Patents
Literature
119 results about "Gastric band" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
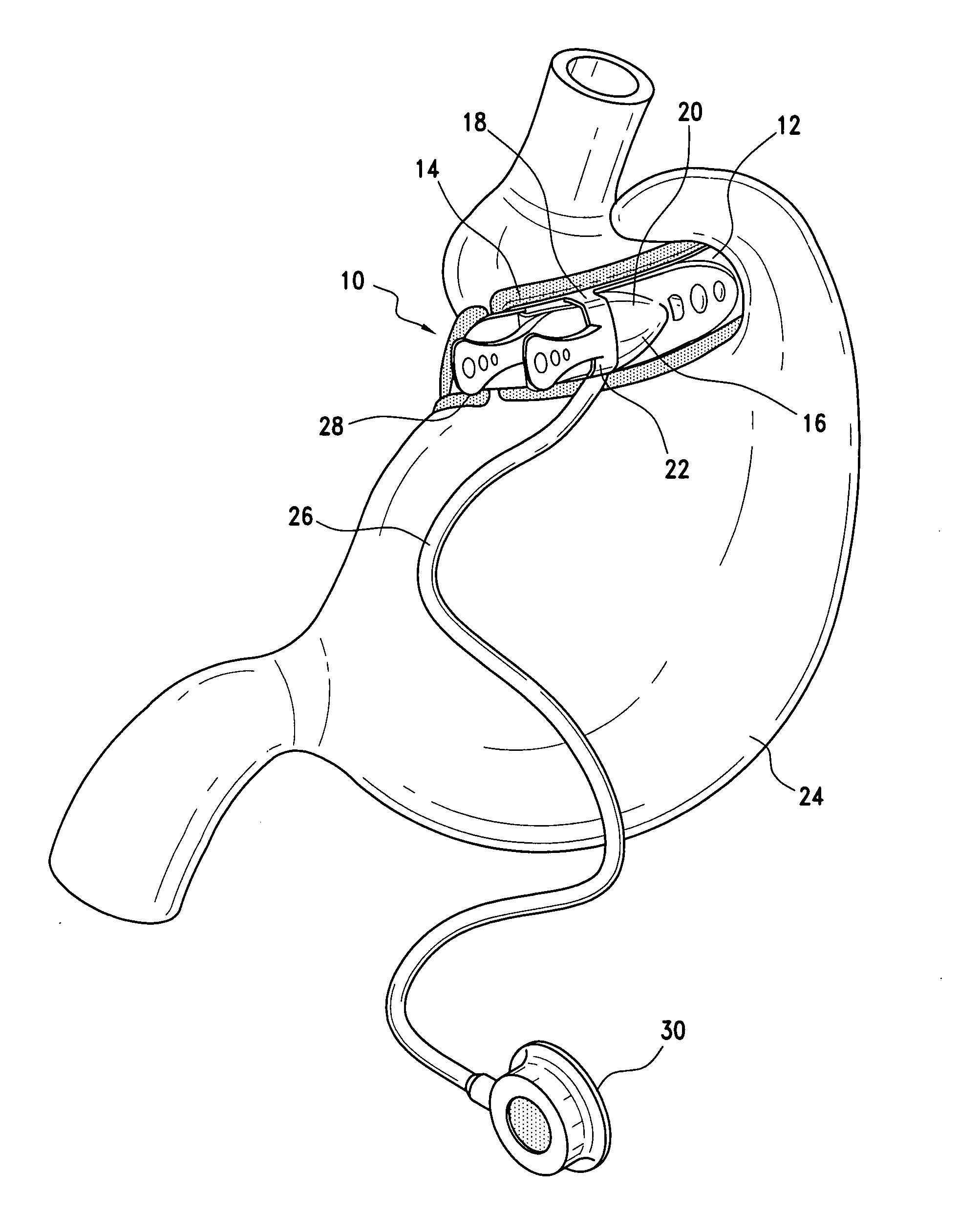
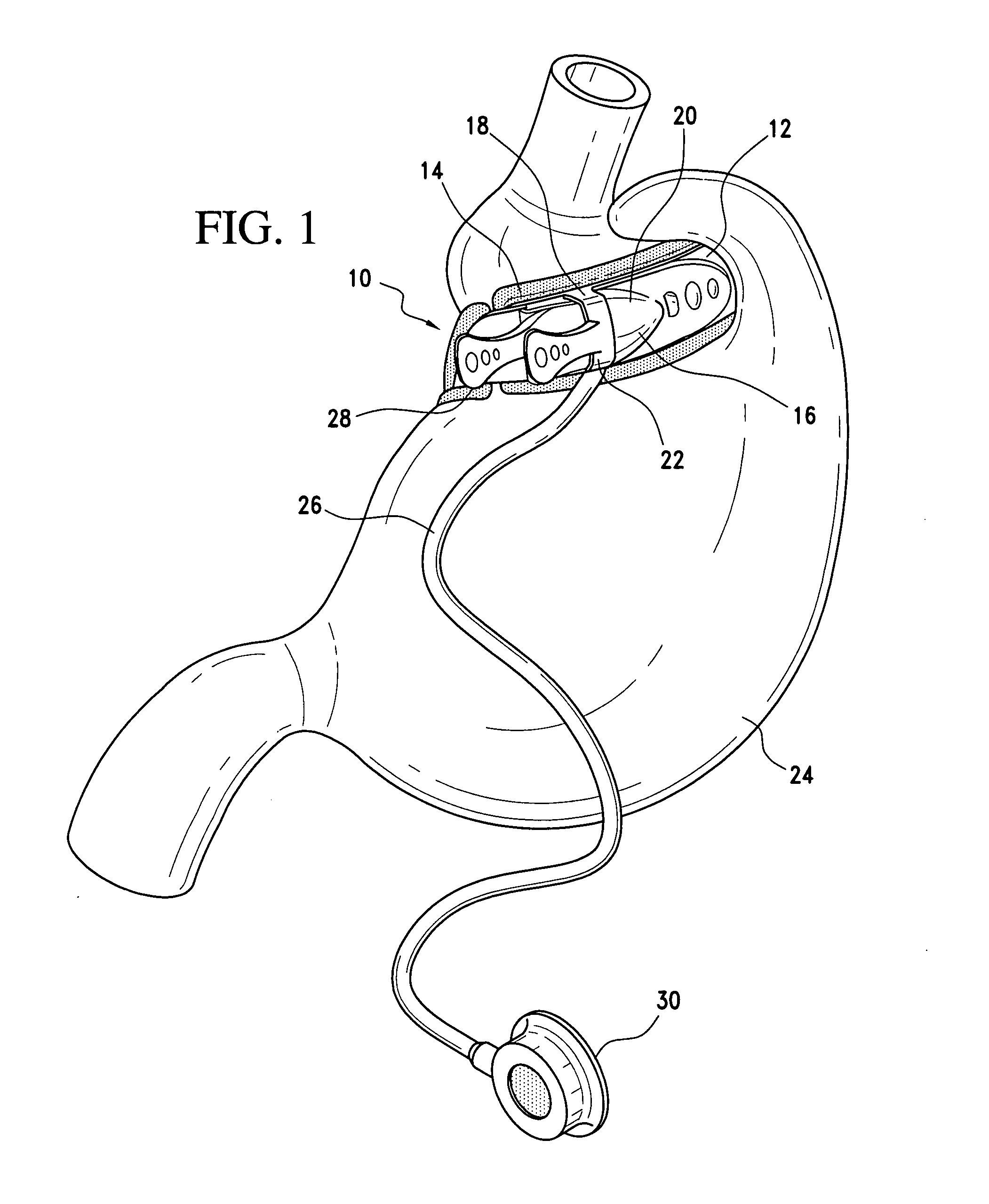
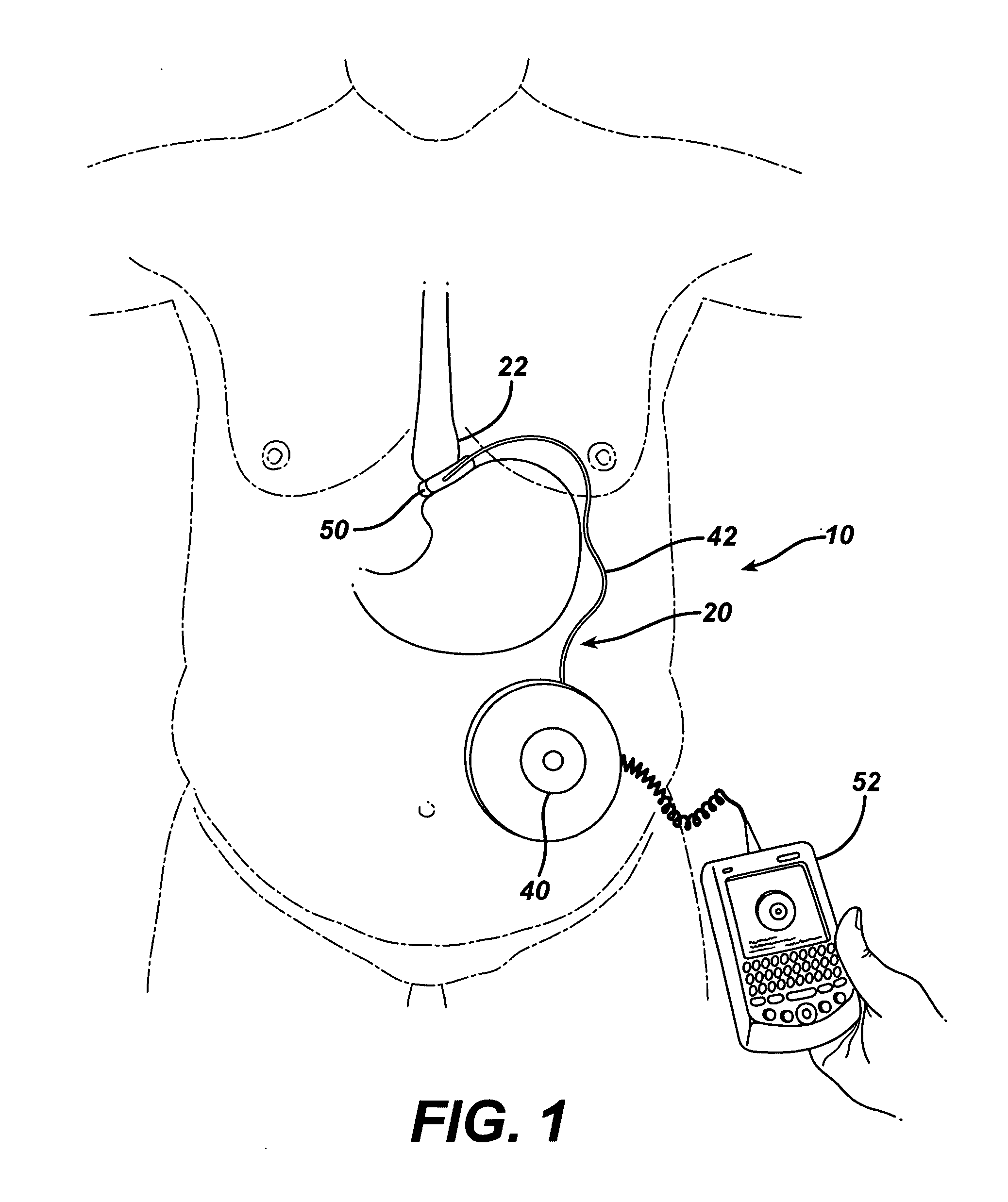
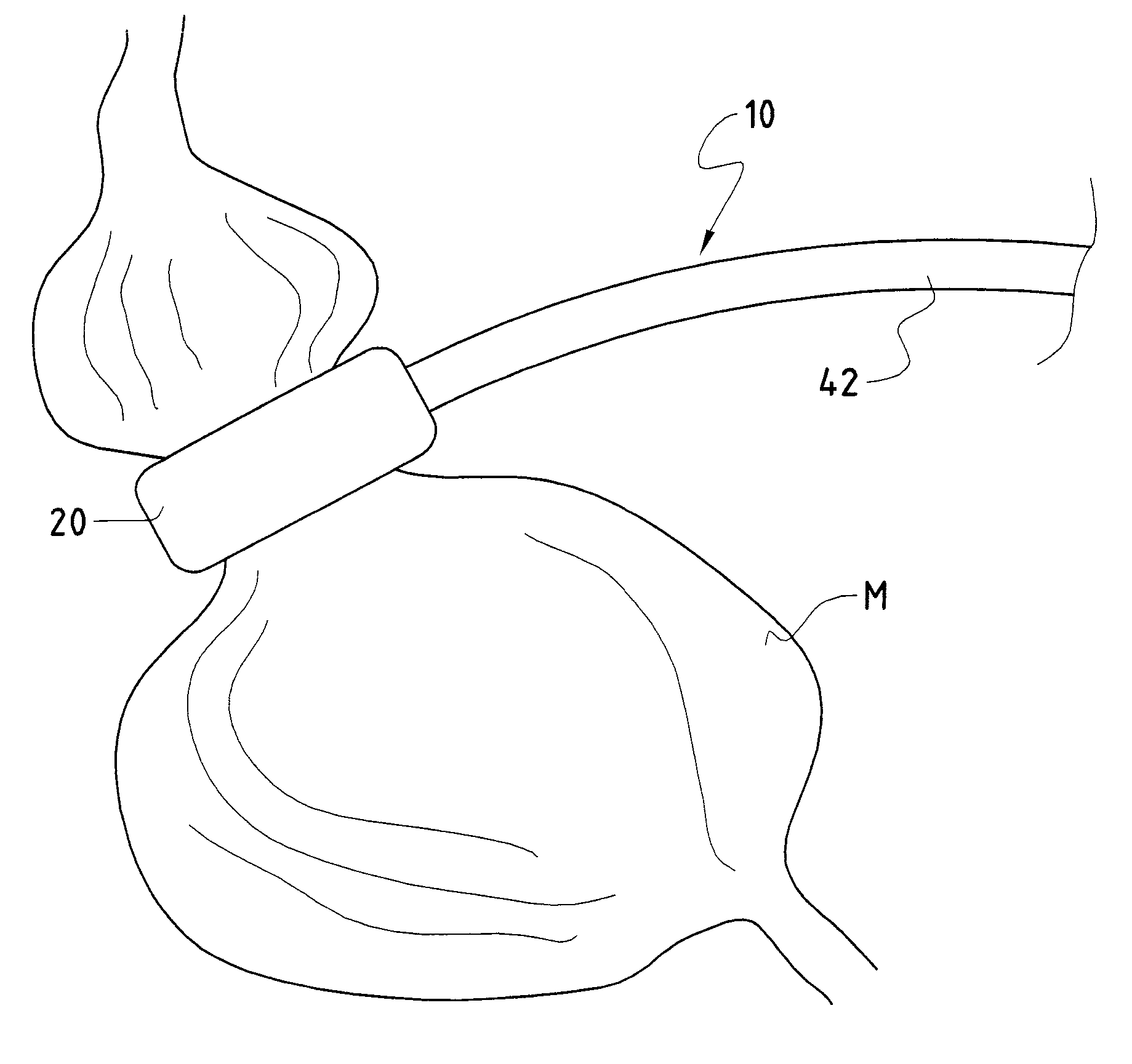
Methods and devices for measuring impedance in a gastric restriction system
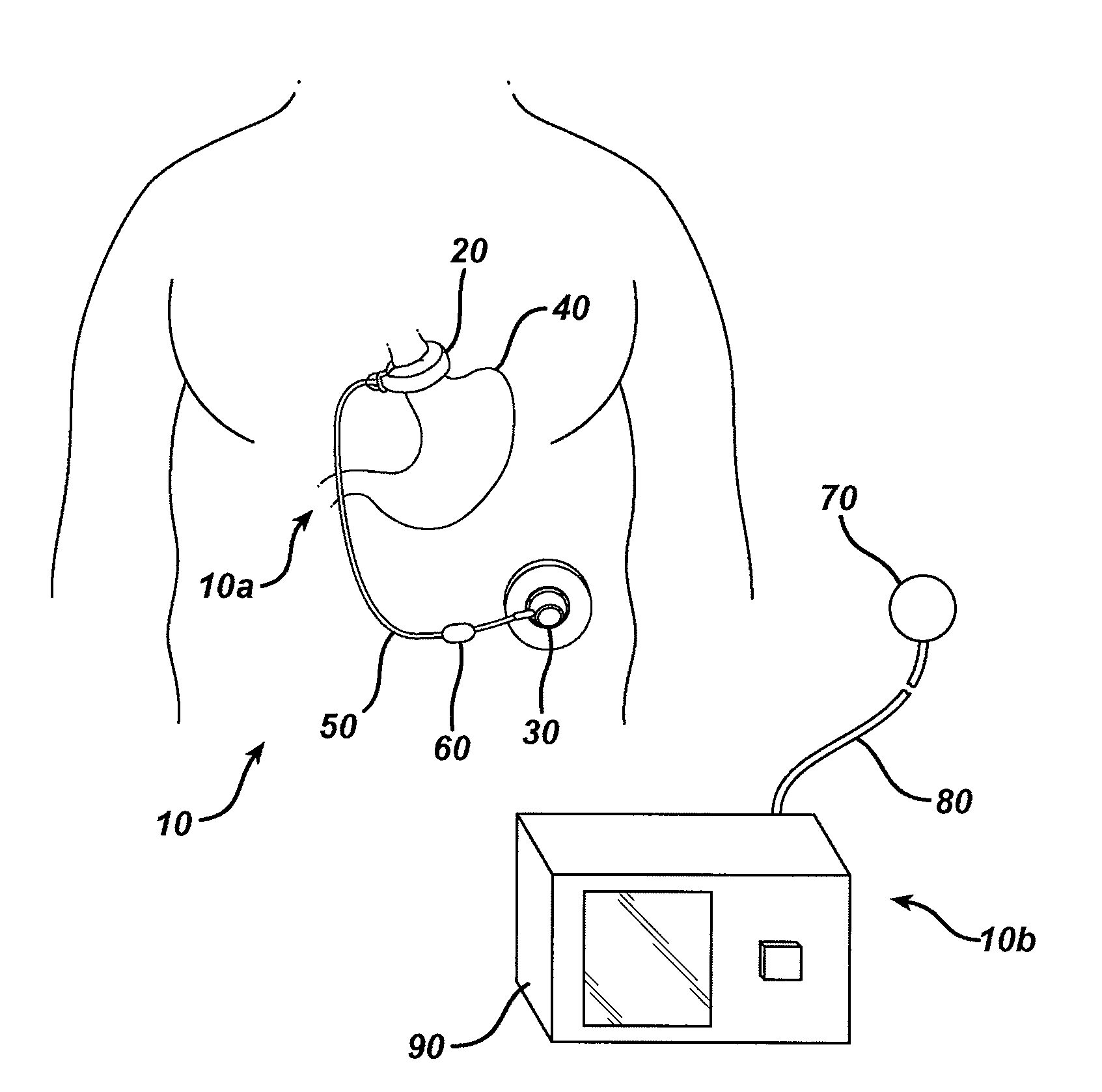
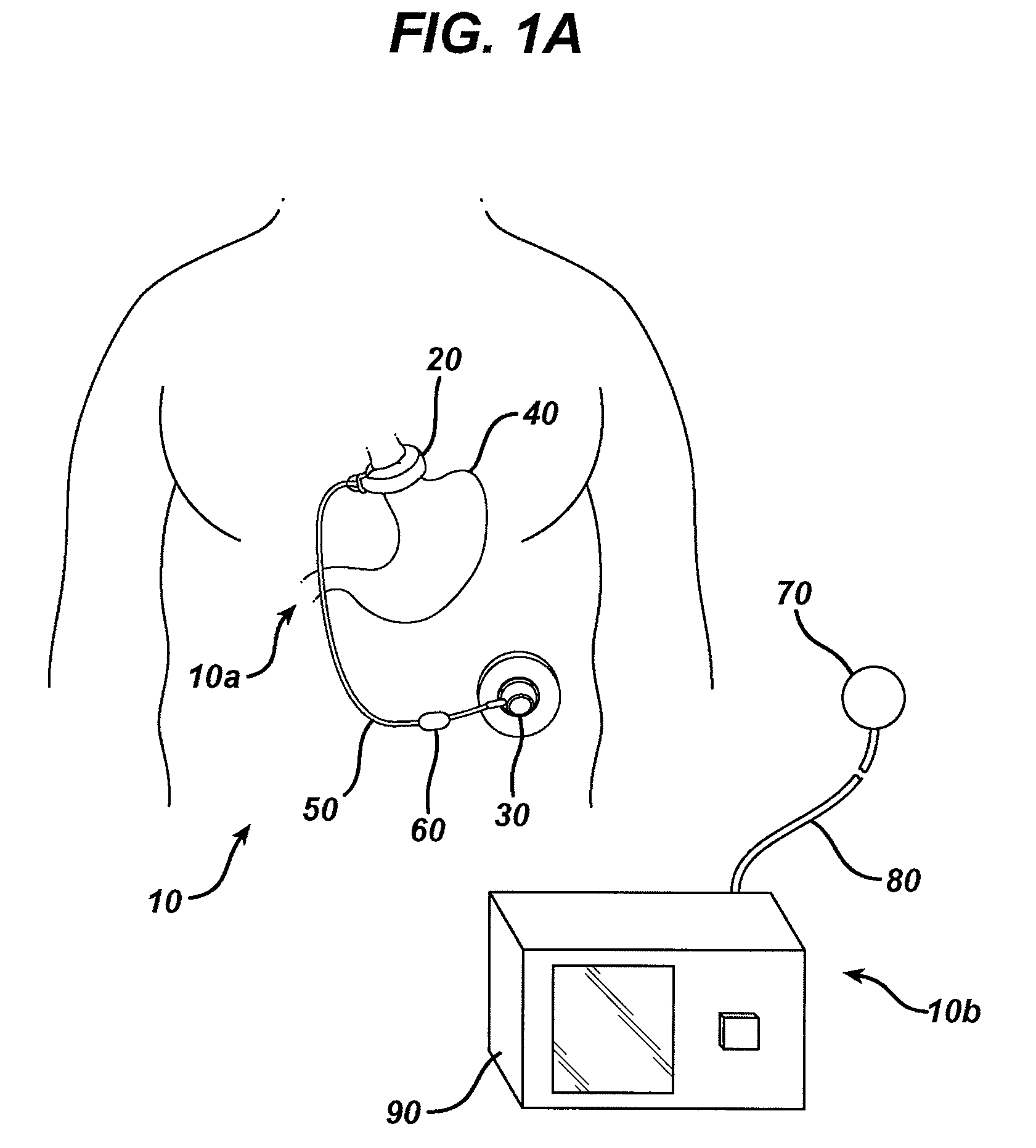
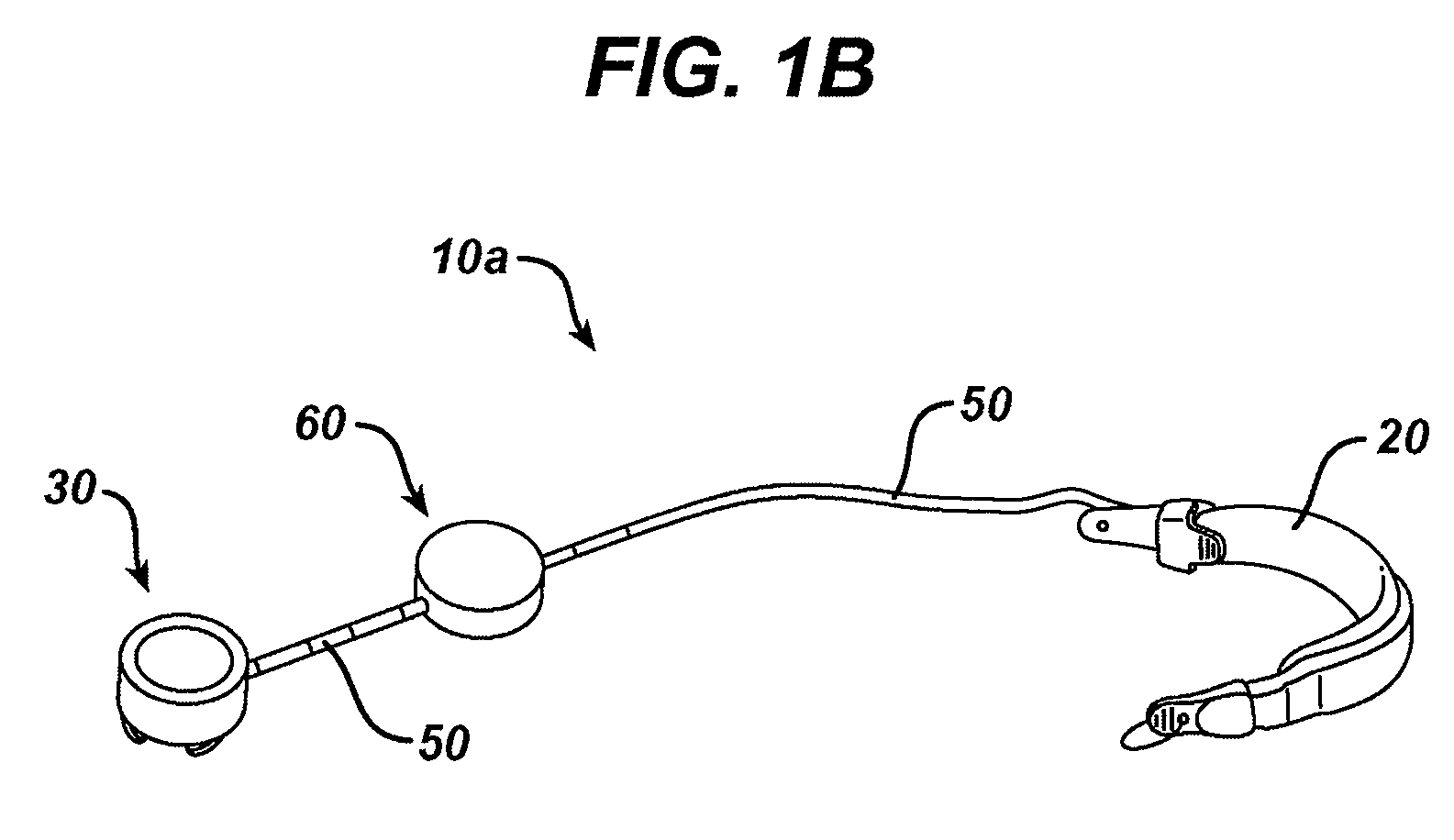
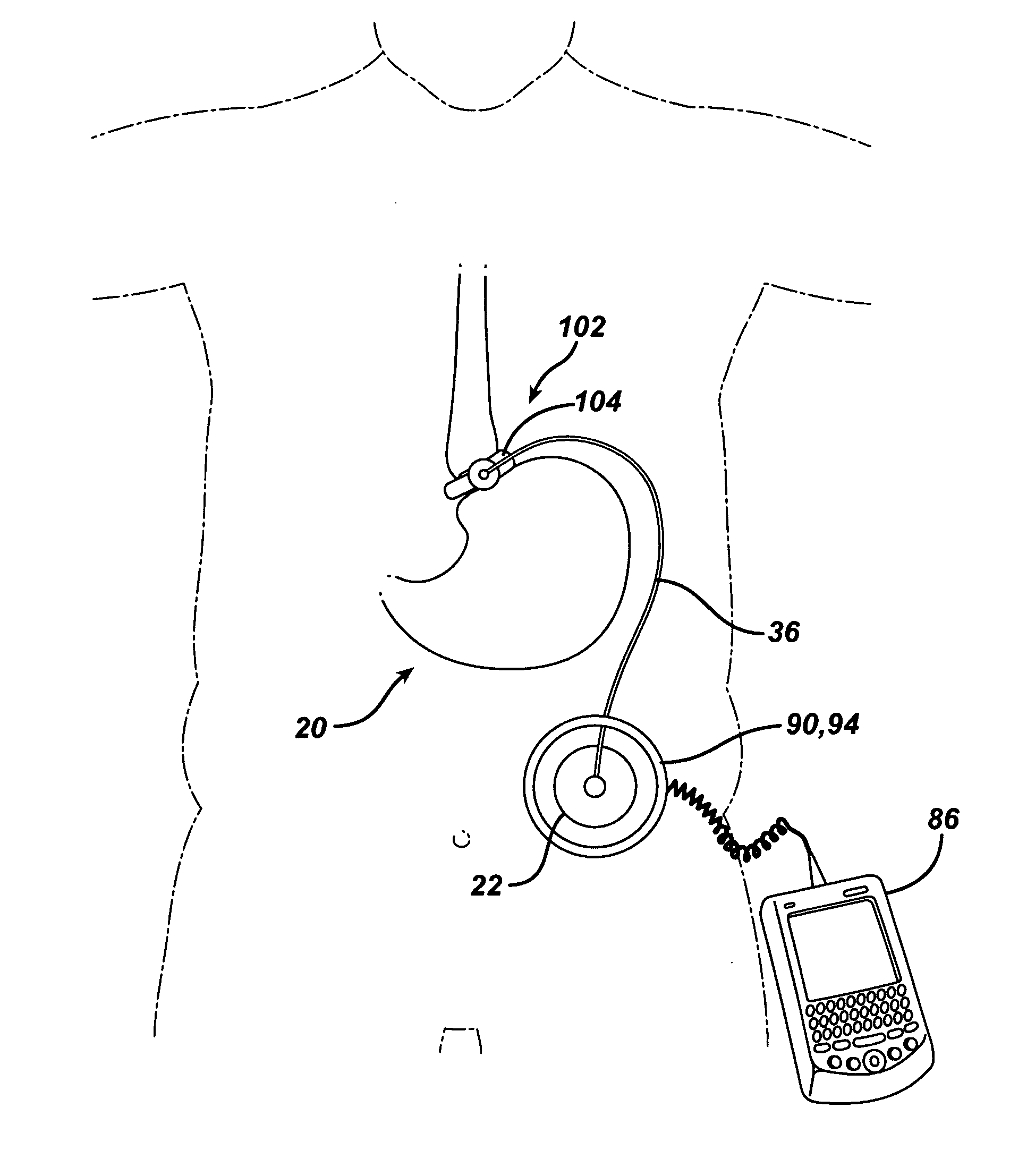
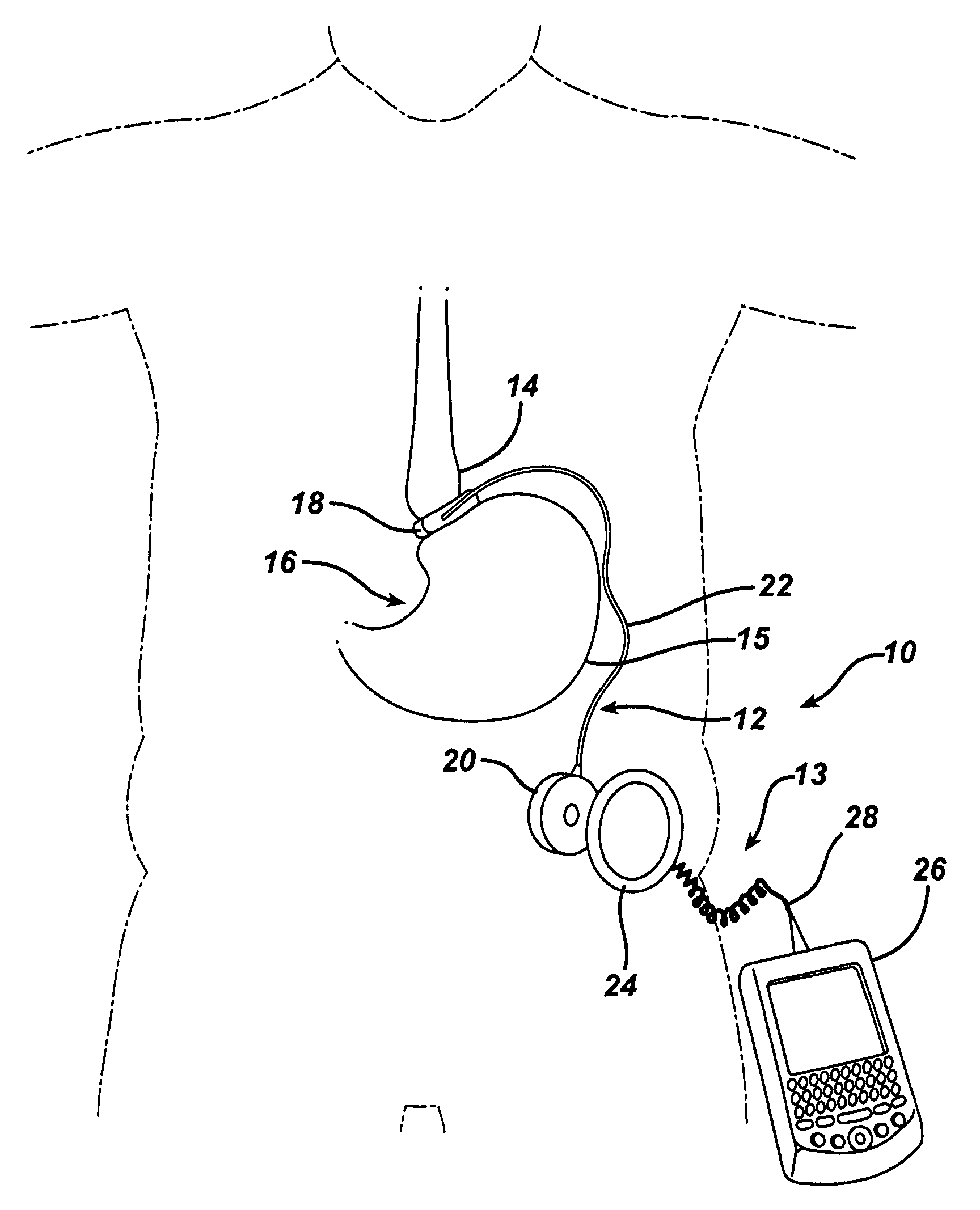
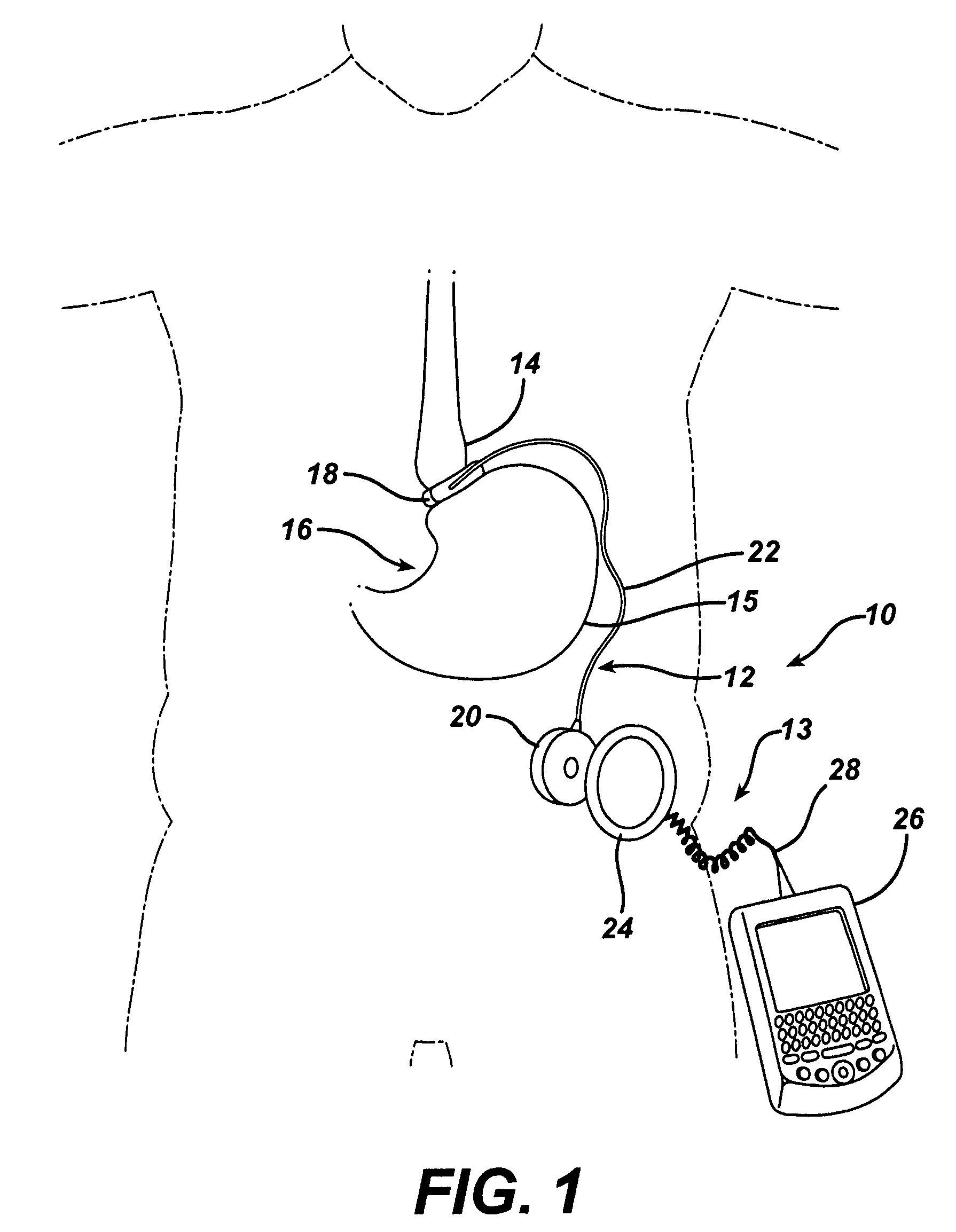
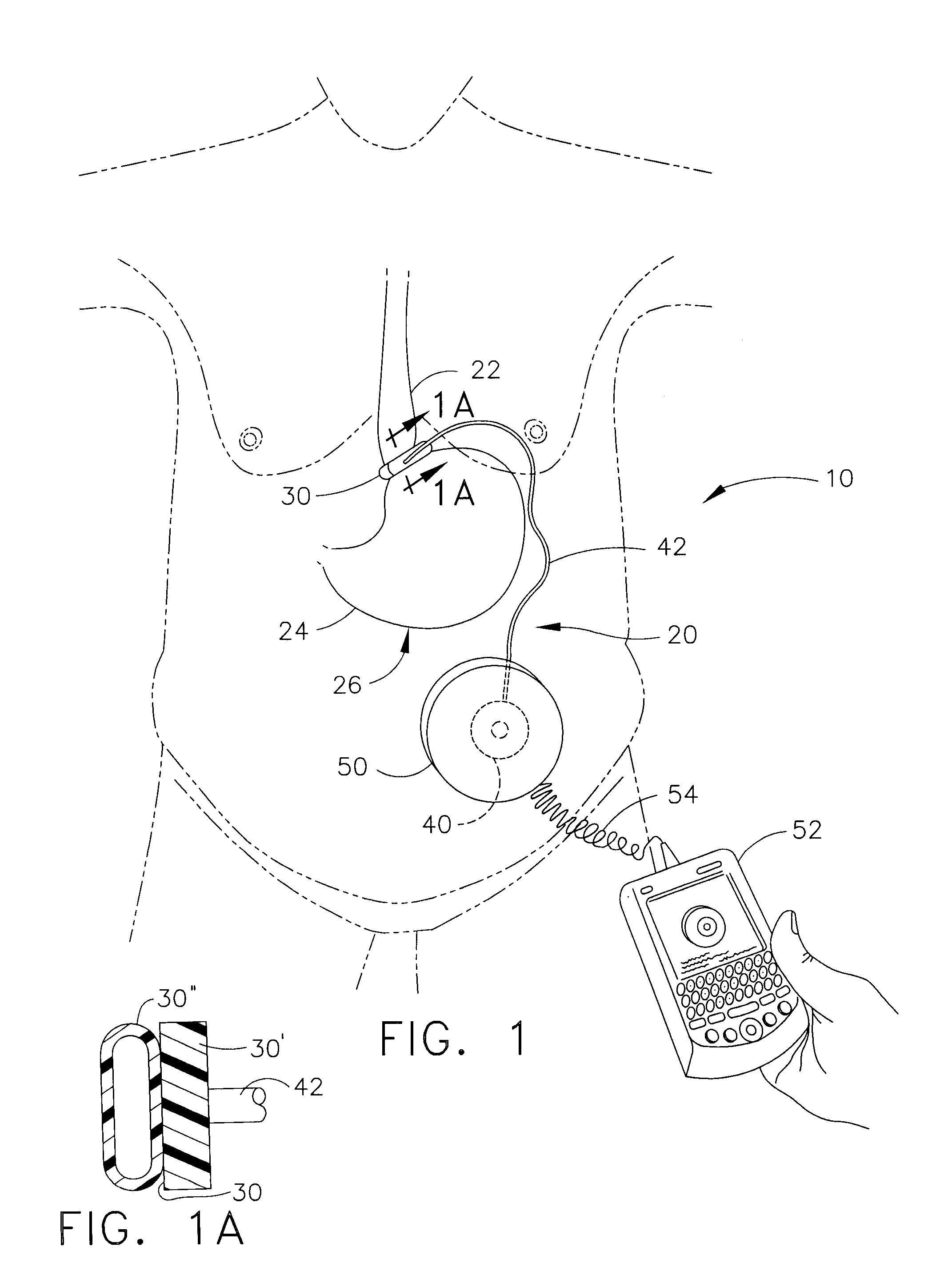
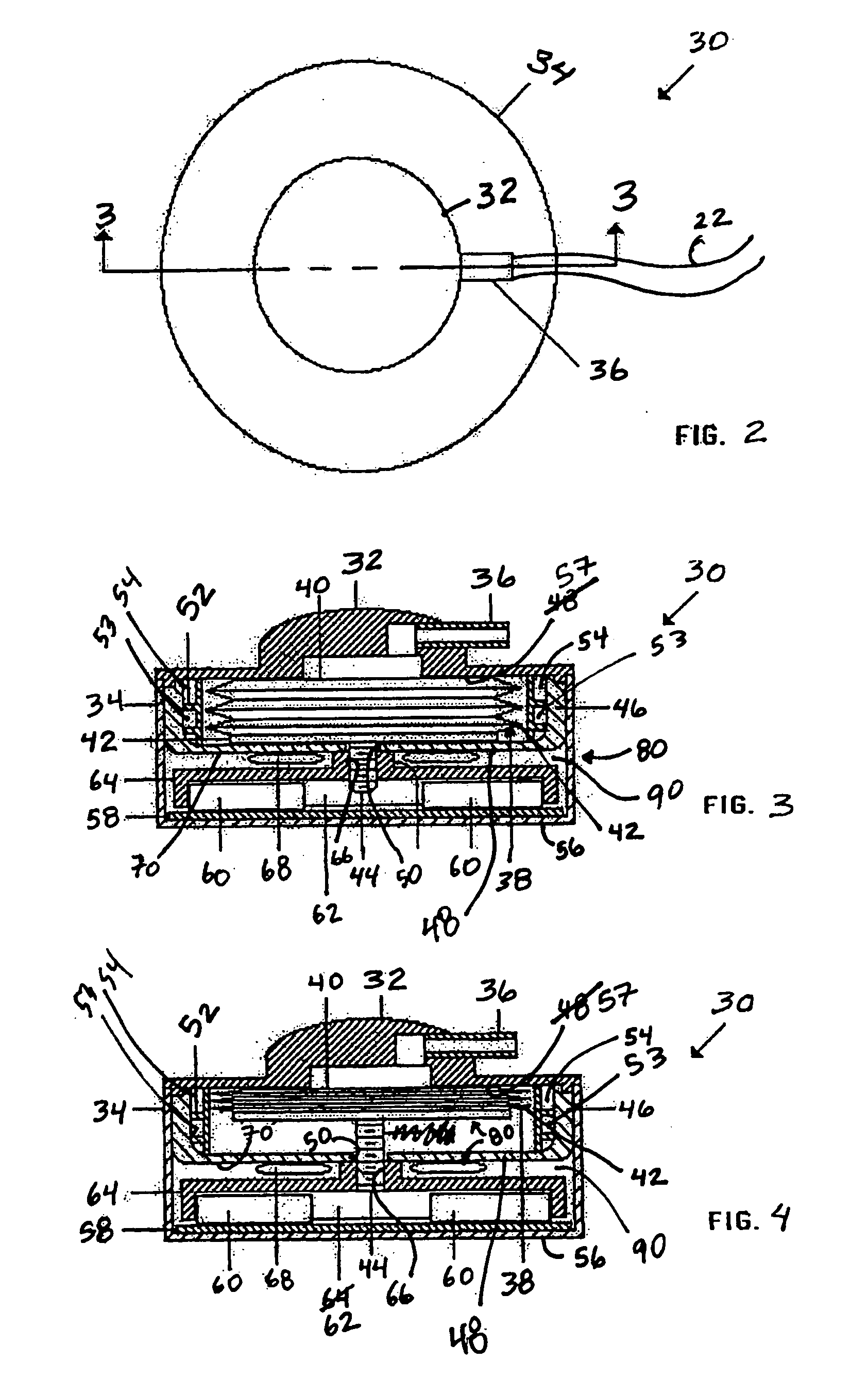
Methods and devices are provided for gathering impedance data related to implantable restriction devices. In general, the methods and devices can enable patients, health care providers, and others to use gathered data as a feedback mechanism to non-invasively monitor efficacy of an implantable restriction device in a patient and to identify, modify, and / or prescribe a treatment plan for the patient considering the gathered data. Impedance data can be gathered and analyzed for tissue proximate to the restriction device, e.g., a fat pad between a gastric band and the patient's stomach. Electrodes in contact with the tissue can measure an impedance of the tissue, with the impedance between the electrodes changing as the tissue reduces in size (e.g., as fat cells shrink) and / or changes configuration.
Owner:ETHICON ENDO SURGERY INC
Adjustable gastric implant
A gastric band implant characterized in that a strap constituted by an elongate piece is secured to the inside of an inflatable bag. The width and thickness of the piece are smaller than the corresponding dimensions of an oblong right cross-section of the bag. The piece possesses convex longitudinal edges and has complementary overlapping connection structures on opposite end portions of the strap.
Owner:MEDICAL INNOVATION DEV
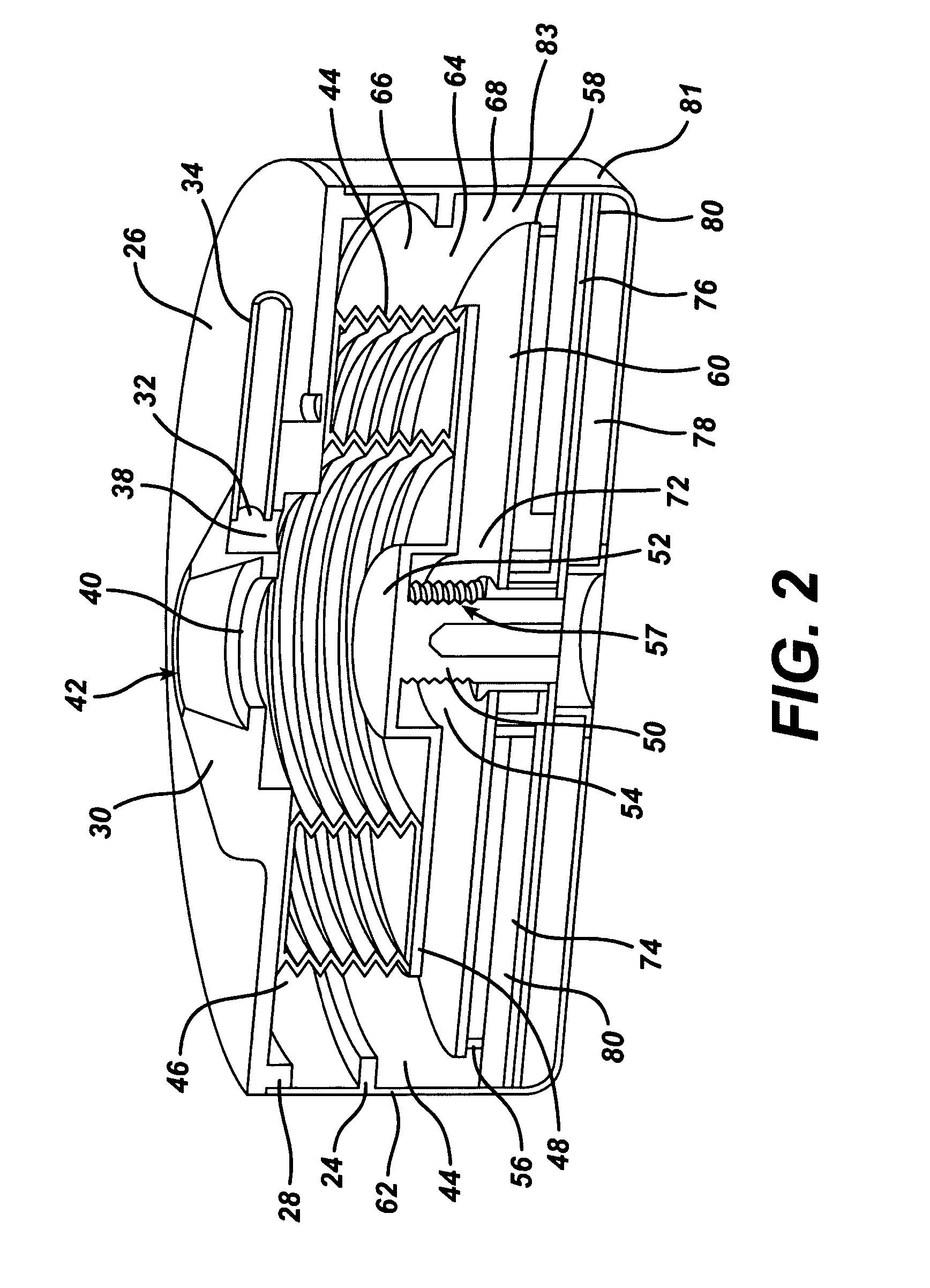
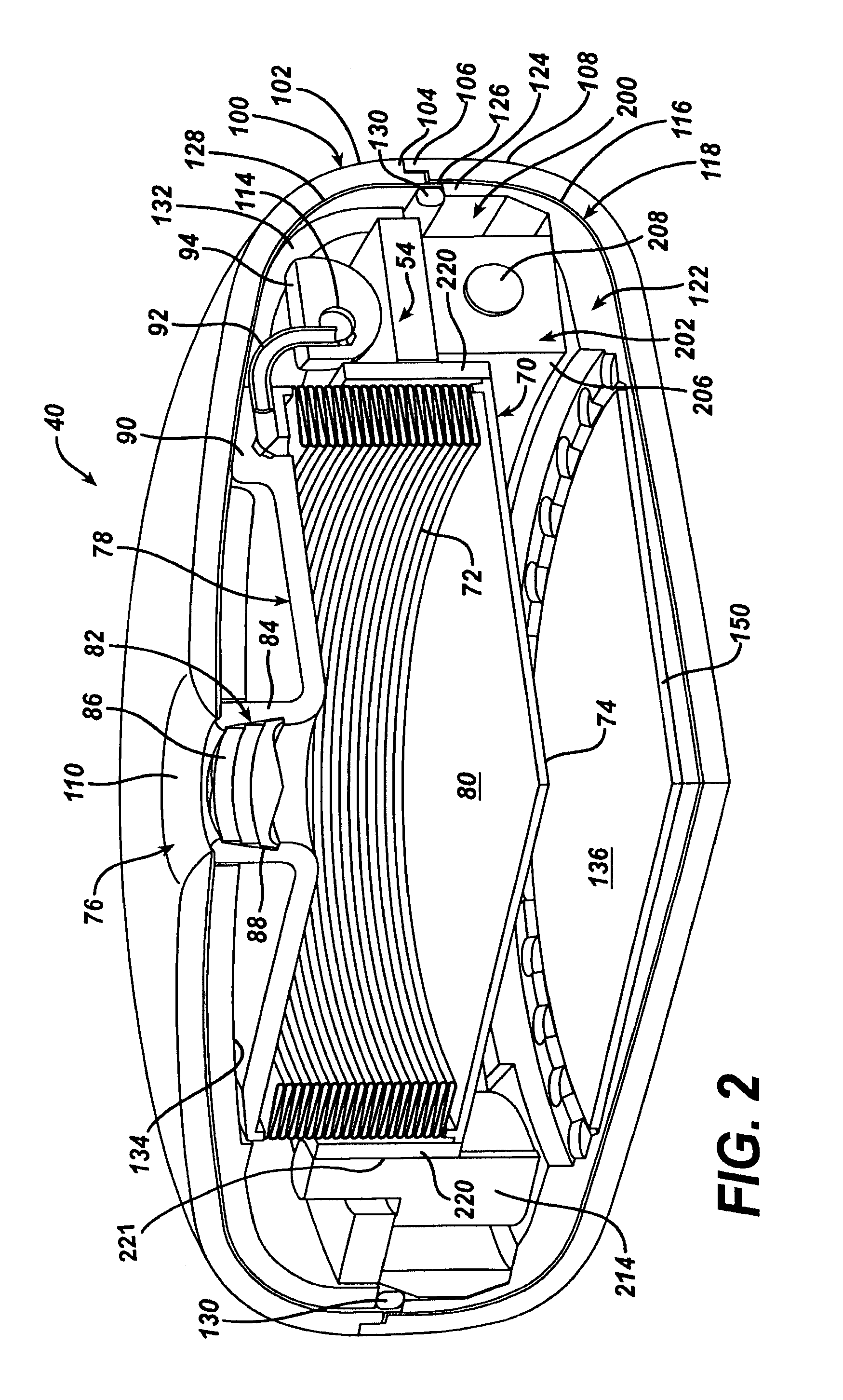
Piezo electrically driven bellows infuser for hydraulically controlling an adjustable gastric band
A remotely controlled gastric band system that is practically immune to external magnetic fields, such as from a Magnetic Resonance Imaging (MRI) machine, incorporates a bi-directional pump and fluid reservoir to adjust fluid volume in a gastric band. A piezoelectrically driven (e.g., rotary actuator, linear actuator) selectively compresses and expands a metal bellows hermetically sealed within a biocompatible and nonferromagnetic case such as titanium.
Owner:ETHICON ENDO SURGERY INC
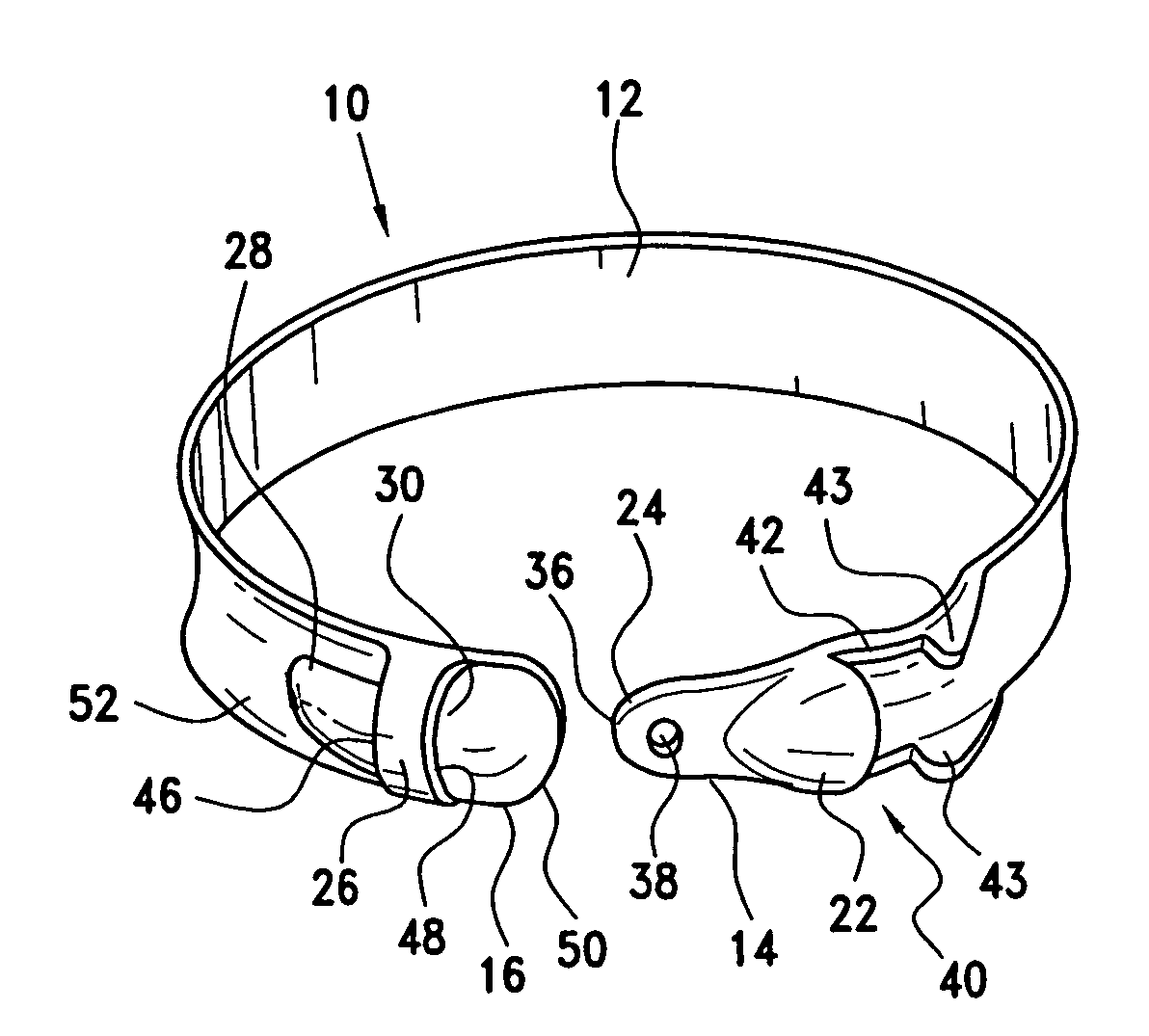
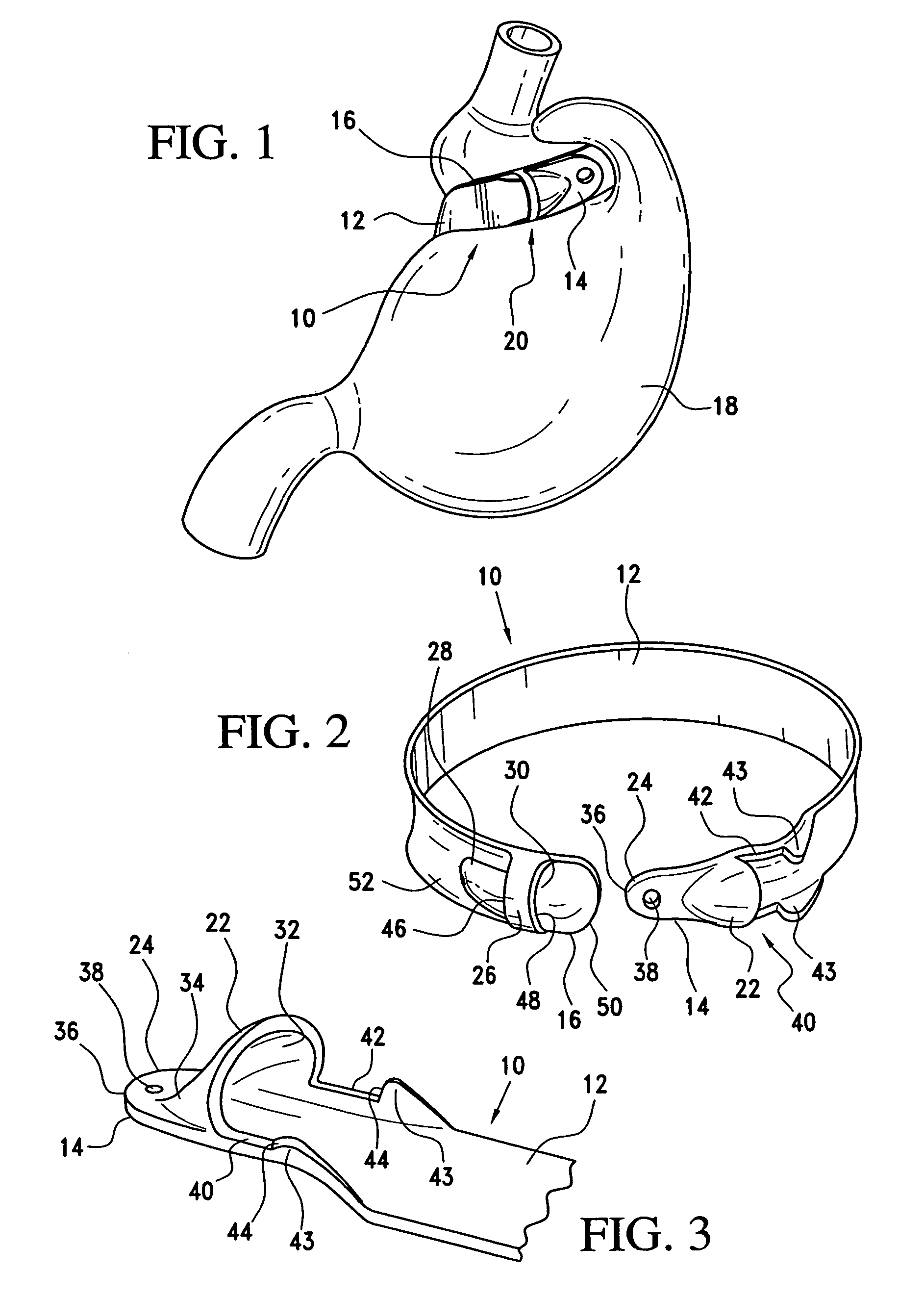
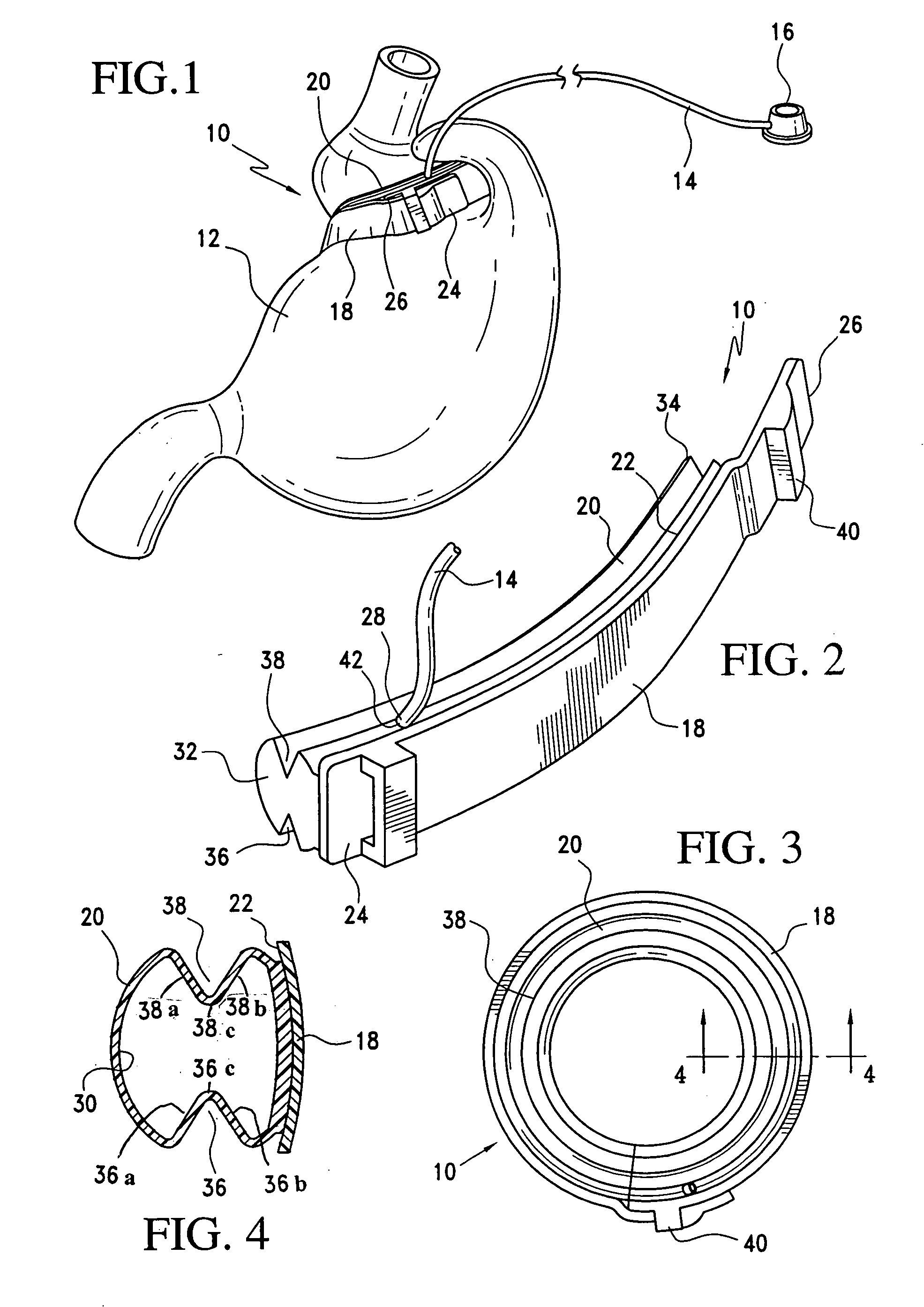
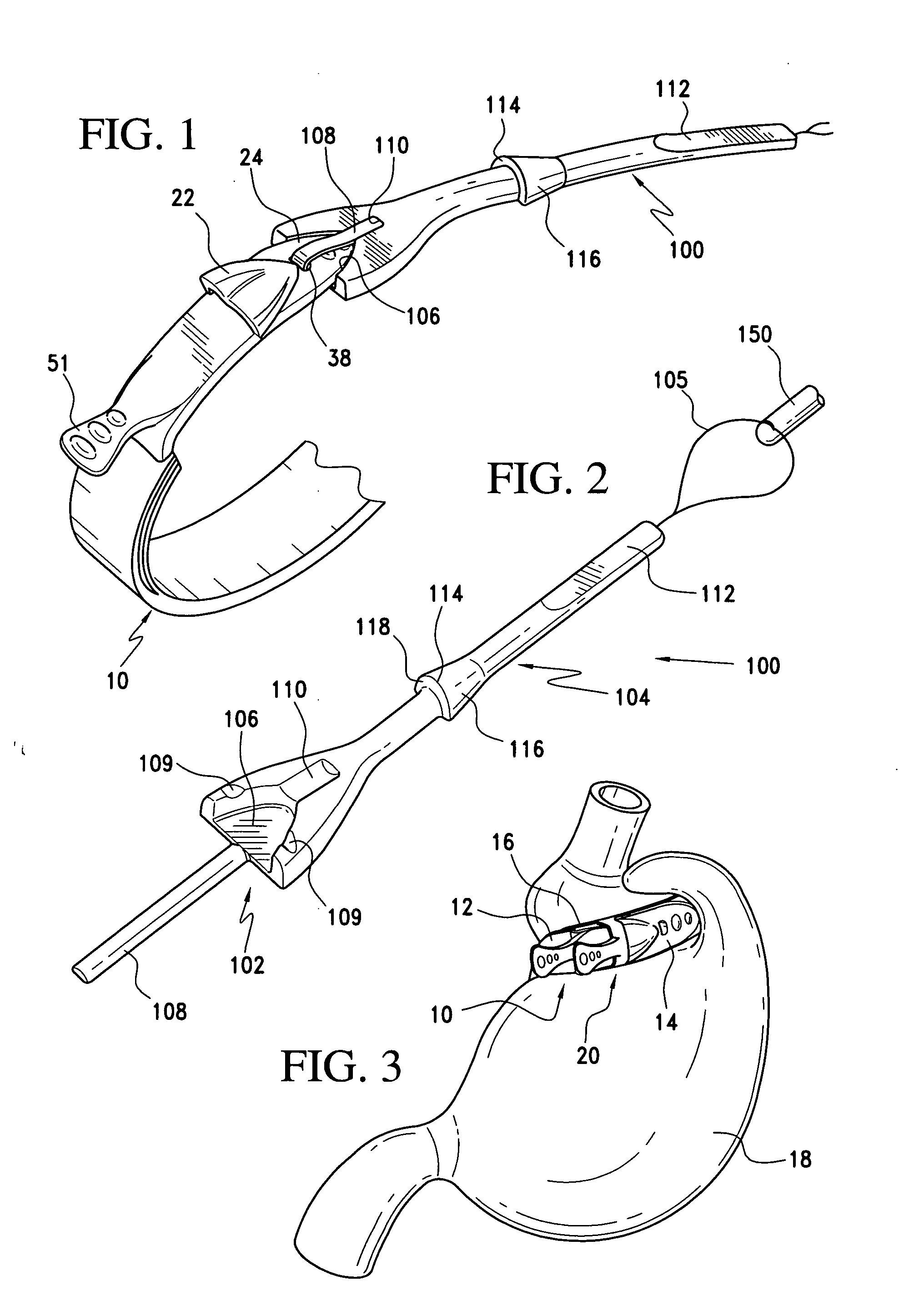
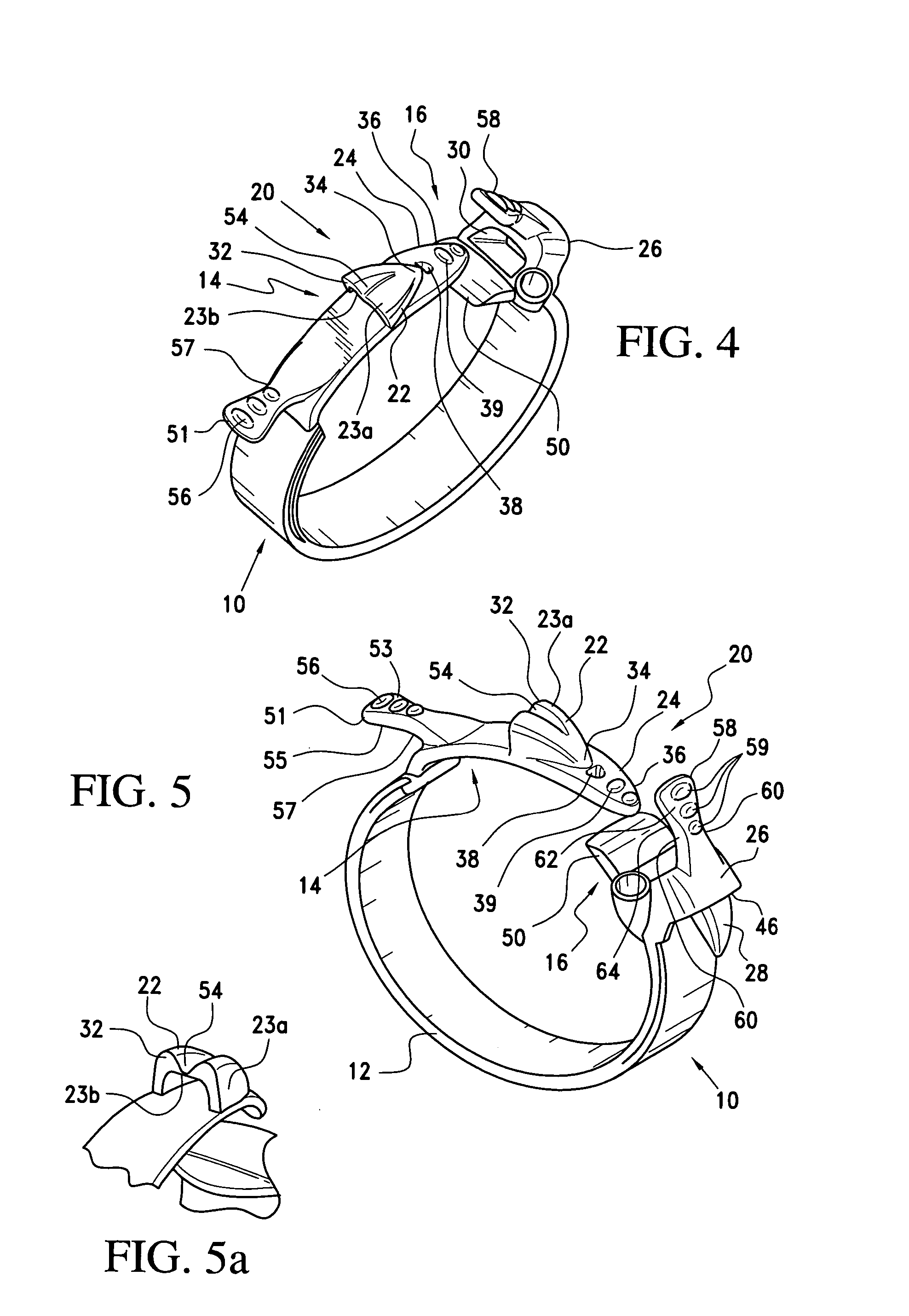
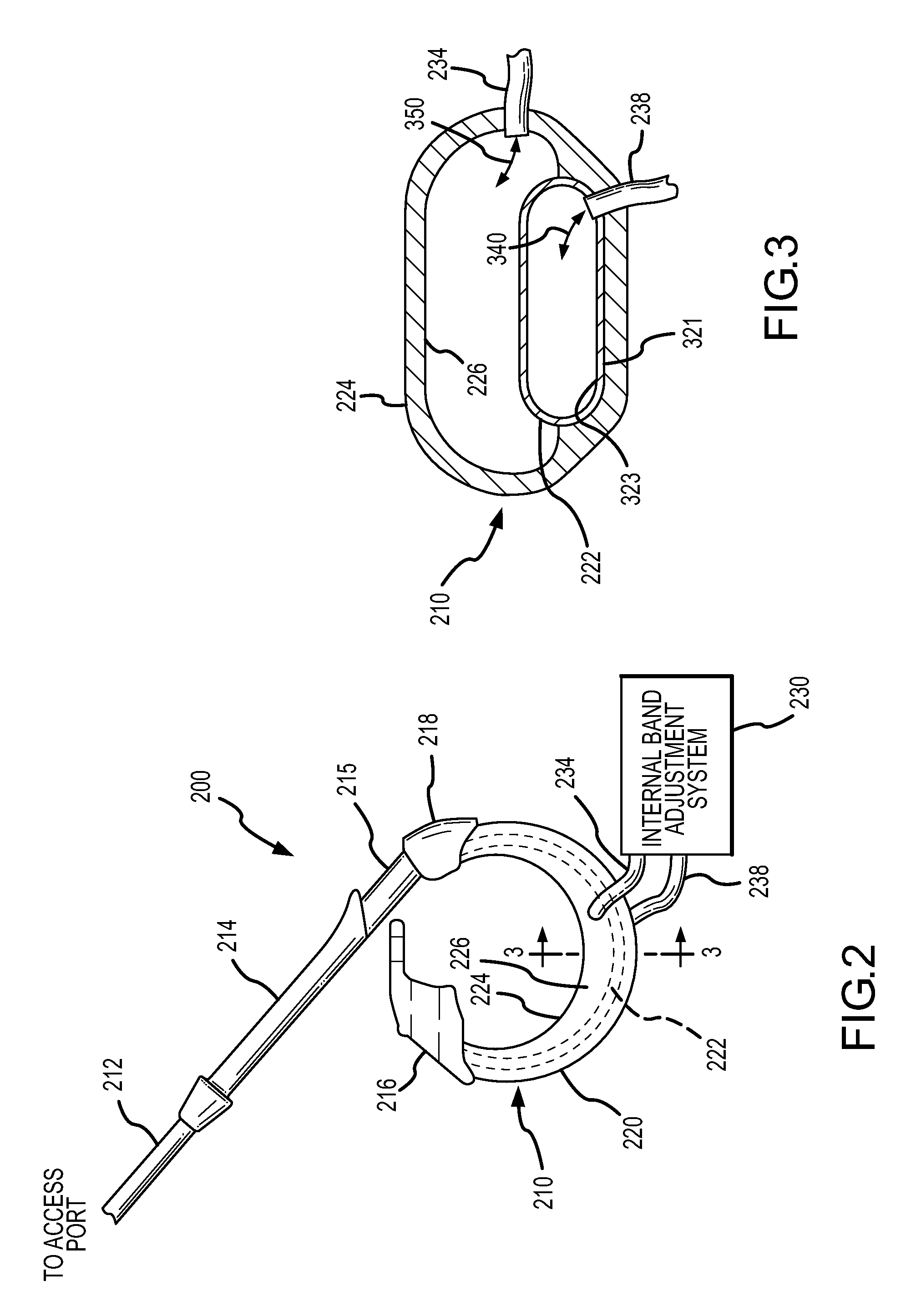
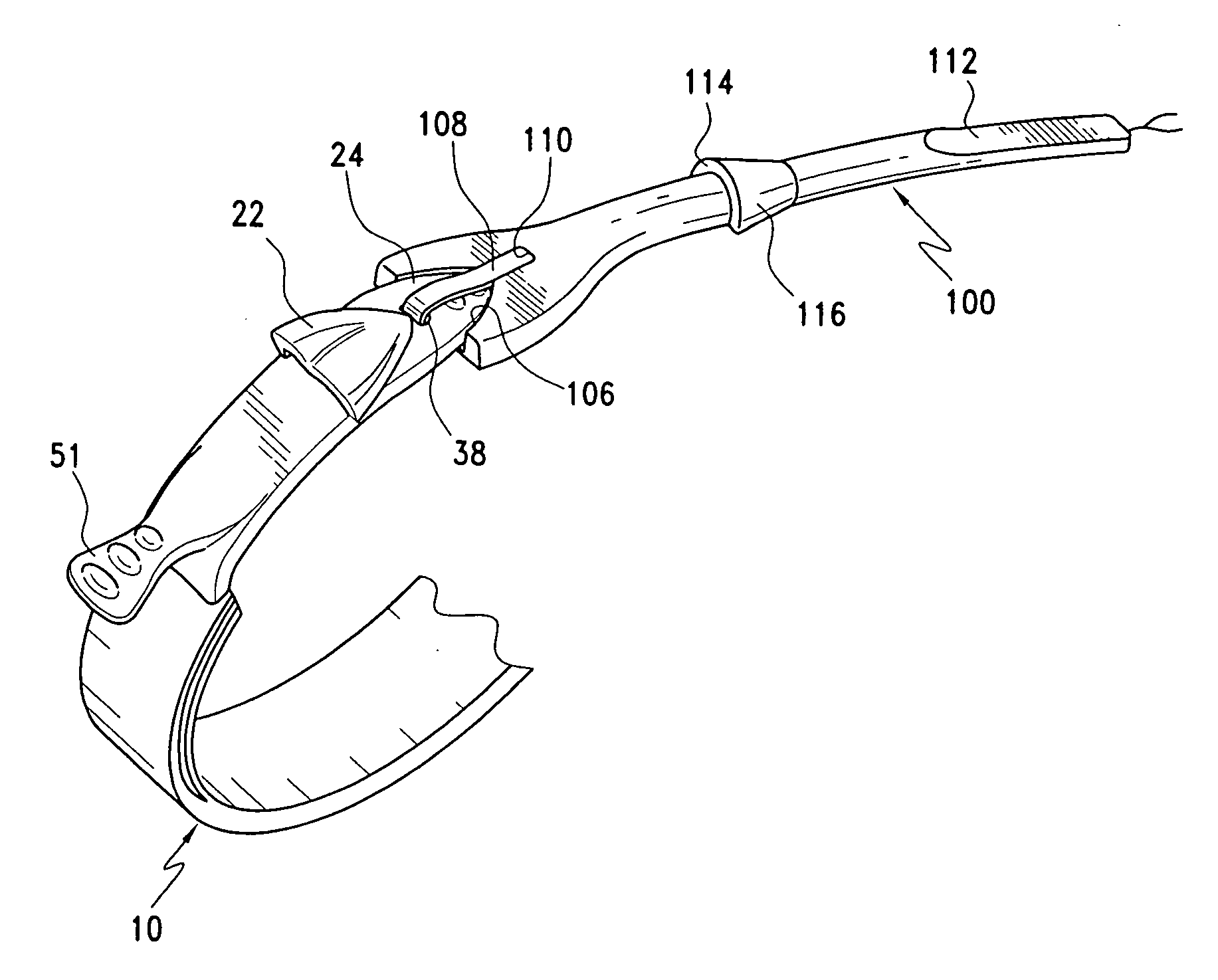
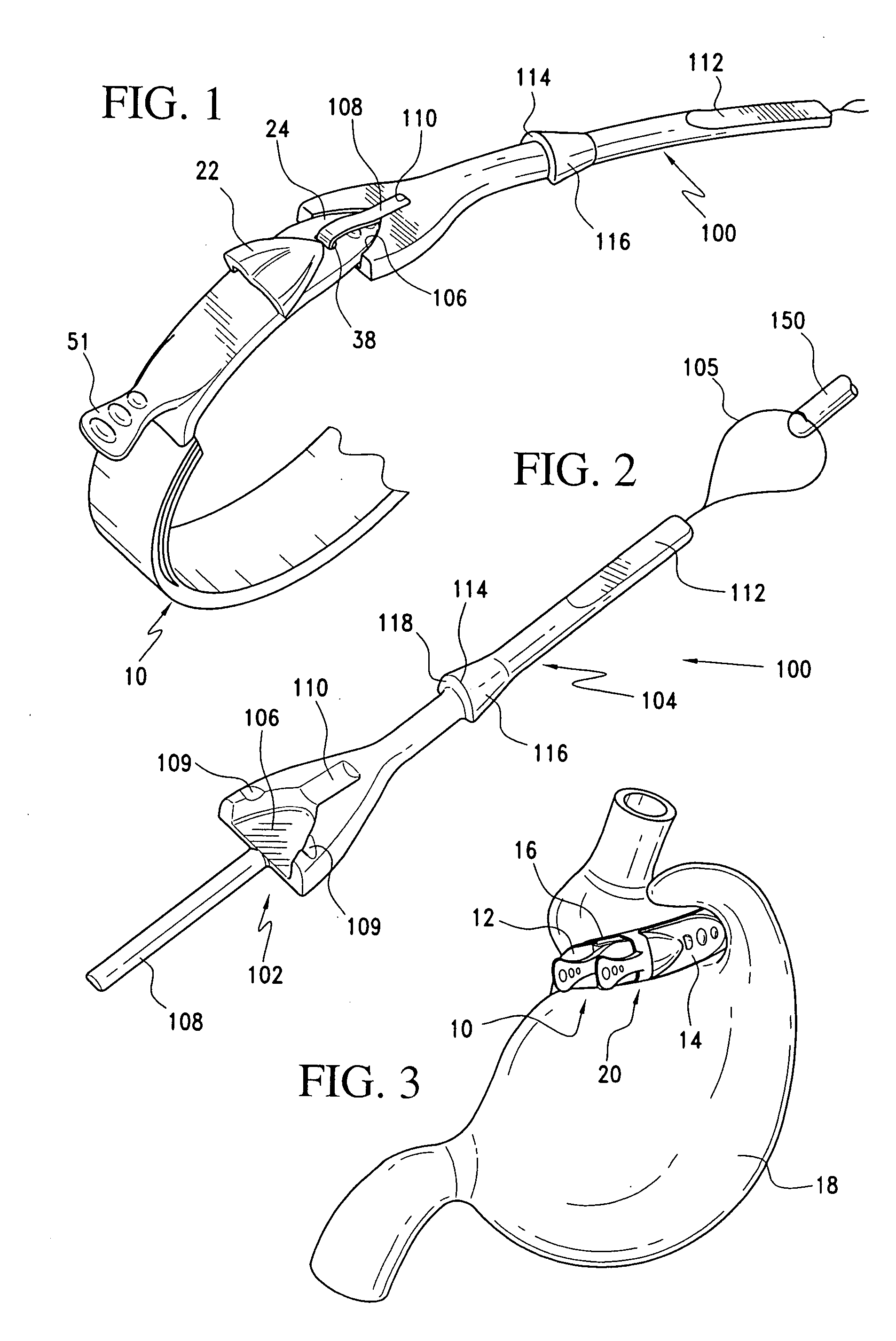
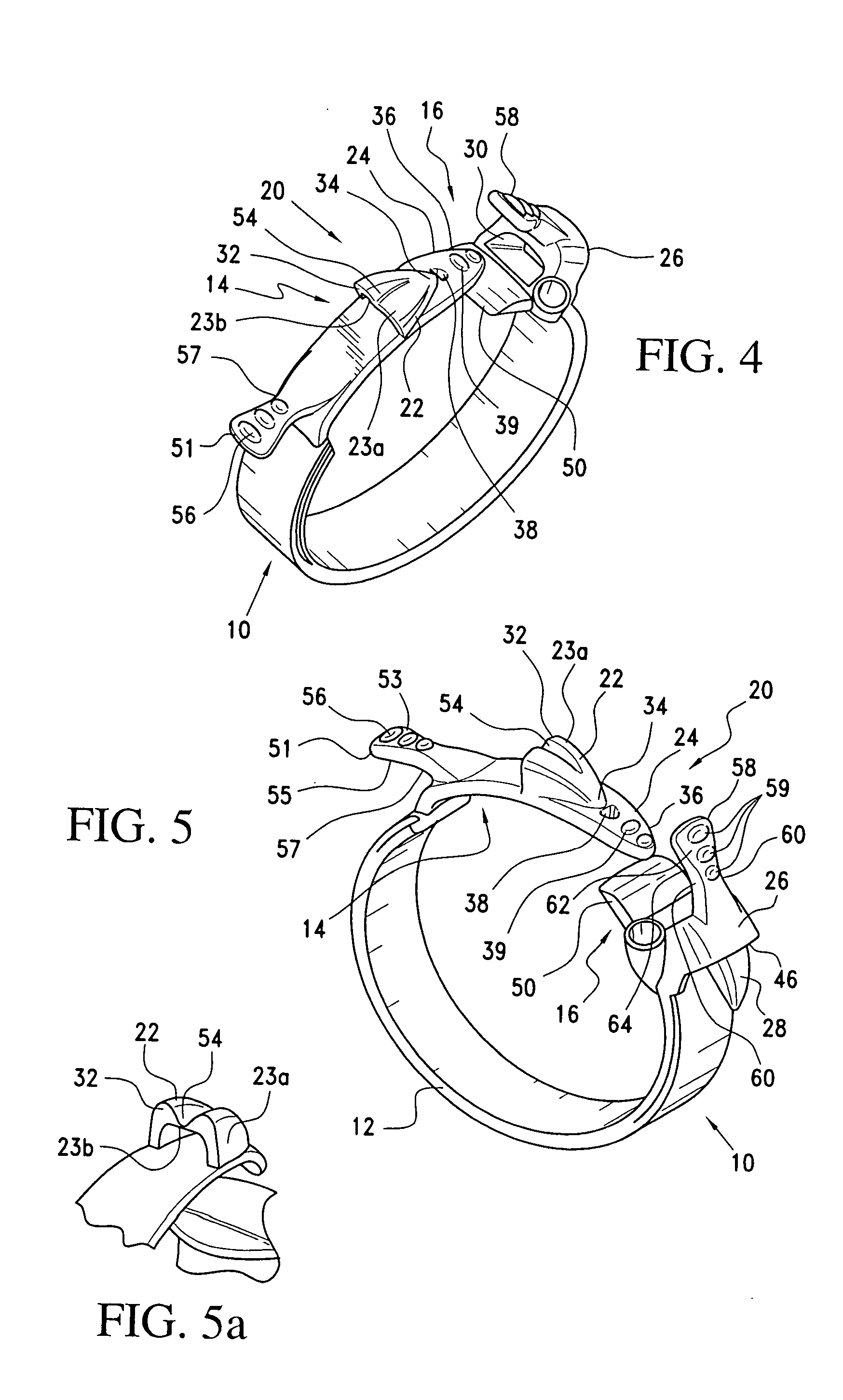
Latching device for gastric band
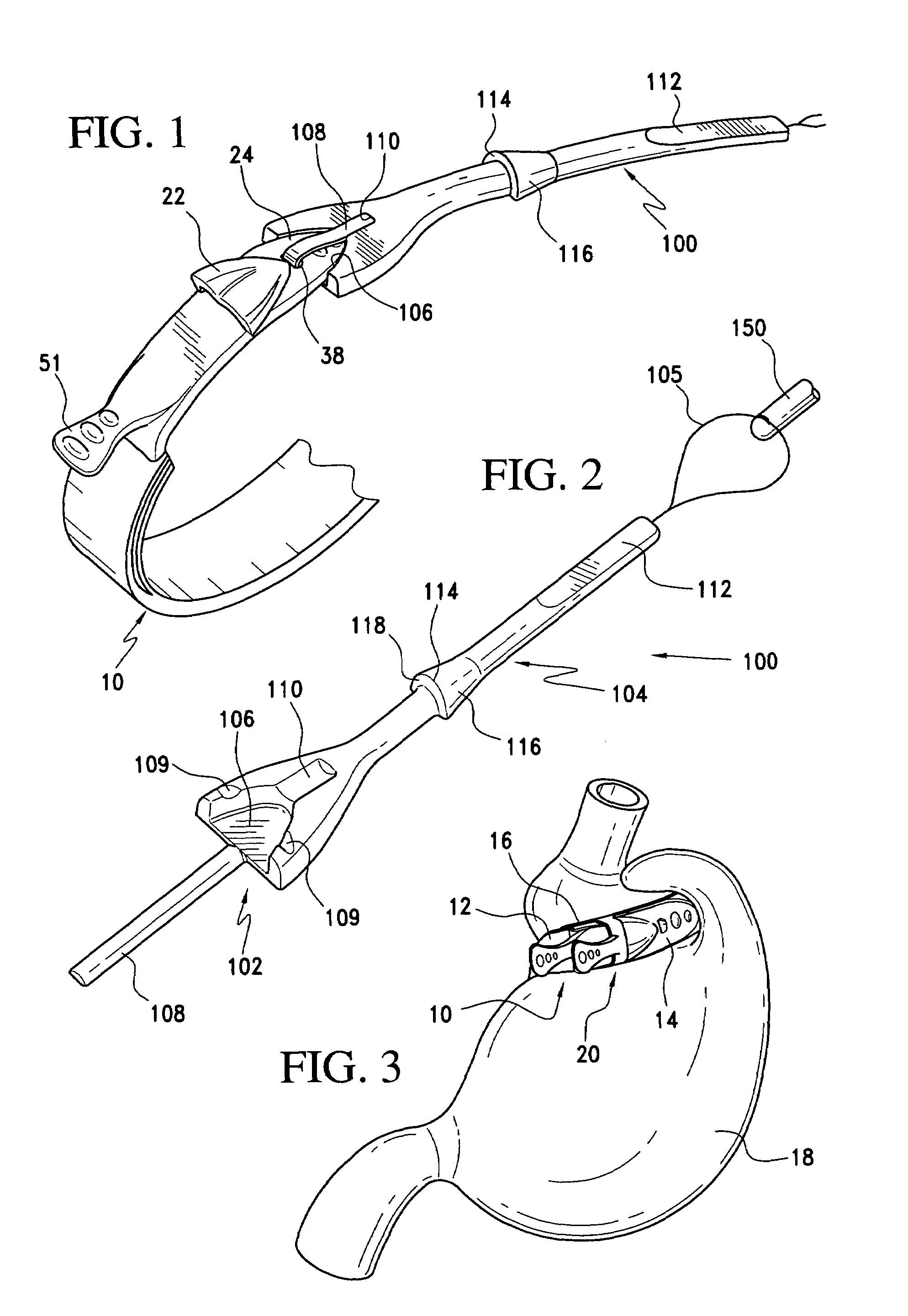
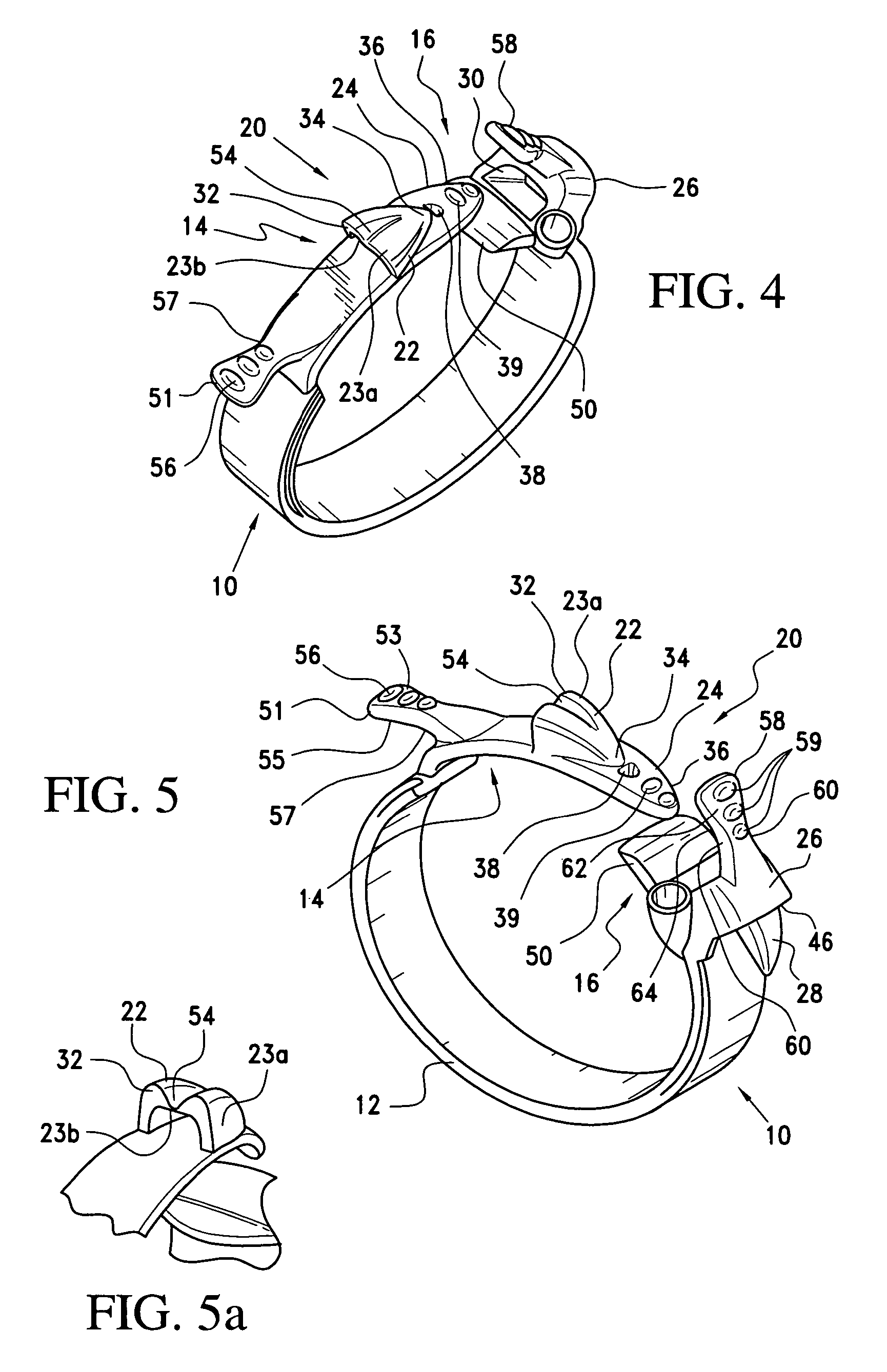
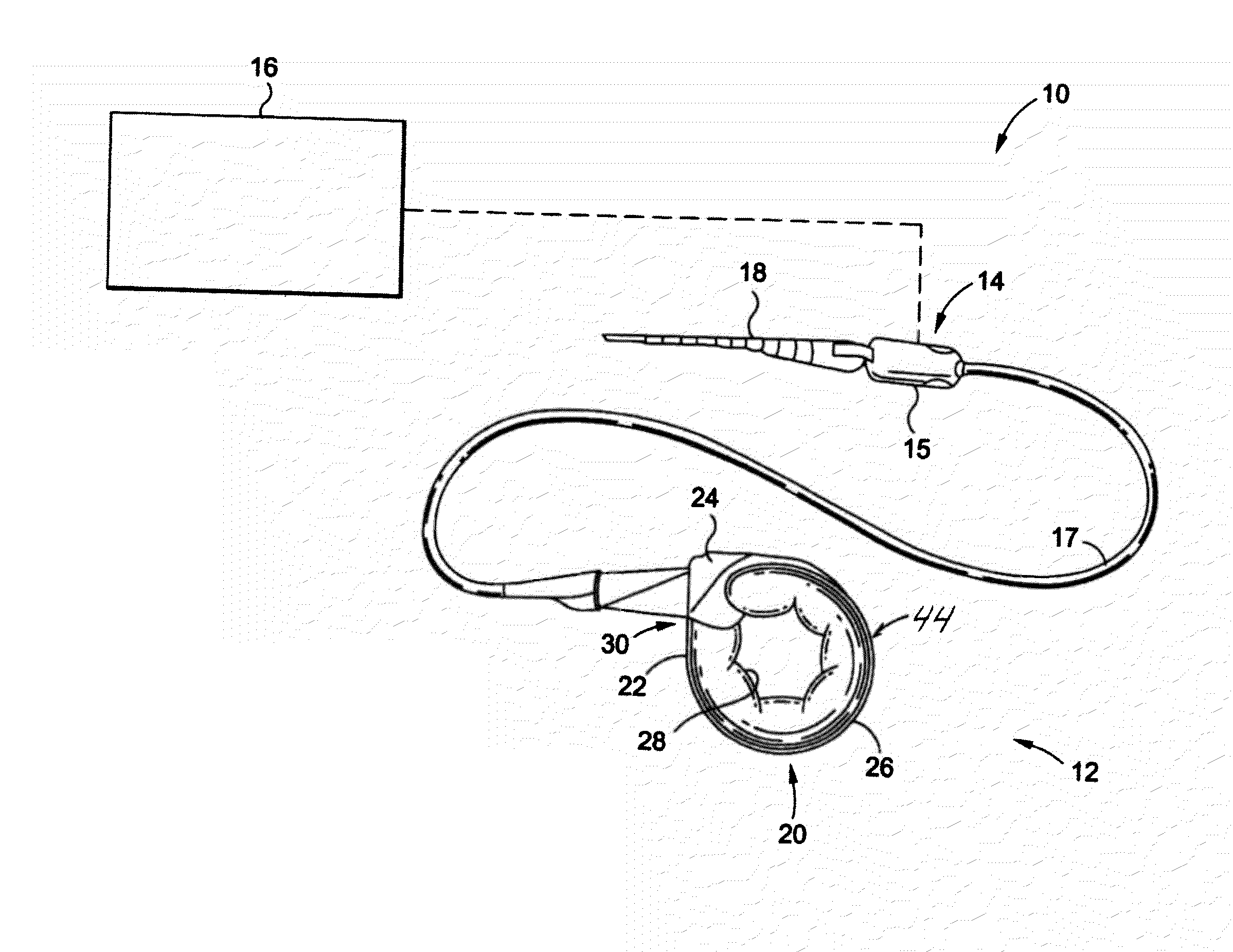
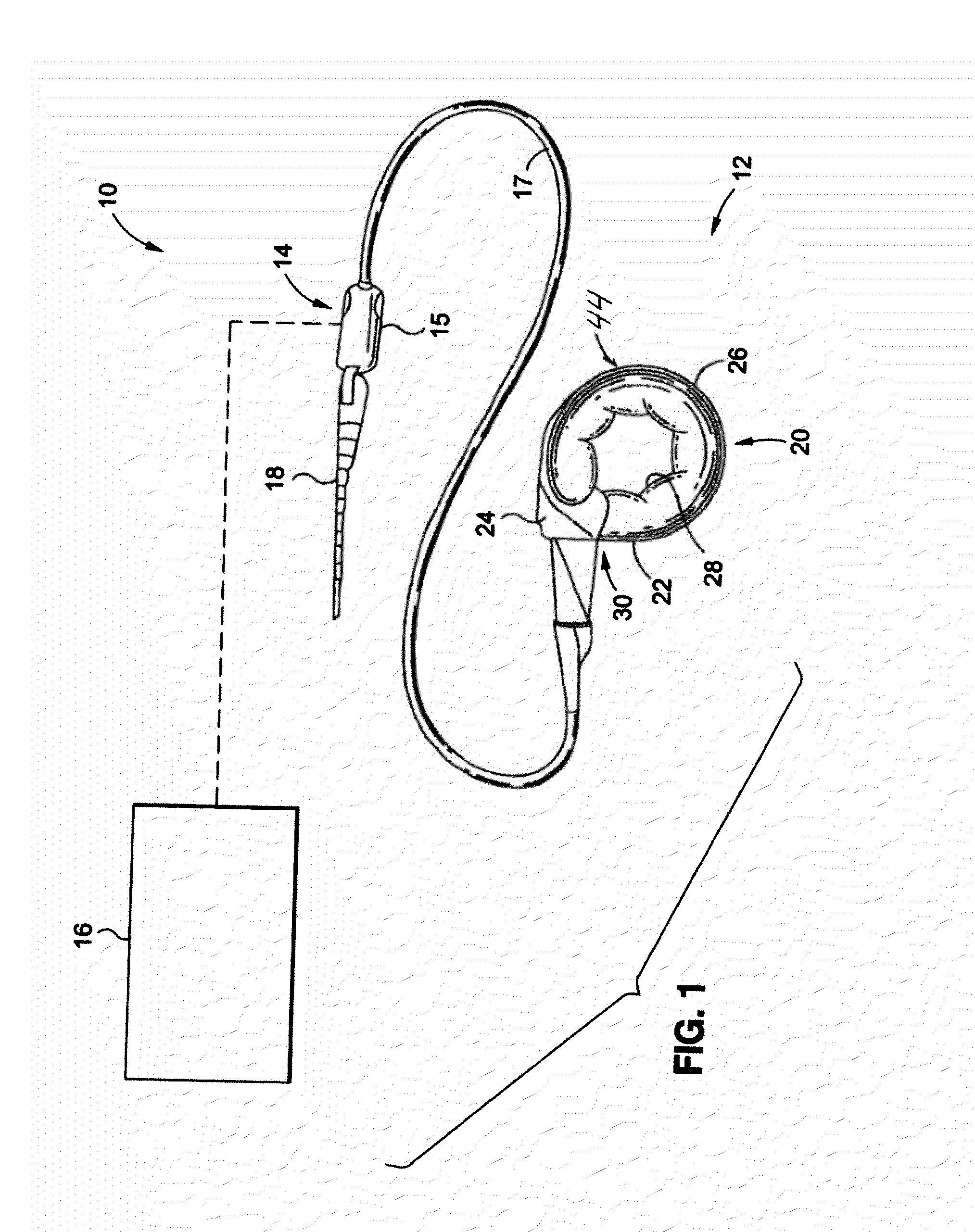
A gastric band includes a band body having a first end and a second end. The band body includes a latching mechanism. The latching mechanism is composed of a shell member at the first end of the band body and a collar member at the second end of the band body, the shell member and collar member being shaped and dimensioned for selective locking and unlocking in a manner creating a loop of the gastric band for positioning about a stomach wall.
Owner:ETHICON ENDO SURGERY INC
Thermodynamically driven reversible infuser pump for use as a remotely controlled gastric band
InactiveUS7351240B2Small volumeAvoid changeAnti-incontinence devicesFlexible member pumpsEngineeringPiezo electric
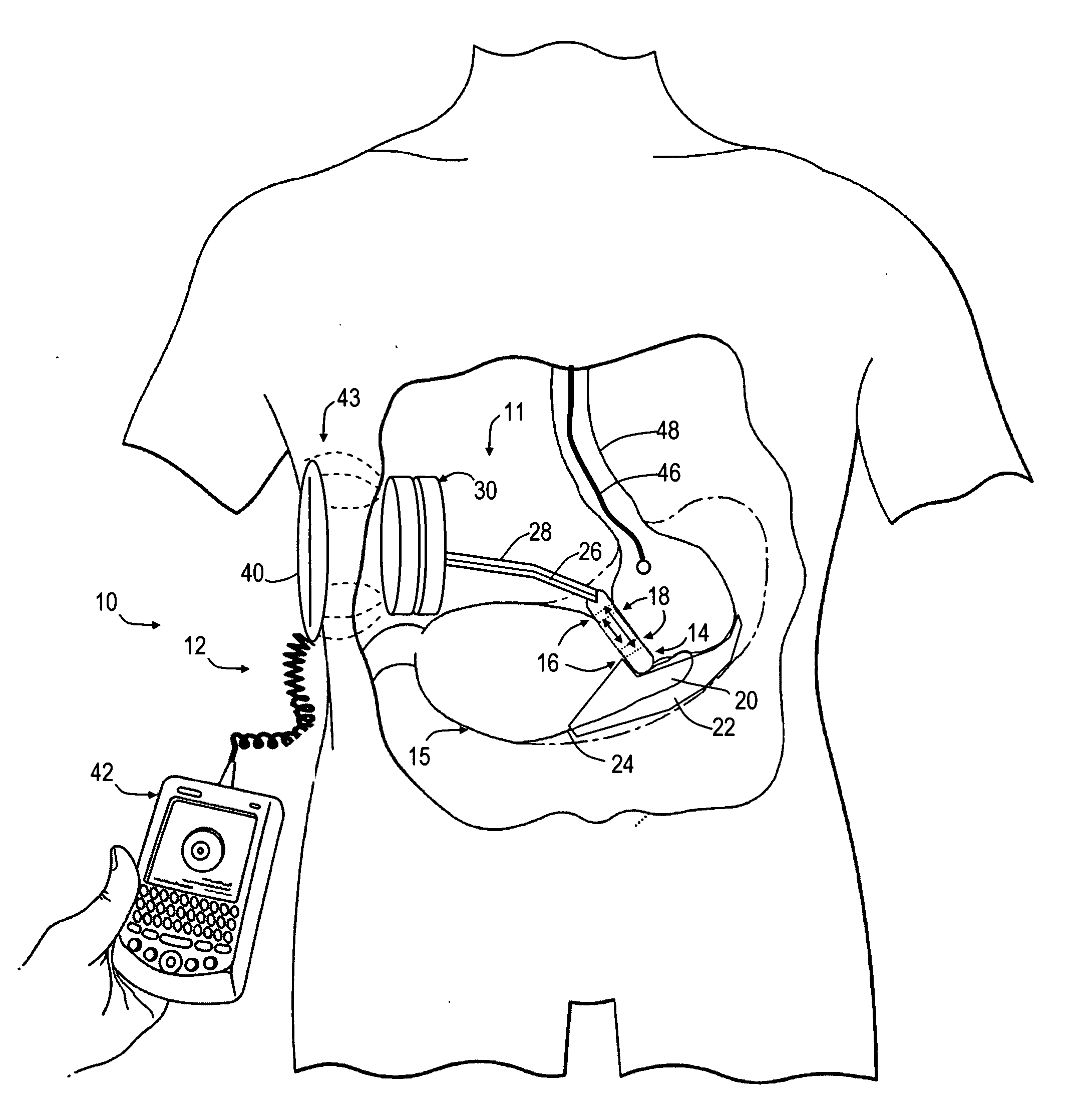
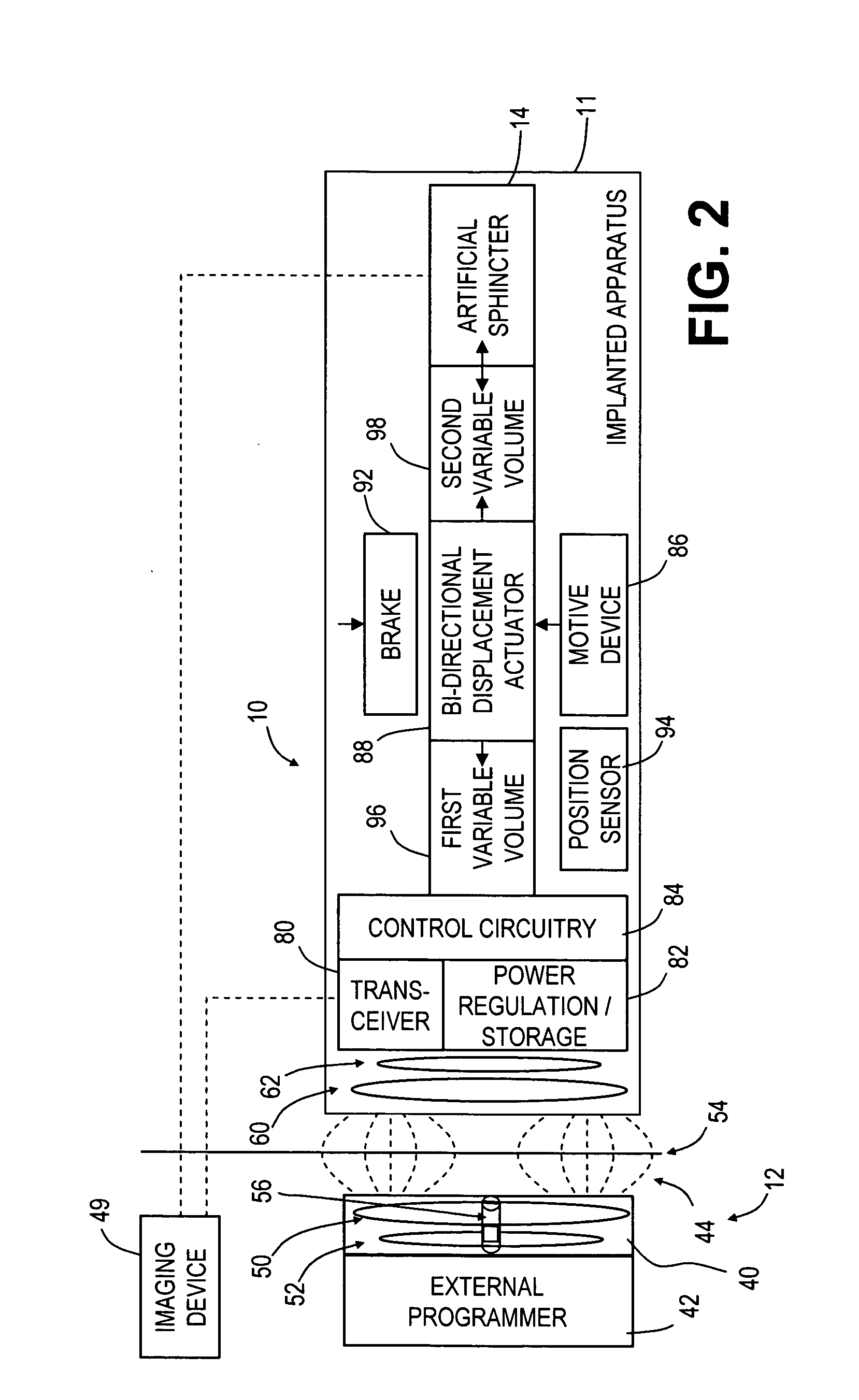
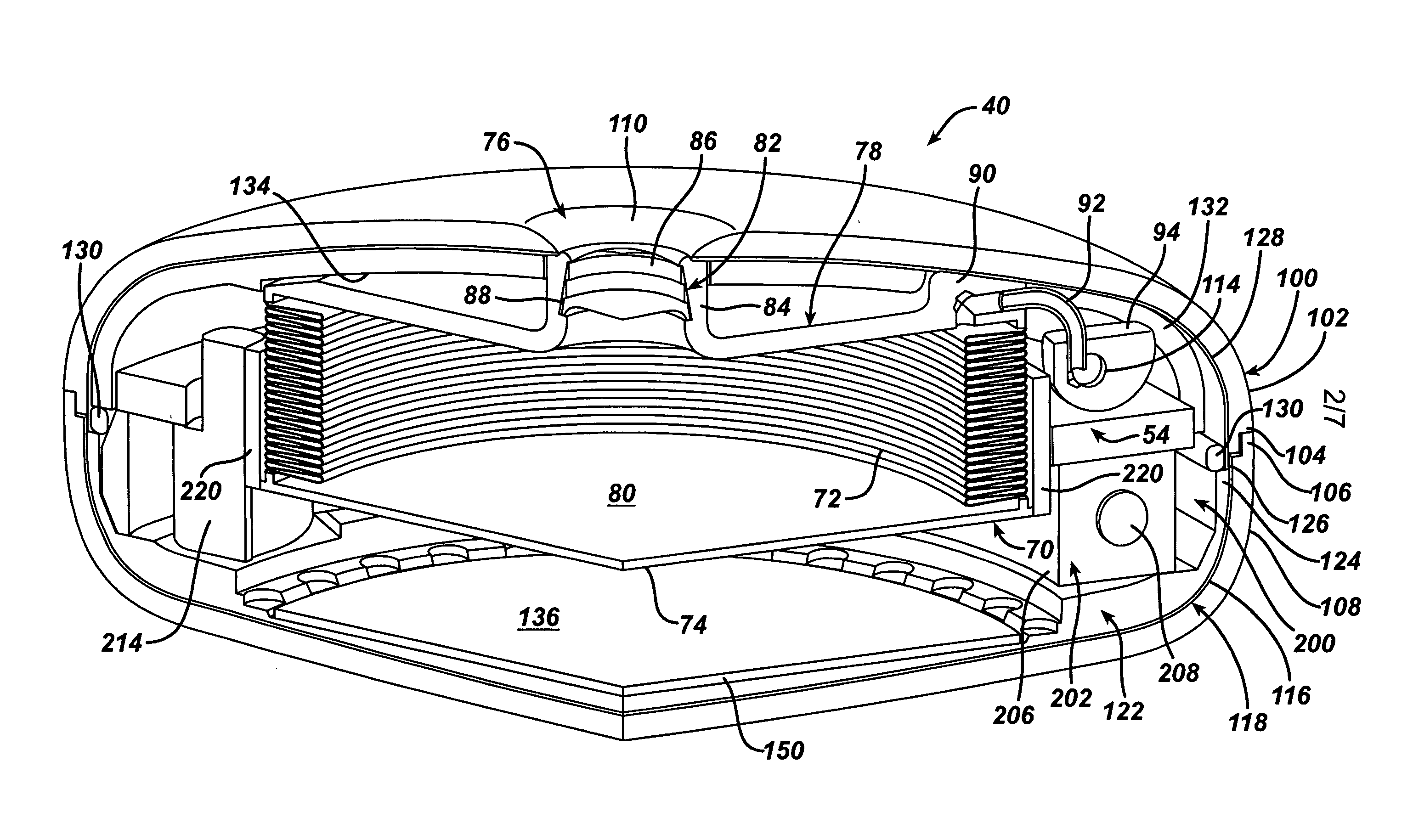
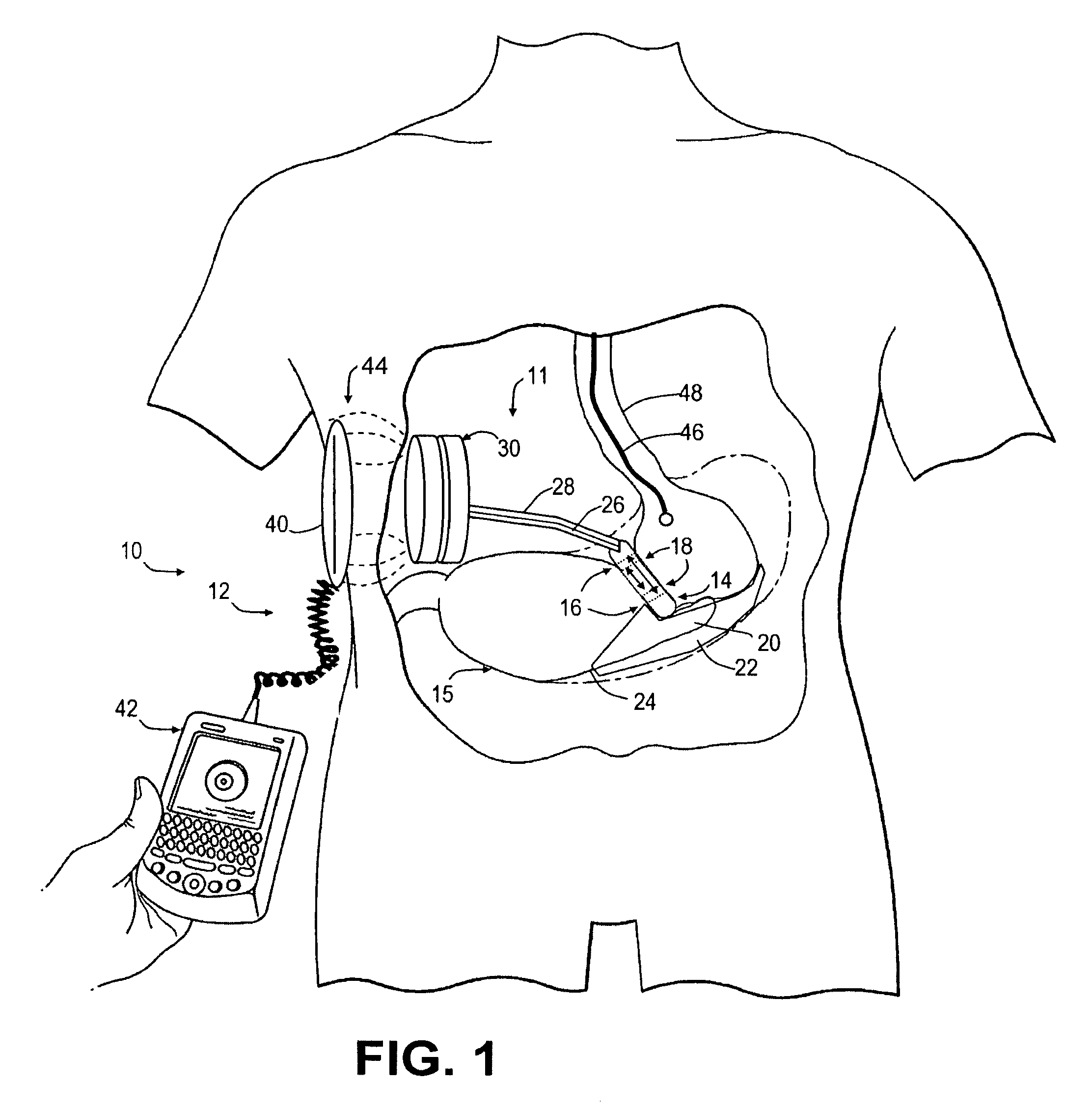
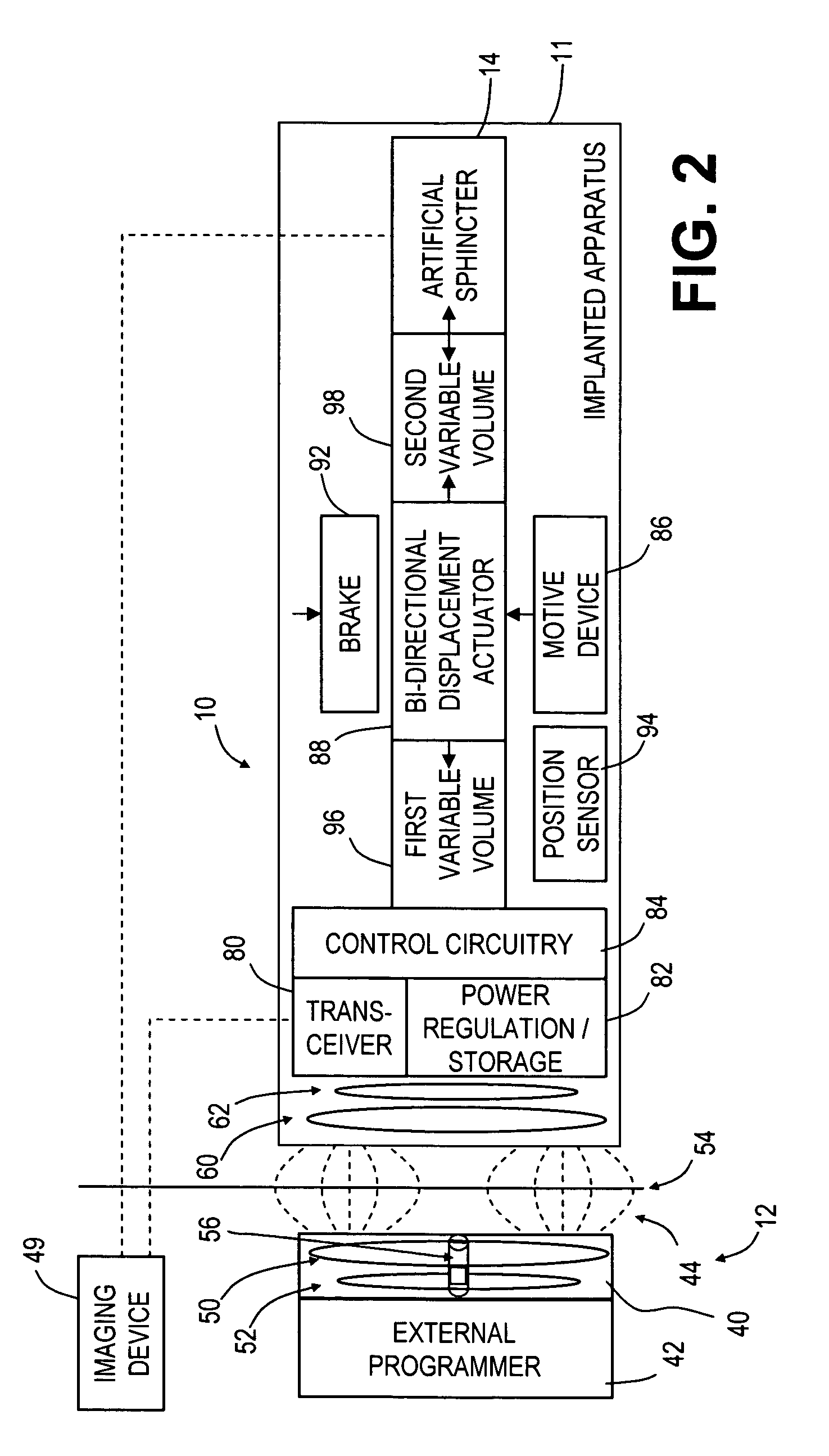
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band, by a combination of thermodynamic actuation and a piezo-electrically disengaged drum brake assembly that thereby achieves a desirable small volume device. A propellant within a propellant cavity surrounds a metal bellows accumulator biased at body temperature to either expand or collapse the bellows accumulator with the opposite direction of movement effected by a thermal element that heats in combination with a negatively-biased propellant or cools in combination with a positively-biased propellant. A drum brake assembly locks the metal bellows accumulator in place between adjustments by thermodynamic actuation by activating piezo-electric stack actuators that disengage calipers from a brake drum attached to the bellows accumulator.
Owner:ETHICON ENDO SURGERY INC
Electroactive polymer actuated gastric band
InactiveUS20070027356A1Easy to openIncrease the diameterObesity treatmentProsthesisElectroactive polymer actuatorsGastric band
Methods and devices are provided for remotely adjusting a size of a gastric band disposed around a patient's stomach. In one exemplary embodiment, a gastric band is provided having a first end and a second end that mate to one another to encircle a stomach. A latch mechanism can be formed on the band, and at least one actuator can be coupled to the latch mechanism and it can be adapted to expand and contract the latch mechanism when energy is applied thereto to adjust a diameter of the band. In one exemplary embodiment, the actuator(s) is an electroactive polymer actuator.
Owner:ETHICON ENDO SURGERY INC
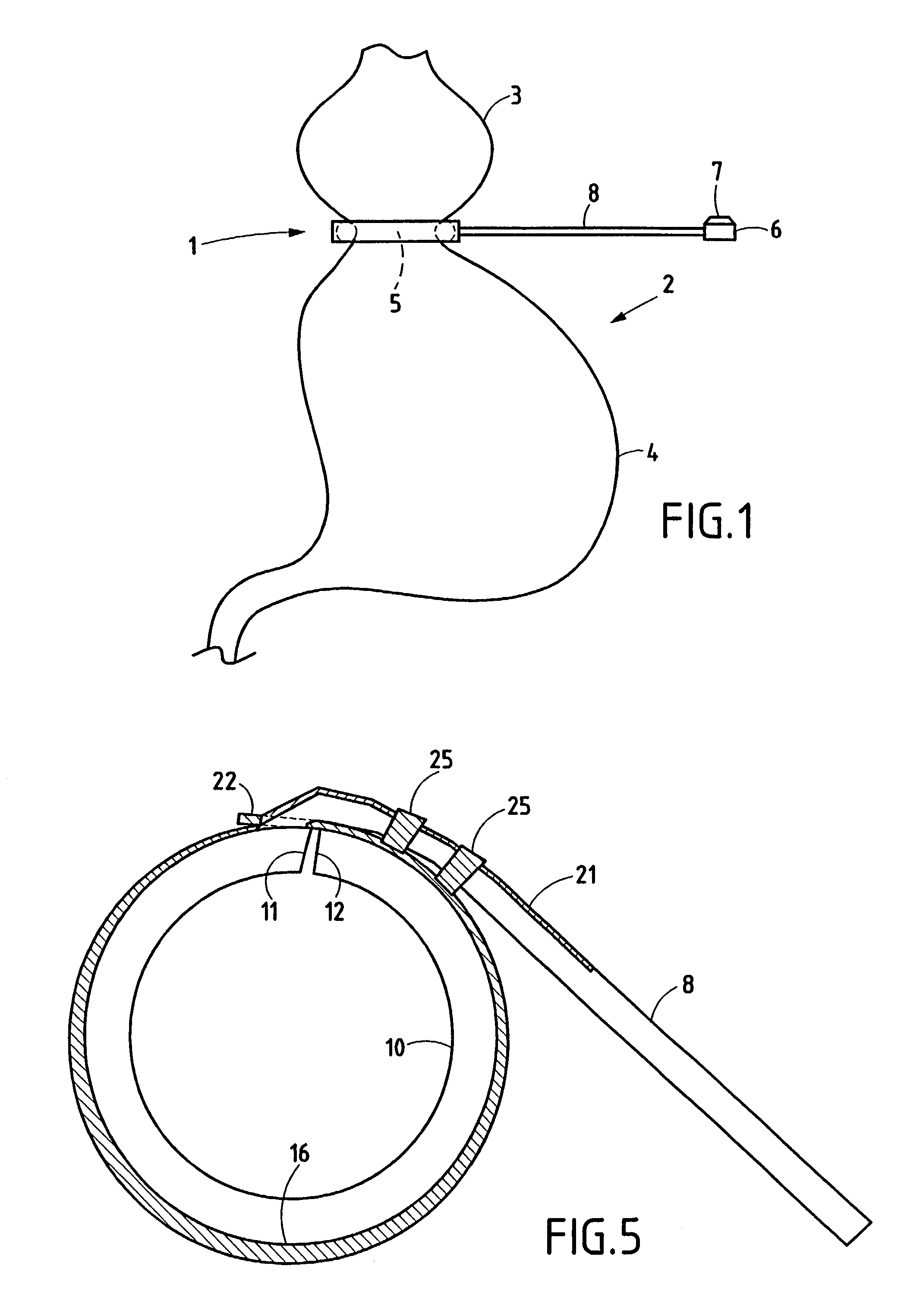
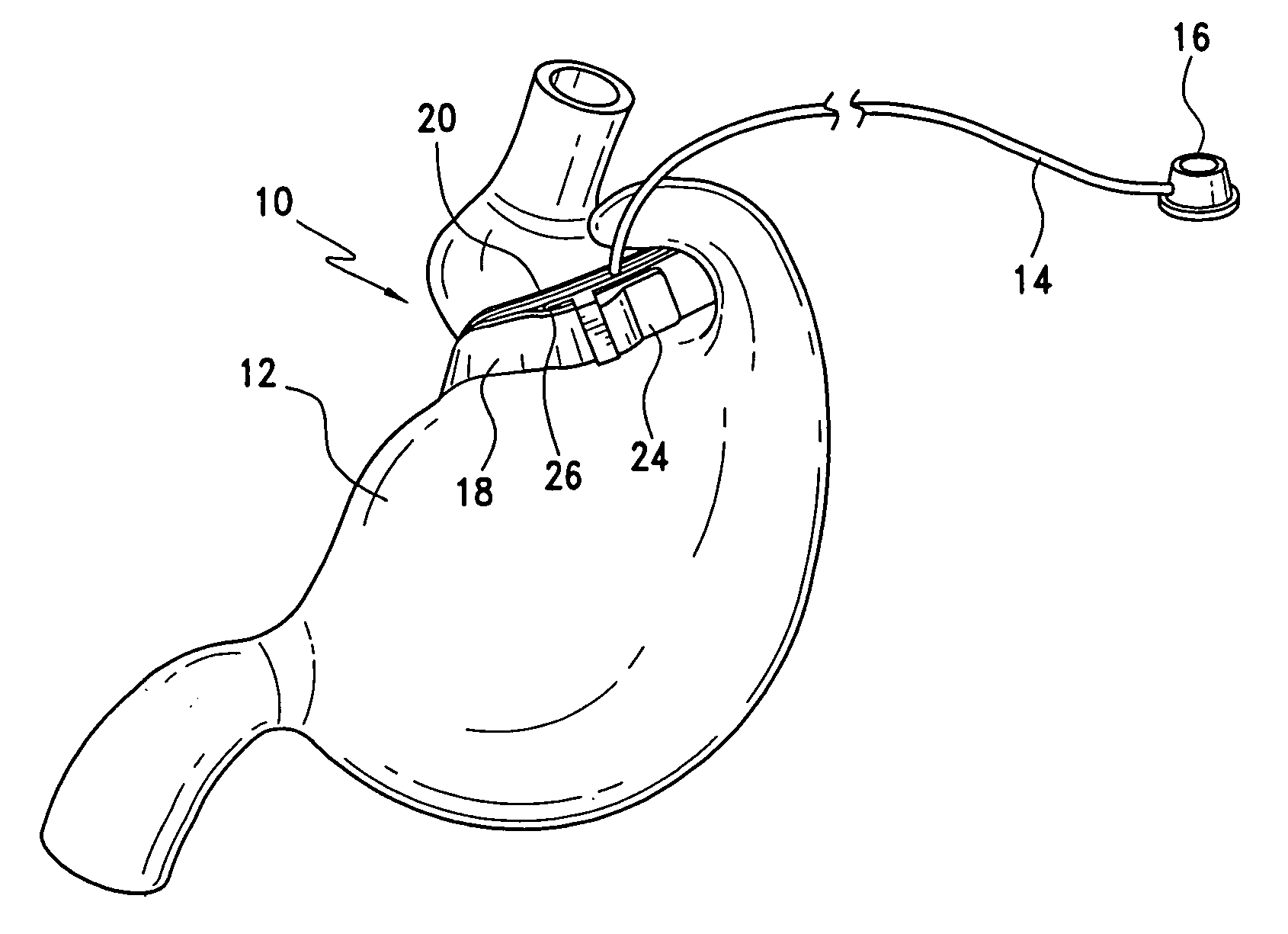
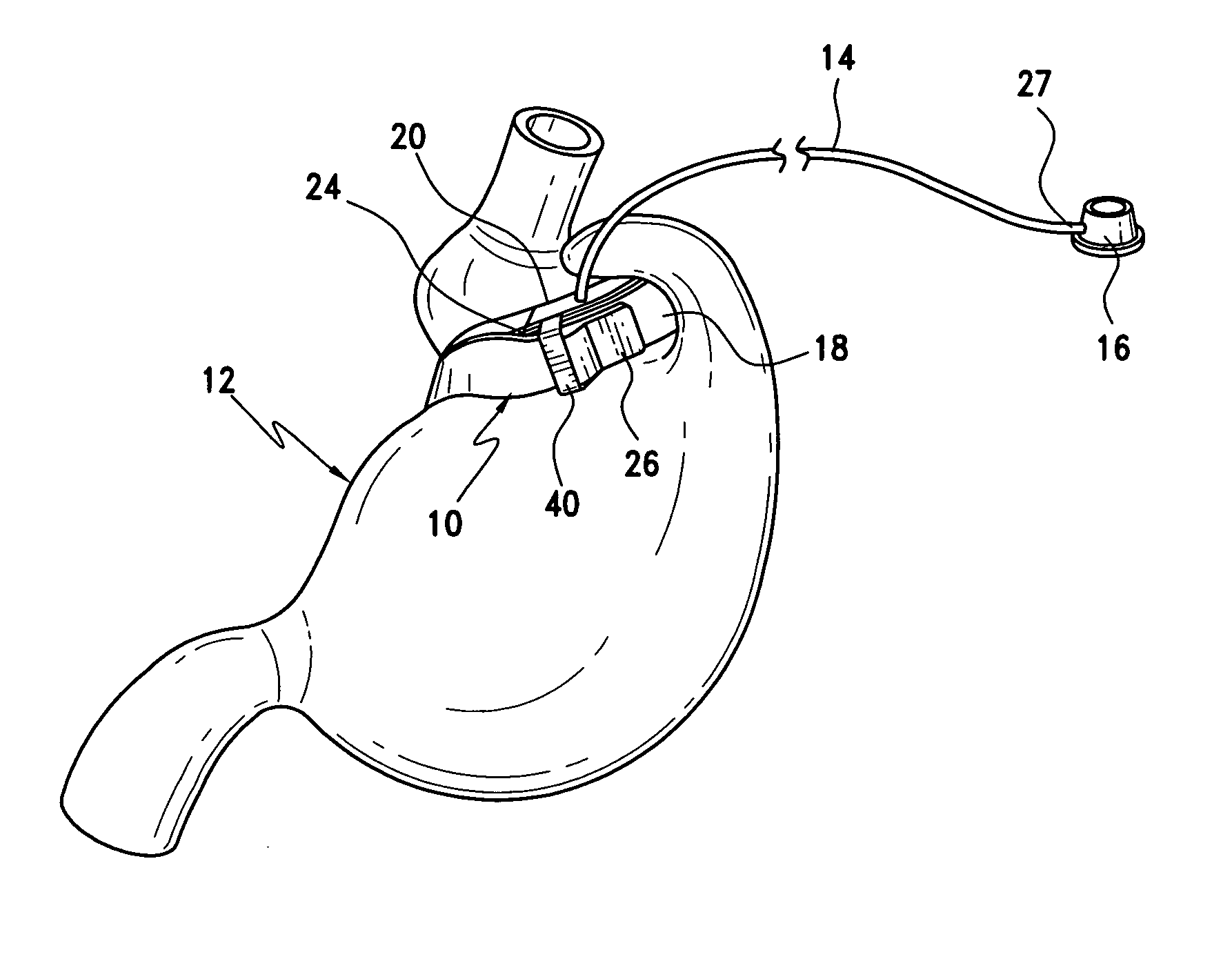
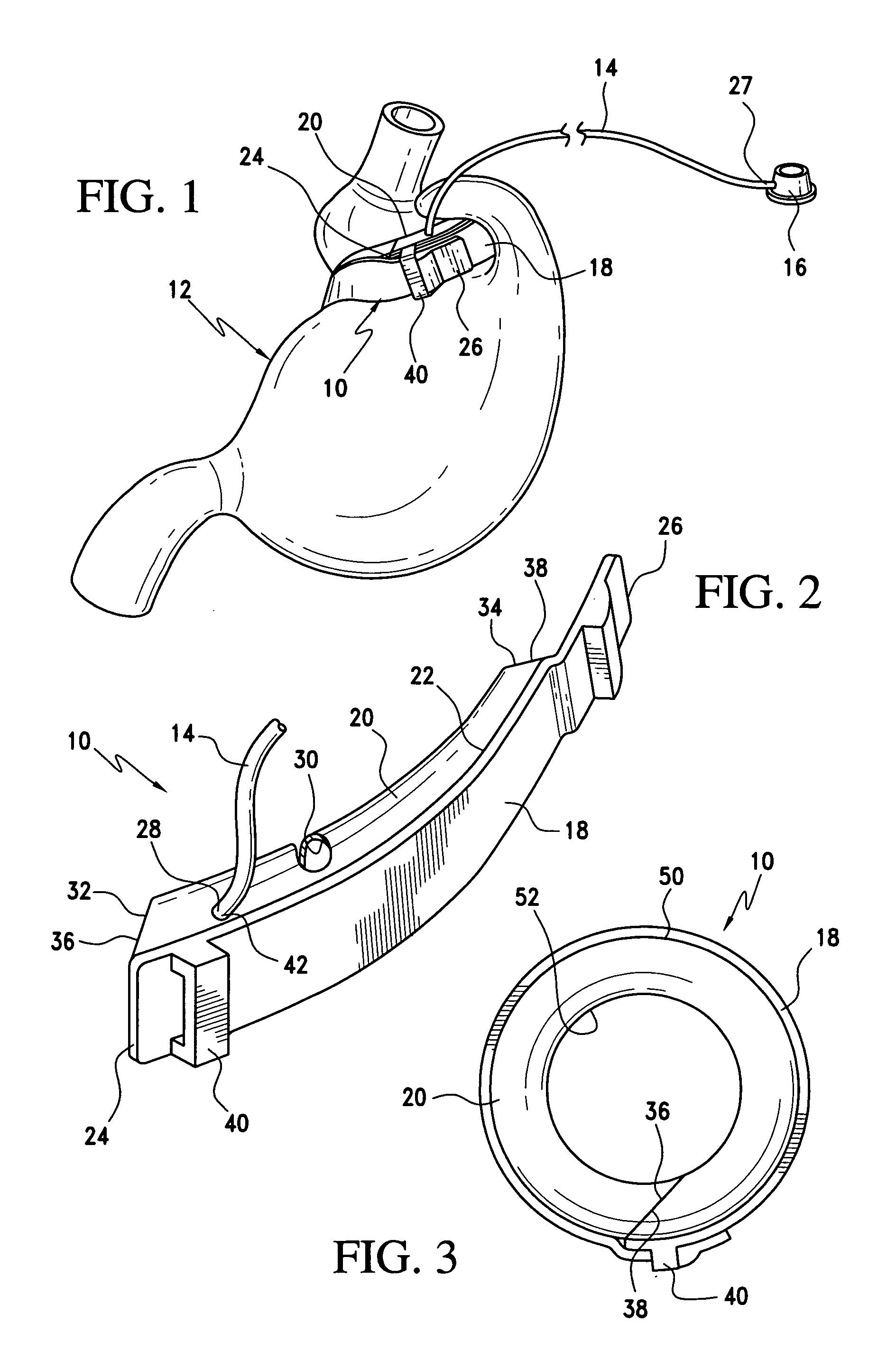
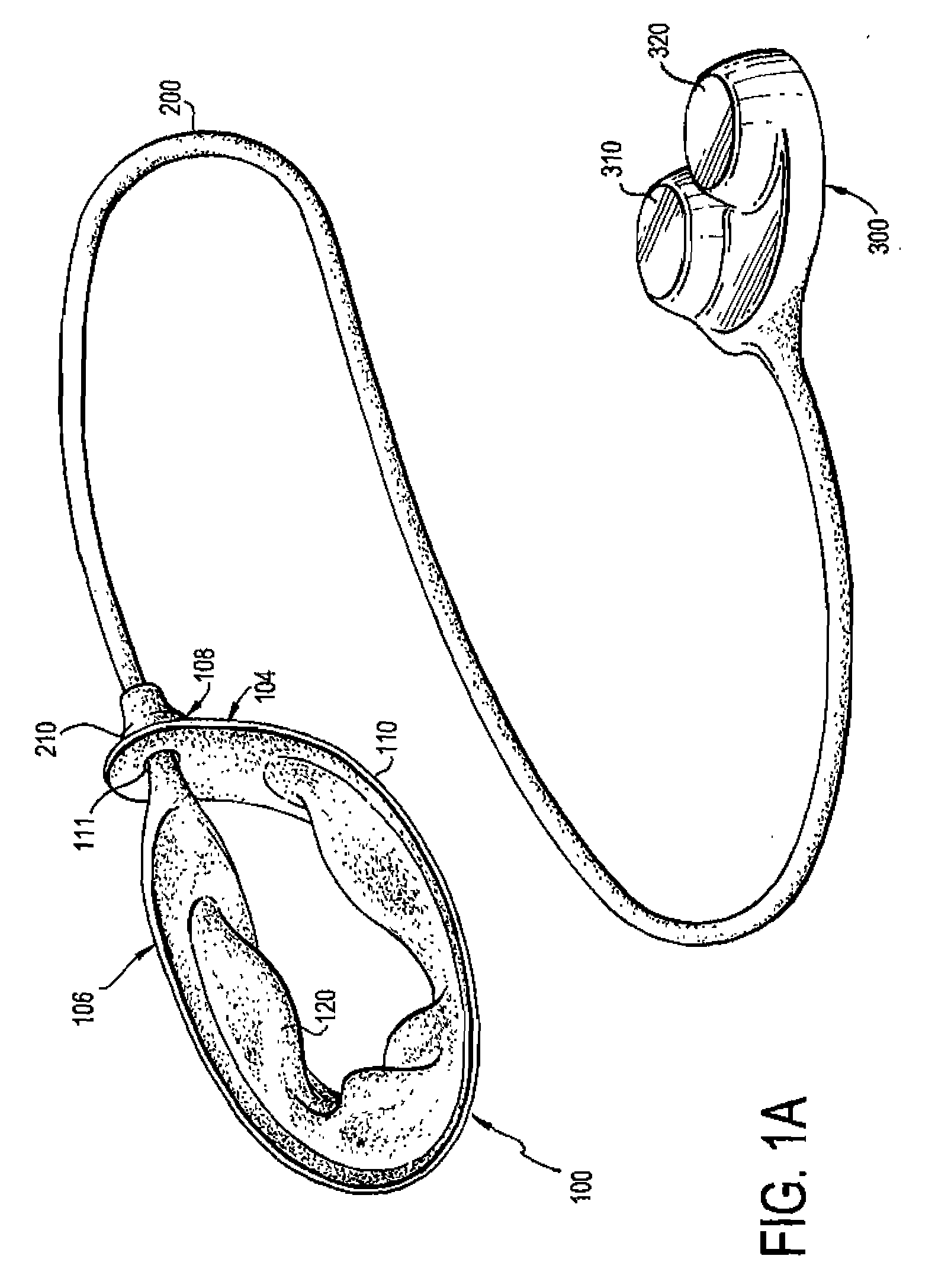
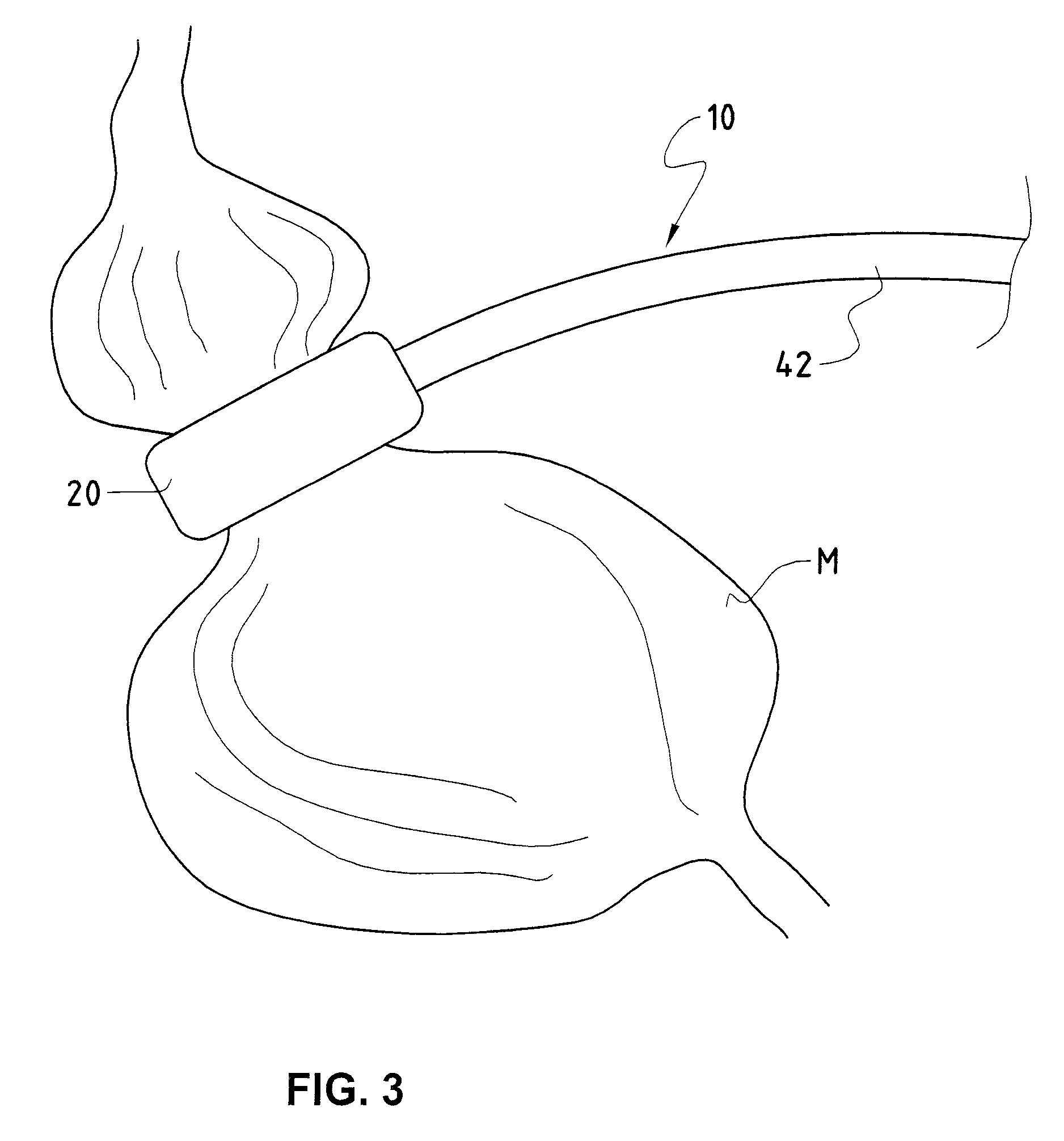
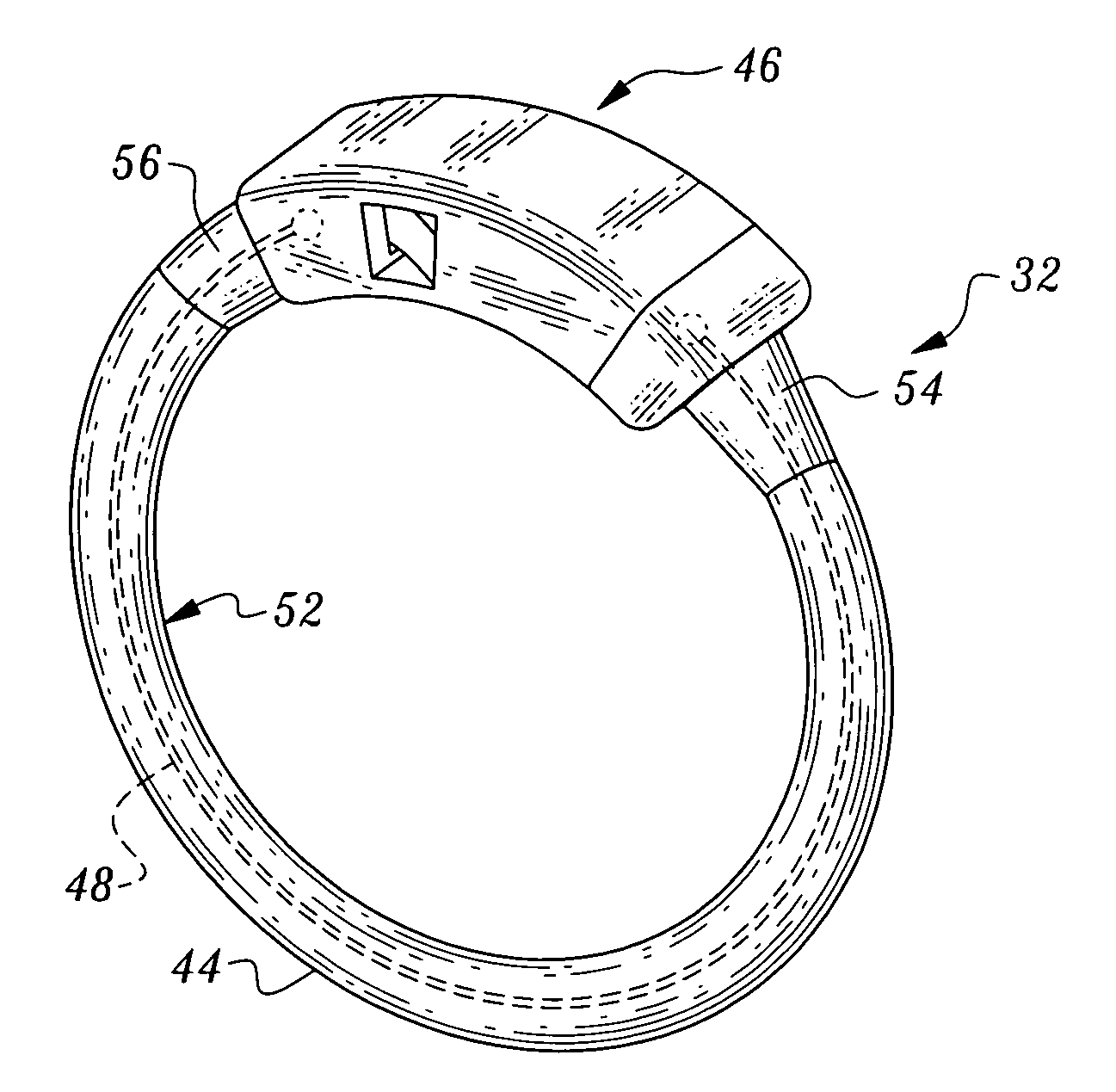
Gastric band
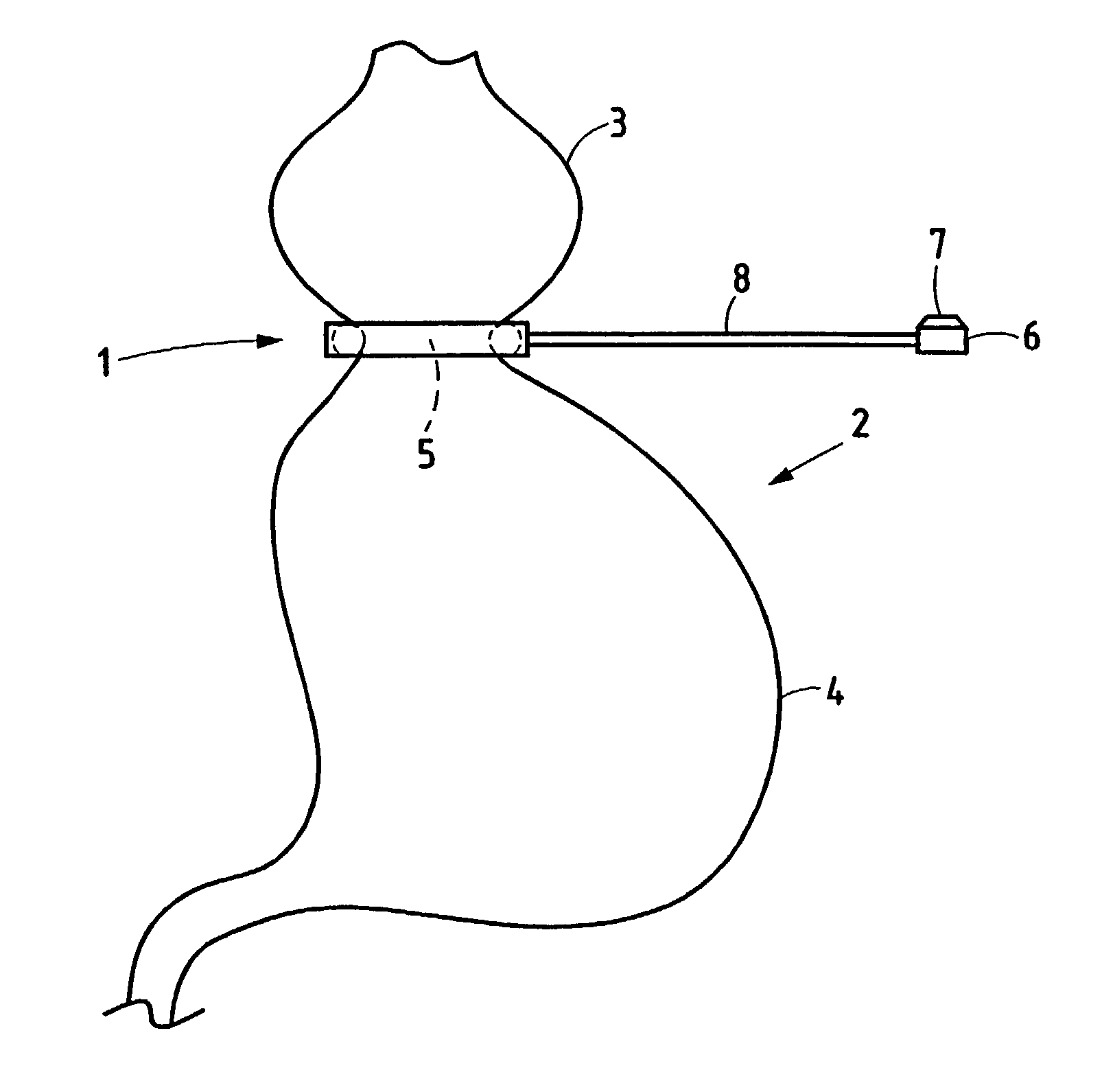
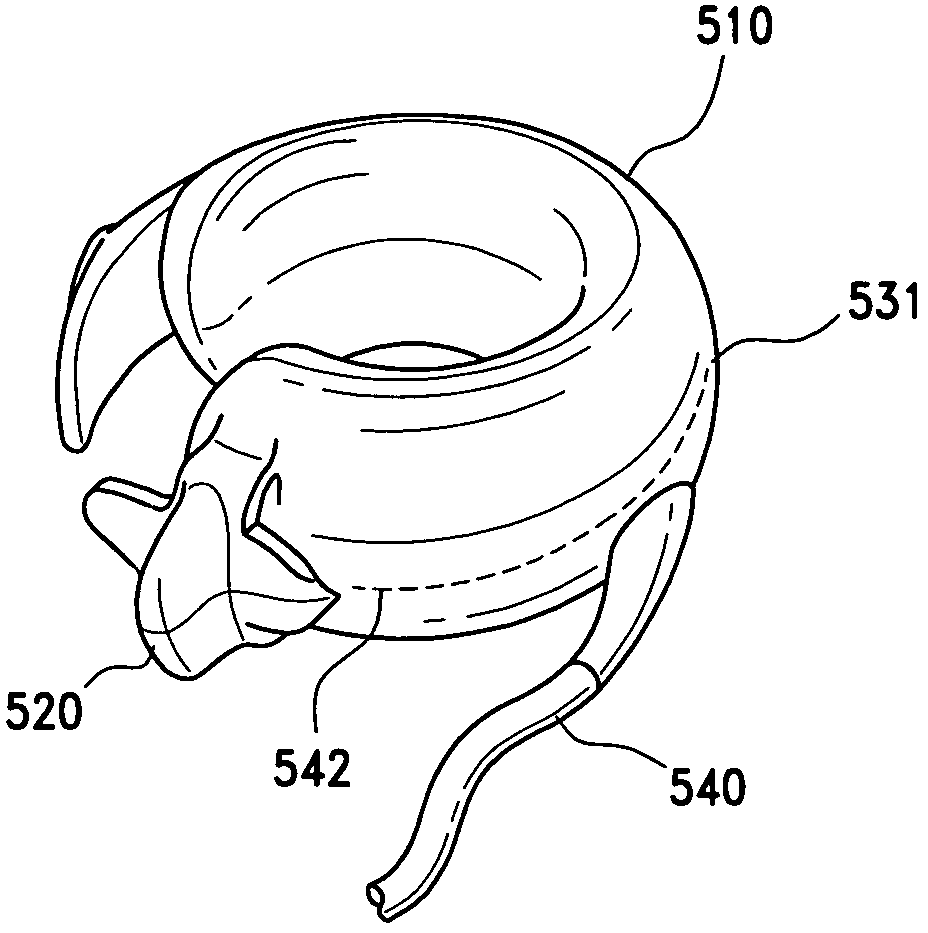
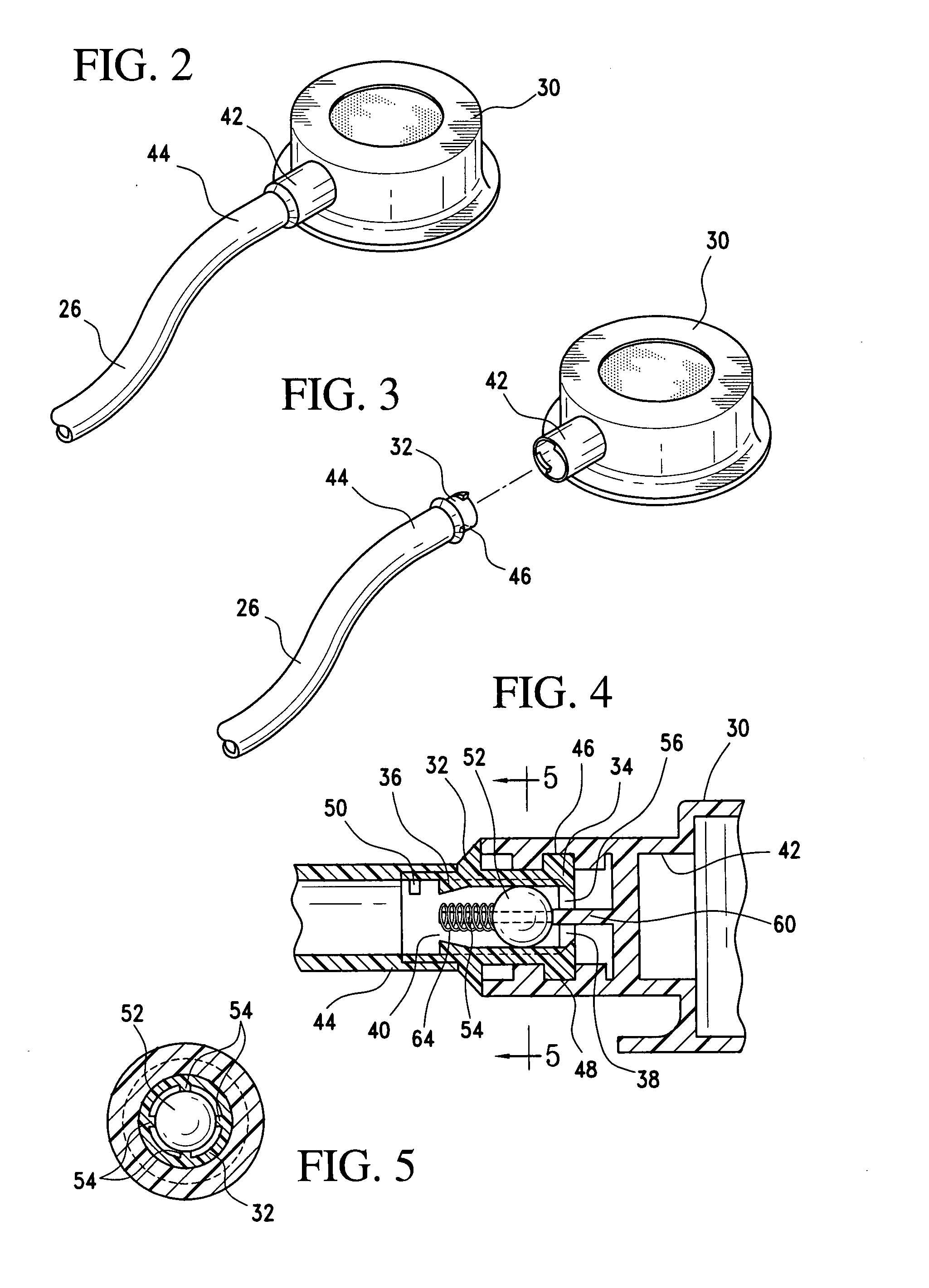
A gastric band includes a balloon shaped and dimensioned to circumscribe the stomach at a predetermined location. The balloon includes a longitudinally extending body and a supply tube is secured to the balloon for fluid communication with an internal cavity of the balloon.
Owner:ETHICON ENDO SURGERY INC
Piezo electrically driven bellows infuser for hydraulically controlling an adjustable gastric band
A remotely controlled gastric band system that is practically immune to external magnetic fields, such as from a Magnetic Resonance Imaging (MRI) machine, incorporates a bi-directional pump and fluid reservoir to adjust fluid volume in a gastric band. A piezoelectrically driven (e.g., rotary actuator, linear actuator) selectively compresses and expands a metal bellows hermetically sealed within a biocompatible and nonferromagnetic case such as titanium.
Owner:ETHICON ENDO SURGERY INC
Metal bellows position feedback for hydraulic control of an adjustable gastric band
InactiveUS7481763B2Avoid the needNon-surgical orthopedic devicesObesity treatmentClosed loopEngineering
A remotely controlled gastric band system that is practically immune to external magnetic fields, such as from a Magnetic Resonance Imaging (MRI) machine, incorporates a bi-directional pump and fluid reservoir to adjust fluid volume for hydraulic control of a gastric band. A piezoelectric driver (e.g., rotary actuator, linear actuator) selectively compresses and expands a metal bellows hermetically sealed within a biocompatible and nonferromagnetic enclosure or case such as titanium. Directly sensing a position of the metal bellows yields an accurate reading of volume contained therein, allowing for closed-loop control of the gastric band.
Owner:ETHICON ENDO SURGERY INC
Bi-directional infuser pump with volume braking for hydraulically controlling an adjustable gastric band
InactiveUS7374565B2Increase varietyAvoid changeAnti-incontinence devicesPharmaceutical delivery mechanismPiezo electricEngineering
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band, by a combination of thermodynamic actuation and a piezo-electrically disengaged drum brake assembly that thereby achieves a desirable small volume device. A propellant within a propellant cavity surrounds a metal bellows accumulator biased at body temperature to either expand or collapse the bellows accumulator with the opposite direction of movement effected by a thermal element that heats in combination with a negatively-biased propellant or cools in combination with a positively-biased propellant. A drum brake assembly locks the metal bellows accumulator in place between adjustments by thermodynamic actuation by activating piezo-electric stack actuators that disengage calipers from a brake drum attached to the bellows accumulator.
Owner:ETHICON ENDO SURGERY INC
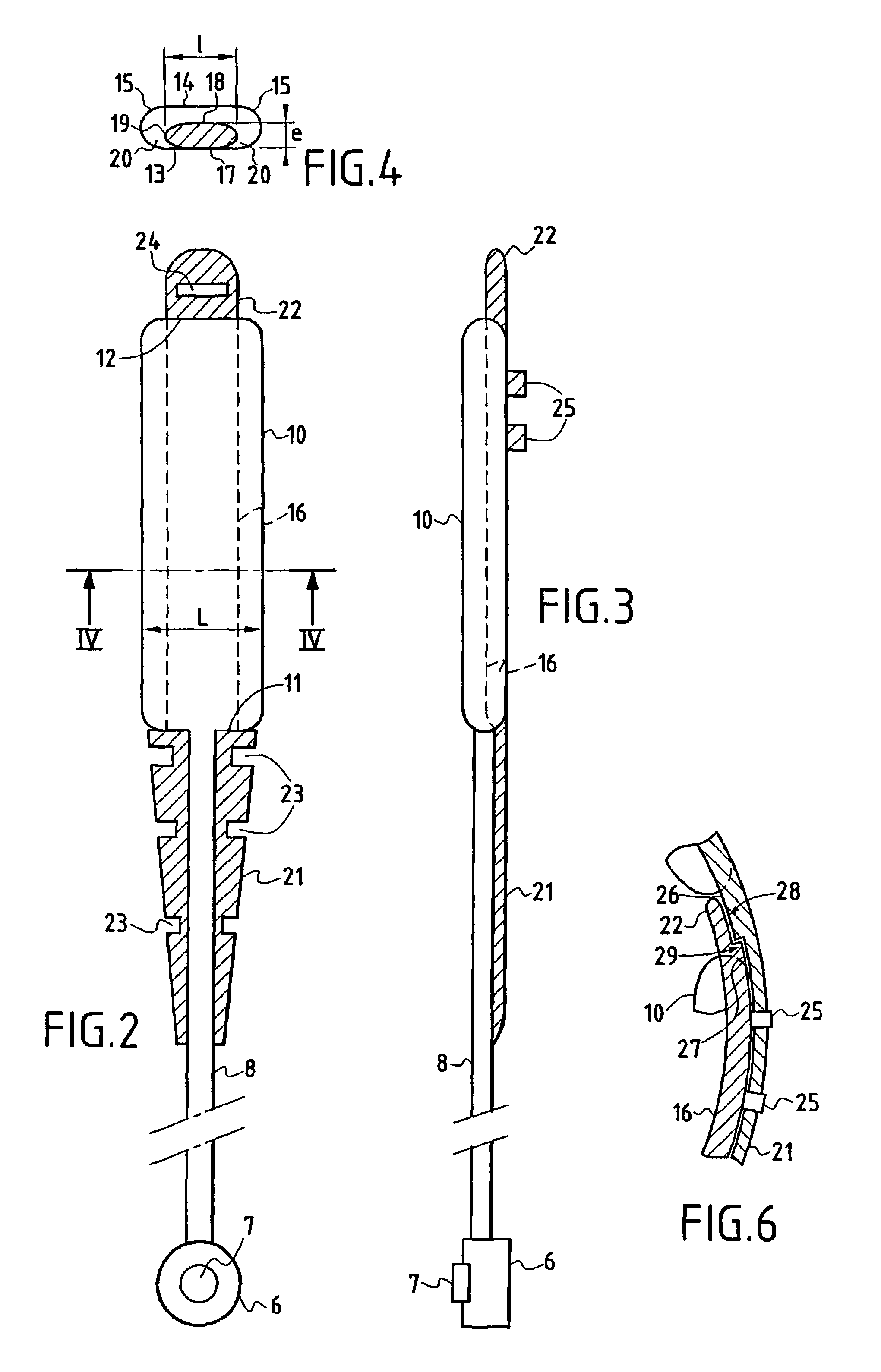
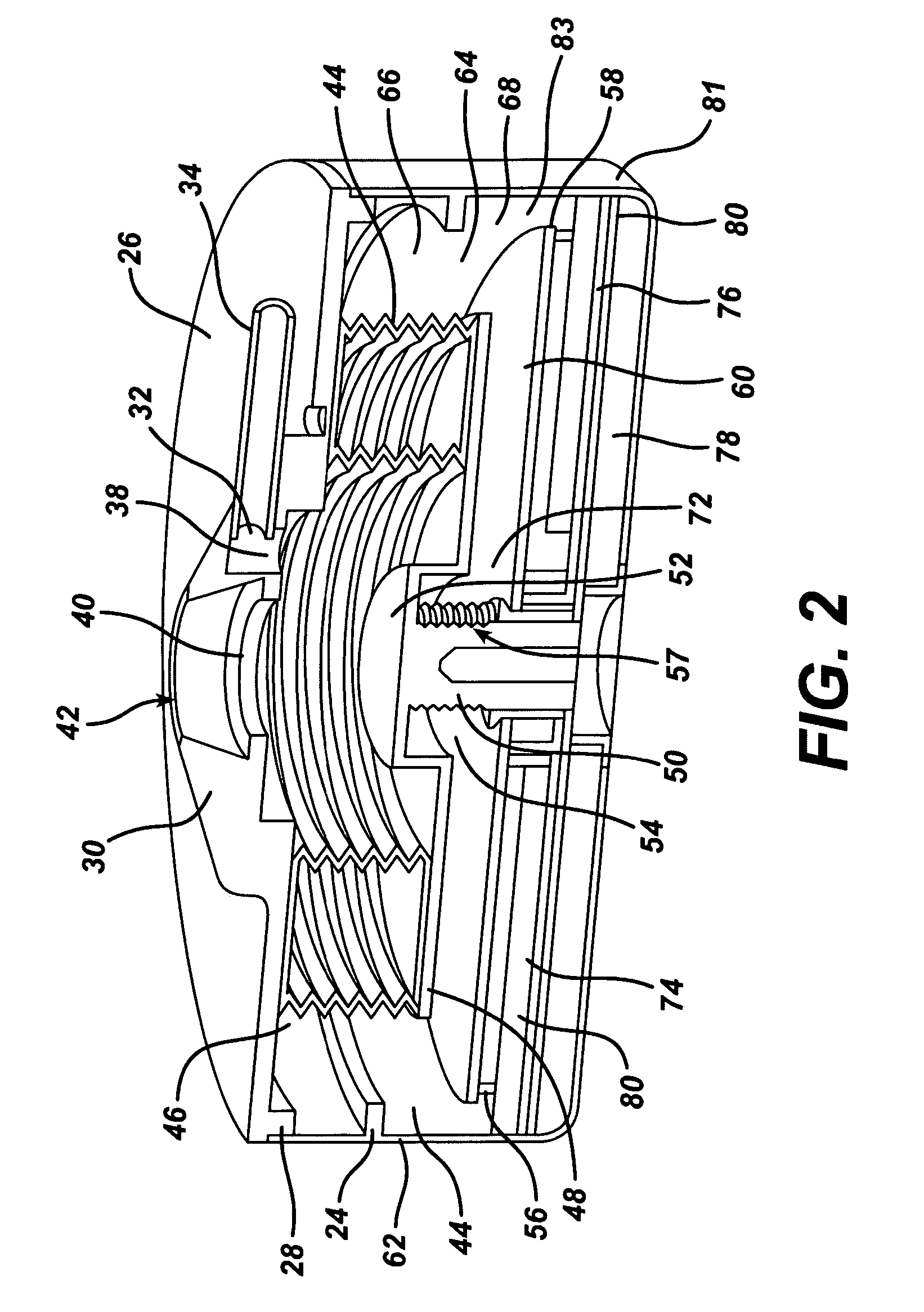
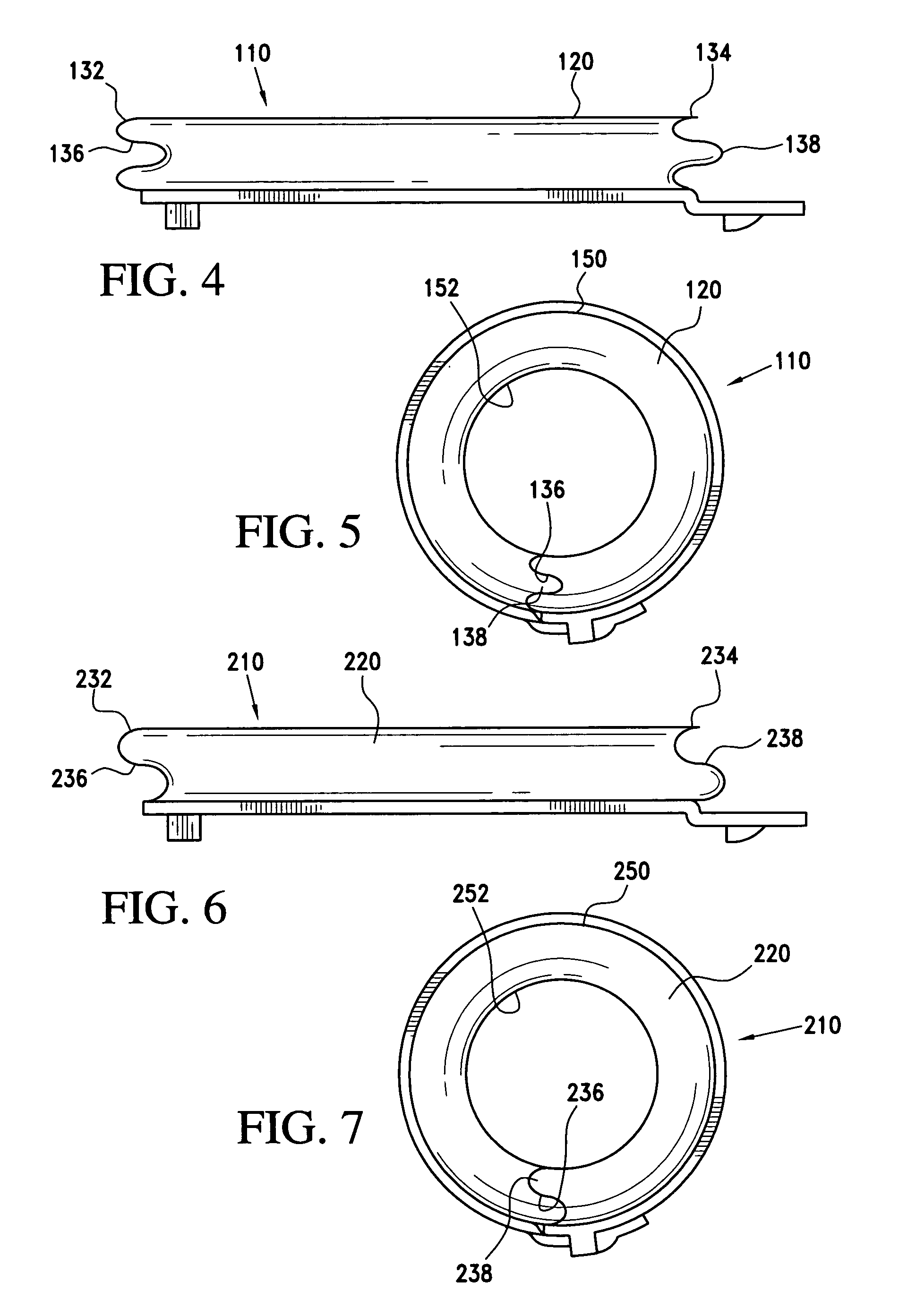
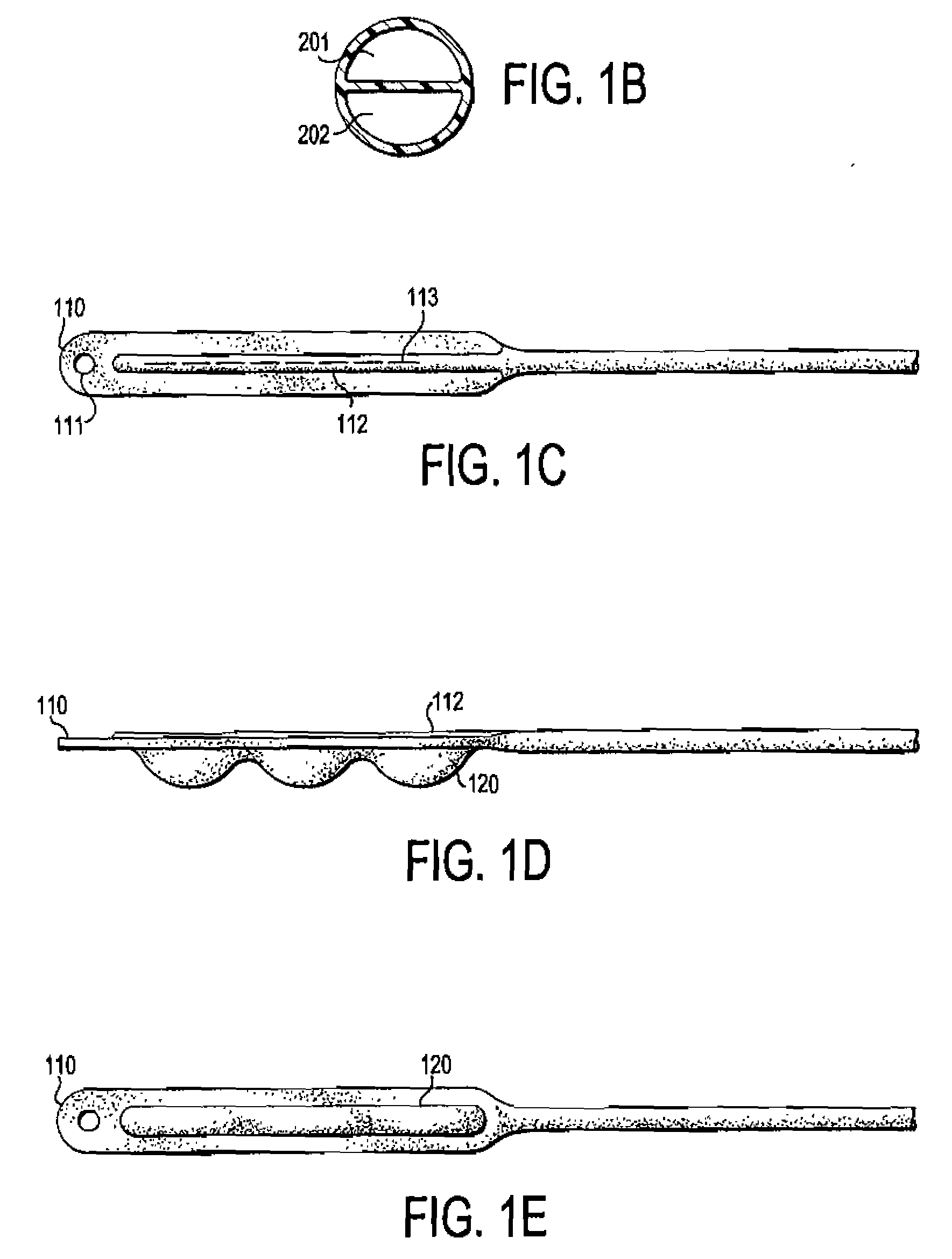
Accordion-like gastric band
A gastric band includes a belt and a balloon secured to the belt. The balloon and belt being shaped and dimensioned to circumscribe the stomach at a predetermined location. The balloon includes a longitudinally extending body with at least one crease formed therein, the crease extending along a longitudinal axis of the body for providing the balloon with a reduced noninflated profile without reducing the fill volume of the fully expanded balloon.
Owner:ETHICON ENDO SURGERY INC
Gastric band with mating end profiles
A gastric band includes a belt and a balloon secured to the belt. The balloon and belt are shaped and dimensioned to circumscribe the stomach at a predetermined location. The balloon includes a longitudinally extending body having a first end and a second end, the first end and the second end respectively including mating profiles which align to create generally continuous surfaces along an outer surface of the gastric band and an inner surface of the gastric band as the outer surface and the inner surface transition between the first and second ends of the balloon.
Owner:ETHICON ENDO SURGERY INC
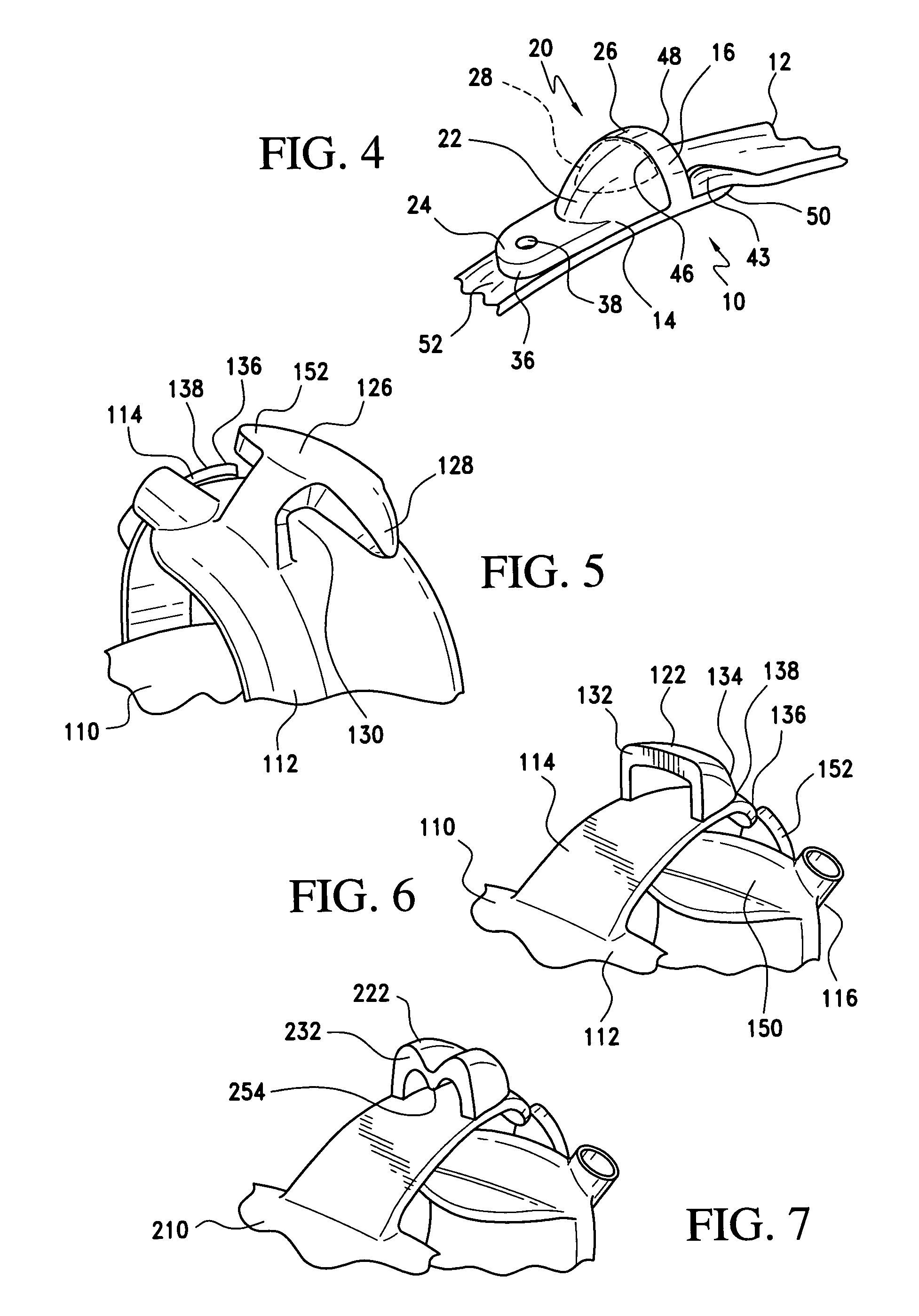
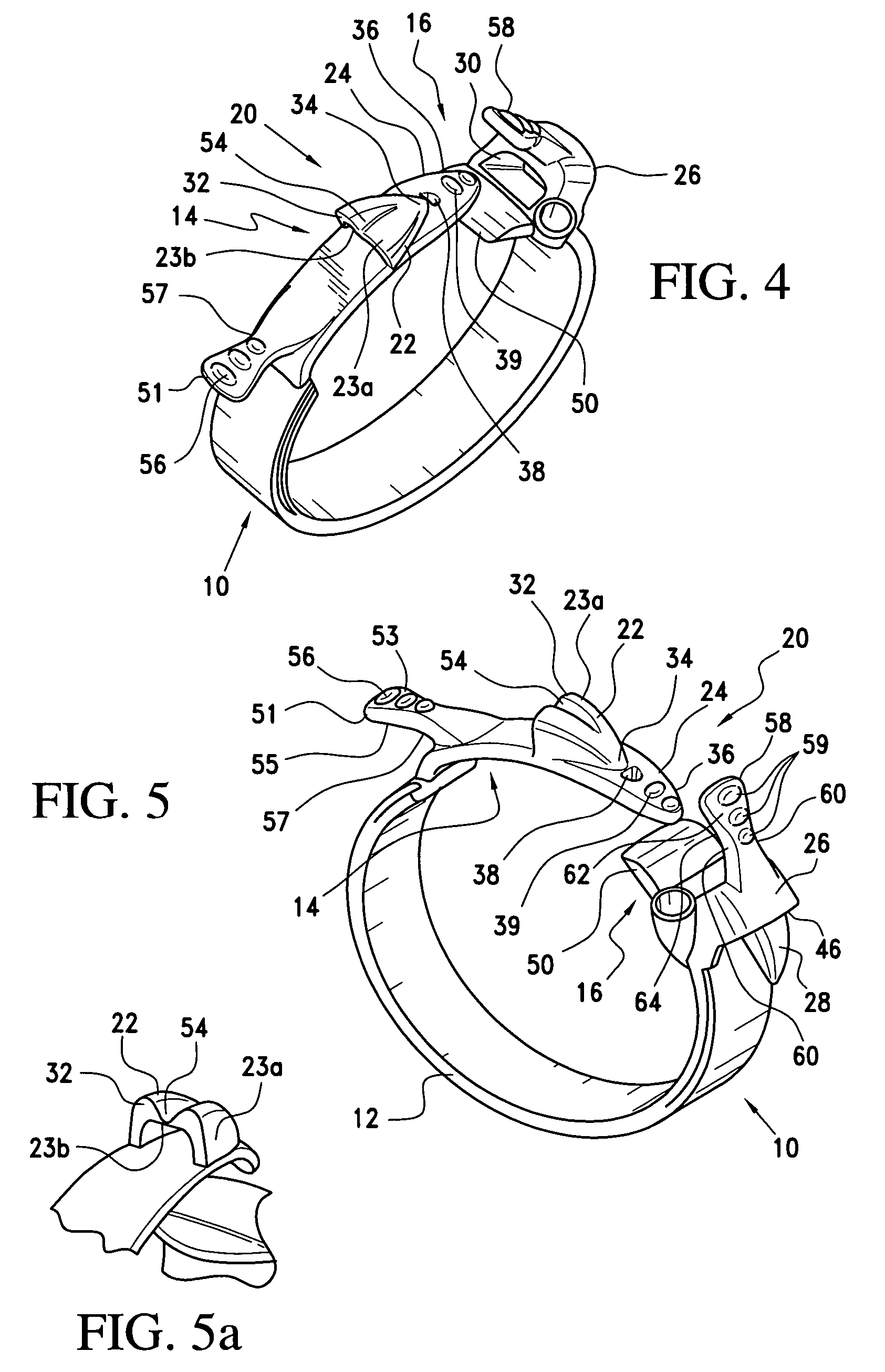
Gastric band suture tab extender
A removable suture tab extender for a gastric band is shaped and dimensioned for selective attachment to a first end of the gastric band, the gastric band including a latching mechanism composed of a first latching member positioned at the first end of the gastric band and a second latching member positioned at a second end of the gastric band. The gastric band extender includes an elongated body member having a first end and a second end. The first end of the elongated body member is shaped and dimensioned for coupling with the first latching member. The second end of the elongated body member includes a gripping section shaped and dimensioned to facilitate gripping thereof as the gastric band extender is passed through the second latching member.
Owner:ETHICON ENDO SURGERY INC
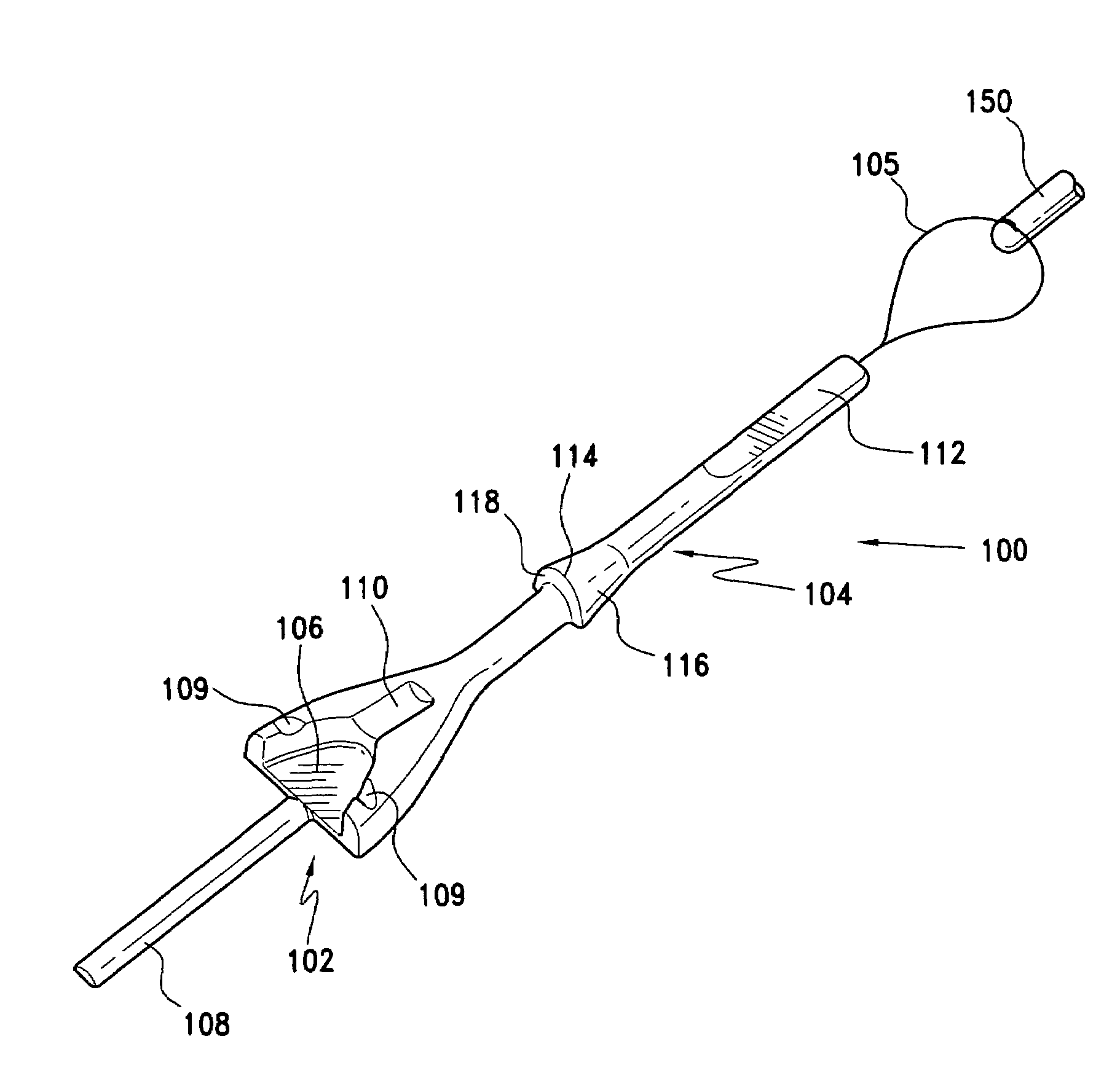
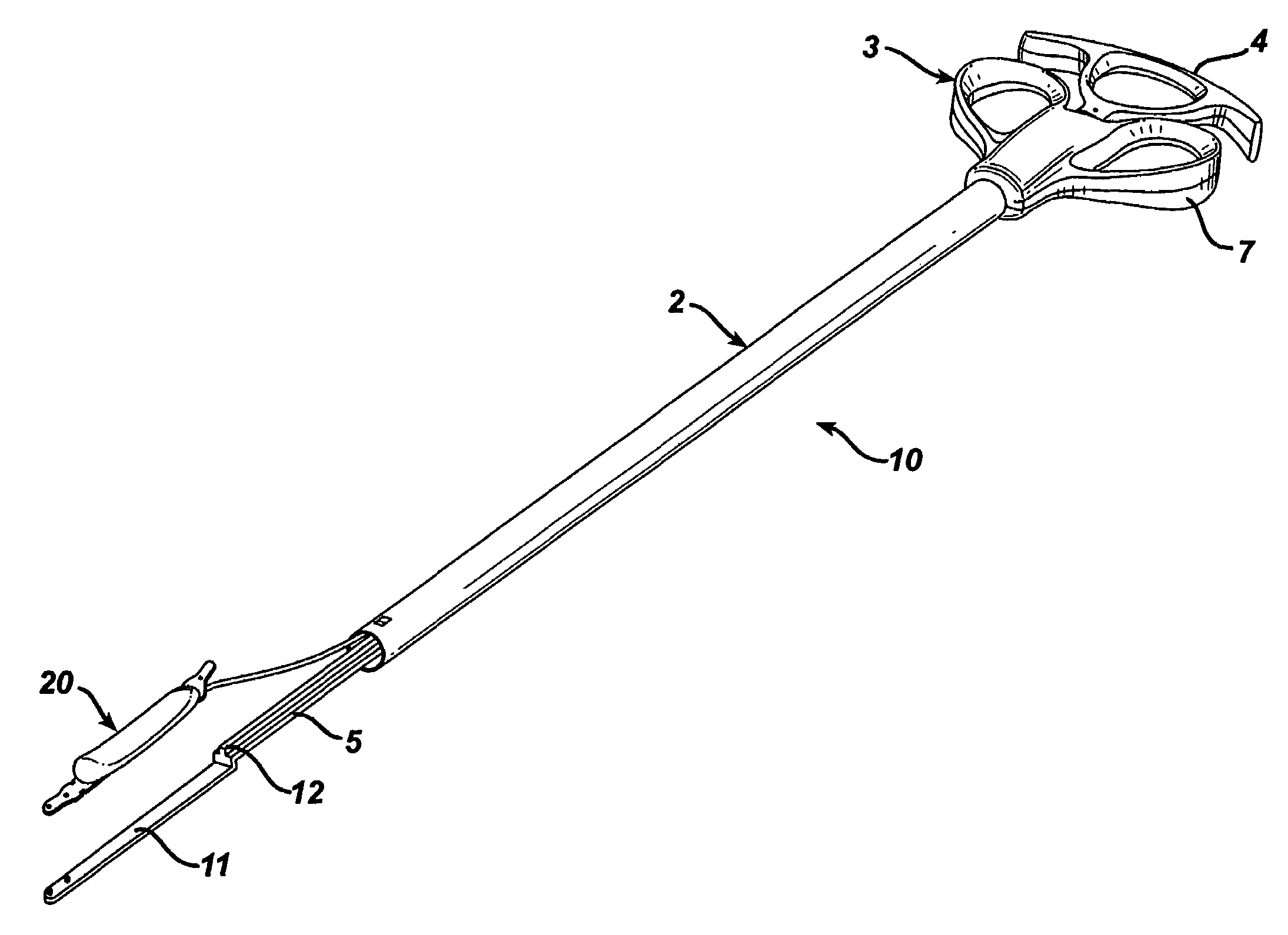
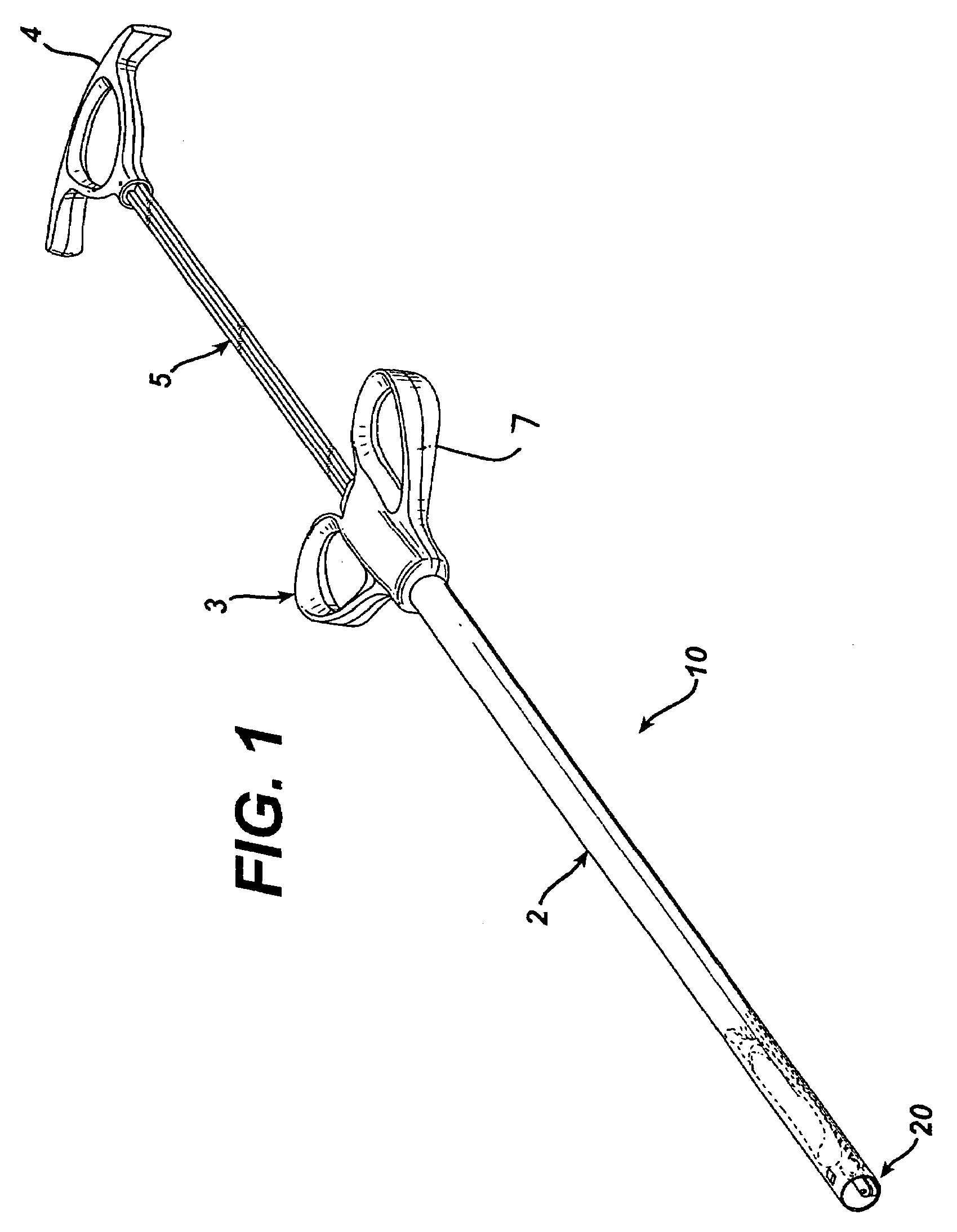
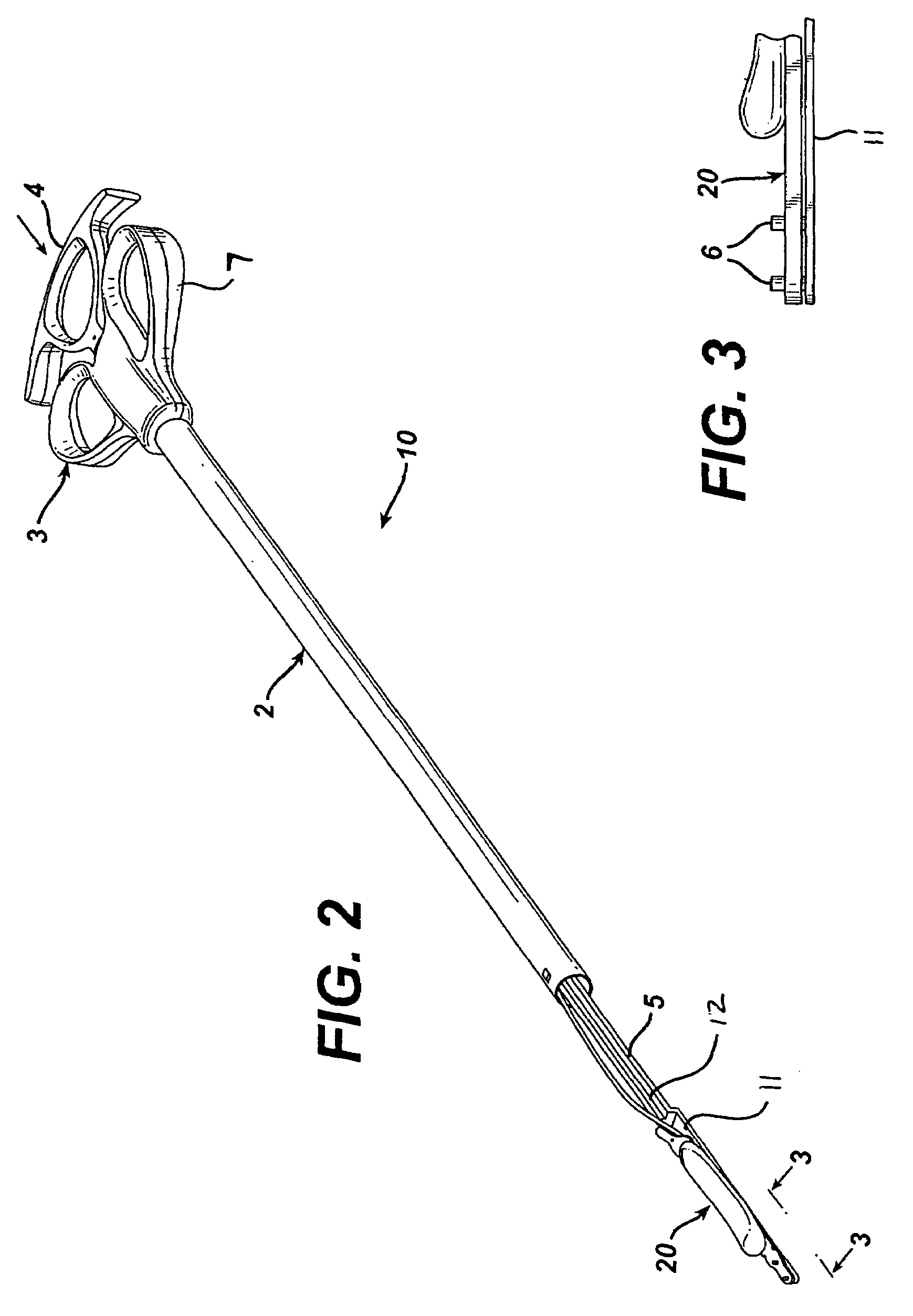
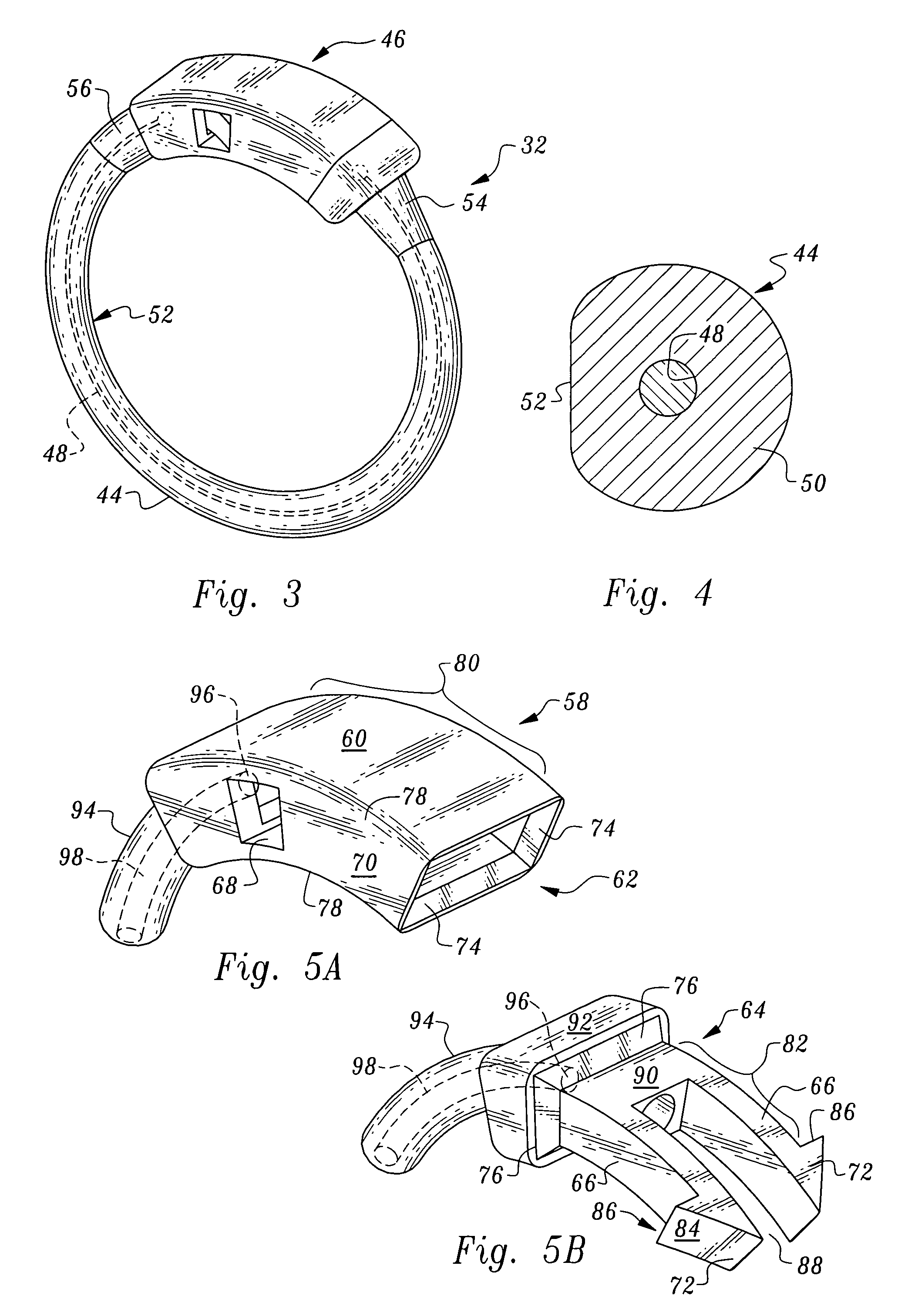
Gastric band introduction device
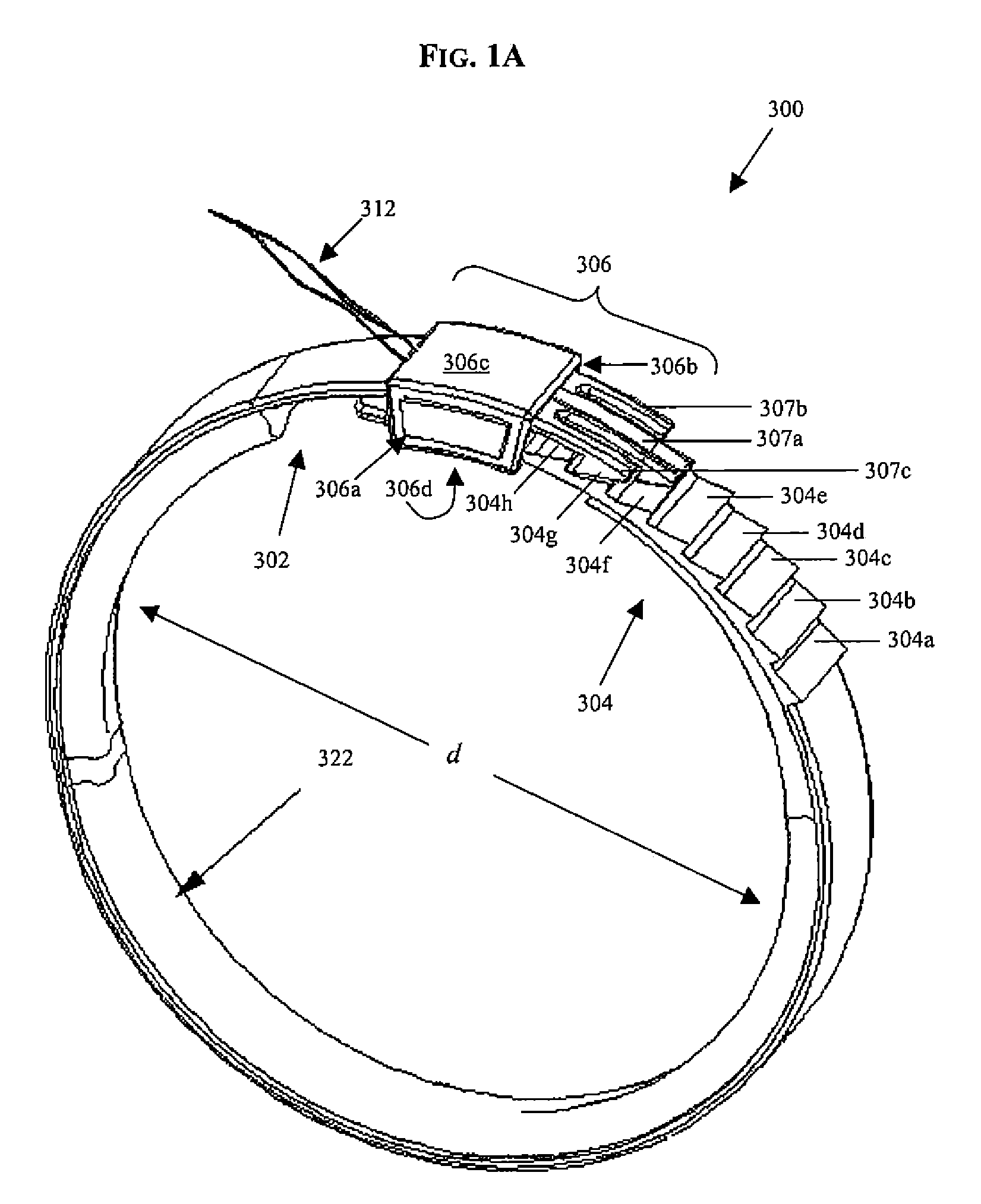
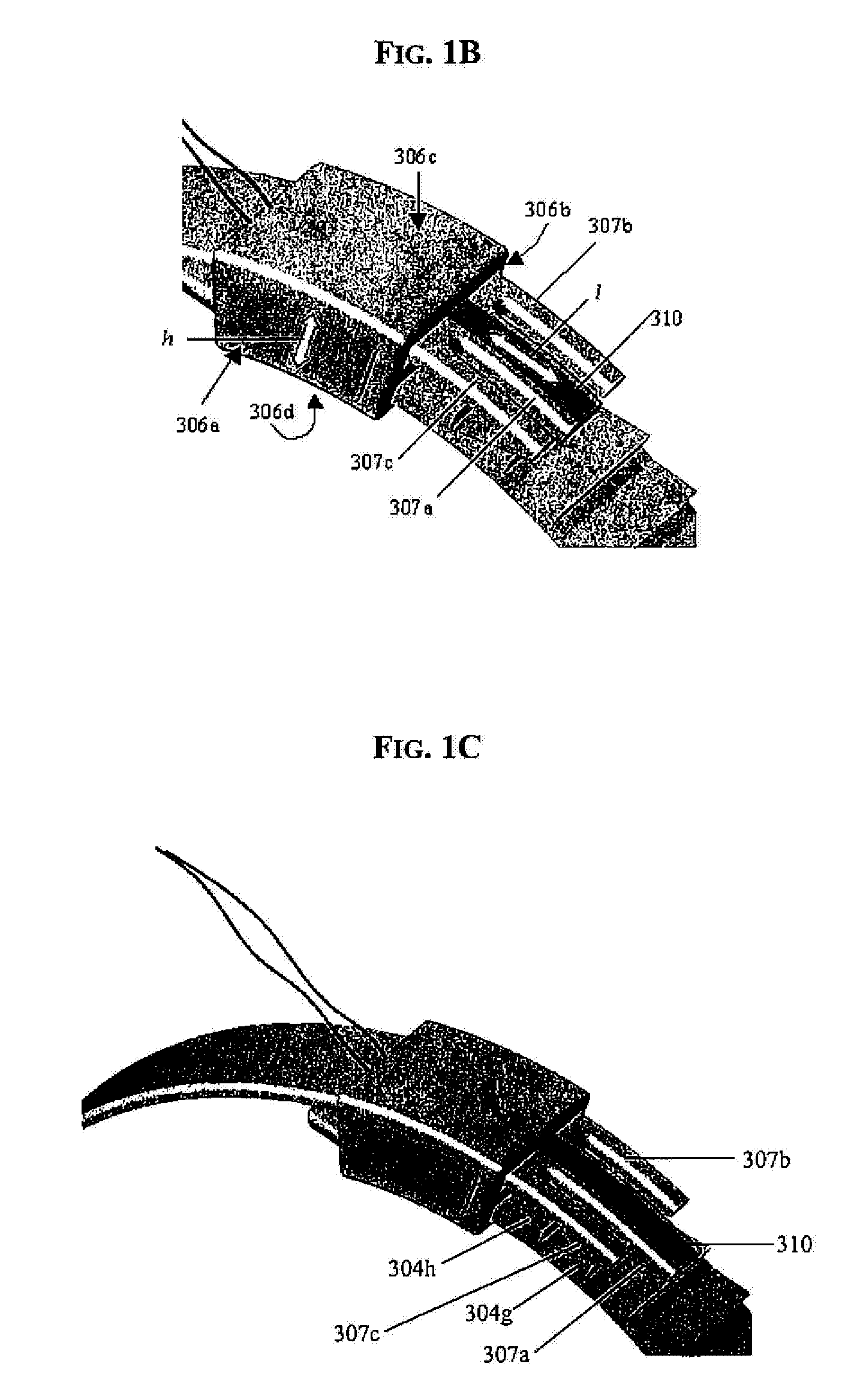
A surgical tool for safely introducing a gastric band into a patient's abdomen is provided. The instrument includes an inner rod slidably and coaxially disposed within a support tube. The inner rod includes a gastric band releasably secured thereon. Distal movement of the inner rod into the support tube exposes said gastric band.
Owner:ETHICON ENDO SURGERY INC
Metal bellows position feedback for hydraulic control of an adjustable gastric band
InactiveUS20050267500A1Precise motion controlAvoid the needNon-surgical orthopedic devicesObesity treatmentClosed loopEngineering
A remotely controlled gastric band system that is practically immune to external magnetic fields, such as from a Magnetic Resonance Imaging (MRI) machine, incorporates a bi-directional pump and fluid reservoir to adjust fluid volume for hydraulic control of a gastric band. A piezoelectric driver (e.g., rotary actuator, linear actuator) selectively compresses and expands a metal bellows hermetically sealed within a biocompatible and nonferromagnetic enclosure or case such as titanium. Directly sensing a position of the metal bellows yields an accurate reading of volume contained therein, allowing for closed-loop control of the gastric band.
Owner:ETHICON ENDO SURGERY INC
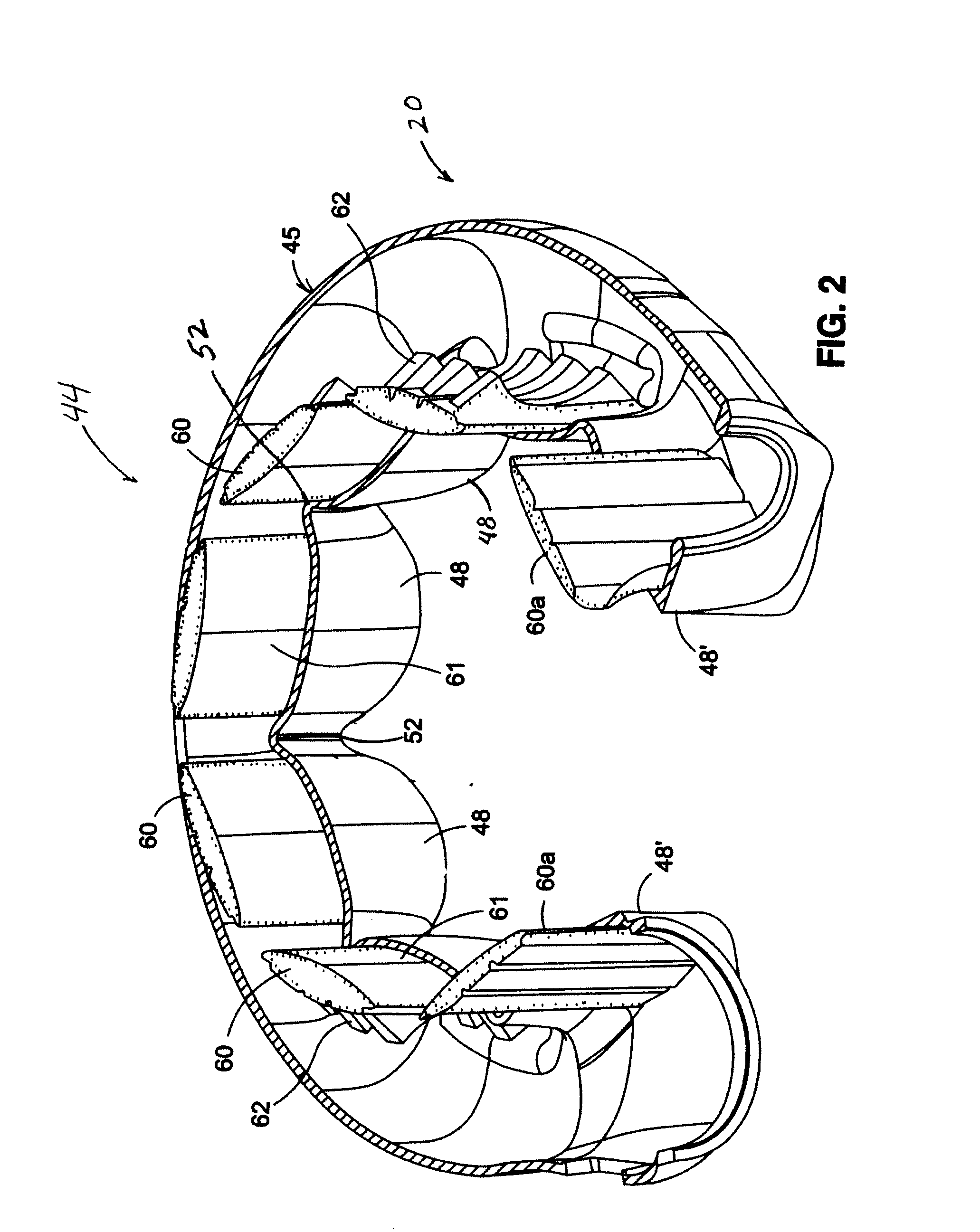
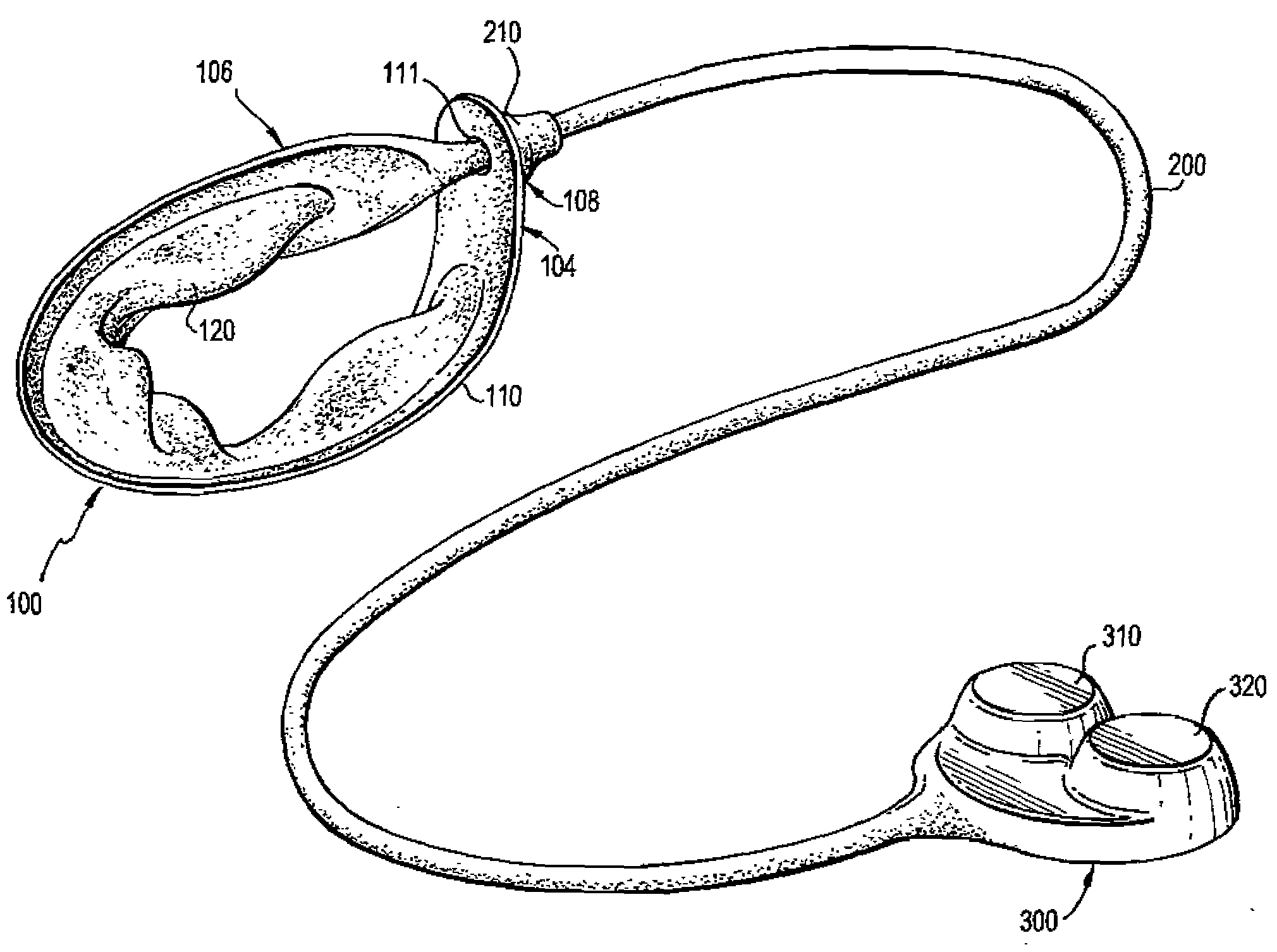
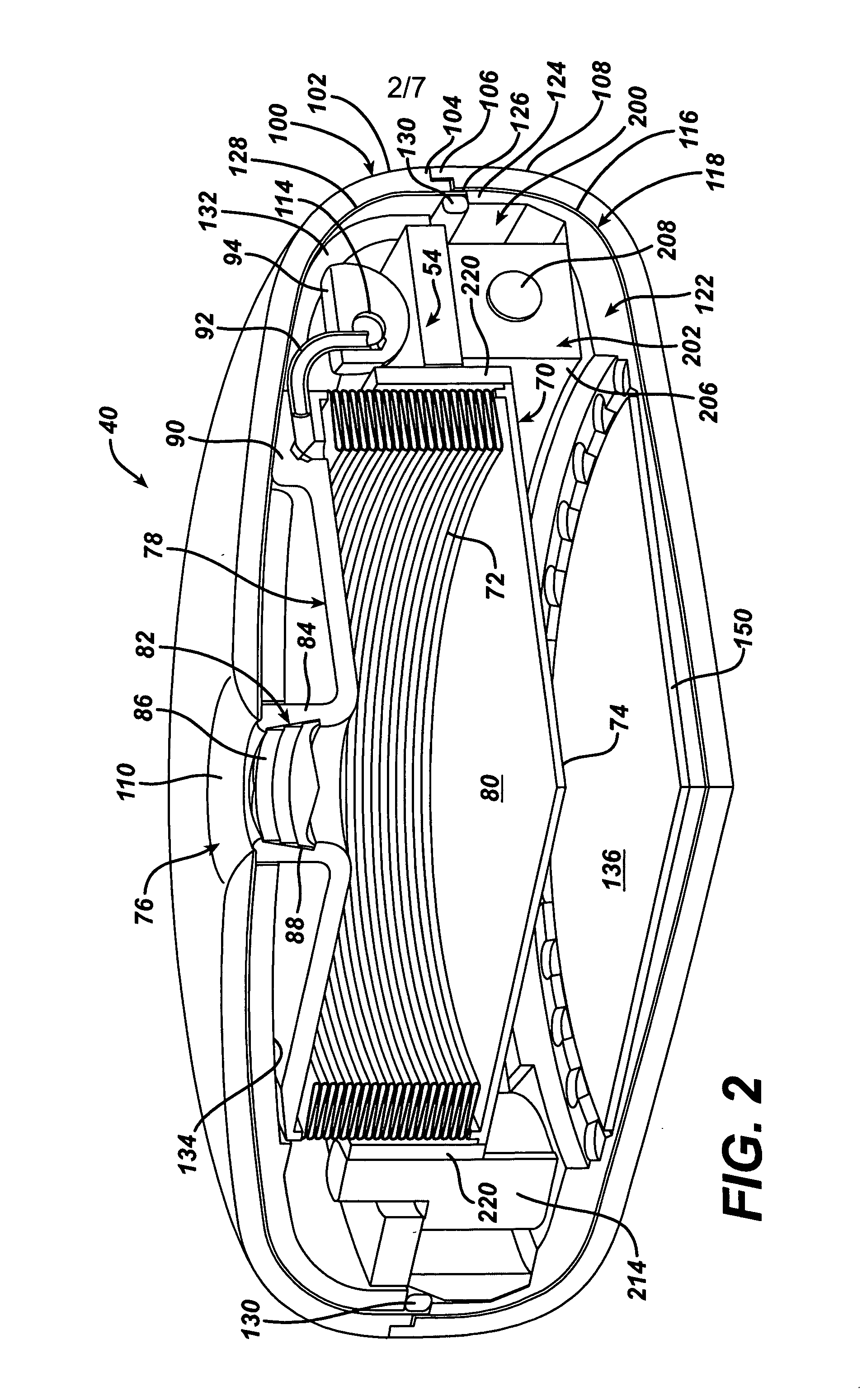
Mechanical Gastric Band With Cushions
ActiveUS20100087843A1Reduce generationAvoid pinchingObesity treatmentProsthesisGastric bandEngineering
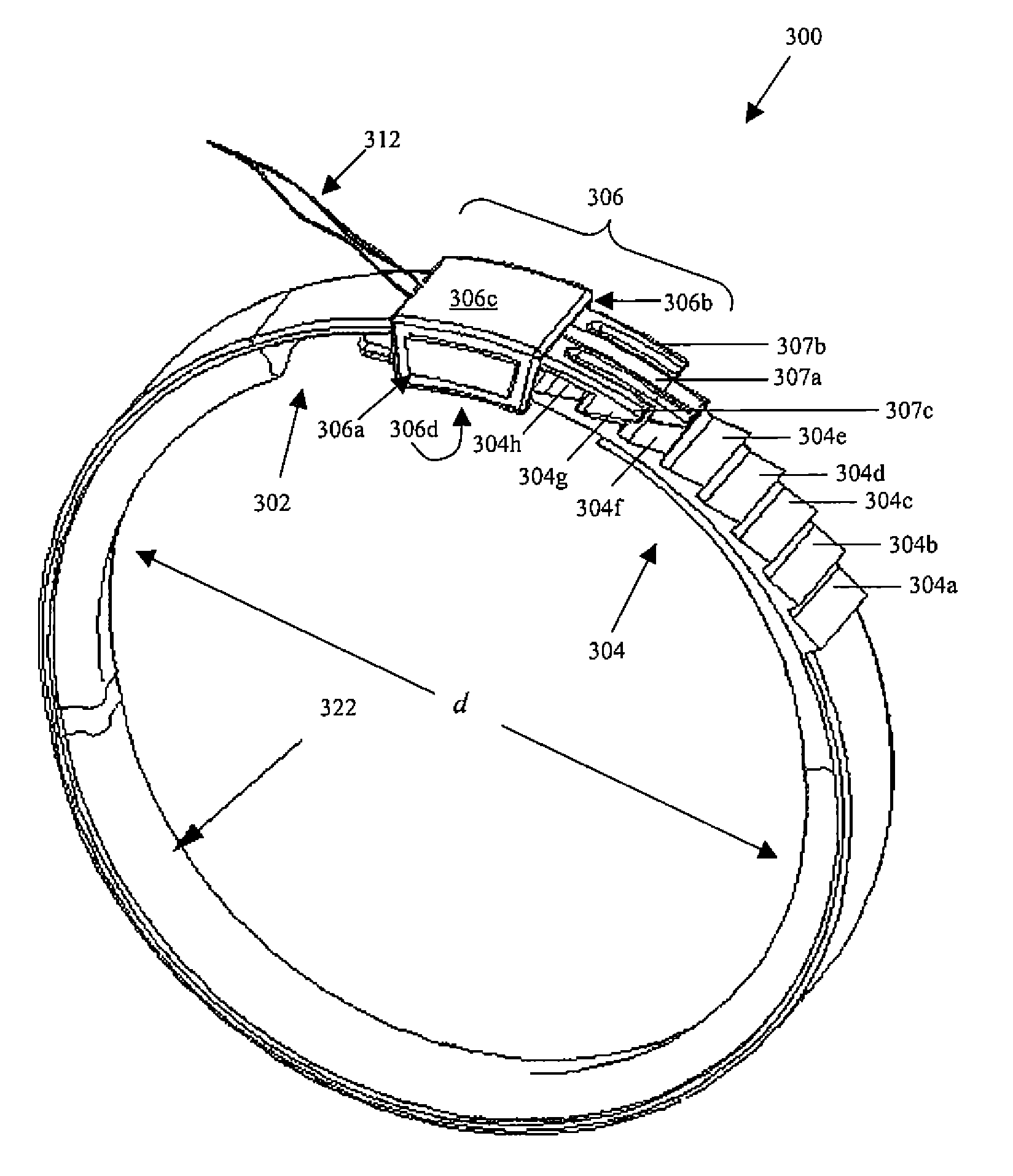
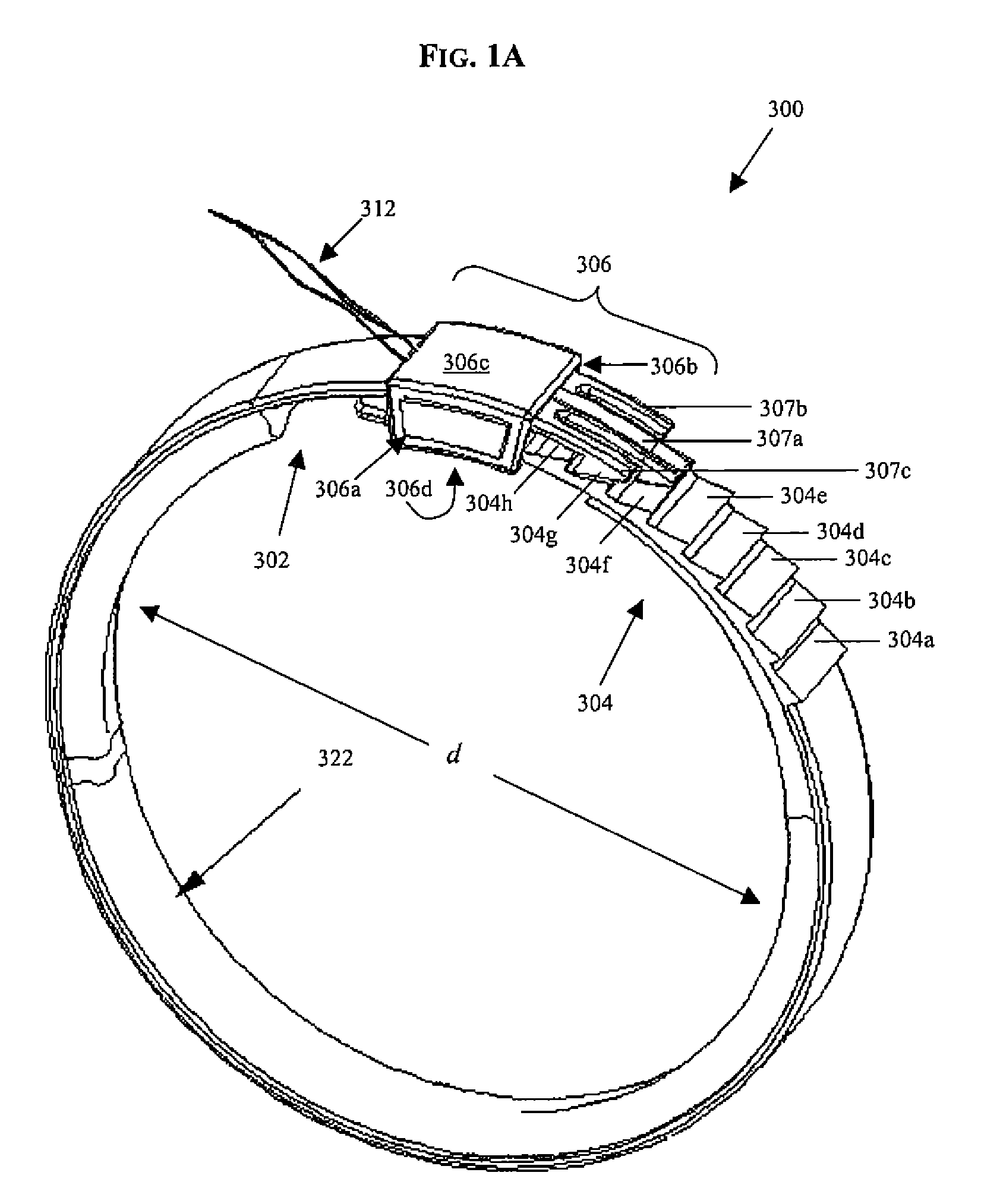
A system for regulating the functioning of an organ or duct generally includes an implantable band structured to at least partially circumscribe an organ or duct and an actuating mechanism operable to effect constriction of the band. The system further includes a plurality of incompressible cushion segments defining a substantially star-shaped inner circumference of the band, the star-shape effective to prevent pinching and necrosis of tissue during adjustment.
Owner:RESHAPE LIFESCIENCES INC
Precurved gastric band
A low pressure, high volume gastric band includes a balloon shaped and dimensioned to circumscribe the stomach at a predetermined location and a belt extending about the balloon. The gastric band is precurved extend about the stomach and the balloon exhibits 360 degree coverage about the stomach.
Owner:ETHICON ENDO SURGERY INC
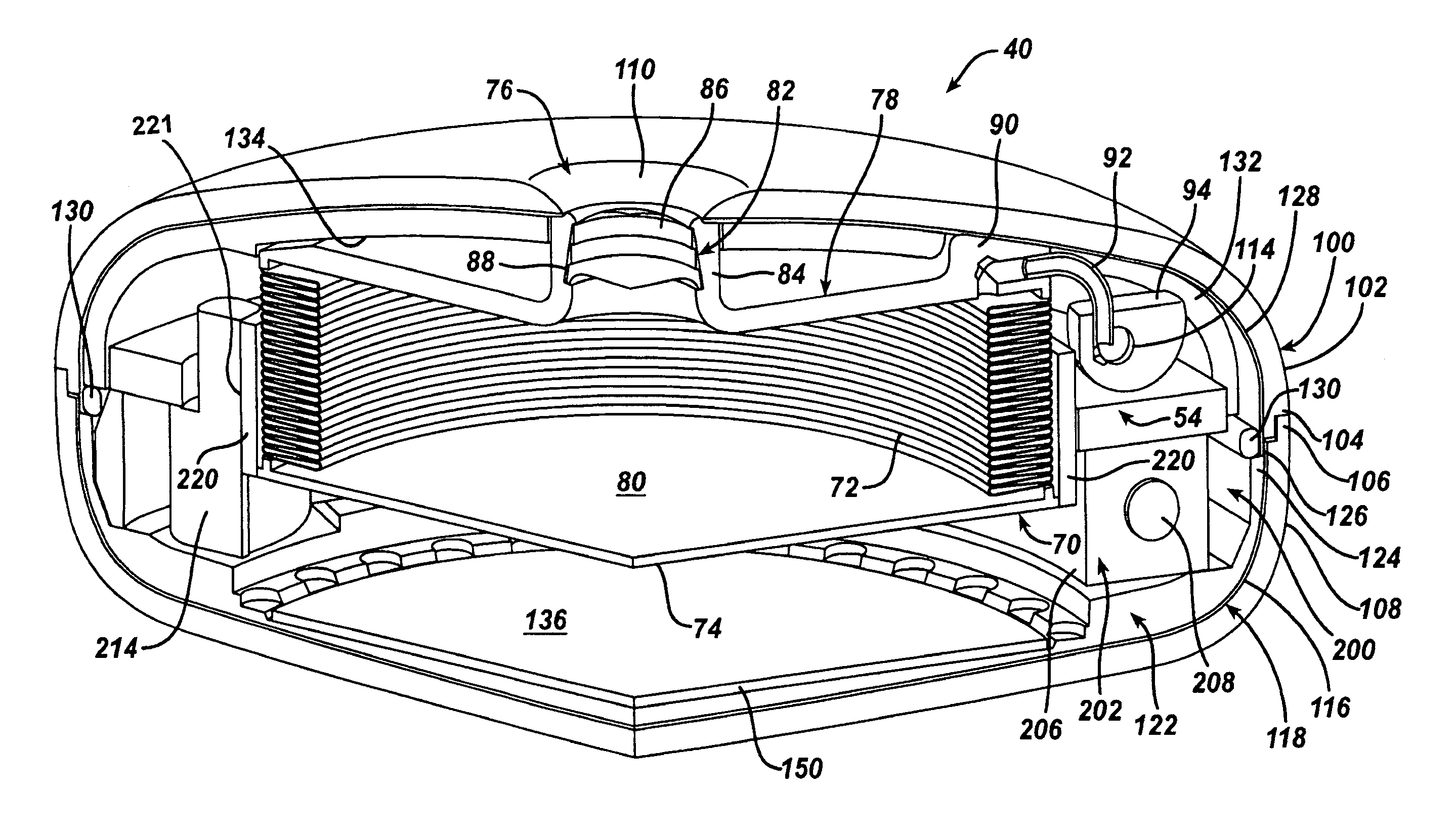
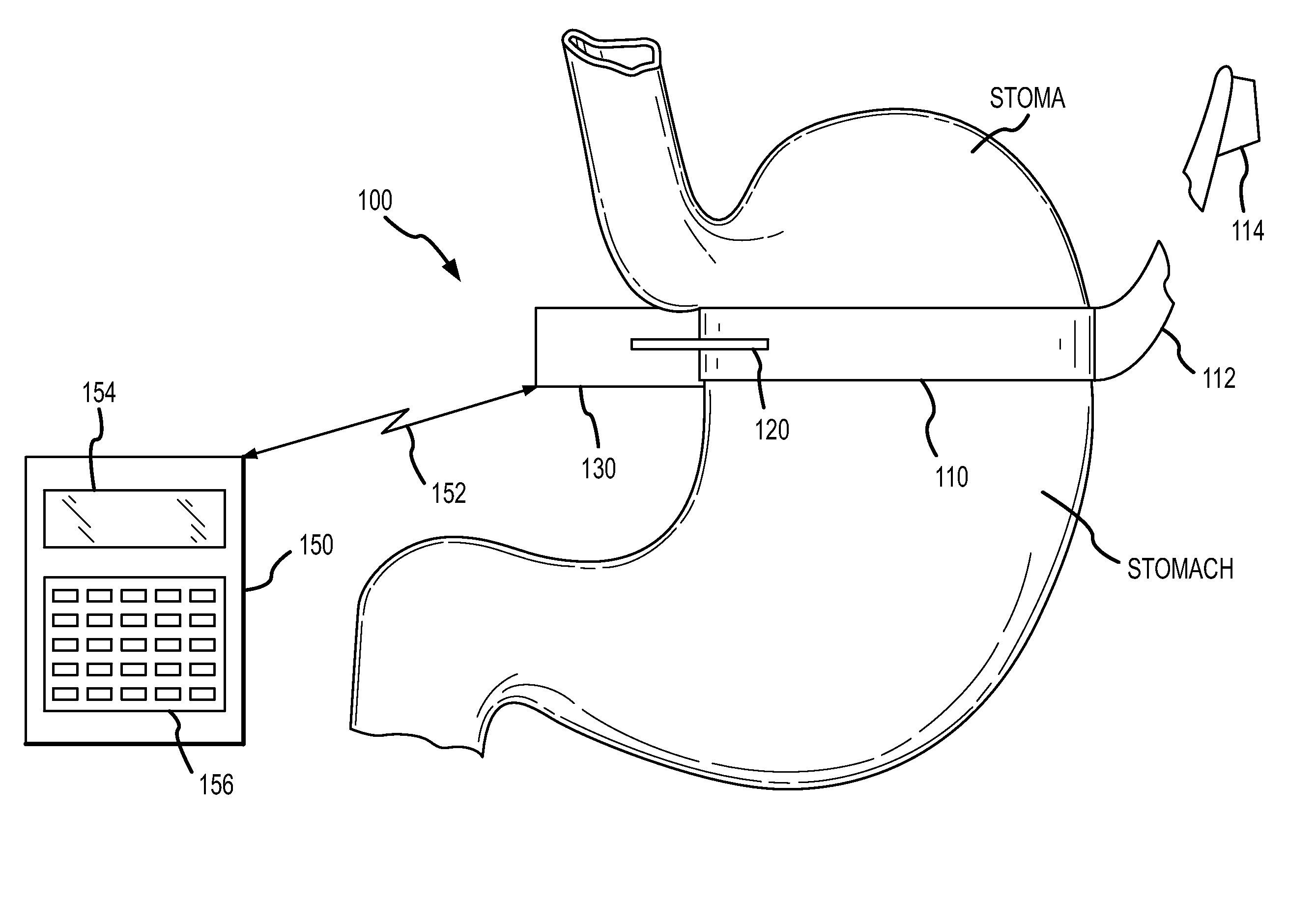
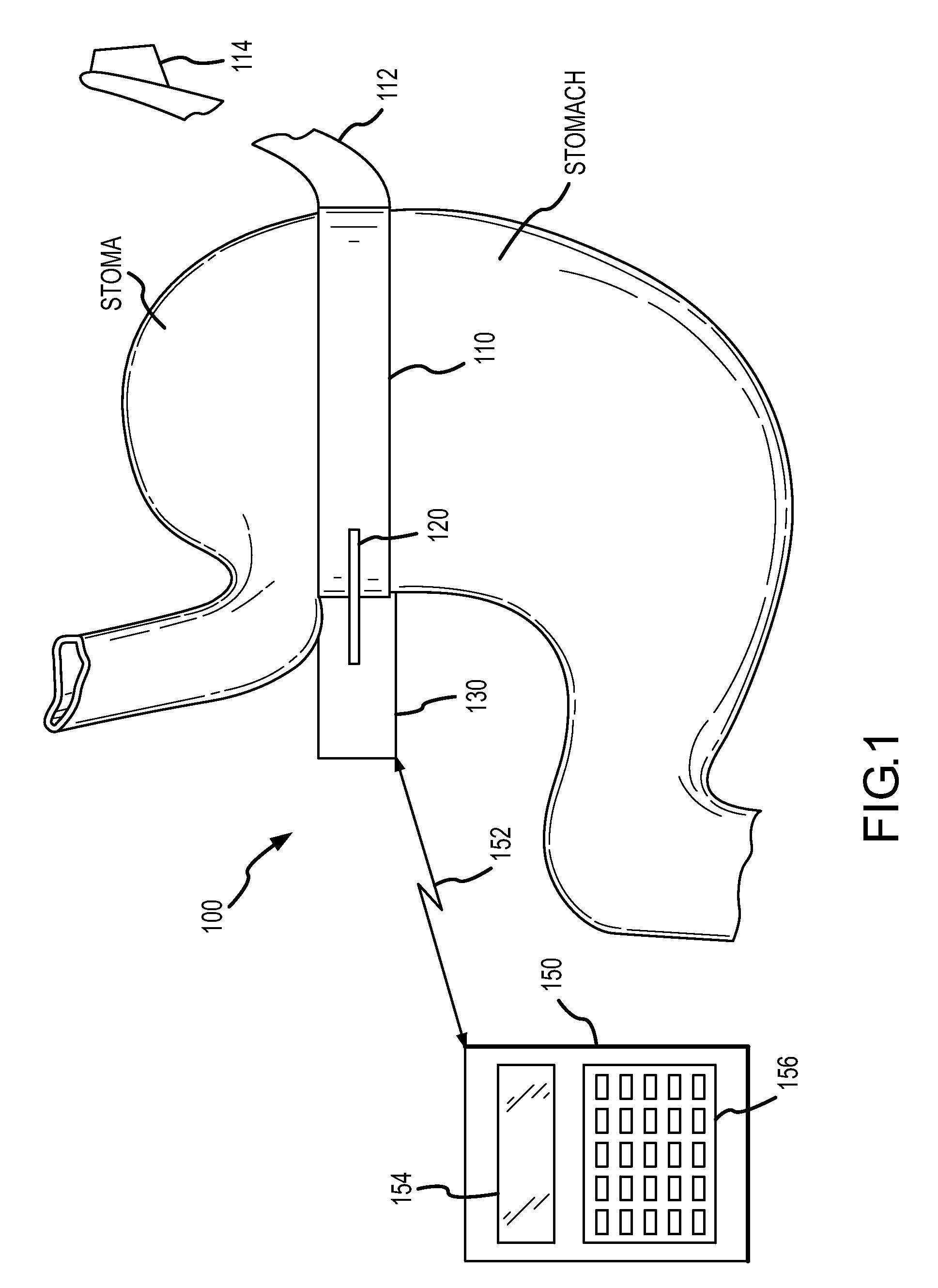
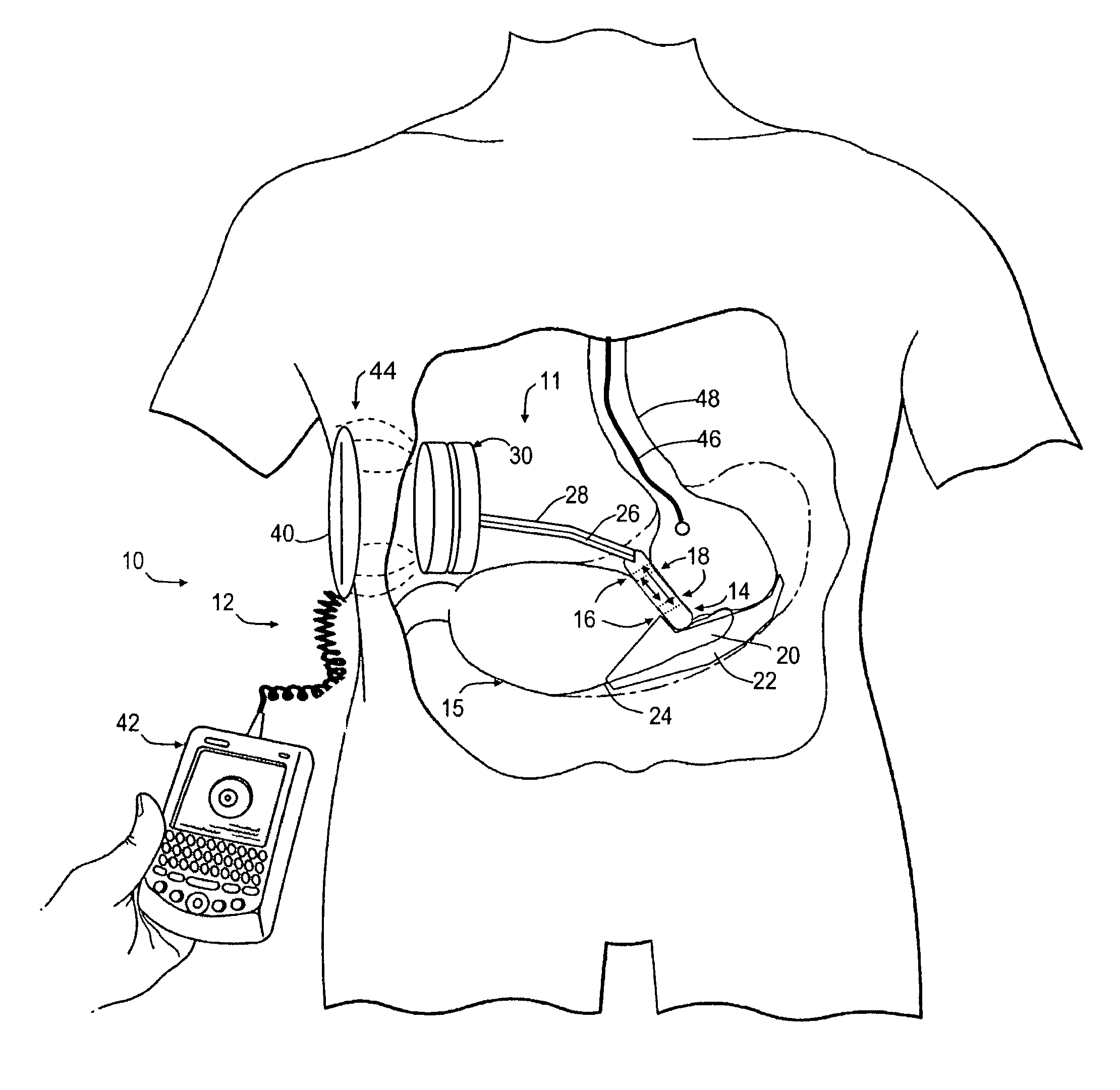
Hydraulic gastric band collapsible reservoir
InactiveUS20070265645A1Protection from damageDiagnostic recording/measuringTourniquetsStomaGastric band
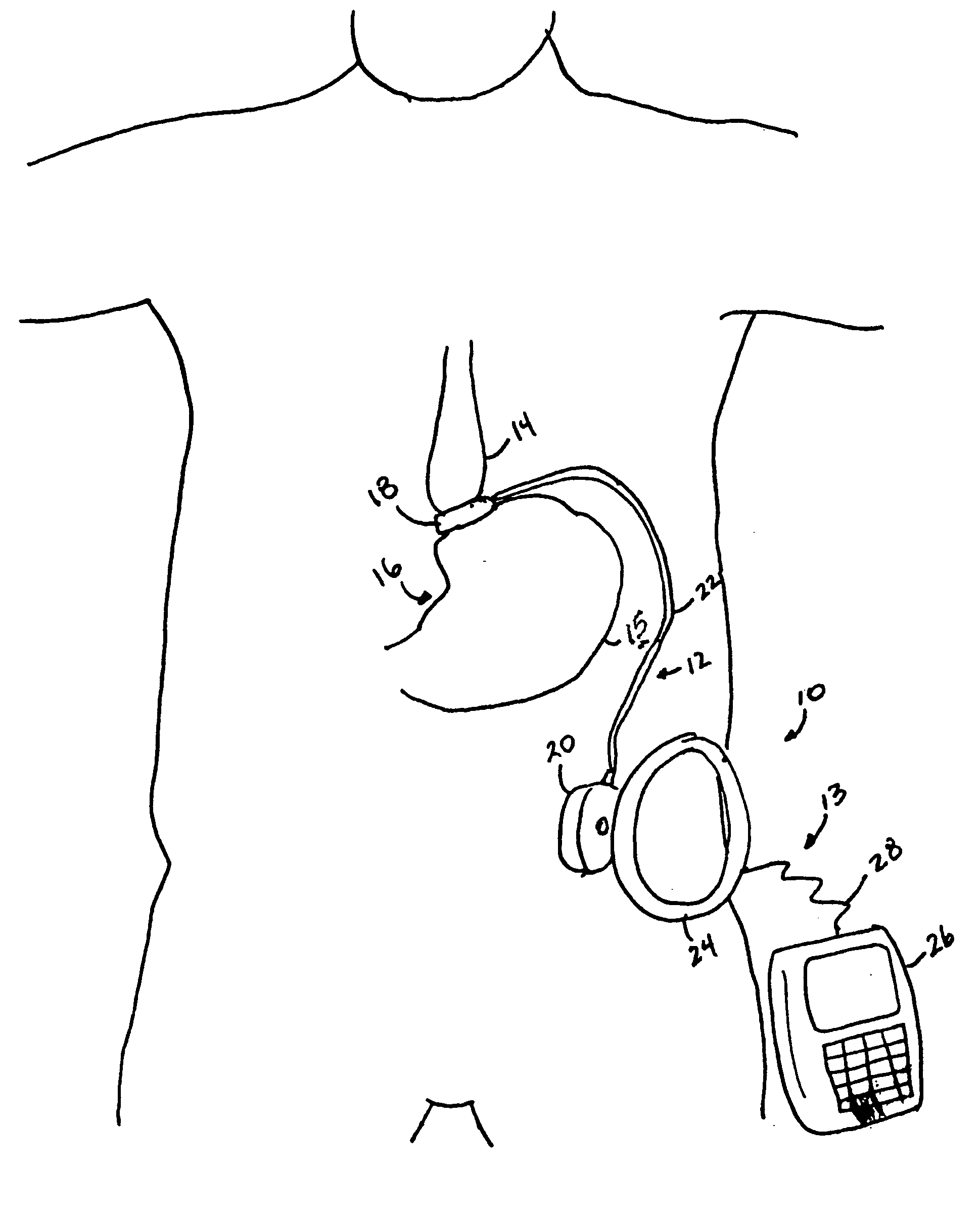
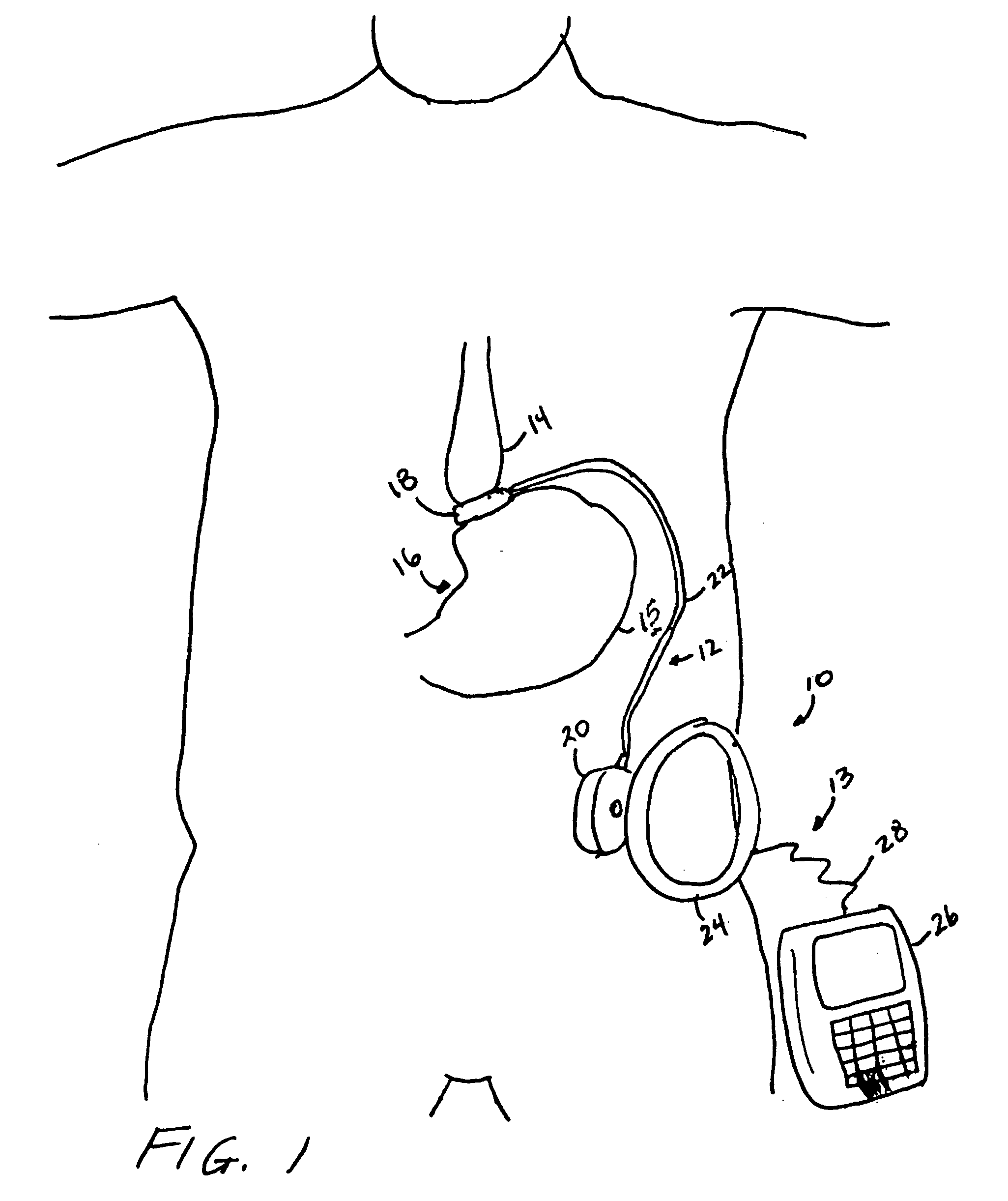
A self-regulating gastric band apparatus for adjusting stoma size. The apparatus includes an adjustable gastric band that has an inner ring expanding with injected fluid. A band adjustment assembly is provided for implanting with the gastric band that includes a sensor for sensing fluid pressure in the inner ring. The band adjustment assembly further includes a pump assembly connected to the expandable inner ring and to a controller that can operate the pump assembly to adjust the volume of the fluid in the band based on the sensed fluid pressure. The band adjustment assembly includes memory storing an operating range relative to a target fluid pressure, and the pump assembly is operated to maintain the sensed band pressure within the operating range. The target pressure being set to maintain pressure variations below a predefined variation limit generally corresponding with satiated fill volumes for a particular patient and implanted band. An elongated fluid reservoir may extend along a substantial part of a fill tube. A balloon-like expandable fluid reservoir in fluid communication with the pump assembly may store a volume of the fluid for adjusting the volume of fluid in the lumen. A protective outer sheath may be provided around the exterior of an expandable fluid reservoir in both a first, deflated state, and a second, inflated state of the reservoir.
Owner:APOLLO ENDOSURGERY INC
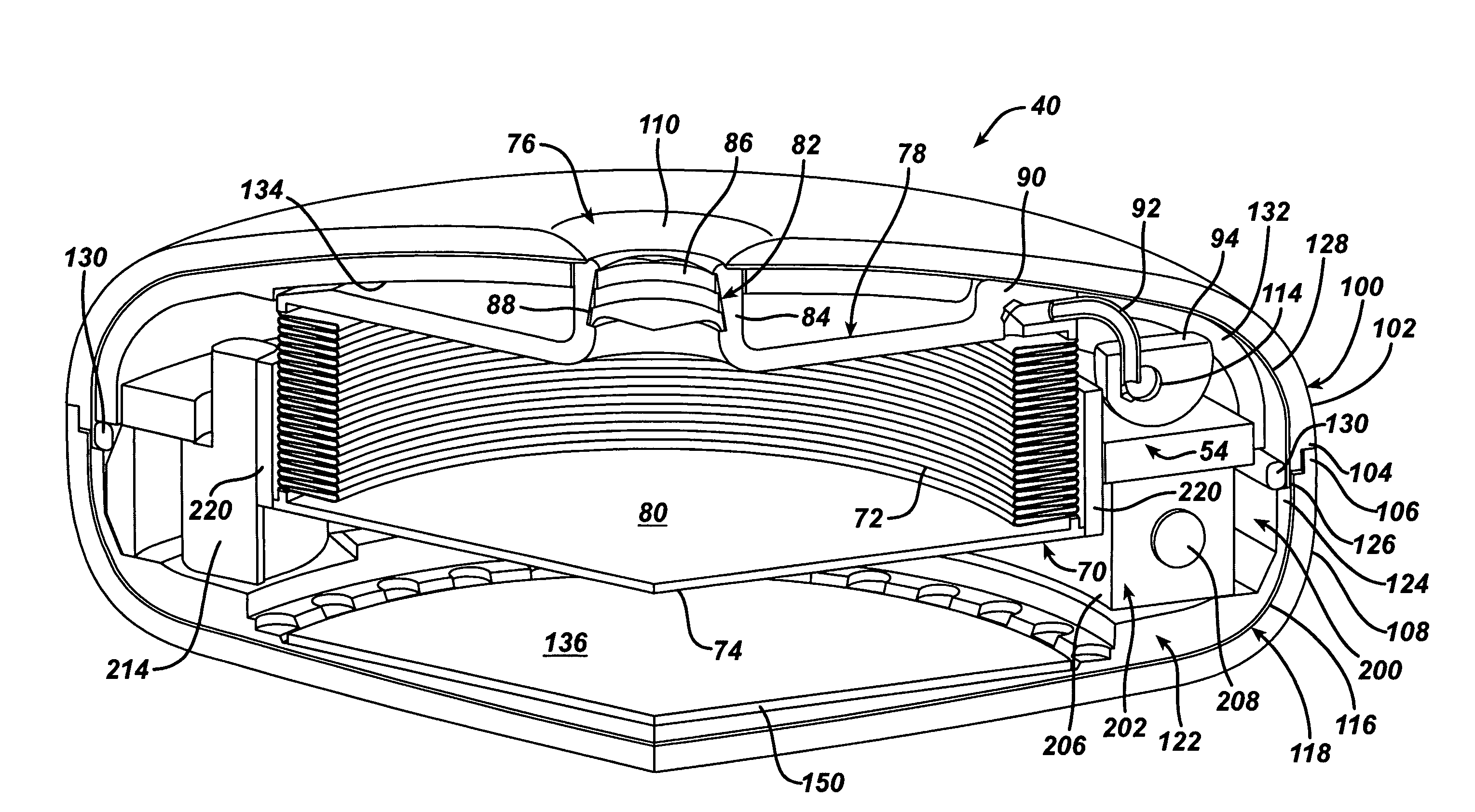
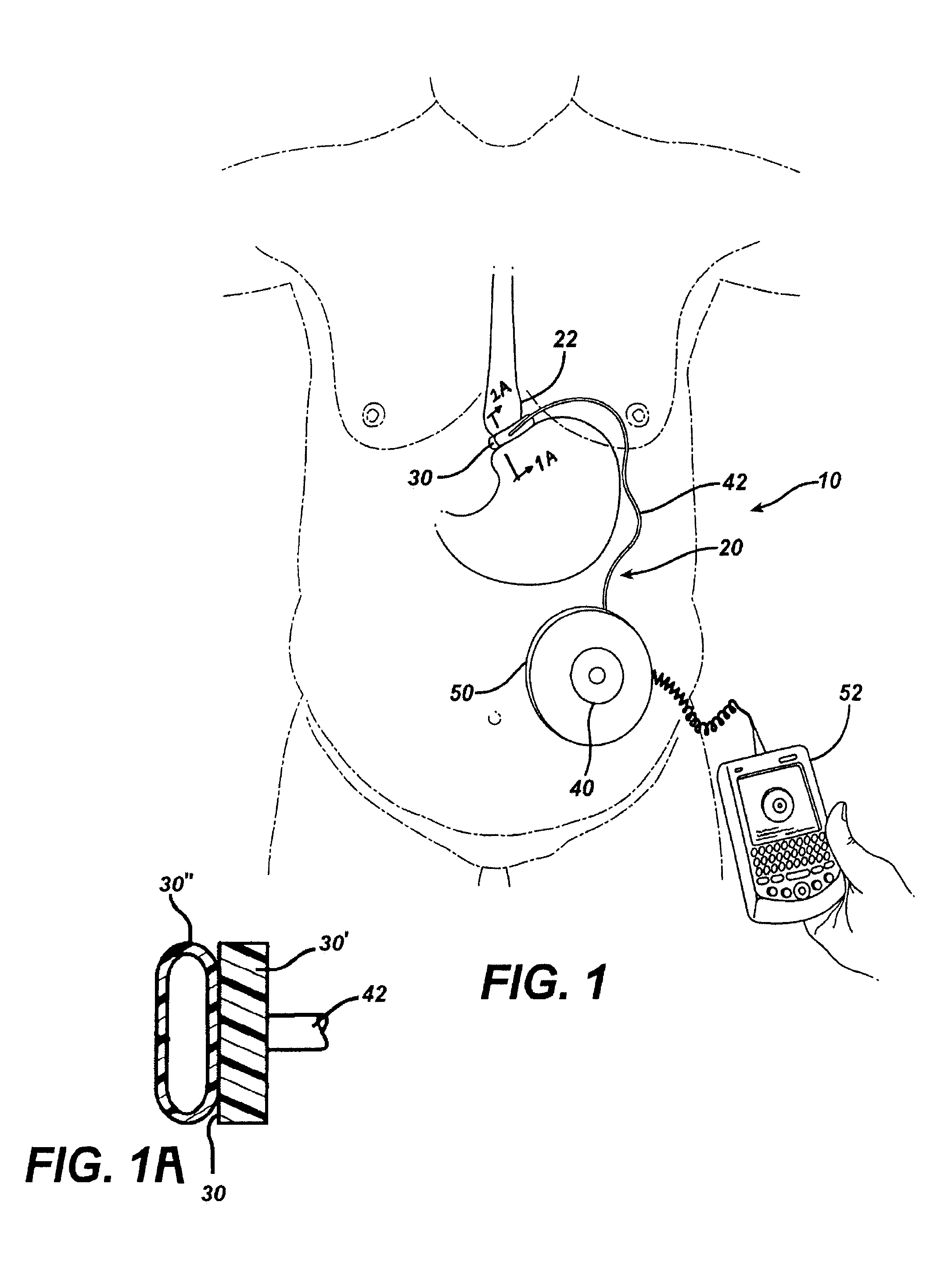
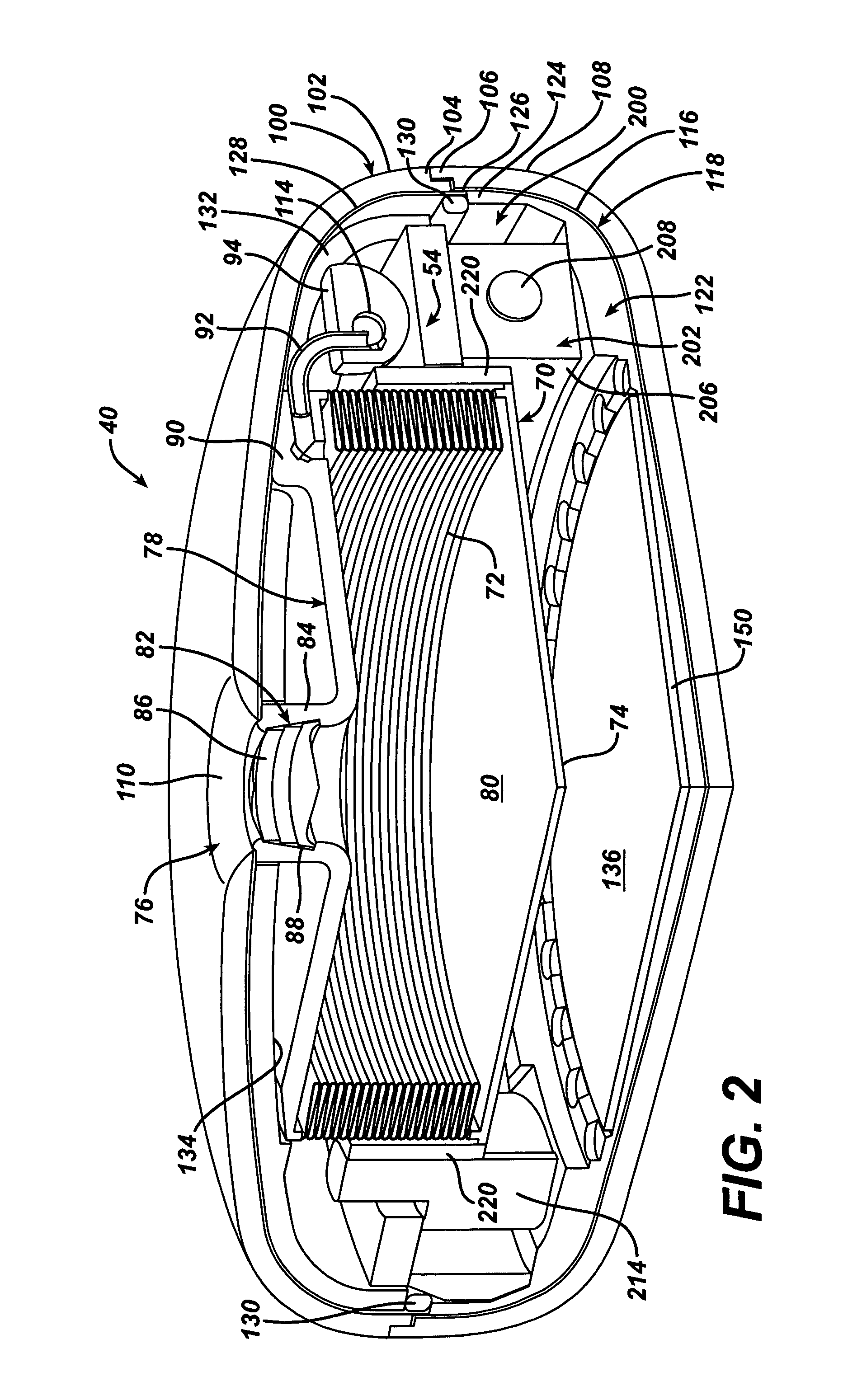
Methods and devices for measuring impedance in a gastric restriction system
Methods and devices are provided for gathering impedance data related to implantable restriction devices. In general, the methods and devices can enable patients, health care providers, and others to use gathered data as a feedback mechanism to non-invasively monitor efficacy of an implantable restriction device in a patient and to identify, modify, and / or prescribe a treatment plan for the patient considering the gathered data. Impedance data can be gathered and analyzed for tissue proximate to the restriction device, e.g., a fat pad between a gastric band and the patient's stomach. Electrodes in contact with the tissue can measure an impedance of the tissue, with the impedance between the electrodes changing as the tissue reduces in size (e.g., as fat cells shrink) and / or changes configuration.
Owner:ETHICON ENDO SURGERY INC
Gastric Inflation Band with Integrated Infusion Catheter
An inflatable gastric band incorporates a fluid channel within an outer surface of the band and an inflatable bladder on a stomach-facing side of the band. The gastric band attaches to a double lumen catheter, which communicates with a dual infusion port. One lumen communicates with the fluid channel and one port, while the other lumen communicates with the inflatable bladder and a second port. Injection of fluid into one chamber of the infusion port expands inflatable bladder, allowing adjustment to the diameter of the stoma. Injection of drugs or therapeutic agents into the second chamber allows the infusion of drugs or agents directly into the abdominal cavity of a gastric band recipient. The inflatable bladder can be continuous or segmented. A segmented bladder reduces pinching and / or folding of the inner surface of the gastric band during expansion / contraction, thereby lessening stomach pinching and / or irritation.
Owner:MEDICAL COMPONENTS INC
Gastric band with supply tube check valve
A balloon-type gastric band includes a balloon shaped and dimensioned to circumscribe the stomach at a predetermined location. The balloon includes a longitudinally extending body. A supply tube is secured to the balloon for fluid communication with an internal cavity of the balloon, wherein the supply tube includes a valve controlling the flow of fluid to and from the balloon.
Owner:ETHICON ENDO SURGERY INC
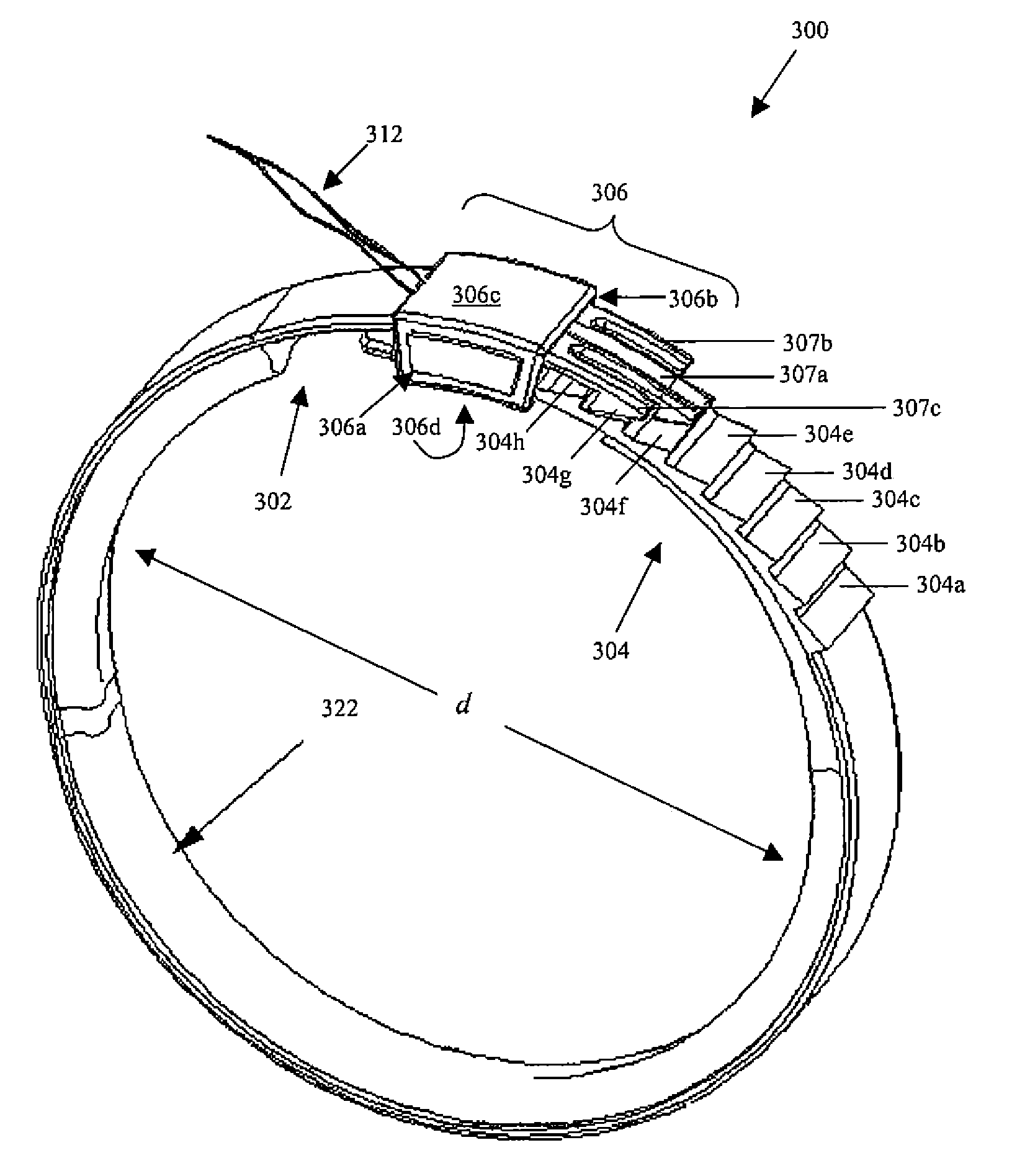
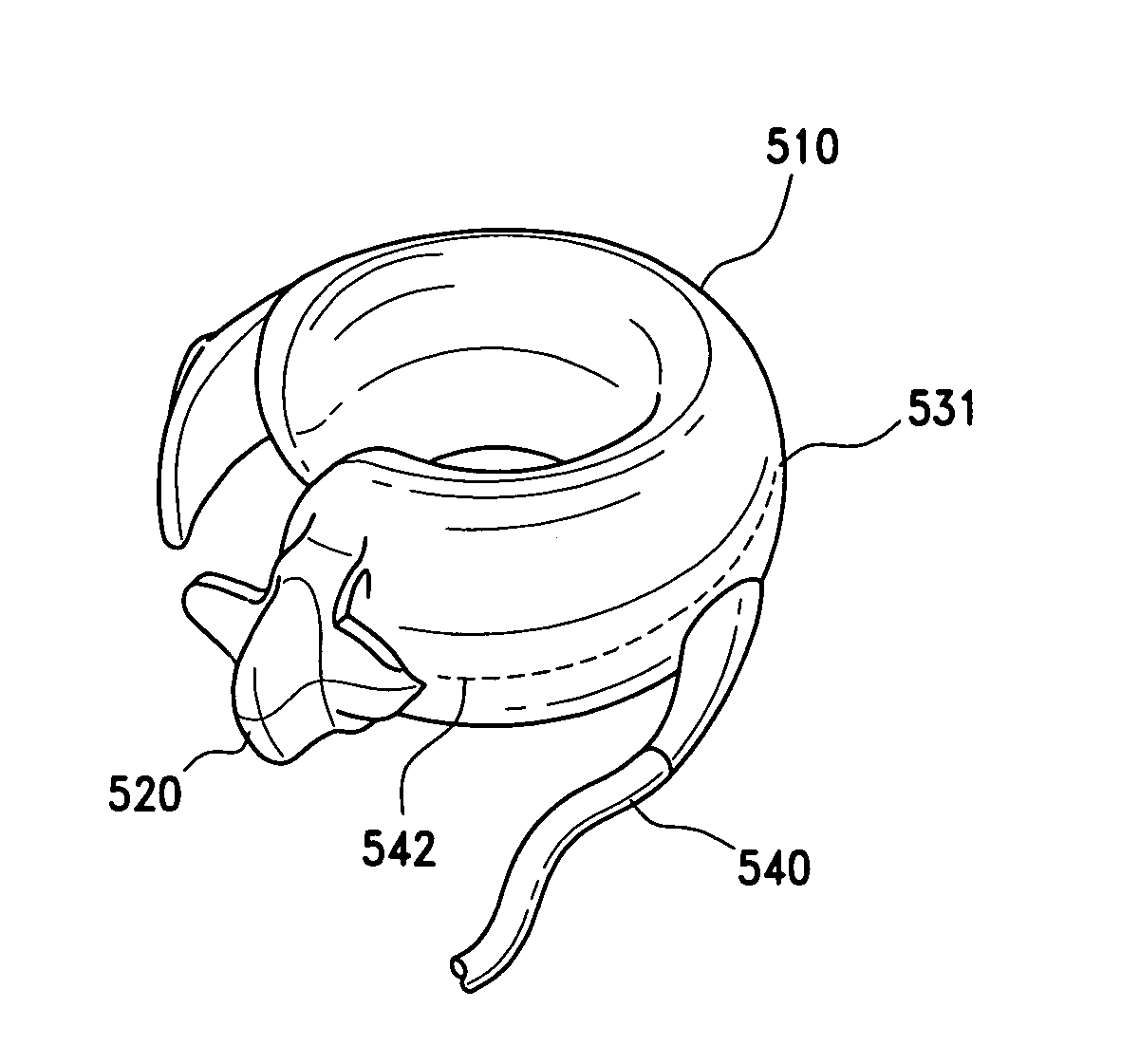
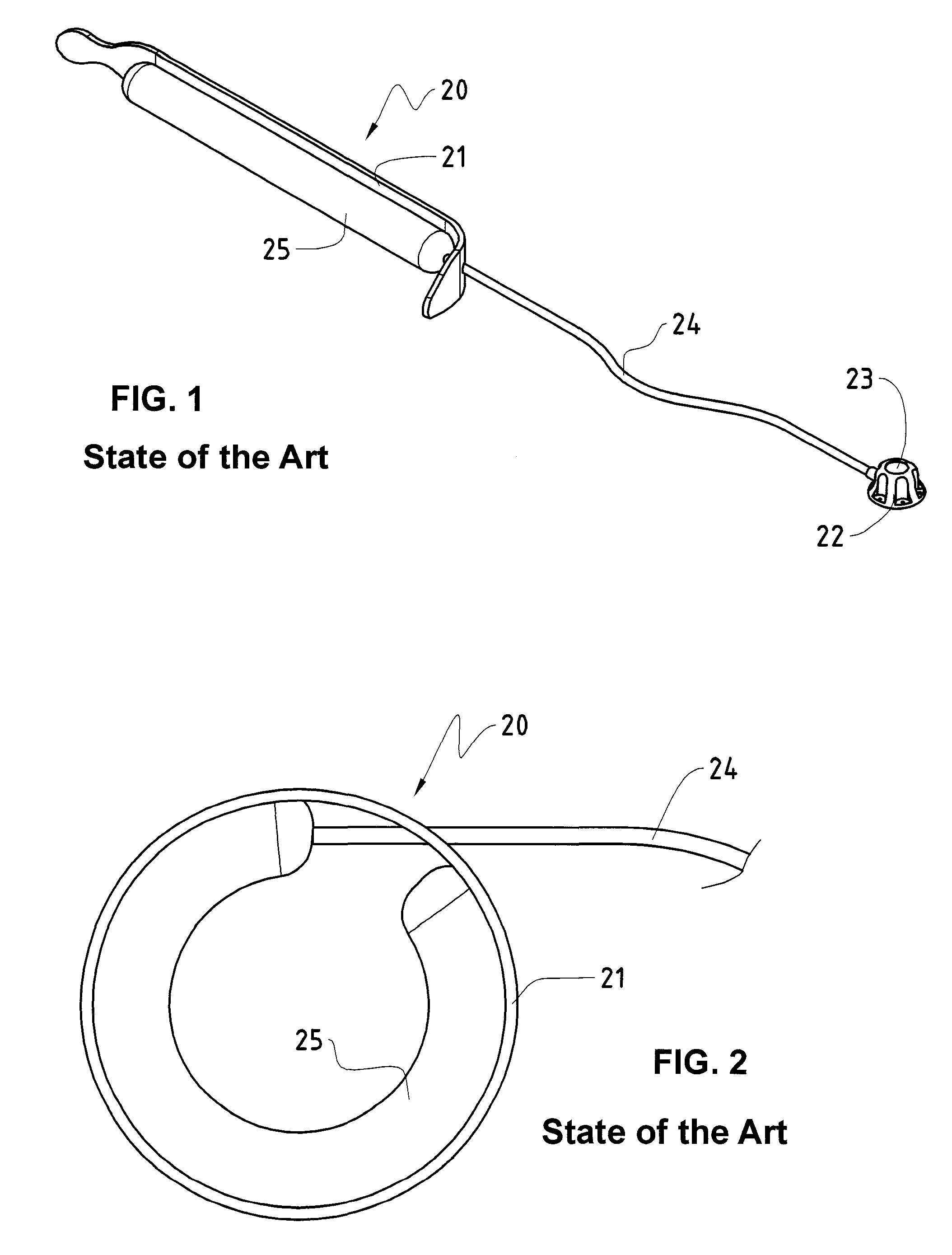
Releasably-securable one-piece adjustable gastric band
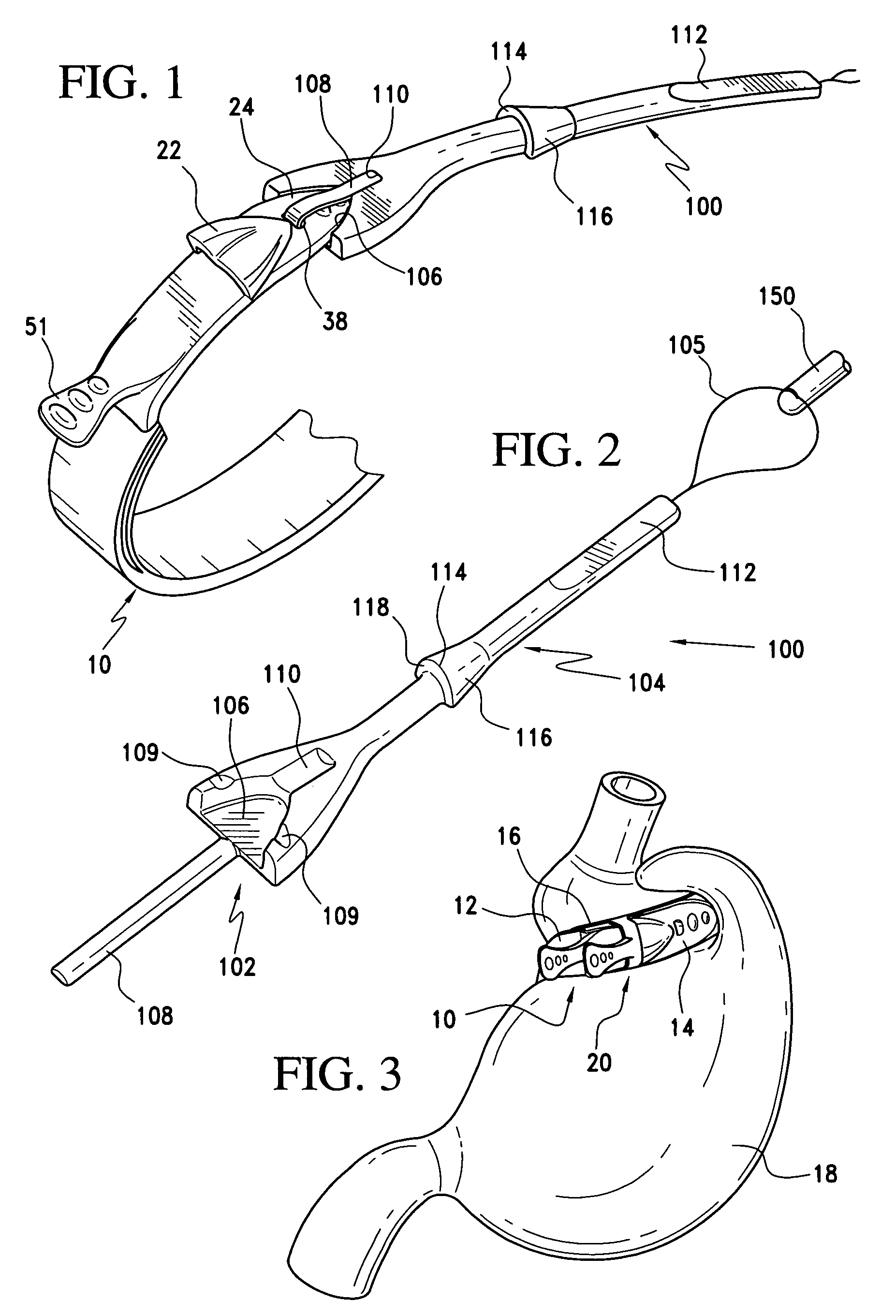
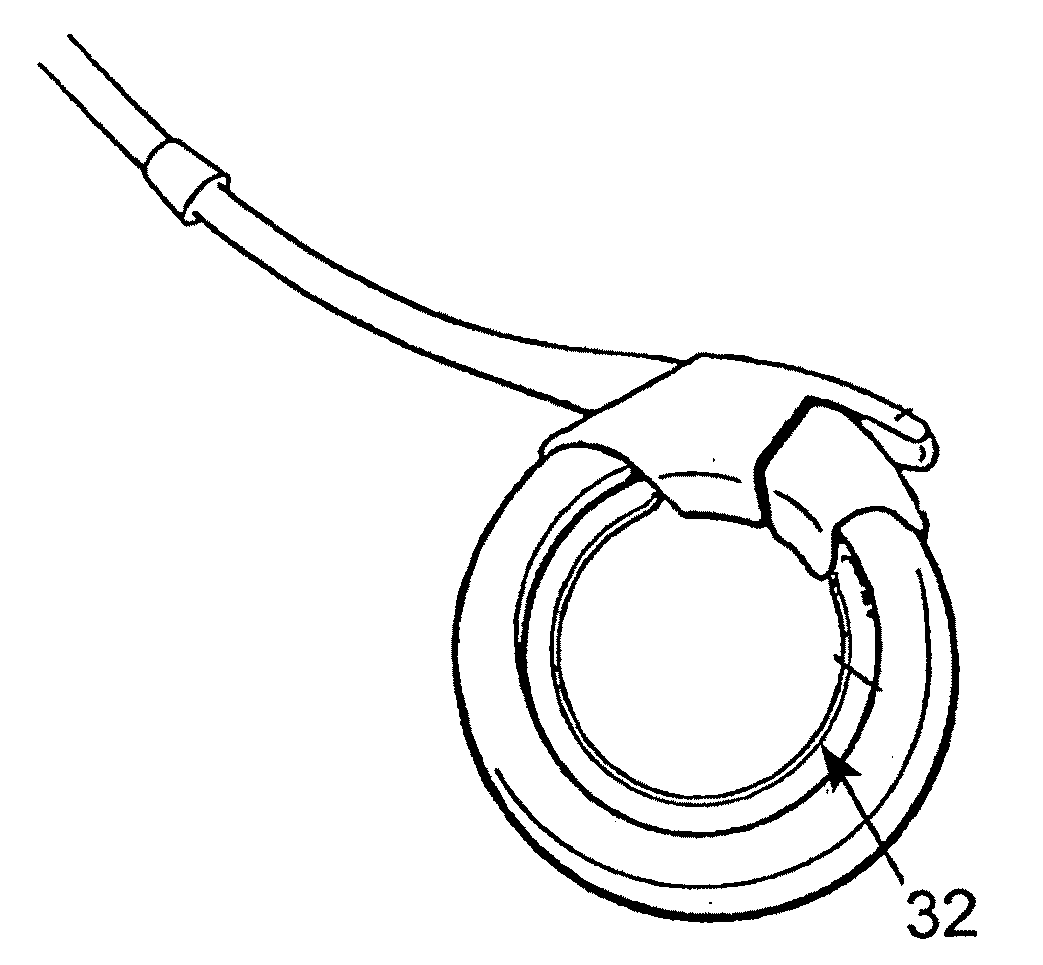
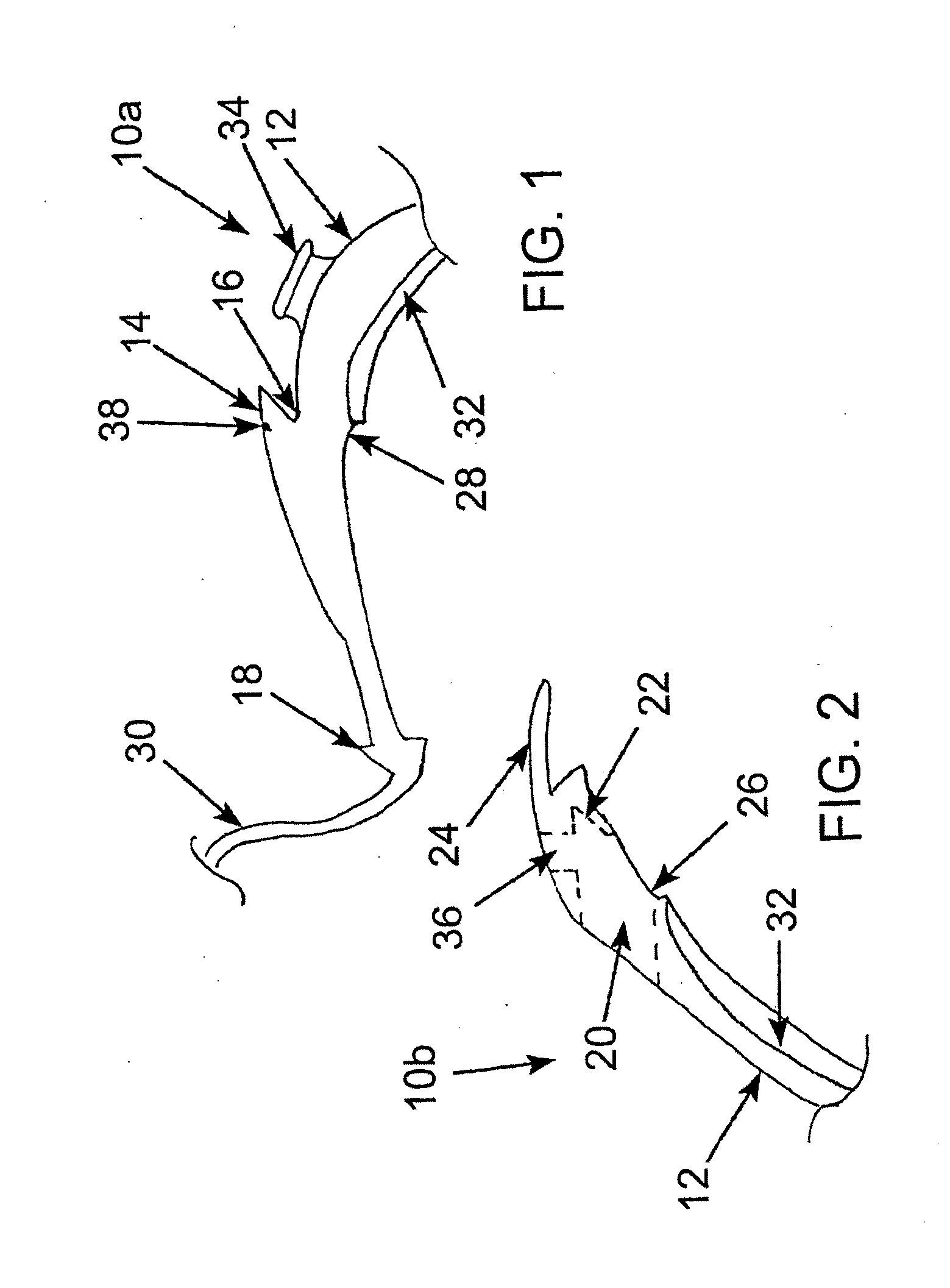
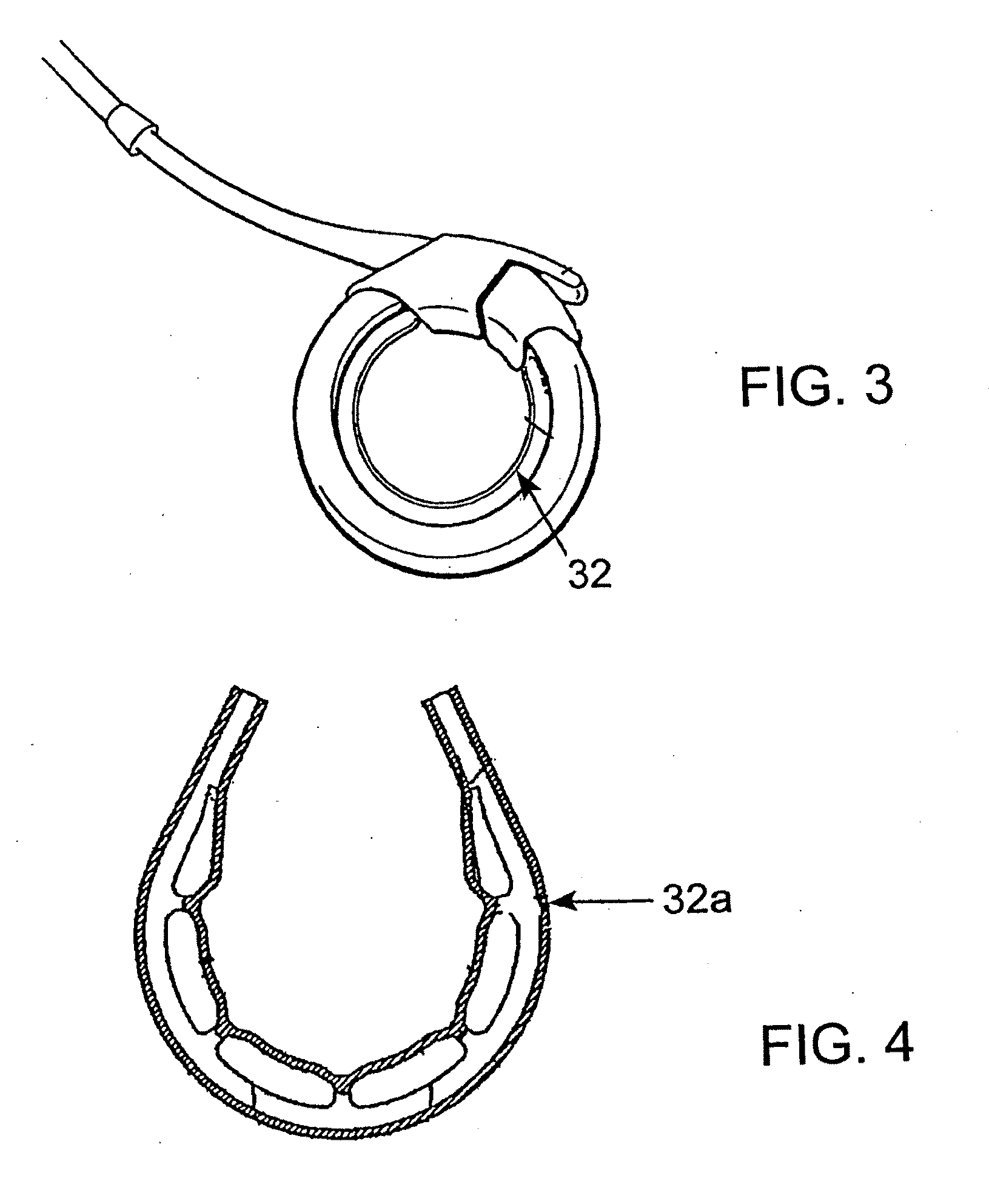
A releasably-securable gastric band (12) having a tail end (10a) and a head end (10b) for receiving the tail end (10a) is disclosed. The gastric band (12) also includes a releasable locking means (20) that releasably secures the head (10b) and tail ends (10a) together. The tail end (10a) may include a tooth (14) and the head end (10b) may include a notch (22) for engaging the tooth (14). Upon insertion of the tail end (10a) into the head end (10b), the tooth (14) mates with the notch (22) and releasably locks the tail end (10a) in the head end (10b). The releasably-securable gastric band (12) includes a release tab (24). When force is applied to the release tab (24) in a direction perpendicular to a central axis of the gastric band (12), the tooth (14) is disengaged from the notch (22) to allow the gastric band (12) to be released.
Owner:APOLLO ENDOSURGERY INC
Magnetic resonance imaging (MRI) safe remotely adjustable artificial sphincter
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band. Materials are nonferrous and nonmagnetic so as to be magnetic resonance imaging (MRI) safe, being substantially immune to strong magnetic fields and not introducing an electromagnetic interference / compatibility (EMIC) hazard.
Owner:ETHICON ENDO SURGERY INC
Thermodynamically driven reversible infuser pump for use as a remotely controlled gastric band
InactiveUS20050277974A1Avoid inconvenienceSmallAnti-incontinence devicesFlexible member pumpsEngineeringPiezo electric
An implantable artificial sphincter system provides long-term adjustment via transcutaneous energy transfer (TET), minimizing invasive adjustment through adding or removing fluid via a syringe. An infuser device provides bi-directional fluid transfer via a flexible conduit to a sphincter band, such as a gastric band, by a combination of thermodynamic actuation and a piezo-electrically disengaged drum brake assembly that thereby achieves a desirable small volume device. A propellant within a propellant cavity surrounds a metal bellows accumulator biased at body temperature to either expand or collapse the bellows accumulator with the opposite direction of movement effected by a thermal element that heats in combination with a negatively-biased propellant or cools in combination with a positively-biased propellant. A drum brake assembly locks the metal bellows accumulator in place between adjustments by thermodynamic actuation by activating piezo-electric stack actuators that disengage calipers from a brake drum attached to the bellows accumulator.
Owner:ETHICON ENDO SURGERY INC
Adaptive device and adaptive method for automatically adapting the stomach opening of a patient
ActiveUS20090062826A1Low costShifting is not possibleDiagnosticsTubular organ implantsGastric bandSelf adaptive
In an adaptive device and an adaptive method for adapting the stomach opening of a patient, a gastric band having a non-elastic back part on the exterior and a first expandable chamber on the interior are placed around the stomach of the patient for adapting the stomach opening, and the stomach opening of the patient is adapted by modifying the amount of fluid in the first expandable chamber. For this purpose, the adaptive device includes a second expandable chamber, so that the second expandable chamber is connected to the first expandable chamber, and whereby the fluid is displaced from the one expandable chamber to the other expandable chamber in order to modify the stomach opening of the patient. The adaptive device can in particular be controlled by changing the position of the body of the patient.
Owner:STEFFEN RUDOLF
Gastric band composed of different hardness materials
A gastric band has a balloon shaped and dimensioned to circumscribe the stomach at a predetermined location and a belt extending about the balloon. Further, a linking layer is positioned between the balloon and the belt, wherein the linking layer is harder than either the balloon or the belt. Additionally, the belt includes a first latching member at a first end of the gastric band and second latching member at a second end of the gastric band, the first latching member and the second latching member being shaped and dimensioned for selective engagement to secure the gastric band about a stomach of a patient, wherein the first latching member and second latching member are composed of different materials.
Owner:ETHICON ENDO SURGERY INC
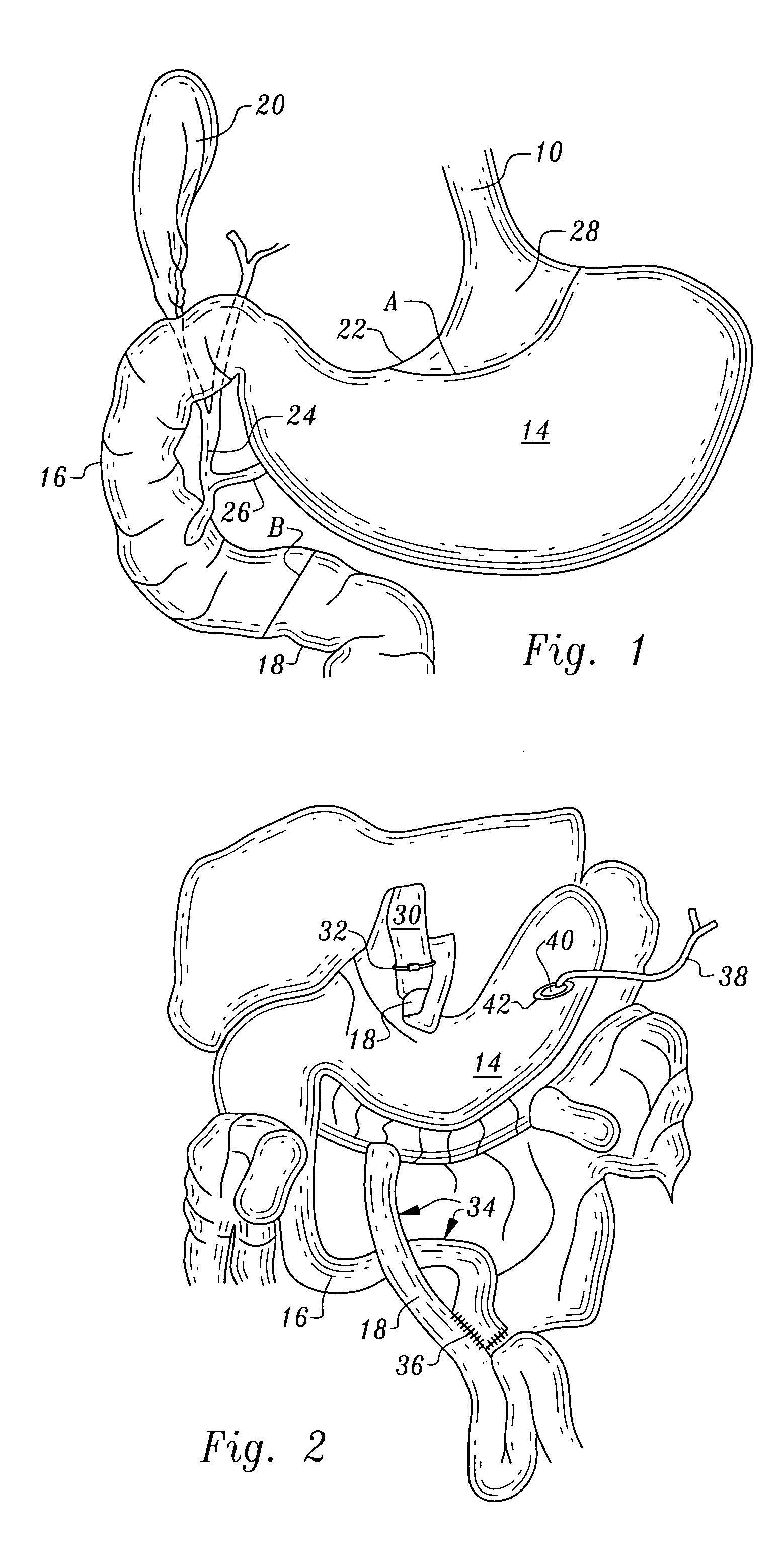
Gastric bypass band and surgical method
InactiveUS20050277963A1Maintain weight lossEasy to placeDiagnostic markersNon-surgical orthopedic devicesDuodenum lengthIngested food
An inventive method for performing gastric bypass surgery and a gastric bypass band that is used in conjunction with the surgical method is disclosed. The inventive method involves separating a top portion of the stomach along with the esophagus from the remainder of the stomach, and re-connecting the separated portion to the small intestine to form a gastric pouch of about 20-30 cc in size. The inventive gastric band is placed midway along the gastric pouch to act as a restrictor valve to limit the amount of food passing through the valve. The band also retains food within the gastric pouch to give the patient a feeling of satiety. The band is comprised of an expansion-resistant section combined with a one-way latch mechanism. The latch has a curved orientation so that the band is formed into a radial profile when placed around the gastric pouch.
Owner:BARIATEC CORP
Magnetic resonance imaging (MRI) safe remotely adjustable artifical sphincter
Owner:ETHICON ENDO SURGERY INC
Electroactive polymer actuated gastric band
InactiveUS7766815B2Easy to openIncrease heightObesity treatmentProsthesisGastric bandElectroactive polymer actuators
Methods and devices are provided for remotely adjusting a size of a gastric band disposed around a patient's stomach. In one exemplary embodiment, a gastric band is provided having a first end and a second end that mate to one another to encircle a stomach. A latch mechanism can be formed on the band, and at least one actuator can be coupled to the latch mechanism and it can be adapted to expand and contract the latch mechanism when energy is applied thereto to adjust a diameter of the band. In one exemplary embodiment, the actuator(s) is an electroactive polymer actuator.
Owner:ETHICON ENDO SURGERY INC
Hydraulic gastric band with collapsible reservoir
A self-regulating gastric band apparatus for adjusting stoma size. The apparatus includes an adjustable gastric band that has an inner ring expanding with injected fluid. A band adjustment assembly is provided for implanting with the gastric band that includes a sensor for sensing fluid pressure in the inner ring. The band adjustment assembly further includes a pump assembly connected to the expandable inner ring and to a controller that can operate the pump assembly to adjust the volume of the fluid in the band based on the sensed fluid pressure. The band adjustment assembly includes memory storing an operating range relative to a target fluid pressure, and the pump assembly is operated to maintain the sensed band pressure within the operating range. The target pressure being set to maintain pressure variations below a predefined variation limit generally corresponding with satiated fill volumes for a particular patient and implanted band. An elongated fluid reservoir may extend along a substantial part of a fill tube. A balloon-like expandable fluid reservoir in fluid communication with the pump assembly may store a volume of the fluid for adjusting the volume of fluid in the lumen. A protective outer sheath may be provided around the exterior of an expandable fluid reservoir in both a first, deflated state, and a second, inflated state of the reservoir.
Owner:APOLLO ENDOSURGERY INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com