Eprinomectin transdermal agent, preparation method and application
An ipremectin and ipremectin-containing technology, applied in the field of ipremectin transdermal agents, can solve the problems of unstable blood drug concentration, increased drug content, and decreased milk quality, and achieve no liver first pass Effect, low partition coefficient, small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] An eprimectin transdermal agent, which consists of the following parts by weight of raw materials:
[0043]
[0044] A preparation method of eprimectin transdermal agent, its steps are:
[0045] The first step: sample preparation, each penetration enhancer has five levels (as shown in the table of penetration enhancer levels), at 25℃, according to L 25 (5 6 ) 25 test samples were prepared in the first four columns, the first column is azone (a), the second column is oleic acid (b), the third column is propylene glycol (c), and the fourth column is dimethyl sulfoxide (d ), add four kinds of penetration enhancers, add 0.1 g of eprimectin to each sample, add 95% medical alcohol to make a 5mL sample, and mix the samples evenly.
[0046] Penetration enhancer level table
[0047]
[0048] Step 2: Perform an in vitro skin penetration test. Fix the skin of a Kunming mouse between the diffusion pool and the receiving pool of the YB-P6 smart transdermal tester, with the inner skin facing...
Embodiment 2-6
[0056] An eprimectin transdermal agent, which is composed of the following parts by weight of raw materials (Table 1):
[0057]
[0058] The preparation steps are the same as in Example 1.
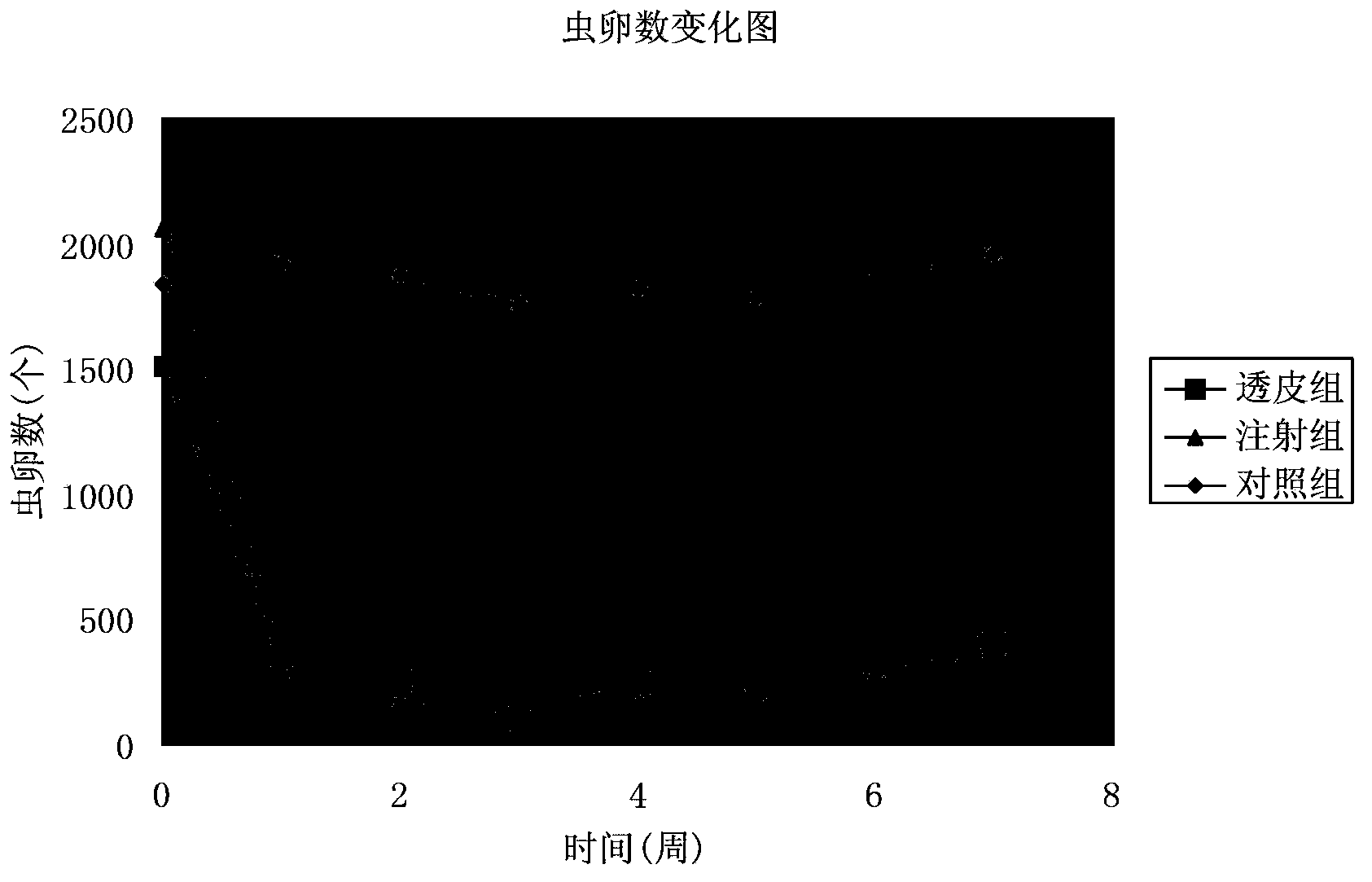
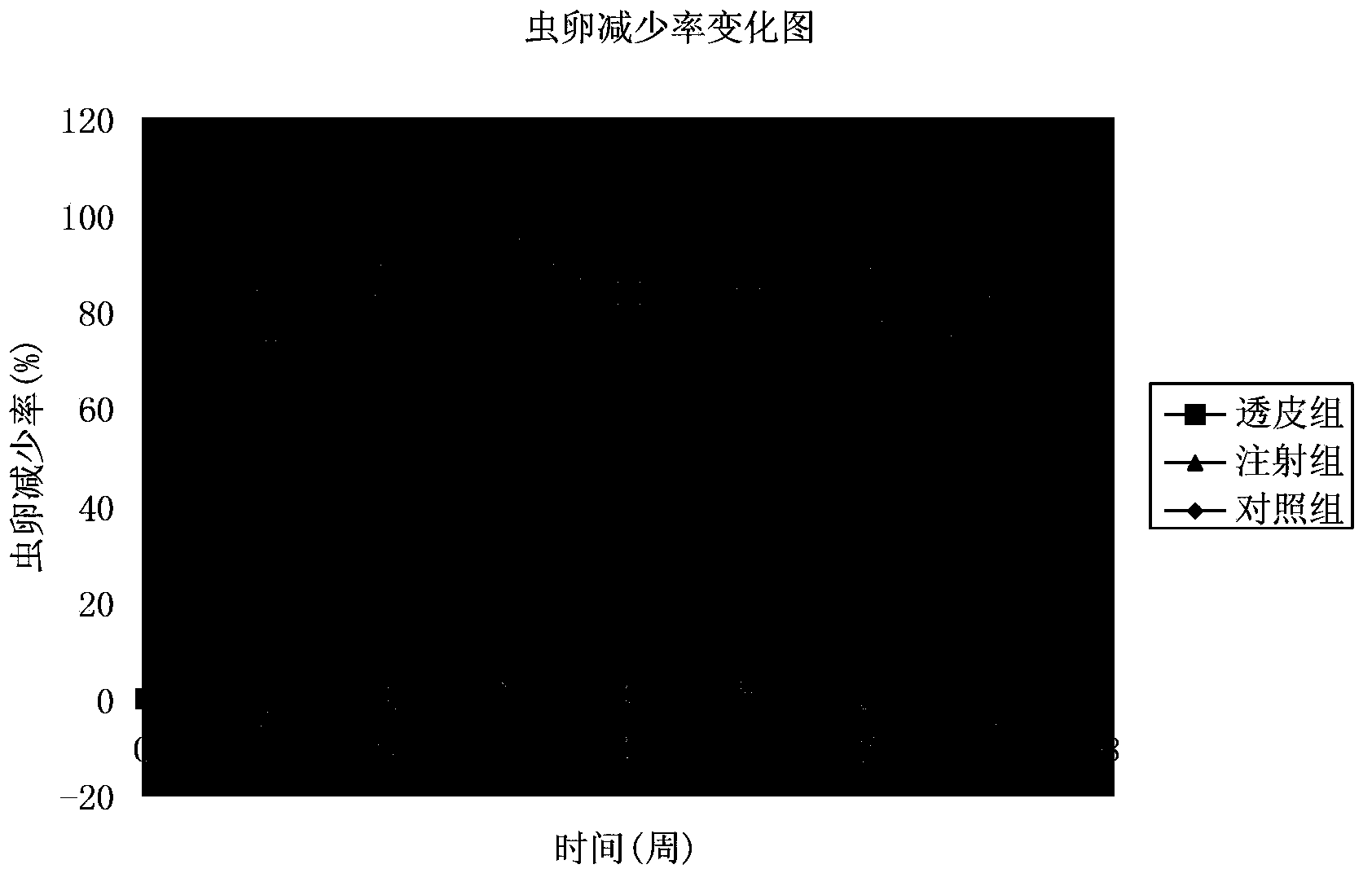
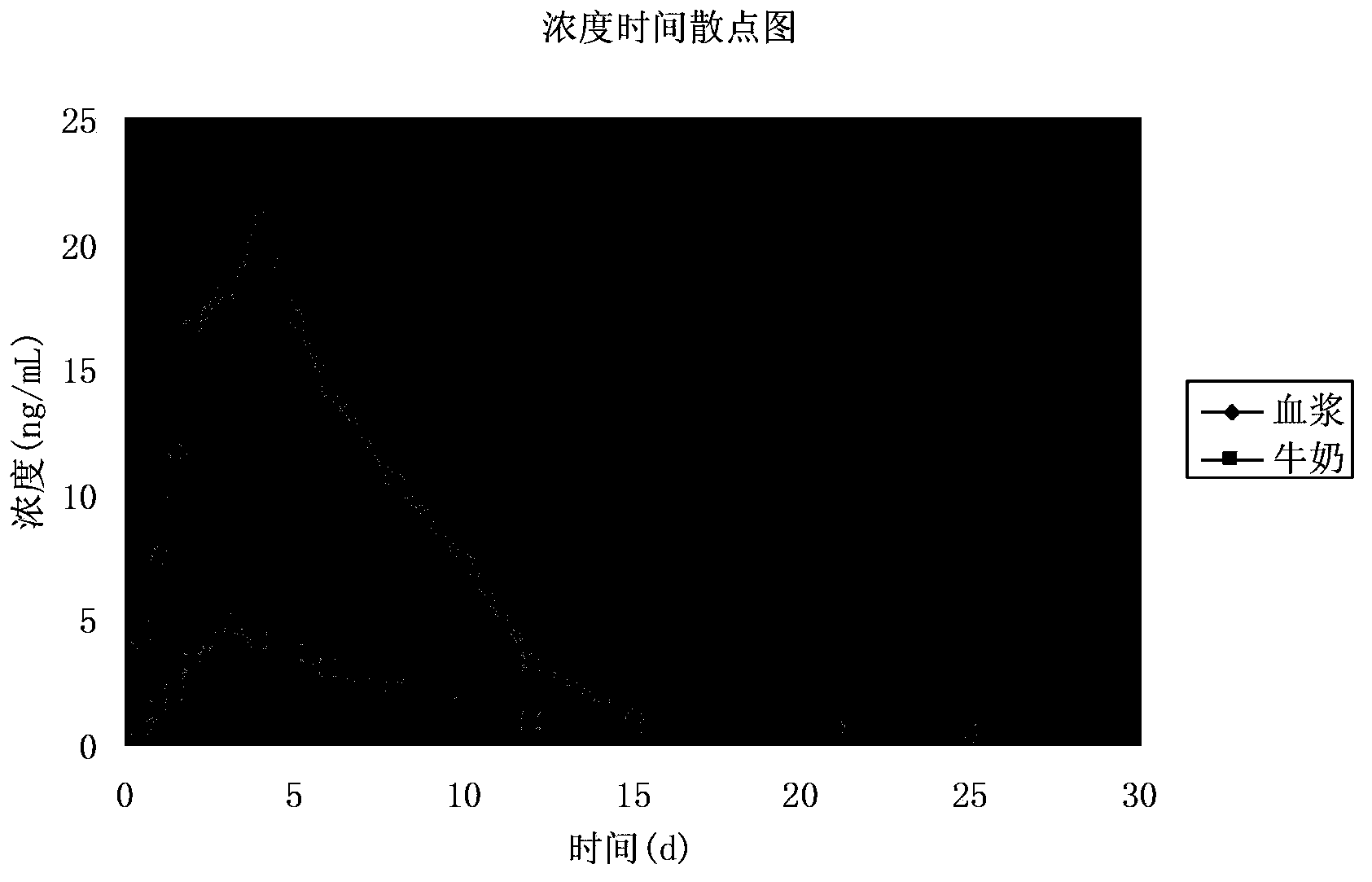
[0059] The application of an eprimectin transdermal agent in the preparation of a medicine for treating or preventing gastrointestinal nematodes in yak, and the application process is:
[0060] Step 1: Test data and results of stability test
[0061] Temperature acceleration test Take 3 batches of samples and place them in a closed and dark container with a temperature of (40±2)℃ and a relative humidity of (75±5)% for 3 months. The three batches of samples decreased by 1.85%, 1.62%, and 2.63% (n=3) respectively after 3 months. There was no layering, precipitation or flocculation in each sample, and they were all slightly yellow and transparent liquids.
[0062] Light acceleration test Place 3 batches of samples in a light box, turn the samples regularly, and place them for 10 days at a temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com