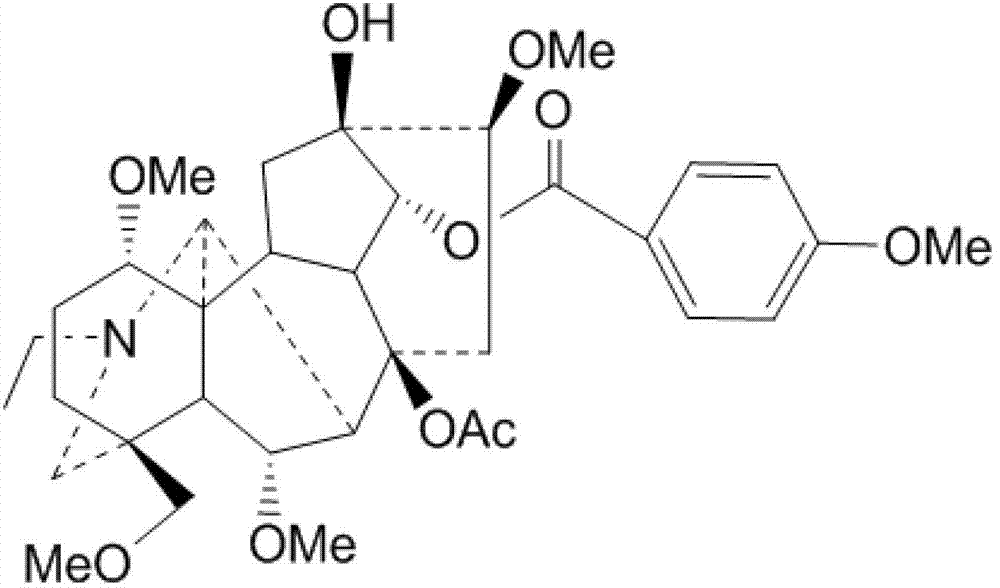
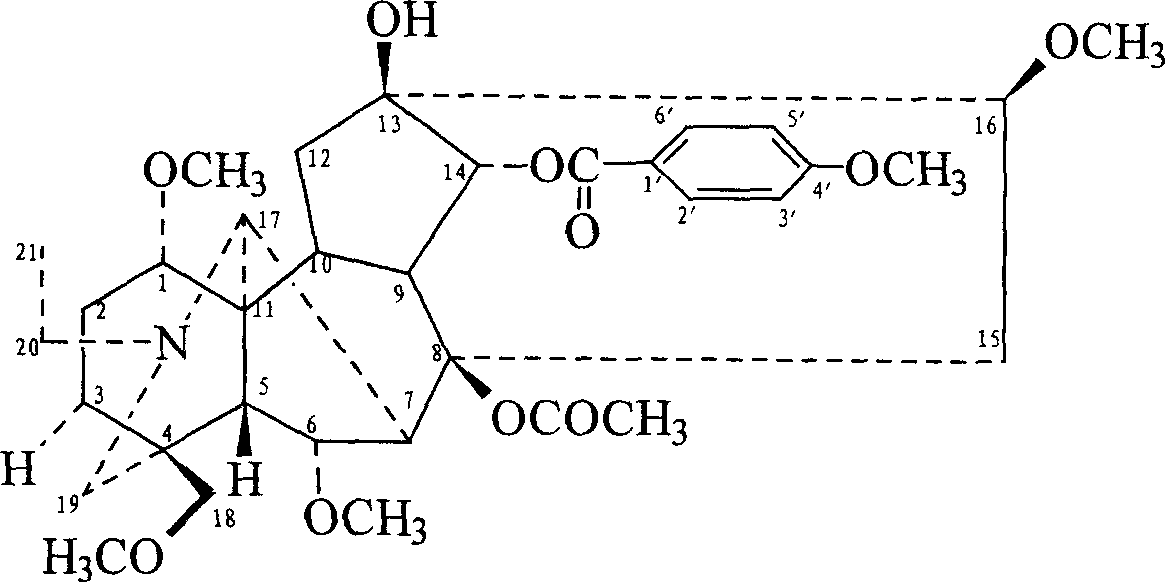
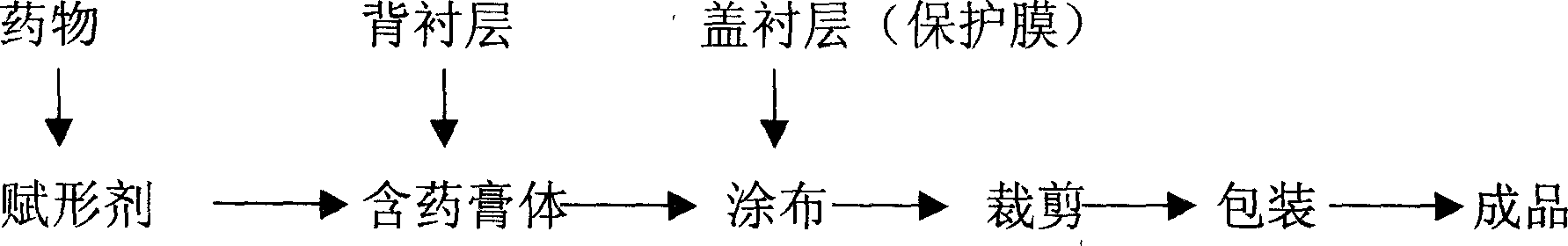
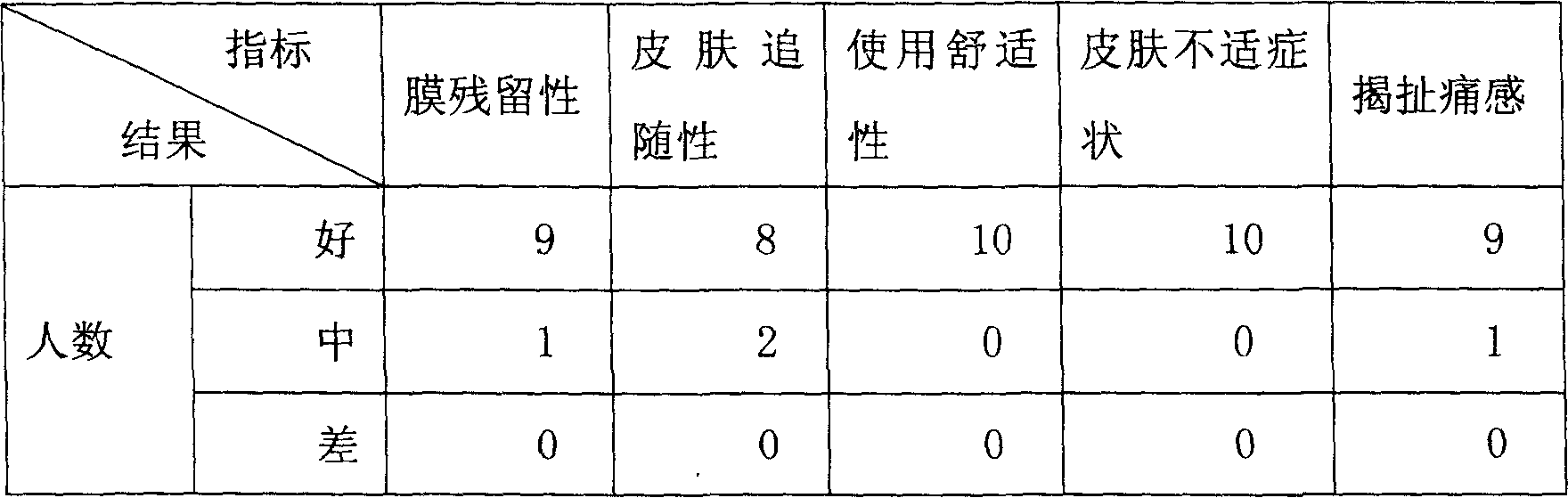
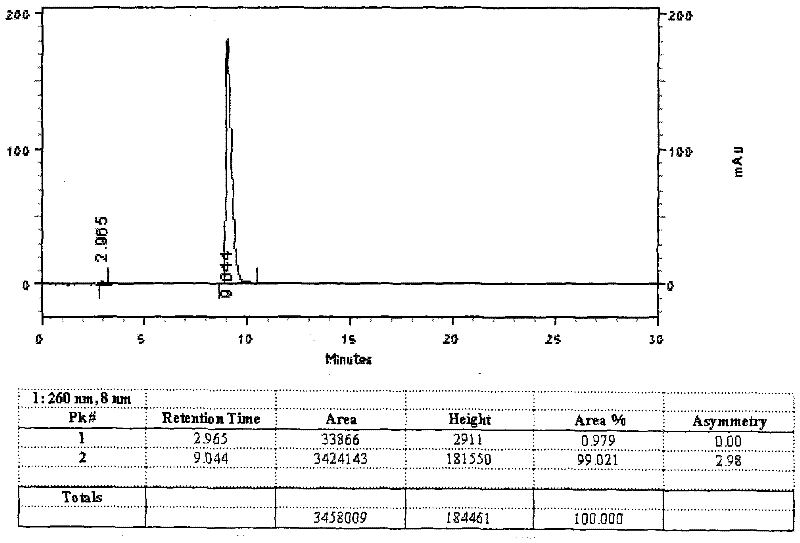
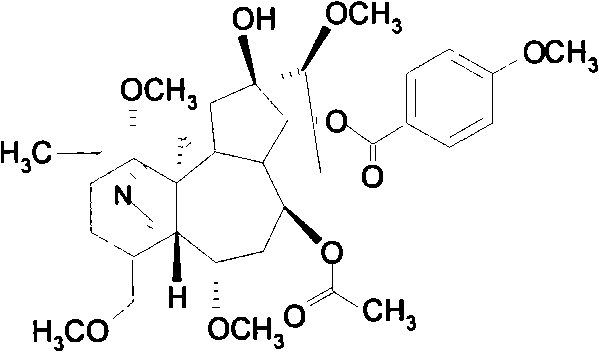
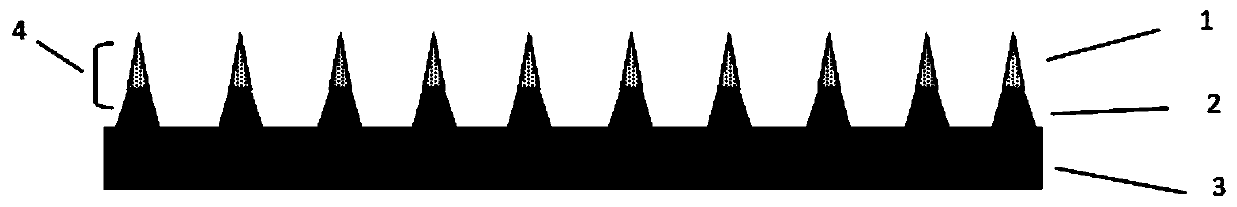
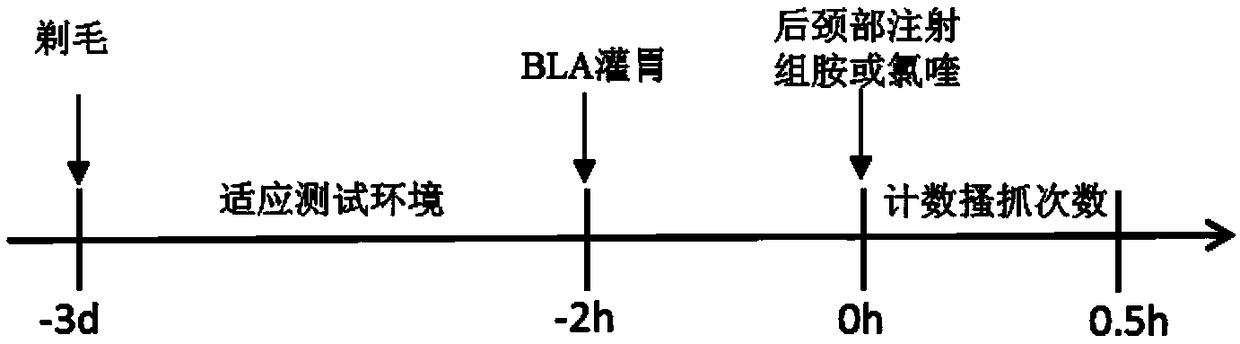
Patents
Literature
46 results about "Bulleyaconitine-A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
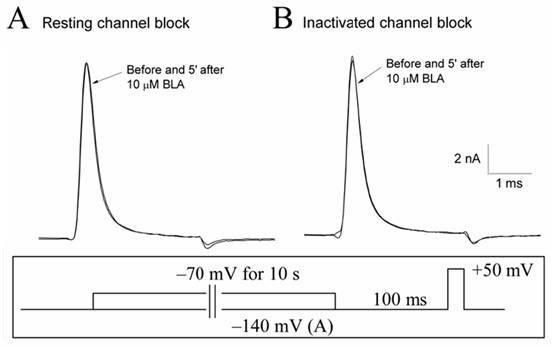
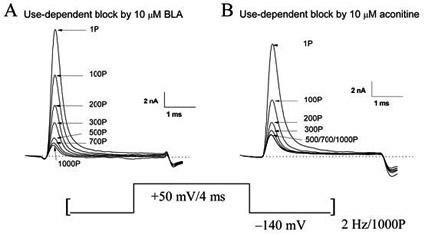
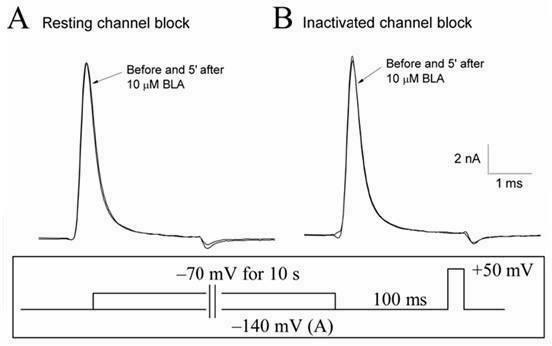
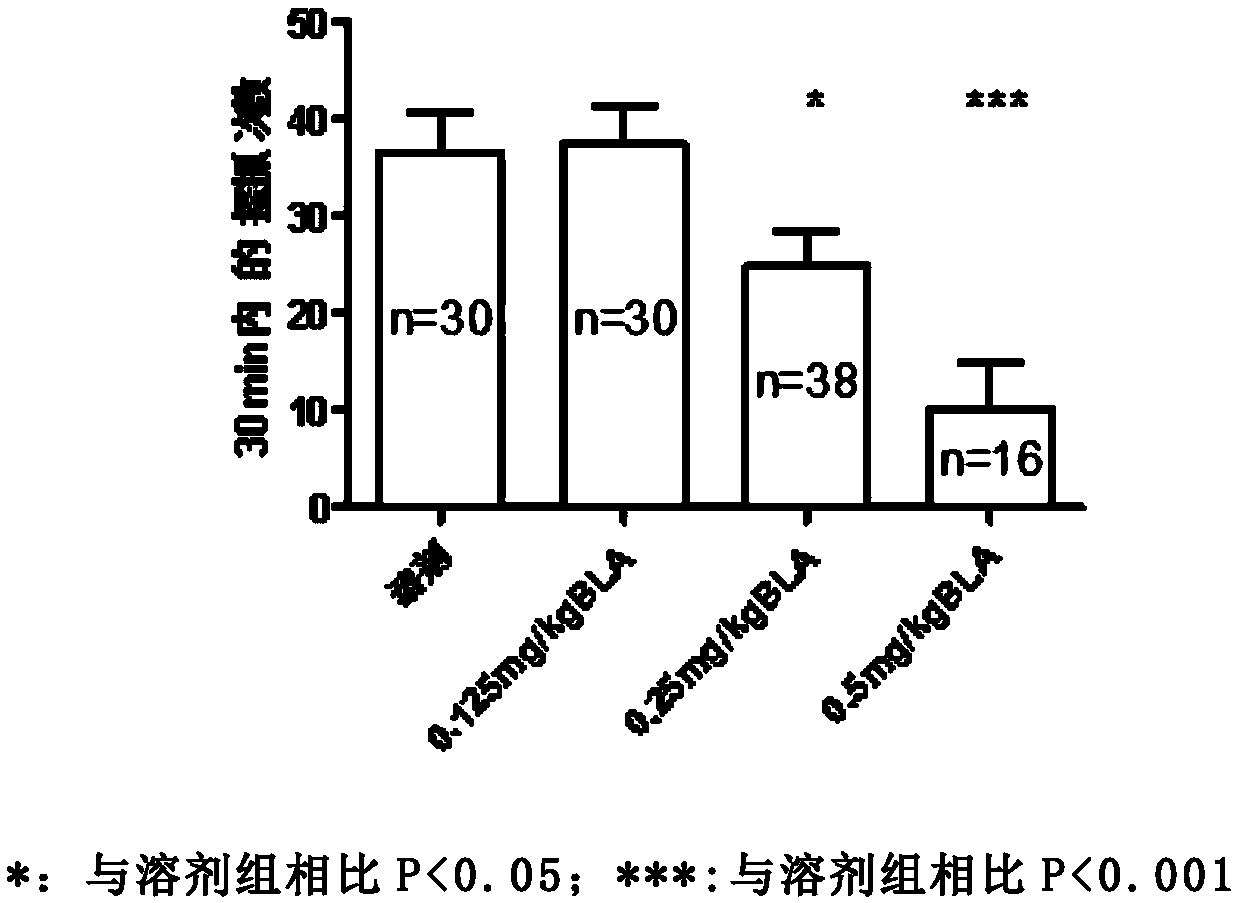
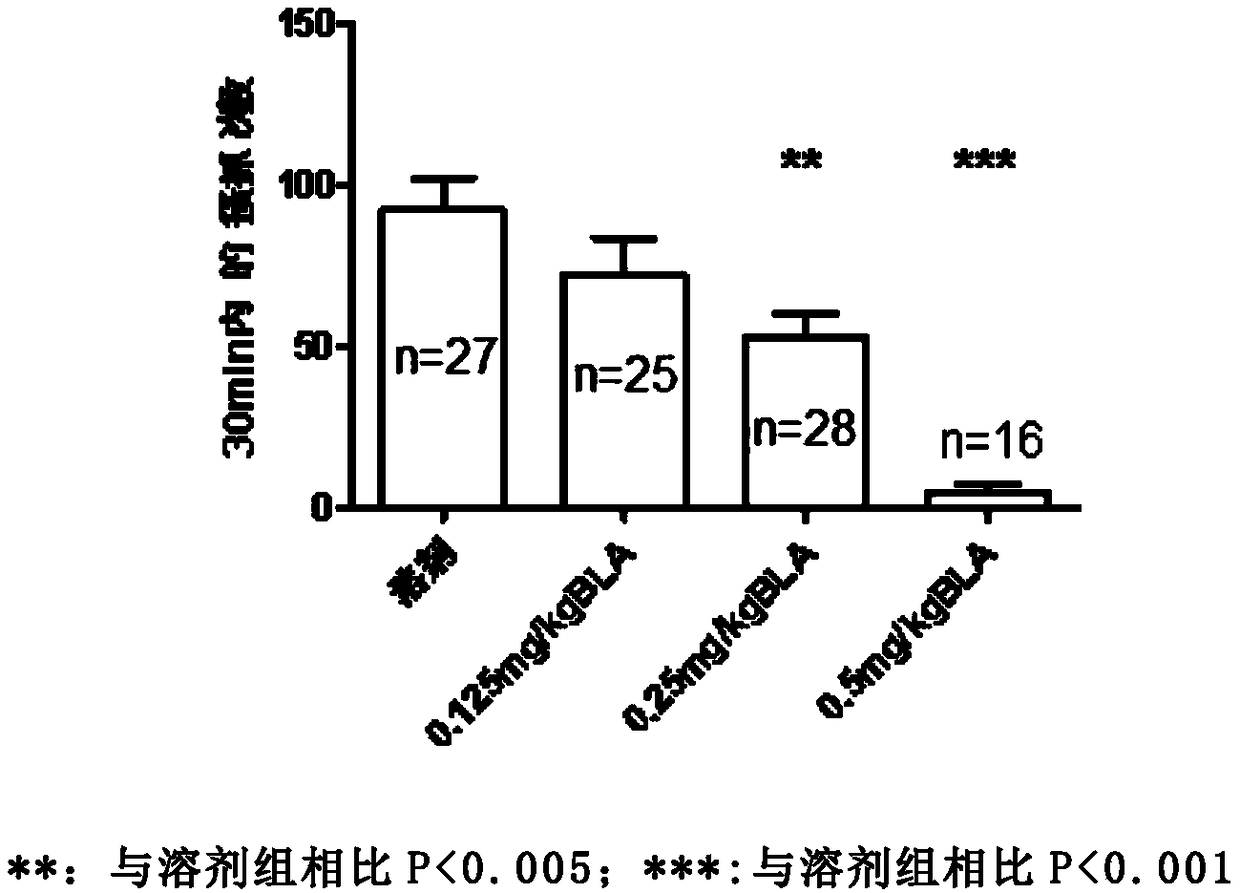
Bulleyaconitine A (BLA) is an analgesic and antiinflammatory drug isolated from Aconitum plants. BLA has several potential targets, including voltage-gated Na + channels. We tested whether BLA elicited long-lasting cutaneous analgesia, when co-injected with lidocaine and epinephrine, as a model for prolonged infiltration anesthesia.
Method for preparing simplified high-purity bulleyaconitine A
InactiveCN101830849AReduce consumptionShorten the production cycleOrganic chemistryWater bathsFiltration
The invention relates to a method for preparing simplified high-purity bulleyaconitine A, which comprises the following steps of: immersing tuberous root of kusnezoff monkshood root in 1 to 2 percent alkaline solution; crushing the tuberous root; performing heat reflux extraction with gasoline or petroleum ether 6 and the like; loading the extract; eluting the tuberous root with solvent gasoline, ethyl acetate and triethylamine or diethylamine in a volume ratio of 90-95 to 1-10 to 1-5; concentrating the eluent under reduced pressure till the eluent is dry; then thermally dissolving the eluent with the solvent gasoline; standing the obtained solution for 20 to 24 hours at normal temperature; crystallizing, filtering and drying the solution to obtain the crude product of the bulleyaconitine A; fully dissolving the crude product in methanol on a water bath of 45 to 55 DEG C; filtering the obtained solution and preserving the heat for 30 minutes on the water bath of 45 to 55 DEG C; adding pure water or distilled water dropwise to assist in the crystallization; standing the solution for 20 to 24 hours at normal temperature to form a crystal; performing filtration to remove the liquid part; washing the crystal for three times; and pumping the crystal and drying the crystal to obtain the finished product. The process has the advantages of simplified production flow, low cost, no three-waste emission, simple operation, high production safety and high extraction rate of the bulleyaconitine A.
Owner:云南珏草生物科技有限公司
Bulleyaconitine A efficient extraction and separation method
A bulleyaconitine A efficient extraction and separation method comprises pretreatment, extraction, concentration, acidification, basification, extraction, column chromatography and crystallization steps. According to the method, 75% acid ethanol solution containing 0.3% of sulfuric acid is used for extraction for three times, concentrated ammonia water is slowly added under stirring during alkalization to adjust pH to 8, loss of bulleyaconitine A is reduced; during column chromatography on silica gel, the flow rate of the column chromatography is reasonably and effectively controlled at 13-20ml / min, the separation effect is better; ethyl acetate is used for extraction, the solvent residue is zero, the bulleyaconitine A highest content can reach 98-99%, the product purity is high, and the rate extraction of the bulleyaconitine A of a radix aconiti kusnezoffii medicinal material can be increased to 0.15 to 0.22 %. The method uses non-toxic or low-toxic organic solvents and reduces organic solvent kinds and usage amount, saves energy, reduces conservation, and reduces personnel hazards and environmental pollution.
Owner:云南大围山生物制药有限公司
Method for preparing high-purity bulleyaconitine A
The invention discloses a method for preparing high-purity bulleyaconitine A. The method comprises the following steps of: soaking a fresh radix aconiti agrestis root in acid aqueous solution, performing homogenate extraction and cavitation mixing solid-liquid extraction, collecting and concentrating the filtrate, and respectively performing gradient elution to obtain a crude bulleyaconitine A product through ion exchange resin column chromatography and macroporous adsorption resin column chromatography; and uniformly mixing crude bulleyaconitine A product and ethanol aqueous solution, dissolving in a water bath, cooling the filtrate to room temperature, standing and crystallizing at the temperature of 4 DEG C, repeatedly washing the crystal by using the ethanol aqueous solution, and obtaining a final product. According to the method, crushing and extracting are finished in one step through a homogenization method, and the method has the advantages of zero dust and simple flow. By adoption of a negative-pressure cavitation extraction technology, the mass transfer rate is improved, heating is not required, and the production period is shortened; and moreover, the reagents used in the whole process are ethanol and water, the organic solvent amount is greatly reduced, the production cost can be reduced, the environmental pollution is reduced, and the method has good application prospect.
Owner:INST OF MEDICINAL PLANTS YUNNAN ACAD OF AGRI SCI
Bulleyaconitine A preparation method
InactiveCN106008344AReduce manufacturing costImprove production efficiencyOrganic chemistrySilica gelNatural medicine
The invention relates to the natural medicine preparation field, and in particular, relates to a bulleyaconitine A preparation method; the preparation method comprises four steps: extraction, extraction separation, column chromatography separation purification, and crystallization purification. According to the bulleyaconitine A preparation method provided by the invention, alumina column chromatography is used for replacing silica gel column chromatography, so that the production cost is saved; a petroleum ether-diethylamine binary system is used as an eluent and is easy to recycle and reuse, so as to reduce environmental pollution.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
Preparation method for bulleyaconitine A
A preparation method for high-purity bulleyaconitine A comprises the steps of extraction, extraction separation, column chromatography separation and purification, crystallization and purification. The content of the bulleyaconitine A in the product detected by a high performance liquid chromatography (HPLC) is greater than 98 percent. According to the preparation method, the extraction is realized at room temperature and the bulleyaconitine A is free from hydrolysis; a silica gel column chromatography eluent only uses a binary system, a solvent is recycled more easily and the adjustment on the polarity trends to be consistent more easily, and thus the production cost is greatly reduced. The preparation method is simple, easy for industrial production and high in streamline degree; and the purity of products is high.
Owner:YUNNAN AGRICULTURAL UNIVERSITY
Cataplasma of bulleyaconitine A
InactiveCN1965814AGood biocompatibilityHigh affinityHydroxy compound active ingredientsAntipyreticIrritationMedicine
The invention relates to an external agent-bab agent of wild aconite root element, wherein it is formed by wild aconite root element and some findings. And it comprises liner layer, drug paste and cover lliner layer. The drug paste comprises wild aconite root element, adhesive, stuff, accelerator, and humectant, while each unit comprises 0.5-30mg wild aconite root element. The invention comprises quick effect, better humidity property and ventilation. It has little excitation on skin.
Owner:KPC PHARM INC
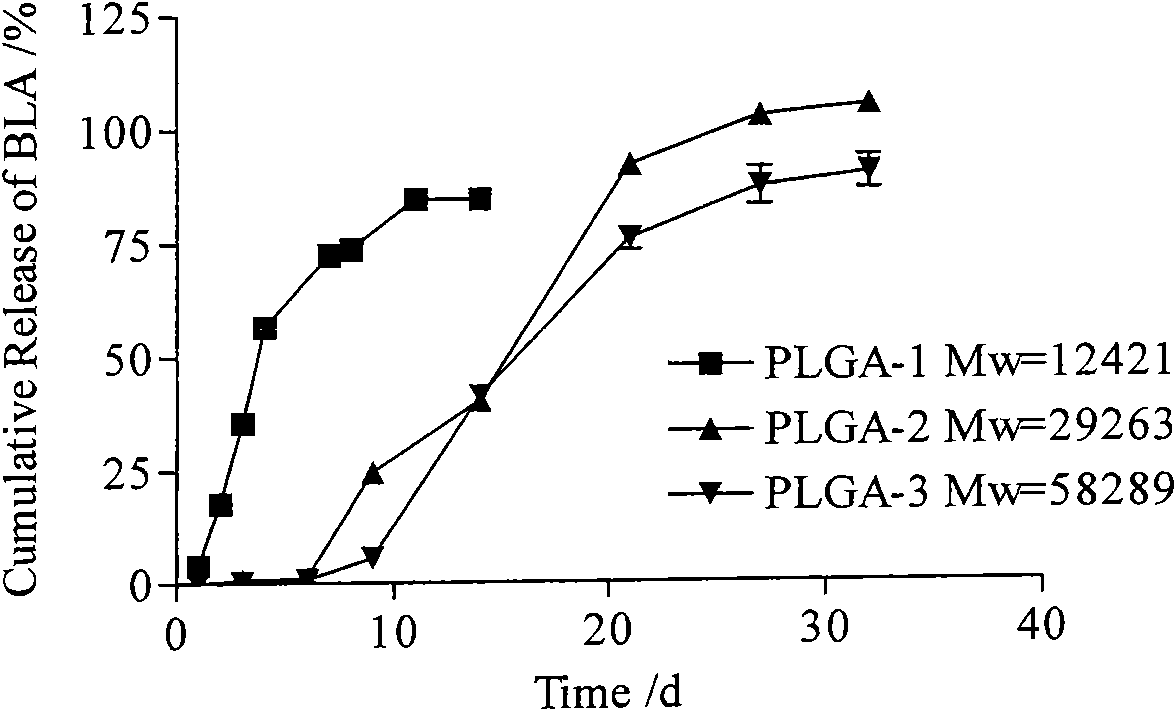
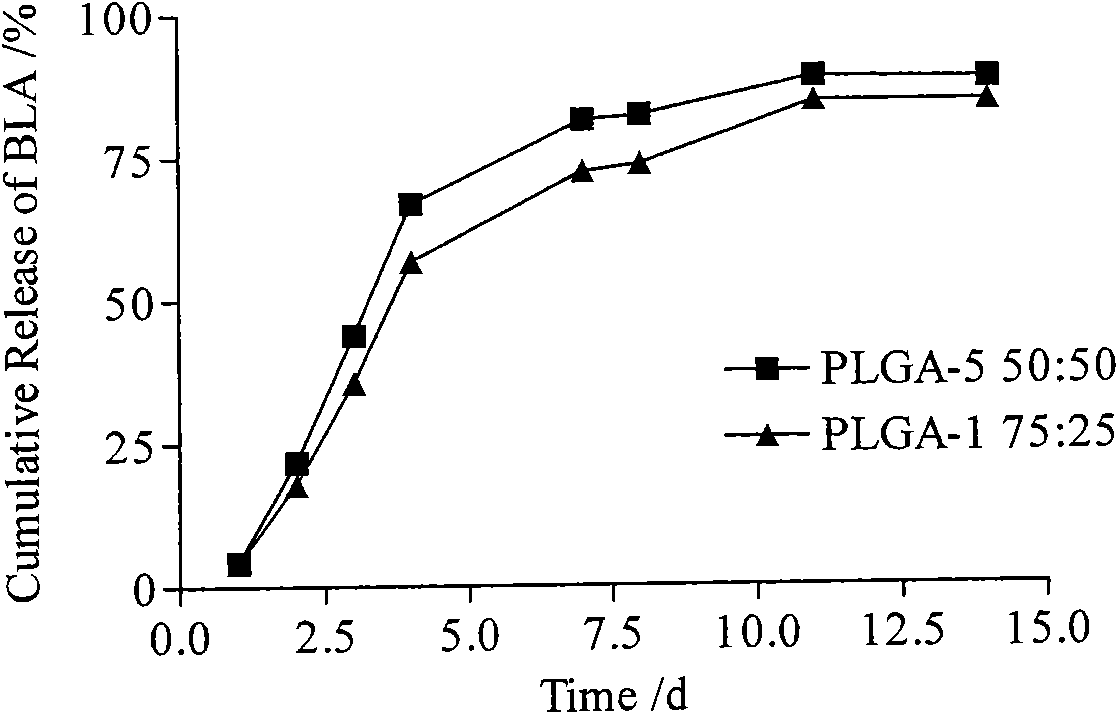
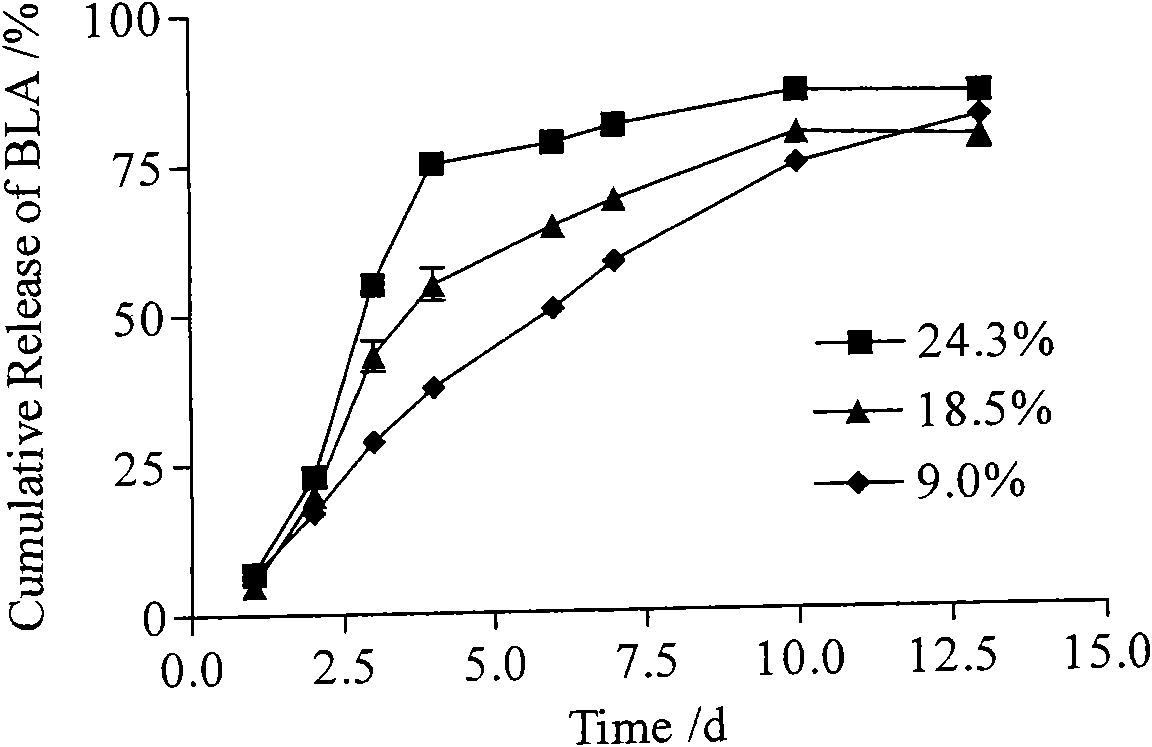
Bulleyaconitine A micro-balloons and bulleyaconitine A long-acting injection and preparation method and application of same
InactiveCN101554364AStable blood concentrationReduce the number of dosesOrganic active ingredientsPowder deliveryBlood concentrationMicrosphere
The invention provides a bulleyaconitine A micro-balloons, bulleyaconitine A long acting injection and a preparation method and application of the same. The micro-balloons contain therapeutically effective dosage of bulleyaconitine A and biodegradable polymer. The bulleyaconitine A long-acting injection has good safety, convenient use, long drug action time and high obedience of patient, can achieve the aim of reducing administration time, prolonging the drug action time, reducing blood concentration fluctuation and increasing the administration obedience of the patient, can achieve a week drug action by administration once and has higher clinical application value.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
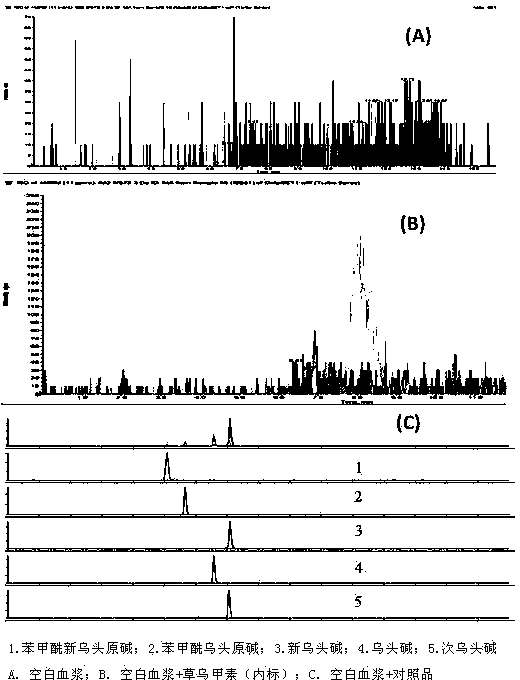
Method for simultaneously determining 5 alkaloids in Sini agent vegetable drug plasma
InactiveCN103512989ATo clarify the law of pharmacokineticsComponent separationElectro sprayEthyl acetate
The invention relates to a method for simultaneously determining 5 alkaloids in Sini agent vegetable drug plasma, which comprises the following steps: (1) taking medicated plasma of a mammal given Sini agent vegetable drugs, adding bulleyaconitine A (interior label), alkalifying with ammonia water, scrolling, adding ethyl acetate for extraction, scrolling, centrifugating, extracting the subnatant with ethyl acetate twice, merging the ethyl acetate layers, blow-drying with nitrogen, and dissolving the residue with a mobile phase; and (2) HPLC-MS-MS (high performance liquid chromatograph-mass spectrometer-mass spectrometer) determination: the mobile phases comprise a phase A acetonitrile-0.1% glacial acetic acid (1:99, v / v) and a phase B acetonitrile-water (99:1, v / v); the mass spectrometer conditions are as follows: electric spray ion source (ESI) and cation detection mode; and the detection ions are as follows: aconitine m / z 646.3->586.3, mesaconine m / z 632.3->572.3, hypaconitine m / z 616.3->556.3, benzoylmesaconine m / z 590.3->558.3, and benzoylaconine m / z 604.3->554.3. The method is suitable for pharmacokinetic research of aconitine, hypaconitine, mesaconine, benzoylmesaconine and benzoylaconine in Sini agent vegetable drugs in a mammal.
Owner:SHANGHAI ZHANGJIANG ENG RES CENT OF MODERN PREPARATION TECH OF TRADITIONAL CHINESE MEDICINE
Application of bulleyaconitine A used as state-independent sodium ion channel blocker to analgesia
InactiveCN102552254AInhibition of Ca2+ influxTreating Neuropathic PainOrganic active ingredientsNervous disorderSide effectBULK ACTIVE INGREDIENT
The invention discloses application of bulleyaconitine A (BLA) used as a state-independent sodium ion channel blocker to the analgesia, and relates to application of a medicine, in particular to application of aconitine alkaloids to the analgesia. The application of the bulleyaconitine A used as the state-independent sodium ion channel blocker to the analgesia is characterized in that the BLA only blocks a neuron sodium ion channel in an open state in a state dependence way, and does not interact with a neuron sodium ion channel in a resting state or an inactivation state. Compared with other analgesic medicines, the medicine in which the bulleyaconitine A is taken as an active ingredient has the advantage that the analgesic side effect is relatively small.
Owner:YUNNAN HAOPY PHARM LTD
Fat emulsion injection of bulleyaconitine A and its manufacturing method
InactiveCN1602865AIndicator qualifiedMeet the requirements for injectionOrganic active ingredientsEmulsion deliveryWhite rabbitEmulsion
The invention provides a bulleyaconitine fat emulsion transfusion and its producing method, including effective component, medicinal auxiliary and emulsifier, and its characteristic: it uses bulleyaconitine as the effective component, contains 0.1-0.8mg of bulleyaconitine per bottle, and mixes in a proper amount of medicinal auxiliary and emulsifier to prepare the medicinally acceptable fat emulsion. At normal temperature, the emulsion can be placed for 1 year, stable and reliable, and through stimulative test on big white rabbits, the stimulative property of the emulsion is grade 0-1 and has better antiphlogistic and analgesic effect and energy supplementing effect and simultaneously, quickly takes effect and has good compliance of patient.
Owner:KUNMING ZIJIAN BIOTECH
Application of bulleyaconitine A in preparing medicament for treating ache related to sodium channel
InactiveCN101480392ANo toleranceNon-addictiveOrganic active ingredientsNervous disorderDiseaseAdditive ingredient
The invention relates to an application of bulleyaconitine A used for preparing medicine for treating pain related to a Nachannel, belonging to medicine containing effective organic ingredients, in particular to medicine using the bulleyaconitine A for treating a disease. The medicine contains excipient which plays roles in padding, bond, disintegration and lubrication; the bulleyaconitine A and the excipient are mixed to be pressed into tablets; and each tablet of bulleyaconitine A contains 0.1mg to 0.5mg of the bulleyaconitine A. The medicine plays obvious roles in resisting pain and inflammation and improving rationalization indexes for wide sodion pain, such as rheumathritis, osteoarthritis, scapulohumeral periarthritis, lumbodorsal fascia inflammation, strain of lumbar muscles, sprain of joints, closed fracture of limbs, sprain, toothache, and the like. The medicine can be rapidly absorbed by oral administration, eliminates the half-life for about 5 hours, and has an excretion pathway by two channels and little toxicity. Compared with opium medicine, the medicine does not generate a tolerant phenomenon in the course of resisting pain, causes no addiction and is convenient for treatment and use as an oral administration preparation.
Owner:YUNNAN HAOPY PHARM LTD
Preparation method of high purity bulleyaconitine A
ActiveCN101555227BHigh purityReduce manufacturing costNervous disorderOrganic chemistrySolvent vaporSilica gel
The invention relates to a preparation method of high purity bulleyaconitine A. The preparation method comprises the following steps: root of radix aconiti feri of Yunnan is crushed, and acid alcoholic solvent or alcohol water solution is used for diacolation, and supersound or thermal refluxing extraction. The extracting solution is decompressed and concentrated to be non-alcoholic at the temperature of 80 to 90 DEG C, cooled down to room temperature, to ensure the relative density to be 1.00 to 1.30; standing is performed for 10 to 20 hours; the extracting solution is filtered; the pH is adjusted to 6.0 to 8.0 with basifier; and then the extracting solution is extracted with ethyl acetate or chloroform. The ethyl acetate or chloroform liquid is contracted, column chromatography is performed to the obtained paste, silica gel or neutral alumina are used as fillers, any one of solvent vapor-acetone-diethylamine, ligarine-acetone-diethylamine or cyclohexane-acetone-diethylamine is used for eluation, the bulleyaconitine A in the collected liquid is constantly checked during the thin layer chromatography, the collected liquid containing the bulleyaconitine A is merged, the solvent is concentrated, carbinol or ethanol is dissolved, filtering is performed, crystallization is performed at room temperature, filtering is performed, the crystal is repeatedly rinsed with carbinol or ethanol, and the final product is obtained. The purity of the obtained product is high, the production cost is low, and the toxicity of the used organic solvent is low.
Owner:KPC PHARM INC
Bulleyaconitine A dissoluble microneedle patch and preparation method thereof
PendingCN111568887AEfficient transdermal drug deliveryImprove complianceOrganic active ingredientsNervous disorderVinyl etherPolyethylene glycol
The invention relates to the technical field of traditional Chinese medicine, and concretely discloses a Bulleyaconitine A dissoluble microneedle patch and a preparation method thereof. The Bulleyaconitine A dissoluble microneedle patch disclosed by the invention comprises needle bodies and a substrate layer, wherein each needle body comprises a support layer and a needle point medicine carrying layer on the support layer; the support layers are bonded on the substrate layer; the composition of medicine in the needle point medicine carrying layers includes high-molecular polymers, Bulleyaconitine A and ethanol; and the high-molecular polymers are one or more of polyvinylpyrrolidone, methyl vinyl ether-cis-butenedioic anhydride copolymers and polyethylene glycol. The microneedle patch disclosed by the invention has the advantages that the Bulleyaconitine A indissoluble in water can be made into a dissoluble microneedle patch by combining with a microneedle technology, the efficient transdermal administration of the Bulleyaconitine A is realized; the administration mode is minimally invasive administration; the compliance of a patient is good; and the autonomous administration can berealized.
Owner:YUNNAN INST OF MATERIA MEDICA
Application of bulleyaconitine A in preparing medicament for treating Nav1.7 ache disease
The invention relates to an application of bulleyaconitine A used for preparing medicine for treating Nav1.7 pain, which belongs to medicine containing effective organic ingredients, in particular to medicine using the bulleyaconitine A for treating a disease. The medicine contains excipient which plays roles in padding, bond, disintegration and lubrication, the bulleyaconitine A and the excipient are mixed to be pressed into tablets, and each tablet of bulleyaconitine A contains 0.1mg to 0.5mg of the bulleyaconitine A. The invention has the characteristics of rapid absorption by oral administration, low content in the brain for the medicine distributed in a body, long half life period, little toxicity, and no tolerant phenomenon or addiction.
Owner:YUNNAN HAOPY PHARM LTD
Application of bulleyaconitine A
ActiveCN109453169ASuppress itchingAvoid situations where itching recursNervous disorderInorganic non-active ingredientsLiver and kidneyScratching
The invention relates to the field of medicines, and particularly relates to application of bulleyaconitine A. Bulleyaconitine A is applied to treating pruritus cutanea, and the situation that drugs such as antihistamines and hormones can produce unwelcome reactions so that the drugs cannot be applied for a long time and can only be applied intermittently, and thus pruritus recurs is avoided. Bulleyaconitine A has the positive effects when being applied to treating pruritus cutanea, has no liver and kidney toxicity side effects, can be applied for a long time, can control pruritus cutanea notto recur, and can avoid pachulosis and lichenification caused by unconscious scratching and secondary lesion such as eczema, neurodermatitis and prurigo nodularis. Bulleyaconitine A is applied to treating pruritus cutanea, solves the problem that a patient is troubled a lot by pruritus so that sleeping and resting of the patient are affected seriously because pruritus recurs at night, and effectively relieves the pain and mental stress of the patient and the family brought by pruritus.
Owner:YUNNAN HAOPY PHARM LTD
Method for preparing simplified high-purity bulleyaconitine A
The invention relates to a method for preparing simplified high-purity bulleyaconitine A, which comprises the following steps of: immersing tuberous root of kusnezoff monkshood root in 1 to 2 percent alkaline solution; crushing the tuberous root; performing heat reflux extraction with gasoline or petroleum ether 6 and the like; loading the extract; eluting the tuberous root with solvent gasoline, ethyl acetate and triethylamine or diethylamine in a volume ratio of 90-95 to 1-10 to 1-5; concentrating the eluent under reduced pressure till the eluent is dry; then thermally dissolving the eluentwith the solvent gasoline; standing the obtained solution for 20 to 24 hours at normal temperature; crystallizing, filtering and drying the solution to obtain the crude product of the bulleyaconitine A; fully dissolving the crude product in methanol on a water bath of 45 to 55 DEG C; filtering the obtained solution and preserving the heat for 30 minutes on the water bath of 45 to 55 DEG C; adding pure water or distilled water dropwise to assist in the crystallization; standing the solution for 20 to 24 hours at normal temperature to form a crystal; performing filtration to remove the liquid part; washing the crystal for three times; and pumping the crystal and drying the crystal to obtain the finished product. The process has the advantages of simplified production flow, low cost, no three-waste emission, simple operation, high production safety and high extraction rate of the bulleyaconitine A.
Owner:云南珏草生物科技有限公司
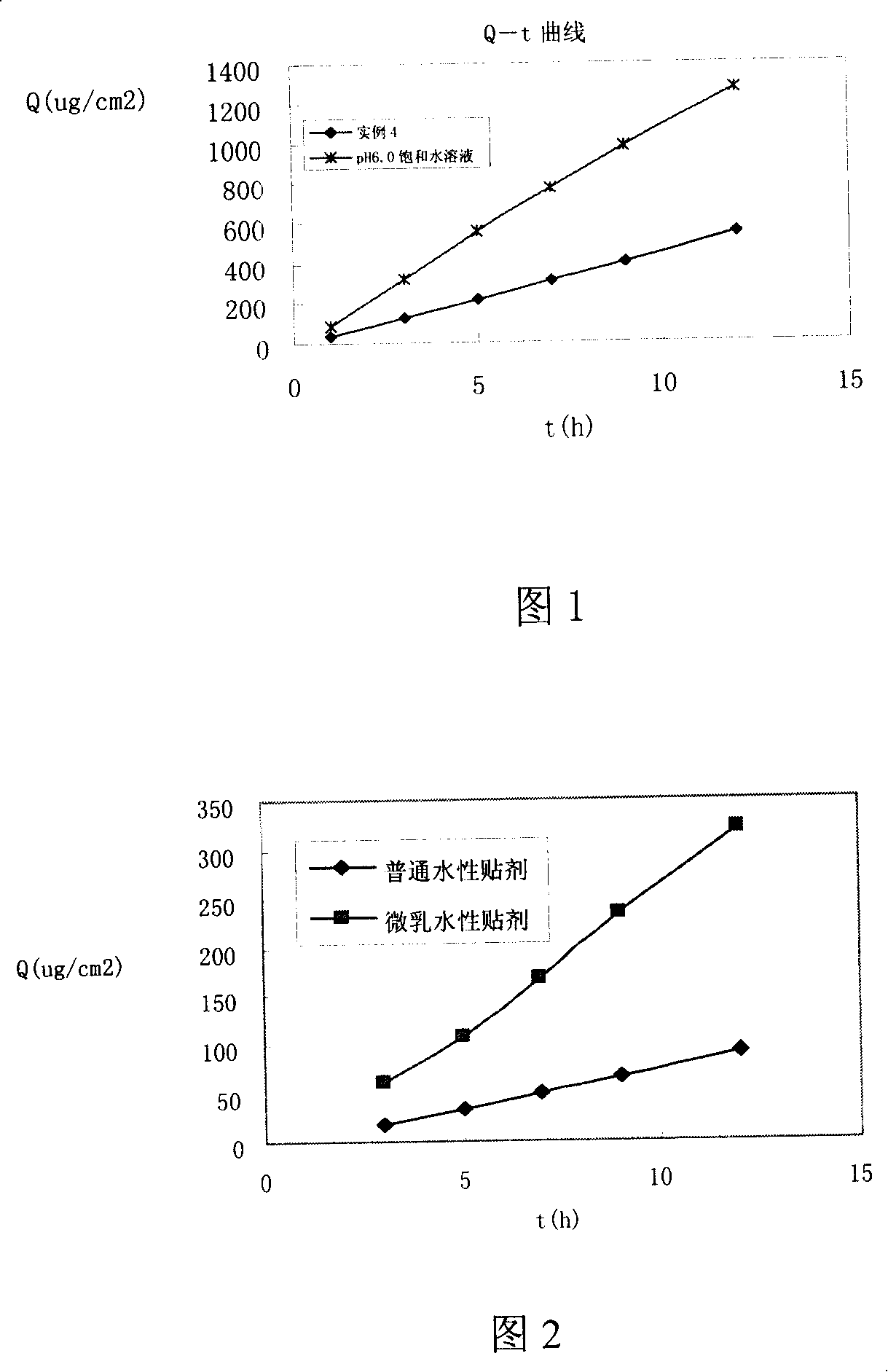
Kusnezoff monkshood root esculin microemulsion and its preparation
InactiveCN101152148AImprove skin penetration rateOrganic active ingredientsAntipyreticNasal cavityMonkshood Root
The present invention discloses a Bulleyaconitine A microemulsion and the preparing method. The Bulleyaconitine A is dissolved in oil phase. Emulsifier, assistant emulsifier and other additives are added. A microemulsion which is transparent or is with slightly turbid light and is with a particle size of less than 100 nm is prepared. Compared with material drug of Bulleyaconitine A, the chemical stability and mucosa permeance capability of the drug-containing microemulsion are significantly improved. The Bulleyaconitine A microemulsion is used as releasing vector and is combined with an excipient which is acceptable on pharmacy. An appropriate preparing method is used to prepare a nasal cavity preparation which is high in biological absorption degree, safe, stable and convenient and various paper release formulation preparation. The present invention is used for the treatment of various pains.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Medicament for treating osteoarticular diseases and preparation method of medicament
InactiveCN104586879AGuaranteed functionNormal functionOrganic active ingredientsAntipyreticDiseaseMetabolite
The invention relates to a medicament for treating osteoarticular diseases and a preparation method of the medicament. The medicament comprises the following components in percentage by weight (W / W): 1%-25% of glucosamine, 1%-25% of chondroitin sulfate and 0.01%-2% of an efficient non-addictive analgesic, wherein the efficient non-addictive analgesic is lappaconitine, bulleyaconitine A or aconine extracted from ranunculaceous aconitum plants. According to the medicament, the defect that an existing preparation cannot be used for relieving pain is overcome; by virtue of the efficient non-addictive analgesic, the nervous impulse transfer of pain is blocked, the activity of injured bone joints can be maintained, and meanwhile, power is provided for the replacement and the expelling of metabolite in cavities of bone joints, so that the metastasis is maintained, the injured bone joint surfaces are repaired, and the normal functions of the bone joints are maintained; by virtue of blood circulation in skeletal muscles, sufficient nutrients are supplied to the muscles, and the amyotrophy is avoided.
Owner:郭曙平
Bulleyaconitine A lipidosome freeze-dried powder injection and preparation method thereof
InactiveCN101579318AImprove bioavailabilityReduce insecurityOrganic active ingredientsPowder deliveryIrritationFreeze-drying
The invention provides a bulleyaconitine A lipidosome freeze-dried powder injection and a preparation method thereof. The injection mainly comprises the following compositions by weight ratio: bulleyaconitine A:phospholipid:cholesterol:freeze-dry protecting agent=1:1-60:1-10:1-360. The prepared freeze-dried powder injection with encapsulation rate of between 75 and 90 percent not only can obviously improve storage stability, but also can lead the bulleyaconitine A lipidosome freeze-dried powder to be re-dissolved after injection water is added. The prepared bulleyaconitine A lipidosome freeze-dried powder injection has light or non irritation in clinic application compared with the prior bulleyaconitine A injection, and has slow release property compared with common preparation.
Owner:KPC PHARM INC
Bulleyaconitine A liniment, preparation method, and applications thereof
InactiveCN106551897AReduce systemic side effectsLong-lasting effectOrganic active ingredientsNervous disorderBulleyaconitine-ADrug
The invention discloses a bulleyaconitine A liniment, a preparation method, and an applications thereof. The bulleyaconitine A liniment is composed of, by mass, 0.1-1.0% of bulleyaconitine A, 4-25% of propylene glycol, 0.2-2.5% of azone, and 74-95% of glycerol triacetate. The preparation method includes the steps of: grinding and sieving the bulleyaconitine A for later use; adding the azone and the glycerol triacetate to the propylene glycol according to the ratio in the formula, and uniformly mixing the components to obtain a mixed solvent; adding the mixed solvent to the bulleyaconitine A, and performing ultrasonic vibration to obtain the target product, bulleyaconitine A liniment. The bulleyaconitine A liniment is applied in preparation of drugs for therapy of inflammation and / or pain. The novel bulleyaconitine A liniment is researched through deeply researching studies of the inventor, wherein the optimum-selected solvent composition satisfies the solvent permeation-assisting system, so that the liniment is quick to absorb and has no irritation and allergy on skin.
Owner:KPC PHARM INC
Bulleyaconitine A liposome and preparation method thereof
InactiveCN101884615ALow toxicityLess irritatingOrganic active ingredientsAntipyreticCholesterolIrritation
The invention discloses a bulleyaconitine A liposome, which comprises the following components in part by mass: 0.01 to 1 part of bulleyaconitine A, 1 part of phospholipid, 0.05 to 1 part of cholesterol and 0.001 to 0.03 part of vitamin E. In the method, the bulleyaconitine A liposome is prepared by adopting a unique two-step method; and the preparation method has the advantages of a few steps, simple and convenient operation and high controllability and reproducibility. The medicament entrapment efficiency of the bulleyaconitine A liposome prepared by the method can reach over 85 percent. The choice of the adopted phospholipids is wide; and natural phospholipids or soybean phospholipids can be used, and synthesized neutral phospholipids or electronegative phospholipids can also be used. The grain size of the bulleyaconitine A liposome is lower than 200 nm; and a preliminary toxicity test shows that the bulleyaconitine A liposome can reduce the toxicity and irritation of the bulleyaconitine A, and improve the compliance of a patient; and therefore, the bulleyaconitine A liposome is particularly suitable to be used as an intramuscular injection.
Owner:SHANGHAI INST OF PHARMA IND
Application of bulleyaconitine A in preparation of drugs for treating irritable bowel syndrome
ActiveCN109718236AExpand indication useRelieve visceral painOrganic active ingredientsNervous disorderVisceral painAnxiety
The present invention belongs to the field of biomedicine and discloses a new application of bulleyaconitine A in preparation of drugs for treating irritable bowel syndrome. When the bulleyaconitine Ais applied to the treatment of the irritable bowel syndrome, the bulleyaconitine A can effectively alleviate visceral pain, mental anxiety and hyperalgesia caused by the irritable bowel syndrome, andis safe and effective.
Owner:SHANGHAI MENTAL HEALTH CENT (SHANGHAI PSYCHOLOGICAL COUNSELLING TRAINING CENT) +1
A kind of preparation method of high-purity aconitin
The invention discloses a method for preparing high-purity bulleyaconitine A. The method comprises the following steps of: soaking a fresh radix aconiti agrestis root in acid aqueous solution, performing homogenate extraction and cavitation mixing solid-liquid extraction, collecting and concentrating the filtrate, and respectively performing gradient elution to obtain a crude bulleyaconitine A product through ion exchange resin column chromatography and macroporous adsorption resin column chromatography; and uniformly mixing crude bulleyaconitine A product and ethanol aqueous solution, dissolving in a water bath, cooling the filtrate to room temperature, standing and crystallizing at the temperature of 4 DEG C, repeatedly washing the crystal by using the ethanol aqueous solution, and obtaining a final product. According to the method, crushing and extracting are finished in one step through a homogenization method, and the method has the advantages of zero dust and simple flow. By adoption of a negative-pressure cavitation extraction technology, the mass transfer rate is improved, heating is not required, and the production period is shortened; and moreover, the reagents used in the whole process are ethanol and water, the organic solvent amount is greatly reduced, the production cost can be reduced, the environmental pollution is reduced, and the method has good application prospect.
Owner:INST OF MEDICINAL PLANTS YUNNAN ACAD OF AGRI SCI
Bulleyaconitine A multivesicular liposome and preparation method thereof
InactiveCN101744765AIncrease profitReduce wasteOrganic active ingredientsNervous disorderEtioplastsActive component
The invention discloses a bulleyaconitine A multivesicular liposome and a preparation method thereof. The bulleyaconitine A multivesicular liposome comprises medicament active components, namely bulleyaconitine A and blank multivesicular liposome which serve as carriers; the blank multivesicular liposome comprises the following components in part by mass: 1 part of lipid component and 0.1 to 50 parts of ion gradient regulator, wherein the lipid component comprises amphiphilic lipid and neutral lipid in a molar ratio of 5-36:1. In the preparation method, the blank multivesicular liposome which is packaged with a gradient regulator is used, bulleyaconitine A permeates a phospholipid film and enters a water phase in the blank multivesicular liposome by a transmembrane gradient active loading method to obtain the bulleyaconitine A multivesicular liposome. The bulleyaconitine A multivesicular liposome has the advantages of high utilization rate of raw materials, high loading concentration, excellent sustained release effect in in-vivo / in-vitro tests and better effect of resisting tumors.
Owner:SHANGHAI INST OF PHARMA IND
Low-toxicity antiarrhythmic medicine and preparation method thereof
ActiveCN113149906AOrganic active ingredientsOrganic chemistry methodsAntiarrhythmic effectChemical compound
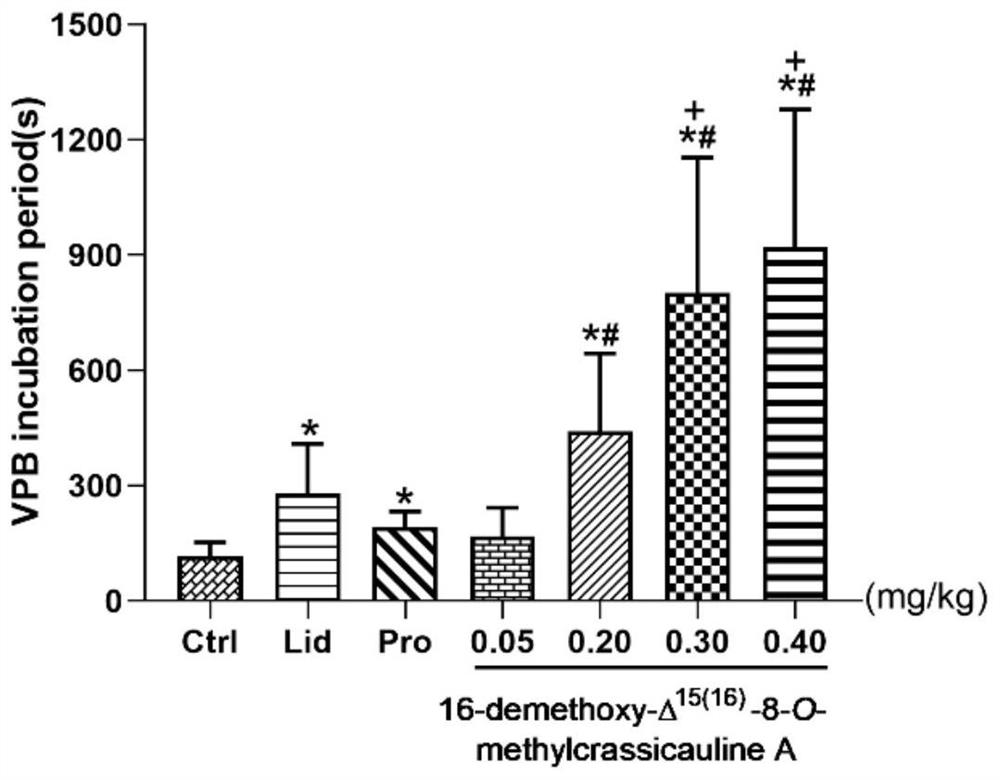
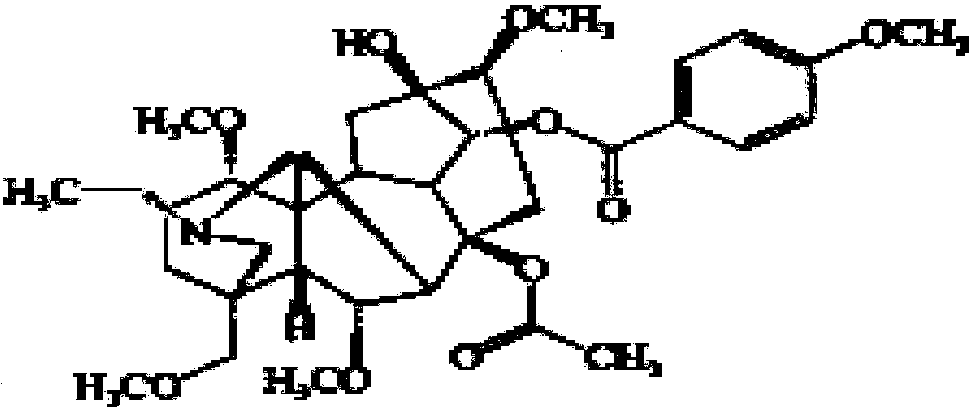
The invention provides a low-toxicity anti-arrhythmia medicine and a preparation method thereof. The structure of the compound is shown as a formula I in the specification. Experimental results show that the compound provided by the invention has excellent anti-arrhythmia activity, can delay the VPB latency period of aconitine induced arrhythmia rats and effectively prevent further development of VPB, and the anti-arrhythmia effect of the compound is even superior to that of a positive drug lidocaine under the dosage of 0.30 mg / kg and 0.40 mg / kg. More importantly, compared with bulleyaconitine A, the cardiotoxicity of the compound disclosed by the invention is obviously reduced; compared with the known sand-fried product delta 15, 16-16-demethoxyindinacitine, the compound disclosed by the invention has the advantages that the acute toxicity is obviously lower, and the safety is higher. The invention provides a new choice for preparing a low-toxicity anti-arrhythmia medicine, and provides a new method for controlling the quality of the sand-fried Aconitum bulleyanum or sand-fried Aconitum crassicaule.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
A high-efficiency extraction and separation method of aconitin
A bulleyaconitine A efficient extraction and separation method comprises pretreatment, extraction, concentration, acidification, basification, extraction, column chromatography and crystallization steps. According to the method, 75% acid ethanol solution containing 0.3% of sulfuric acid is used for extraction for three times, concentrated ammonia water is slowly added under stirring during alkalization to adjust pH to 8, loss of bulleyaconitine A is reduced; during column chromatography on silica gel, the flow rate of the column chromatography is reasonably and effectively controlled at 13-20ml / min, the separation effect is better; ethyl acetate is used for extraction, the solvent residue is zero, the bulleyaconitine A highest content can reach 98-99%, the product purity is high, and the rate extraction of the bulleyaconitine A of a radix aconiti kusnezoffii medicinal material can be increased to 0.15 to 0.22 %. The method uses non-toxic or low-toxic organic solvents and reduces organic solvent kinds and usage amount, saves energy, reduces conservation, and reduces personnel hazards and environmental pollution.
Owner:云南大围山生物制药有限公司
Bulleyaconitine A and lidocaine compounded sterile powder for injection
InactiveCN101744810AMake up for the shortcomings of the hysteresisReduce systemic toxicityOrganic active ingredientsPowder deliverySterile waterPharmacology
The invention relates to bulleyaconitine A and lidocaine compounded sterile powder for injection, belonging to a medicinal preparation containing effective organic components, in particular to a medicinal preparation containing bulleyaconitine A. A lidocaine solution with a concentration of 80mM is added to a bulleyaconitine A solution with a concentration of 0.20-0.35mM to prepare the sterile powder suitable for preparing sterile solutions, wherein the volume of the lidocaine solution accounts for 0.5-1.5 percent of that of the bulleyaconitine A solution; and the sterile powder is subpackaged in ampoules or other appropriate containers in specifications of 2ml, 5ml or 10ml according to a standard of 0.2-0.5mg and prepared into injections by using sterile solutions before using. The invention provides the bulleyaconitine A and lidocaine compounded sterile powder for injection, which is used as a non-narcotic painkiller and can be expected to reduce adverse reactions of the bulleyaconitine A to the human body.
Owner:李彪
Transdermal paster containing bulleyaconitine A and its preparation method
The present invention provides an external patch for improving pain and inflammation, which particularly relates to a transdermal patch containing the bulleyacinitine A and the corresponding production method.
Owner:KPC PHARM INC
Application of bulleyaconitine A
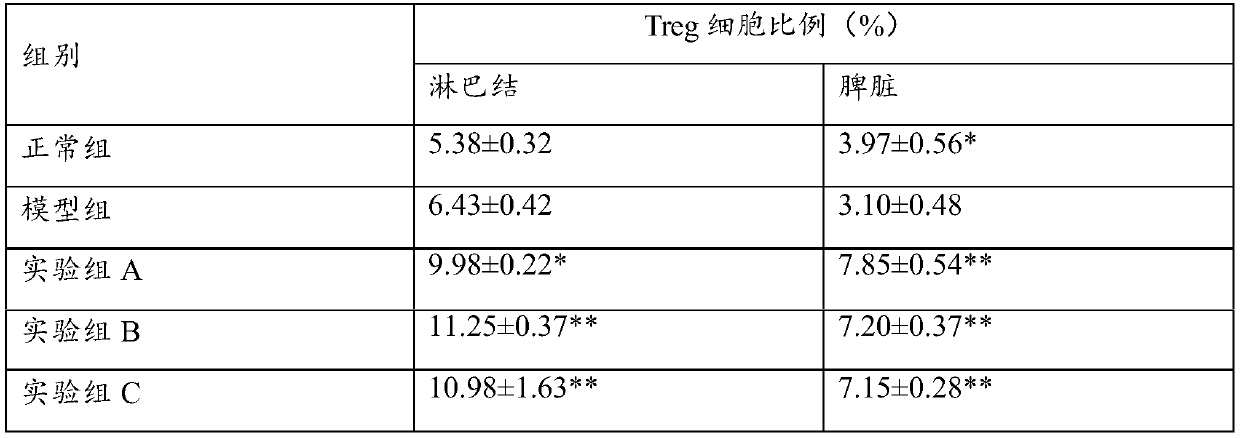
ActiveCN110742888ACause some damagesAchieve anti-psoriasis effectsOrganic active ingredientsDermatological disorderPharmaceutical drugClinical tests
The invention relates to the technical field of medicines, and discloses application of bulleyaconitine A. According to the application of the bulleyaconitine A, through an animal model test and a clinical test, it is verified that the bulleyaconitine A can effectively reduce thicknesses of skin lesions, make color of the skin lesions pale, reduce an area of skin rash, relieve pruritus and improvevarious physiological indexes, relevant results are all statistically different, the curative effect of the bulleyaconitine A in treatment of psoriasis is obvious, the safety is high, and therefore,the invention provides related application of the bulleyaconitine A in the treatment of the psoriasis.
Owner:YUNNAN HAOPY PHARM LTD
Medicinal composition for treating cold wet numbness and its production
The invention is concerned with the method of preparation and the quality testing process of the compound medicine for wind cold dampness arthralgia syndrome therapy. The compound contents hydrochloric acid green vine base and bulleyaconitine A. It can use for several symptoms, such as muscle soreness, articulation swelling, aching, numbness of body parts, etc.
Owner:SICHUAN KELUN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com