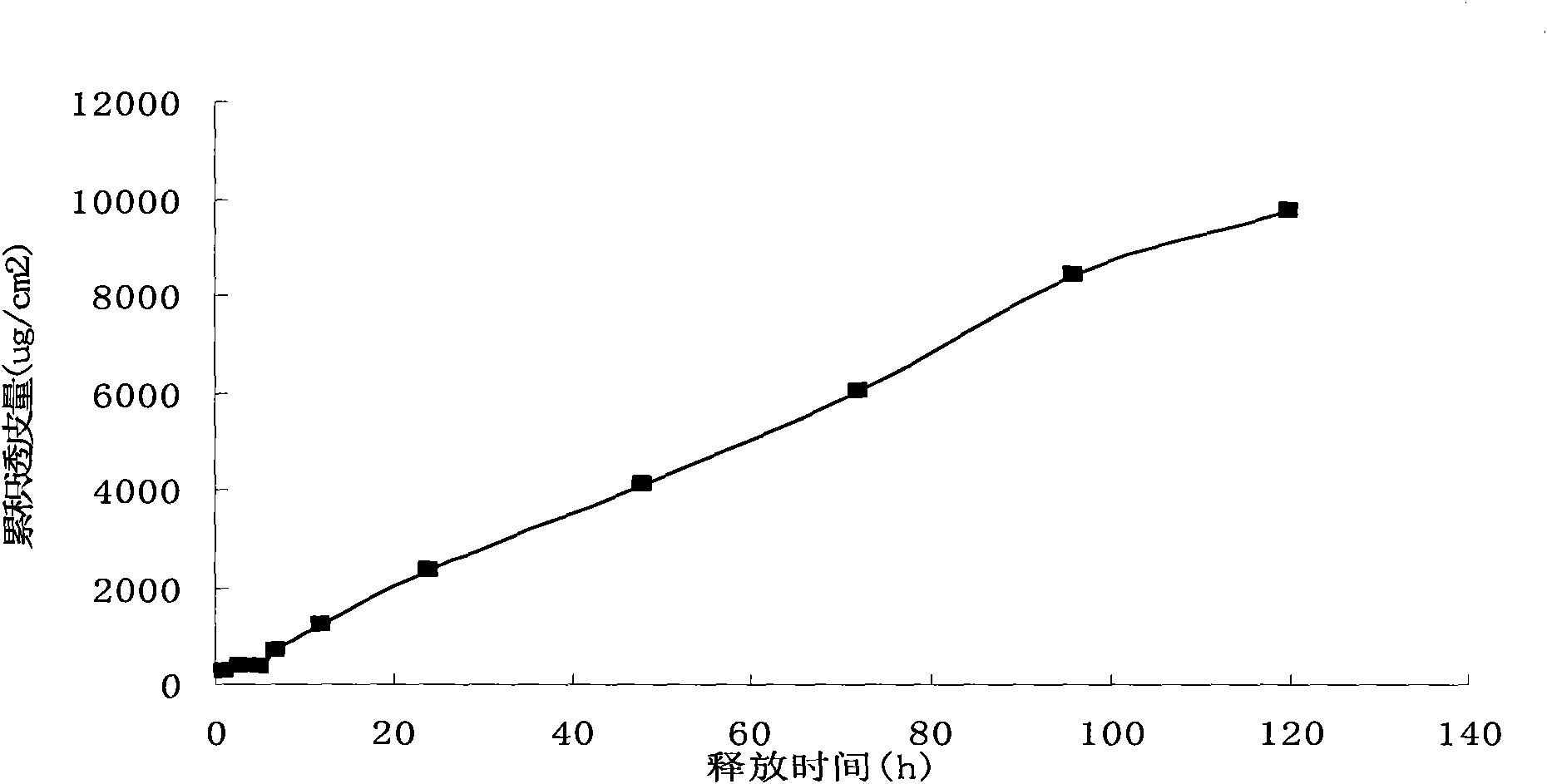
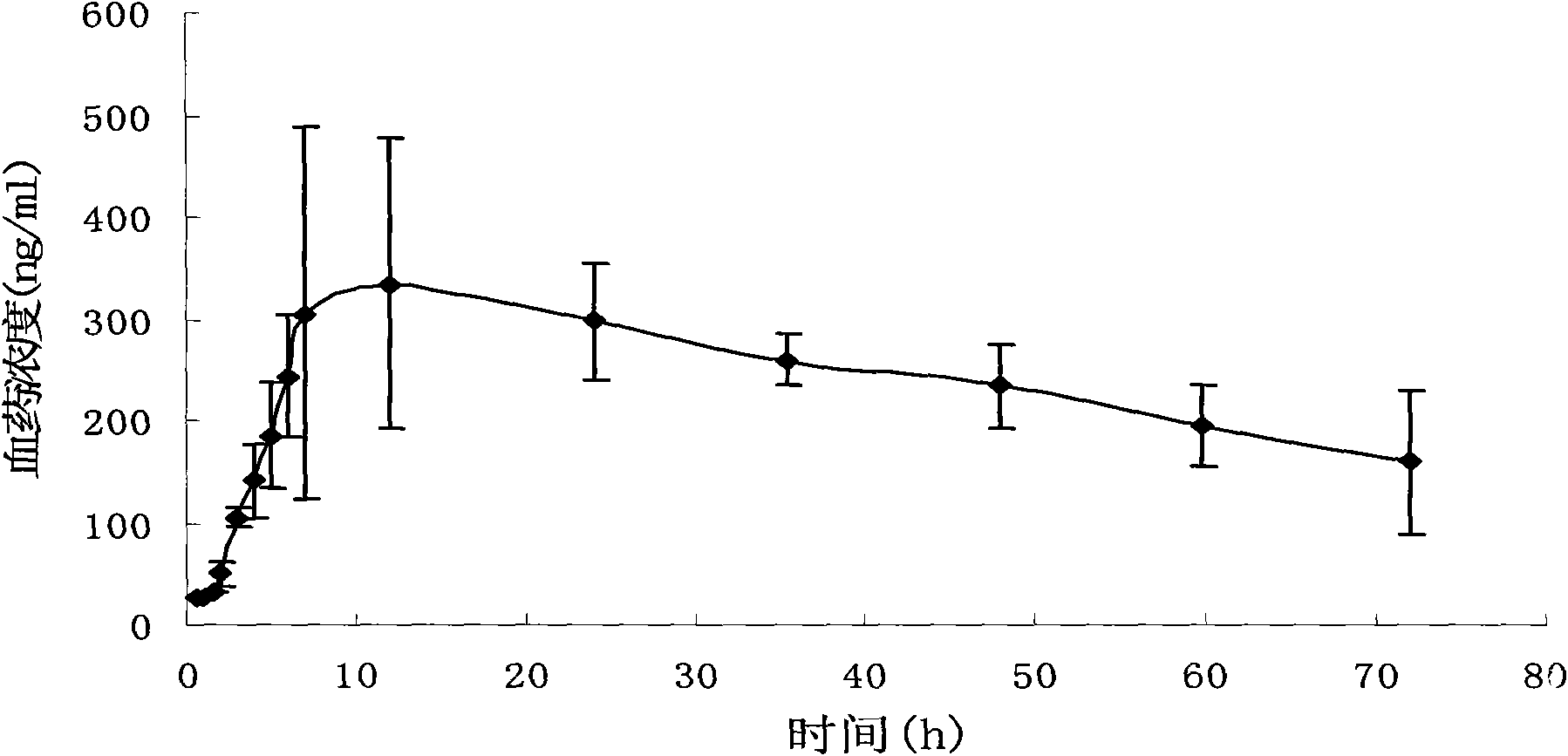
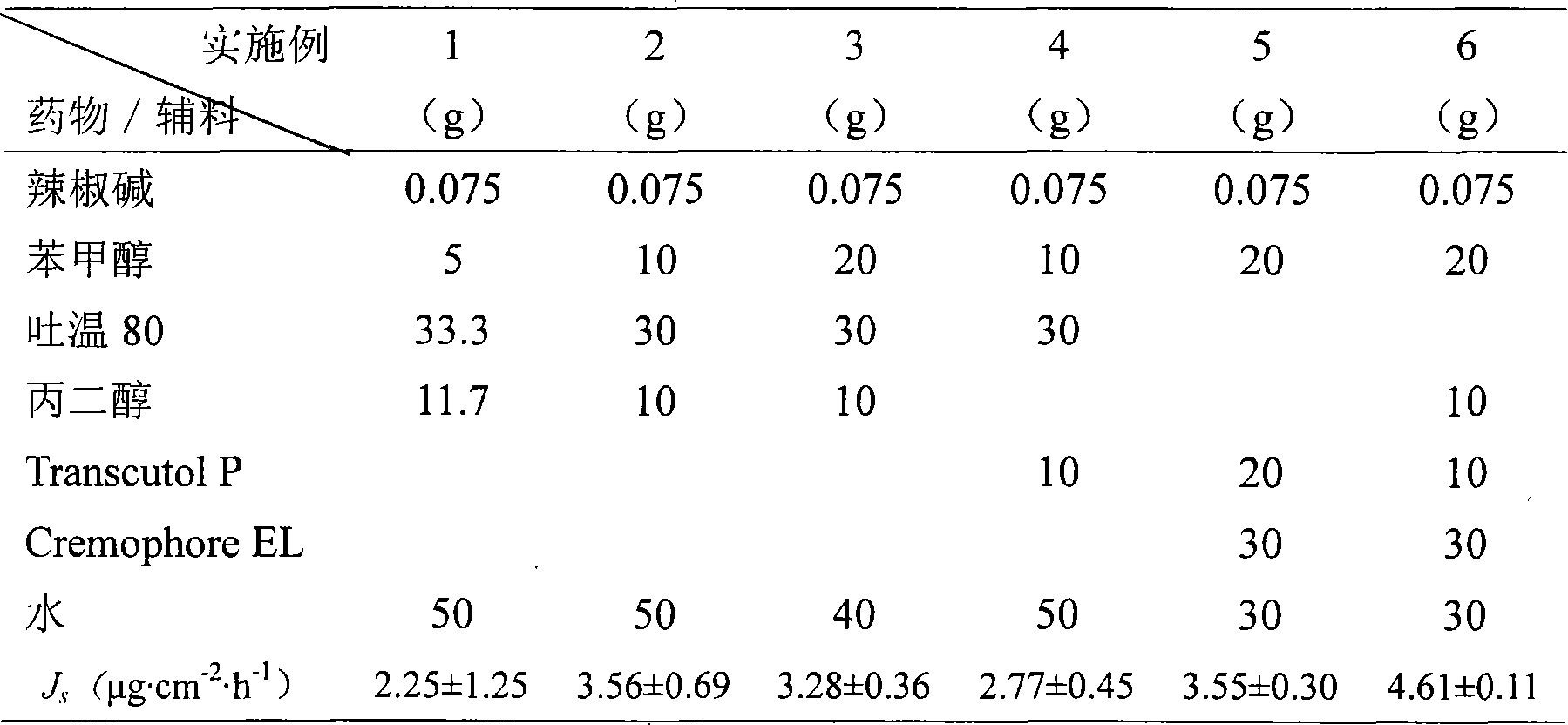
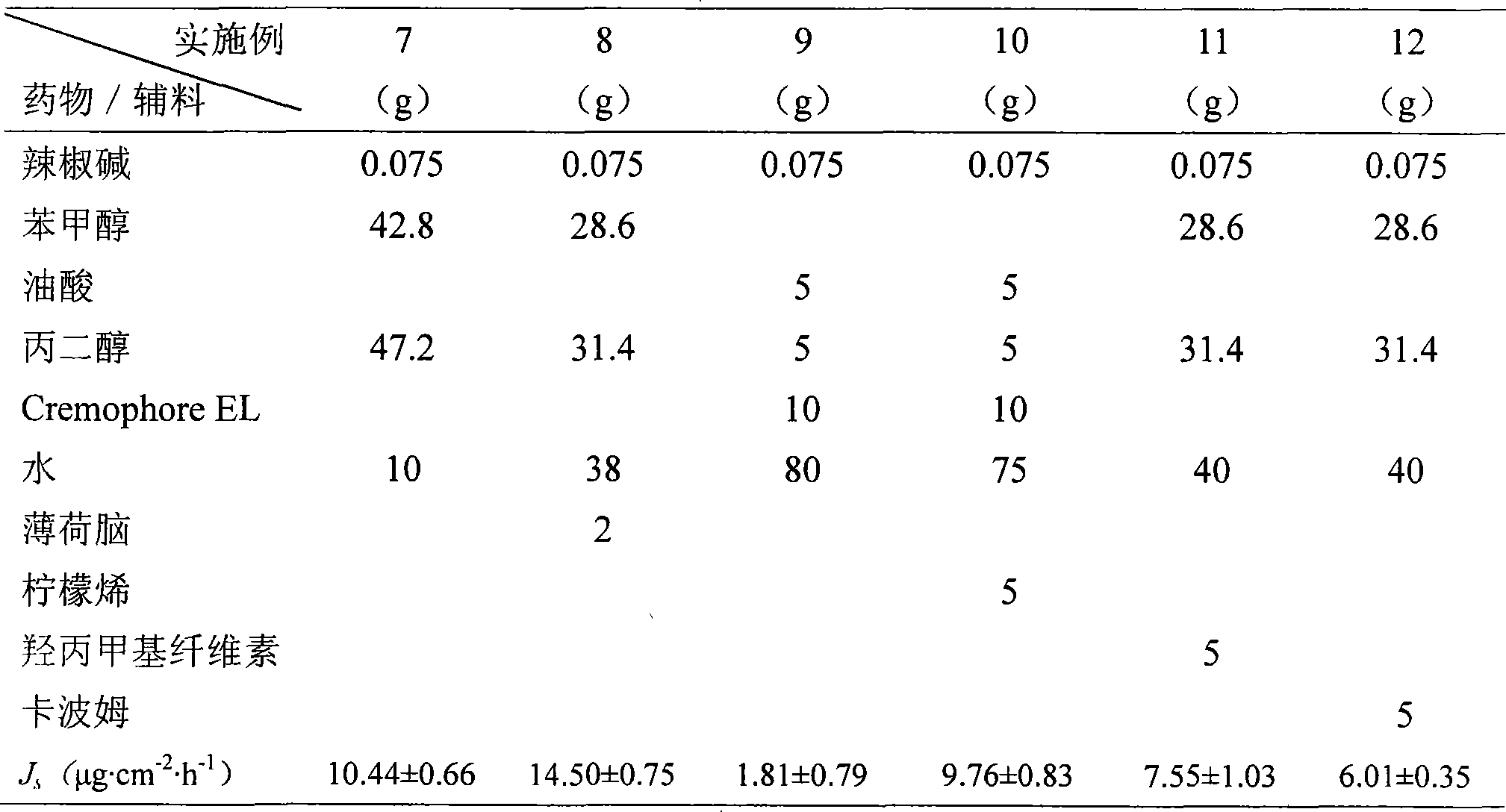
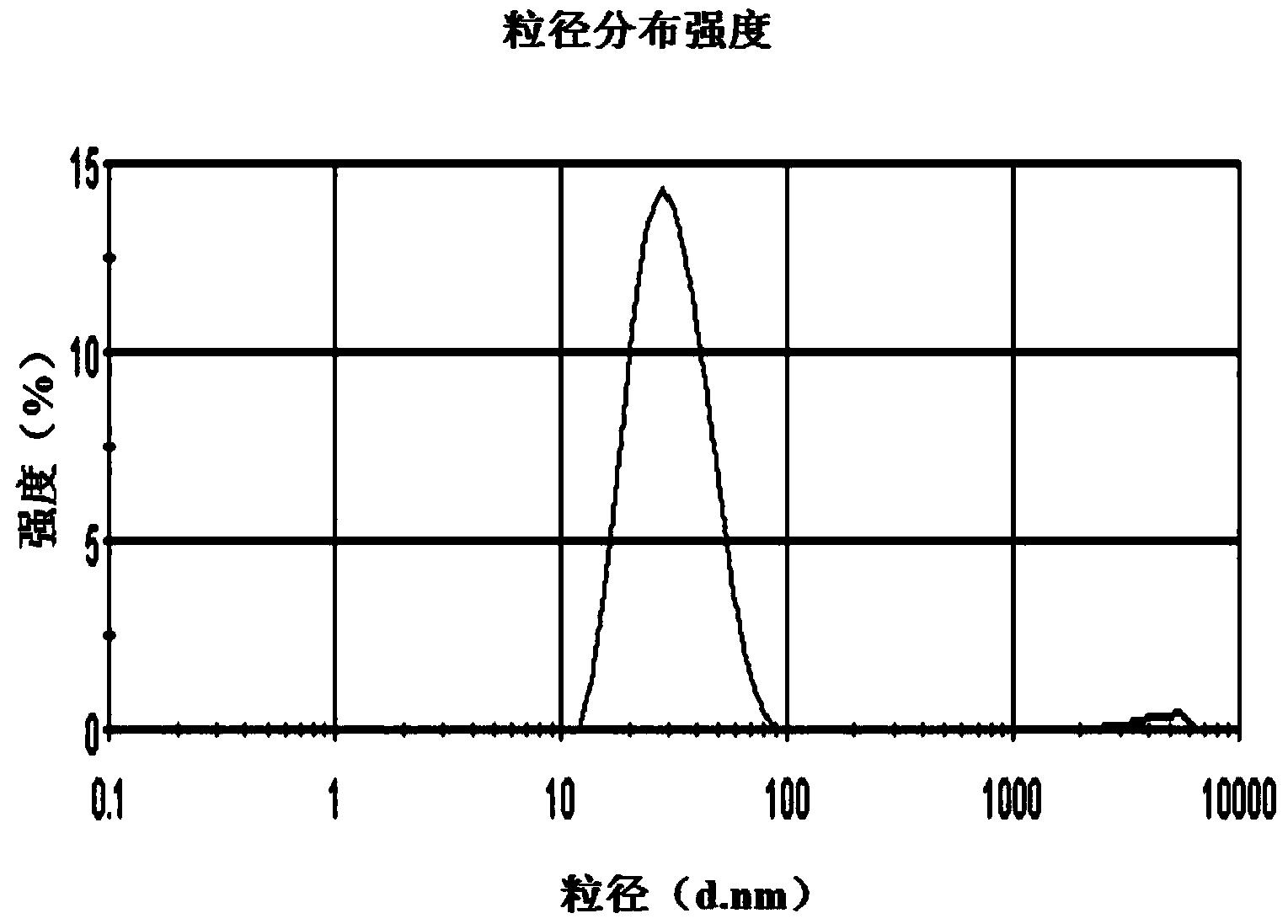
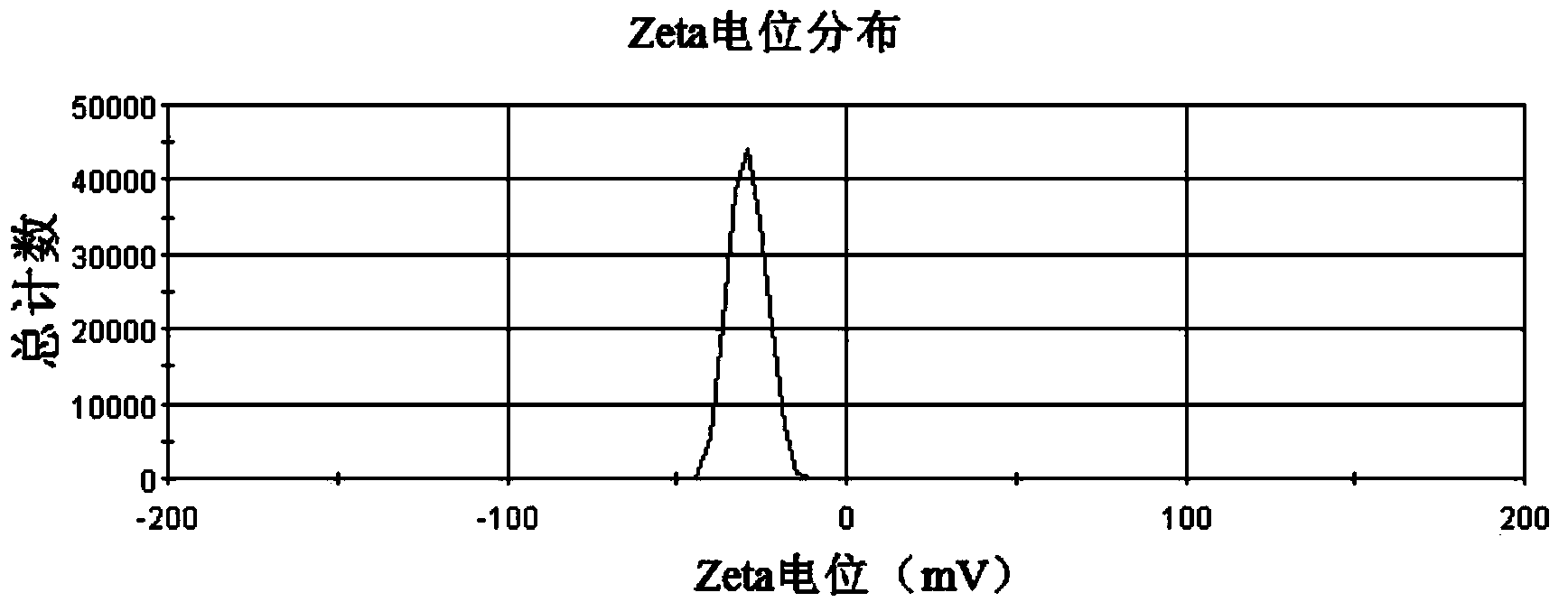
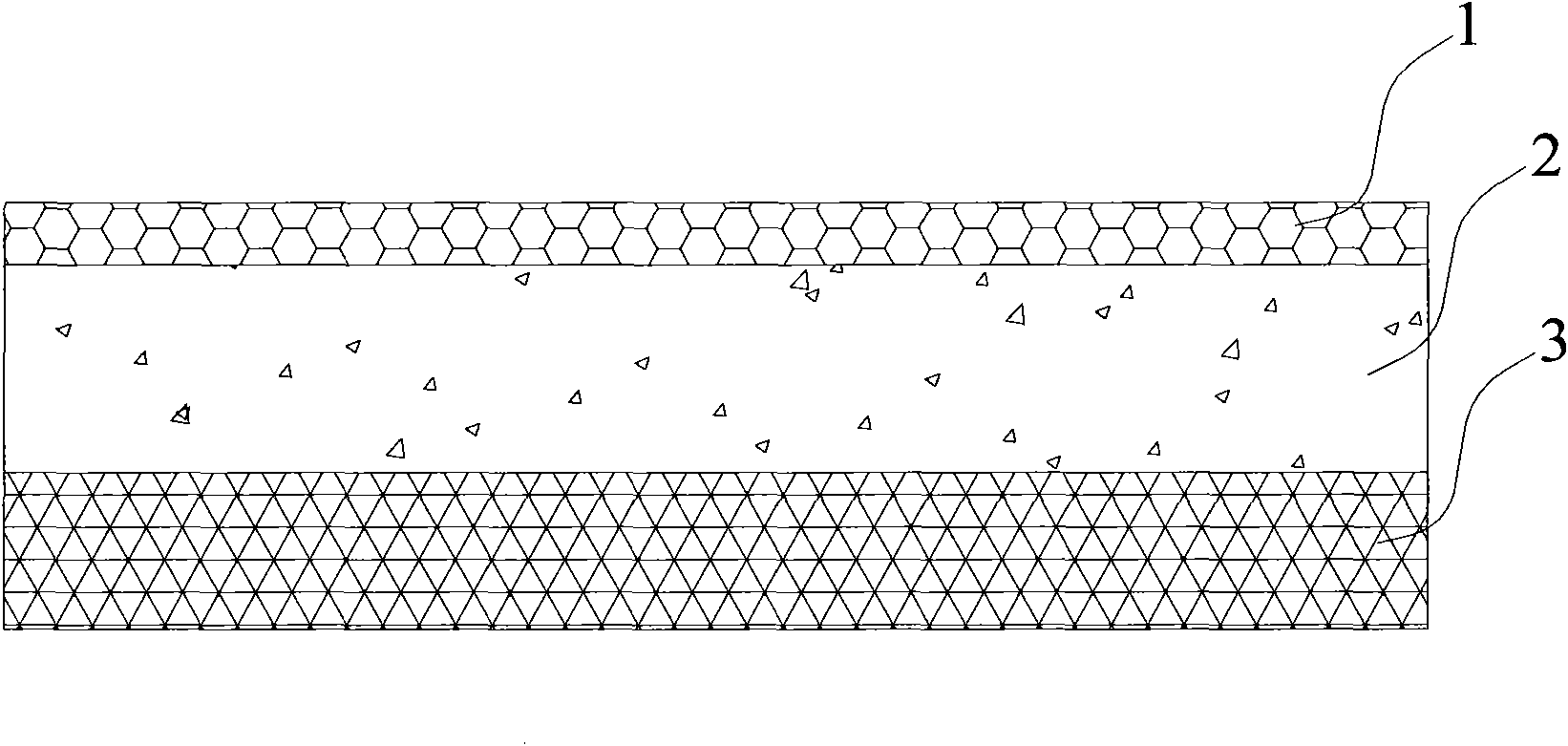
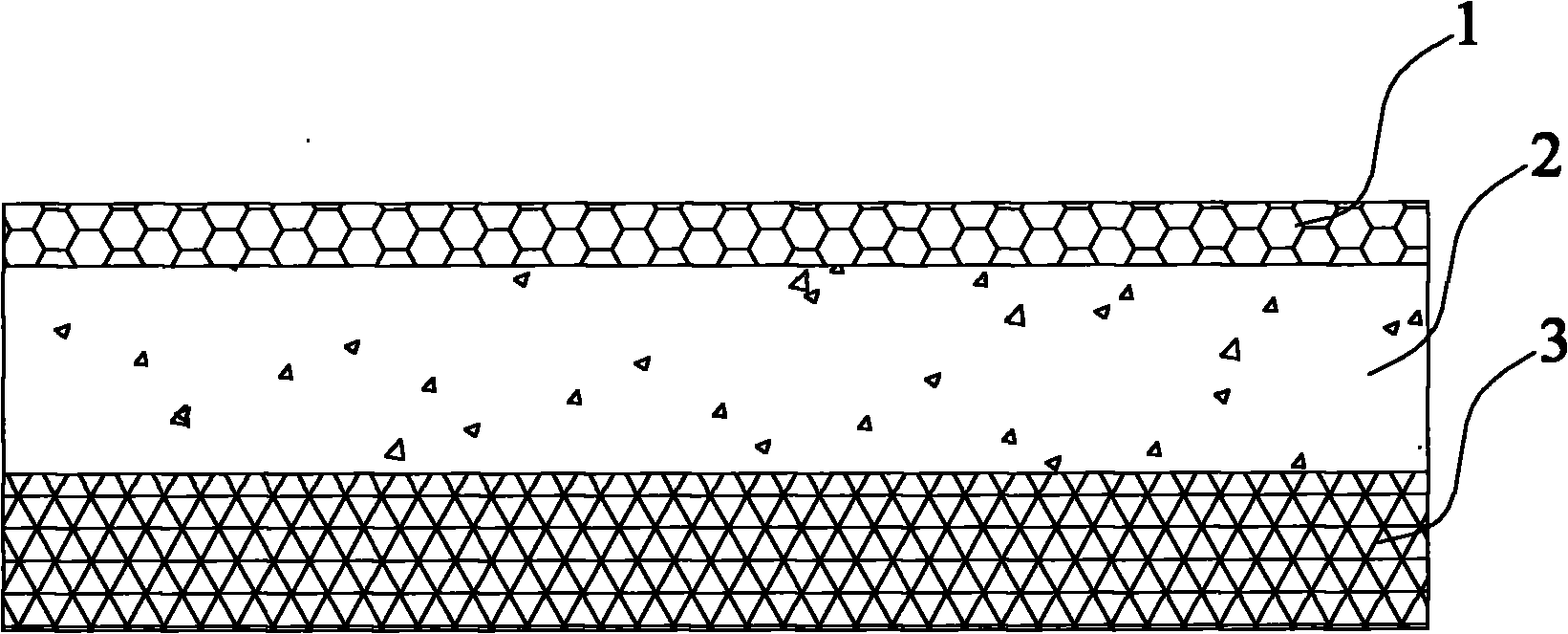
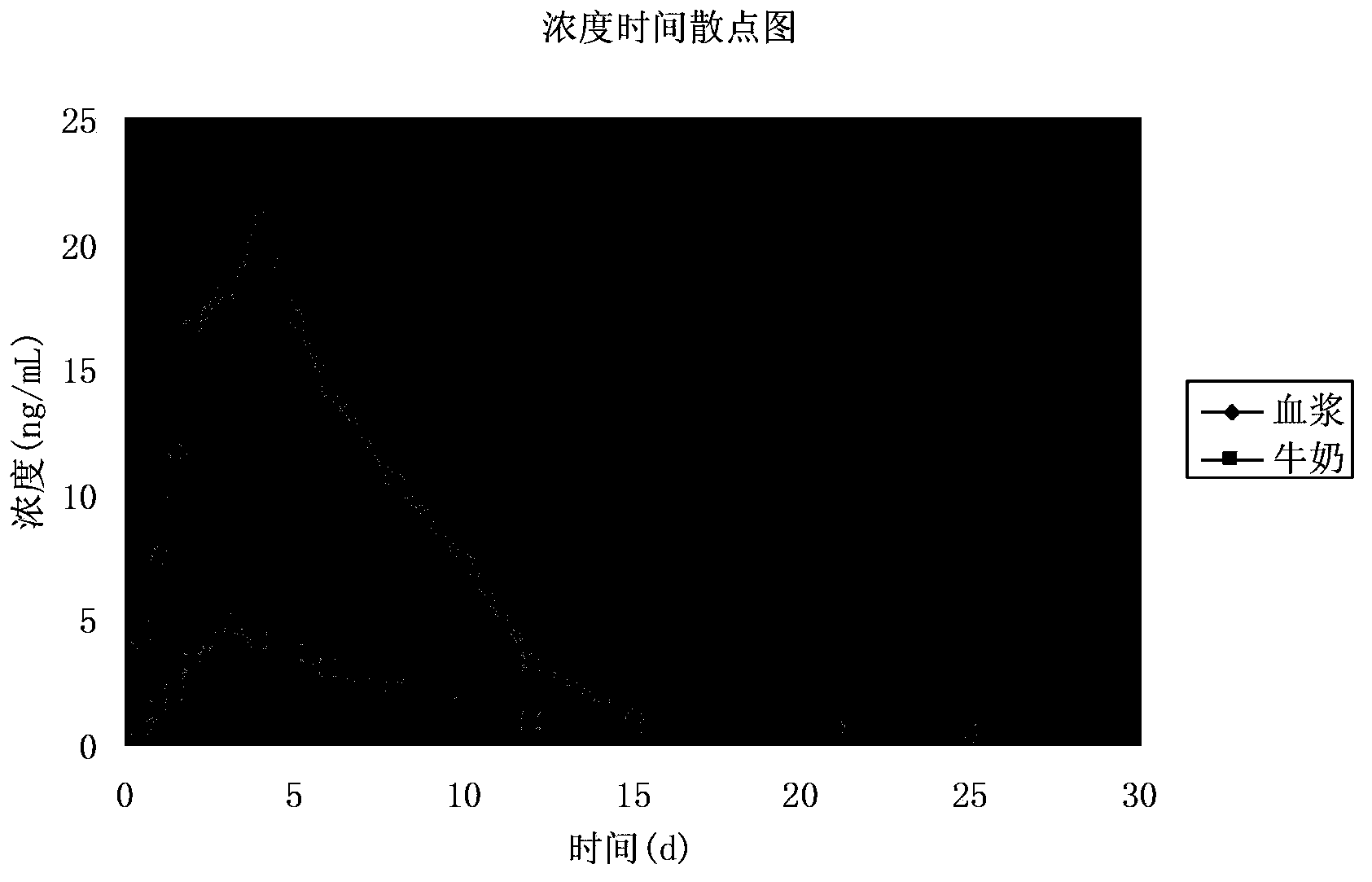
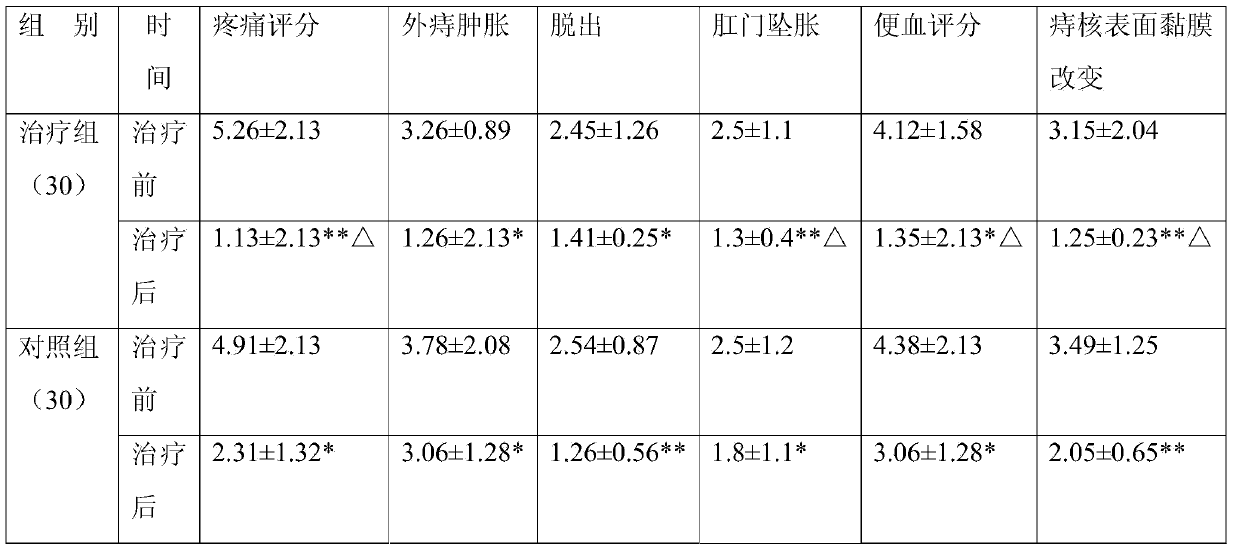
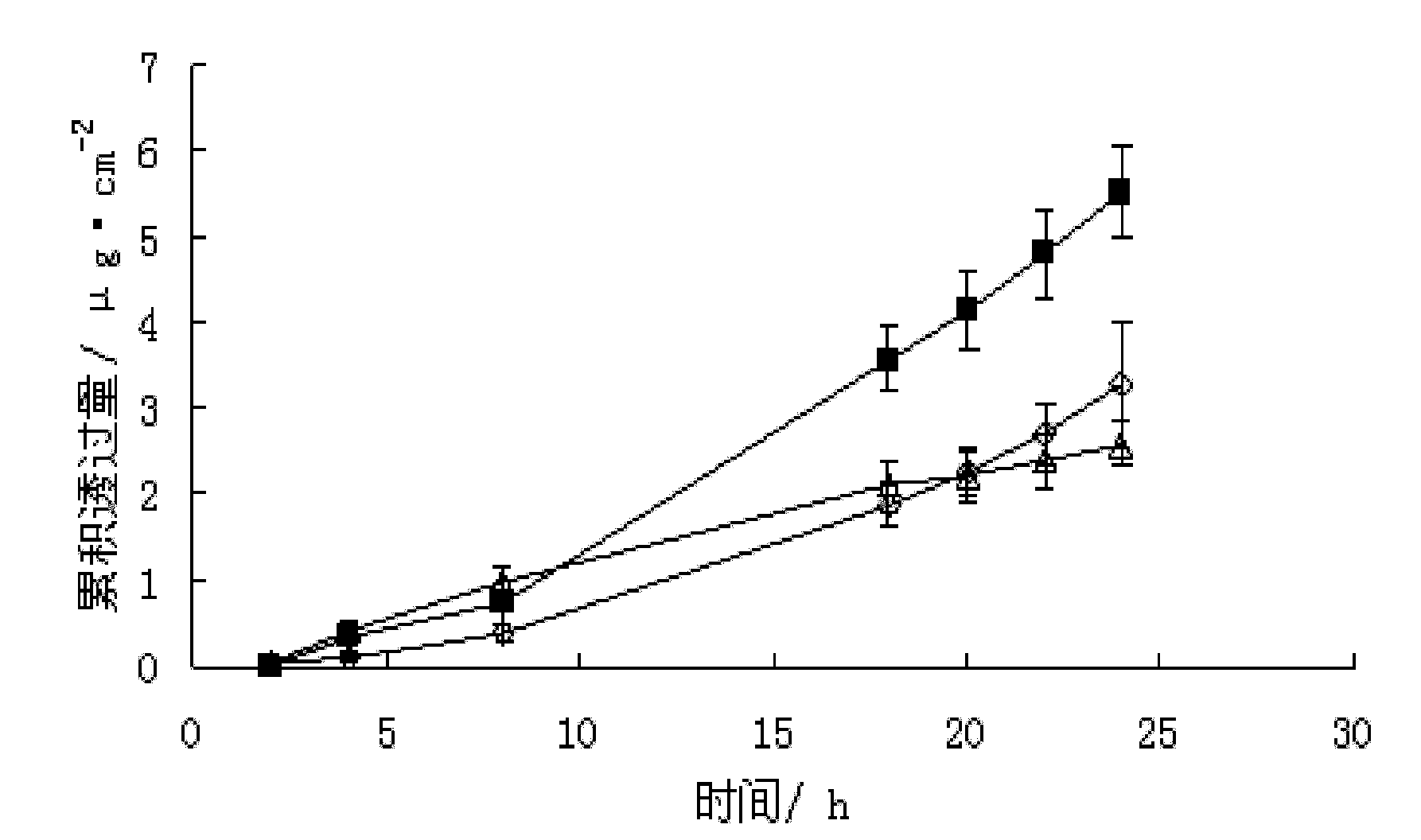
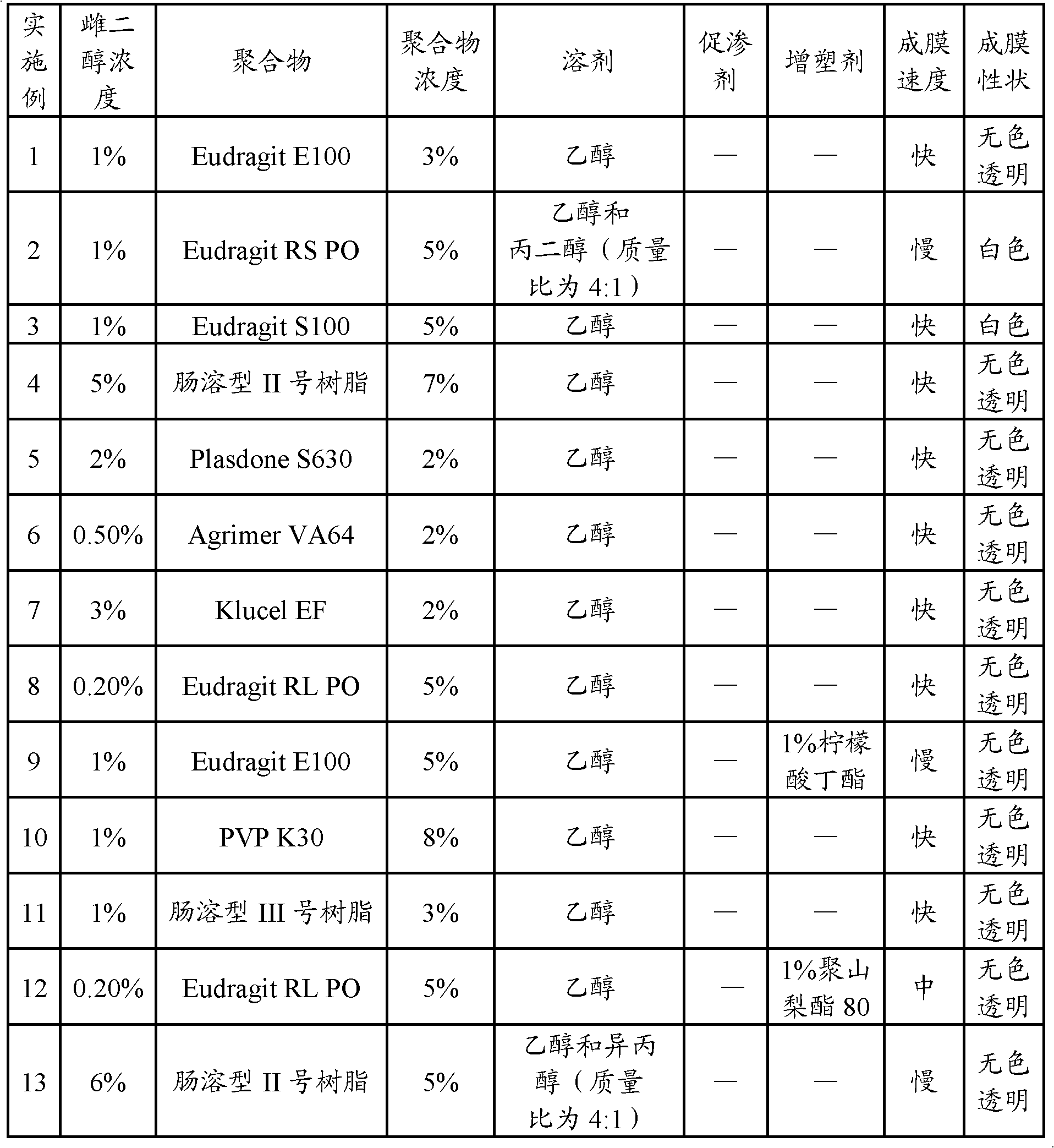
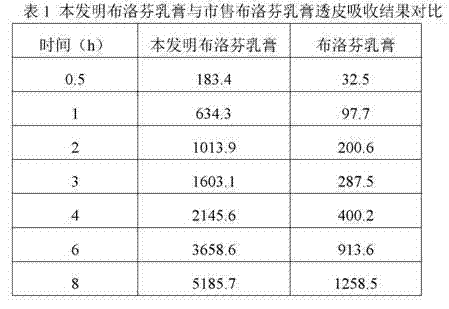
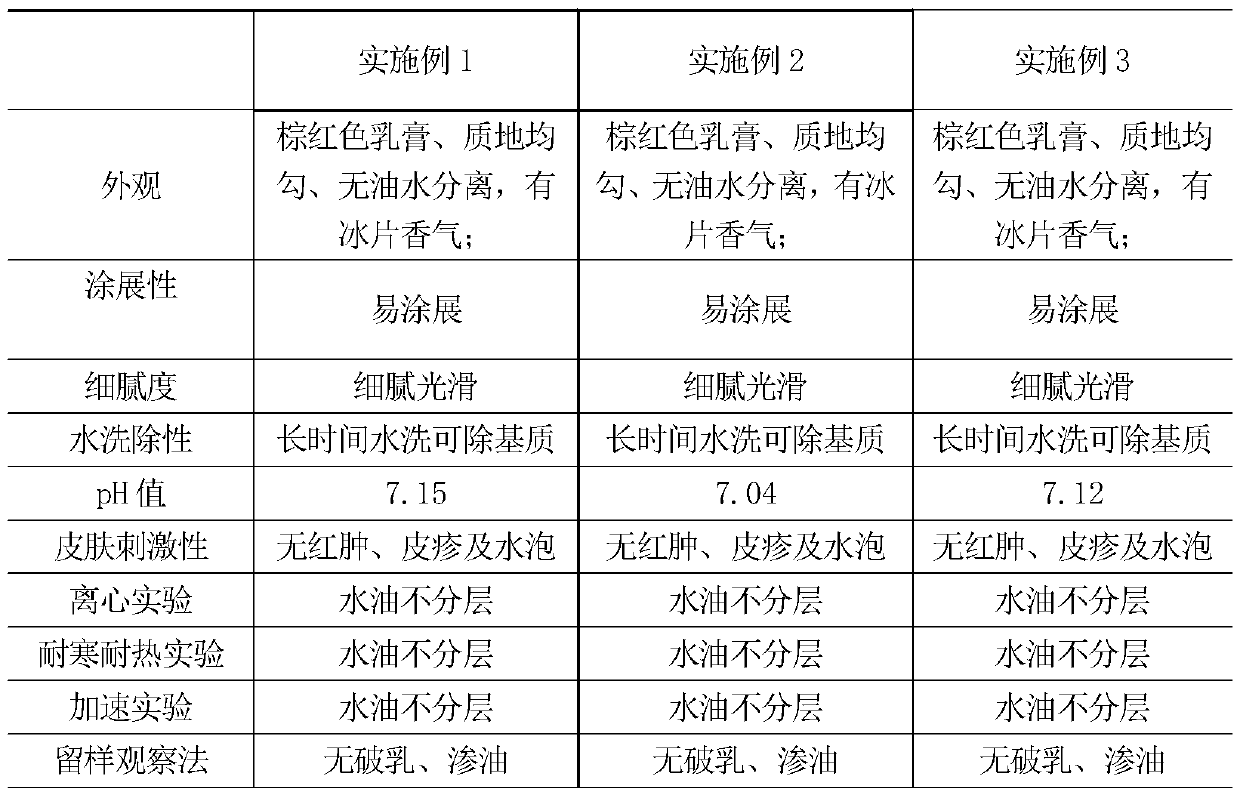
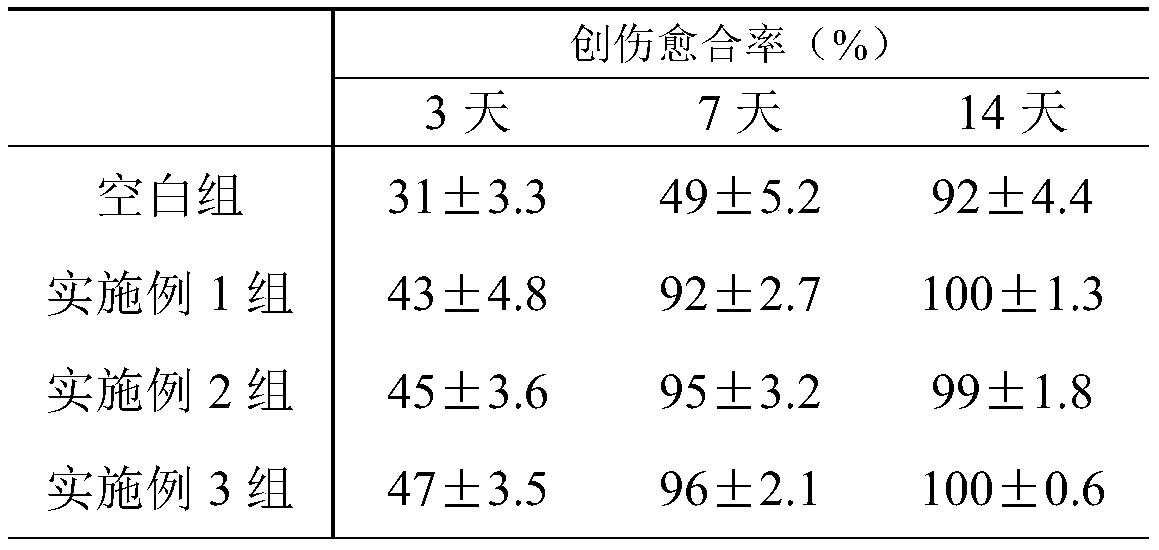
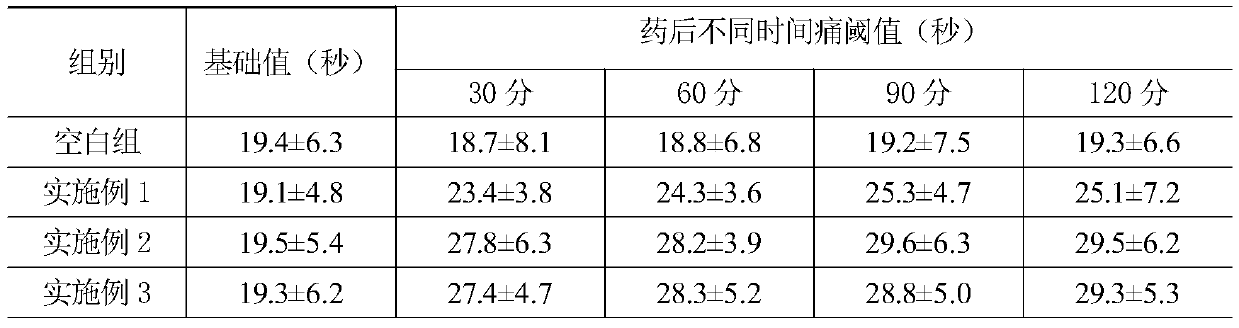
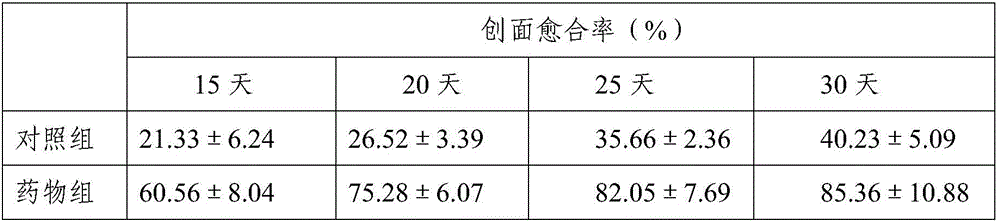
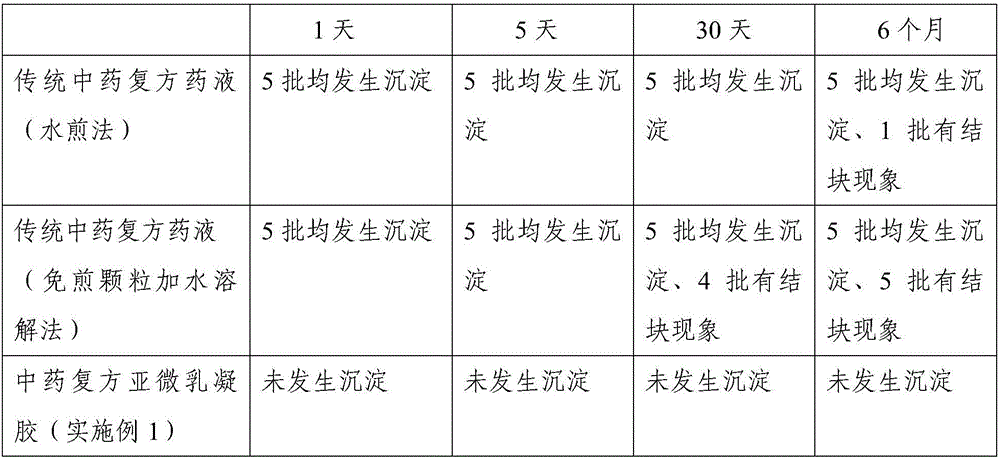
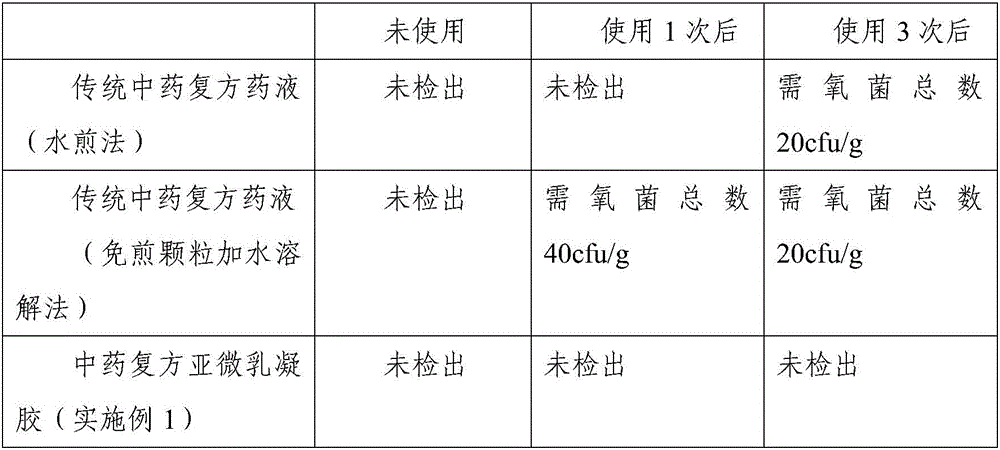
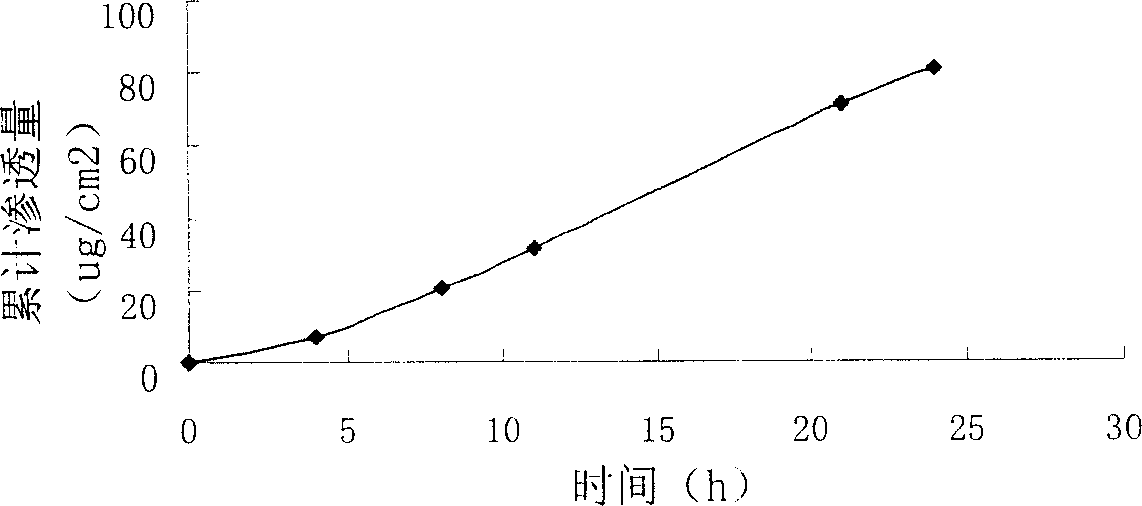
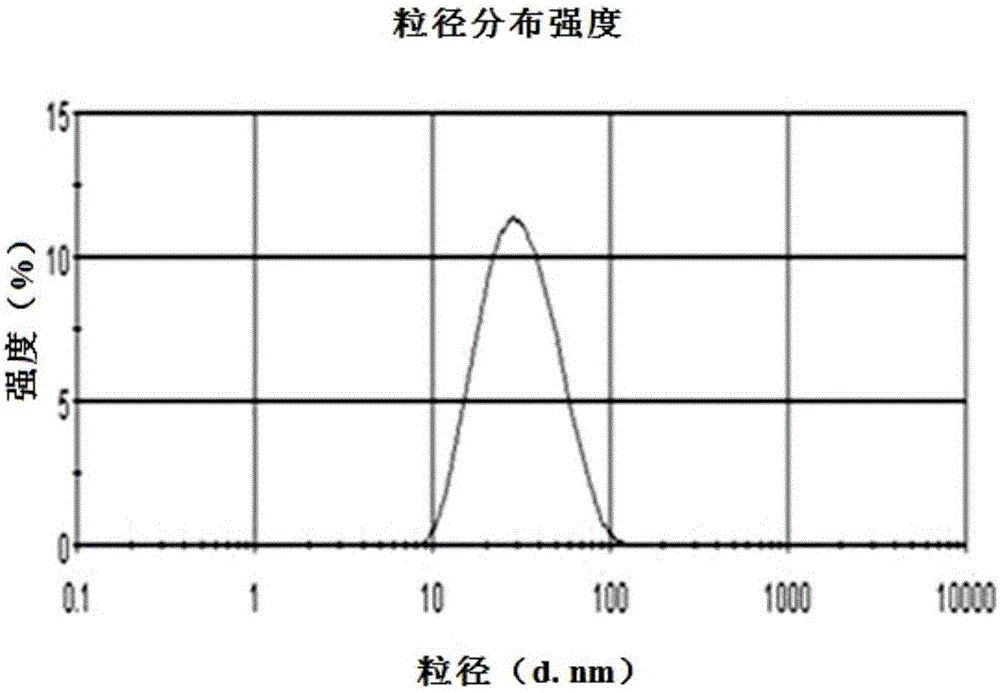
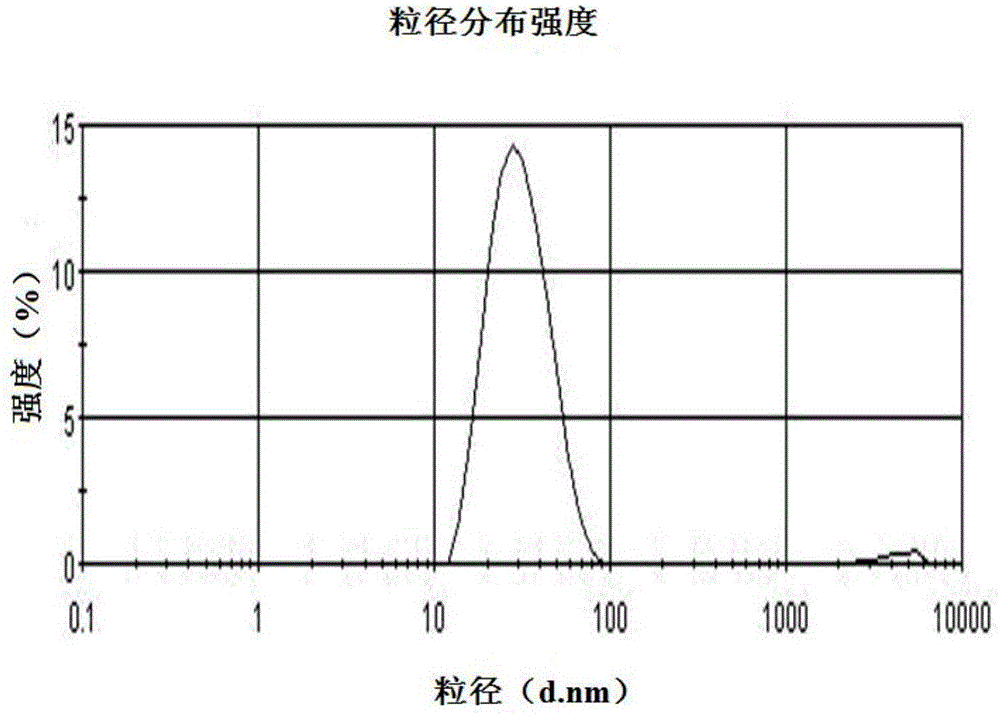
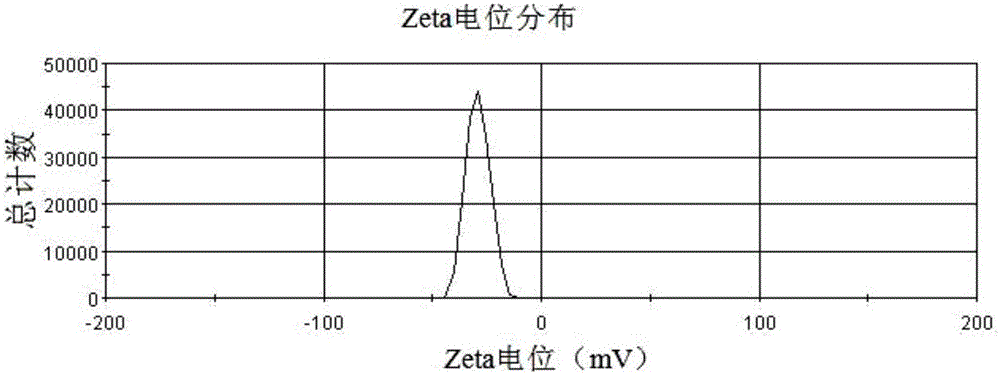
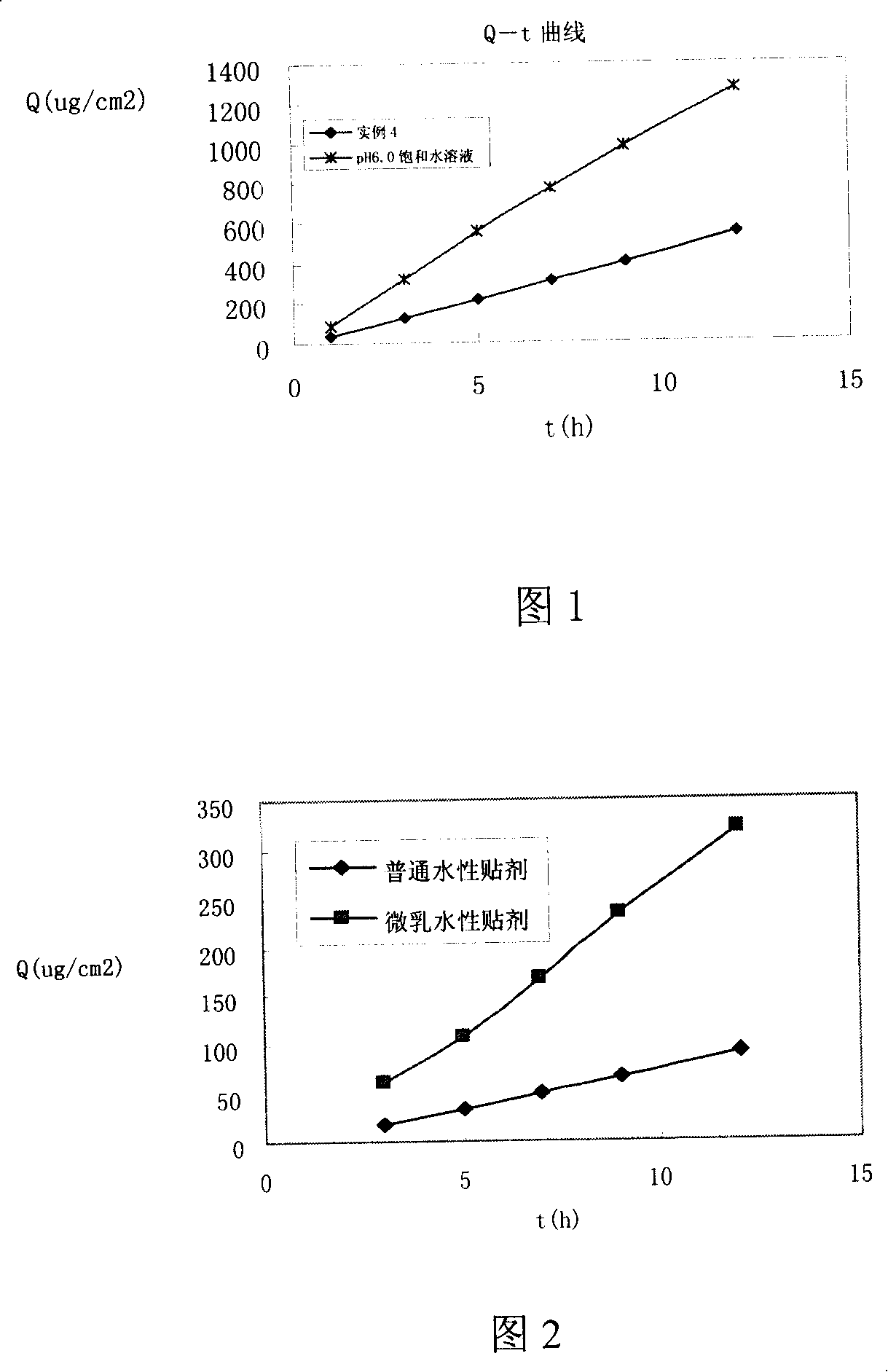
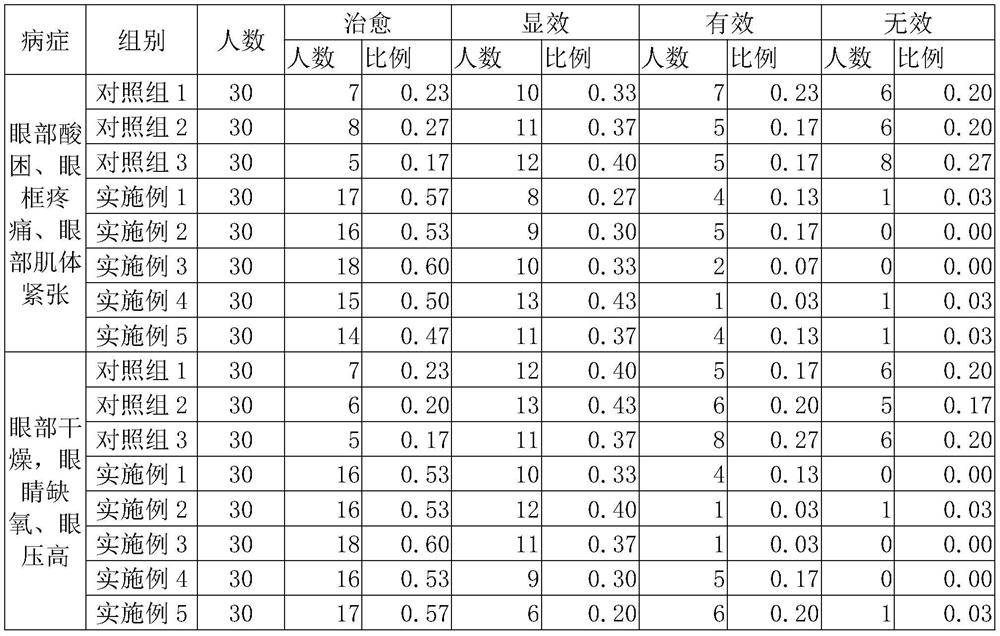
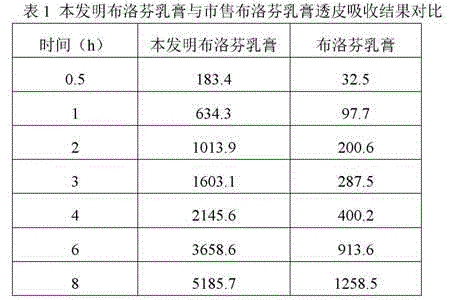
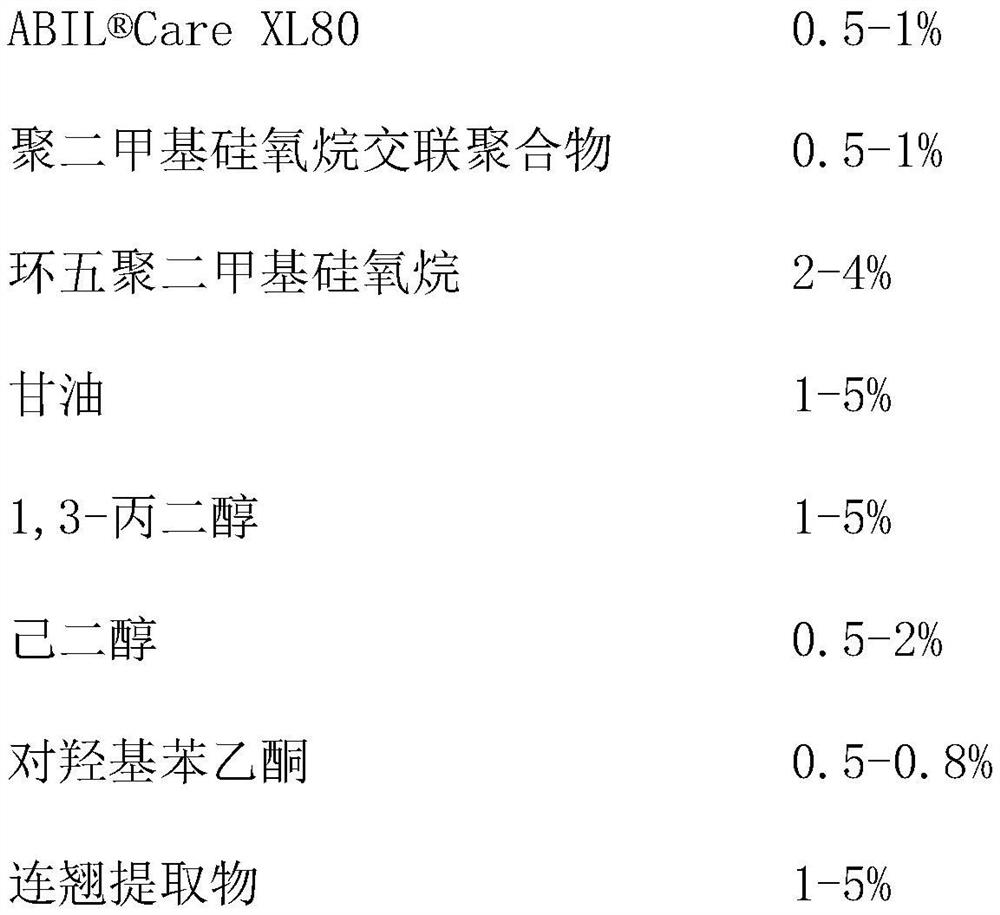
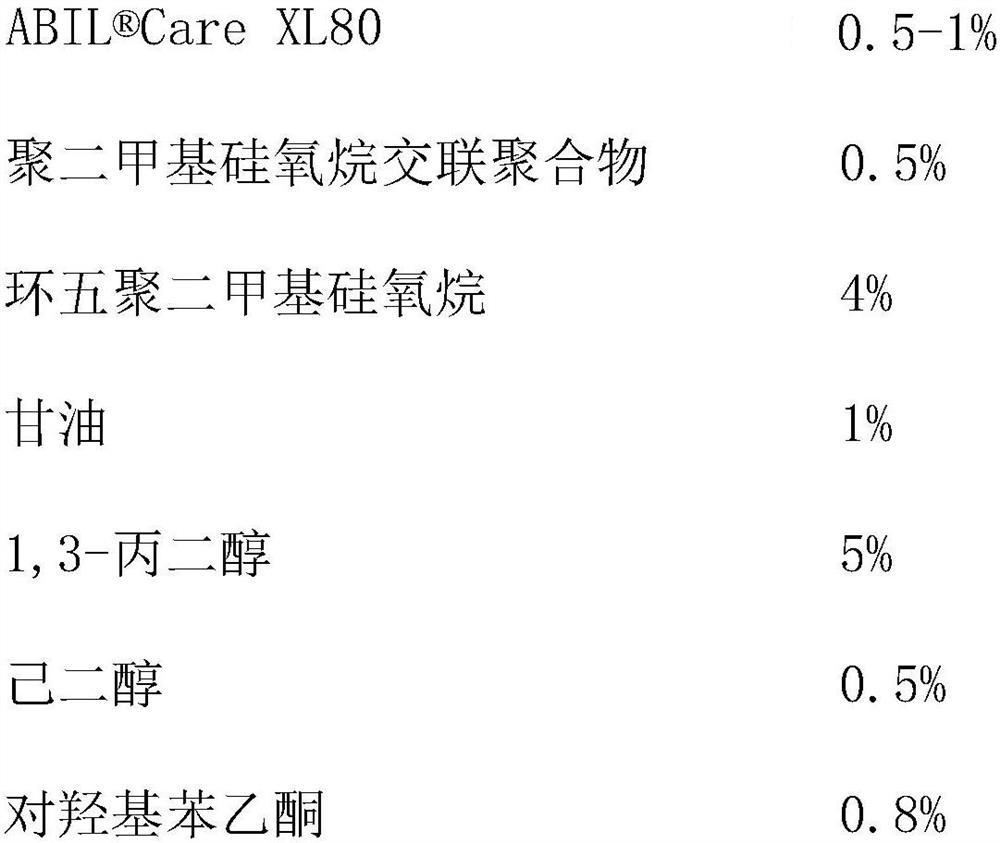
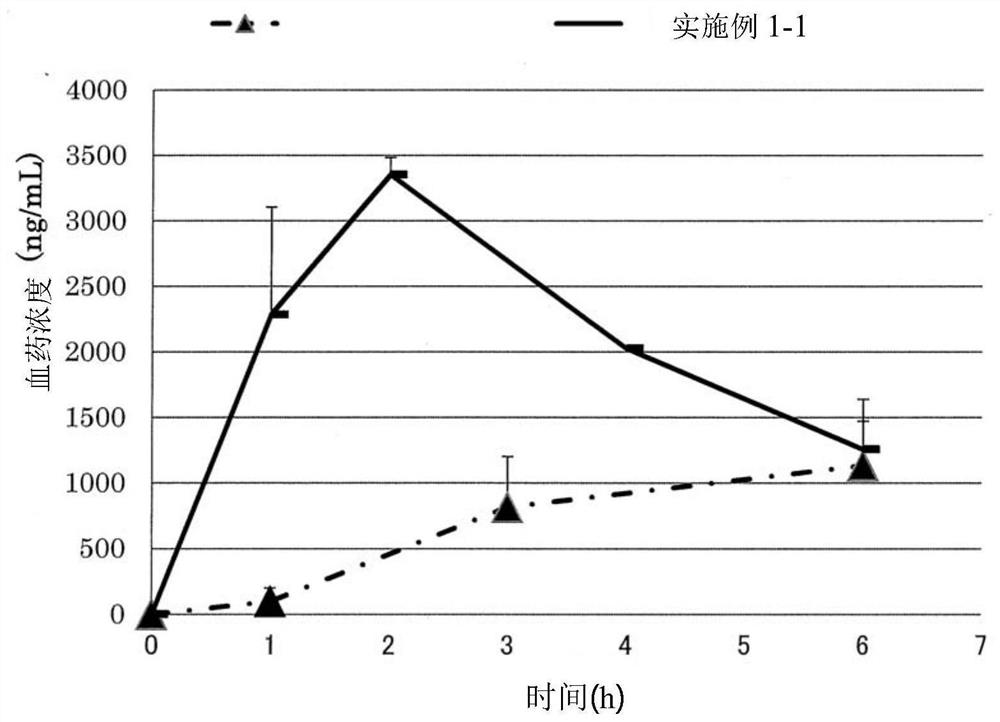
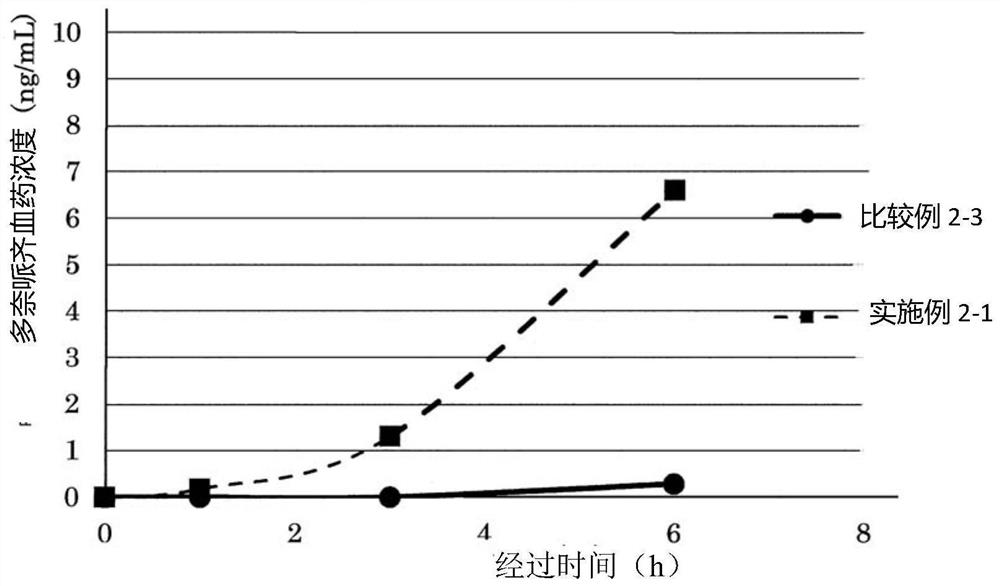
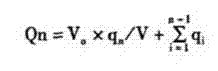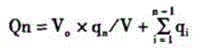Patents
Literature
51results about How to "Improve skin penetration rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
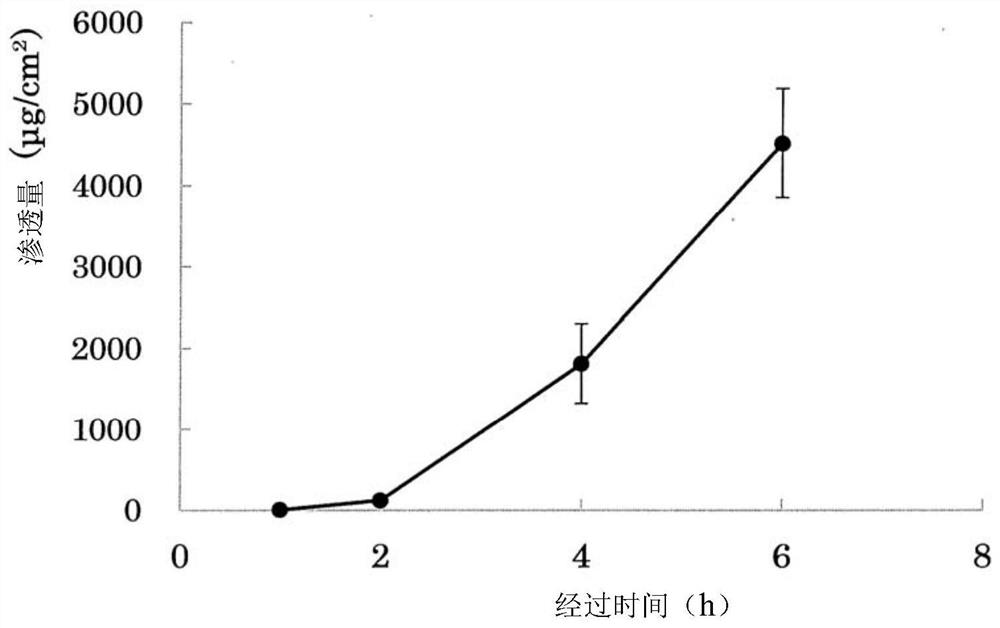
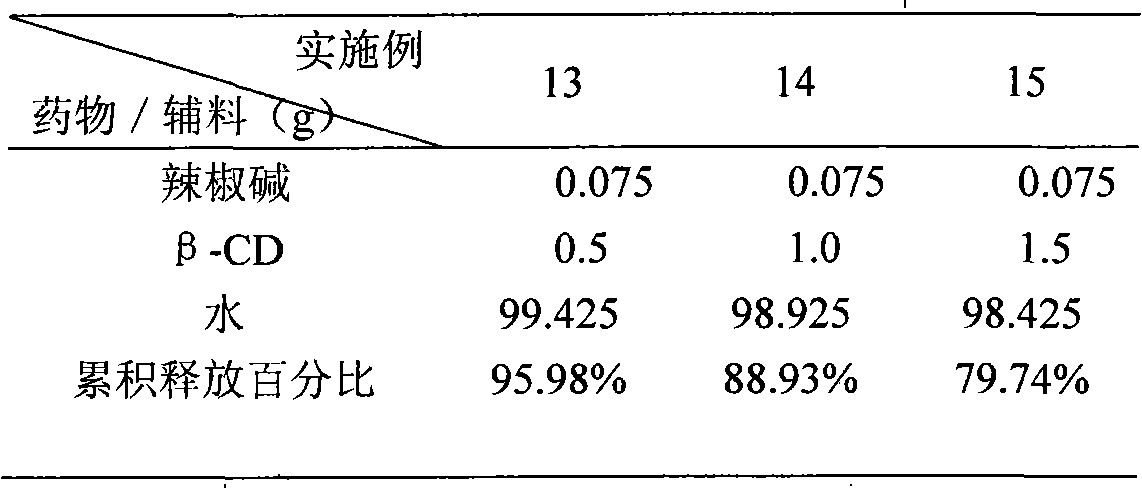
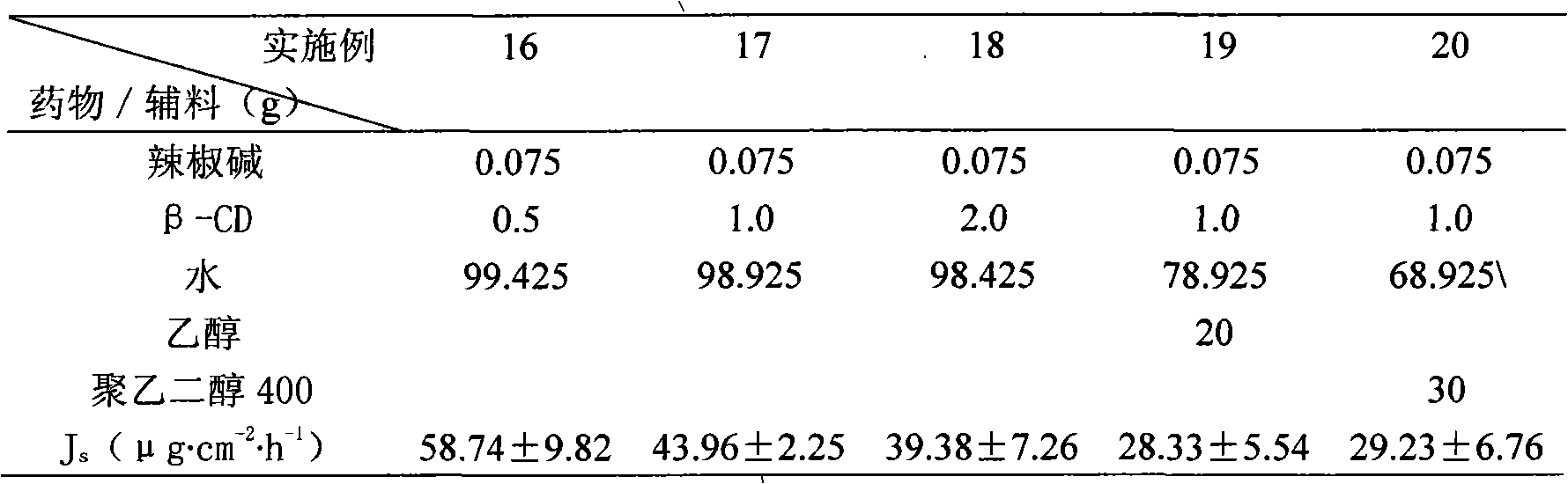
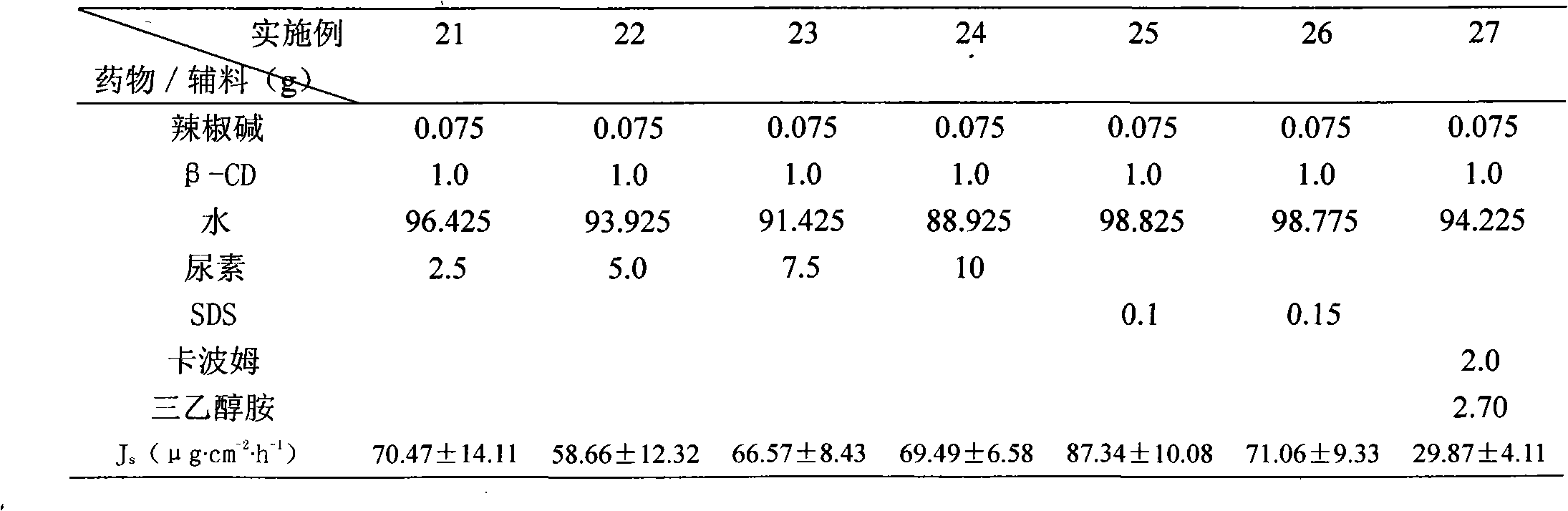
Capsaicin beta-cyclodectrin inclusion-compound and liposome and gel of inclusion-compound
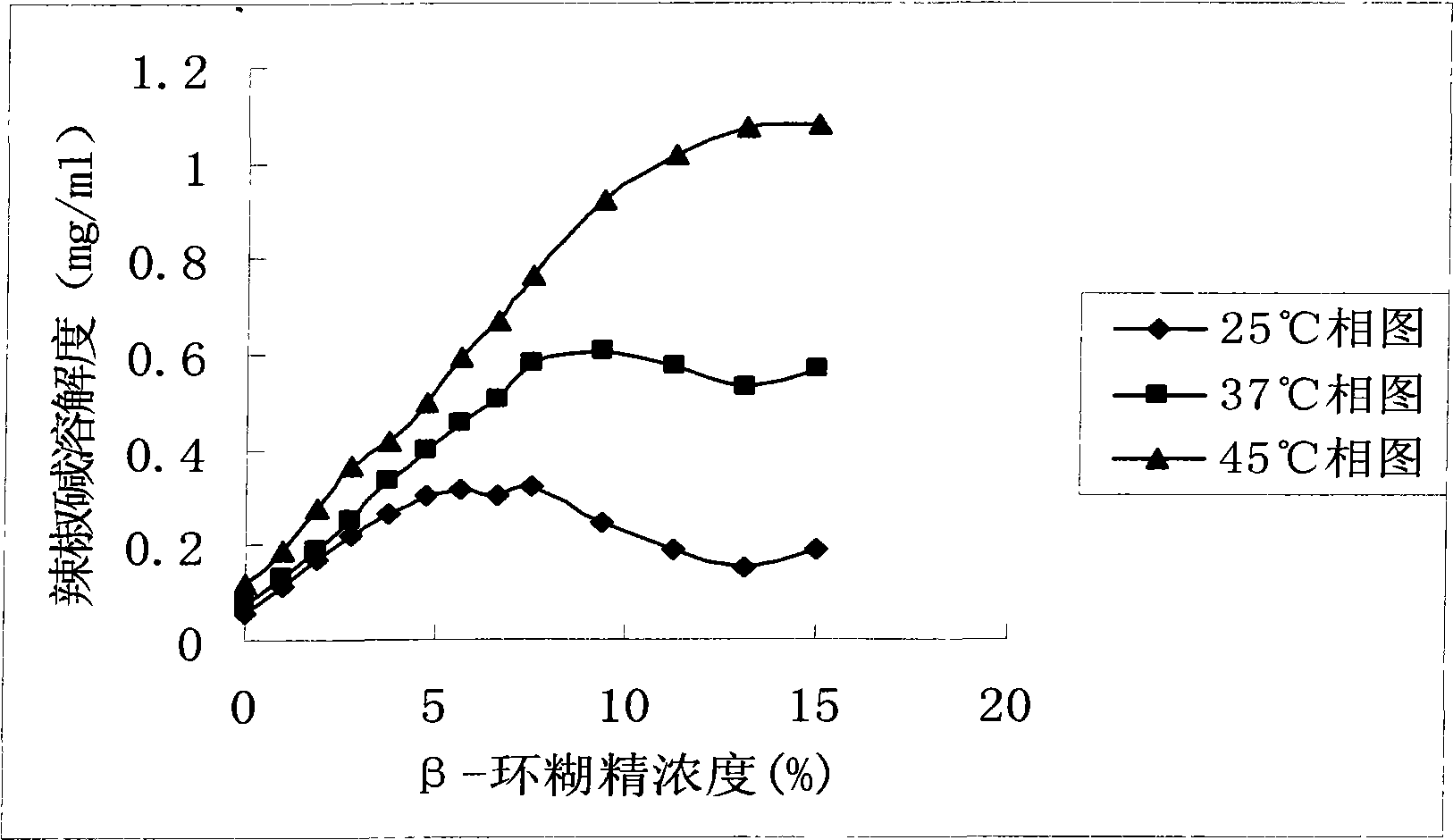
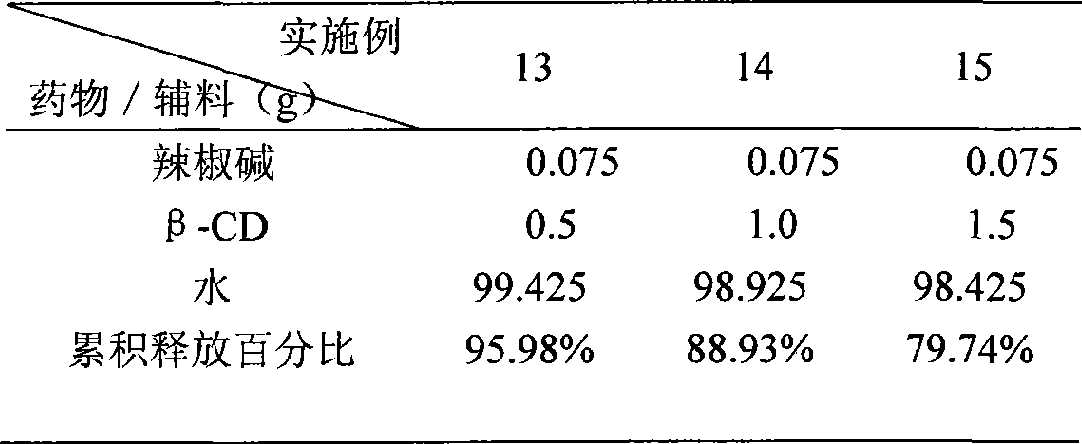
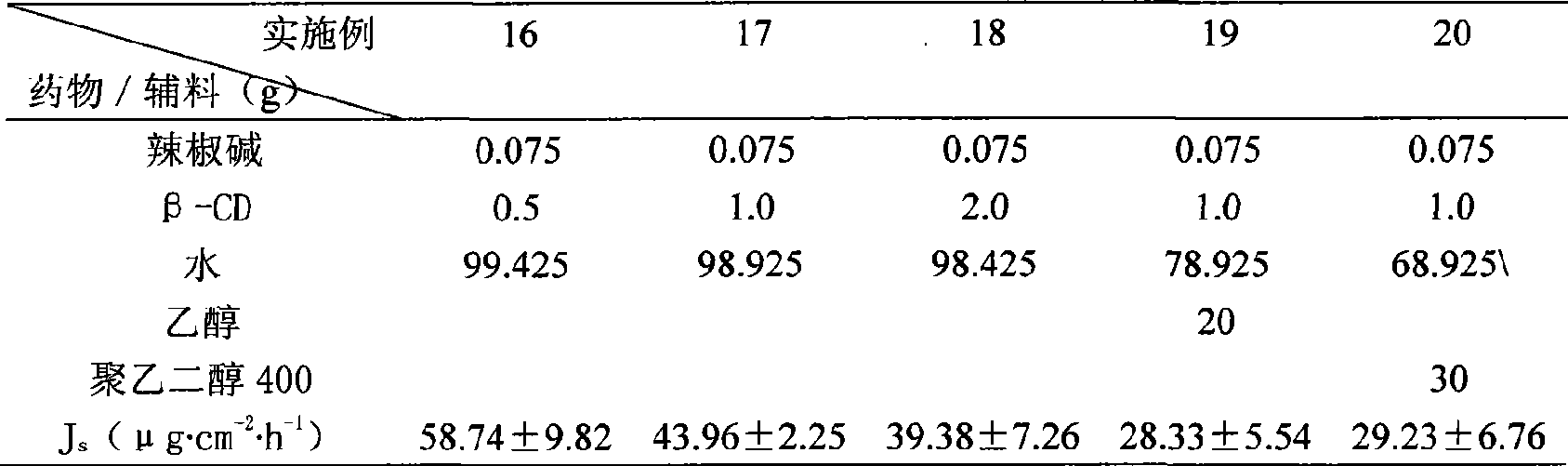
InactiveCN101507818ALess irritatingImprove apparent solubilityOrganic active ingredientsAntipyreticSolubilityIrritation
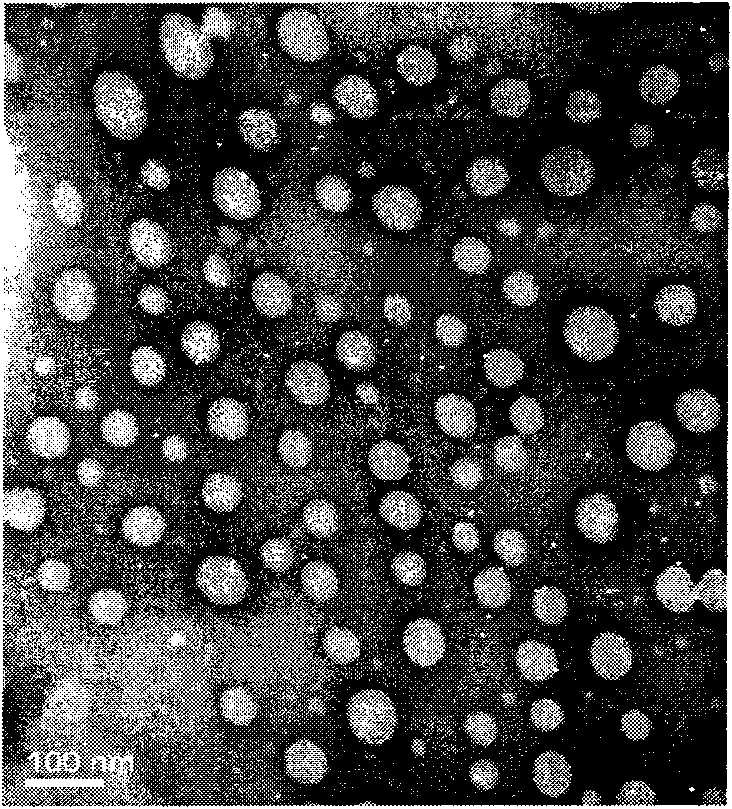
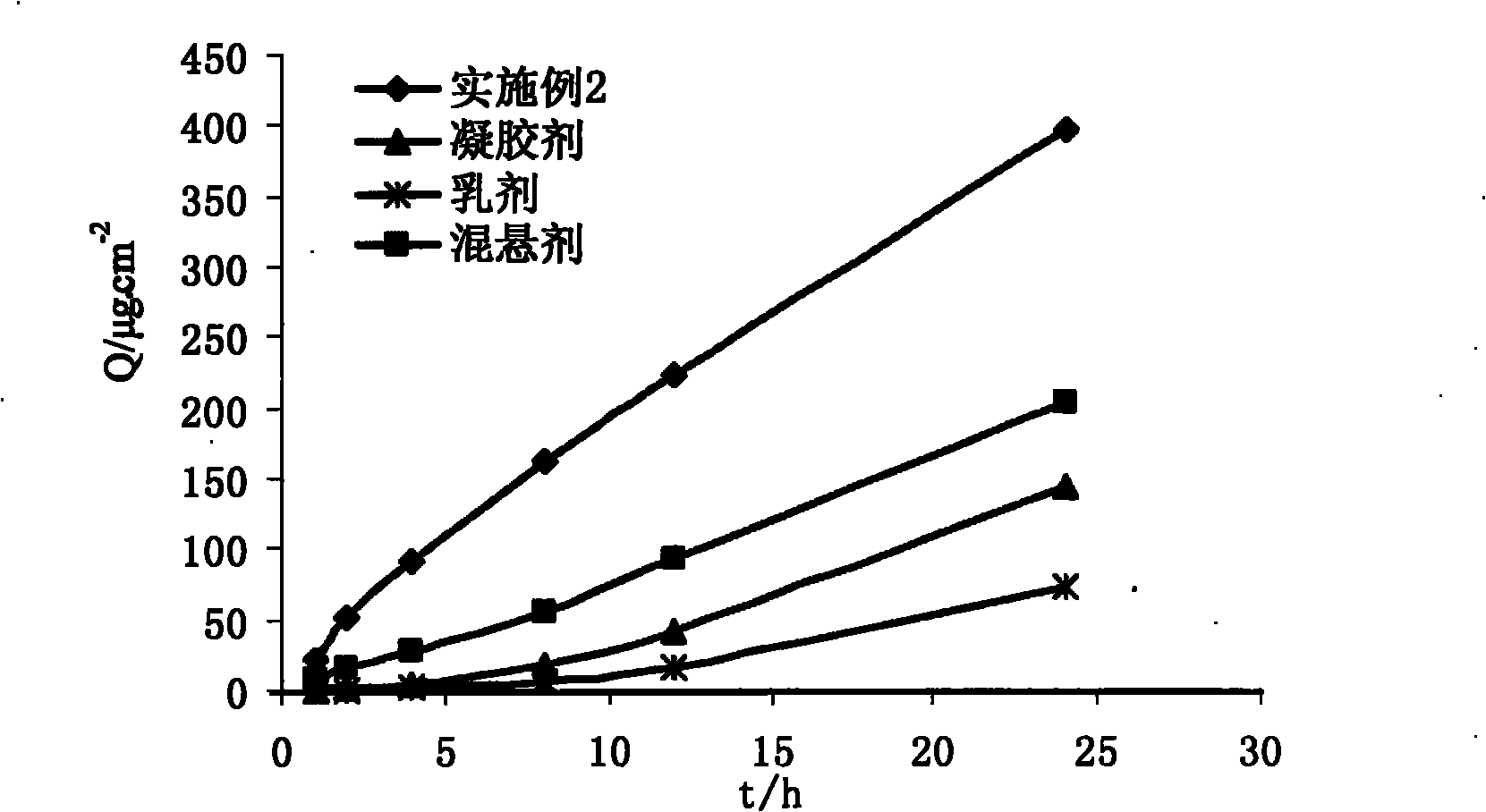
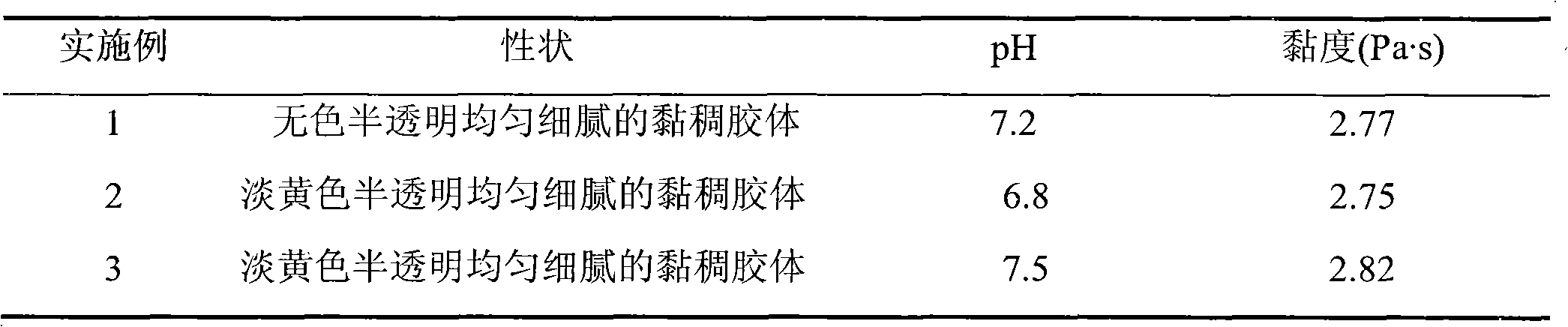
The invention discloses a capsaicin beta-cyclodextrin inclusion compound, as well as liposome and gel thereof. The capsaicin beta-cyclodextrin inclusion compound is prepared by the following method: (1) beta-cyclodextrin is dissolved in an aqueous phase and prepared into a solution, and the solution is added with capsaicin or a capsaicin solution so as to obtain a mixed solution of capsaicin and beta-cyclodextrin; and (2) the solution-type capsaicin beta-cyclodextrin inclusion compound is obtained from the mixed solution prepared in a step (1) through at least one treatment mode of standing, heating, stirring, ultrasound or grinding. The apparent solubility of the capsaicin of the inclusion compound increases by 2 to 20 times. In vitro transdermal study shows that the capsaicin inclusion compound can obviously raise the transdermal rate of medicine by 5 to 20 times as compared with cream and hydrogel, and obviously reduce the irritation of capsaicin at skin. Besides transdermal drug administration, the prepared inclusion compound and the inclusion compound liposome can also be orally taken, injected or administrated in bladders. In addition, the invention is simple in production process, and the quality is stable and easy to control.
Owner:LOGISTICS UNIV OF CAPF
Risperidone percutaneous absorption paster
InactiveCN101366705AProlong the action timeLittle side effectsOrganic active ingredientsNervous disorderTectorial membraneSide effect
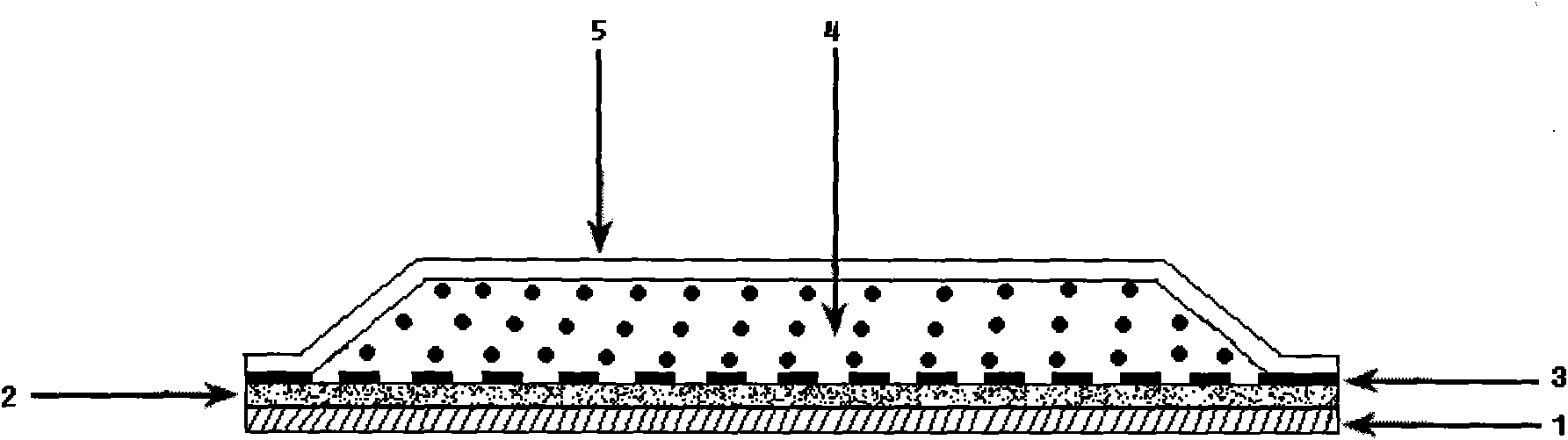
The invention discloses a risperidone transdermal absorbing patch, which consists of a back lining layer, a protective film and a substrate layer which is arranged between the back lining layer and the protective film and contains medicines. The compositions of the substrate layer containing the medicines by weight percent are 65 to 95 percent of polyacrylate pressure sensitive adhesive, 4 to 15 percent of risperidone and 1 to 20 percent of transdermal enhancer. The patch delivers the risperidone by means of transdermal impregnation, can prolong the acting time of the medicine, maintains stable blood drug level, reduces the side effect of the medicine, is convenient to use, and can be taken as a medicine for treating various mental sickness such as schizophrenia, mania or dementia and so on.
Owner:ZHEJIANG UNIV
Transdermal delivery type hydrogel as well as preparation method and application thereof
InactiveCN108653198AGood transdermal absorptionImprove skin penetration ratePeptide/protein ingredientsMetabolism disorderPhotoinitiatorChemistry
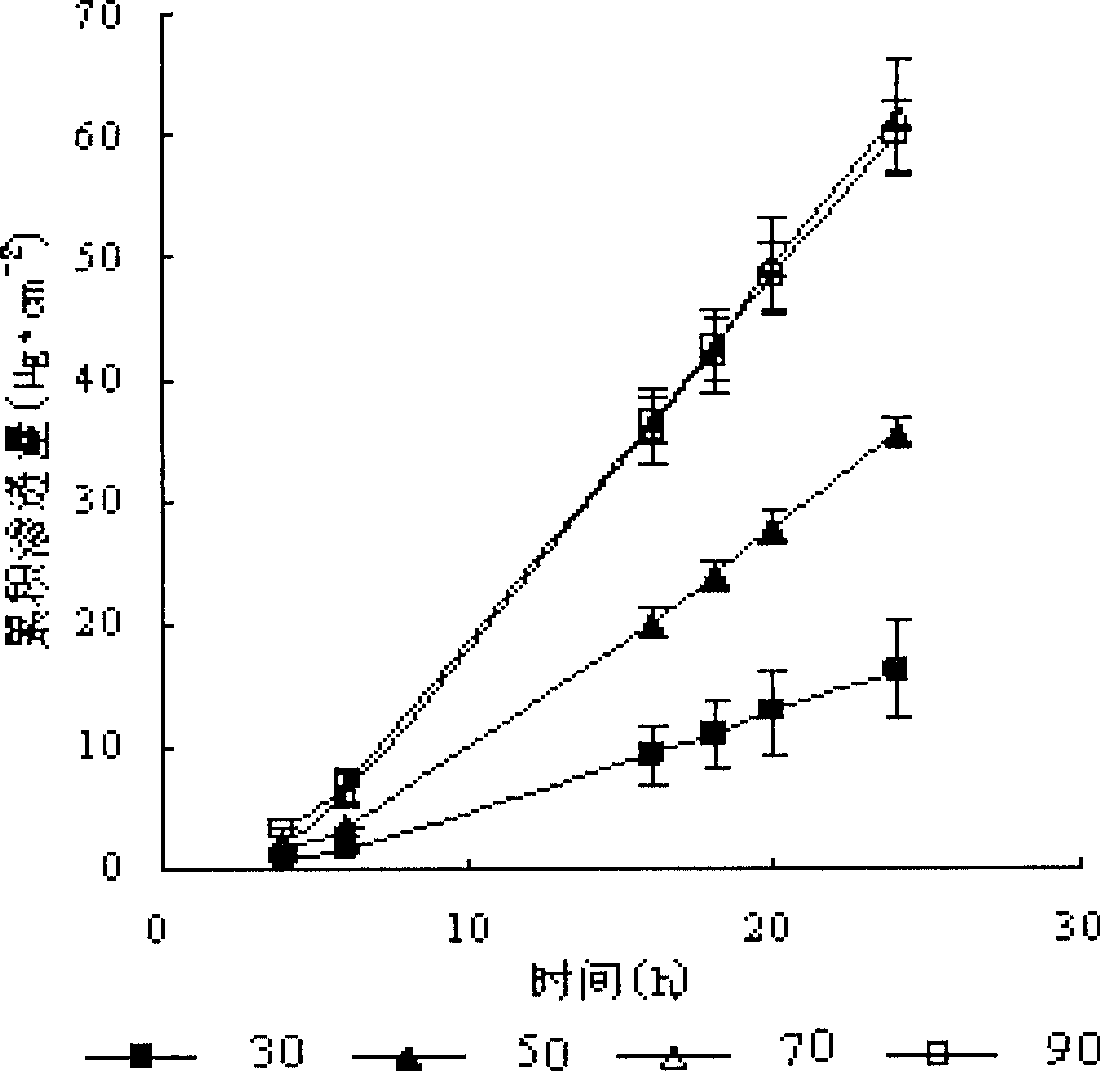
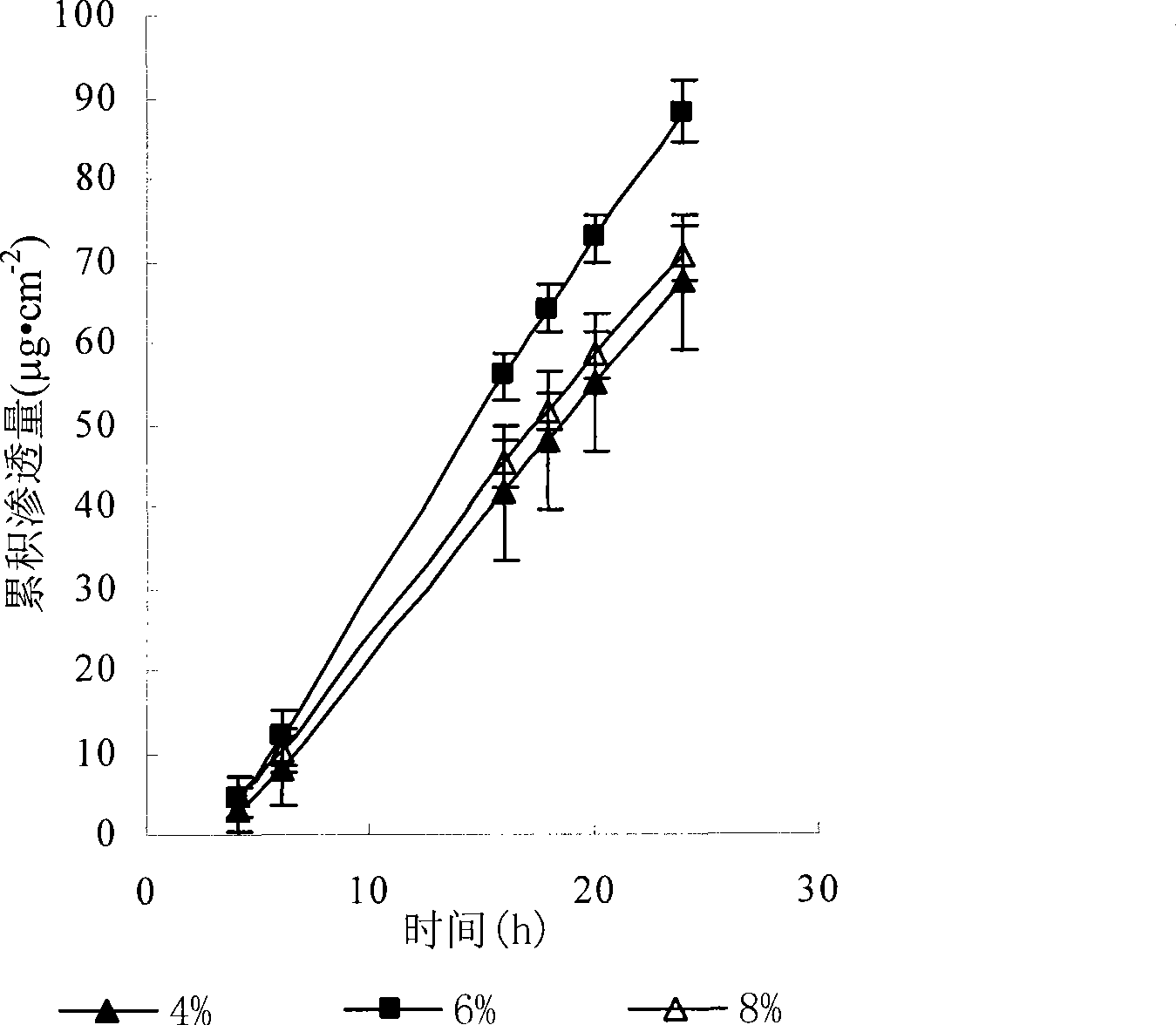
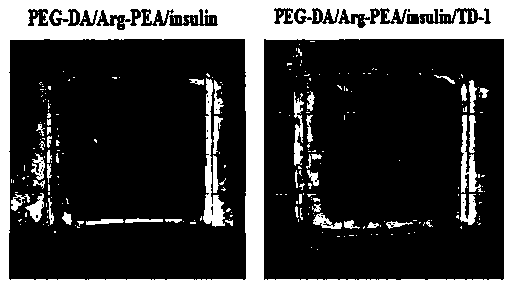
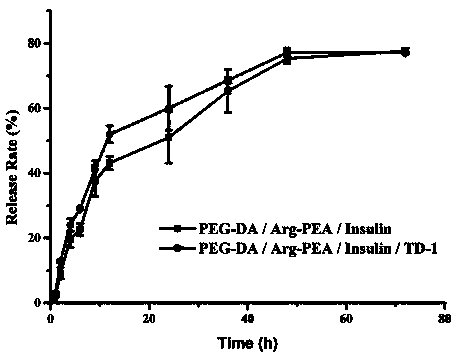
The invention discloses transdermal delivery type hydrogel as well as a preparation method and application thereof. The hydrogel is prepared from an active component, permeation enhancing peptide, unsaturated polyesteramide of arginine, polyethylene glycol diacrylate, a photoinitiator and water, wherein the sum of the mass percent of the unsaturated polyesteramide of arginine and polyethylene glycol diacrylate is 10 percent to 20 percent; the sum of the mass percent of the active component, the permeation enhancing peptide, the photoinitiator and the water is 80 percent to 90 percent. The hydrogel disclosed by the invention has the advantages of easiness for transdermal absorption, good biocompatibility and no cytotoxicity; a protein-type drug can be loaded very well and insulin is given to patients with diabetes mellitus in a transdermal delivery manner, so that the hydrogel has an efficient blood glucose lowering effect and the utilization dosage of the drug can be greatly reduced; controllable slow release drug administration is also realized in a disease treatment process; the transdermal delivery type hydrogel has high safety and is convenient to use, so that the transdermal delivery type hydrogel is an ideal transdermal patch for clinically treating the diabetes mellitus and has a good application prospect.
Owner:SUN YAT SEN UNIV
Granisetron hydrochloride microemulsion-based gel and preparation method thereof
InactiveCN101933902APromote transdermal absorptionImprove skin penetration rateOrganic active ingredientsDigestive systemOil phaseMicroemulsion
The invention discloses a granisetron hydrochloride microemulsion-based gel and a preparation method thereof. The preparation contains microemulsion and blank gel, wherein the microemulsion consists of granisetron hydrochloride or free alkali thereof, oil phase, emulsifier, co-emulsifier and water phase; and the blank gel consists of gel matrix, neutralizer, humectant and water. The preparation method of the preparation uses the conventional pharmaceutical equipment and comprises the following steps: evenly mixing microemulsion and blank gel in a certain ratio, adding a certain amount of penetration enhancer, and adjusting the pH value to obtain the granisetron hydrochloride microemulsion-based gel. The preparation has simple preparation technology, high production efficiency, low cost, stable quality and easy industrial production. The product of the invention has good effect of transdermal absorption, hardly has any stimulation on the skin and can satisfy the clinical medication requirements basically, thus the product is a novel preparation with good compliance and high therapeutic index.
Owner:CHONGQING MEDICAL UNIVERSITY
Pravastatin transdermal administration preparation and preparation method thereof
ActiveCN101856342ALittle side effectsLower total cholesterolMetabolism disorderEster active ingredientsIrritationRelease time
The invention relates to a pravastatin transdermal administration preparation and a preparation method thereof. In the invention, pravastatin or a pharmaceutically acceptable salt of the pravastatin serves as a medicament active ingredient, and the medicament active ingredient is a filling closed transdermal preparation which consists of a protective layer, an adhesive layer, a controlled-release membrane layer, a medicament reservoir and a backing layer; the medicament reservoir comprises 1 to 20 percent of pravastatin, and the balance of reservoir substrate; the adhesive layer comprises 0 to 10 percent of pravastatin, and the balance of pressure-sensitive adhesive; the reservoir substrate comprises 5 to 50 percent of transdermal enhancer, while the adhesive layer comprises 0 to 20 percent of transdermal enhancer; and the medicament release area of the preparation is 1 to 100 cm<2>. The pravastatin transdermal administration preparation is convenient to use and carry; the first pass effect of a liver and a gastrointestinal tract is avoided; and an in-vivo experiment of an animal proves that the preparation has no irritation or sensitization to skin. The pravastatin transdermal administration preparation can keep stable and persistent blood concentration, the continuous medicament releasing time is 1 to 7 days, so that the preparation provides the more convenient and safer treatment method for a patient.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Capsicine microemulsion and microemulsion gel rubber
InactiveCN101108165ASkin irritationImprove skin penetration rateOrganic active ingredientsAntipyreticPolymer scienceSkin penetration
The invention discloses a capsicine microemulsion and microemulsion gelatin. The capsicine microemulsion is prepared with the following methods: (1) according to quality percentage, measure: 0.001 per cent to 5 per cent of capsicine, 1 per cent to 90 per cent of oil phase, 0 to 80 per cent of surfactant, 1 per cent to 90 per cent of cosurfactant, 1 per cent to 90 per cent of water pahse and 0 to 40 per cent of energizer; (2) mix the components evenly, or mix them with a mixer, or emulsify them with an emulsifying machine, or grind them with a colloid mill. Compared with capsicine cream and hydrogel, the microemulsion in the invention can outstanding improve the drug skin penetration rate by 2 to 6 times, effectively reduce the capsicine's pungency to skin and can be administrated via skin penetration, oral taking, injection or intravesical medication with simple production process, stable and controllable quality.
Owner:LOGISTICS UNIV OF CAPF
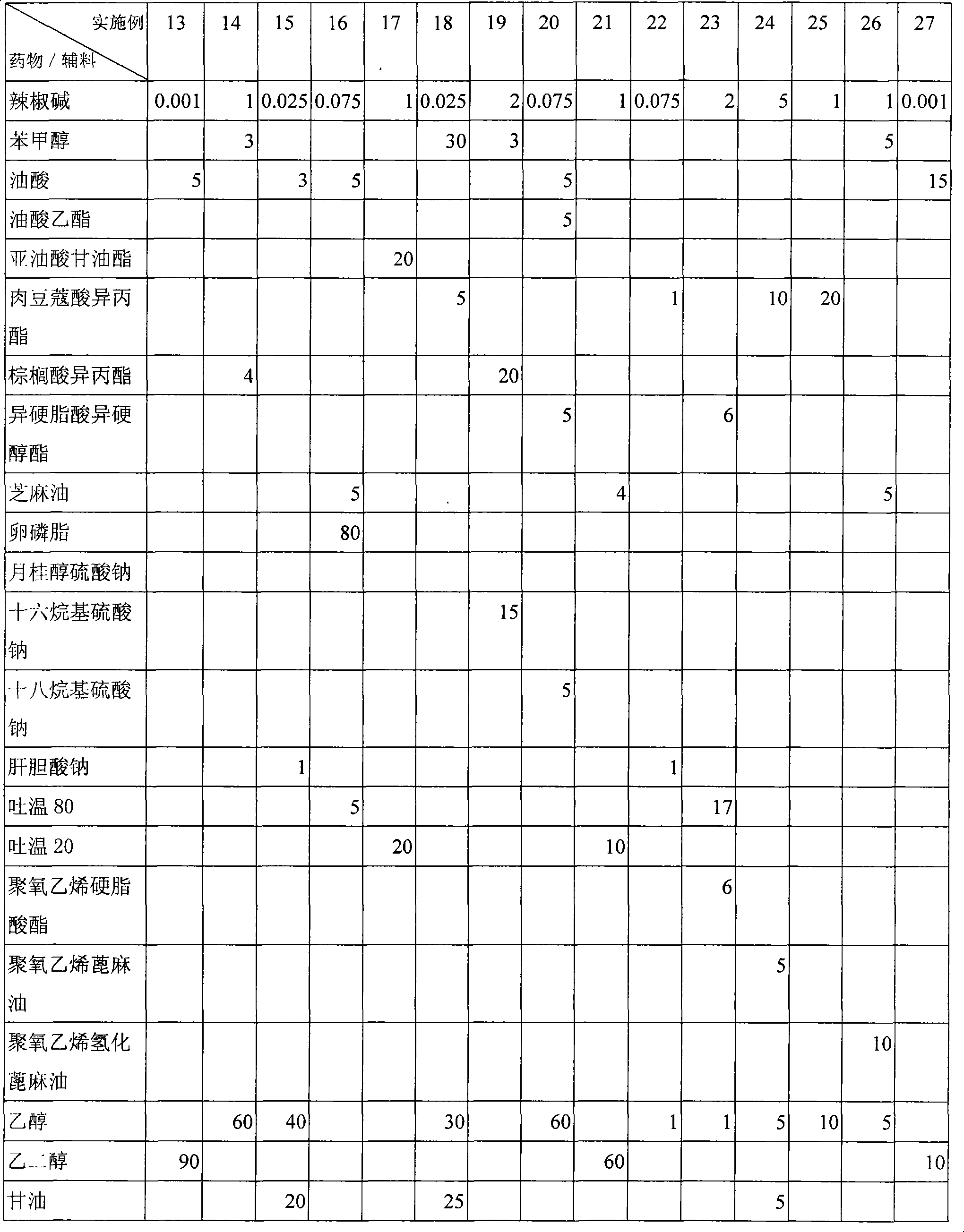
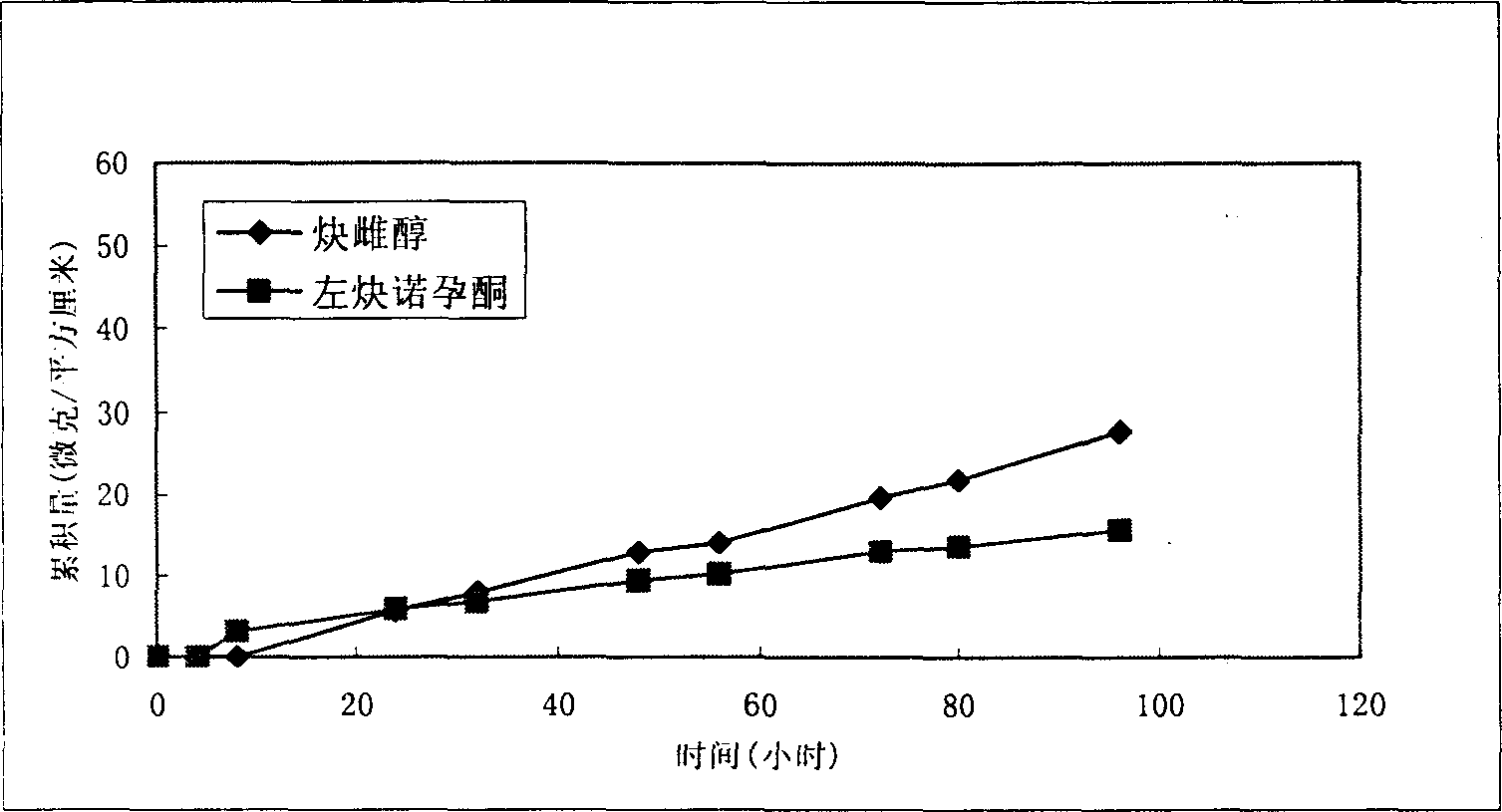
Percutaneous contraceptive drugs delivery system and method
ActiveCN1633995AImprove skin penetration rateHigh hormone contentOrganic active ingredientsMedical devicesDiethylene glycolDelivery system
The invention provides a percutaneous contraceptive drugs delivery system and method, wherein the delivery system (TCDS) comprises a back lining layer, a viscous polymeric medicine storage layer adhered on the back lining layer, a strippable protective layer adhered on the viscous polymeric medicine storage layer, the viscous polymeric medicine storage layer comprises skin permeable accelerant composition, humectant, viscous polymer, active dosage of one or more steroid hormones, wherein the skin permeable accelerant composition is a mixture of dimethyl sulphoxide, 2-hydroxypropanoic aliphatic alcohol ester, diethylene glycol alkyl ether and oleinic acid by the initial weight ratio of 2 : 1 : 1 : 0.8 to 5 : 1 : 1 : 0.8. The invention can substantially improve steroid hormone transdermal speed and increase TCDS effectiveness and convenience.
Owner:RUNBIO BIOTECH CO LTD
Compound antimalarial composition
InactiveCN104224787ASmall particle sizeIncrease the speed of transdermalOrganic active ingredientsAerosol deliveryOrganic solventMedicine
The invention relates to a compound antimalarial composition. Particularly, the invention relates to a compound antimalarial composition containing artesunate and febrifugine and in particular relates to a compound antimalarial ethosome composition containing artesunate ethosomes and febrifugine ethosomes, a compound antimalarial ethosome gel paste as well as a preparation method and an application of the compound antimalarial ethosome gel paste. The compound antimalarial ethosome gel paste is prepared from the following components including a gel paste matrix, the artesunate ethosomes, the febrifugine ethosomes, a backing and an antisticking layer. According to the compound antimalarial ethosome gel paste disclosed by the invention, by utilizing the carrier permeation enhancing property of the ethosomes, the percutaneous permeability of an antimalarial medicament and the antimalarial effect of the medicament are improved; in addition, with the adoption of the compound antimalarial ethosome gel paste, the preparation technology is simple, high-temperature heating is not needed in the preparation process, and a harmful organic solvent is avoided.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Novel penetration-promoting agent composition and application thereof to transdermal administration system
ActiveCN101940790AImprove solubilityImprove skin penetration rateOrganic active ingredientsOrganic non-active ingredientsOsteoporosisSide effect
The invention provides a novel penetration-promoting agent composition and the application thereof to a transdermal administration system. The penetration-promoting agent provided by the invention contains an etheric compound and an alcohol compound and more preferably contains fatty acid having 6 to 18 carbon atoms or fatty alcohol having 6 to 18 carbon atoms. In the penetration-promoting agent of the invention, dimethylsulfoxide (DMSO) with controversial potential toxicity to a human body is not used; and an obtained product has the advantages of high transdermal rate, high adhesion, no potential toxic or side effect to the human body and safe and comfortable use. The transdermal administration system provided by the invention is applied by being stuck to the skin of a woman so as to fulfill the aims of effectively controlling birth and realizing conception control. The novel penetration-promoting agent composition can also be used for preventing and treating osteoporosis, climacteric metancholia of women and the like.
Owner:广东伊康纳斯生物医药科技股份有限公司 +1
Chinese herbal compound microemulsion gel emplastrum, and its preparation method and application
InactiveCN107412699AIncrease moisture contentHigh strengthHydroxy compound active ingredientsAntipyreticCross-linkAnaphylaxis
The invention relates to a Chinese herbal compound microemulsion gel emplastrum, and its preparation method and application. In particular, the invention relates to a microemulsion gel emplastrum composition. The microemulsion gel emplastrum composition comprises methyl salicylate, menthol, camphor, borneol, traditional Chinese medicine extract containing active ingredients such as paeonol, eugenol and piperine, a microemulsion matrix, a hydrophilic polymer framework material, a binder, a cross-linking agent, a cross-linking regulator, etc., wherein the active ingredients are entrapped in the microemulsion. The invention further relates to a preparation method for the microemulsion gel emplastrum composition and application of the microemulsion gel emplastrum composition to blood circulation invigoration and pain relieving. The microemulsion gel emplastrum composition in the invention not only ensures high-degree integration of drugs and the matrix, and also has the characteristics of low irritation to skin, little anaphylaxis, good transdermal absorption performance, simple preparation process, etc.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI +1
Eprinomectin transdermal agent, preparation method and application
ActiveCN104095866AInsect repellent effect is efficientImprove insect repellent effectOrganic active ingredientsDigestive systemWhite mouseNematode
The invention discloses an eprinomectin transdermal agent, a preparation method and application. The eprinomectin transdermal agent is composed of azone, oleic acid, propylene glycol, dimethyl sulfoxide and alcohole with a certain weight amount. The preparation method comprises: A, performing sample preparation, namely, setting five levels for each transdermal promoting agent, and adding alcohol for preparing samples; B, performing in-vitro transdermal experiments, fixedly disposing skin of a white mouse between a dispersion pool and a receiving pool of a YB-P6 intelligent transdermal tester, enabling the inner layer surface of skin to face to the receiving pool, enabling the whole dispersion pool to be full with the eprinomectin transdermal-agent formula sample, and adding acetonitrile to fill the receiving pool as a receiving solution; C, detecting the receiving solution obtained in the step B by using high performance liquid chromatographic instrument, and selecting a formula through visual analysis and variance analysis on an orthogonal experiment result; D, preparing the formula and verifying according to the front steps; and E, preparing a final product, and preparing a transdermal agent containing 0.025-0.1 g of eprinomectin. The formula is reasonable, convenient to use and capable of continuously expelling parasites without needing withdrawal time, and has the expelling rate of 90% or more on nematode and ectoparasites.
Owner:HUAZHONG AGRI UNIV
Nano-emulsion in-situ gel for treating haemorrhoids
InactiveCN103735620AImprove solubilityImprove skin penetration rateHydroxy compound active ingredientsAntipyreticSalvia miltiorrhizaHemorrhoids
The invention relates to nano-emulsion in-situ gel for treating haemorrhoids. The gel comprises the following components by weight: 30-100g of sophora flavescens, 30-120g of salviae miltiorrhizae, 30-100g of wild chrysanthemum, 1-8g of borneol, 1-8g of menthol, 30-160g of polyoxyethylated castor oil, 10-80g of absolute ethyl alcohol, 5-60g of isopropyl myristate, 60-160g of poloxamer 407, and 5-80g of poloxamer 188. The nano-emulsion in-situ gel can infiltrate into a mucous membrane to play the effects of clearing heat, removing toxicity, drying dampness, relieving itching, activating blood circulation to remove stasis, diminishing edema and relieving pain, and can be used for promoting local blood circulation, and improving nutrition of peripheral tissues to achieve the aims of diminishing edema and inflammation, and relieving pain.
Owner:HENAN UNIV OF CHINESE MEDICINE
Estradiol transdermal spray and preparation method thereof
InactiveCN102018671AImprove complianceImproves transdermal penetrationOrganic active ingredientsNervous disorderBlood concentrationPatient compliance
The invention discloses an estradiol transdermal spray, composed of the following raw materials in percentage by weight: 0.1-10% of estradiol, 0.5-10% of polymer, 0-20% of penetration enhancer, 0-10% of plasticizer and balance of solvent. The estradiol transdermal spray provided by the invention is applied to skin in a spray from, a uniform polymer thin film is formed after the solvent is volatilized, and medicine is dispersed in the film in a molecule form, so that not only the medicine transdermal penetration effect is improved and the blood concentration is sustained and stable, but also the patient compliance is good. The estradiol transdermal spray is filled in a spraying device and can be directly used, thus being easy and simple to use, and the dosage can be freely adjusted as required. The invention also discloses a preparation method of the estradiol transdermal spray, which has strong operability and low technological conditional requirements, and is simple and easy to realize and easy for industrialized production.
Owner:ZHEJIANG UNIV
Method for preparing ibuprofen cream
ActiveCN103690474AIncrease penetration rateQuick effectOrganic active ingredientsAntipyreticBenzoic acidMenthol
The invention discloses a method for preparing ibuprofen cream. The method comprises the following steps: mixing white vaseline, lanolin, beewax and menthol and dissolving to obtain an oil phase; dissolving carboxymethyl-ethyl-beta-cyclodextrin in water to obtain carboxymethyl-ethyl-beta-cyclodextrin solution, dissolving ibuprofen in ethyl acetate to obtain an ibuprofen ethyl acetate solution; adding the ibuprofen ethyl acetate solution into the carboxymethyl-ethyl-beta-cyclodextrin solution to prepare an ibuprofen cyclodextrin inclusion solution, adding ethyl p-hydroxybenzoate into the ibuprofen cyclodextrin inclusion solution to obtain a water phase; adding the oil phase into the water phase, stirring to prepare the ibuprofen cream, and flavoring and filling. By virtue of the ibuprofen cream prepared by the method, the infiltration speed of the ibuprofen cream can be improved, so that the characteristics of quick acting and good pain relieving effect can be achieved.
Owner:郑州大明药物科技有限公司
Ointment for clearing heat, cooling blood, stopping bleeding, diminishing swelling and promoting wound healing and preparation method thereof
InactiveCN110787261ARelieve painReduce economyHydroxy compound active ingredientsAerosol deliveryWound healingMyrrh
The invention discloses ointment for clearing heat, cooling blood, stopping bleeding, diminishing swelling and promoting wound healing and a preparation method thereof. The ointment is prepared from the following raw materials: rheum officinale, rhizoma coptidis, radix notoginseng, radix angelicae dahuricae, colla corii asini, calcined dragon bones, rhizoma bletillae, myrrh, cuttlebone, radix rubiae, dragon's blood, pearl, borneol and liquorice roots. The ointment has very good curative effects on various skin trauma bleeding, ulcer, ulceration and the like, the treatment period is short, andthe pain and economic burden of a patient are greatly reduced. The ointment directly acts on an affected part, improves the absorption efficiency of medicines, promotes rapid healing of bleeding points and ulcer surfaces, has the advantages of rapid curative effect, short course of treatment, pure traditional Chinese medicine preparation, no toxic or side effect, wide and easily available medicinesource, convenient application, external application to the affected part, small dosage, no pain and no recurrence after healing.
Owner:XIAN CHIHO PHARMA
Medicine extract submicron emulsion gel and preparation method of medicine extract submicron emulsion gel
InactiveCN106728227AImprove efficiencyShort course of treatmentAerosol deliveryOintment deliverySide effectPreservative
The invention discloses medicine extract submicron emulsion gel. The medicine extract submicron emulsion gel is prepared from the following raw materials in parts by weight: 20 to 50 parts of cortex moutan, 20 to 50 parts of rhizoma chuanxiong, 10 to 40 parts of safflower, 5 to 60 parts of herba selaginellae and 5 to 60 parts of herba geranii; the medicine extract submicron emulsion gel further comprises 50mg / g to 300mg / g of a medical oil phase, 5mg / g to 50mg / g of an emulsifier, 1mg / g to 50mg / g of an assistant emulsifier, 0.1mg / g to 35mg / g of a stabilizer, 0.1mg / g to 20mg / g of an antioxygen, 5mg / g to 50mg / g of a gel matrix and 0.5mg / g to 3mg / g of a preservative. The medicine extract submicron emulsion gel has the effects of promoting flow of qi and blood circulation, expelling wind and detoxifying, clearing heat and expelling dampness and is used for treating targeted medicine erythra, radiodermatitis and chemotherapeutic extravasation and has high effective rate, small toxic side effect and short course treatment.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
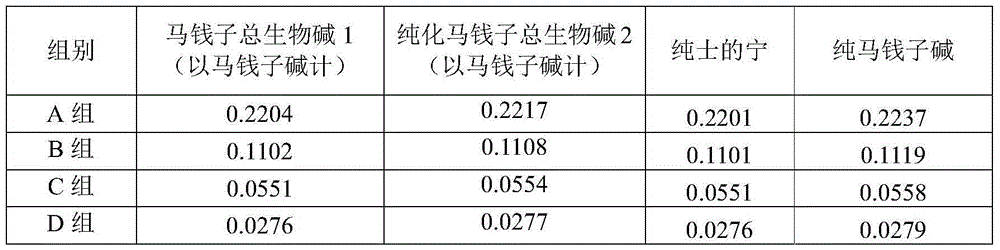
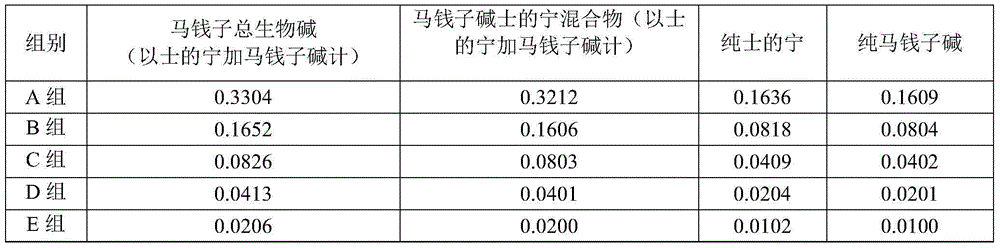
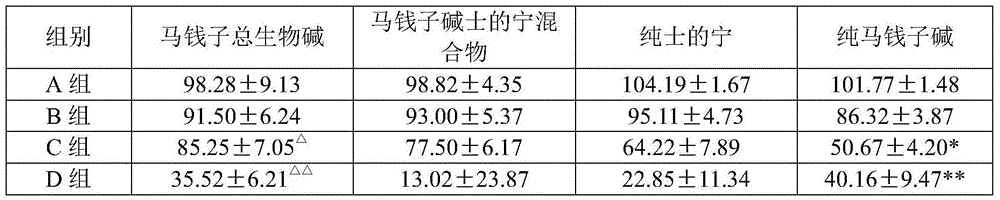
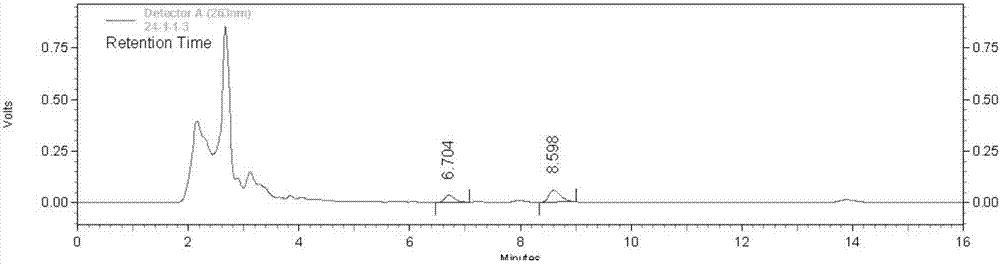
Preparation method and pharmaceutical application of nux vomica total alkaloids
InactiveCN103908493AHigh yieldImprove skin penetration rateAntipyreticAnalgesicsJoint arthralgiaEthyl acetate
The invention discloses a preparation method and a pharmaceutical application of nux vomica total alkaloids. The preparation method of the invention comprises the following steps: (1) crushing nux vomica, sieving with a sieve of 10-40 meshes to prepare coarse powder; (2) weighing 100g-200g of the coarse powder, performing heating reflux extraction with 1000mL-2000mL of alcohol acid solution, performing centrifugation, removing precipitates, recovering the alcohol acid solution at a reduced pressure to obtain a concentrate; (3) adding a pH adjusting agent, adjusting the pH value of the concentrate to 9-14, extracting with 3000mL-4000mL of an extraction solvent, recovering the solvent at a reduced pressure, drying at a reduced pressure to obtain nux vomica total alkaloids powder; (4) dissolving the nux vomica total alkaloids powder in a mixed acid solution, performing centrifugation to obtain the supernatant; (5) adding the pH adjusting agent, adjusting the pH value of the supernatant to 10-13, extracting with dichloromethane or ethyl acetate, recovering the solvent at a reduced pressure, and drying at a reduced pressure to obtain nux vomica total alkaloids. The purified product of nux vomica total alkaloids prepared in the invention can increase the transdermal rate of main drug components rapidly, improve the bioavailability, is low in toxicity, and has significant effect on treating rheumatic arthralgia.
Owner:ZHEJIANG UNIV OF TECH +2
Transdermal-absorption preparation containing compound cortex phellodendri liquid and preparing method and application of transdermal-absorption preparation
ActiveCN106983789APromote transdermal absorptionImprove skin penetration rateHydroxy compound active ingredientsAntipyreticMentholBioavailability
The invention relates to a transdermal-absorption preparation containing compound cortex phellodendri liquid and a preparing method and application of the transdermal-absorption preparation. The transdermal-absorption preparation comprises the compound cortex phellodendri liquid, borneol and menthol. The transdermal-absorption preparation can be used to prepare infection-preventing drugs and can be prepared into a lotion, an aerosol, paste or gel and the like. The transdermal-absorption preparation has the advantages that the borneol and the menthol can have a synergic effect, the transdermal-absorption rate of the drug effective component berberine hydrochloride can be enhanced evidently, the effects of clearing away heat and toxic materials and relieving swelling and removing necrotic tissue of the compound cortex phellodendri liquid are increased, and drug bioavailability is further increased.
Owner:NO 411 HOSPITAL OF PLA
Ethinyl estradiol gas permeable absorbing gel
InactiveCN1679590AImprove skin penetration rateLittle side effectsOrganic active ingredientsUrinary disorderSkin permeabilityRadiology
A percutaneous absorbing gel of eston-E for treating amenorrhoea, climacteric syndrome and prostatic cancer, or for contraceptive purpose is prepared from eston-E, percutaneous absorption promoter, and carbomer.
Owner:ZHEJIANG UNIV
Compound antimalarial composition
ActiveCN105147689ALow toxicityEnhance antimalarial effectOrganic active ingredientsAerosol deliveryOrganic solventSkin penetration
The invention relates to a compound antimalarial composition. Particularly, the invention relates to a compound antimalarial composition containing artesunate and febrifugine, particularly to a compound antimalarial ethosome composition containing artesunate ethosomes and febrifugine ethosomes, and a compound antimalarial ethosome gel cataplasm, as well as the preparation method and usage thereof. The compound antimalarial ethosome gel cataplasm is composed of a gel substrate, artesunate ethosomes, febrifugine ethosomes, a backing and an anti-sticking layer. According to the compound antimalarial composition provided by the invention, carriers of the ethosomes are used for promoting percolation properties, the release and the skin penetration of the antimalarial are improved, and the anti-malarial effect of the antimalarial is enhanced; in addition, the ethosome gel cataplasm provided by the invention is simple in preparation process, high temperature heating is not required during the preparation process, and the use of a harmful organic solvent can be avoided.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Kusnezoff monkshood root esculin microemulsion and its preparation
InactiveCN101152148AImprove skin penetration rateOrganic active ingredientsAntipyreticNasal cavityMonkshood Root
The present invention discloses a Bulleyaconitine A microemulsion and the preparing method. The Bulleyaconitine A is dissolved in oil phase. Emulsifier, assistant emulsifier and other additives are added. A microemulsion which is transparent or is with slightly turbid light and is with a particle size of less than 100 nm is prepared. Compared with material drug of Bulleyaconitine A, the chemical stability and mucosa permeance capability of the drug-containing microemulsion are significantly improved. The Bulleyaconitine A microemulsion is used as releasing vector and is combined with an excipient which is acceptable on pharmacy. An appropriate preparing method is used to prepare a nasal cavity preparation which is high in biological absorption degree, safe, stable and convenient and various paper release formulation preparation. The present invention is used for the treatment of various pains.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Cold-hot medicinal moxibustion capable of relieving fatigue and reducing fine lines and having eye protection effect and preparation method of medicinal moxibustion
InactiveCN111905023AImprove permeabilityGood curative effectDevices for heating/cooling reflex pointsSenses disorderCassiaLavandula
The invention discloses cold-hot medicinal moxibustion capable of relieving fatigue and reducing fine lines and having eye protection effect and a preparation method of the medicinal moxibustion, andrelates to the technical field of moxibustion. The medicinal moxibustion includes a cold-hot bag and a medicament patch; the medicament patch includes a medicament bag and medicament; the medicament includes a medicament composition I and a medicament composition II; the medicament composition I includes, by weight in parts, 10-16 parts of wild chrysanthemum flowers, 6-12 parts of boxthorn leaves,8-12 parts of lavender, 12-14 parts of compound essential oil and 16-20 parts of a ethosome matrix; and the medicament composition II includes, by weight in parts, 12-14 parts of honeysuckle flowers,6-8 parts of cassia seeds, 5-7 parts of argy wormwood leaf, 4-6 parts of peppermint and 18-22 parts of a microemulsion matrix. The medicinal moxibustion is convenient in using, comfort and simple, can be used when people have a rest during work and study, has good effects when being used during sleep and is suitable for populations who overuse eyes and have large work stress to use.
Owner:成都蜜儿堂职业技能培训学校有限公司
The preparation method of ibuprofen cream
ActiveCN103690474BIncrease penetration rateQuick effectOrganic active ingredientsAntipyreticBenzoic acidMenthol
The invention discloses a method for preparing ibuprofen cream. The method comprises the following steps: mixing white vaseline, lanolin, beewax and menthol and dissolving to obtain an oil phase; dissolving carboxymethyl-ethyl-beta-cyclodextrin in water to obtain carboxymethyl-ethyl-beta-cyclodextrin solution, dissolving ibuprofen in ethyl acetate to obtain an ibuprofen ethyl acetate solution; adding the ibuprofen ethyl acetate solution into the carboxymethyl-ethyl-beta-cyclodextrin solution to prepare an ibuprofen cyclodextrin inclusion solution, adding ethyl p-hydroxybenzoate into the ibuprofen cyclodextrin inclusion solution to obtain a water phase; adding the oil phase into the water phase, stirring to prepare the ibuprofen cream, and flavoring and filling. By virtue of the ibuprofen cream prepared by the method, the infiltration speed of the ibuprofen cream can be improved, so that the characteristics of quick acting and good pain relieving effect can be achieved.
Owner:郑州大明药物科技有限公司
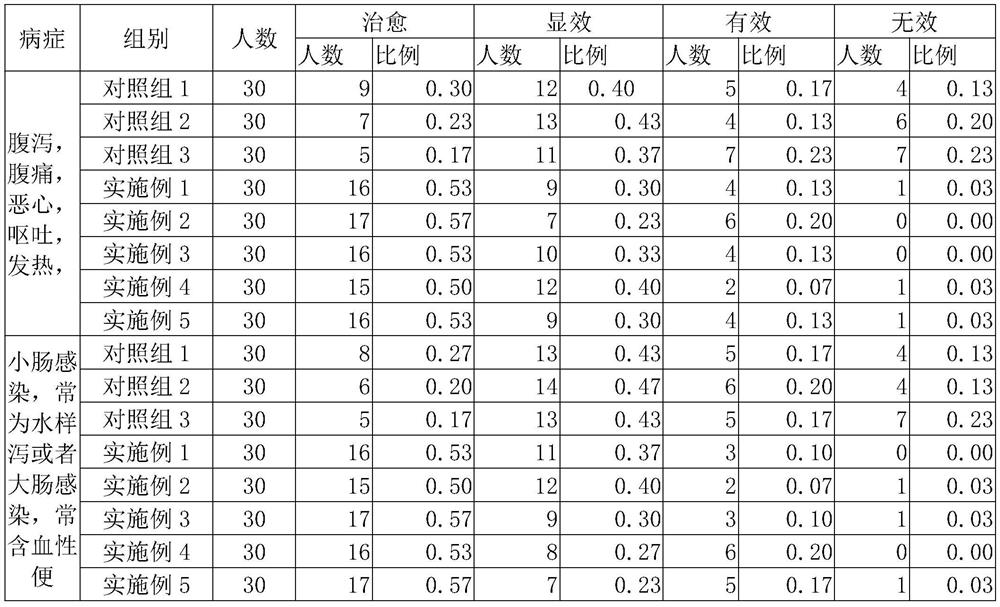
Medicinal moxibustion for acute diarrhea and preparation method thereof
InactiveCN111939205AImprove permeabilityGood curative effectDevices for heating/cooling reflex pointsDigestive systemAcute diarrheaDiarrhea symptoms
The invention discloses medicinal moxibustion for acute diarrhea and a preparation method thereof, and relates to the technical field of medical treatment and health care. The medicinal moxibustion comprises a heating bag and a medicament patch, wherein the medicament patch comprises a medicament bag and a medicament; the medicament comprises a medicament composition I and a medicament compositionII; the medicament composition I is prepared from the following raw materials in parts by weight: 13 to 15 parts of fructus evodiae, 8 to 14 parts of cortex cinnamomi, 8 to 10 parts of flos caryophylli, 12 to 14 parts of compound essential oil and 16 to 20 parts of ethosome matrix; the medicament composition II is prepared from the following components in parts by weight: 10 to 14 parts of semenmyristicae, 6 to 10 parts of fructus piperis, 5 to 7 parts of folium artemisiae argyi, 4 to 8 parts of radix bupleuri and 18 to 22 parts of microemulsion matrix. The medicinal moxibustion for the acute diarrhea, disclosed by the invention, can be used for rapidly adjusting water metabolism of a human body, recovering normal functions and alleviating and eliminating diarrhea symptoms; the treatmentcourse is obviously shortened and no toxic side effect is caused; and the medicinal moxibustion has no abnormal medicinal taste and is low in cost.
Owner:国人健康管理(杭州)有限公司
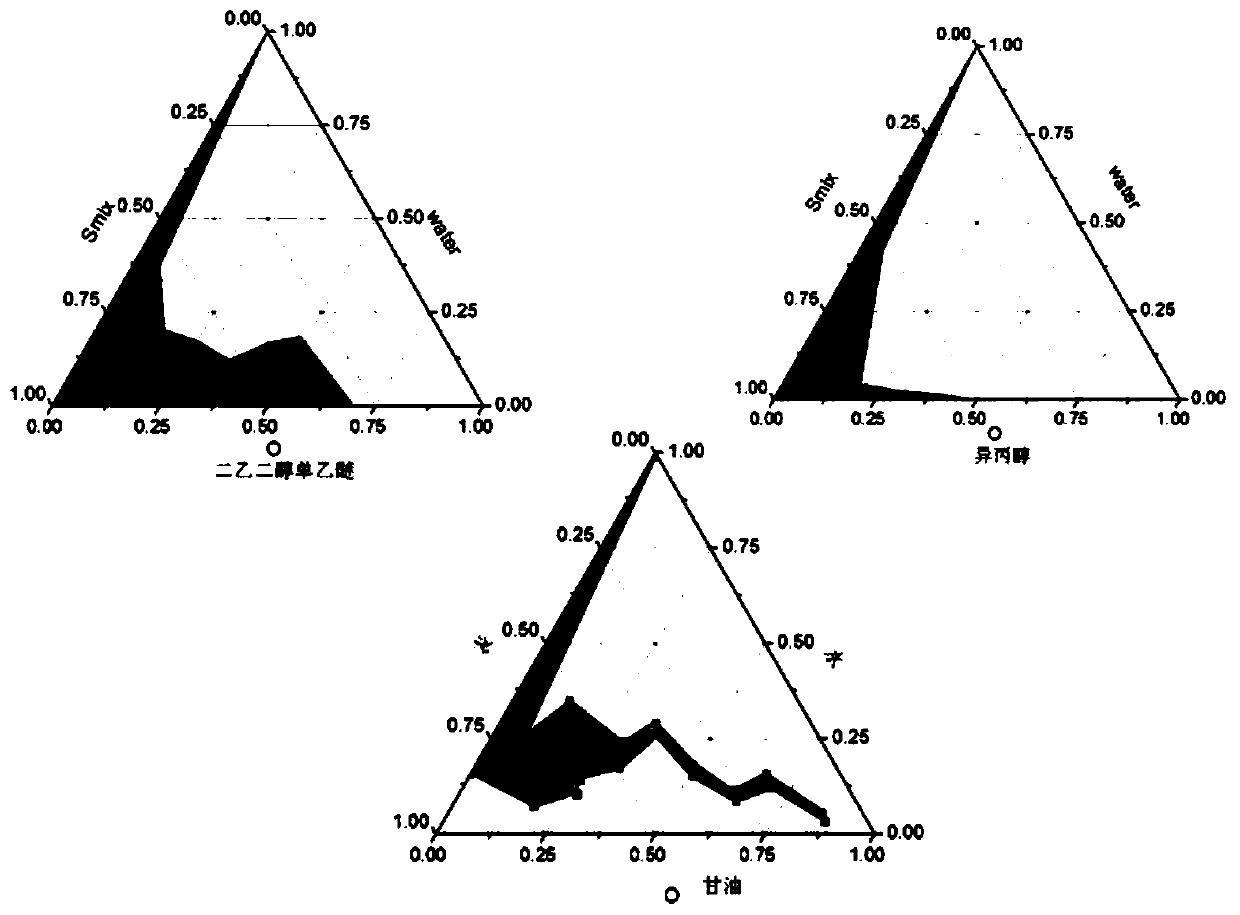
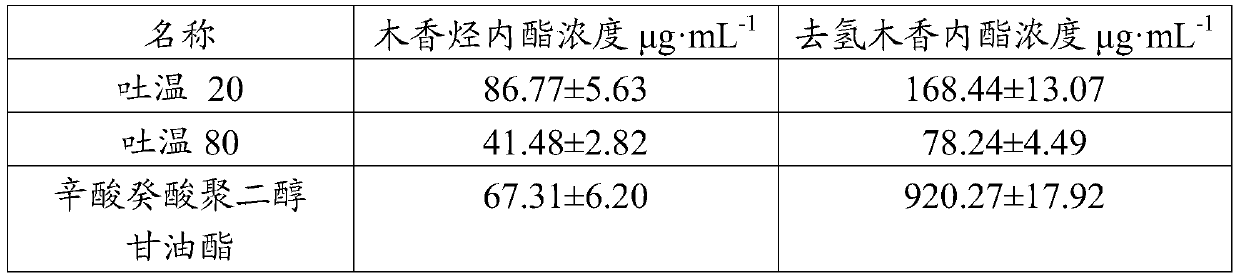
Radix aucklandiae extract microemulsion as well as preparation method and application thereof
ActiveCN110859804AImprove skin penetration rateReduce adverse reactionsOrganic active ingredientsAntipyreticDiethylene glycol monoethyl etherEthylene glycol monoethyl ether
The invention provides a radix aucklandiae extract microemulsion as well as a preparation method and an application thereof. The radix aucklandiae extract microemulsion provided by the invention is prepared from a radix aucklandiae extract, isopropyl myristate, Tween 80, diethylene glycol monoethyl ether and deionized water. The invention also provides the preparation method of the radix aucklandiae extract microemulsion, which comprises the following steps: 1) proportionally weighing isopropyl myristate, Tween 80, diethylene glycol monoethyl ether, deionized water and the radix aucklandiae extract to prepare a suspension; and 2) uniformly stirring the suspension obtained in the step 1) to obtain the microemulsion. In the microemulsion provided by the invention, the transdermal rates of costunolide and dehydrocostunolide are obviously improved, and compared with an aqueous solution of the radix aucklandiae extract, the permeation enhancing ratio of costunolide and dehydrocostunolide ofthe radix aucklandiae extract microemulsion is obviously improved.
Owner:天津市尖峰天然产物研究开发有限公司
Acne-removing composition and application thereof, and acne-removing nanogel emulsion comprising the acne-removing composition
ActiveCN113171322BPropionibacteriumInhibition formationCosmetic preparationsToilet preparationsEpitheliumBacillus acnes
The invention discloses an acne-removing composition, which is characterized in that, the acne-removing composition comprises forsythia extract, walnut spring flower extract and wintergreen extract. It can effectively inhibit Propionibacterium acnes, balance skin oil secretion, accelerate the shedding of stratum corneum cells, loosen keratinocytes accumulated at the opening of sebaceous glands, correct abnormal keratinization of hair follicle epithelium, smooth the excretion of sebaceous gland secretions, and inhibit the formation of acne. The results of crowd trials have confirmed that it has a good acne-removing effect.
Owner:广东中科卓原生物科技有限公司
transdermal liquid formulation
ActiveCN107613961BShort lag timeReduce stimulationNervous disorderSolution deliveryOctanolPropanediol
The object of the present invention is to provide a liquid preparation for external use which shortens the lag time of transdermal absorption and has excellent percutaneous permeability, and provides a liquid preparation for external use which reduces skin irritation. A percutaneous absorption type liquid preparation that can solve the above problems, which contains: (a) propylene glycol 40-98w / w%; (b) phosphatidylcholine 0.1-5w / w%; (c) oleyl alcohol and / or isodeca Octaconol 0.1-10w / w%; (d) drug 0.1-25w / w%. Furthermore, it is preferable to contain alkanolamine. The alkanolamine is preferably triethanolamine.
Owner:MEDRX CO LTD
Capsaicin beta-cyclodectrin inclusion-compound and liposome and gel of inclusion-compound
InactiveCN101507818BImprove skin penetration rateLess irritatingOrganic active ingredientsAntipyreticSolubilityIrritation
The invention discloses a capsaicin beta-cyclodextrin inclusion compound, as well as liposome and gel thereof. The capsaicin beta-cyclodextrin inclusion compound is prepared by the following method: (1) beta-cyclodextrin is dissolved in an aqueous phase and prepared into a solution, and the solution is added with capsaicin or a capsaicin solution so as to obtain a mixed solution of capsaicin and beta-cyclodextrin; and (2) the solution-type capsaicin beta-cyclodextrin inclusion compound is obtained from the mixed solution prepared in a step (1) through at least one treatment mode of standing, heating, stirring, ultrasound or grinding. The apparent solubility of the capsaicin of the inclusion compound increases by 2 to 20 times. In vitro transdermal study shows that the capsaicin inclusion compound can obviously raise the transdermal rate of medicine by 5 to 20 times as compared with cream and hydrogel, and obviously reduce the irritation of capsaicin at skin. Besides transdermal drug administration, the prepared inclusion compound and the inclusion compound liposome can also be orally taken, injected or administrated in bladders. In addition, the invention is simple in production process, and the quality is stable and easy to control.
Owner:LOGISTICS UNIV OF CAPF
External preparation with analgesic and anti-inflammatory effects and preparation method thereof
InactiveCN110917227AHigh drug loadingImprove skin penetration rateOrganic active ingredientsAntipyreticAnti-inflammatoryPolyacrylamide
The invention discloses an external preparation with analgesic and anti-inflammatory effects. The external preparation comprises a drug composition and a hydrogel matrix; the drug composition consistsof 80-100g of Sichuan lovage rhizome oil and 5-10g of ibuprofen; and 1000g of the hydrogel matrix consists of the following components in percentage by weight: 10-15g of polyacrylamide, 15-20g of sodium alginate, 80-100g of a transdermal absorption enhancer, 100-150g of a humectant, 10-15ml of a preservative and the balance of distilled water. According to the external preparation with the analgesic and anti-inflammatory effects, the hydrogel matrix is used as a carrier to be added with the drug composition, so that a drug loading amount of the hydrogel matrix is increased, the transdermal rate of drugs can be improved, and the drugs are transferred in a transdermal drug delivery mode. In addition, the external preparation is convenient to use, the action effect of the drug is improved toa great extent, and meanwhile, the analgesic and anti-inflammatory treatment effect can be obviously improved.
Owner:武汉市蔡氏福宁中草药有限公司
Negative pressure skin penetration administration instrument and using method thereof
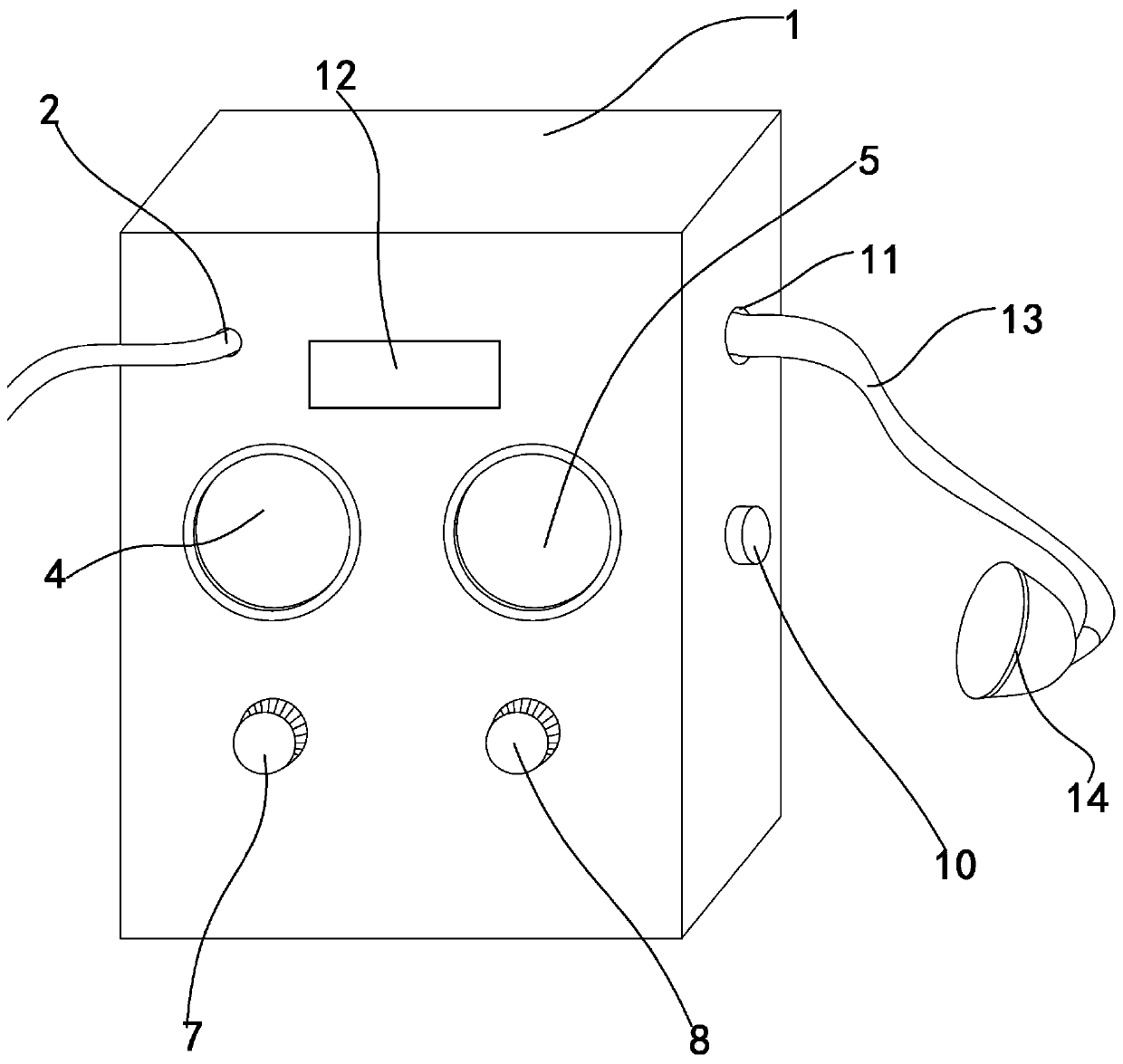
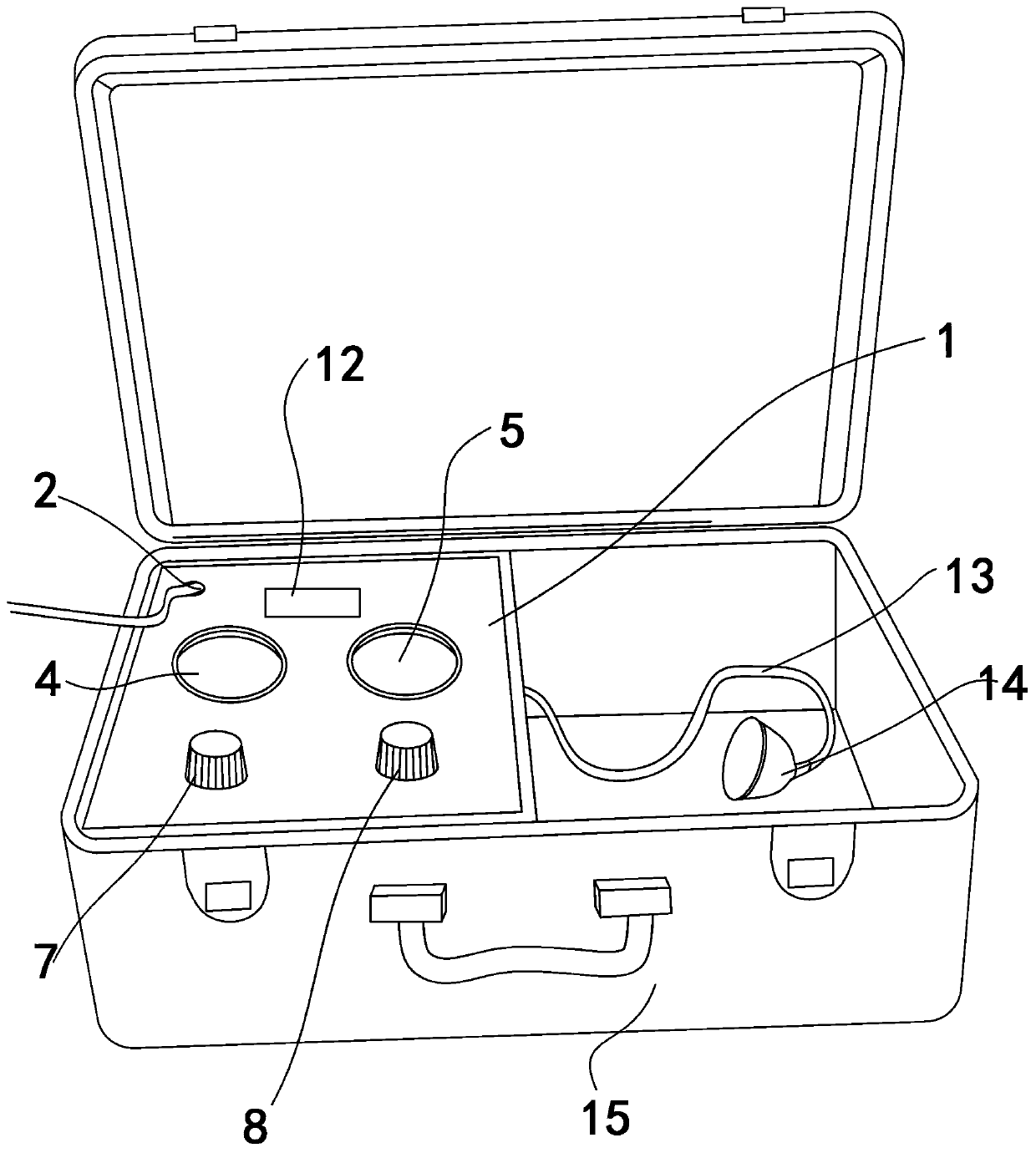
PendingCN110755740APlay a feedback rolePlay the effect of automatic controlMedical devicesVacuum pumpingPharmacy medicine
The invention discloses a negative pressure skin penetration administration instrument and a using method thereof, and relates to the technical field of skin penetration administration. The negative pressure skin penetration administration instrument comprises an administration instrument box body, a power source interface and an external-connected administration end, wherein the power source interface is formed in the administration instrument box body; and the external-connected administration end is connected to the administration instrument box body. The negative pressure skin penetrationadministration instrument also comprises an electricity leak protecting machine, a vacuum display gauge, a time display gauge, a vacuum pump, a vacuity regulating device, a time regulating device, a control circuit board, a vacuum exhaust adjusting valve and a vacuum output opening. According to the negative pressure skin penetration administration instrument disclosed by the invention, the opening and the closing of the vacuum pump and the closing and opening of the vacuum pumping adjusting valve are controlled and adjusted by a time relay through a circuit under the situation of negative pressure, so that the size of negative pressure is adjusted, the form of the skin is continuously changed under the effect of different vacuity, pores and tissue gaps of the skin are continuously enlarged and contracted, the breathe effect of the skin is generated, and medicines can better enter the skin to be absorbed by the human body.
Owner:柴雪青
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com