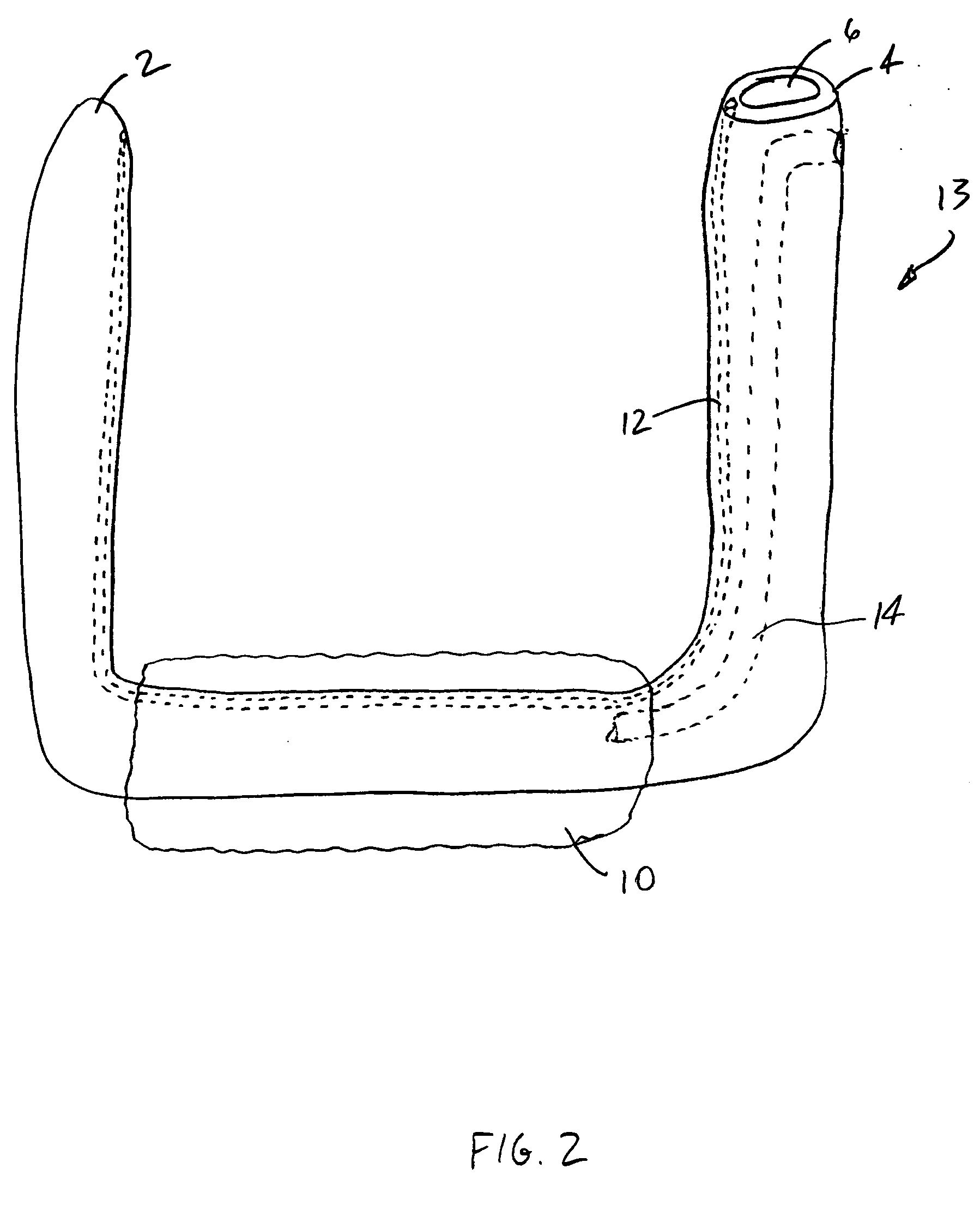
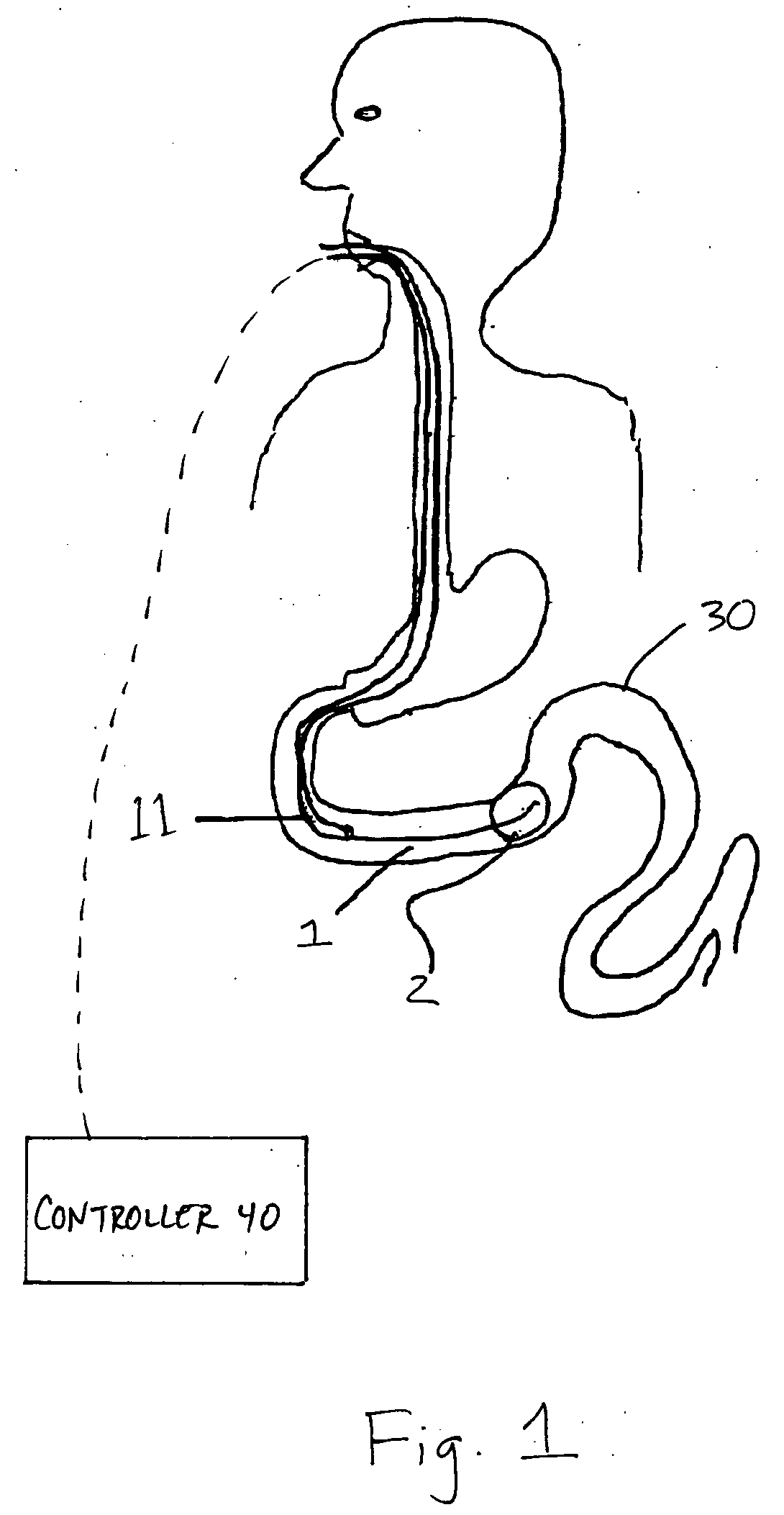
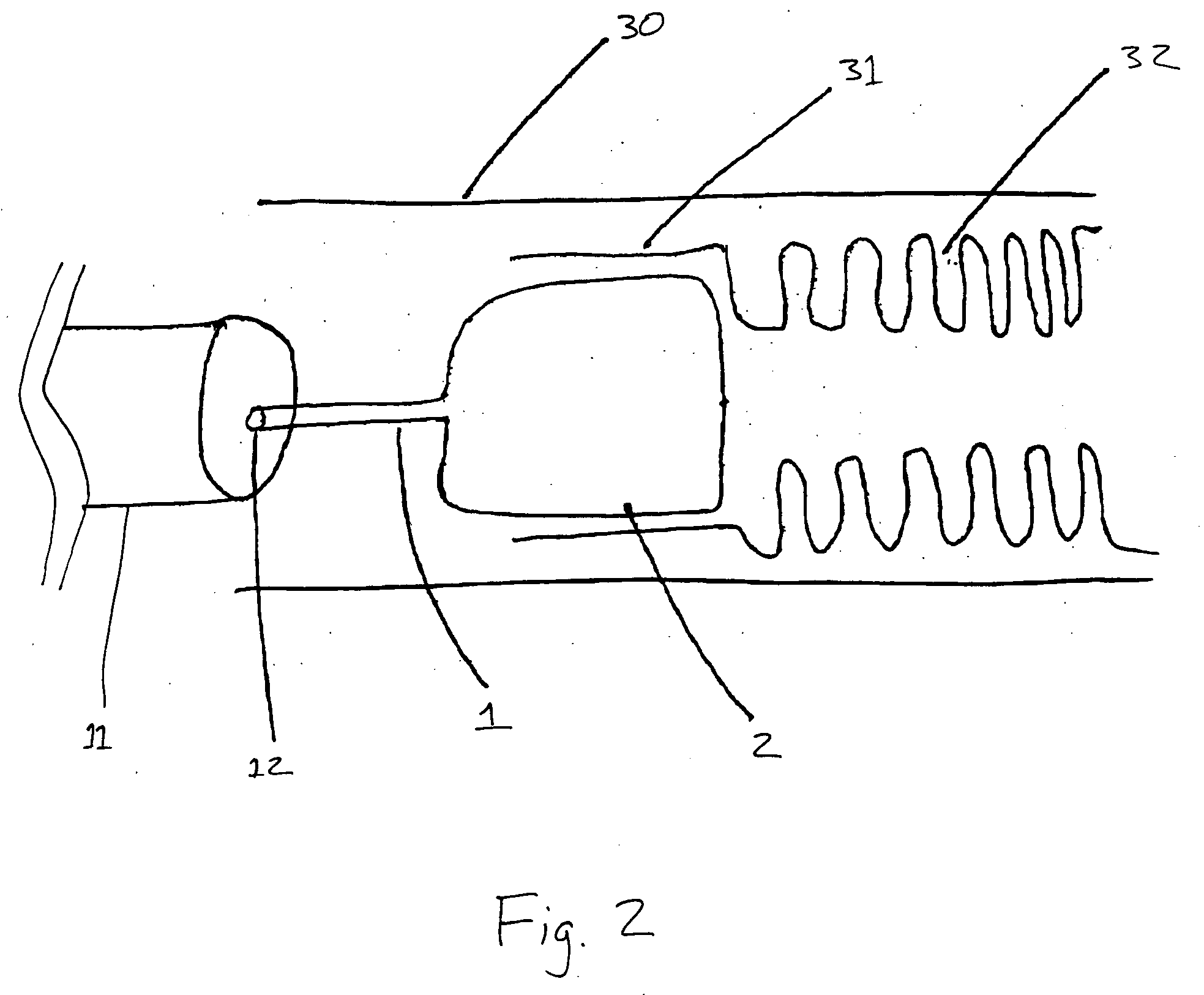
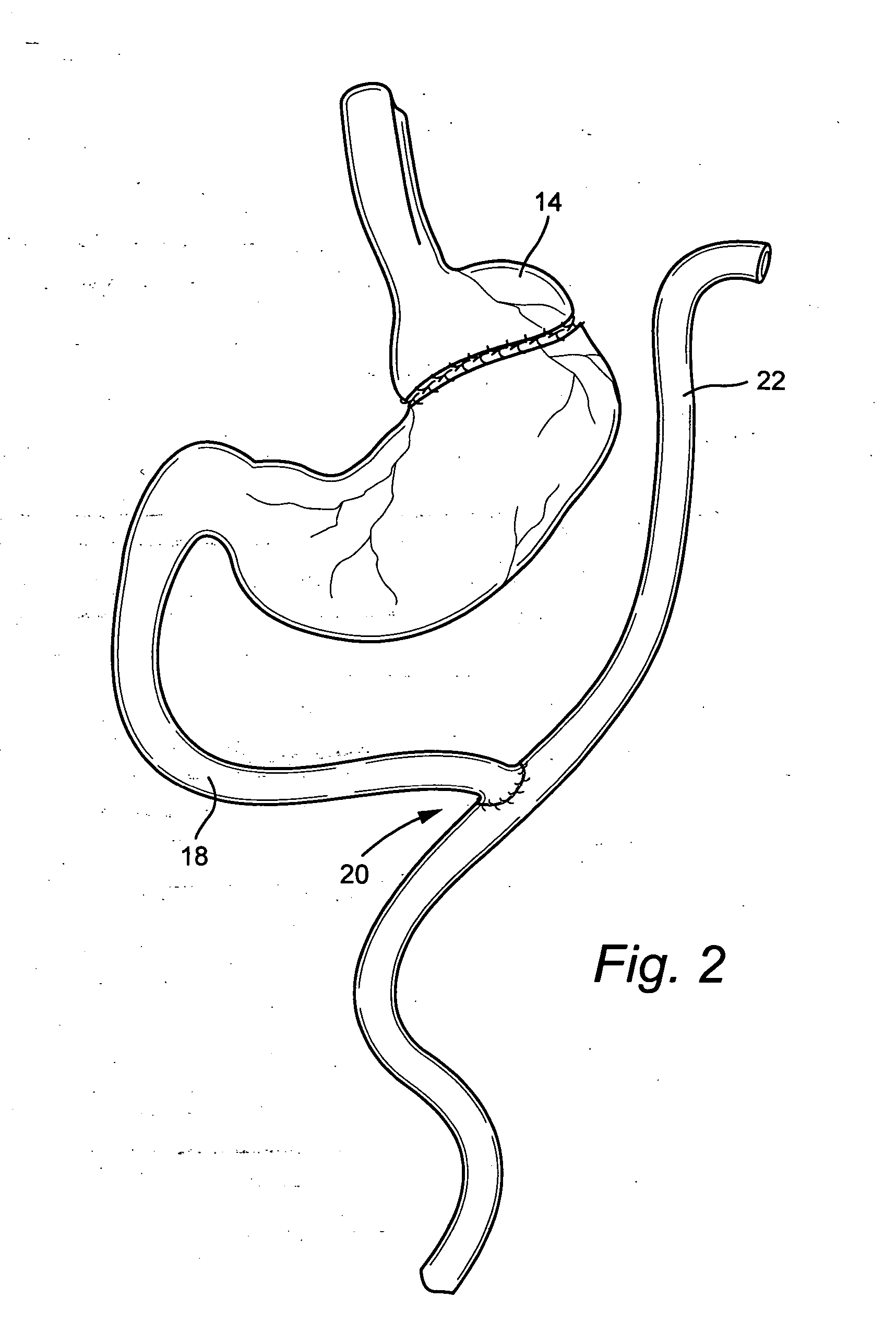
InactiveUS20050277963A1Maintain weight lossEasy to placeDiagnostic markersNon-surgical orthopedic devicesDuodenum lengthIngested food
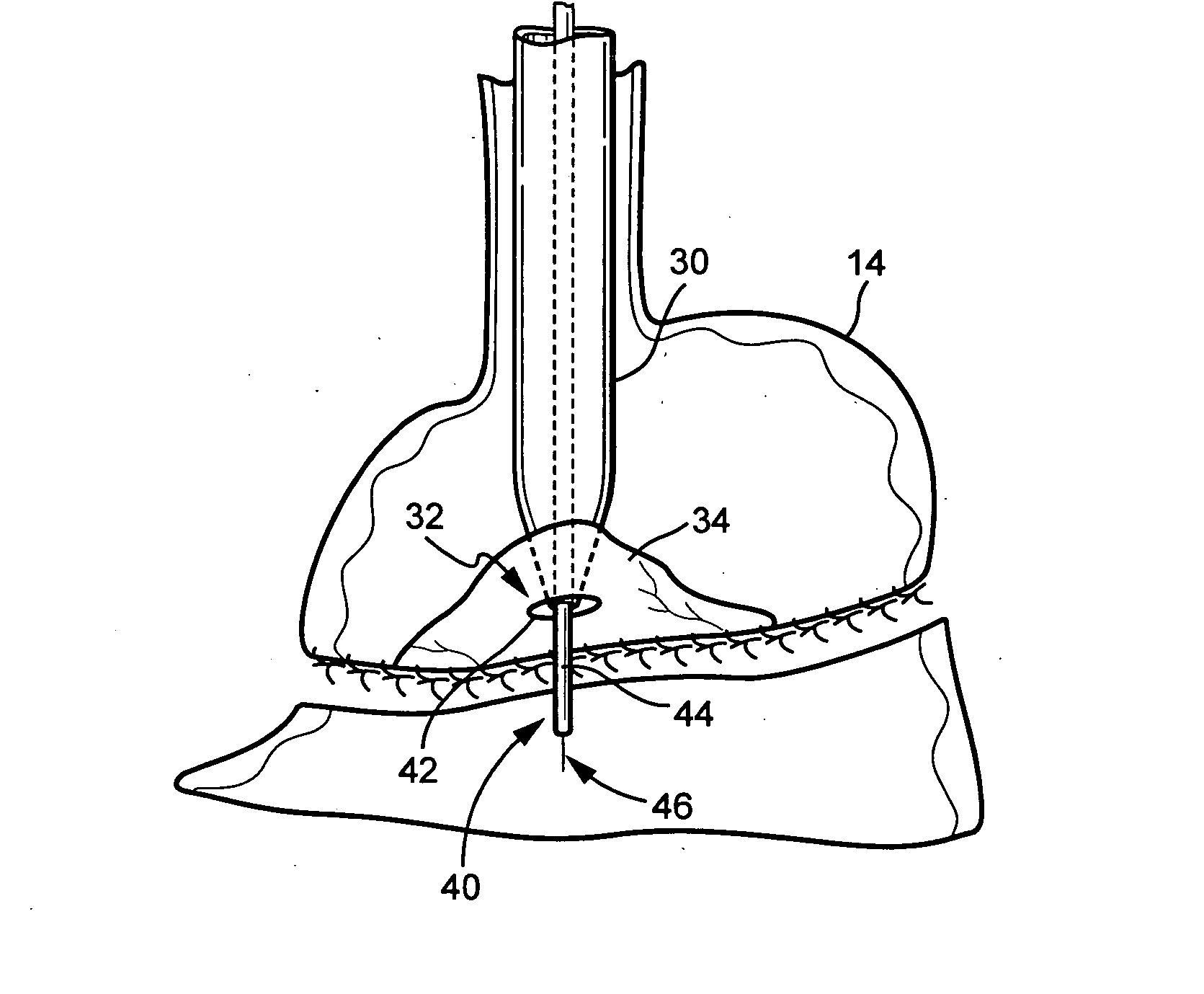
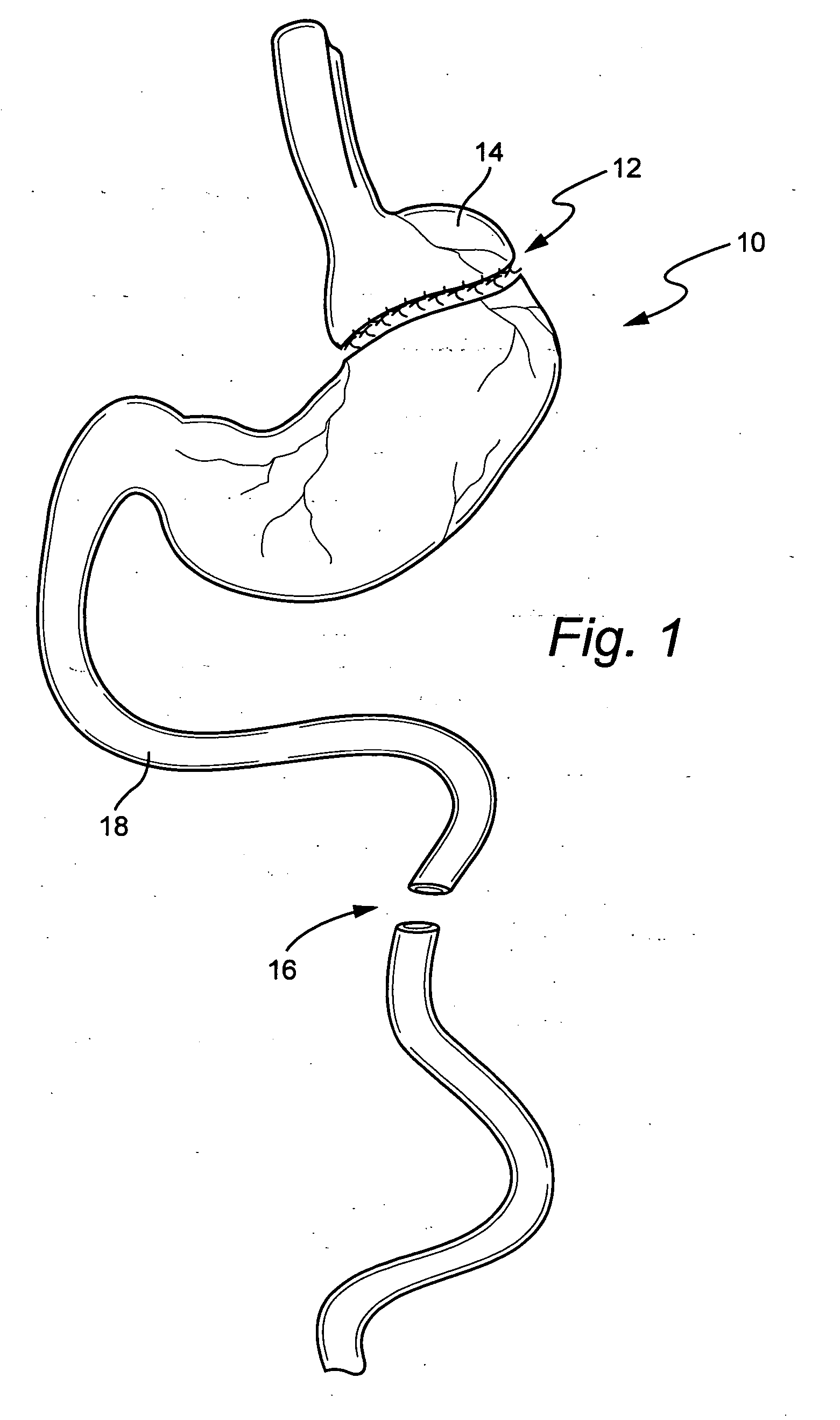
An inventive method for performing gastric bypass surgery and a gastric bypass band that is used in conjunction with the surgical method is disclosed. The inventive method involves separating a top portion of the stomach along with the esophagus from the remainder of the stomach, and re-connecting the separated portion to the small intestine to form a gastric pouch of about 20-30 cc in size. The inventive gastric band is placed midway along the gastric pouch to act as a restrictor valve to limit the amount of food passing through the valve. The band also retains food within the gastric pouch to give the patient a feeling of satiety. The band is comprised of an expansion-resistant section combined with a one-way latch mechanism. The latch has a curved orientation so that the band is formed into a radial profile when placed around the gastric pouch.
Owner:BARIATEC CORP
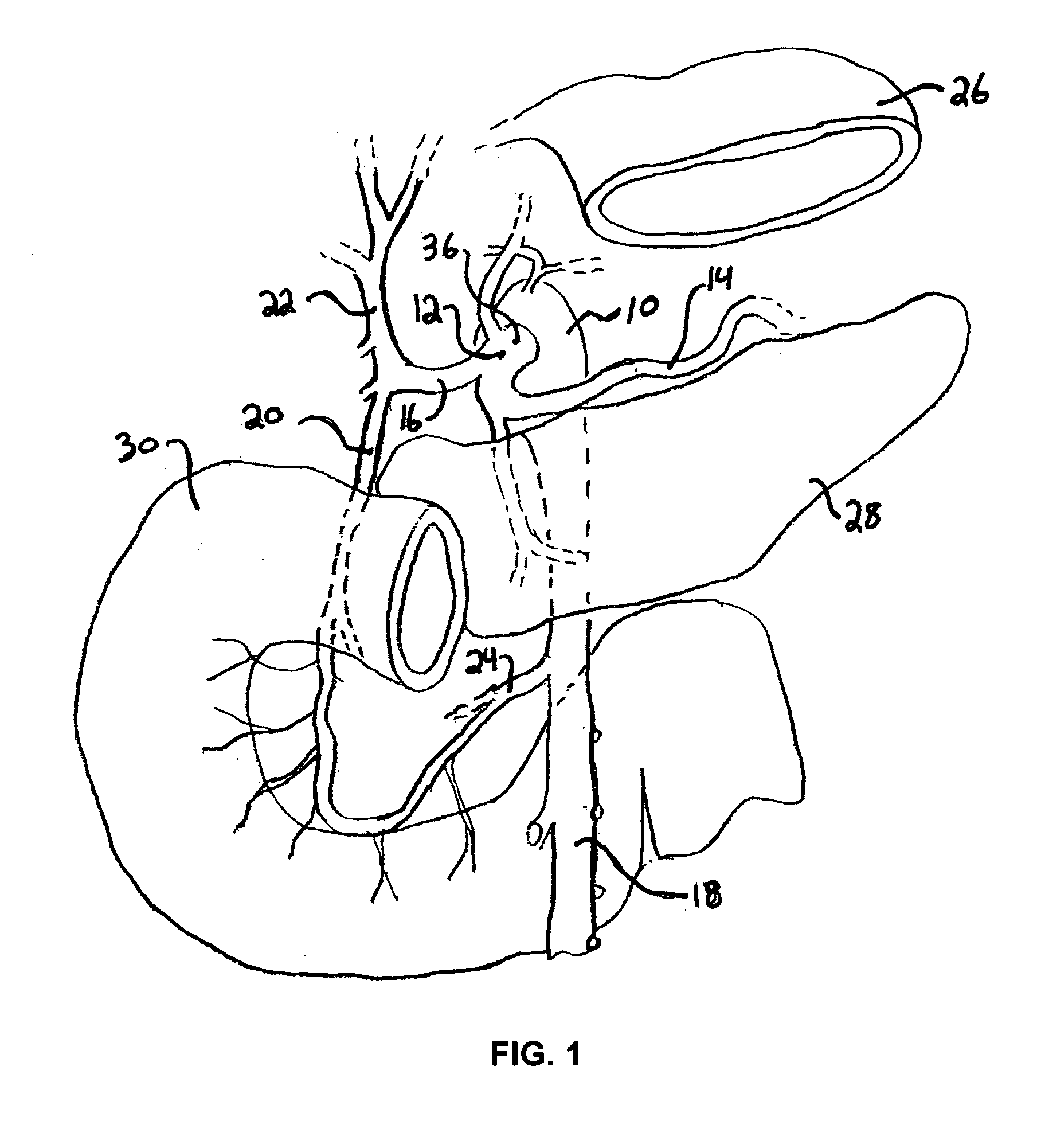
Pancreatic exocrine secretion diversion apparatus and method
A method and apparatus for treating a patient's health condition by diverting pancreatic exocrine secretions include a flow diverter of material compatible with chronic residence within a small intestine of the patient. The flow diverter has a cover end and a discharge end. The flow diverter is sized to be placed within the small intestine with the discharge end placed distally from said cover end and with said flow diverter further sized so permit passage of chyme through the small intestine and past the flow diverter. The cover end is sized to cover a discharge papilla of the pancreatic duct. The diverter is adapted to divert at least a portion of pancreatic exocrine secretion from the papilla to the distal discharge end.
Owner:RESHAPE LIFESCIENCES INC
Systems and methods for treating obesity
A system and methods useful for treating morbid obesity include the installation of a stenosis into an artery of a morbidly obese patient, the artery selected to be one that supplies blood to the small intestine of the patient.
Owner:ANAXIOM
Gastrointestinal-specific multiple drug release system
InactiveUS20050208133A1Reduce dosing frequencyAntibacterial agentsPowder deliveryMedicineSmall intestine
The present invention provides compositions and methods for the multiple release of a drug in the gastrointestinal tract of a subject through the use of an oral multiple drug release system. The system provides site-specific release of the drug to both the small intestine and the colon in the form of multiple controlled doses for long-lasting efficacy, thereby reducing the drug dosing frequency.
Owner:ASTELLAS PHARMA INC
Hydrodynamically balancing oral drug delivery system with biphasic release
InactiveUS20060099245A1Promote absorptionMaintain dimensional stabilityBiocidePowder deliveryControlled releaseSmall intestine
The present invention relates to an oral drug delivery system with biphasic release characteristics comprising a porous matrix comprising at least one drug substance, sugar(s), a release retarding polymer, gas generating components and optionally, pharma-ceuti-cally acceptable auxiliary components wherein the pharmaceutical composition further comprises a coating of said drug substance. The pharmaceutical composi-tion, either in the form of pellets (multiparticulate or single unit dosage form), beads, granules, capsules or tablets, is retained in the stomach while selectively delivering the drug(s) at gastrointestinal levels and upper parts of the small intestine over an extended period of time. The release of the drug from the said pharmaceutical composition is characterized by a biphasic release profile of the drug substance, which exhibits both immediate and controlled release characteristics.
Owner:RANBAXY LAB LTD
Floating gastro-intestinal anchor
InactiveUS20060142731A1SecurityIncrease satietyBalloon catheterSurgeryLarge intestineSmall intestine
The present invention is directed to a floating anchor, which can be inserted into the esophagus, stomach, small intestine, large intestine, or rectal cavity and reverts to a bent shape when placed therein.
Owner:SPATZ FGLA INC (US)
Bifidobacterium bifidum and application thereof
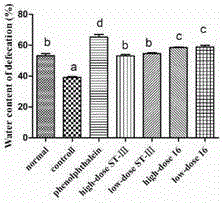
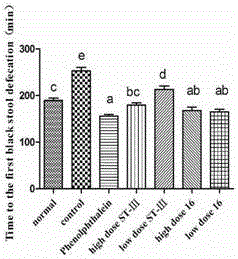
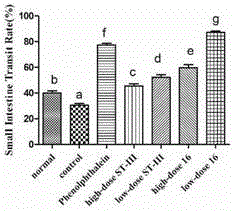
ActiveCN106834187AImprove toleranceIncrease the water content of fecesBacteriaDigestive systemSide effectFeces
The invention discloses bifidobacterium bifidum capable of remarkably relieving constipation and application thereof and belongs to the technical field of microorganisms. The bifidobacterium bifidum CGMCC NO.13632 can well tolerate simulative gastrointestinal fluid, can be well attached to colon cancer cells HT-29, and can remarkably increase the water content of excrement of constipation mice and shorten the first tarry stool time. On the aspect of increasing the small intestine driving rate, the bifidobacterium bifidum CGMCC NO.13632 is superior to cathartic phenolphthalein, a best effect is shown, secretion of gastrointestinal regulatory peptide relevant to constipation in serum is also adjusted while constipation pathological factors are remarkably improved, and the bifidobacterium bifidum does not have toxic and side effects of medicine for treating constipation, and is a first choice for preventing and treating constipation. The bifidobacterium bifidum CGMCC NO.13632 is used for preparing pharmaceutical compositions and fermented food for relieving constipation, and has very wide application prospects.
Owner:JIANGNAN UNIV
Duodenal internal covering membrane made of degradable biocompatible material and application thereof
ActiveCN102626330ANo allergic reactionNo toxic reactionSurgeryNon-surgical orthopedic devicesBile JuiceMedical equipment
The invention provides a duodenal internal covering membrane, which is made of degradable shape memory biocompatible material and relates to degradable medical equipment internally installed in a digestive tract. After the internal covering membrane is implanted into a duodenum, chyme can be split from bile and pancreatic juice, so stomach exudates are prevented from being directly digested, absorbed and metabolized in the duodenum, and the histocompatibility is good; after being implanted into a human body, the duodenal internal covering membrane is steady, is not easy to slide off to be incarcerated and can be gradually degraded in the human body after 2 months to 5 years, so complicated operation and the injury to organs and tissue, which are caused when the duodenal internal covering membrane is removed from the human body in the future, are avoided, a rebound effect achieved after the original barrier is instantaneously and thoroughly dismounted can be slowed down, and the duodenal internal covering membrane can be prepared into medical equipment which is used for curing obesity and diabetes mellitus and does not need to be removed from the human body in the future; and tube-shaped part sinking induced by gastrointestinal motility and jejunum content backflow caused by the decrease of a gap between a tube-shaped part of the internal covering membrane and the inner wall of the duodenum can be avoided. The prepared medical equipment for curing the obesity and the diabetes mellitus can achieve the effects of dropping prevention, removal exemption, rebound inhibition and injury reduction.
Owner:万平
Intestinal ablation to limit food absorption
A method for treating obesity in a human patient is provided whereby an ablation source is introduced into the small intestine of the patient via a working channel of an endoscope, and the intestinal mucosa of the patient is ablated over a length of the small intestine in a range of approximately 40 cm to 100 cm.
Owner:SOLOMON STEPHEN
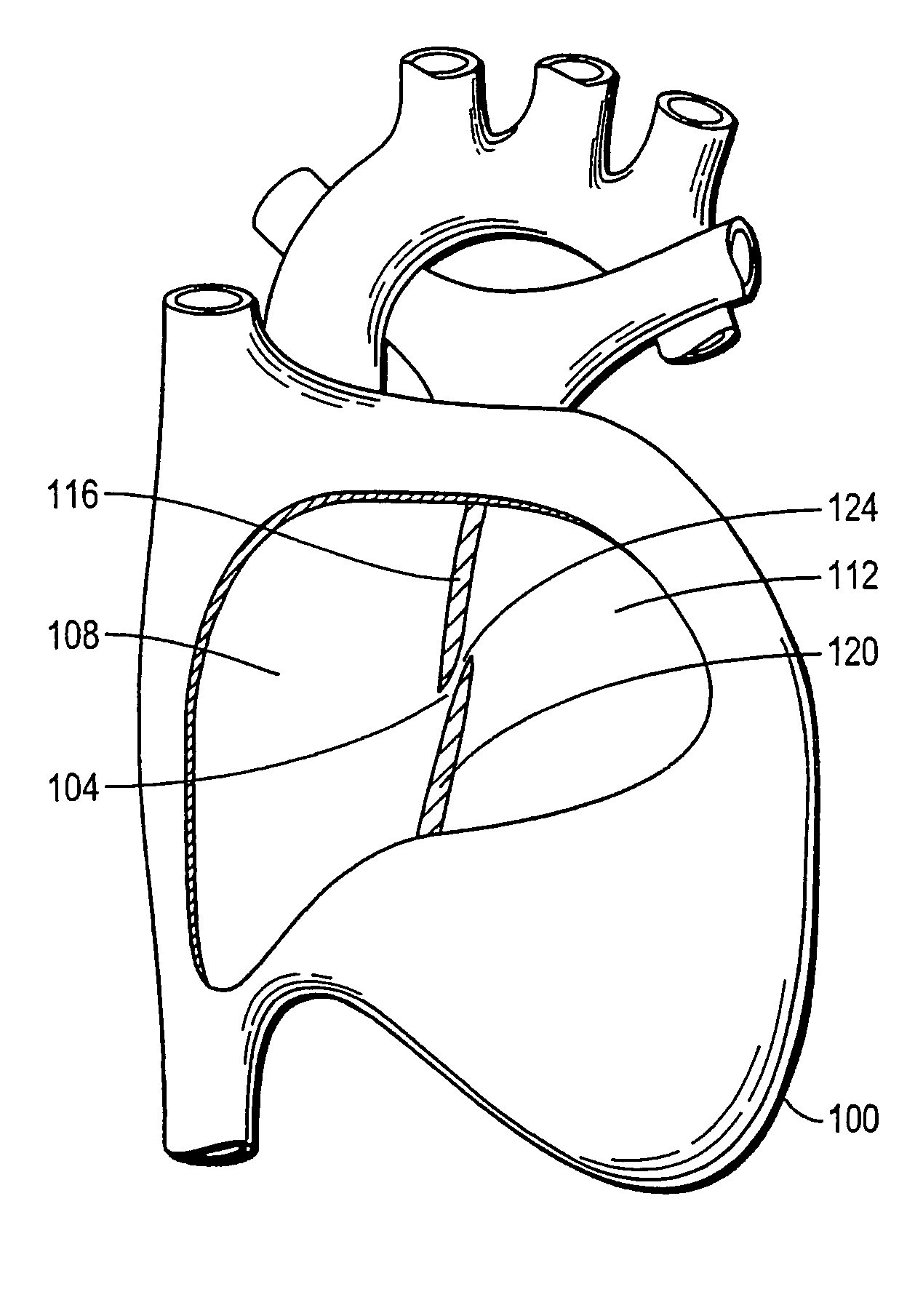
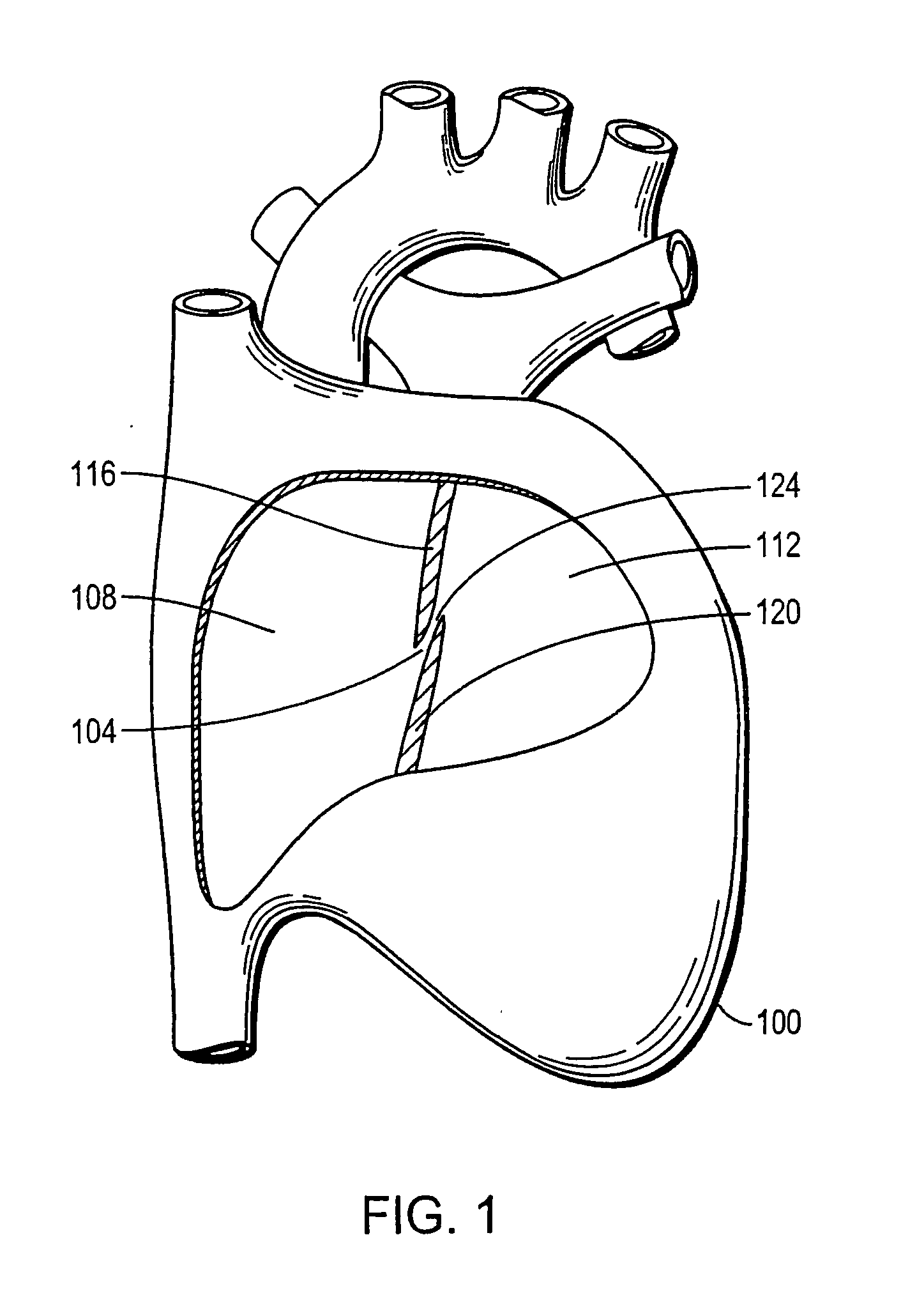
Device with biological tissue scaffold for percutaneous closure of an intracardiac defect and methods thereof
The invention provides an intracardiac occluder, which has biological tissue scaffolds as occlusion shells, for the percutaneous transluminal treatment of an intracardiac defect. The intracardiac occluder includes a proximal support structure supporting the proximal occlusion shell and a distal support structure supporting the distal occlusion shell. In one embodiment, biological tissue derived from the tunica submucosa layer of the porcine small intestine forms the occlusion shells.
Owner:WL GORE & ASSOC INC
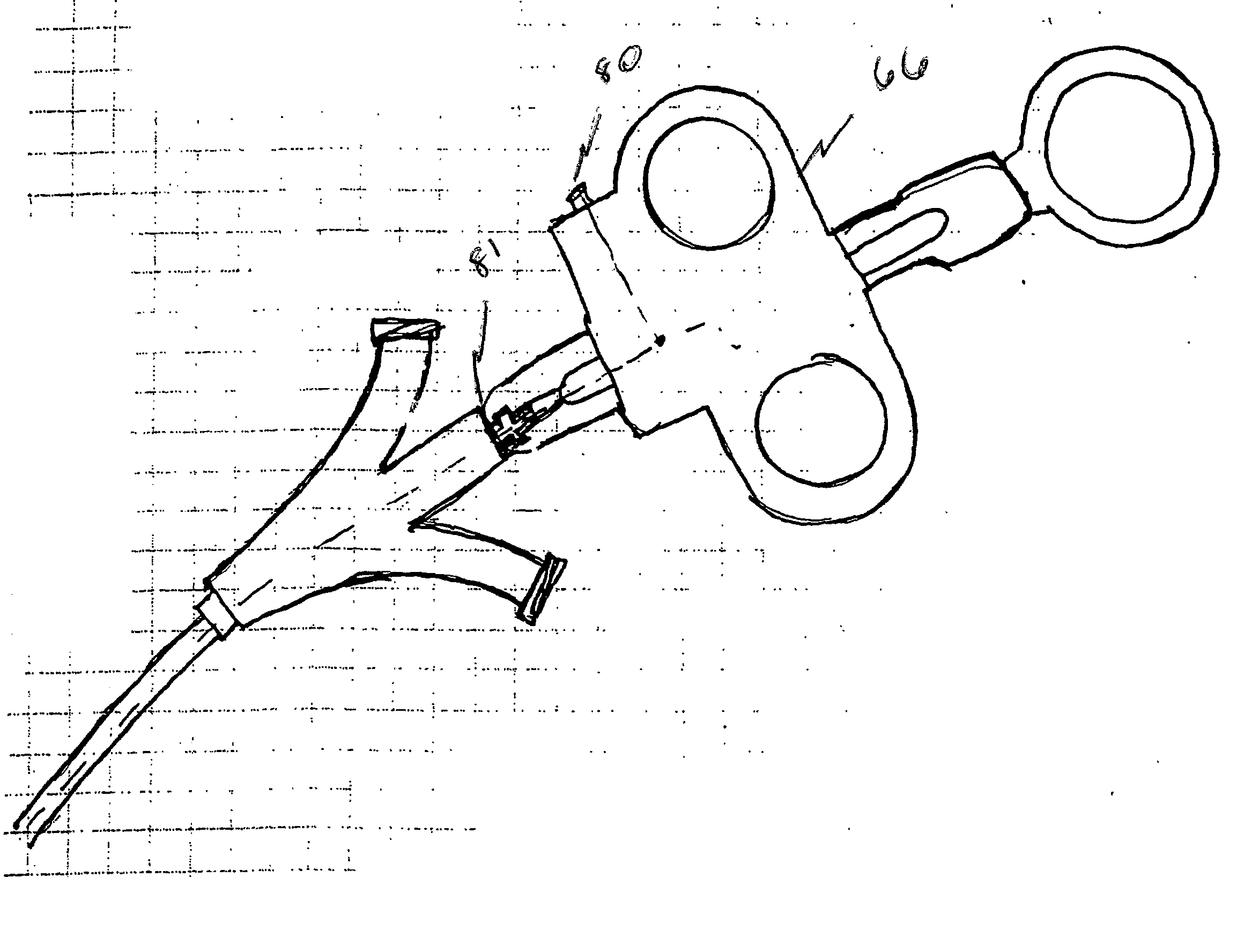
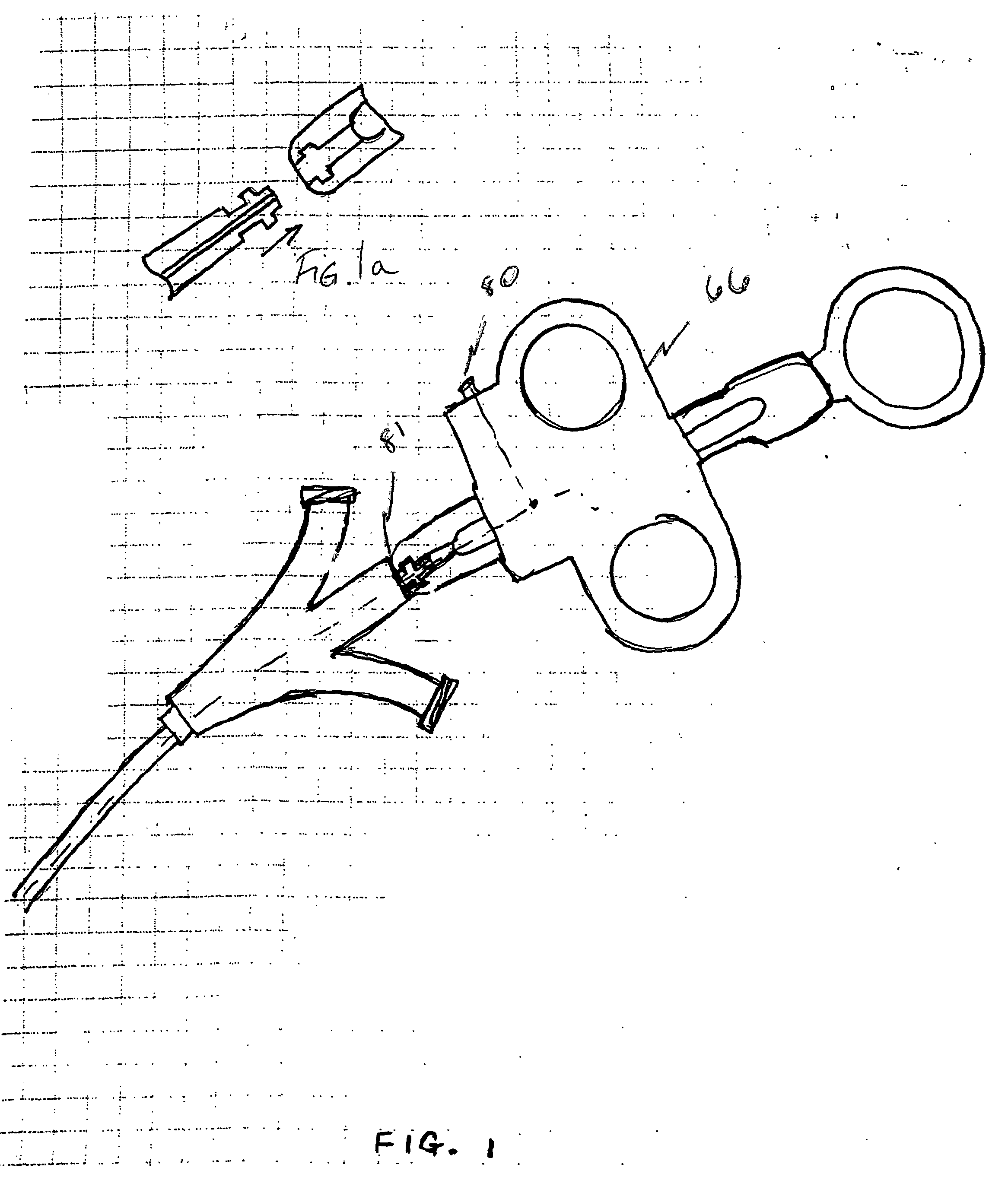
Steerable sphincterotome and methods for cannulation, papillotomy and sphincterotomy
The present invention relates to methods and devices for performing endoscopic cannulation, papillotomy and sphincterotomy and similar procedures. According to the present state of the art, endoscopic cannulation of the common bile duct and papillotomy and similar procedures are accomplished by advancing the device into an endoscope / duodenoscope so that the distal tip of the device exits the endoscope adjacent the sphincter muscles at the Papilla of Vater. The endoscope mechanisms are then manipulated to orient the distal tip of the device to the desired position for proper cannulation of the duct. Due to inconsistencies in, for example, the sphincterotome, anatomy, and endoscope manipulation, it is difficult to accurately and consistently position the sphincterotome for proper cannulation. The steerable sphincterotome of the present invention allows the physician to control the position of the distal tip of the device independently of the endoscope and adjust for inconsistencies in the device and the anatomy. According to the present invention, the handle to which the cutting wire is attached is freely rotatable relative to the catheter. The handle, secured to the cutting wire but rotatable relative to the shaft of the catheter, provides a mechanism to rotate the wire, transmitting the force to rotate the device tip. With the handle rotating independently of the shaft at the proximal end, the force can be applied directly to the distal tip without twisting the entire shaft. Also a rotation lock to maintain the orientation of the tip and / or a rotation marking, to indicate the amount of rotation may be included.
Owner:BOSTON SCI SCIMED INC
Systems and methods for bariatric therapy
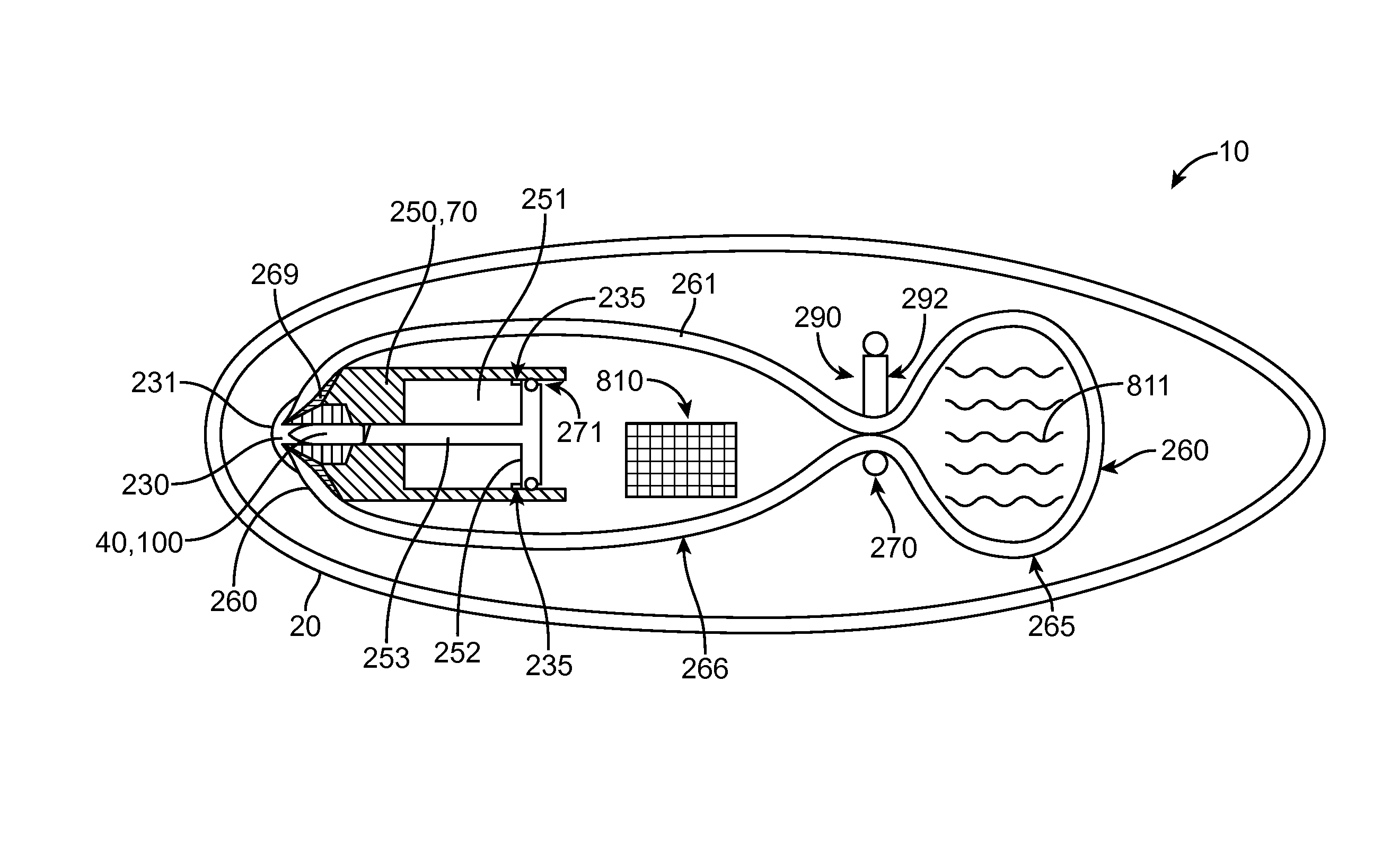
InactiveUS20110172584A1Minimize absorptionReduce penetrationIntravenous devicesStomachImplanted deviceSmall intestine
The present invention provides bariatric therapy systems. One system includes a gastrointestinal implant device and a delivery mechanism therefor. The device can include a sleeve for placement into a small intestine and to minimize absorption of nutrients by its walls. An anchoring mechanism coupled to a proximal end of the sleeve and designed to be secured within the stomach can be provided. A passageway extending through the anchoring mechanism and the sleeve can also be provided, along which food can be directed from the stomach to the small intestine. The delivery mechanism can include a housing for accommodating the device, and a deploying balloon situated within the housing and which can be actuated to direct the sleeve of the device from within the housing to the site of implantation. Methods for providing bariatric therapy are also provided by the present invention.
Owner:PAVILION MEDICAL INNOVATIONS
Dosage forms of risedronate
ActiveUS20060110452A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyImmediate release
Oral dosage forms of a risedronate comprised of a safe and effective amount of a pharmaceutical composition comprising risedronate, a chelating agent, and, means for effecting delayed release of the risedronate and the chelating agent in the small intestine provide immediate release of the pharmaceutical composition to the small intestine of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between risedronate and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of risedronate and the chelating agent to the small intestine, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Kit and method for EEA anvil placement in bariatric surgery
InactiveUS20070088389A1Facilitating and simplifying anvil passageFacilitating and simplifying and placementSuture equipmentsStapling toolsAbdominal cavitySmall intestine
A system of catheters, guidewires filaments, loops and laparoscopic instruments to simplify the placement of the anastomosis device to connect a stomach pouch to the small intestine in bariatric surgery, the surgical reduction of stomach size to treat morbid obesity.
Owner:MIAMI UNIVERISTY OF
Prokinetic drugs assistance to small intestine imaging
InactiveUS20050095200A1Increase forward motionEnhanced informationBiocideGeneral/multifunctional contrast agentsCost effectivenessCurative effect
The present invention discloses a novel method of increasing the efficacy of small intestine wall video-capsule imaging, by means of controlled administration of pro-kinetic medications. This cost effective method is adapted inter alia for in vivo imaging of the cavities in the digestive tract by means of pro-kinetic medication, comprising among other means administering an predetermined dose of a pro-kinetic drug to increase the forward push of the video-capsule and / or segmentary peristaltic contractions; and obtaining an image of the digestive tract wall, resulting in an increase percentage of visual information about the internal gastrointestinal cavity.
Owner:SCHWARZBERG MOSHE
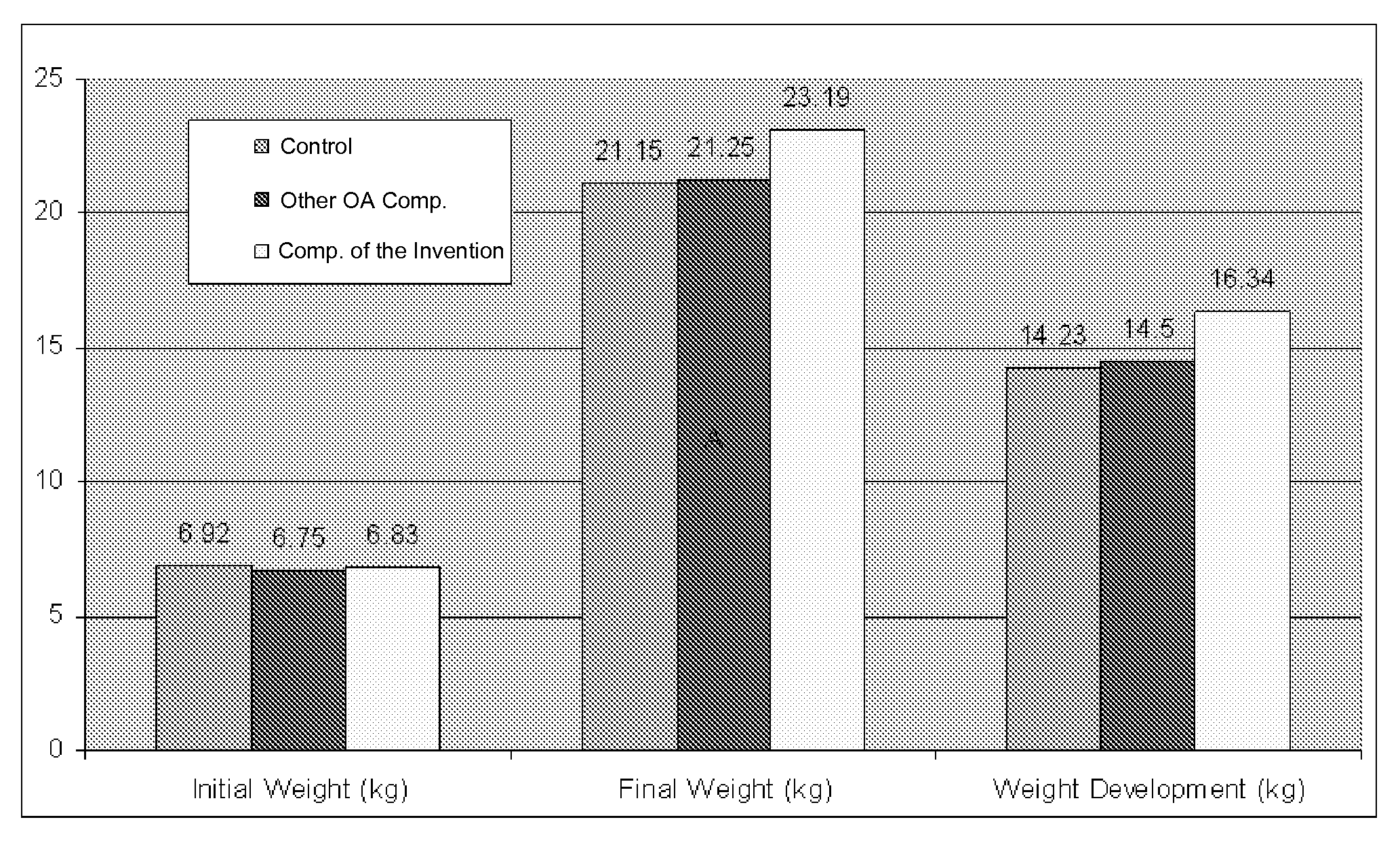
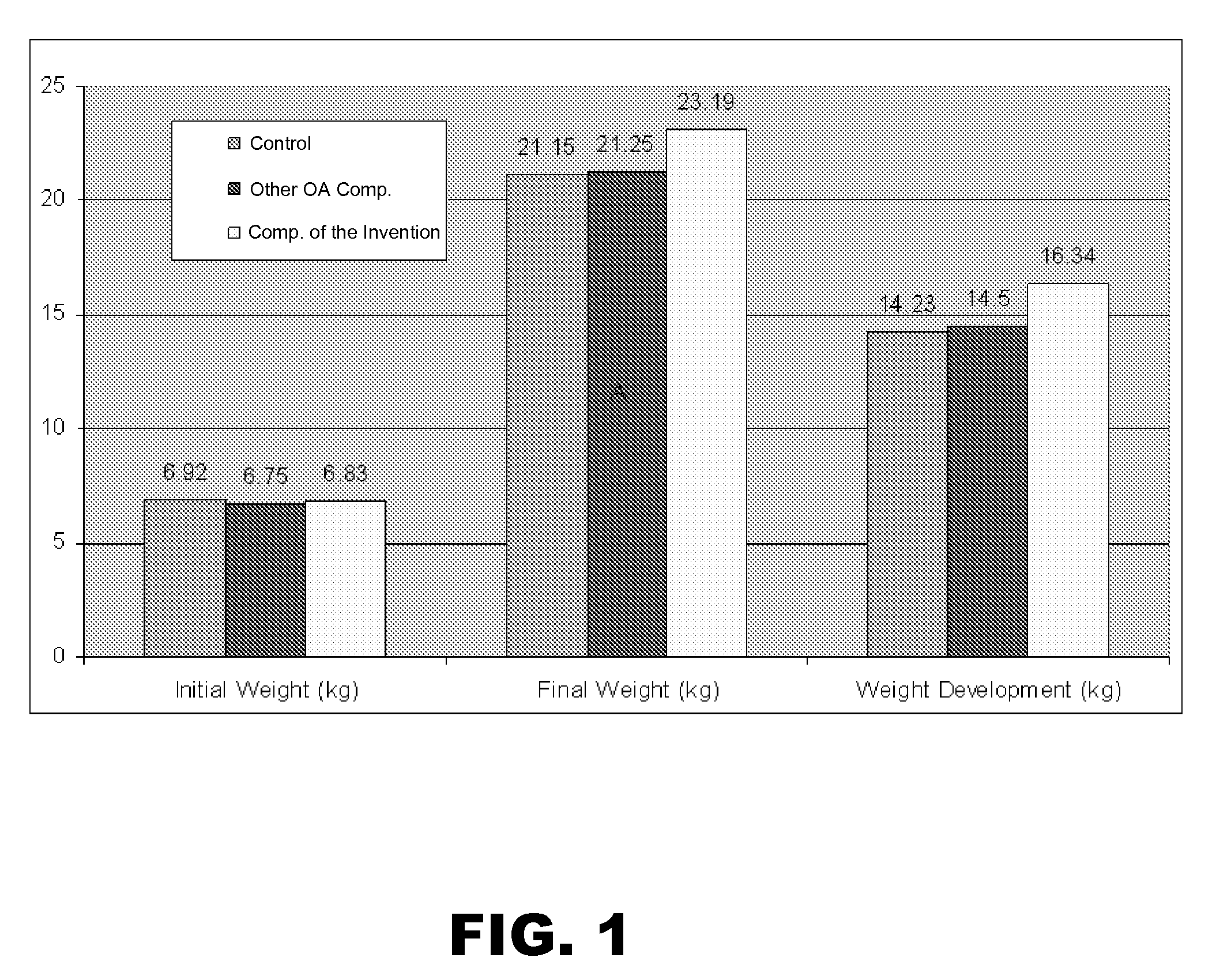
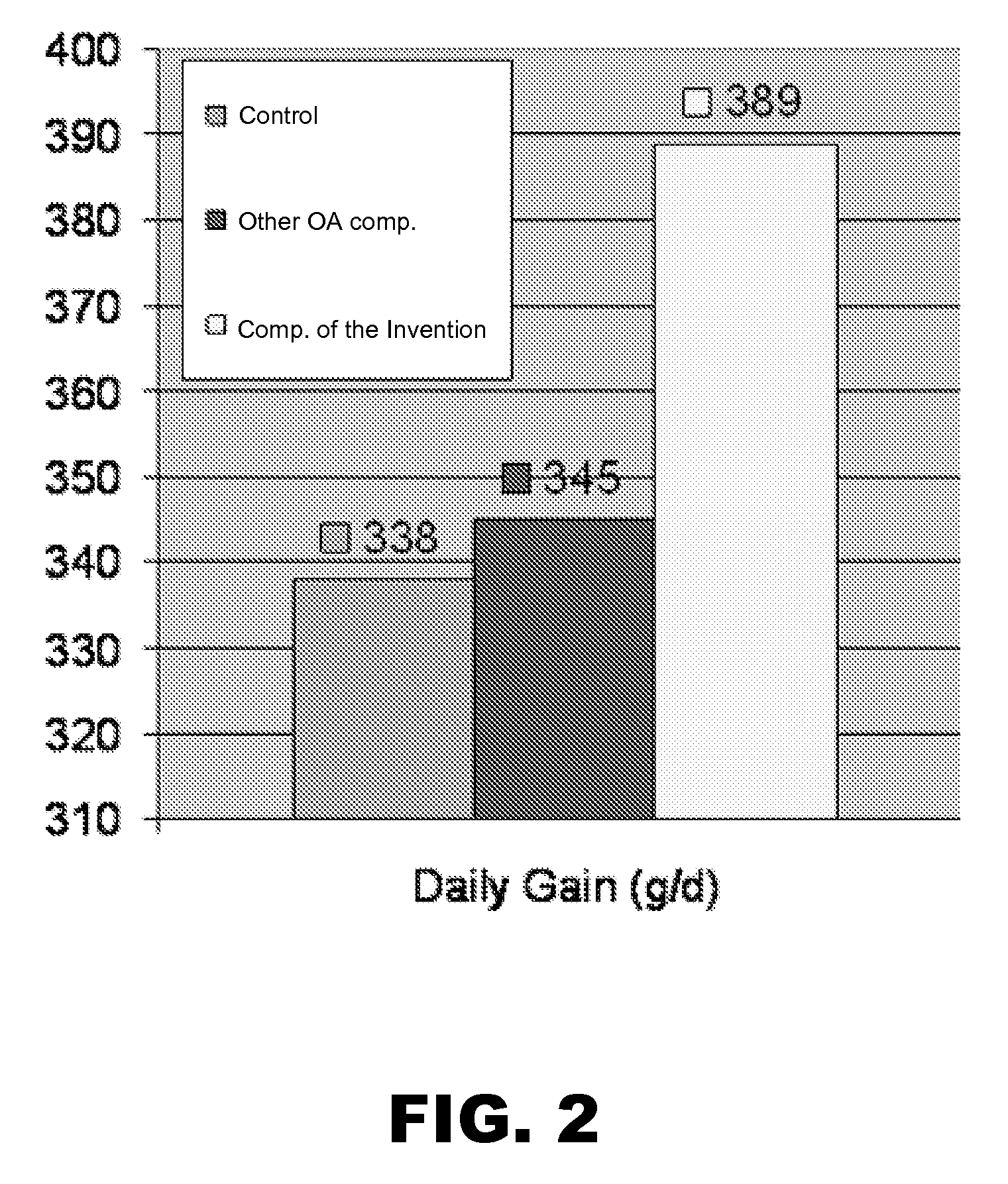
Matrix-embedded compositions having organic acids and fatty acids
The invention generally provides matrix-embedded compositions having organic acids and fatty acids. The compositions may be administered to an animal to deliver intact organic acids and fatty acids to the animal's small intestine. The invention also provides monograstric feed rations comprising the matrix-embedded compositions.
Owner:NOVUS INTERNATIONAL INC
Probiotic double layer embed microcapsule process
The invention discloses a probiotics double-embedded microcapsule technology. The acid-proof double-embedded enteric-coated microcapsule is prepared with 75 % probiotics enriched solution, 15 % milk protein, 10 % capsule-core findings and enteric solubility colloid comprising 12-20 % converted starch, 4-8 % acacia gum, 6-10 % carbowax, 4-8 % sodium alginate, 3-5 % chitose and 60 % common salt with concentration of 0.9 % by fluidized spray-on process. The products by this double-embedded microcapsule technology can resist acid stomach and bile acid and is shelf stable, and can immediately dissolve and release after reaching small intestine and can colonize on adsorption intestinal canal mucous membrane, and the process is much easier and the cost is much lower.
Owner:湖南科尔生物技术有限公司
Method for improving enzymolysis efficiency of crude heparin sodium extraction technology
The invention relates to a method for improving enzymolysis efficiency of a crude heparin sodium extraction technology. The method can effectively improve enzymolysis efficiency, decompose impurity proteins, improve crude product quality, improve a yield and increase economic benefits. The method comprises the following steps of 1, dissolution: preparing mucous membrane water, 2, acid protease catalysis: heating the mucous membrane water, adding acid protease into the mucous membrane water based on the number of small intestines of the pig, adjusting a pH, adding metal ions into the mixed solution based on the number of small intestines of the pig, adjusting salinity by sodium chloride, and carrying out a thermal insulation reaction process, 3, alkaline protease catalysis: carrying out heating, adding two alkaline proteases into the reaction produce based on the number of small intestines of the pig, adding a pH value and salinity, carrying out a thermal insulation reaction process, carrying out heating and carrying out a thermal insulation reaction process, and 4, filtration on the enzymolysis mother liquor by a combined filter cloth, introduction of the filtered enzymolysis mother liquor into an adsorption tank, and follow-up processes.
Owner:杭州惠顺生物科技有限公司
Pharmaceutical formulations for carrier-mediated transport statins and uses thereof
InactiveUS20050119331A1Improve bioavailabilityMechanism is preventedBiocideOrganic chemistryLipid formationCholesterol
The present invention relates to formulations comprising therapeutically effective amounts of at least one acid-stable, carrier-mediated transport statin, at least one poorly water-soluble, carrier-mediated transport statin, or at least one large molecular weight, carrier-mediated transport statin, such as atorvastatin and rosuvastatin, or a pharmaceutically acceptable salt thereof, and methods of their use. The present formulations and methods are designed to exhibit a controlled-release of a therapeutic amount of the statin in the small intestine, thereby limiting systemic exposure of the statin and maximizing liver-specific absorption of the drug. The formulations and methods of the present invention are particularly useful for treating and / or preventing conditions that are benefited by decreasing levels of lipids and / or cholesterol in the body.
Owner:CIRC PHARM RES & DEV LTD
Methods for diagnosis and treatment of crohn's disease
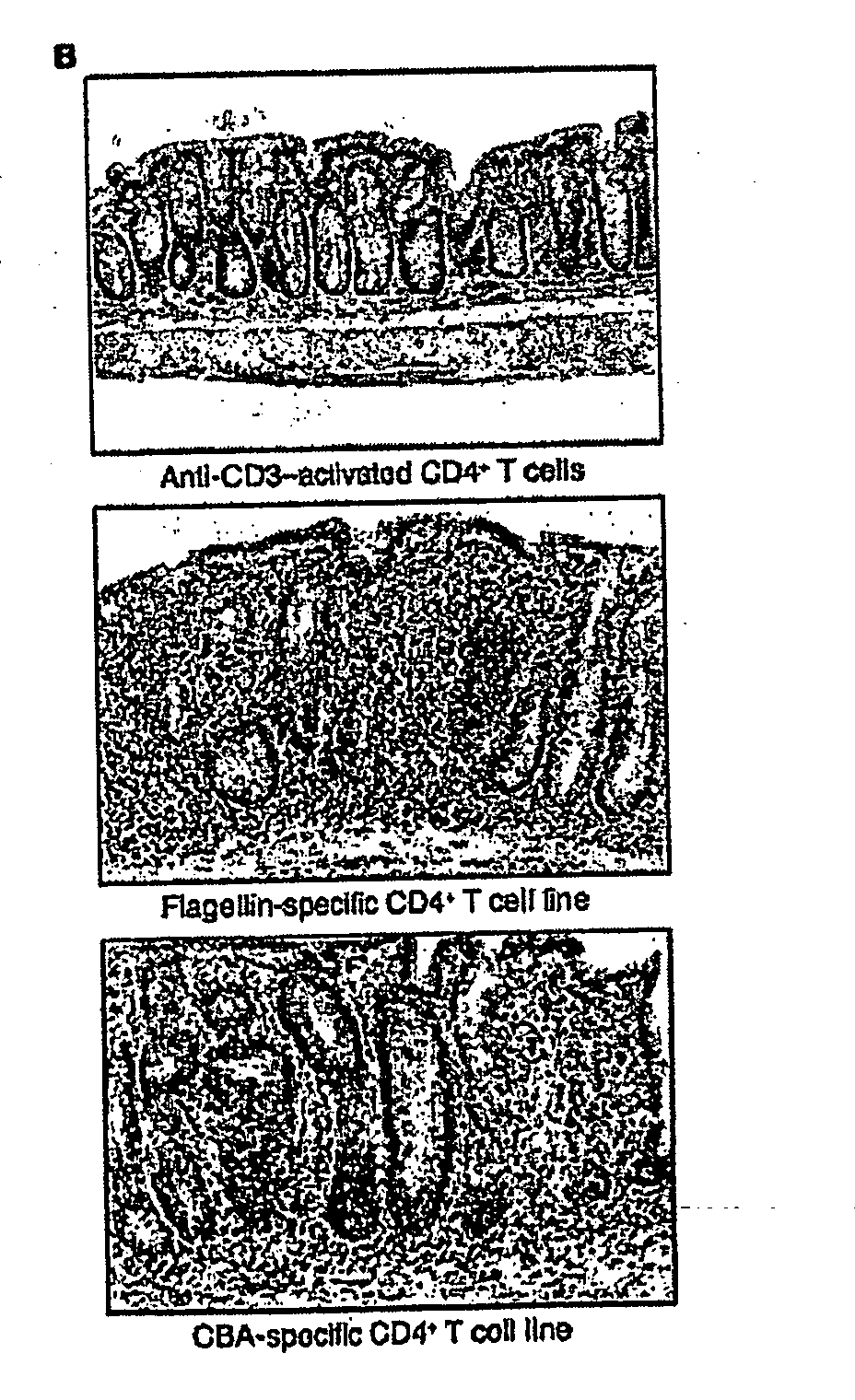
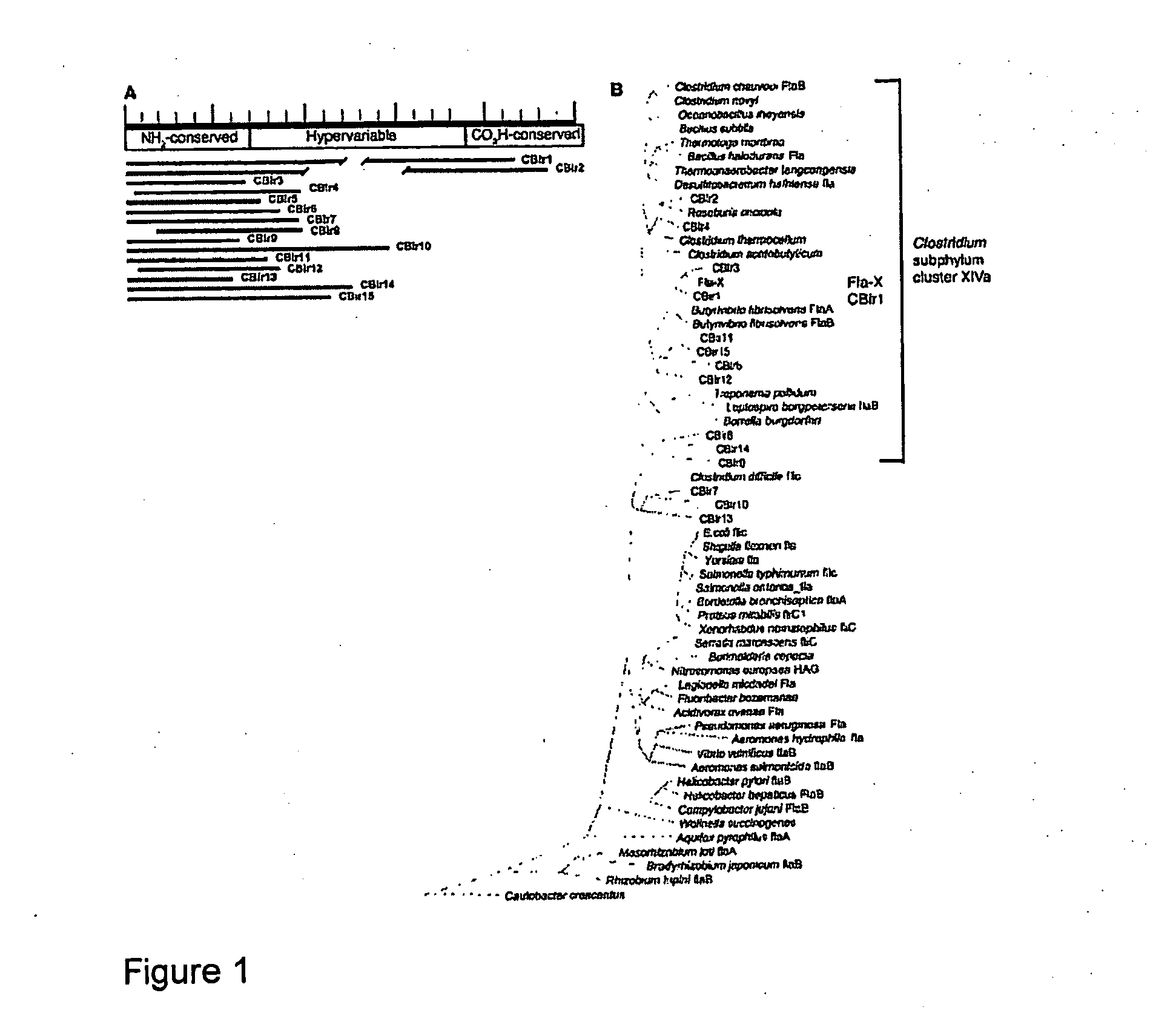
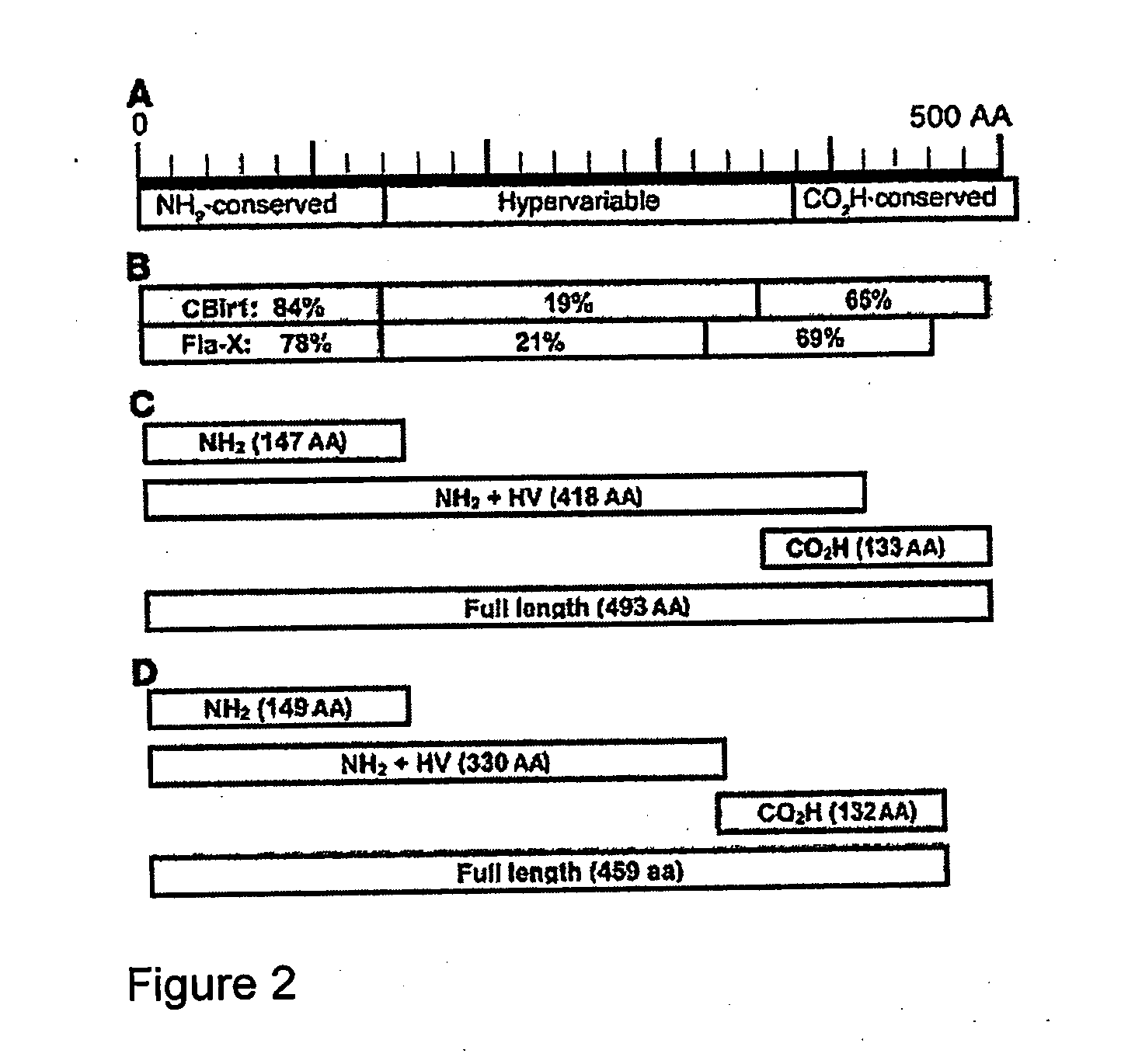
The inventors have discovered an elevated serum response to CBir1 flagellin in Crohn's disease patients. The present invention relates to methods for diagnosis and treatment of Crohn's disease and / or subtypes of Crohn's disease. Diagnosis is accomplished by determining the presence of the anti-CBir1 expression or determining the presence of anti-CBir1 expression and detection of the presence of pANCA. Treatment methods include antigen-directed therapy targeting CBir1 flagellin and manipulating the bacteria in the colon and / or small intestine.
Owner:CEDARS SINAI MEDICAL CENT
Methods of using gamma cyclodextrin to control blood glucose and insulin secretion
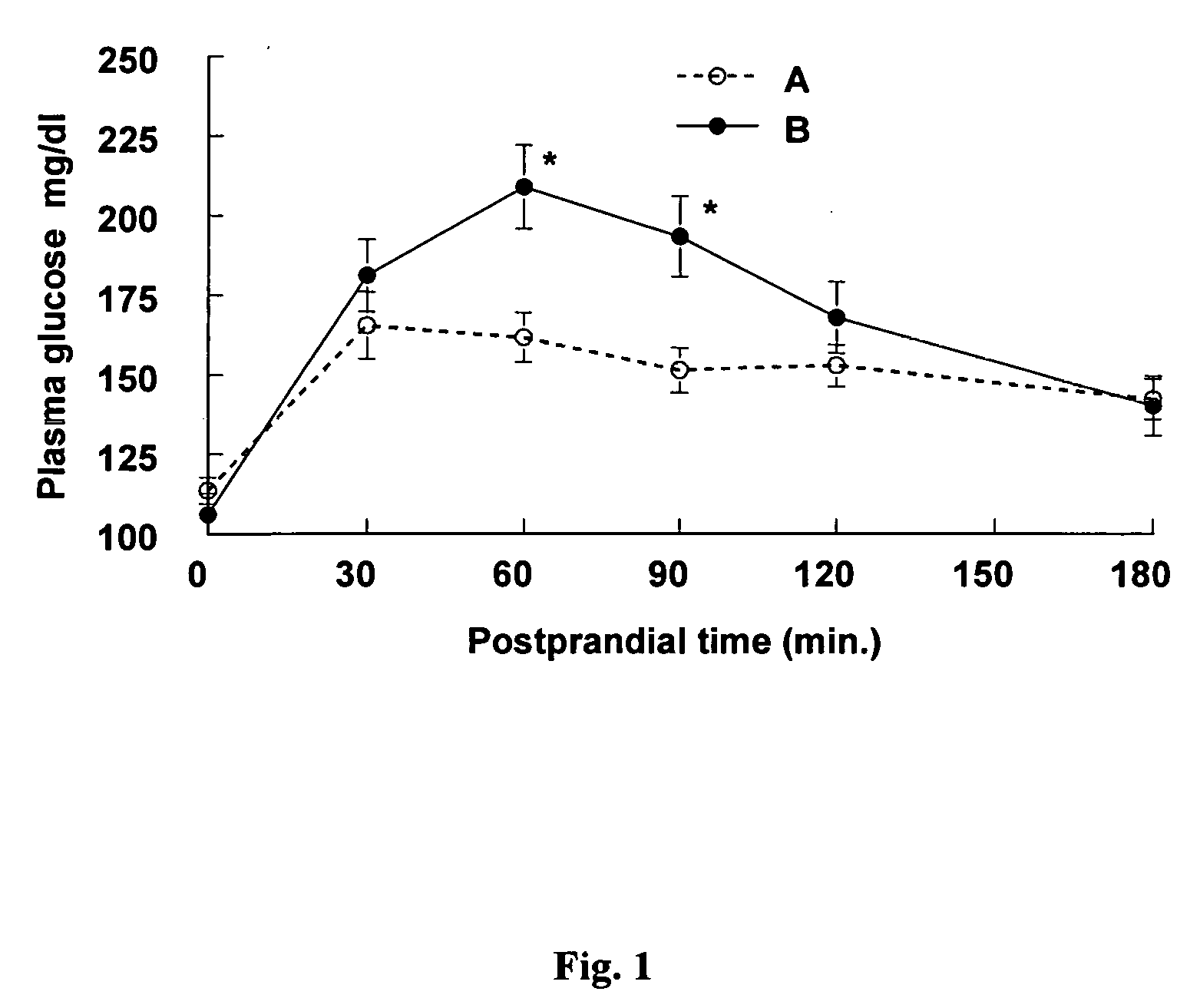
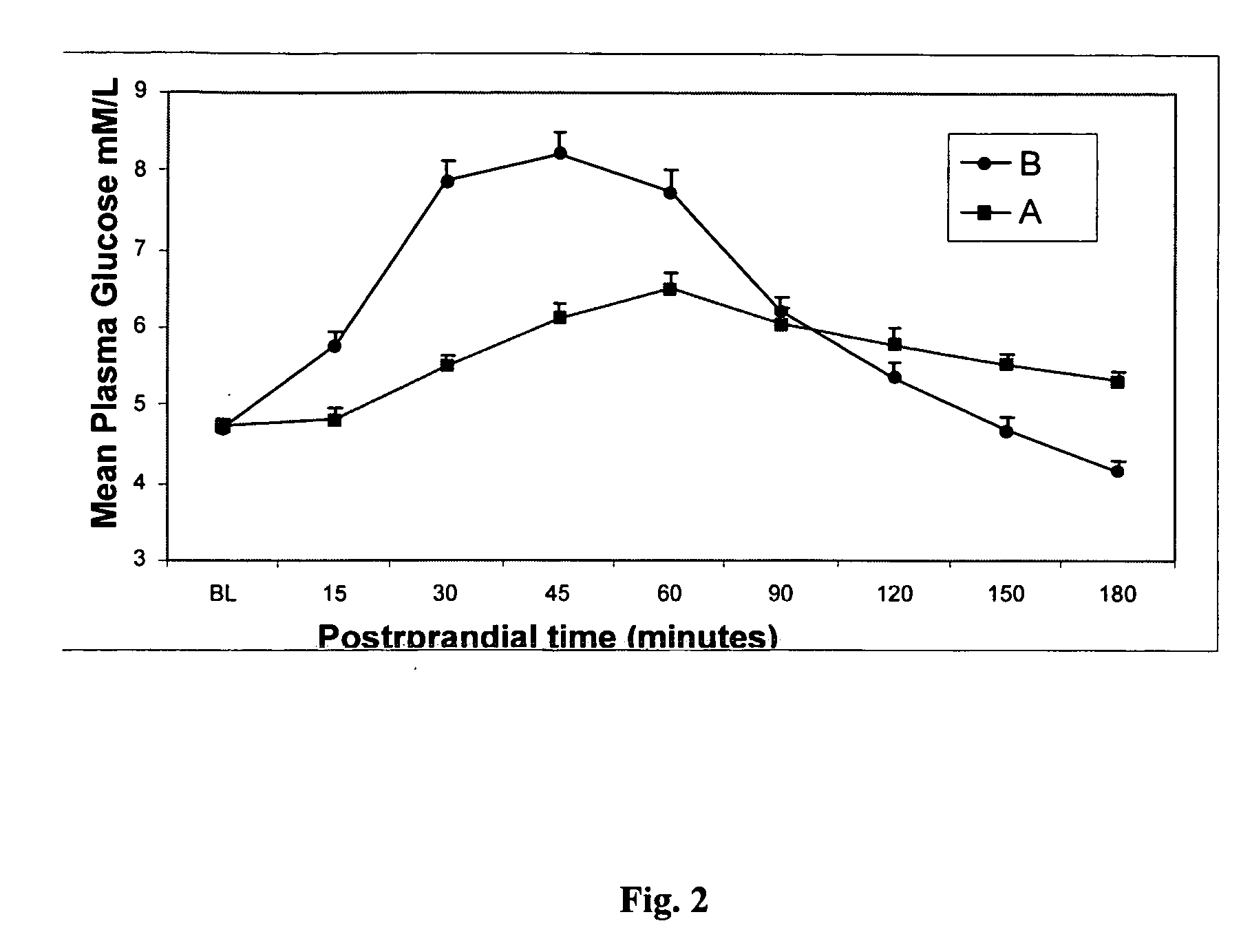
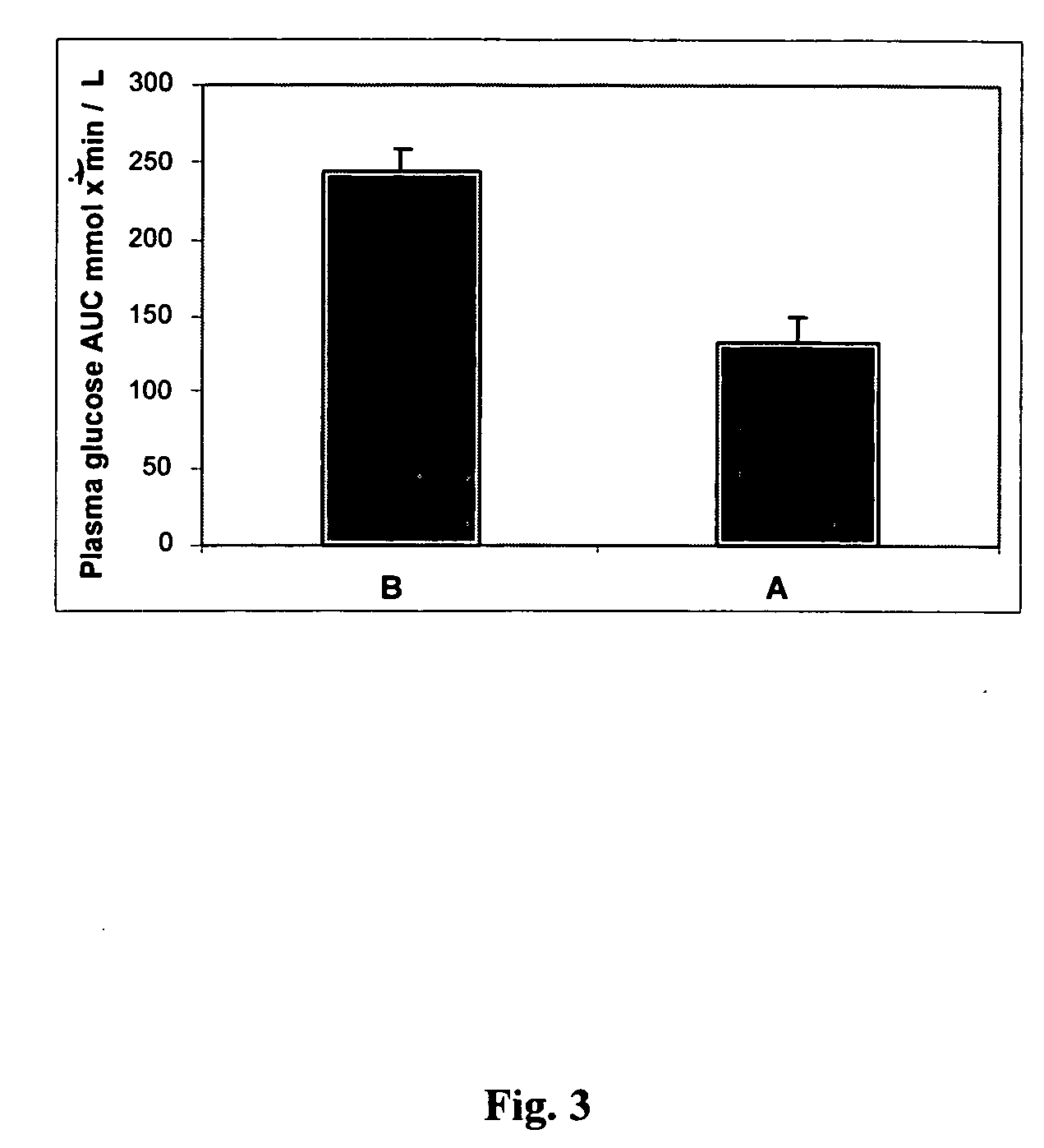
InactiveUS20050215523A1Blood glucose levelProlonged glycemic responseOrganic active ingredientsBiocideCyclodextrinDuodenum length
Disclosed are methods of producing a blunted postprandial glycemic response in an individual, and / or reducing postprandial insulin secretion, said methods comprising administering to the individual a nutritional or other product comprising gamma-cyclodextrin. Also disclosed are similar other methods directed toward the use of such products to provide weight and appetite control, to normalize blood glucose levels in individuals with impaired glucose tolerance, to minimize nighttime hypoglycemia in diabetic and non-diabetic patients, to prevent reactive hypoglycemia in susceptible non-diabetics, to normalize blood glucose levels in individuals with gestational diabetes or impaired glucose tolerance during gestation, and / or to provide a prolonged glycemic response during exercise. The methods are based upon the discovery that gamma-cyclodextrins are rapidly metabolized and absorbed in the small intestine, but subsequently result in a surprisingly blunted postprandial glycemic response and reduced insulin secretion.
Owner:ABBOTT LAB INC
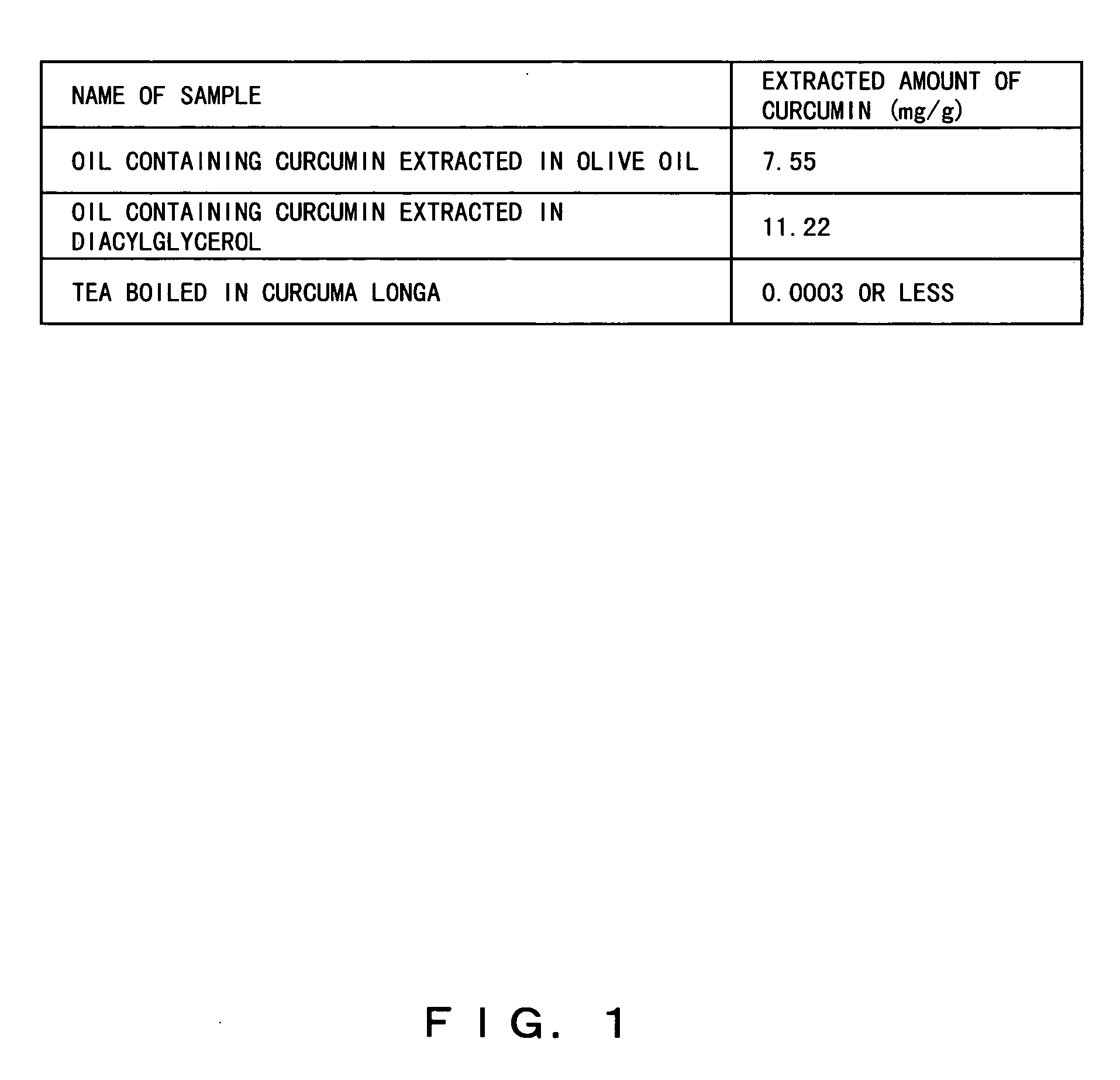
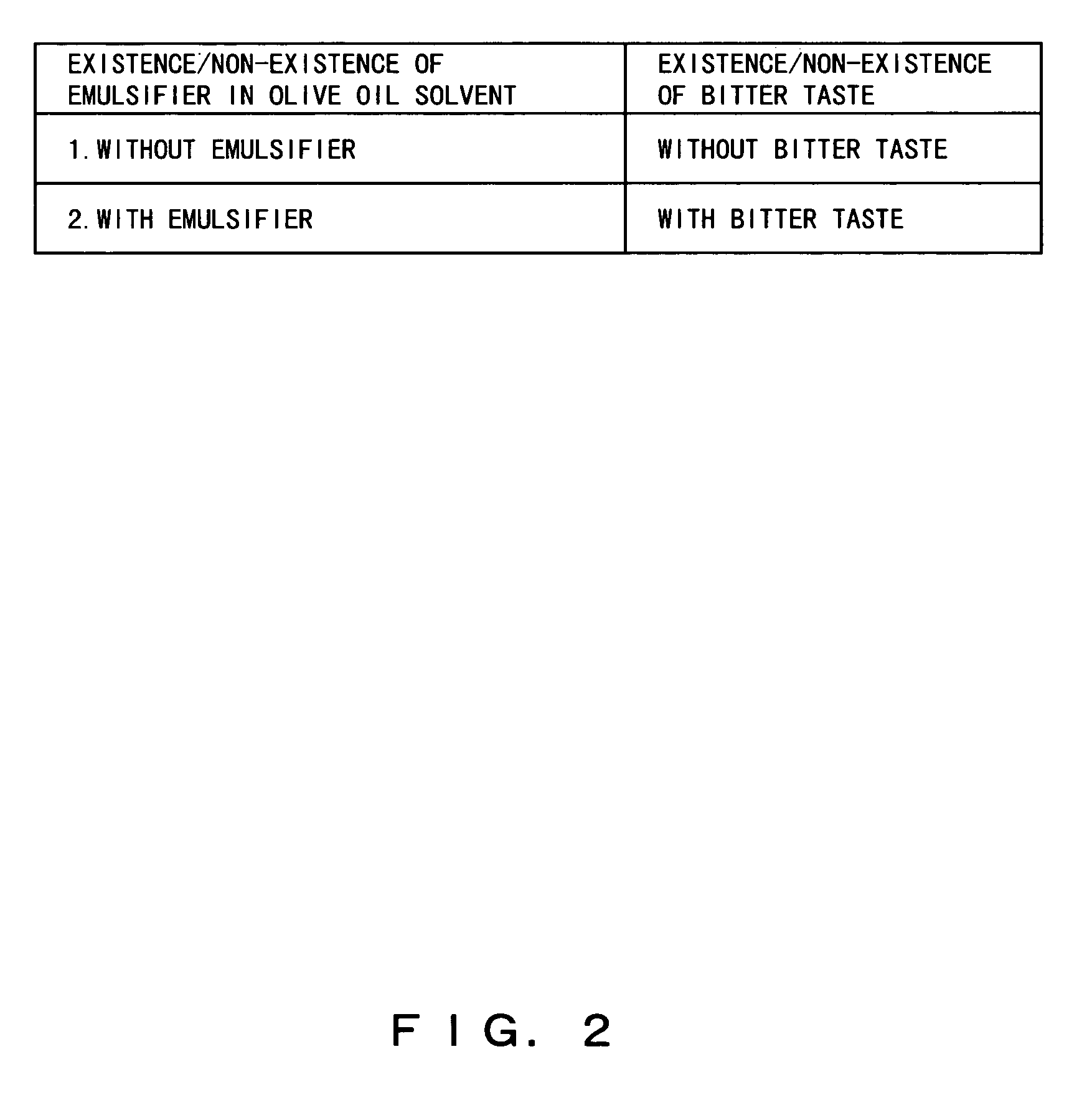
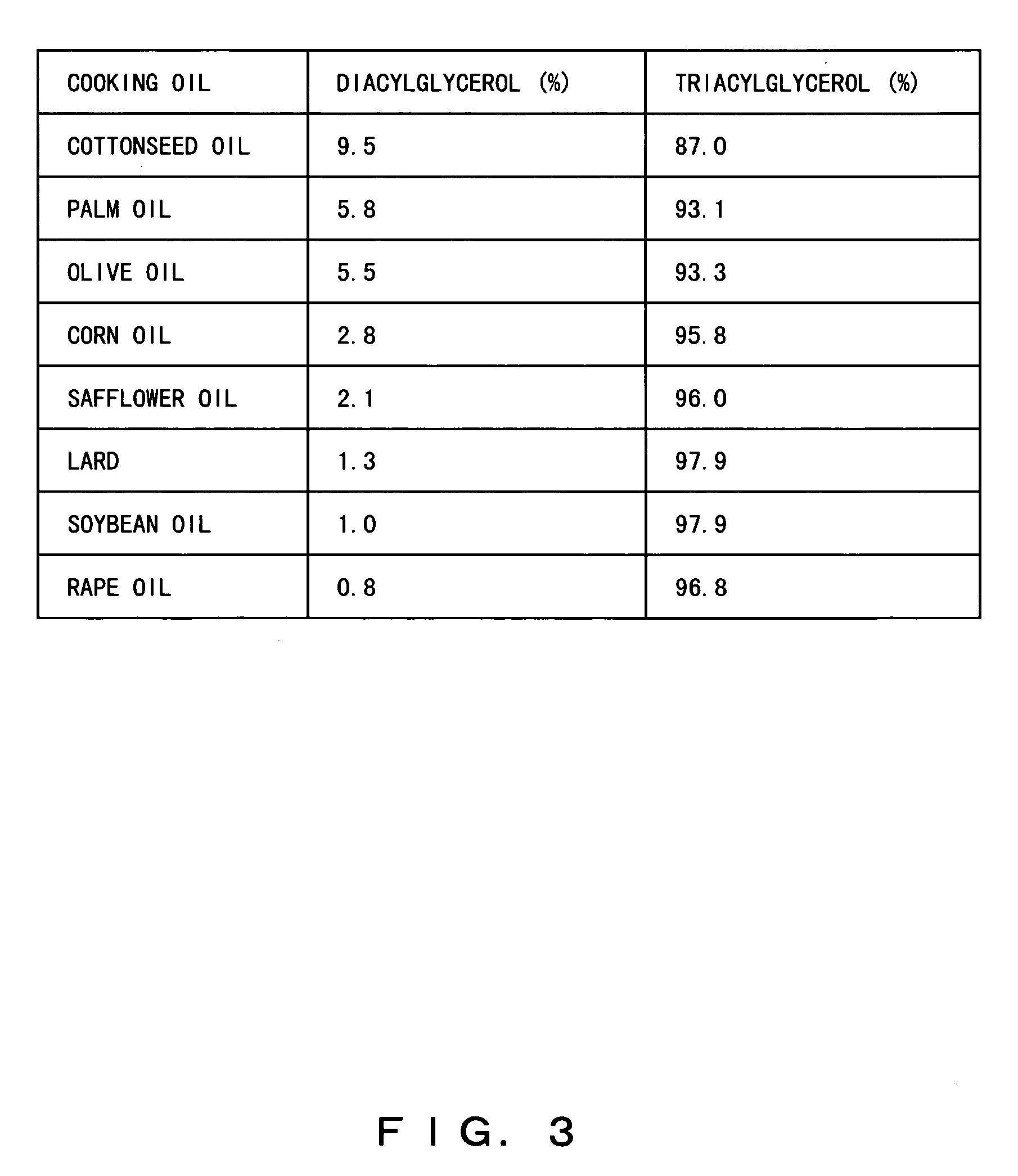
Composition containing herbal medicine component for promoting absorption and production method thereof
To make diacylglycerol obtained by adding an emulsifier contain both an effective oil-soluble component and an effective water-soluble component which are contained in herbal medicines, a herbal medicine component for promoting the absorption of the oil-soluble component in the small intestine and a production method thereof are objects of the present invention. The powder of the herbal medicines is added to solvent oil selected from diacylglycerol and / or triacylglycerol cooking oil to which lecithin and / or a W / O type emulsifier are added, is heated and an herbal medicine component is extracted or dissolved. By such a method, drugs and healthy food which contain all effective oil-soluble and water-soluble components contained in the herbal medicines and which promote the absorption of the oil-soluble components in the small intestine can be produced.
Owner:OSHIRO SEIRI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com