Application of bulleyaconitine A in preparation of drugs for treating irritable bowel syndrome
A technology for irritable bowel syndrome and fenugreek, which is applied in the field of biomedicine, can solve the problems of increasing constipation intractability, drug dependence, and no effective drugs, and achieves expanded indications, high safety, safety and effectiveness. The effect of drug choice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
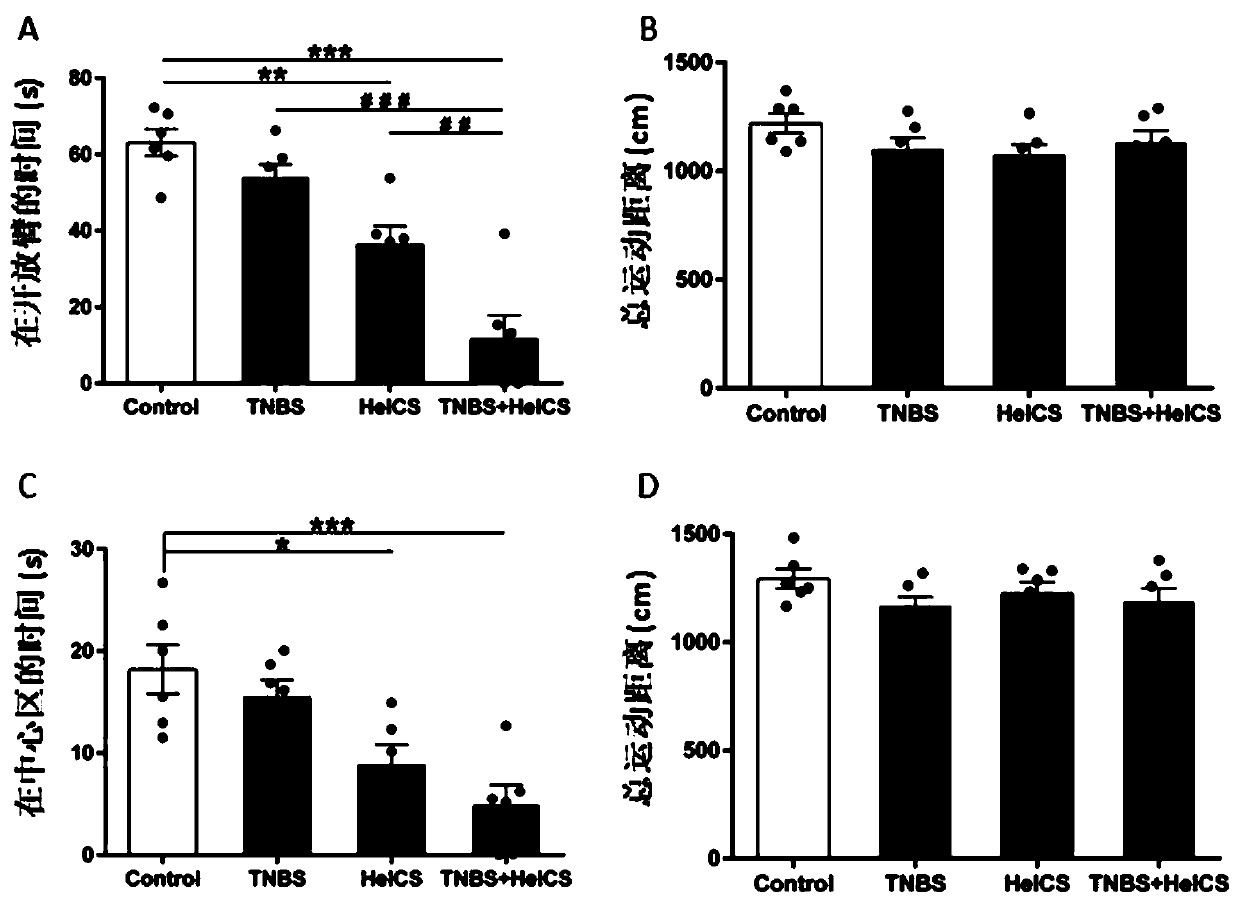
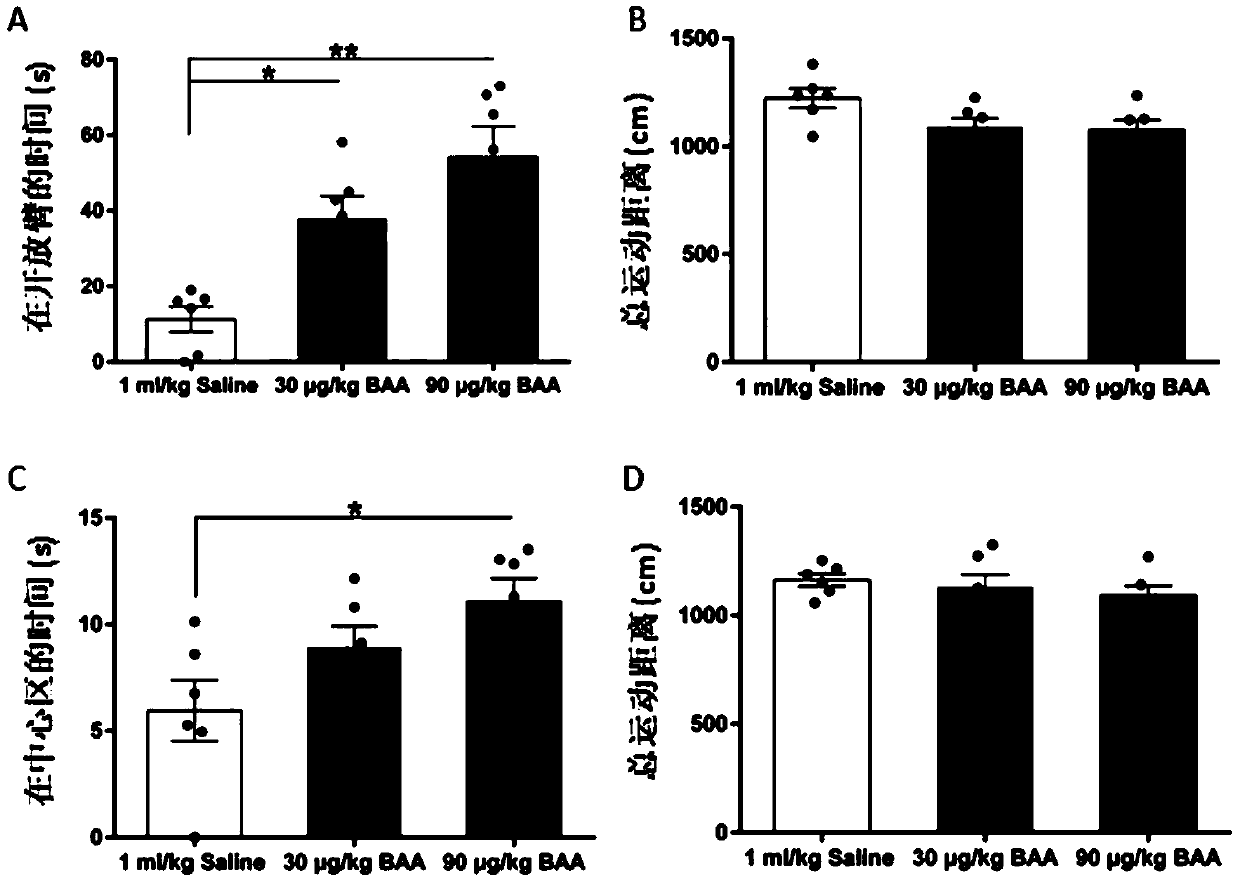
[0018] Example 1: The effect of orientalin on anti-anxiety, pain sensitivity and visceral pain caused by IBS.
[0019] 1. Experimental animals:
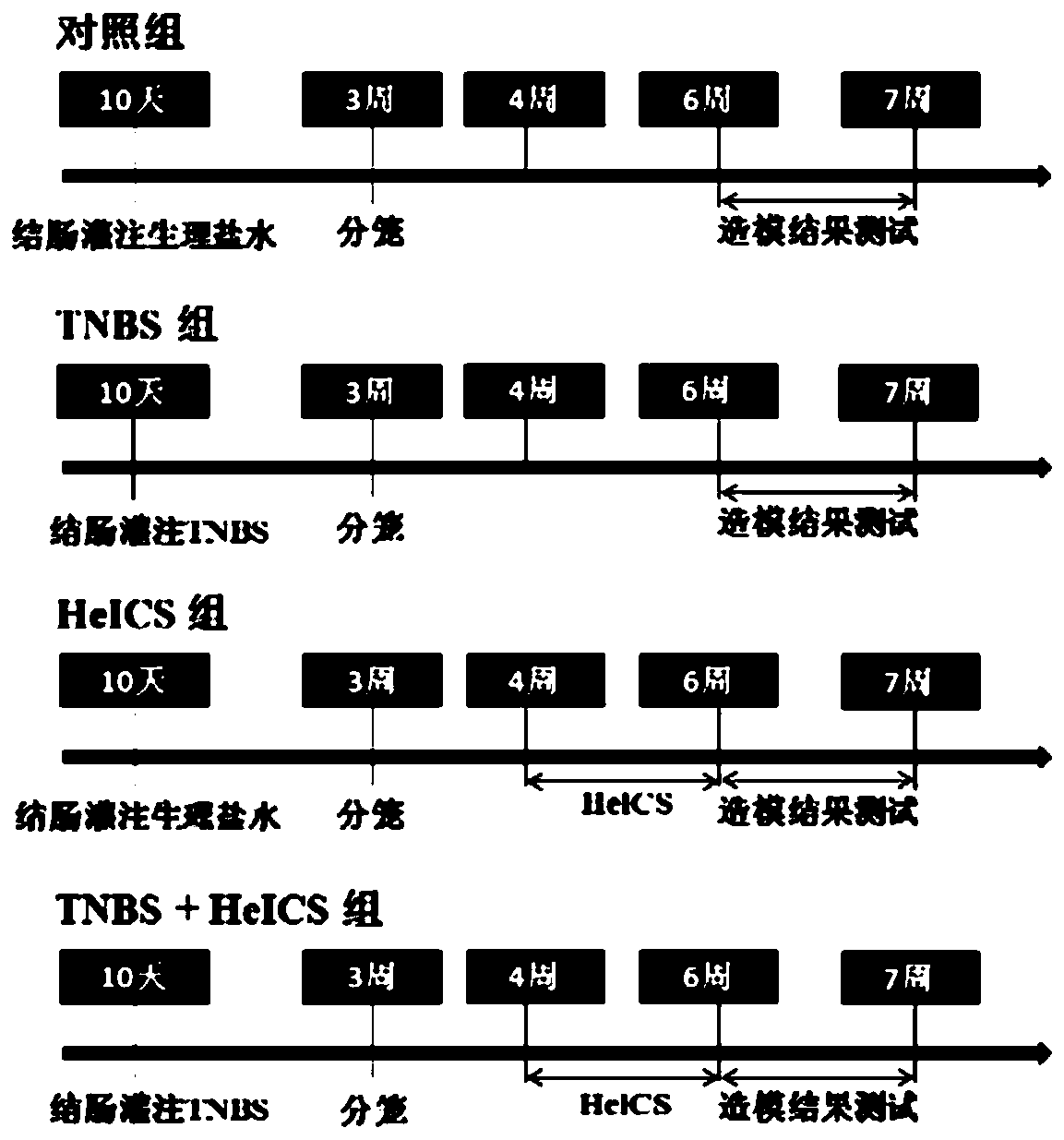
[0020] IBS can be divided into constipation type (IBS-C), diarrhea type (IBS-D), indeterminate type (IBS-U) and mixed type (IBS-M), and IBS-D is the most common type. In order to simulate a more suitable clinical pathogenesis and the most common IBS animal model, the present invention combines five heterologous chronic stress simulated IBS rat models on the basis of neonatal enteritis, which is equivalent to the IBS postinflammation (IBS-PI) model, and detects large Effects on visceral sensitivity, changes in mental activity, feces score, and the number of defecated pellets in rats. In the experiment, female SD rats, which are more prone to IBS symptoms, were used for the experiment. SD rats were bred and raised in the Department of Experimental Animal Science and the Central Key Laboratory of Shanghai Jiaotong University. The feed...
Embodiment 2
[0044] Effects of sativa on intestinal defecation habit and gastrointestinal motility function in SD rats with chronic visceral pain.
[0045] Because females in infancy are prone to stress and anxiety symptoms, the inventor divided female SD rats into experimental groups (two groups) and control groups. The weight of SD rats was 80 ± 2 to 100 ± 2g, and there were ten rats in each group. . The experimental process is as follows Figure 7 As shown in A, the SD rats in the experimental group and the control group underwent colonic perfusion with 2,4,6-trinitrobenzenesulfonic acid 10 days after birth. When the rats were 3 weeks old (the SD rats in this age group were in juvenile to adolescence), the experimental group was administered gavage with aconitin (two administration concentrations: 0.3mg / kg, 0.9mg / kg; 10ml / kg / day), the control group was given the same volume of normal saline / day. Then SD rats in the experimental group and the control group were restrained in a fix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com